Abstract
Wearing clothes and using sun protection products are effective ways of preventing non-melanocytic skin cancer. Sun protection products are classified as cosmetics in Europe. The number of filters authorized by Regulation (EC) No 1223/2009 amended by Regulation (EU) No 344/2013 stands at a total of 27 (26 organic filters and one inorganic filter-titanium dioxide). After the development of methods for determining the efficacy of sun protection products (both in vivo and in vitro), a certain number of authors took an interest in the parameters involved in the efficacy of this category of products. The nature of the filter, the concentration used and the influence of certain ingredients in the formula are all criteria to be taken into account. Concerning titanium dioxide, considerable progress has been made in order to increase its efficacy and to facilitate its implementation. The reduction of the size of the particles used has allowed the products to be more transparent (the pale clown’s mask of days passed is just a bad memory) and above all, to be more effective. The study of a large number of commercial forms of titanium dioxide enables to conclude that nanoparticular titanium dioxide is far superior to pigmentary titanium dioxide. An emulsion composed of 25% pigmentary titanium dioxide only enables Sun Protection Factor (SPF) 5 to be obtained. The same emulsion but with 25% coated nanoparticular titanium dioxide (Tayca MT-100TV) enables a Sun Protection Factor of around 40 to be reached. The reduction of the size of the filtering particles thus proves to be indispensable for the development of highly protective sun protection products.
1. Introduction
The incidence of skin cancer has increased over the past decades and according to the World Health Organization (WHO), two to three million new cases of non-melanocytic skin cancer and 132,000 cases of melanomas are reported every year in the world. The involvement of ultraviolet radiation in the process of carcinogenesis noticed by a certain number of dermatologists as early as the end of the 19th century has now been clearly demonstrated [1]. In 1894, the German dermatologist Paul Gerson Unna clearly stated that the sun was the cause of degenerative lesions on the skin of sailors who were chronically exposed to ultraviolet radiation [2]. Then, it was the American dermatologist James Nevins Hyde who established the same fact. He published an article entitled “On the influence of light on the production of cancer of the skin” in 1906 in “The American Journal of Medical Science” [3]. The Bordeaux dermatologist William Dubreuilh followed the same logic, diagnosing cancerous lesions on the uncovered areas of grape-pickers’ skin who were exposed professionally and therefore, chronically to the sun [4]. The end of the 19th century and the beginning of the 20th century saw an increase in studies showing the consequences of repeated exposure to the sun. Gradually, more and more voices made themselves heard, warning against what could be considered as excessive sun exposure. It should be noted that in spite of all the awareness campaigns designed to encourage people to avoid the sun and to use different means of sun protection (clothing, cosmetics, etc.), the assessment is still largely pessimistic in the 21st century. Different explanations can be sought for these alarming statistics. Firstly, the bad habits of consumers who keep on exposing themselves either to natural ultraviolet (UV) rays or to artificial ones [5], which can even go as far as developing a real dependence on the sun, even though they admit that they are perfectly aware of the risks [6]. Is the sun a friend or foe? That is a question man has been asking since the dawn of time. Is it a friend who warms the Earth and gives life? A friend who is idolized to such as point as they become a demi-god or even a god? A friend who gives us the perfect skin color? Or is the sun rather an enemy who is responsible for the development of some kinds of skin cancer? An enemy who gives the skin the color of the manual laborer who is continuously exposed to its whims? Depending on the era or the individual, the sun has been considered as a friend and as foe. The sun was idolized by the Incas and the Ancient Egyptians and adored by sunbathers as early as the beginning of the 20th century—Jean Cocteau was one of them. He did not accept his physical appearance and was totally devoted to the sun thinking that it was the only way to make himself look more handsome. “Sting my body, burn it brown/beat my load of sorrow down. […] Sun, your sharp blows have not missed/On my neck your weighted fist” [7]. “You make me more tipsy than opium will” affirms the famous opium user, hereby showing that exposure to the sun is more of an addiction than merely worrying about one’s appearance—the sun seems to have two faces according to which side is being considered. Tanning can be seen both as an aesthetic asset and also as the visible sign of cell damage. This exposure to the sun is not necessarily linked to leisure activities; it must be remembered that a great number of people (gardeners, farmers, builders, sailors, etc.) all work outside, which means that they are chronically exposed to ultraviolet rays. These groups of people are generally not inclined to use topical sun protection products mainly due to psychological reasons (cosmetics, in their minds, are more intended for women) or for reasons of safety (it is difficult to work safely on a building site if your hands are covered in sun cream) [8]. In the case of consumers using sun protection products (SPP), it can be observed that there is largely poor compliance in terms of the SPP applied. It is considered that consumers only apply a quarter or half of the required dose at the time of the efficacy test, that is to say 2 mg/cm2 [9]. Furthermore, even in the case of subjects who apply their SPP with care, it is observed that certain areas of the skin are less well protected, due to the fact that it is difficult to systematically obtain a uniform film of product on the whole of the body’s surface. This partly explains the possible appearance of sunburn on those areas in the case of prolonged exposure [10]. Finally, some people seem to be wary of SPPs because they are afraid of potential endocrine disrupting effects linked to certain ingredients in the SPPs [11]. We can also ask ourselves the question of the use of certain anti-inflammatory ingredients that inhibit the actinic erythema formation without any photoprotective activity.
We are going to study here the nanomaterials that can be found in sun protection products, considering their efficacy and their toxicology. We will address the two mineral ingredients on the one hand (titanium dioxide and zinc oxide) and two organic filters on the other, namely Tinosorb® M (methylene bis-benzotriazolyl tetramethylbutylphenol or Bisoctrizole) and Tinosorb® A2B (Tris-Biphenyl Triazine) (BASF, Ludwigshafen, Germany). The latter can also be found in nano-particle form, depending on the supplier. The size of the particles is in the region of 100 nm.
2. Regulatory Aspects and Definition
It is important to remember that in Europe, sun protection products are considered as cosmetics, apart from very rare exceptions where they are considered medical devices. For the sake of this study, we will consider sun protection products only as cosmetics.
Article 2 “Definitions” of Regulation (EC) No 1223/2009 [12], which came into effect in July 2013 and regulates cosmetic products in Europe, defines a nanomaterial as “an insoluble or bio-persistent material, manufactured intentionally and which is characterized by one or several external dimensions or an internal structure on a scale 1 to 100 nm”. It is specified that the consumer must be informed of the fact that there are nanomaterials in a product. This is done via the list of ingredients: nano should be written in brackets after the name of the ingredient concerned. For example, in the case of TiO2, Titanium dioxide (nano) will be written. The particular case of zinc oxide will be mentioned. It should be noted that this ingredient should not be used as a filter in SPPs as is it neither on Appendix VI of Regulation (EC) No 1223/2009 [12] nor is it in Regulation (EU) No 344/2011 [13].
3. Influence of Nanomaterials on the Level of Efficacy of Sun Protection Products
At the beginning of the 20th century, René Cerbelaud, a pharmacist from Paris, made a list in a formulary of a certain number of formula said to have photo-protective properties [14]. On the basis of current knowledge, it appears that most of them are not very effective or even not effective at all (Table 1).

Table 1.
Cream presented as being “for use when spending time on snow or glaciers—recommendable formula”.
| Ingredients | Quantity (g) |
|---|---|
| Esculine | 5 |
| Distilled rose water | 25 |
| Washed Kaolin or better Colloidal Kaolin | 3 |
| Ground titanium oxide | 2 |
| Sublimated zinc oxide | 5 |
| Vaseline oxycholestérinée | 60 |
| Concentrated extract of eau de cologne | 0.40 |
| Madagascan Ylang-Ylang essence | 0.10 |
Others could prove to be dangerous due to presence of a photo-sensitizing agent in their formula (Table 2).

Table 2.
Cream presented as “not letting ultraviolet rays pass”.
| Ingredients | Quantity (g) |
|---|---|
| Ground β Methylumbelliferone | 5 |
| Distilled rose water | 30 |
| René Cerbelaud Hydrocarbon | 65 |
| Concentrated extract of eau de cologne | 0.40 |
| Madagascan Ylang-Ylang essence | 0.10 |
Again in this formulary, a reference can be found for a cream designed to be used in extreme conditions (mountains, glaciers), which has two screens, titanium oxide and zinc oxide, two ingredients which we will talk about later. These two ingredients are pigmentary powders which leave an opaque white film on the skin and continued to be used until recently, before nano-particle forms came on to the market. Their high covering power is well known. They are used, for example, in foundation powders to cover up skin blemishes. This high covering power is considered, in the framework of SPP formulation, as being a major disadvantage known as the “Pierrot’s mask”.
It is important to remember that the first SPPs were launched onto the market at a time when no method of determining their efficacy had been developed. The 1970s heralded a double-edged revolution in the field of cosmetics. Indeed, firstly, regulations were being brought out after the talc Morhange Scandal, which caused the deaths of around 30 infants after talc containing a very high level of hexachlorophene was used on them [15]. Directive 76/768/CEE would have a direct impact on the formulation process of cosmetics in general and of SPPs in particular. A list of UV filters was drawn up; it comprised the list of authorized filters and their maximum usable dose. Then, methods for determining the efficacy of SPPs, both in vivo and in vitro increased, allowing products on the market to be compared with each other in terms of the level of photoprotection provided. The in vivo method developed by the German dermatologist Schulze, which was based on the erythemal power of ultraviolet B (UVB) rays, involved the radiation of around ten volunteers. The relationship between the Minimal Erythema Dose (MED) obtained on skin protected by a SPP and on unprotected skin enables the SPF (Sun Protection Factor) value to be obtained which is a universal indicator used to quantify the efficacy of SPPs. Although it is not very ethical and is marred by a certain number of biases which all tend to overestimate the determined SPF values. A certain number of incorporated ingredients are enable to artificially increased the SPF in vivo determined. This is the case of molecules with anti-inflammatory properties like allantoin, bisabolol, sodium glycyrrhizinate [16], filters themselves [17,18] and vasoconstrictors (aluminum salts, plant extracts, etc.). It should be noted that aluminum oxide, which has a well-known power as an astringent, is a very frequently used additive for coating titanium dioxide particles. This ingredient is particularly used to prevent the phenomenon of particles agglomerating together, thus leading to the improvement of the performances of the product which is created. It is also used in order to stop the reactive oxygen species (ROS) generation by the photocatalytic TiO2. The raw materials, which tend to slow down the appearance of sunburn without actually having a photoprotective effect per se are a danger for the consumer who is no longer warned by the appearance of sunburn. Numerous in vitro methods using various supports (human skin explants, cardboard, quartz, plastic, etc.) have been implemented over the last forty years in order to find a substitute for the in vivo method which is still very widely used. The easiest to use and the cheapest material is polymethylmethacrylate (PMMA). The formula established by Sayre then used by Diffey and Robson puts the SPF in relation to the transmission value obtained for the sample (this is proportional to the fraction of the transmitted incident ray), a weighting factor which takes into account the more or less erythemal character of the incident (UVB rays are much more erythemal than ultraviolet A (UVA) rays) and a factor taking into account the spectrum of the lamp used. The dose-effect relationships were thus obtained for 18 organic filters, which were authorized at the time (para-aminobenzoic acid (PABA) has since been banned due to its allergenic potential). Titanium dioxide exists in around a hundred specialties, which vary according to the nature of the coating used and by the grain size of the particles (15, 40, 50, 60 and 80 nm).
In order to compare the filters with each other, they were incorporated into an O/W emulsion made by our lab. Concentration ranges were carried out in order to study the behavior of the filters in question. The emulsions were applied with a powder-free finger cot on PMMA plates at the rate of 2 mg/cm2. The transmission of the sample was determined with a spectrophotometer equipped with an integrating sphere. According to the area of integration considered, the SPF (290–400 nm) and/or the UVA-PF (320–400 nm) can be obtained.
3.1. Titanium Dioxide Nanoparticles
Concerning titanium dioxide, we can see that there is a very wide variety of raw materials on the market which contain this active material (Table 3).
When we consider the different specialties of coated titanium dioxide nanoparticles available on the market, it is difficult to quantify the impact of grain size compared to the impact of the coating insofar as the specialties differ from each other in terms of both particle size and the nature of the coating used. Besides, according to the amount of coating, the percentage of active material is likely to vary in large proportions (Table 3).

Table 3.
Example of commercial forms of titanium dioxide.
| Trade Name (Supplier) | INCI Name | AM (%) | Size (nm) | Coating |
|---|---|---|---|---|
| Standard titanium oxide (LCW) | Titanium dioxide (CI 77891) | 100 | 200 | no |
| Eusolex T-Oleo (Merck) | Titanium Dioxide, Butylene Glycol, Dicaprylate/Dicaprate, Silica (and) Polyglyceryl-2 Dipolyhydroxystearate | 30 | 20 | yes |
| Eusolex T-Aqua (Merck) | Water (for EU: Aqua), Titanium dioxide, Alumina, Sodium hexametaphosphate, Phenoxyethanol, Sodium methylparaben | 25.8 | 20 | yes |
| Eusolex T-Avo (Merck) | Titanium dioxide, Silica | 79.6 | 20 | yes |
| Eusolex T-2000 (Merck) | Titanium dioxide, Alumina, Simethicone | 80.3 | 14 | yes |
| Eusolex TS (Merck) | Titanium dioxide, Alumina, Stearic acid | 73–79 | 20 | yes |
| Optisol (Croda) | Titanium dioxide | >99 | <150 | no |
| UV-Titan M111 (Merck) | Alumina, Titanium dioxide | 70–100 | 14 | yes |
| UV-Titan X140 (Merck) | Titanium dioxide, Silica, Glycerin | 80–100 | 14 | yes |
| Tayca MT-100TV | Titanium dioxide, Alumina, Stearic acid | 82 | 15 | yes |
| Tego Sun T805 (Merck) | Titanium Dioxyde, Trimethoxycaprylylsilane | 95 | 20 | yes |
| Tego Sun TDEC 45 (Merck) | Titanium Dioxide, Diethylhexyl Carbonate, Polyglyceryl-6 Polyhydroxystearate | 45 | 20 | yes |
| Tego Sun TAQ 40 (Evonik) | Titanium Dioxide, Glycerin, Isolaureth-4 Phosphate, Vinyl Buteth-25/Sodium Maleate Copolymer | 36.3–37.3 | 10–50 | yes |
| Parsol TX (DSM) | Titanium Dioxide, Silica, Dimethicone | 84.9 | <150 | yes |
INCI: International Nomenclature of Cosmetic Ingredients; AM: Active Matter.
The reduction in particle size is an essential element for the efficacy of the products that are formulated (Table 4).

Table 4.
Efficacy of different commercial forms of titanium dioxide compared in terms of photoprotection [19].
| Trade Name | SPF at 25% (w/w) |
|---|---|
| LCW Standard Titanium Oxide | 5 |
| Eusolex T-Oleo | 7 |
| Eusolex T-aqua | 6 |
| Eusolex T-Avo | 28 |
| Eusolex T-2000 | 25 |
| Eusolex TS | 39 |
| Optisol | 25 |
| UV-Titan M111 | 27 |
| UV-Titan X140 | 12 |
| Tayca MT-100 TV | 41 |
| Tego Sun T805 | 30 |
| Tego Sun TDEC 45 | 9 |
| Tego Sun TAQ 40 | 7 |
| Parsol TX | 24 |
However, the coating nature is very important because it inhibits the agglomeration of the particles. It appears that uncoated pigmentary size titanium dioxide is only of minor interest in terms of topical photoprotection (Table 4). However, it is the form that was used until nanoparticle forms were launched on the market. Incorporated into a lab-made O/W emulsion (the same formula whatever the filter being tested), uncoated pigmentary titanium dioxide (LCW standard titanium dioxide) only enables an SPF of 5 to be obtained for a usage dose of 25%. At such a dose, it is indeed impossible to obtain an acceptable emulsion from a cosmetic point of view as the texture is like a paste. We can thus conclude that the preparations formulated in the 1930s, which left a white film on the skin did not have any significant photoprotective effect. In the same way, currently, the high covering quality of a SPP signals the presence of pigmentary titanium dioxide and not a nanoparticle one in the preparation being used, which considerably influences the efficacy of the product in question. The resurgence of this type of product comes from the fear of organic filters, which has spread amongst certain categories of consumers. This situation comes from the fact that an estrogenic effect was highlighted, even though it is thousands or millions of times less than the reference molecule, namely 17 beta-estradiol [20]. It should be reminded that this “endocrine disrupting” factor, which is far less significant than the disrupting effect of using a contraceptive hormone replacement therapy, should not make people reluctant to use SPPs. The fear of consumers regarding organic filters may also be due to the mediatization of certain publications that affirm that these UV-filters have negative impact to the environment [21]. This distrust is shown concretely in the rejection of cosmetics in general and SPPs in particular or even resorting to the use of cosmetics which are presented as being safe for health, as organic SPPs only contain inorganic filters. In no way do these filters enable high protection levels to be reached [22]. A way of effective photoprotection is thus necessary in a general prevention policy of photo-induced skin-cancer. It has been clearly demonstrated nowadays that effective protection against sunburn is also effective against photo-induced skin cancer [23]. These SPPs contain organic filters used in combination with or without nano-particular inorganic filters incorporated into an excipient. The reduction of the size of the filtering particles is a process that should not be neglected when seeking to maximize the efficacy of a SPP. From a psychological point of view, the consumer will feel better protected if he sees a white film on his skin because he supposes that this provides protection rather than a transparent film, which he judges to be inefficient as it cannot be seen by the naked eye. A small number of companies which market mineral pigmentary titanium dioxide-based SPPs gamble on this psychological aspect in order to sell SPPs which are nevertheless ineffective. Fortunately, these companies are not in the majority. Most companies use nano-particle titanium dioxide ((nano) written next to the name of the ingredient shows that this form has been used) in order to increase the efficacy of the formulated product. Considering that it is quite difficult to incorporate more than 15% of nano-particle titanium dioxide in an emulsion, it clearly appears that a SPP, which only has this product, cannot provide an SPF of 30 or higher. Aluminum oxide is sometimes presented as being an SPF booster. This is not entirely true. Let us note that aluminum oxide (alumina) is an active astringent which tightens the vessels and thus, interferes with the appearance of an erythema adding bias to the method of determining the SPF of SPPs in vivo determined.
From a toxicological point of view, although we know that pigmentary titanium dioxide is less photo-catalytic than nano-particular forms [24]. Certain authors consider that adding titanium dioxide nanoparticles in SPPs causes an increase in the production of reactive oxygen species (ROS) involved in the process of carcinogenesis, which is of course not desirable. We can hereby see the importance of creating a coating. Certain authors advise that the filter should be encapsulated in particles of zeolite [25]. Numerous studies on skin penetration carried out in vitro both on human and animal skin show the presence TiO2 nanoparticles in the Stratum corneum, as well as in the pilosebaceous infudibulum. Concerning the in vivo studies on animals, the study conducted by the Food and Drug Administration remains the most relevant concerning possible cutaneous penetration by TiO2 nanoparticles, especially thanks to the animal that was chosen, namely the pig. It should be noted that during this study, the formulae only contained 5% of TiO2, whilst the maximum concentration is 25%. Applications were carried out on mini-pigs’ skin four times a day, five days a week for 22 days [26]. TiO2 nanoparticles (coated and uncoated) and submicronic TiO2 particles (300–500 nm) were found in the Stratum corneum and a few isolated particles of TiO2 were even found in the dermis in the case of animals treated with the three types of particles. Nevertheless, the Scientific Committee on Consumer Safety (SCCS), the committee in charge of the safety of ingredients at a European level [27], confirmed in April 2014 that titanium dioxide nanoparticles used at a concentration of 25% maximum as a UV filter in SPPs could be considered as causing no particular problems for human health after application on healthy, intact or sunburnt skin. Previously, in December 2014, the SCCS had drawn attention to the possible risks in the case of incorporating TiO2 into sprayable forms.
Concerning the impact on the environment, linked to the salting-out of TiO2 nanoparticles associated with the use of cosmetic products and more particularly with sunscreen products, there is almost no data at the present time [28,29,30,31]. As this risk cannot currently be assessed, for the same reason, neither can it be totally excluded. With this in mind, it must be remembered that the quantity of nanoparticles corresponding to their use in SPP only represents 0.1% of the total production of TiO2, in France for example, according to the Afsset (French Agency for Environmental and Occupational Health Safety).
3.2. Zinc Oxide Nanoparticles
The case of zinc oxide is very distinctive insofar as this substance has never been considered as a sun filter in Europe. It is still not mentioned in Appendix VI of Regulation (EC) No 1223/2009 [12] establishing the list of UV filters authorized in Europe. On this subject, let us remember that in 2011 the French health authorities issued an opinion concerning zinc oxide, indicating that it cannot be incorporated as a UV filter in cosmetic products until it is listed in the regulatory appendix listing authorized UV filters [32]. In spite of these recommendations, zinc oxide is found in organic SPPs only containing mineral filters. If we compare with what was mentioned for titanium dioxide, we can see that there are far fewer nanoparticle forms of zinc oxide (Table 5).

Table 5.
Example of commercial forms of nanoparticle zinc oxide.
| Trade Name (Supplier) | ZnO Content (%) | Size (nm) | Coating |
|---|---|---|---|
| Zinc oxide neutral (Symrise) | 95 | 41 | – |
| Zinc oxide NDM (Symrise) | 92 | <50 | Dimethicone |
| Z-Cote (BASF) | – | 80 | – |
| Z-Cote max (BASF) | 96–99 | – | Dimethoxydiphenylsilane, Triethoxycaprylylsilane |
| Z-Cote HP1 (BASF) | 98 | – | Triethoxycaprylylsilane |
| Tego Sun Z500 (Evonik) | 99.5 | 10–60 | – |
| Tego Sun Z800 (Evonik) | >94 | 10–60 | Triethoxycaprylylsilane |
| Nanox 200 (Elementis Specialties) | 99 | 60 | – |
| Nanox gel 200 TN (Elementis Specialties) | 55 | 60 | C12–15 alkyl benzoate, Polydydroxystearic acid |
Zinc oxide, even in its nanoparticle state, only provides a very mediocre level of efficacy both in the UVA and UVB ranges (Table 6), lower than the level that is likely to be obtained with titanium dioxide.

Table 6.
Efficacy of different commercial forms of zinc oxide compared in terms of photoprotection [19].
| Trade Name | SPF at 25% (w/w) |
|---|---|
| Zinc oxide neutral | 8.33 |
| Zinc oxide NDM | 6.35 |
| Z-Cote | 6.17 |
| Z-Cote max | 10.13 |
| Z-Cote HP1 | 6.04 |
| Tego Sun Z500 | 6.55 |
| Tego Sun Z800 | 8.10 |
| Nanox 200 | 5.25 |
| Nanox gel 200 TN | 5.00 |
The most effective commercial form is Z-Cote Max, which only gives SPF 10 for a usage dose of 25%. This dose has been tested insofar as it is the maximum dose authorized in the United States.
Concerning the toxicological aspects, few studies are available about zinc oxide nanoparticles. It is, for example, possible to use interesting optical ways like tomography to study possible ZnO nanoparticle in humans in vivo [33]. Some studies on skin absorption carried out in vitro, not only on animal and human skin models but also on volunteers, highlight the presence of ZnO nanoparticles in the superficial layers of the skin following the application of products containing them. It can be shown that there is a statistically significant increase in the rate of ZnO in the blood and urine of volunteers [34]. This increase remains low in view of zinc levels normally present in humans. It is not known if ZnO was absorbed in the form of particles of ZnO or in the form of soluble Zn2+ ions or indeed in both of those forms. It is not currently possible to conclude as to the absence of skin penetration of ZnO nanoparticles. It would be important to increase the number of studies in order to make a decision on this point, especially as a photocatalytic effect is also to be feared, as in the case of titanium dioxide.
3.3. Bisoctrizole
Bisoctrizole or 2,2'-methylene-bis-(6(2H-benotriazol-2-yl)-4-(1,1,3,3-tetramethylbutyl)phenol) (CAS No 103597-45-1) (Tinosorb® M, BASF, Ludwigshafen, Germany) is a hydrophilic filter—which is an advantage as the vast majority of UV filters available are lipophilic-, broad spectrum, and launched onto the market in the late 1990s. Its maximum dose of use is fixed at 10% (w/w). At this percentage, high levels of protection in the UVB region cannot be obtained (Figure 1). It is considered as a mediocre filter as it does not enable one SPF unit per percentage of use to be obtained, as is the case, for example, with ethylhexylmethoxycinnamate.
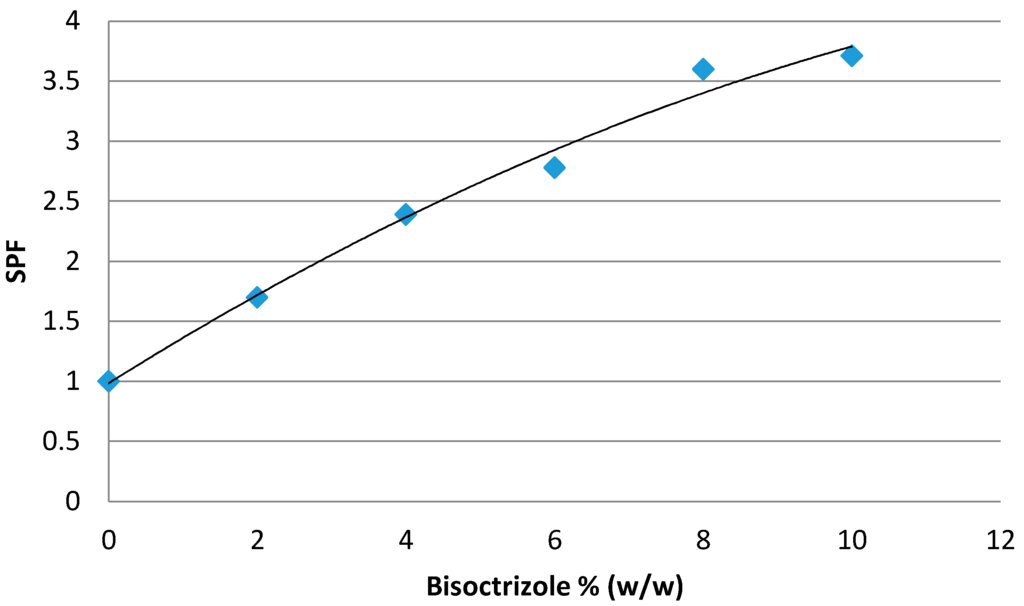
Figure 1.
Efficacy of bisoctrizole in the UVB range.
In contrast, it turns out to be interesting in the UVA range (Figure 2) all the more so, as the number of available UVA filters is more and more restricted [35].
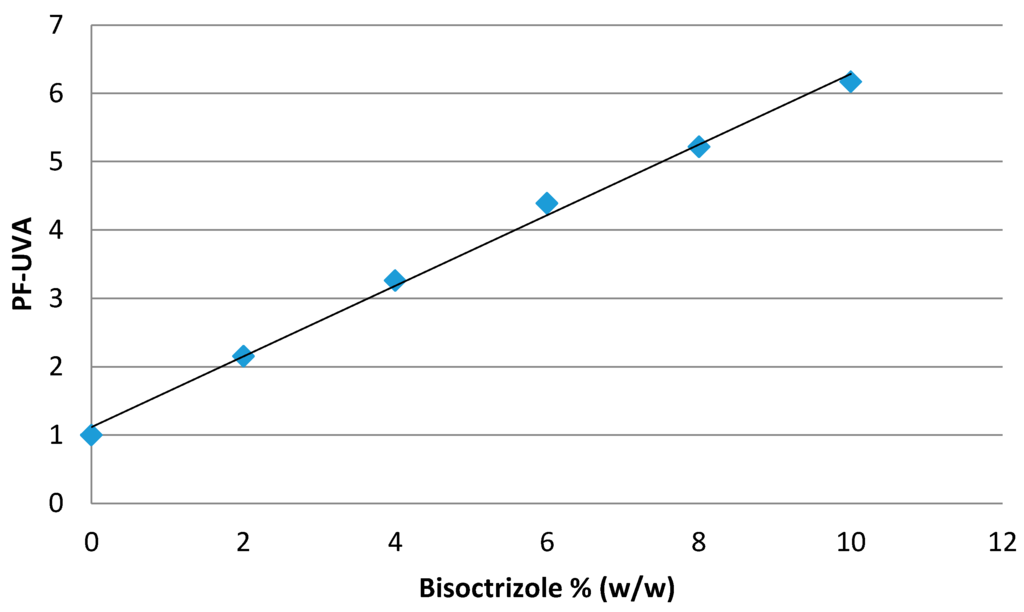
Figure 2.
Efficacy of bisoctrizole in the UVA range.
In terms of photostability, bisoctrizole presents an advantage compared to the vast majority of commercially-available filters, as it is one of the rare filters which is photostable [36].
From the point of view of its harmlessness, very rare cases of allergies and/or photo-allergies [37,38,39] can be noted, occurring a few years after it was launched on the market. The particles of bisoctrizole are stabilized by a surfactant called decyl glucoside. The patch tests carried out seem to show that the real allergen is decylglucoside [40]. Tests carried out on rats have enabled it to be highlighted that there is no endocrine disrupting effect [41]. This broad-spectrum filter presents a certain interest because of its harmlessness and its photostable character. Combinations could obtain high-index SPPs. Using bisoctrizole in combination with all of the organic filters available on the market is interesting [22] as it enables in particular to limit the production of sunburn cells and P53 tumor-suppressor gene [42]. Studies carried out on rats came to the conclusion that bisoctrizole has neither an oestrogenic nor androgenic activity [41].
3.4. Tris-Biphenyl Triazine
Tris-Biphenyl Triazine or 1,3,5-Triazine, 2,4,6-tris[1,1'-biphenyl]-4-yl (Tinosorb® A2B, BASF) is a substance which is now on Appendix VI of Regulation (EC) No 1223/2009 [12]. This hydrodispersible filter presents a remarkable level of efficacy in both the UVA (Figure 3) and UVB (Figure 4) range [43].
Concerning the photostability of emulsions formulated from this filter, we can note that for high percentages of use (8% or 10%), the emulsions prove to be stable in terms of efficacy. Indeed, no significant differences are seen between the efficacy indicator values determined at t0 (before radiation) and those at t2h (after 2 h of radiation) [43].
There is currently no hindsight in terms of harmlessness, as this molecule has just been launched on the market.
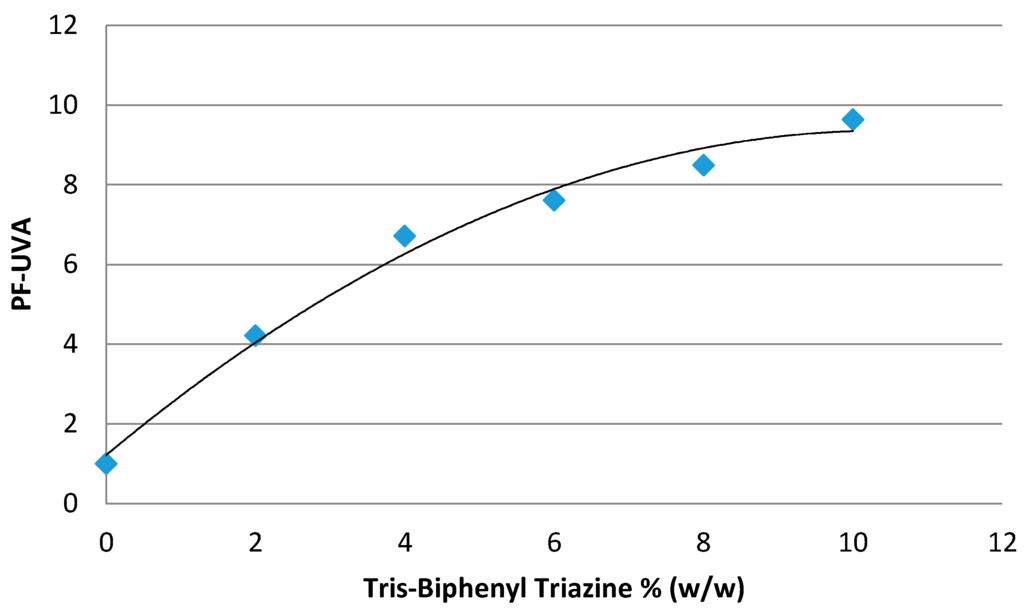
Figure 3.
Efficacy of Tris-Biphenyl Triazine in UVA range.
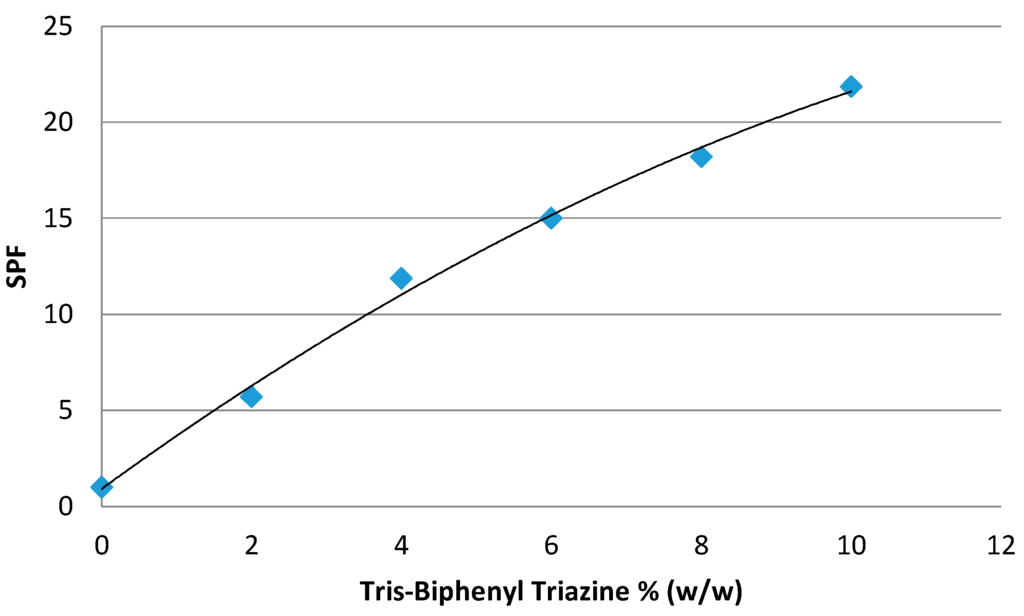
Figure 4.
Efficacy of Tris-Biphenyl Triazine in UVB range.
4. Conclusions
The introduction of nanoparticles in cosmetics in general and more particularly in SPPs must be considered from different aspects. From a point of view of efficacy, it is undeniable that the reduction of the size of particles has allowed efficacy to be increased, particularly in the case of titanium dioxide. From a toxicological point of view, a certain number of questions remain to be answered. Presented for years as being perfectly well-tolerated filters as they are inert, non-allergenic and therefore, indicated for use on young children, inorganic nanoparticle filters began to be singled out because of their photocatalytic character. The question should also be asked regarding transdermal passage. Research must be done to ensure that these nanoparticle filters are totally harmless. In the case of titanium dioxide, it is not a question of choosing between ineffective but toxicologically safe SPPs on the one hand and effective but potentially toxic SPPs on the other. In the field of topical photo-protection, as for any other public health problem, there is no room for controversy [44] but a need for active research in order to check out the assumption being made. It is not necessary to put in opposition inorganic and organic filters. They should be used together in order to obtained very efficiency sunscreens.
Author Contributions
Laurence Coiffard and Celine Couteau both conceived the idea, designed experiments, performed the experiments, analyzed the data and wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guy, G.P., Jr.; Thomas, C.C.; Thompson, T.; Watson, M.; Massetti, G.M.; Richardson, L.C. Vital signs: Melanoma incidence and mortality trends and projections—United States, 1982–2030. Morb. Mortal. Wkly. Rep. 2015, 64, 591–596. [Google Scholar]
- Hollander, A.W. Development of Dermatopathology and Paul Gerson Unna. J. Am. Acad. Dermatol. 1986, 15, 727–734. [Google Scholar] [CrossRef]
- Hyde, J.N. On the influence of light on the production of cancer of the skin. Am. J. Med. Sci. 1906, 131, 1–22. [Google Scholar] [CrossRef]
- Chang, C.; Murzaku, E.C.; Penn, L.; Abbasi, N.R.; Davis, P.D.; Berwick, M.; Polsky, D. More skin, more sun, more tan, more melanoma. Am. J. Public Health 2014, 104, e92–e99. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, C.; Grenier, M.; Maire, C.; Cottencin, O.; Bonnevalle, A.; Behal, H.; Duhamel, A.; Glantenet, R.; Mortier, L. Indoor tanning: Motivations and beliefs among users and non-users in the population of Lille (Northern France). Ann. Dermatol. Venereol. 2015, 142, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Fell, G.L.; Robinson, K.C.; Mao, J.; Woolf, C.J.; Fisher, D.E. Skin β-endorphin mediates addiction to UV light. Cell 2014, 157, 1527–1534. [Google Scholar] [CrossRef] [PubMed]
- Cocteau, J. Batterie in Poèmes; Gallimard: Paris, France, 1920. [Google Scholar]
- Boniol, M.; Koechlin, A.; Boniol, M.; Valentini, F.; Chignol, M.C.; Doré, J.F.; Bulliard, J.L.; Milon, A.; Vernez, D. Occupational UV exposure in French outdoor workers. J. Occup. Environ. Med. 2015, 57, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Stokes, R.; Diffey, B. How well are sunscreen users protected? Photodermatol. Photoimmunol. Photomed. 1997, 13, 186–188. [Google Scholar] [CrossRef] [PubMed]
- Pissavini, M.; Diffey, B. The likelihood of sunburn in sunscreen users is disproportionate to the SPF. Photodermatol. Photoimmunol. Photomed. 2013, 29, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Maipas, S.; Nicolopoulou-Stamati, P. Sun lotion chemicals as endocrine disruptors. Hormones 2015, 14, 32–46. [Google Scholar] [PubMed]
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:342:0059:0209:en:PDF (accessed on 20 June 2015).
- Commission Implementing Regulation (EU) No 344/2011 of 8 April 2011. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:096:0015:0016:EN:PDF (accessed on 20 June 2015).
- Cerbelaud, R. Formulaire des Principales Spécialités de Parfumerie et de Pharmacie; En vente à la Pharmacie Bayard & Cerbelaud: Paris, French, 1920. (In French) [Google Scholar]
- Martin-Bouyer, G.; Toga, M.; Lebreton, R.; Stolley, P.D.; Lockhart, J. Outbreak of accidental hexachlorophene poisoning in France. Lancet 1982, 319, 91–95. [Google Scholar] [CrossRef]
- Couteau, C.; Chauvet, C.; Paparis, E.; Coiffard, L.J. Influence of anti-inflammatory ingredients on the SPF determined in vitro. Arch. Dermatol. Res. 2012, 304, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Couteau, C.; Chauvet, C.; Paparis, E.; Coiffard, L.J. UV filters, ingredients with a recognized anti-inflammatory effect. PLoS ONE 2012, 7, e46187. [Google Scholar] [CrossRef] [PubMed]
- Couteau, C.; Couteau, O.; Chauvet, C.; Paparis, E.; Coiffard, L.J. The effect of ultraviolet radiation on the anti-inflammatory effect of filters. Int. J. Pharm. 2013, 452, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Couteau, C.; Alami, S.; Guitton, M.; Paparis, E.; Coiffard, L.J. Mineral filters in sunscreen products—Comparison of the efficacy of zinc oxide and titanium dioxide by in vitro method. Pharmazie 2008, 63, 58–60. [Google Scholar] [PubMed]
- Schlumpf, M.; Schmid, P.; Durrer, S.; Conscience, M.; Maerkel, K.; Henseler, M.; Gruetter, M.; Herzog, I.; Reolon, S.; Ceccatelli, R.; et al. Endocrine activity and developmental toxicity of cosmetic UV filters—An update. Toxicology 2004, 205, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Danovaro, R.; Bongiorni, L.; Corinaldesi, C.; Giovannelli, D.; Damiani, E.; Astolfi, P.; Greci, L.; Pusceddu, A. Sunscreens cause coral bleaching by promoting viral infections. Environ. Health Perspect. 2008, 116, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Couteau, C.; Chammas, R.; Alami, S.; Paparis, E.; Coiffard, L.J. Combination of UVB and UVA organic filters—Influence on SPF and PF-UVA determined by using in vitro method. J. Dermatol. Sci. 2008, 50, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Couteau, C.; Couteau, O.; Alami, S.; Coiffard, L.J. Sunscreen products: What do they protect us from? Int. J. Pharm. 2011, 415, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Kockler, J.; Oelgemöller, M.; Robertson, S.; Glass, B.D. Influence of titanium dioxide particle size on the photostability of the chemical UV-filters butyl methoxy dibenzoylmethane and octocrylene in a microemulsion. Cosmetics 2014, 1, 128–139. [Google Scholar] [CrossRef]
- Shen, B.; Scaiano, J.C.; English, A.M. Zeolite encapsulation decreases TiO2-photosensitized ROS generation in cultured human skin fibroblasts. Photochem. Photobiol. 2006, 82, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Sadrieh, N.; Wokovich, A.M.; Gopee, N.V.; Zheng, J.; Haines, D.; Parmiter, D.; Siitonen, P.H.; Cozart, C.R.; Patri, A.K.; McNeil, S.E.; et al. Lack of significant dermal penetration of titanium dioxide from sunscreen formulations containing nano- and submicron-size TiO2 particles. Toxicol. Sci. 2010, 115, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Vinardell, M.P. The use of non-animal alternatives in the safety evaluations of cosmetics ingredients by the Scientific Committee on Consumer Safety (SCCS). Regul. Toxicol. Pharmacol. 2015, 71, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Labille, J.; Feng, J.; Botta, C.; Borschneck, D.; Sammut, M.; Cabie, M.; Auffan, M.; Rose, J.; Bottero, J.Y. Aging of TiO2 Nanocomposites Used in Sunscreen. Dispersion and Fate of the Degradation Products in Aqueous Environment. Environ. Pollut. 2010, 158, 3482–3489. [Google Scholar] [CrossRef] [PubMed]
- Gondikas, A.P.; von der Kammer, F.; Reed, R.B.; Wagner, S.; Ranville, J.F.; Hofmann, T. Release of TiO2 nanoparticles from sunscreens into surface waters: A one-year survey at the old Danube recreational Lake. Environ. Sci. Technol. 2014, 48, 5415–5422. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Sánchez, A.; Sánchez-Quiles, D.; Basterretxea, G.; Benedé, J.L.; Chisvert, A.; Salvador, A.; Moreno-Garrido, I.; Blasco, J. Sunscreen products as emerging pollutants to coastal waters. PLoS ONE 2013, 8, e65451. [Google Scholar] [CrossRef] [PubMed]
- Foltête, A.S.; Masfaraud, J.F.; Bigorgne, E.; Nahmani, J.; Chaurand, P.; Botta, C.; Labille, J.; Rose, J.; Férard, J.F.; Cotelle, S. Environmental impact of sunscreen nanomaterials: Ecotoxicity and genotoxicity of altered TiO2 nanocomposites on Vicia faba. Environ. Pollut. 2011, 15, 2515–2522. [Google Scholar] [CrossRef] [PubMed]
- Recommandations Relatives à L’utilisation des Nanoparticules de Dioxyde de Titane et d’oxyde de Zinc en Tant que Filtres UV dans les Produits Cosmétiques. Available online: http://ansm.sante.fr/var/ansm_site/storage/original/application/07fee639ffe2915fd26d91d42a9487d8.pdf (accessed on 20 June 2015). (In French)
- Breunig, H.; Weinigel, M.; König, K. In vivo imaging of ZnO nanoparticles from sunscreen on human skin with a mobile multiphoton tomograph. BioNanoScience 2015, 5, 42–47. [Google Scholar] [CrossRef]
- Gulson, B.; McCall, M.; Korsch, M.; Gomez, L.; Casey, P.; Oytam, Y.; Taylor, A.; McCulloch, M.; Trotter, J.; Kinsley, L.; et al. Small amounts of zinc from zinc oxide particles in sunscreens applied outdoors are absorbed through human skin. Toxicol. Sci. 2010, 118, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Couteau, C.; Pommier, M.; Paparis, E.; Coiffard, L.J. Study of the efficacy of 18 sun filters authorized in European Union tested in vitro. Pharmazie 2007, 62, 449–452. [Google Scholar] [PubMed]
- Couteau, C.; Faure, A.; Fortin, J.; Paparis, E.; Coiffard, L.J. Study of the photostability of 18 sunscreens in creams by measuring the SPF in vitro. J. Pharm. Biomed. Anal. 2007, 44, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.E.; Soter, N.A.; Cohen, D.E. Positive patch- and photopatch-test reactions to methylene bis-benzotriazolyl tetramethylbutylphenol in patients with both atopic dermatitis and chronic actinic dermatitis. Dermatitis 2011, 22, 106–111. [Google Scholar] [PubMed]
- González-Pérez, R.; Trébol, I.; García-Río, I.; Arregui, M.A.; Soloeta, R. Allergic contact dermatitis from methylene-bis-benzotriazolyl tetramethylbutylphenol (Tinosorb M). Contact Dermatitis 2007, 56, 121. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.; Kirk, S.; Wilkinson, M.S. Allergic contact dermatitis caused by Tinosorb® M. Contact Dermat. 2011, 65, 48–49. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P.; Gonçalo, M.; Figueiredo, A. Allergic contact dermatitis to decyl glucoside in Tinosorb M. Contact Dermat. 2010, 62, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Ashby, J.; Tinwell, H.; Plautz, J.; Twomey, K.; Lefevre, P.A. Lack of binding to isolated estrogen or androgen receptors, and inactivity in the immature rat uterotrophic assay, of the ultraviolet sunscreen filters Tinosorb M-active and Tinosorb S. Regul. Toxicol. Pharmacol. 2001, 34, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Gélis, C.; Girard, S.; Mavon, A.; Delverdier, M.; Paillous, N.; Vicendo, P. Assessment of the skin photoprotective capacities of an organo-mineral broad-spectrum sunblock on two ex vivo skin models. Photodermatol. Photoimmunol. Photomed. 2003, 19, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Couteau, C.; Chauvet, C.; Paparis, E.; Coiffard, L.J. Tris-Biphenyl Triazine, a new very interesting ultraviolet filter in terms of photoprotective efficiency. Int. J. Pharm. 2015, 487, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Burnett, M.E.; Wang, S.Q. Current sunscreen controversies: A critical review. Photodermatol. Photoimmunol. Photomed. 2011, 27, 58–67. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).