Under Persistent Assault: Understanding the Factors that Deteriorate Human Skin and Clinical Efficacy of Topical Antioxidants in Treating Aging Skin
Abstract
:1. Introduction
2. Ultraviolet Irradiation
3. Infrared A Radiation
4. Pollution
5. Topical Antioxidants as Treatment
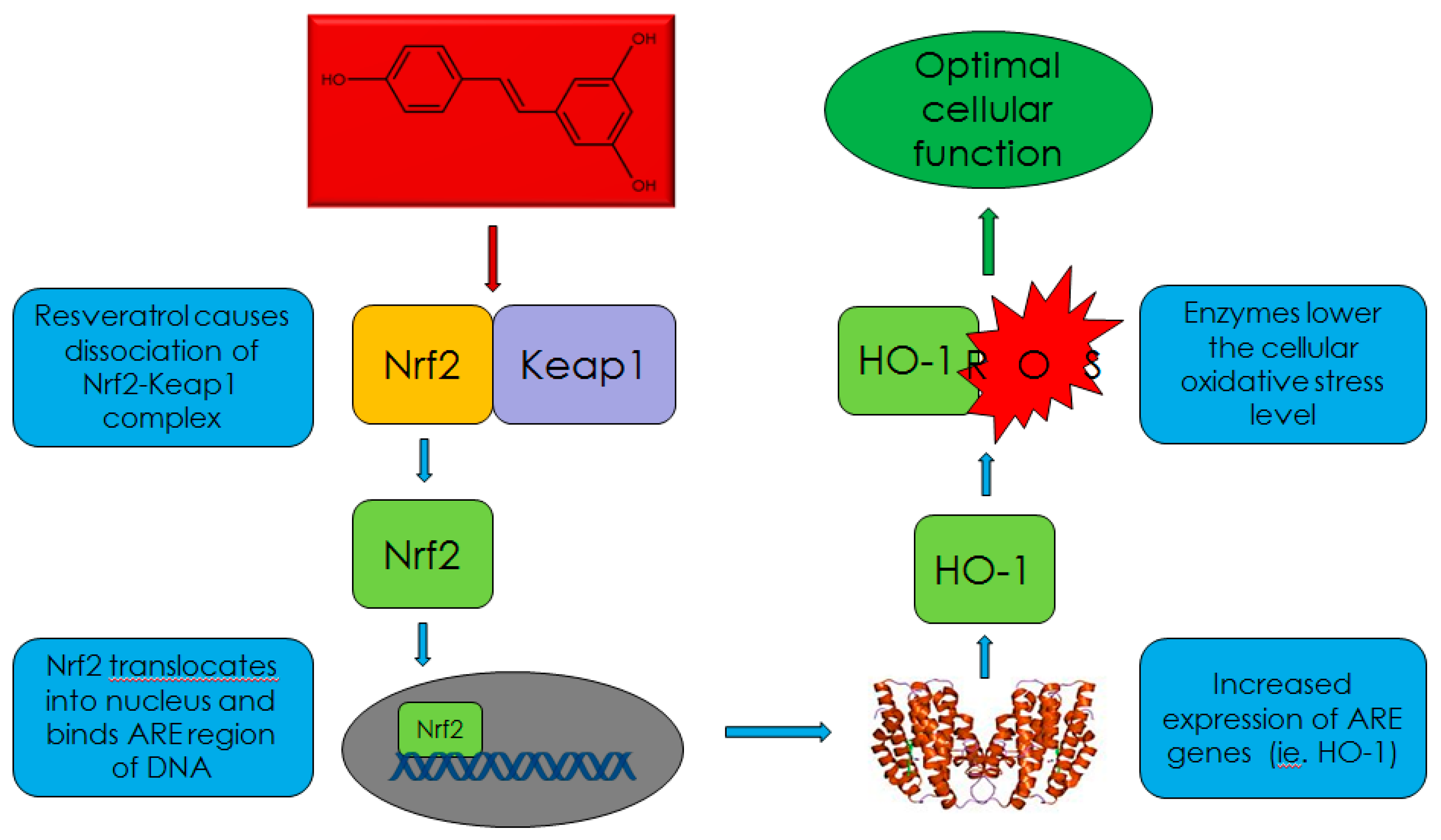
6. Countermeasures by Antioxidants
7. Conclusions
Author Contributions
Conflicts of Interest
References
- Schroeder, P.; Schieke, S.M.; Morita, A. Premature Skin Aging by Infrared Radiation, Tobacco Smoke and Ozone. In Skin Aging; Springer Berlin Heidelberg: Berlin, Germany, 2006; pp. 45–53. [Google Scholar]
- Schroeder, P.; Lademann, J.; Darvin, M.E.; Stege, H.; Marks, C.; Bruhnke, S.; Krutmann, J. Infrared Radiation-Induced Matrix Metalloproteinase in Human Skin: Implications for Protection. J. Investig. Dermatol. 2008, 128, 2491–2497. [Google Scholar] [CrossRef] [PubMed]
- Kvam, E.; Tyrrell, R.M. Induction of oxidative DNA base damage in human skin cells by UV and near visible radiation. Carcinogenesis 1997, 18, 2379–2384. [Google Scholar] [CrossRef] [PubMed]
- Thiele, J.J.; Podda, M.; Packer, L. Tropospheric ozone: An emerging environmental stress to skin. Biol. Chem. 1997, 378, 1299–1305. [Google Scholar] [PubMed]
- Baudouin, C.; Charveron, M.; Tarroux, R.; Gall, Y. Environmental pollutants and skin cancer. Cell Biol. Toxicol. 2002, 18, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Darr, D.; Fridovich, I. Free radicals in cutaneous biology. J. Investig. Dermatol. 1994, 102, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Unna, P. Histopathologie der Hautkrankheiten; August Hirschwald: Berlin, Germany, 1984. (In German) [Google Scholar]
- Dubreuilh, W. Des hyperkeratoses circonscrites. Ann. Dermatol. Venereol. 1896, 7, 1158–1204. (In French) [Google Scholar]
- Scharffetter-Kochanek, K.; Wlaschek, M.; Brenneisen, P.; Schauen, M.; Blaudschun, R.; Wenk, J. UV induced reactive oxygen species in photocarcinogenesis and photoaging. Biol. Chem. 1997, 378, 1247–1257. [Google Scholar] [PubMed]
- Liochev, S.I.; Fridovich, I. The role of O2 in the production of HO: In vitro and in vivo. Free Radic. Biol. Med. 1994, 16, 29–33. [Google Scholar] [CrossRef]
- Dixit, R.; Mukhtar, H.; Bickers, D.R. Studies on the role of reactive oxygen species in mediating lipid peroxide formation in epidermal microsomes of rat skin. J. Investig. Dermatol. 1983, 81, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Briganti, S.; Ricardo, M. Antioxidant activity, lipid peroxidation and skin disease. What’s new? JEADV 2003, 17, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Rittie, L.; Fischer, G.J. UV-light-induced signal cascades and skin aging. Ageing Res. Rev. 2002, 1, 705–720. [Google Scholar] [CrossRef]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechaisms of photoaging and chronological skin aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Varani, J.; Voorhees, J.J. Looking older: Fibroblast collapse and therapeutic implications. Arch. Dermatol. 2008, 144, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Traikovich, S.S. Use of Topical Ascorbic acid and its effect on photo damaged skin topography. Arch. Otorhinol. Head Neck Surg. 1999, 125, 1091–1098. [Google Scholar] [CrossRef]
- Fisher, G.J.; Wang, Z.; Datta, S.C.; Varani, J.; Kang, S.; Voorhees, J.J. Pathophysiology of premature skin aging induced by ultraviolet light. N. Engl. J. Med. 1997, 337, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Breitenbach, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richer, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Krutmann, J. Photoprotection of human skin beyond ultraviolet radiation. Photodermatol. Photoimmunol. Photomed. 2014, 30, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Vierkotter, A.; Krutmann, J. Environmental influences on skin aging and ethnic-specific manifestations. Dermatoendocrinology 2012, 4, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Grether-Beck, S.; Krutmann, J. Involvement of lipid rafts and caveolins in UVA signaling. Open Dermatol. J. 2009, 3, 153–159. [Google Scholar] [CrossRef]
- Karu, T.I. Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem. Photobiol. 2008, 84, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Calles, C.; Schneider, M.; Macaluso, F.; Benesova, T.; Krutmann, J.; Schroeder, P. Infrared a radiation influences the skin fibroblast transcriptome: Mechanisms and consequences. J. Investig. Dermatol. 2010, 130, 1524–1536. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Lee, M.J.; Lee, S.R.; Kim, K.H.; Cho, K.H.; Eun, H.C.; Chung, J.H. Augmentation of UV-induced skin wrinkling by infrared irradiation in hairless mice: Implications for protection. Mech. Ageing Dev. 2005, 126, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, Y.K.; Cho, K.H.; Chung, J.H. Regulation of type 1 procollagen and MMP-1 expression after single or repeated exposure to infrared radiation in human skin. Mech. Ageing Dev. 2006, 127, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.; Cho, S.; Shin, M.H.; Kim, Y.K.; Seo, J.-E.; Lee, Y.M.; Park, C.-H. Effects of Infrared Radiation and Heat on Human Skin Aging in Vivo. J. Investig. Dermatol. 2009, 14, 15–19. [Google Scholar]
- Vierkotter, A.; Schikowski, T.; Ranft, U.; Sugiri, D.; Matsui, M.; Kramer, U.; Krutmann, J. Airborne Particle Exposure and Extrinsic Skin Aging. J. Investig. Dermatol. 2010, 130, 2719–2726. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; van der Vliet, A.; Schock, B.C.; Okamoto, T.; Obermuller-Jevic, U.; Cross, C.E.; Packer, L. Ozone exposure activates oxidative stress response in murine skin. Toxicology 2002, 179, 163–170. [Google Scholar] [CrossRef]
- Valacchi, G.; Pagnin, E.; Okamoto, T.; Corbacho, A.M.; Olano, E.; Davis, P.A.; van der Vliet, A.; Packer, L.; Cross, C.E. Induction of stress proteins and MMP-9 by 0.8 ppm of ozone in murine skin. Biochem. Biophys. Res. Commun. 2003, 305, 741–746. [Google Scholar] [CrossRef]
- Valacchi, G.; Weber, S.U.; Luu, C.; Cross, C.E.; Packer, L. Ozone potentiates vitamin E depletion by ultraviolet radiation in murine stratum corneum. FEBS Lett. 2000, 466, 165–168. [Google Scholar] [CrossRef]
- Pryor, W.A. Mechanisms of radical formation from reactions of ozone with target molecules in the lung. Free Radic. Biol. Med. 1984, 17, 451–465. [Google Scholar] [CrossRef]
- Donaldson, K.; Tran, L.; Jimenez, L.A.; Duffin, R.; Newby, D.E.; Mills, N.; MacNee, W.; Stone, V. Combustion-derived nanoparticles: A review of their toxicology following inhalation exposure. Part Fibre Toxicol. 2005, 2. [Google Scholar] [CrossRef] [PubMed]
- Hamanaka, H.; Miyachi, Y.; Imamura, S. Photoprotective effect of topically applied SOD on sunburn reaction in comparison with sunscreen. J. Dermatol. 1990, 17, 595–598. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, Y.; Hoshi, K.; Yamagawa, A.; Takana, K. Topical application of superoxide dismutase cream. Drugs Exp. Clin. Res. 1991, 17, 127–131. [Google Scholar] [PubMed]
- Lopez-Torres, M.; Thiele, J.J.; Shindo, Y.; Han, D.; Packer, L. Topical application of lpha-tocopherol modulates the antioxidant network and diminishes ultraviolet-induced oxidative damage in murine skin. Br. J. Dermatol. 1998, 138, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Selim, M.A.; Shea, C.R.; Grichnik, J.M.; Omar, M.M.; Monteiro-Riviere, N.A.; Pinnell, S.R. UV photoprotection by combining topical antioxidants vitamin C and vitamin E. J. Am. Acad. Dermatol. 2003, 48, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.C.; Burch, J.A.; Streilein, R.D.; Iannacchione, M.A.; Hall, R.P.; Pinnell, S.R. A topical antioxidant solution containing vitamin C and E stabilized by ferulic acid provides protection for human skin against damage caused by ultraviolet irradiation. J. Am. Acad. Dermatol. 2008, 59, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Drake, L.A.; Dinehart, S.M.; Farmer, E.R.; Goltz, R.W.; Graham, G.F.; Hordinsky, M.K.; Lewis, C.W.; Pariser, D.M.; Webster, S.B.; Whitaker, D.C.; et al. Guidelines of care for photoaging/photodamaged. J. Am. Acad. Dermatol. 1996, 35, 462–464. [Google Scholar] [PubMed]
- Davies, M.B.; Austin, J.A.; Patridge, D.A. Vitamin C: Its Chemistry and Biochemistry; Royal Society of Chemistry: London, UK, 1991. [Google Scholar]
- Oresajo, C.; Pillai, S.; Manco, M.; Yatskayer, M.; McDaniel, D. Antioxidants and the skin: Understanding formulation and efficacy. Dermatol. Ther. 2012, 25, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Balagula, Y.; Osterwalder, U. Photoprotection: A review of the current and future technologies. Dermtol. Ther. 2010, 23, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Marrot, L.; Jones, C.; Perez, P.; Meunier, J.P. The significance of Nrf2 pathway in (photo)-oxidative stress response in melanocytes and keratinocytes of the human epidermis. Pigment Cell Melanoma Res. 2008, 21, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; An, C.; Gao, Y.; Leak, R.K.; Chen, J.; Zhang, F. Emerging Roles of Nrf2 and Phase II Antioxidant Enzymes in Neuroprotection. Prog. Neurobiol. 2013, 100, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Baqi, Z.; Feher, A.; Recchia, F.A.; Sonntaq, W.E.; Pearson, K.; de Cabo, R.; Csiszar, A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H18–H24. [Google Scholar] [CrossRef] [PubMed]
- Kode, A.; Rajendrasozhan, S.; Caito, S.; Yang, S.R.; Megson, I.L.; Rahman, I. Resveratrol induces glutathione synthesis by activation of Nrf2 and protects against cigarette smoke-mediated oxidative stress in human lung epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 294, L478–L488. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Fan, C.; Chen, N.; Huang, J.; Yang, Q. Resveratrol pretreatment attenuates cerebral ischemic injury by upregulating expression of transcription factor Nrf2 and HO-1 in rats. Neurochem. Res. 2011, 36, 2352–2362. [Google Scholar] [CrossRef] [PubMed]
- Farris, P.; Yatskayer, M.; Chen, N.; Krol, Y.; Oresajo, C. Evaluation of Efficacy and Tolerance of a Nightime Topical Antioxidant Containing Resveratrol, Baicalin, and Vitamin E for Treatment of Mild to Moderately Photodamaged Skin. J. Drugs Dermatol. 2014, 13, 1467–1472. [Google Scholar] [PubMed]
- Pinnell, S.R.; Yang, H.; Omar, M.; Monteiro-Riviere, N.; DeBuys, H.V.; Walker, L.C.; Wang, Y.; Levine, M. Topical l-ascorbic acid: Percutaneous absorption studies. Dermatol. Surg. 2001, 27, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Shindo, Y.; Witt, E.; Han, D.; Epstein, W.; Packer, L. Enzymic and non-enzymic antioxidants in epidermis and dermis of human skin. J. Investig. Dermatol. 1994, 102, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Frei, B.; England, L.; Ames, B.N. Ascorbate is an outstanding antioxidant in human blood plasma. Proc. Natl. Acad. Sci. USA 1989, 86, 6377–6381. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Asada, K. Monodehydroascorbate reductase from cucumber is a flavin adenine dinucleotide enzyme. J. Biol. Chem. 1985, 260, 12920–12926. [Google Scholar] [PubMed]
- Neuzil, J.; Thomas, S.R.; Stocker, R. Requirement for, promotion, or inhibition by alpha-tocopherol of radical-induced initiation of plasma lipoprotein lipid peroxidation. Free Radic. Biol. Med. 1997, 22, 57–71. [Google Scholar] [CrossRef]
- Lin, F.H.; Lin, J.Y.; Gupta, R.D.; Tournas, J.A.; Burch, J.A.; Selim, M.A.; Monteiro-Riviere, N.A.; Grichnik, J.M.; Zielinski, J.; Pinnell, S.R. Ferulic acid stabilizes a solution of vitamins C and E and doubles its photoprotection of skin. J. Investig. Dermatol. 2005, 125, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Manstein, D.; Herron, G.S.; Sink, R.K.; Tanner, H.; Anderson, R.R. Fractional photothermolysis: A new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg. Med. 2004, 34, 426–438. [Google Scholar] [CrossRef] [PubMed]
- Waibel, J.; Beer, K.; Narurkar, V.; Alster, T. Preliminary observations on fractional ablative resurfacing devices: Clinical impressions. J. Drugs Dermatol. 2009, 8, 481–485. [Google Scholar] [PubMed]
- Pinnell, S.R.; Murad, S.; Darr, D. Induction of Collagen Synthesis by Ascorbic Acid. A Possible Mechanism. Arch. Dermatol. 1987, 123, 1684–1686. [Google Scholar] [CrossRef]
- Nusgens, B.V.; Humbert, P.; Rougier, A.; Richard, A.; Lapiere, C.M. Stimulation of collagen biosynthesis by topically applied vitamin C. Eur. J. Dermatol. 2002, 12, XXXII–XXXIV. [Google Scholar] [PubMed]
- Waibel, J.S.; Mi, Q.S.; Wulkan, A. Split Face Comparison of the Effect of 15% Vitamin C, 1% Vitamin E & Ferulic Acid Serum to Decrease Post-Operative Recovery in Fractional Ablative Laser Resurfacing for Photodamage. In Proceedings of AAD 2014, Denver, CO, USA, 21–25 March 2014.
- Campos, V.; Steiner, D.; Arbache, S.; Ferrara, F.; Santos, K.; Capucho, L. A Prospective Split-Face Double-Blind Randomized Placebo-Controlled Trial to Assess the Efficacy of Vitamin C and Ferulic Acid Serum Post-Fractional Ablative Laser for Skin Rejuvenation. In Proceedings of AAD 2014, Denver, CO, USA, 21–25 March 2014.
- Campos, V.; Steiner, D.; Arbache, S.; Ferrara, F.; Santos, K.; Capucho, L. A Prospective Split-Face Clinical Study to Evaluate the Long-Term Efficacy of Vitamin C and Ferulic Acid Serum Post-Fractional Ablative Laser for Skin Rejuvenation. In Proceedings of AAD 2015, San Francisco, CA, USA, 20–24 March 2015.
- Maeda, K.; Naganuma, M.; Fukuda, M. Effect of chronic exposure to ultraviolet-A including 2% ultraviolet-B on free radical reduction systems in hairless mice. Photochem. Photobiol. 1991, 54, 575–582. [Google Scholar] [CrossRef]
- Thiele, J.J.; Traber, M.G.; Tsang, K.G.; Cross, C.E.; Packer, L. In vivo exposure to ozone depletes vitamin C and E and induces lipid peroxidation in epidermal layers of murine skin. Free Radic. Biol. Med. 1997, 23, 85–91. [Google Scholar] [CrossRef]
- Shindo, Y.; Wit, E.; Han, D.; Packer, L. Dose-response effect of acute ultraviolet irradiation on antioxidants and molecular markers of oxidation in murine epidermis and dermis. J. Investig. Dermatol. 1994, 23, 470–475. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farris, P.K.; Krol, Y. Under Persistent Assault: Understanding the Factors that Deteriorate Human Skin and Clinical Efficacy of Topical Antioxidants in Treating Aging Skin. Cosmetics 2015, 2, 355-367. https://doi.org/10.3390/cosmetics2040355
Farris PK, Krol Y. Under Persistent Assault: Understanding the Factors that Deteriorate Human Skin and Clinical Efficacy of Topical Antioxidants in Treating Aging Skin. Cosmetics. 2015; 2(4):355-367. https://doi.org/10.3390/cosmetics2040355
Chicago/Turabian StyleFarris, Patricia K., and Yevgeniy Krol. 2015. "Under Persistent Assault: Understanding the Factors that Deteriorate Human Skin and Clinical Efficacy of Topical Antioxidants in Treating Aging Skin" Cosmetics 2, no. 4: 355-367. https://doi.org/10.3390/cosmetics2040355
APA StyleFarris, P. K., & Krol, Y. (2015). Under Persistent Assault: Understanding the Factors that Deteriorate Human Skin and Clinical Efficacy of Topical Antioxidants in Treating Aging Skin. Cosmetics, 2(4), 355-367. https://doi.org/10.3390/cosmetics2040355




