Abstract
Problems associated with bleaching hair include damage to the hair and the pungent smell of ammonium hydroxide. Many consumers dislike the stiffness and smell of bleached hair. In this study, we investigated the suppression of both the damage and pungent smell of bleach by using an aqueous solution of 2-amino-2-methyl-1,3-propanediol (AMPD) as an alkaline agent. The test results focused on scanning electron microscope observations, antioxidant activity and protein loss, and showed that the use of AMPD aqueous solution as an alkaline agent suppressed both hair damage and undesirable odor compared with the use of ammonium hydroxide. AMPD aqueous solution is considered more useful than ammonium hydroxide as an alkaline agent in the hair-bleaching process.
1. Introduction
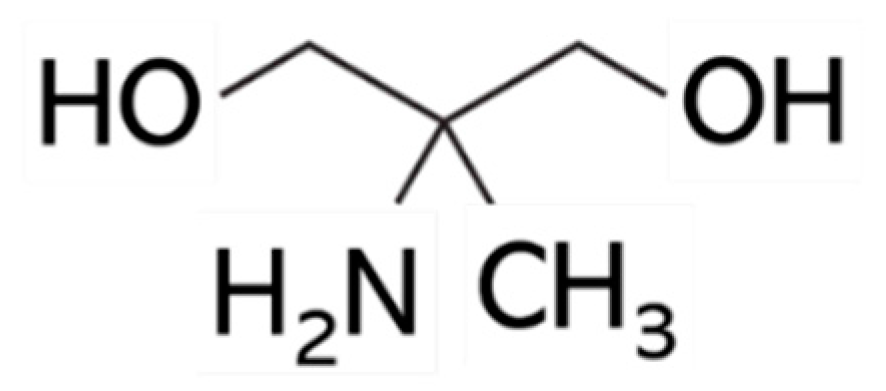
Melanin is a polyphenol-like macromolecule composed primarily of 5,6-dihydroxyindole (DHI) and 5,6-dihydroxyindole-2-carboxylic acid (DHICA) units. Some hair eumelanins contain more DHICA than DHI. On the other hand, blond hair contains pheomelanin (composed of benzothiazine units) rather than dihydroxyindoles [1]. Ordinarily, the removal of melanin coloring from hair through bleaching begins with treatment with an alkaline agent, followed by treatment with an oxidizing agent. In many commercial types of bleach, ammonium hydroxide is used as the alkaline agent, and hydrogen peroxide is employed as the oxidizing agent. The proposed mechanism suggests that under alkaline conditions, hydrogen peroxide produces HOO−, which undergoes a nucleophilic addition to the carbonyl carbon of the catechol in melanin. The melanin catechol is cleaved, and the melanin structure undergoes oxidative degradation [2]. However, the bleaching process damages hair [3,4,5,6,7,8]. The purpose of this study was to evaluate what changes occur in hair damage when ammonium hydroxide, used in the first treatment step, is replaced by another alkaline agent. The present study focused on 2-amino-2-methyl-1,3-propanediol (AMPD; Figure 1) as a replacement for ammonium hydroxide. Hair bleached with AMPD aqueous solution and hydrogen peroxide was evaluated by color analysis, scanning electron microscope observations, oxidation tests, and protein loss tests [9,10,11]. It was noted that hair that was bleached with AMPD aqueous solution showed outstanding decoloration, low antioxidant activity and low protein loss and cuticle damage compared with hair that was bleached using ammonium hydroxide. This suggests that a bleaching process using AMPD aqueous solution minimizes damage to the hair compared with previous ammonium hydroxide-based processes for melanin decoloration. Studies of the hair bleaching effect of hydrogen peroxide using either ammonium hydroxide or AMPD aqueous solution as an alkaline agent demonstrated that AMPD aqueous solution is more useful as an alkaline agent than ammonium hydroxide based on the results of hair color, field emission scanning electron microscope (FE-SEM) observations, antioxidant activity and protein loss.
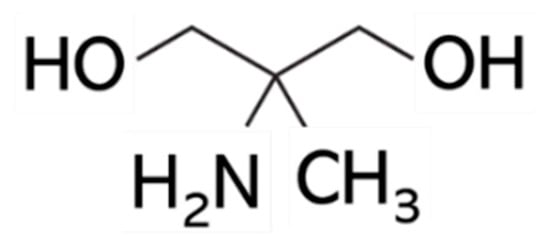
Figure 1.
Chemical structure of 2-amino-2-methyl-1,3-propanediol (AMPD).
AMPD is an aliphatic dihydric alcohol derivative and is used as a pH regulator in cosmetics [12]. It permeates into hair and has a moisturizing effect. In cosmetics, it is included in a variety of products, including toilet water, lotions and sunscreens. It is essentially odorless on its own, and the smell becomes imperceptible when dissolved in water; therefore, it addresses the concerns of customers who dislike pungent smells.
2. Materials and Methods
2.1. Materials
Hydrogen peroxide (35%), ethanol, ammonium hydroxide (28%), hydrochloric acid (20%), l-(+)-ascorbic acid sodium salt, sodium disulfite, 1,1-diphenyl-2-picrylhydrazyl (DPPH), zinc acetate, iron (III) chloride hexahydrate and sodium hydroxide were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). The reagents used were guaranteed reagents of Japanese Industrial Standards (JIS) grade.
2-Amino-2-methyl-1,3-propanediol was purchased from Tokyo Chemical Industry, Ltd. (Tokyo, Japan). N,N-Dimethyl-p-phenylenediamine dihydrochloride was purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA). BCATM Protein Assay Kit—reducing agent-compatible was purchased from Thermo Fisher Scientific Inc. (Waltham, MA, USA). Phosphate-buffered saline (PBS) tablets were purchased from Takara Bio Inc. (Shiga, Japan).
2.2. Comparison of Smell and Human Hair Bleaching Results Using 2-amino-2-methyl-1,3-propanediol (AMPD) Aqueous Solution or Ammonium Hydroxide as an Alkaline Agent
Pungent smell was evaluated in five grades by three expert evaluators. Comparison of human hair bleaching study employed hair from Japanese men in their 20s that had not hitherto been exposed to chemical treatment. The reagents used were 30% hydrogen peroxide, 10% AMPD aqueous solution, and 25% ammonium hydroxide. Decolorization was not complete at 30 and 60 min; therefore, a 90 min experiment was performed. The AMPD aqueous solution and ammonium hydroxide were adjusted to pH 10.75. Samples of 200 mg of hair were weighed out and then immersed for 90 min at room temperature in one of the following solutions: tap water used as a control, a mixed solution of 8% hydrogen peroxide and 2% AMPD, or a mixed solution of 8% hydrogen peroxide and 5% ammonium hydroxide. The hair was then removed from the mixed solutions and thoroughly washed several times in tap water.
2.3. Gloss Meter Measurement of Human Hair Glossiness Following Treatment with AMPD Aqueous Solution or Ammonium Hydroxide as an Alkaline Agent
The gloss meter measures the glossiness of a sample by measuring the quantity of light reflected off the sample. The gloss of a surface can be represented as the quantity of light incident on the surface that is directly reflected. Parallel white light is emitted by the probe head at an angle of 60° to the surface to be measured. A portion of this light is reflected directly at the same angle, and a portion is scattered or reflected after being adsorbed by the surface. The Skin-Glossymeter GL200 (Courage + Khazaka Electronic GmbH, Köln, Germany) measures both directly reflected light (reflection channel) and light from the surface associated with gloss and scattering (diffuse channel). The Skin-Glossymeter GL200 is designed specifically for evaluating the gloss of skin and hair. Although the structure, brightness and color of skin and hair are different, the gloss of different skin types and hair colors can be easily measured with high precision as these differences are accounted for and essentially eliminated by a scattering correction.
2.4. Measurement of the Antioxidant Activity of Human Hair after Using AMPD Aqueous Solution or Ammonium Hydroxide as an Alkaline Agent
Samples of 30 mg of hair added to 300 μL of a 150 μmoL/L DPPH-ethanol solution and 300 µL of pure water, 30 mg of hair added to 300 μL of ethanol and 300 µL of pure water, and 300 µL of pure water added to 300 μL of a 150 μmoL/L DPPH-ethanol solution were prepared, and absorbance (517 nm) measurements were taken after 30 min of treatment using a microplate reader (Multi-Detection Microplate POWERSCAN HT; BioTek, Winooski, VT, USA).
The absorbance of 30 mg of hair added to 300 μL of a 150 μmoL/L DPPH-ethanol solution and 300 µL of pure water was used as the absorbance of each sample solution (A), the absorbance of 30 mg of hair added to 300 μL of ethanol and 300 µL of pure water was used as the absorbance blank of each sample solution (B), and the absorbance of 300 µL of pure water added to 300 μL of a 150 μmoL/L DPPH-ethanol solution was used as the absorbance of the control solution (C). DPPH radical elimination was confirmed based on the deep violet color of DPPH. DPPH radical elimination = {C − (A − B)}/C × 100 (%).
2.5. Determination of Relative Protein Loss from Human Hair on Which AMPD Aqueous Solution or Ammonium Hydroxide was Used as an Alkaline Agent
The protein concentration in the solution of each 100 mg of hair treated with or without an alkaline component was determined. The amount of protein that eluted from the hairs in the solution after the bleaching experiment was measured using a BCA protein assay kit. Reagents A and B were mixed at 50:1, and 1 µL of the post-bleaching solution was added to 99 μL of the mixed solution and incubated at 37 °C for 30 min. Absorbance (562 nm) was measured using the microplate reader to determine the relative quantities.
2.6. Detection of Thiol Group (–SH) Concentration Produced by Disulfide Bond Cleavage in Human Hair after Using AMPD Aqueous Solution or Ammonium Hydroxide as an Alkaline Agent
Each 100 mg hair sample was treated with or without alkaline agents (800 μL). After 24 h, the solutions were recovered and centrifuged for 5 min, and 200 μL of supernatant was collected. Six hundred microliters of 1% aqueous zinc acetate was added, the mixture was centrifuged for 5 min, and the supernatant was discarded. This procedure was used to precipitate the hydrogen sulfide ions as zinc sulfide. One milliliter of pure water was added, and the precipitate was suspended by vortexing and pipetting; it was then centrifuged for 5 min, and the supernatant was discarded. This washing procedure was carried out a total of three times. The supernatant was then discarded, and 200 μL of pure water was added. The precipitate was suspended; 50 μL of a 20 mmoL/L N,N-dimethyl-p-phenylenediamine dihydrochloride solution and 50 μL of a 30 mmoL/L FeCl3 solution were added, followed by vortexing and incubation for 30 min at room temperature. After centrifugation, 100 μL aliquots of the supernatant were added to the wells of three 96-well plates, and the absorbance (665 nm) measurements were taken using the microplate reader. The concentration of hydrogen sulfide ions generated by the reduction of thiol groups (–SH), which were produced by cleavage of disulfide bonds in human hair, was calculated.
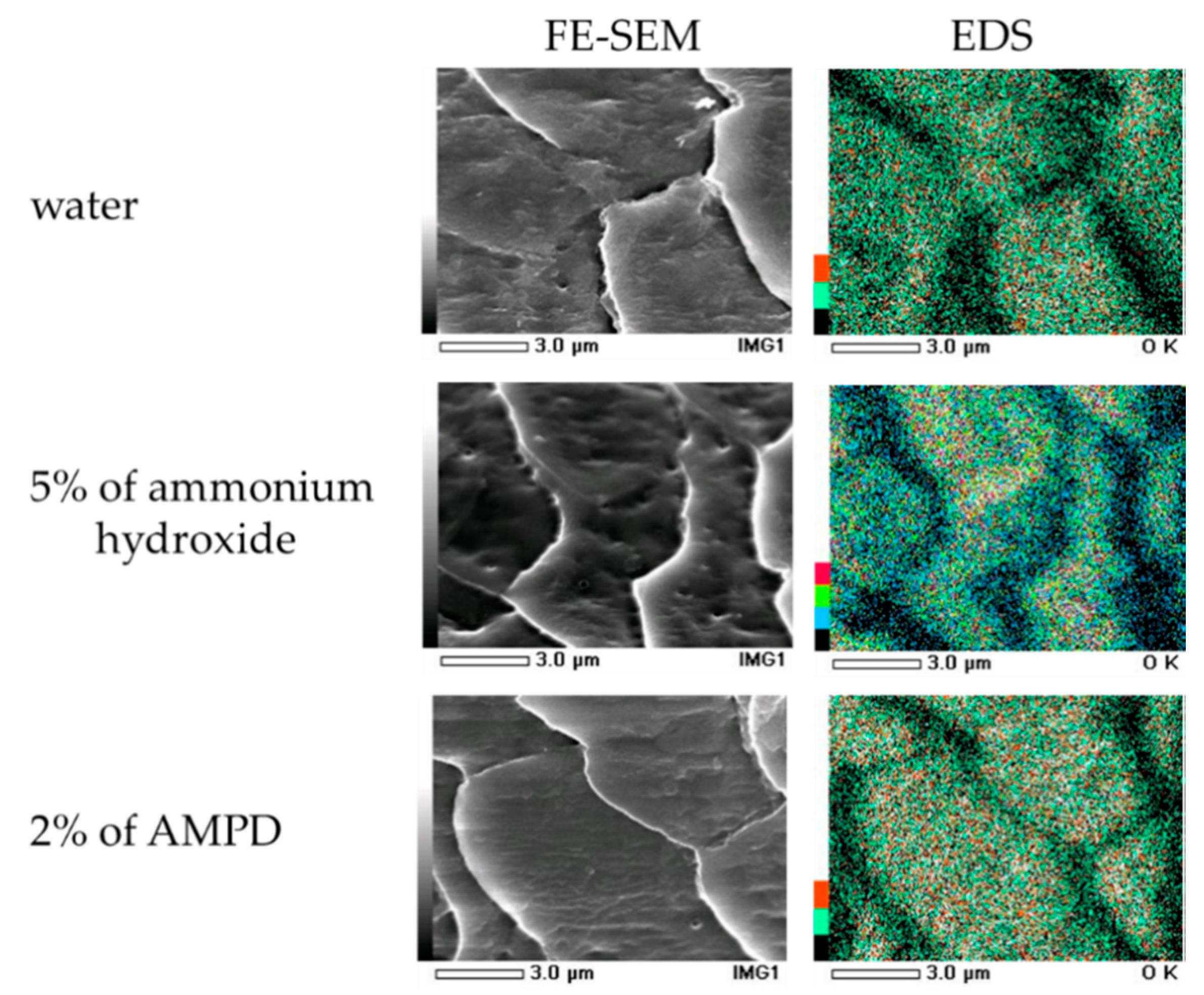
2.7. Field Emission Scanning Electron Microscope (FE-SEM) Observations on Human Hair Following the Use of AMPD Aqueous Solution or Ammonium Hydroxide as an Alkaline Agent
Each hair sample was cut to a length of approximately 2 mm and fixed on the sample stand. Platinum coating was carried out four times at 20 s intervals and was followed by standing for one day under vacuum. The samples were then observed using a field emission scanning electron microscope and energy-dispersive X-ray spectroscopy (EDS, JEOL Ltd., Tokyo, Japan).
2.8. Oxygen Mapping of Human Hair by Energy-dispersive X-ray Spectroscopy (EDS) Following the Use of AMPD Squeous Solution or Ammonium Hydroxide as an Alkaline Agent
Oxygen mapping by EDS was carried out for 70 min on the surface of the hair samples produced for FE-SEM.
2.9. Statistical Analysis
Mean values and standard deviations were calculated, and unpaired t-tests were carried out using Microsoft Excel. Evaluation: +: p < 0.1 was taken as a tendency; *: p < 0.05, **: p < 0.01, ***: p < 0.001, and ***: p < 0.0001 were taken to be significant differences.
3. Results
3.1. Comparison of Smell and Appearance When AMPD Aqueous Solution or Ammonium Hydroxide Was Used as an Alkaline Agent for Dyeing Human Hair
The odor of ammonia was apparent when ammonium hydroxide was used as an alkaline agent, but when AMPD aqueous solution was used, there was no noticeable smell. The appearance was also transparent.
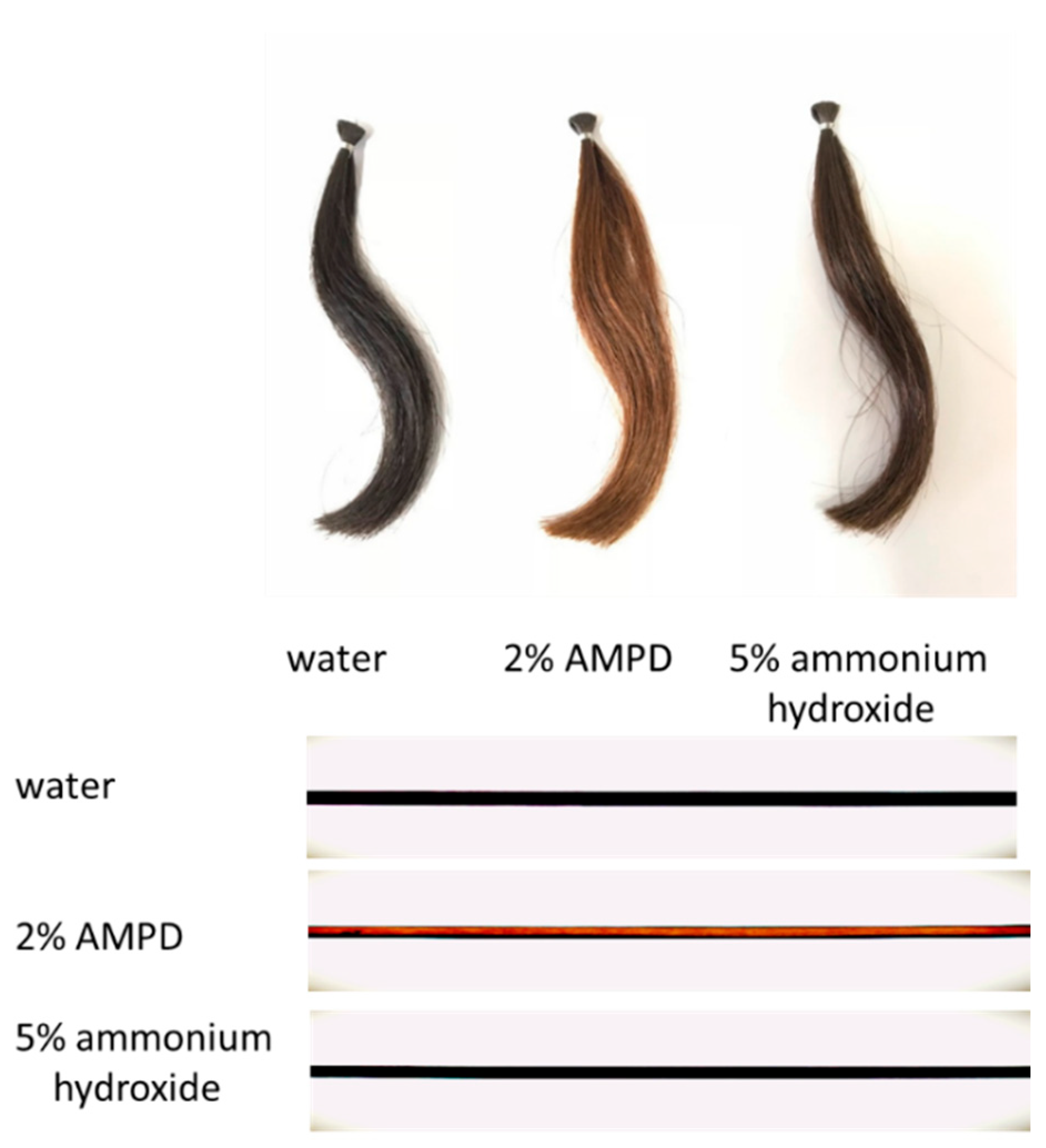
Figure 2 shows images of a bundle of human hair immersed in pure water (control), a bundle of human hair bleached with H2O2 and AMPD aqueous solution, and a bundle of human hair bleached with H2O2 and ammonium hydroxide. The various images were taken with a digital camera, a BX51 upright optical microscope (Olympus Corp., Tokyo, Japan) and BP72 microscope digital camera (Olympus Corp., Tokyo, Japan). It is evident that the hair bundle bleached using AMPD aqueous solution as the alkaline agent shows more intense decoloration than the hair bundle bleached using ammonium hydroxide.
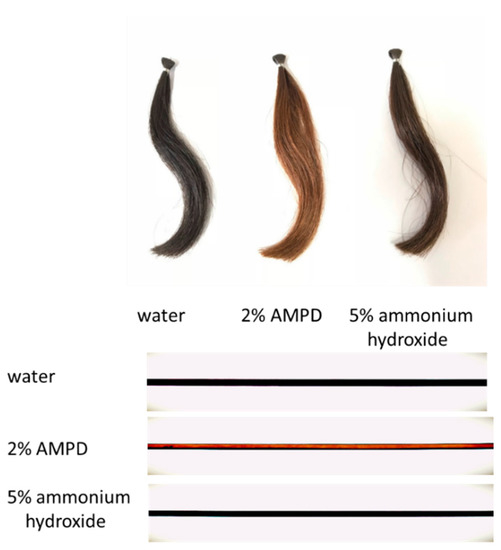
Figure 2.
Images of human hair bleached with H2O2 and 2% AMPD aqueous solution and human hair bleached with H2O2 and 5% ammonium hydroxide. The experimental conditions are described in Section 2.2. (Above) Images taken with a digital camera of same person’s hairs; (Below) images taken with an optical microscope. Top: human hair bundle immersed in pure water (control); middle: human hair bundle bleached with H2O2 and AMPD aqueous solution; bottom: human hair bundle bleached with H2O2 and ammonium hydroxide.
3.2. Comparison of Gloss Meter Measurements of Human Hair after Using AMPD Aqueous Solution or Ammonium Hydroxide as an Alkaline Agent
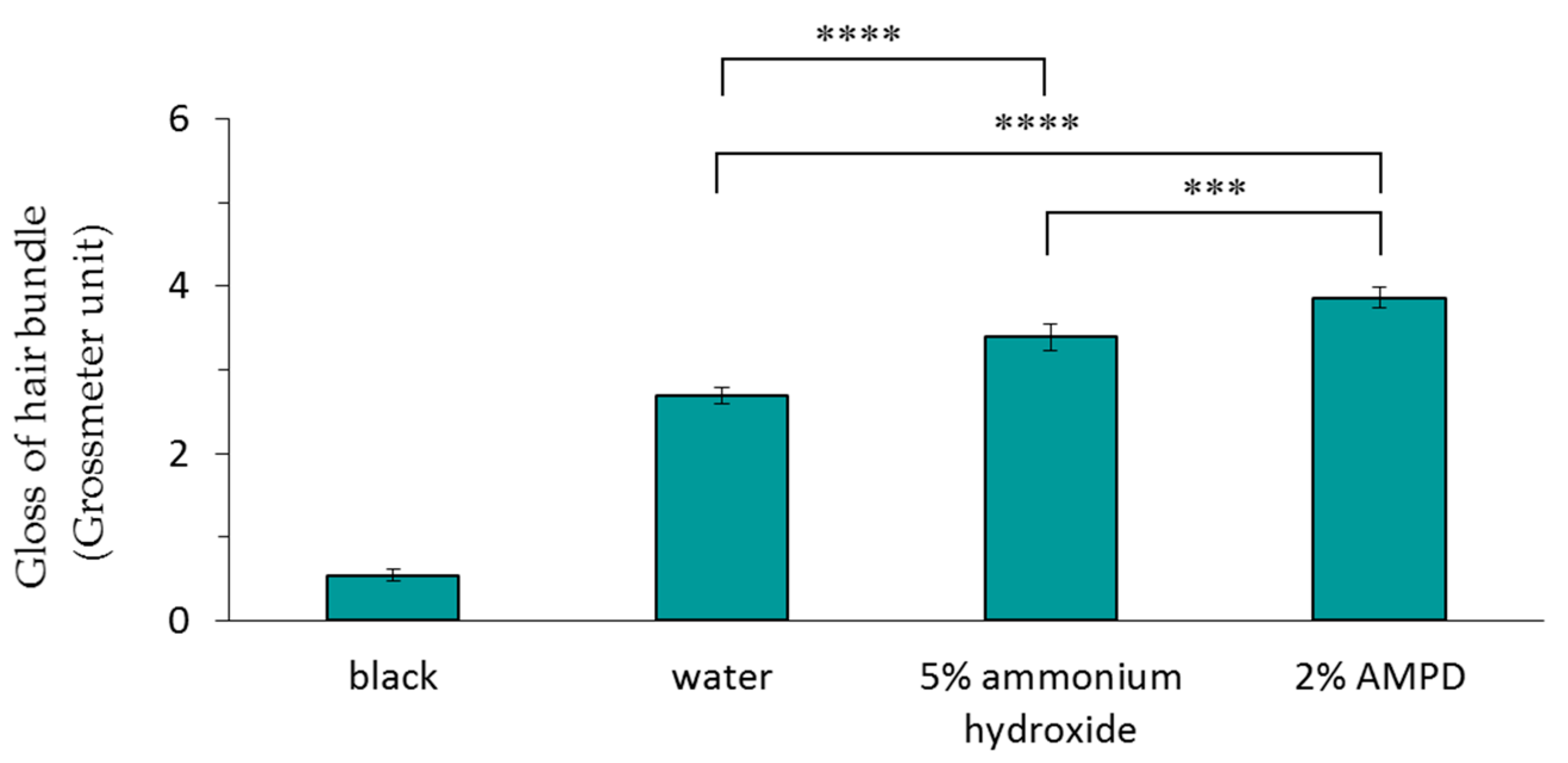
Figure 3 shows the results of the Glossymeter measurements (quantity of directly reflected light) of a human hair bundle immersed in pure water (control), a human hair bundle bleached with H2O2 and AMPD aqueous solution, and a human hair bundle bleached with H2O2 and ammonium hydroxide. The gloss measurement was higher for hair bleached using AMPD aqueous solution than for hair bleached using ammonium hydroxide.
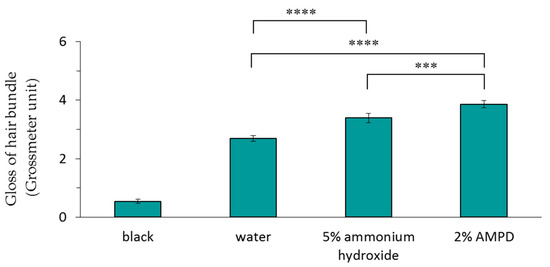
Figure 3.
Comparison of the gloss measurements of a human hair bundle immersed in pure water (control), a human hair bundle bleached with H2O2 and 5% ammonium hydroxide, and a human hair bundle bleached with H2O2 and 2% AMPD aqueous solution. The results are expressed as the mean ± standard deviation of five experiments. *** p < 0.001, **** p < 0.0001.
3.3. Comparison of the Antioxidant Activity of Human Hair Following Treatment with AMPD Aqueous Solution or Ammonium Hydroxide as an Alkaline Agent
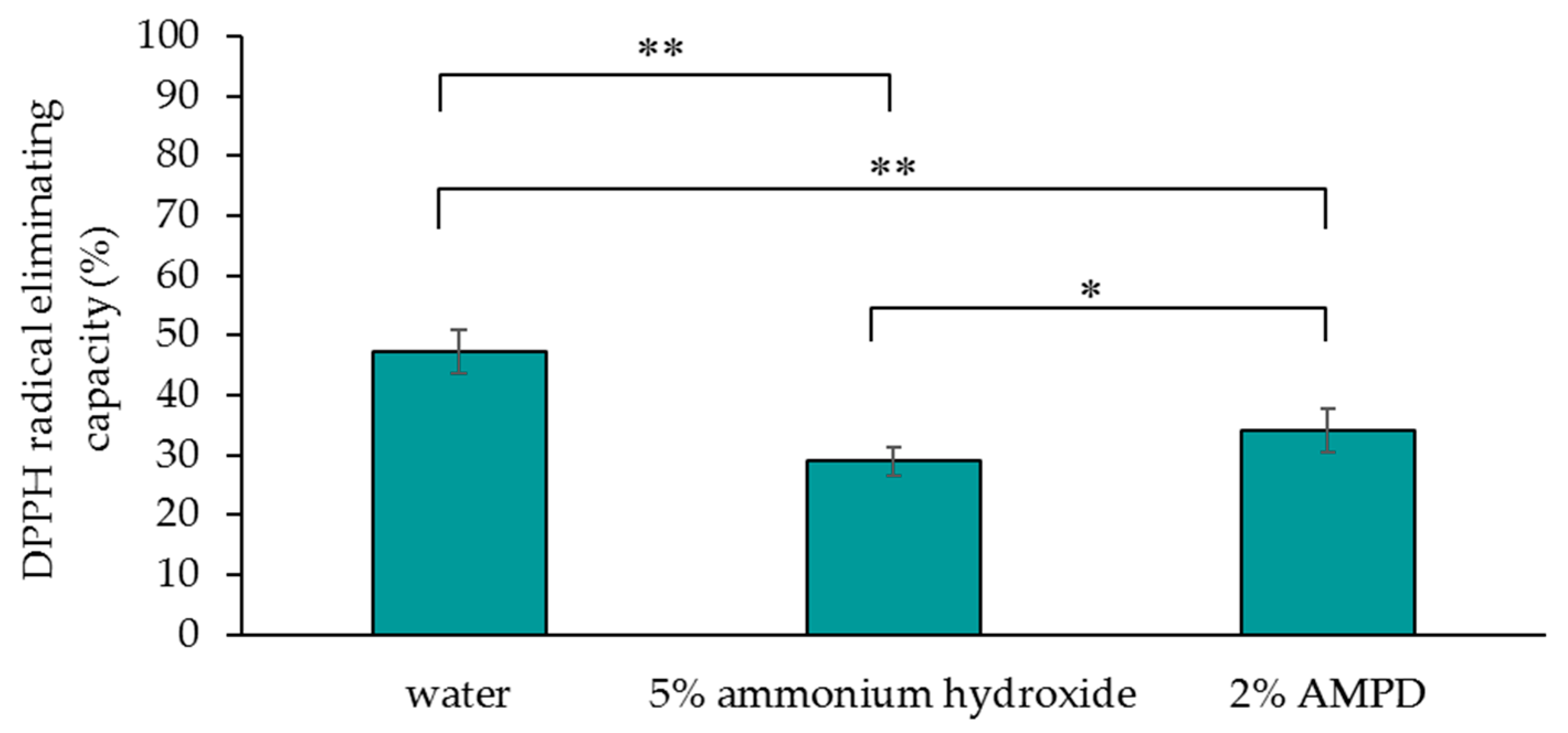
Figure 4 shows the DPPH radical-eliminating capacity of a human hair bundle immersed in pure water (control), a human hair bundle bleached with H2O2 and AMPD aqueous solution, and a human hair bundle bleached with H2O2 and ammonium hydroxide. The DPPH radical-eliminating capacity of the control was 47.3%, the DPPH radical-eliminating capacity of hair treated with AMPD aqueous solution as an alkaline agent was 34.0%, and the DPPH radical-eliminating capacity of hair treated with ammonium hydroxide was 29.0% (Figure 4). It is evident that hair bleached using AMPD had a greater DPPH radical-eliminating capacity than hair bleached using ammonium hydroxide. Thus, use of AMPD as an alkaline agent caused less of a decrease than ammonium hydroxide in the antioxidant activity of bleached hair.
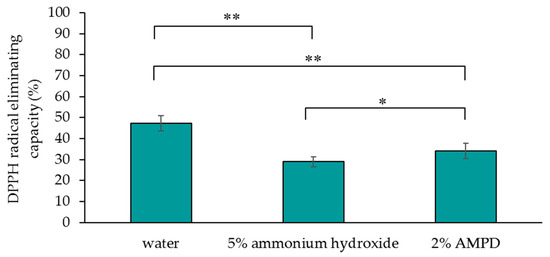
Figure 4.
Comparison of the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical-eliminating capacity of human hair immersed in pure water (control), human hair bleached with H2O2 and 5% ammonium hydroxide, and human hair bleached with H2O2 and 2% AMPD aqueous solution. The results are expressed as the mean ± standard deviation of fifteen experiments. * p < 0.05, ** p < 0.01.
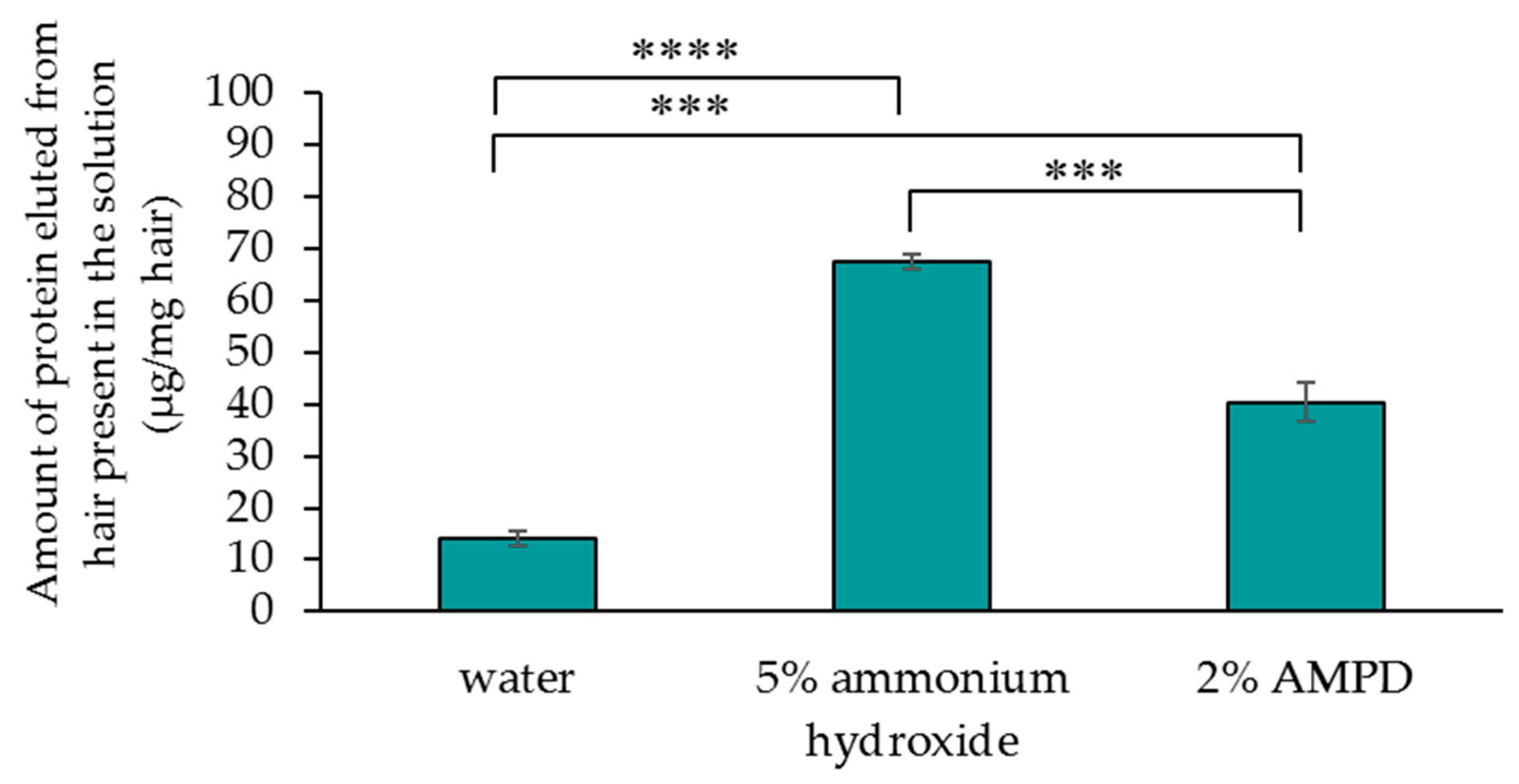
3.4. Comparison of Quantities of Protein Lost From Human Hair after Treatment with AMPD Aqueous Solution or Ammonium Hydroxide as an Alkaline Agent
Figure 5 compares the quantities of protein lost from human hair immersed in pure water (control), human hair bleached with H2O2 and AMPD aqueous solution, and human hair bleached with H2O2 and ammonium hydroxide. After bleaching human hair using ammonium hydroxide, the quantity of protein eluted from the hair in the solution was 67.4 μg/mg hair, while the quantity of protein eluted from the hair present in the solution after using AMPD aqueous solution was 40.4 μg/mg hair. Protein loss was halved by using AMPD aqueous solution as an alkaline agent compared with the use of ammonium hydroxide.
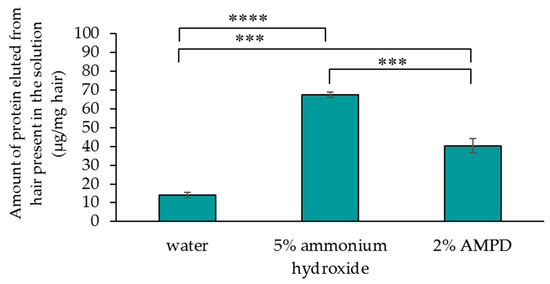
Figure 5.
Determination of quantities of protein lost from human hair after using 5% ammonium hydroxide or 2% AMPD aqueous solution as an alkaline agent. The results are expressed as the mean ± standard deviation of three experiments. *** p < 0.001, **** p < 0.0001.
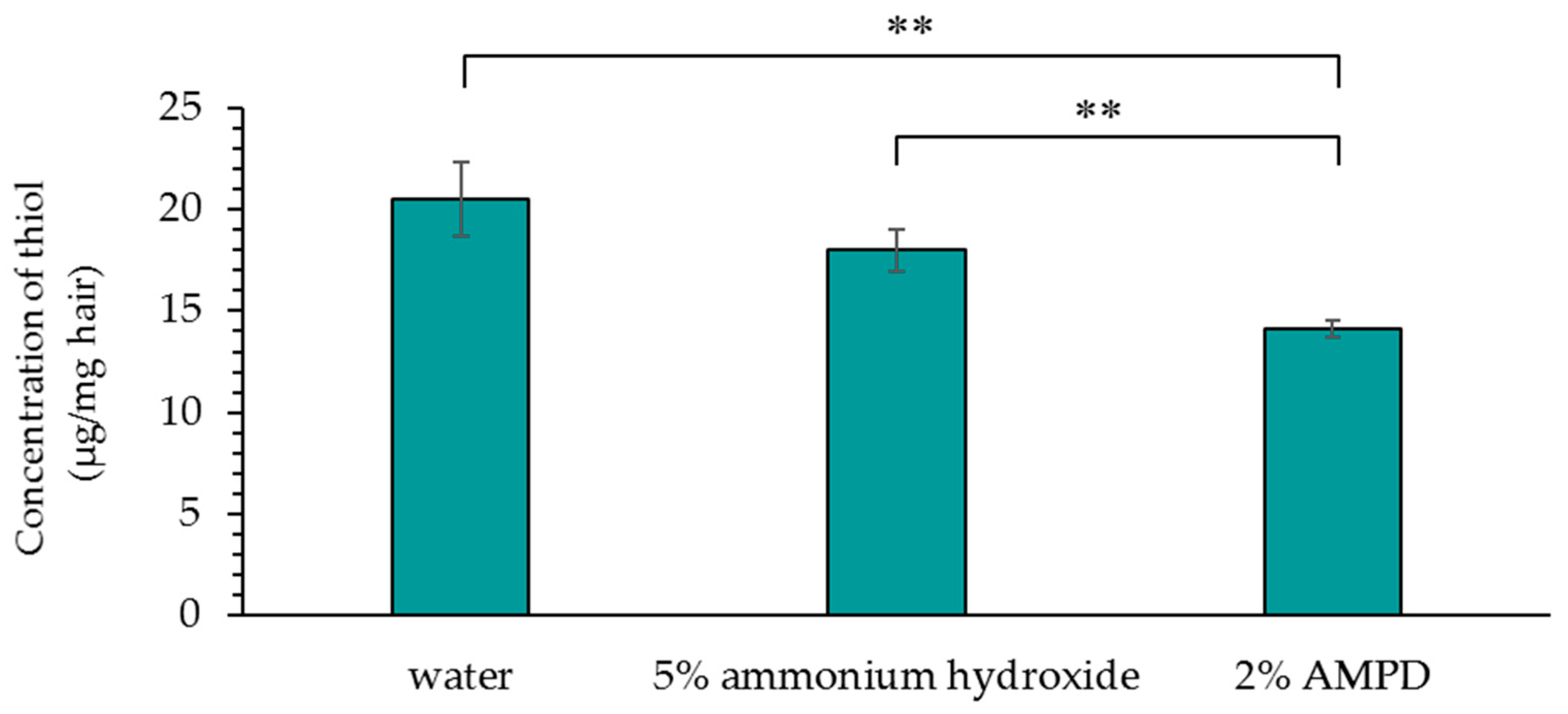
3.5. Concentration of Thiol Groups (-SH) Produced by Disulfide Bond Cleavage in Human Hair after Treatment with AMPD Aqueous Solution or Ammonium Hydroxide as an Alkaline Agent
Figure 6 shows a comparison of the concentration of thiol groups (–SH) produced by cleavage of disulfide bonds in human hair immersed in pure water, human hair bleached with H2O2 and AMPD aqueous solution, and human hair bleached with H2O2 and ammonium hydroxide. The concentration of thiol groups (–SH) produced by the cleavage of disulfide bonds in AMPD-treated hair was lower than the concentration of thiol groups (–SH) produced by disulfide bond cleavage in ammonium hydroxide-treated hair.
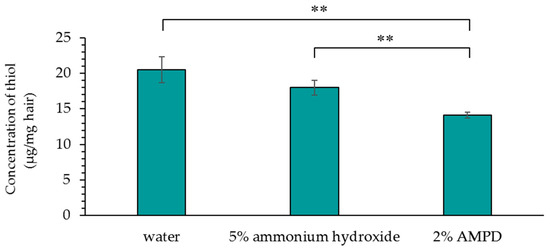
Figure 6.
The concentration of thiol groups (−SH) produced by cleavage of disulfide bonds in human hair after using 5% ammonium hydroxide or 2% AMPD aqueous solution as an alkaline agent. The results are expressed as the mean ± standard deviation of three experiments. ** p < 0.01 vs. ammonium hydroxide.
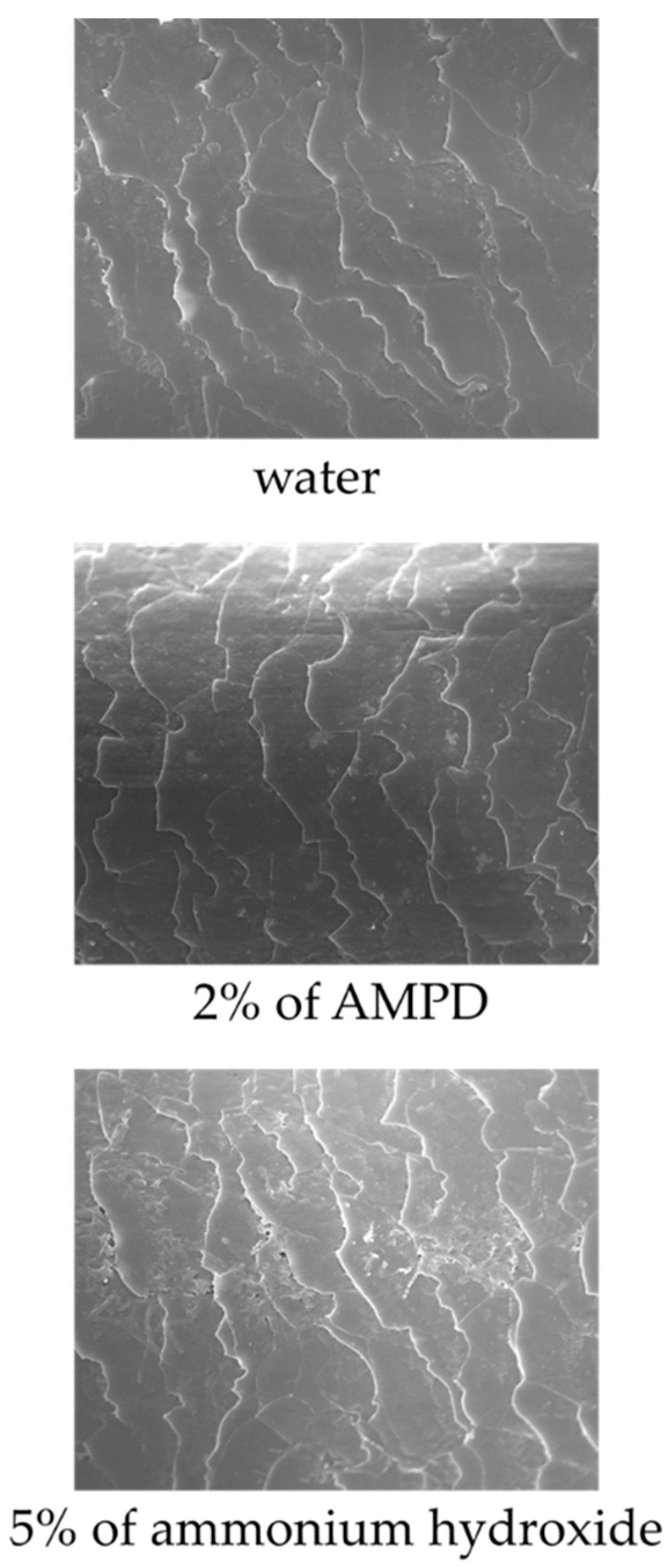
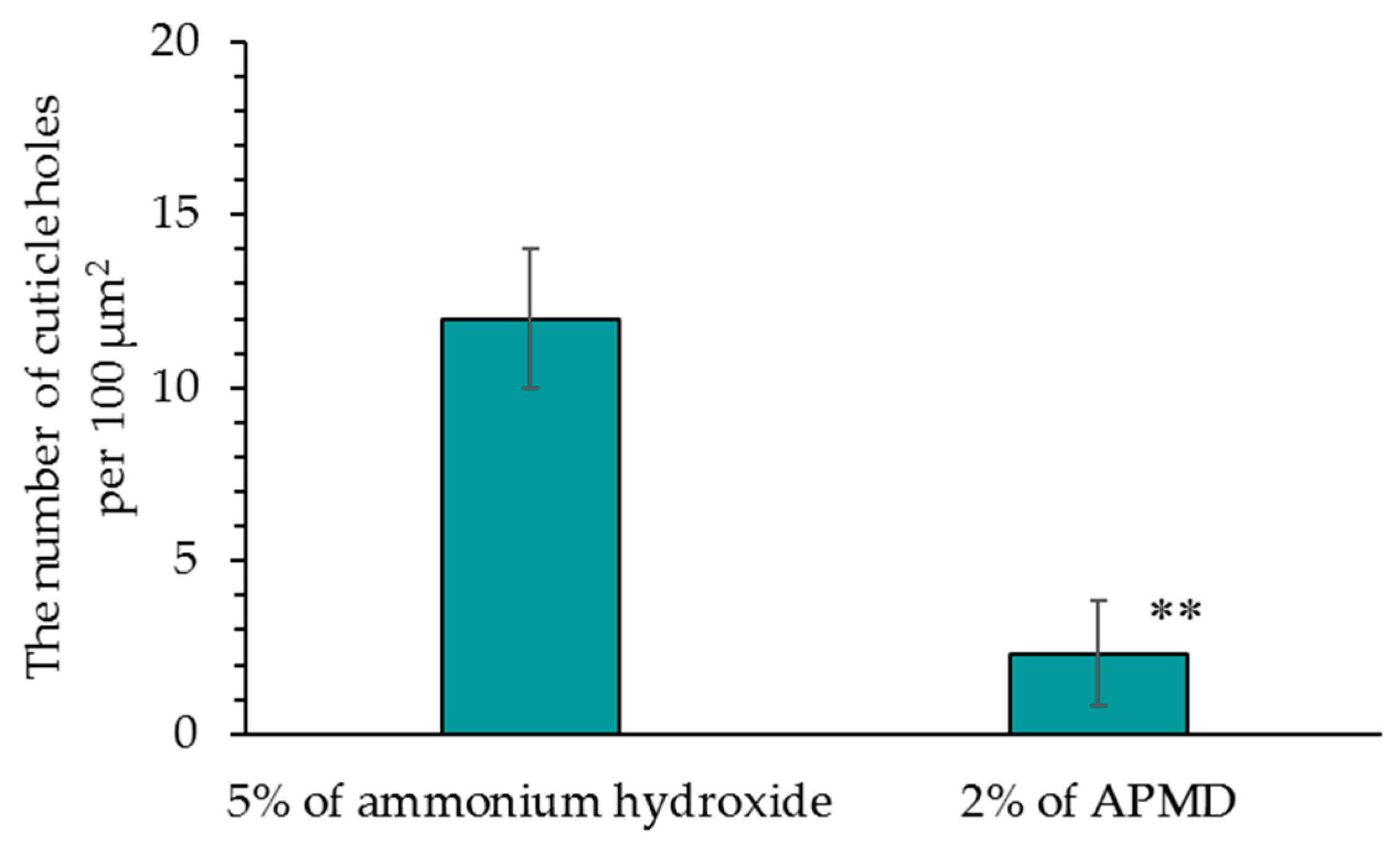
3.6. Field Emission Scanning Electron Microscope (FE-SEM) Observations
Figure 7 shows images of the hair surface obtained by FE-SEM at ×10,000 magnification. Surface unevenness and the number of cuticle holes decreased in hair bleached with AMPD aqueous solution compared with hair bleached using ammonium hydroxide. Unevenness and curling of the cuticle edge were also suppressed. Figure 8 presents a graph with the calculated number of cuticle holes per 100 μm2. The number of cuticle holes observed in the surfaces of human hair was significantly less after using AMPD aqueous solution as an alkaline agent than when using ammonium hydroxide.
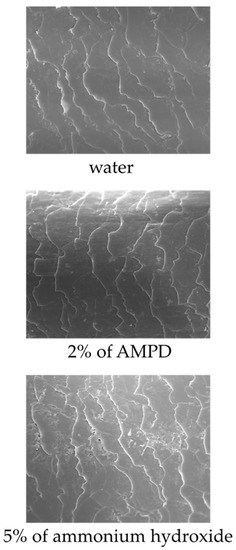
Figure 7.
FE-SEM observations of the surface of human hair after using ammonium hydroxide or AMPD aqueous solution as an alkaline agent (×2000). Top: the surface of human hair immersed in pure water; middle: the surface of human hair after using 2% AMPD aqueous solution as an alkaline agent; bottom: the surface of human hair after using 5% ammonium hydroxide as an alkaline agent.
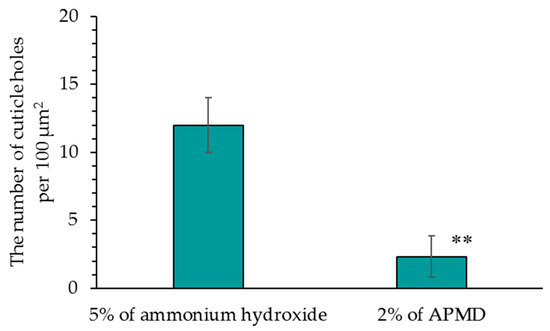
Figure 8.
Comparison of the number of cuticle holes in FE-SEM images (×10,000). The results are expressed as the mean ± standard deviation of three experiments. ** p < 0.01 vs. ammonium hydroxide.
3.7. Oxygen Mapping of Human Hair after Using AMPD Aqueous Solution or Ammonium Hydroxide as an Alkaline Agent Using EDS
The results of EDS-mediated oxygen mapping in human hair after using AMPD aqueous solution or ammonium hydroxide as an alkaline agent are shown in Figure 9. When human hair was immersed in pure water, oxygen atoms were segregated around the surface cuticle layer. The oxygen atoms on the surface of the hair bleached using AMPD aqueous solution were present over the entire cuticle, whereas when ammonium hydroxide was used, the oxygen atoms were localized at the cuticle edge.
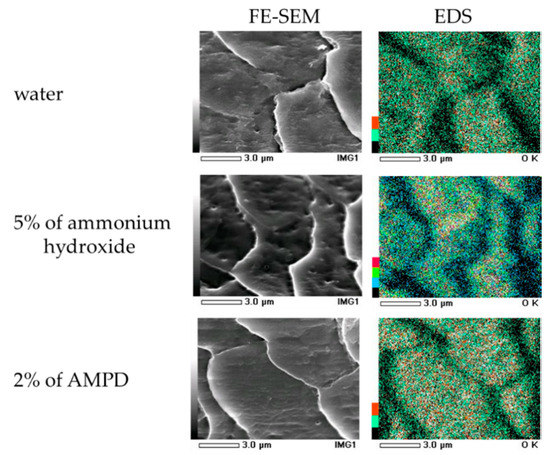
Figure 9.
Oxygen mapping by EDS on the surface of human hair following treatment with 2% AMPD aqueous solution or 5% ammonium hydroxide as an alkaline agent. Strength is in order of strong, red, green, blue, black.
4. Discussion
Compared with hair that was bleached using ammonium hydroxide as an alkaline agent, hair that was bleached using AMPD aqueous solution exhibited improved bleaching properties, decreased antioxidant activity, lower protein loss and less cuticle damage. This suggests that a bleaching process using AMPD aqueous solution suppresses damage to the hair when compared with the conventional process for melanin decoloration that uses ammonium hydroxide as an alkaline agent.
Concentration measurements of thiol groups (–SH) produced by cleavage at identical pH revealed that lower concentrations were present with the use of AMPD aqueous solution than when ammonium hydroxide was used. This suggests that disulfide bonds in hair are cleaved when bleached using an alkaline agent. It is thought that cysteine thiol groups contribute to the formation of hair by forming disulfide bonds and crosslinking keratin protein chains. Therefore, the fact that the concentration of thiol groups (–SH) produced by disulfide bond cleavage in hair was lower when using AMPD aqueous solution than when using ammonium hydroxide in the bleaching process suggests that the effect on crosslinking between keratin protein chains is lower with AMPD than with ammonium hydroxide. This theory is supported by the observed maintenance of hair shape, suppression of cuticle hole formation, and lower protein loss. Additionally, the results of oxygen mapping by EDS indicated that oxygen atoms were present on the surface of the entire cuticle of hair bleached using AMPD aqueous solution, whereas when ammonium hydroxide was used, oxygen atoms were localized at the cuticle edge; therefore, hydrogen peroxide coats the surface of the hair more thoroughly and remains for longer periods of time when AMPD aqueous solution is used in place of ammonium hydroxide in the bleaching process. This is thought to be why the effect of bleaching is stronger using AMPD aqueous solution than when using ammonium hydroxide. AMPD promotes decomposition of desmosome [13] and has a 1,3-propanediol structure, promotes osmosis and has a moisturizing effect on hair. Desmogleins are important proteins in the hair follicle [14]. Adjusting the pH to 8 using aminomethyl propanediol (AMPD) promotes the decomposition of desmogleins by stimulating the activity of exfoliation enzymes, such as kallikrein 5 and 7, which are optimal at this pH [13]. However, AMPD had no effect on the decomposition of desmogleins during bleaching because the pH is 10.75 in a bleaching solution. AMPD is a substituted aliphatic alcohol that functions as a pH adjuster in cosmetic products at concentrations less than 10%. The dermal toxicity data that are presented demonstrate, for example, that mascara with 1.92% AMPD does not cause dermal irritation or allergic contact sensitization, suggesting that the maximum reported concentration of 2% AMPD in mascara would be safe for use [12]. Therefore, AMPD can be expected to have a high affinity for hair, and the bleaching effect of the alkali/hydrogen peroxide can be expected to be persistent. The number of bleaching sessions and the bleach processing times using hydrogen peroxide and AMPD aqueous solutions are topics to be studied in the future.
5. Conclusions
The results of this study indicated that in terms of perceived chemical odor, bleaching effect on hair, scanning electron microscope observations of cuticle damage, antioxidant activity of hair and protein loss, AMPD aqueous solution is considered to be more useful than ammonium hydroxide as an alkaline agent.
Author Contributions
K.I. and K.M. designed the study and performed the experiments and data analysis. K.I. and K.M. interpreted the data and drafted the manuscript. K.M. supervised the study and critically revised the manuscript. All authors read and approved the final manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Borges, C.R.; Roberts, J.C.; Wilkins, D.G.; Rollins, D.E. Relationship of melanin degradation products to actual melanin content: Application to human hair. Anal. Biochem. 2001, 290, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Sawaki, Y.; Foote, C.F. Mechanism of carbon-carbon cleavage of cyclic 1,2-diketones with alkaline hydrogen peroxide. The acyclic mechanism and its application to the basic autoxidation of pyrogallol. J. Am. Chem. Soc. 1983, 105, 5035–5040. [Google Scholar] [CrossRef]
- Inoue, T.; Ito, M.; Kizawa, K. Labile proteins accumulated in damaged hair upon permanent waving and bleaching treatments. J. Cosmet. Sci. 2002, 53, 337–344. [Google Scholar] [PubMed]
- Hessefort, Y.; Holland, B.T.; Cloud, R.W. True porosity measurement of hair: A new way to study hair damage mechanisms. J. Cosmet. Sci. 2008, 59, 303–315. [Google Scholar] [PubMed]
- Jeong, M.S.; Lee, C.M.; Jeong, W.J.; Kim, S.J.; Lee, K.Y. Significant damage of the skin and hair following hair bleaching. J. Dermatol. 2010, 37, 882–887. [Google Scholar] [CrossRef] [PubMed]
- Imai, T. The influence of hair bleach on the ultrastructure of human hair with special reference to hair damage. Okajimas Folia Anat. Jpn. 2011, 88, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dyer, J.M.; Bringans, S.D.; Bryson, W.G. Determination of photo-oxidation products within photoyellowed bleached wool proteins. Photochem. Photobiol. 2006, 82, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.L.; Nunes, A.S.; Gesztesi, J.L. Protein loss quantification of abraded virgin and abraded bleached hair according to Bradford assay. J. Cosmet. Sci. 2004, 55 (Suppl. 2), S175–S179. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.M.; Davis, M.G.; Lucas, R.L.; Reilman, R.; Styczynski, P.B.; Li, C.; Mamak, M.; McComb, D.W.; Williams, R.E.; Godfrey, S.; et al. Preserving fibre health: Reducing oxidative stress throughout the life of the hair fibre. Int. J. Cosmet. Sci. 2015, 37 (Suppl. 2), 16–24. [Google Scholar] [CrossRef] [PubMed]
- França-Stefoni, S.A.; Dario, M.F.; Sá-Dias, T.C.; Bedin, V.; Almeida, A.J.; Baby, A.R.; Velasco, M.V. Protein loss in human hair from combination straightening and coloring treatments. J. Cosmet. Dermatol. 2015, 14, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, N.S.; Davis, J.; Compton, R.G. Analytical strategies for the detection of sulfide: A review. Talanta 2000, 52, 771–784. [Google Scholar] [CrossRef]
- Burnett, C.L.; Bergfeld, W.F.; Belsito, D.V.; Klaassen, C.D.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; Andersen, F.A. Final amended report on safety assessment on aminomethyl propanol and aminomethyl propanediol. Int. J. Toxicol. 2009, 28, 141–161. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Katsuta, Y. Composition for External USE. U.S. Patent 2003/0152599 A1, 14 August 2003. [Google Scholar]
- Kljuic, A.; Bazzi, H.; Sundberg, J.P.; Martinez-Mir, A.; O’Shaughnessy, R.; Mahoney, M.G.; Levy, M.; Montagutelli, X.; Ahmad, W.; Aita, V.M.; et al. Desmoglein 4 in hair follicle differentiation and epidermal adhesion: Evidence from inherited hypotrichosis and acquired pemphigus vulgaris. Cell 2003, 113, 49–60. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).