Efficiency of Nisin as Preservative in Cosmetics and Topical Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms Used
2.2. Tested Product
2.3. In Vitro Sensitivity Test by Disk Diffusion Method
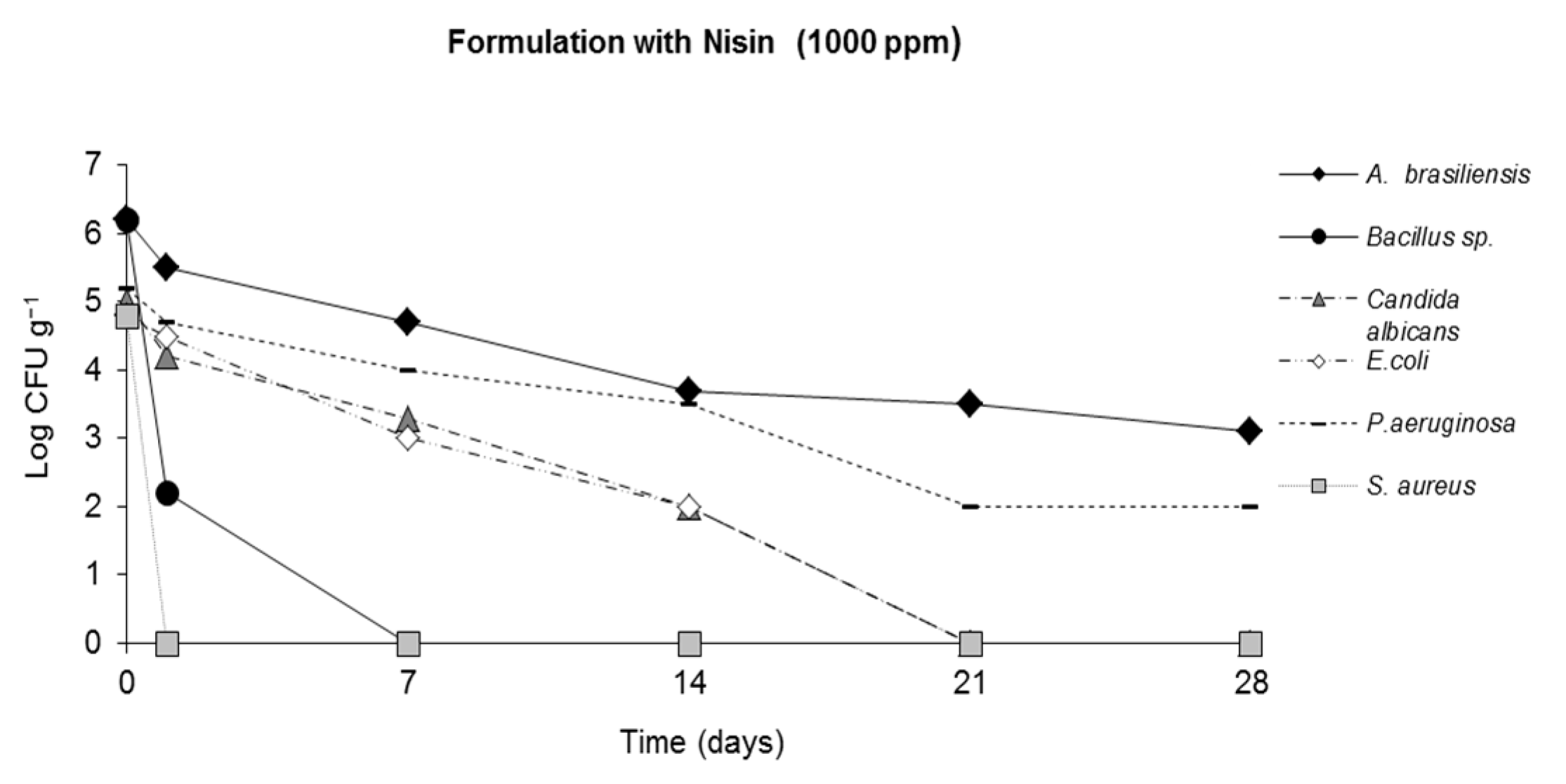
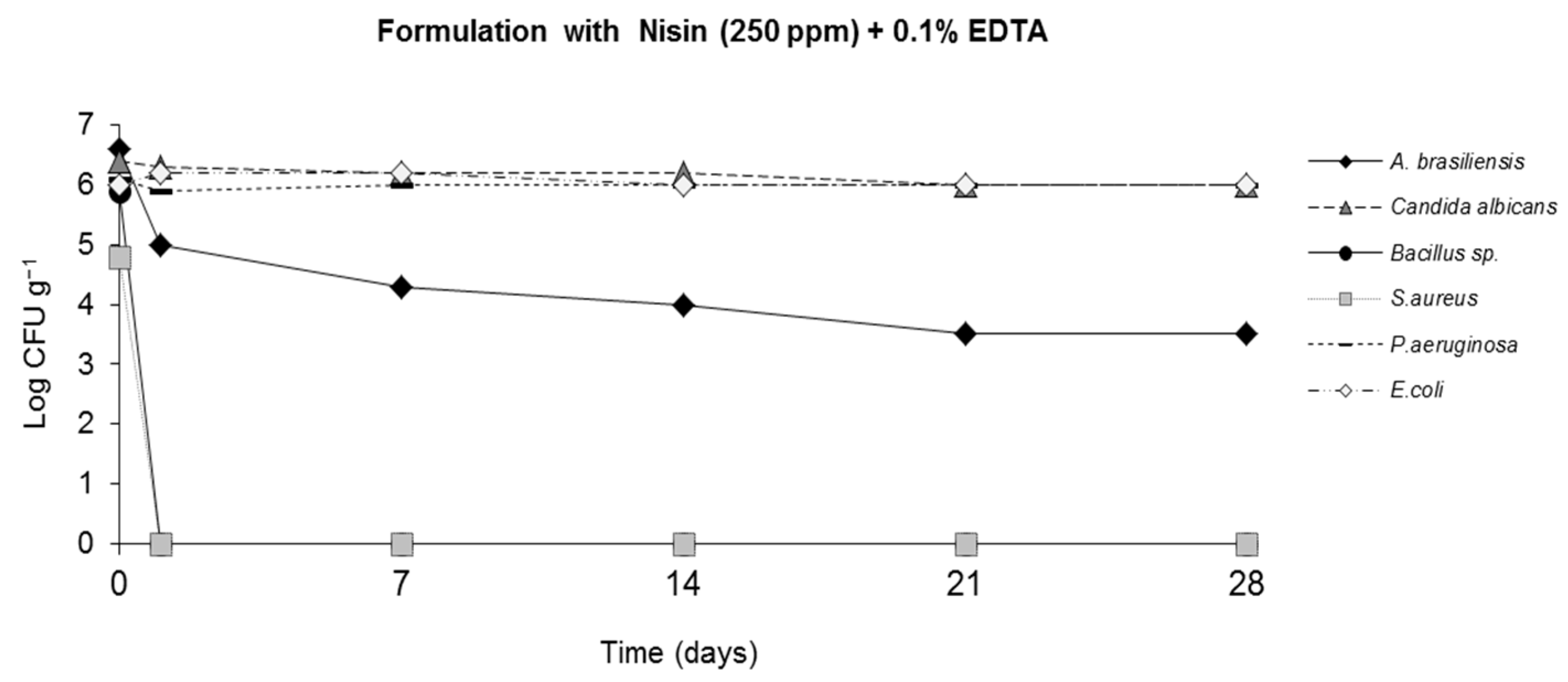
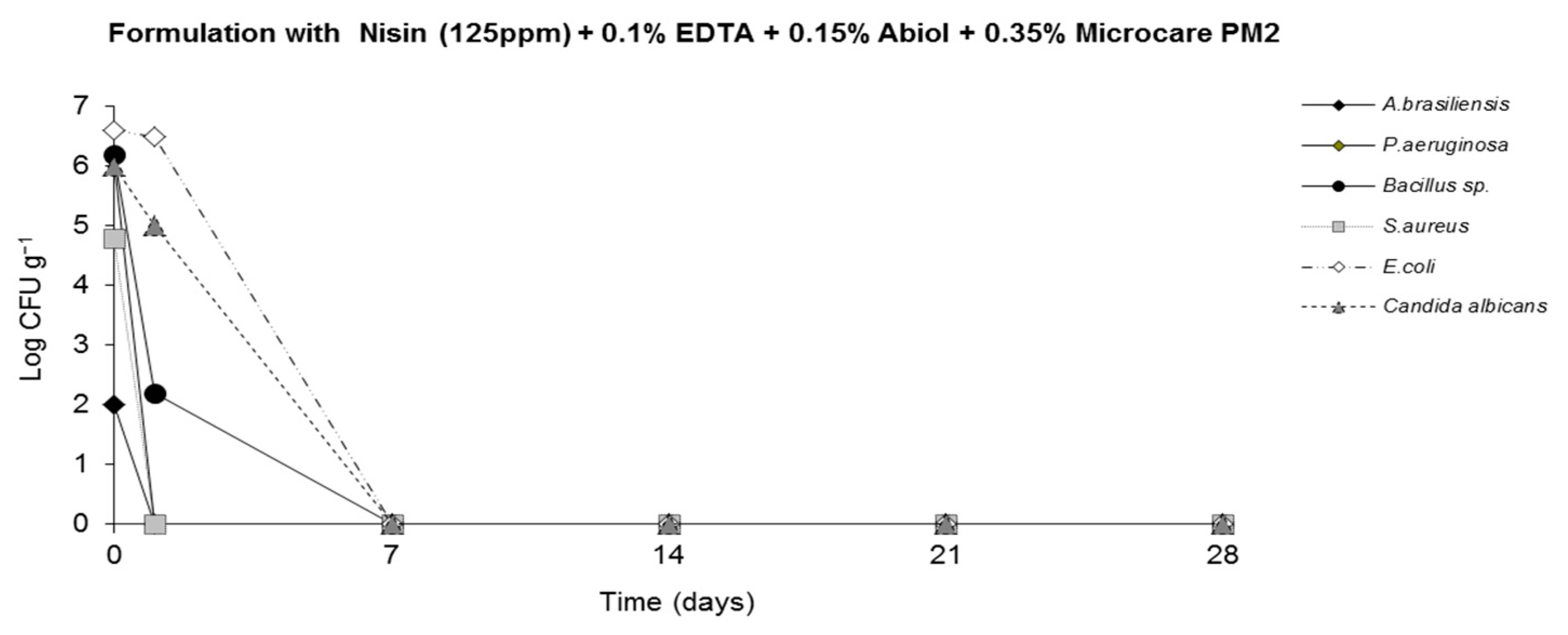
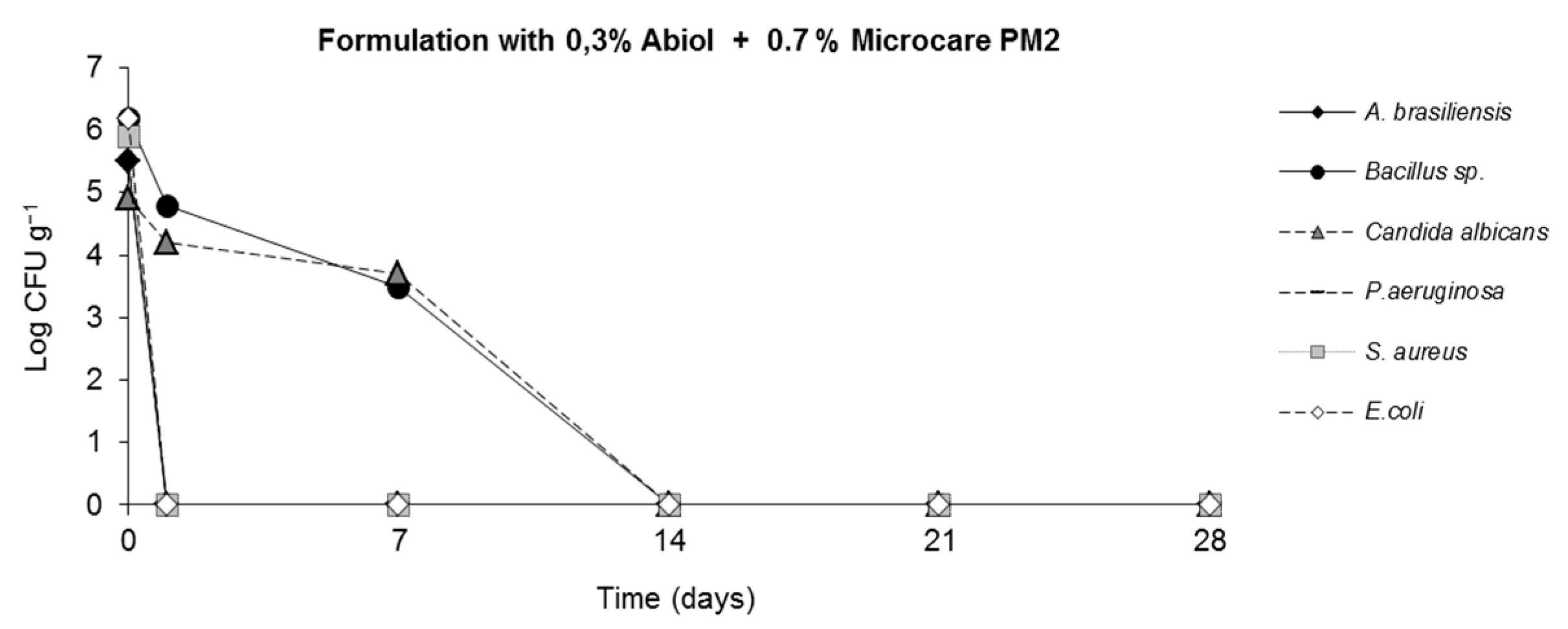
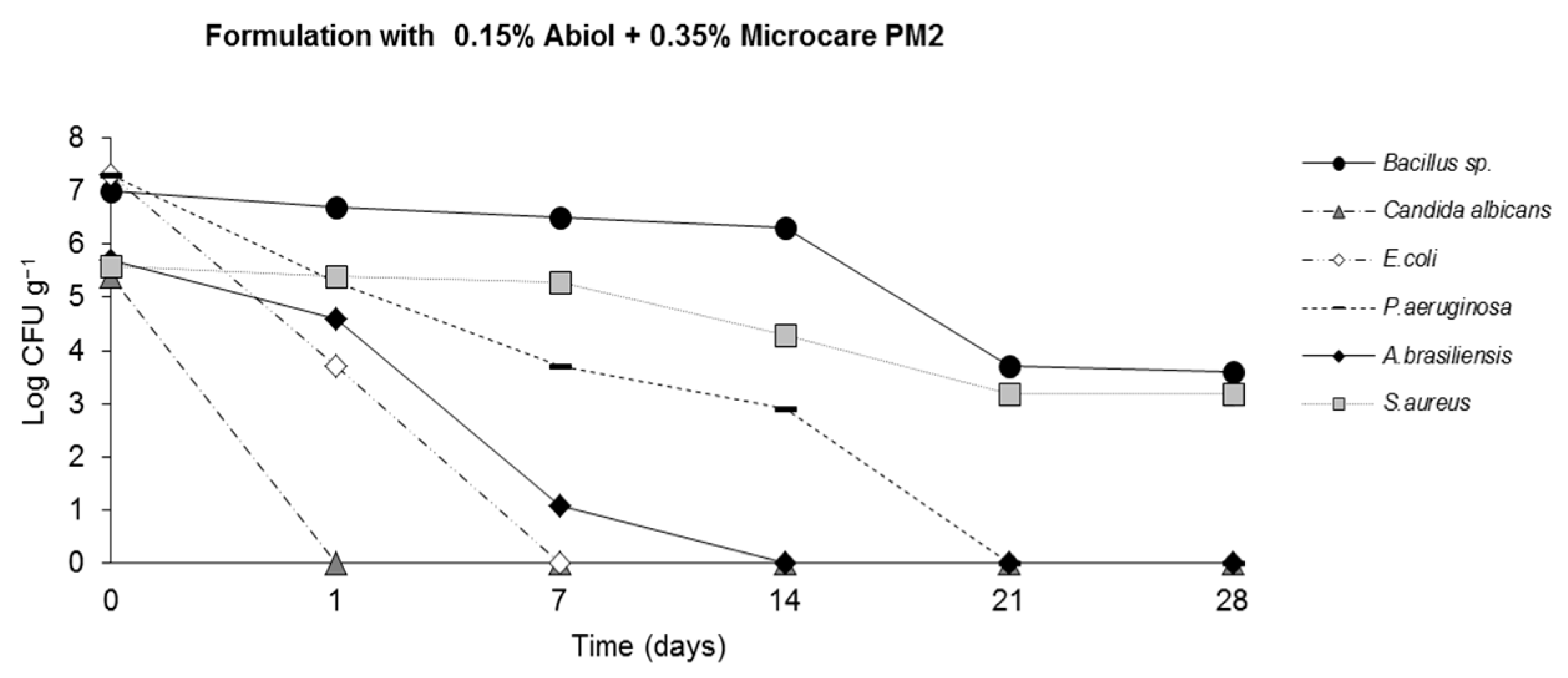
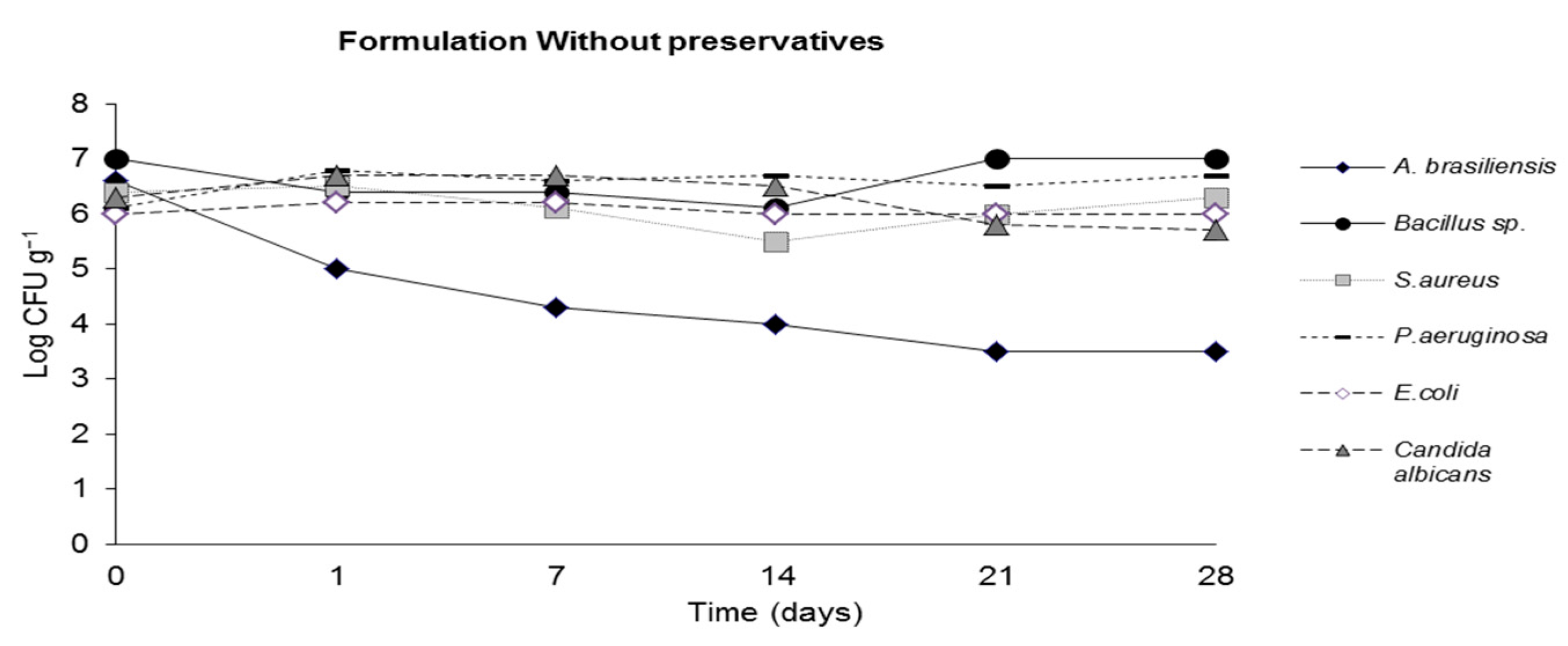
2.4. Challenge Test (ISO 11930 (2012))
3. Results
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- De Groot, A.C.; Bruynzeel, D.P.; Bos, J.D.; van der Meeren, H.L.; van Joost, T.; Jagtman, B.A.; Weyland, J.W. The allergens in cosmetics. Arch. Dermatol. 1988, 124, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Lundov, M.D.; Moesby, L.; Zachariae, C.; Johansen, J.D. Contamination versus preservation of cosmetics: A review on legislation, usage, infections, and contact allergy. Contact Dermat. 2009, 60, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Zacharof, M.P.; Lovitt, R.W. Bacteriocins produced by lactic acid bacteria a review article. APCBEE Procedia 2012, 2, 50–56. [Google Scholar] [CrossRef]
- Campana, R.; Scesa, C.; Patrone, V.; Vittoria, E.; Baffone, W. Microbiological study of cosmetic products during their use by consumers: Health risk and efficacy of preservative systems. Lett. Appl. Microbiol. 2006, 43, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Lundov, M.D.; Johansen, J.D.; Zachariae, C.; Moesby, L. Low-level efficacy of cosmetic preservatives. Int. J. Cosmet. Sci. 2011, 33, 190–196. [Google Scholar] [CrossRef] [PubMed]
- De Arauz, L.J.; Jozala, A.F.; Mazzola, P.G.; Penna, T.C.V. Nisin biotechnological production and application: A review. Trends Food Sci. Technol. 2009, 20, 146–154. [Google Scholar] [CrossRef]
- Sinclair, D. Naturals vs. synthetics in preservatives-the debate continues: Preservatives: Debate. S. Afr. Pharm. Cosmet. Rev. 2010, 37, 30–32. [Google Scholar]
- Nawal, H.B. Study of Preservative Effect of “Propolis” on the Storage Quality of Mashed Potatoes. Food Sci. Technol. Int. 2013, 1, 17–20. [Google Scholar] [CrossRef]
- Varvaresou, A.; Papageorgiou, S.; Tsirivas, E.; Protopapa, E.; Kintziou, H.; Kefala, V.; Demetzos, C. Self-preserving cosmetics. Int. J. Cosmet. Sci. 2009, 31, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Gwak, J.W.; Kamarajan, P.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. Biomedical applications of nisin. J. Appl. Microbiol. 2016, 120, 1449–1465. [Google Scholar] [CrossRef] [PubMed]
- Breukink, E.; de Kruijff, B. The lantibiotic nisin, a special case or not? BBA-Biomembr. 1999, 1462, 223–234. [Google Scholar] [CrossRef]
- Cheigh, C.I.; Pyun, Y.R. Nisin biosynthesis and its properties. Biotechnol. Lett. 2005, 27, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Heng, N.C.; Wescombe, P.A.; Burton, J.P.; Jack, R.W.; Tagg, J.R. The diversity of bacteriocins in Gram-positive bacteria. In Bacteriocins; Riley, M.A., Chavan, M.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 45–92. ISBN 978-3-540-36603-4. [Google Scholar]
- Stevens, K.A.; Sheldon, B.W.; Klapes, N.A.; Klaenhammer, T.R. Nisin treatment for inactivation of Salmonella species and other gram-negative bacteria. Appl. Environ. Microb. 1991, 57, 3613–3615. [Google Scholar]
- Piper, C.; Hill, C.; Cotter, P.D.; Ross, R.P. Bioengineering of a Nisin A-producing Lactococcus lactis to create isogenic strains producing the natural variants Nisin F, Q and Z. Microb. Biotechnol. 2011, 4, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Field, D.; Begley, M.; O’Connor, P.M.; Daly, K.M.; Hugenholtz, F.; Cotter, P.D.; Ross, R.P. Bioengineered nisin A derivatives with enhanced activity against both Gram positive and Gram negative pathogens. PLoS ONE 2012, 7, e46884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Safety Evaluation of Certain Food Additives and Contaminants; Prepared by the Seventy-Seventh Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); WHO Food Additives Series; WHO: Geneva, Switzerland, 2013; Volume 68, pp. 91–114. ISBN 92-41-660562. [Google Scholar]
- Food and Drug Administration. Nisin preparation: Affirmation of GRAS status as a direct human food ingredient. Fed. Regist. 1988, 53, 11247. [Google Scholar]
- Gharsallaoui, A.; Oulahal, N.; Joly, C.; Degraeve, P. Nisin as a food preservative: Part 1: Physicochemical properties, antimicrobial activity, and main uses. Crit. Rev. Food Sci. 2016, 56, 1262–1274. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food on a request from the Commission related to the use of nisin (E 234) as a food additive. EFSA J. 2006, 314, 1–16. [Google Scholar] [CrossRef]
- Penna, T.C.V.; Jozala, A.F.; De Lencastre Novaes, L.C.; Pessoa, A.; Cholewa, O. Production of nisin by Lactococcus lactis in media with skimmed milk. Appl. Biochem. Biotechnol. 2005, 122, 619–637. [Google Scholar] [CrossRef]
- Parente, E.; Ricciardi, A. Production, recovery and purification of bacteriocins from lactic acid bacteria. Appl. Microbiol. Biot. 1999, 52, 628–638. [Google Scholar] [CrossRef]
- Deegan, L.H.; Cotter, P.D.; Hill, C.; Ross, P. Bacteriocins: Biological tools for bio-preservation and shelf-life extension. Int. Dairy J. 2006, 16, 1058–1071. [Google Scholar] [CrossRef]
- International Organization for Standardization. Cosmetics—Microbiology—Evaluation of the Antimicrobial Protection of a Cosmetic Product; ISO 11930; International Organization for Standardization: Geneva, Switzerland, 2012. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved standard M2-A10; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009. [Google Scholar]
- Clinical and Laboratory Standards Institute. Method for Antifungal Disk Diffusion Susceptibility Testing of Nondermatophyte Filamentous Fungi: Approved Guideline; Document M51-A; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2010. [Google Scholar]
- Clinical and Laboratory Standards Institute. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts: Approved Standard; M44-A; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2004. [Google Scholar]
- Alani, J.I.; Davis, M.D.P.; Yiannias, J.A. Allergy to Cosmetics. Dermatitis 2013, 24, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Yim, E.; Baquerizo Nole, K.L.; Tosti, A. Contact dermatitis caused by preservatives. Dermatitis 2014, 25, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Warshaw, E.M.; Maibach, H.I.; Taylor, J.S.; Sasseville, D.; DeKoven, J.G.; Zirwas, M.J.; Fransway, A.F.; Mathias, C.G.T.; Zug, K.A.; de Leo, V.A.; et al. North American contact dermatitis group patch test results: 2011–2012. Dermatitis 2015, 26, 49–59. [Google Scholar] [CrossRef] [PubMed]
- European Commission Press Release Database. Consumers: Commission Improves Safety of Cosmetics. Available online: http://europa.eu/rapid/press-release_IP-14–1051_en.htm (accessed on 8 August 2017).
- Epstein, H. Cosmetics preservation: Sense and nonsense. Clin. Dermatol. 2006, 24, 551–552. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, S.; Varvaresou, A.; Tsirivas, E.; Demetzos, C. New alternatives to cosmetics preservation. J. Cosmet. Sci. 2010, 61, 107. [Google Scholar] [CrossRef] [PubMed]
- Muyima, N.Y.O.; Zulu, G.; Bhengu, T.; Popplewell, D. The potential application of some novel essential oils as natural cosmetic preservatives in an aqueous cream formulation. Flavour. Frag. J. 2002, 17, 258–266. [Google Scholar] [CrossRef]
- Maccioni, A.M.; Anchisi, C.; Sanna, A.; Sardu, C.; Dessi, S. Preservative systems containing essential oils in cosmetic products. Int. J. Cosmet. Sci. 2002, 24, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Nostro, A.; Cannatelli, M.A.; Morelli, I.; Musolino, A.D.; Scuderi, F.; Pizzimenti, F.; Alonzo, V. Efficiency of Calamintha officinalis essential oil as preservative in two topical product types. J. Appl. Microbiol. 2004, 97, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Maurya, S.; De Lampasona, M.P.; Catalan, C.A. A comparison of chemical, antioxidant and antimicrobial studies of cinnamon leaf and bark volatile oils, oleoresins and their constituents. Food Chem. Toxicol. 2007, 45, 1650–1661. [Google Scholar] [CrossRef] [PubMed]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Jovin, E. Antimicrobial and antioxidant properties of rosemary and sage (Rosmarinus officinalis L. and Salvia officinalis L.; Lamiaceae) essential oils. J. Agric. Food Chem. 2007, 55, 7879–7885. [Google Scholar] [CrossRef] [PubMed]
- Oskay, M.; Sari, D. Antimicrobial screening of some Turkish medicinal plants. Pharm. Biol. 2007, 45, 176–181. [Google Scholar] [CrossRef]
- Herman, A.; Tambor, K.; Herman, A. Linalool Affects the Antimicrobial Efficacy of Essential Oils. Curr. Microbiol. 2016, 72, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Zhou, L.; Jiang, W.; Kuang, R.; Li, J.; Tao, R.; Ni, L. An in vitro synergetic evaluation of the use of nisin and sodium fluoride or chlorhexidine against Streptococcus mutans. Peptides 2011, 32, 2021–2026. [Google Scholar] [CrossRef] [PubMed]
- Lequeux, I.; Ducasse, E.; Jouenne, T.; Thebault, P. Addition of antimicrobial properties to hyaluronic acid by grafting of antimicrobial peptide. Eur. Polym. J. 2014, 51, 182–190. [Google Scholar] [CrossRef]
- Valenta, C.; Bernkop-SchnüRch, A.; Rigler, H.P. The antistaphylococcal effect of nisin in a suitable vehicle: A potential therapy for atopic dermatitis in man. J. Pharm. Pharmacol. 1996, 48, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A.B. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 2016, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Patrone, V.; Campana, R.; Vittoria, E.; Baffone, W. In vitro synergistic activities of essential oils and surfactants in combination with cosmetic preservatives against Pseudomonas aeruginosa and Staphylococcus aureus. Curr. Microbiol. 2010, 60, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Nostro, A.; Cannatelli, M.A.; Morelli, I.; Cioni, P.L.; Bader, A.; Marino, A.; Alonzo, V. Preservative properties of Calamintha officinalis essential oil with and without EDTA. Lett. Appl. Microbiol. 2002, 35, 385–389. [Google Scholar] [CrossRef] [PubMed]
| Microorganisms | Culture Media Oxoid, U.K. | Incubation Conditions (Time/Temperature) |
|---|---|---|
| Aspergillus brasiliensis ATCC16404 | Potato dextrose agar | 5–7 days/25 °C |
| Bacillus sp. EC A02 | Tryptic soy agar | 24 h/37 °C |
| Candida albicans ATCC10231 | Sabouraud dextrose medium agar | 24 h/37 °C |
| Escherichia coli ATCC8739 | Tryptic soy agar | 24 h/37 °C |
| Pseudomonas aeruginosa ATCC9027 | Tryptic soy agar | 24 h/37 °C |
| Staphylococcus aureus ATCC25992 | Tryptic soy agar | 24 h/37 °C |
| Tested Preservatives | Aspergillus brasiliensis | Bacillus sp. | Candida albicans | Escherichia coli | Pseudomonas aeruginosa | Staphylococcus aureus |
|---|---|---|---|---|---|---|
| Nisin 125 ppm | - | + | - | - | - | + |
| Nisin 250 ppm | - | + | - | - | - | + |
| Nisin 500 ppm | - | + | - | - | - | + |
| Nisin 1000 ppm | - | + | - | - | - | + |
| Nisin 125 ppm + EDTA 0.1% | - | + | - | - | - | + |
| Nisin 250 ppm + EDTA 0.1% | - | + | - | - | - | + |
| Nisin 500 ppm + EDTA 0.1% | - | + | + | + | - | + |
| Nisin 1000 ppm + EDTA 0.1% | - | + | + | + | - | + |
| Nisin 125 ppm + Abiol 0.15% + Microcare PM2 0.35% | + | + | + | + | + | + |
| Nisin 250 ppm + Abiol 0.15% + Microcare PM2 0.35% | + | + | + | + | + | + |
| Nisin 500 ppm + Abiol 0.15% + Microcare PM2 0.35% | + | + | + | + | + | + |
| Nisin 1000 ppm + Abiol 0.15% + Microcare PM2 0.35% | + | + | + | + | + | + |
| Abiol 0.15% + Microcare PM2 0.35% | - | - | + | + | - | - |
| Abiol 0.3% + Microcare PM2 0.7% | + | + | + | + | + | + |
| Sterile water | - | - | - | - | - | - |
| Propylene glycol | - | - | - | - | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maurício, E.; Rosado, C.; Duarte, M.P.; Verissimo, J.; Bom, S.; Vasconcelos, L. Efficiency of Nisin as Preservative in Cosmetics and Topical Products. Cosmetics 2017, 4, 41. https://doi.org/10.3390/cosmetics4040041
Maurício E, Rosado C, Duarte MP, Verissimo J, Bom S, Vasconcelos L. Efficiency of Nisin as Preservative in Cosmetics and Topical Products. Cosmetics. 2017; 4(4):41. https://doi.org/10.3390/cosmetics4040041
Chicago/Turabian StyleMaurício, Elisabete, Catarina Rosado, Maria Paula Duarte, Joana Verissimo, Sara Bom, and Laura Vasconcelos. 2017. "Efficiency of Nisin as Preservative in Cosmetics and Topical Products" Cosmetics 4, no. 4: 41. https://doi.org/10.3390/cosmetics4040041
APA StyleMaurício, E., Rosado, C., Duarte, M. P., Verissimo, J., Bom, S., & Vasconcelos, L. (2017). Efficiency of Nisin as Preservative in Cosmetics and Topical Products. Cosmetics, 4(4), 41. https://doi.org/10.3390/cosmetics4040041






