Non-Invasive Imaging to Detect the Effects of Topical N-Butanoyl Glutathione (GSH-C4) and Hyaluronic Acid in Inflammatory Eczematous Dermatitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
2.3. Endpoints
2.4. Compliance with Ethical Standards
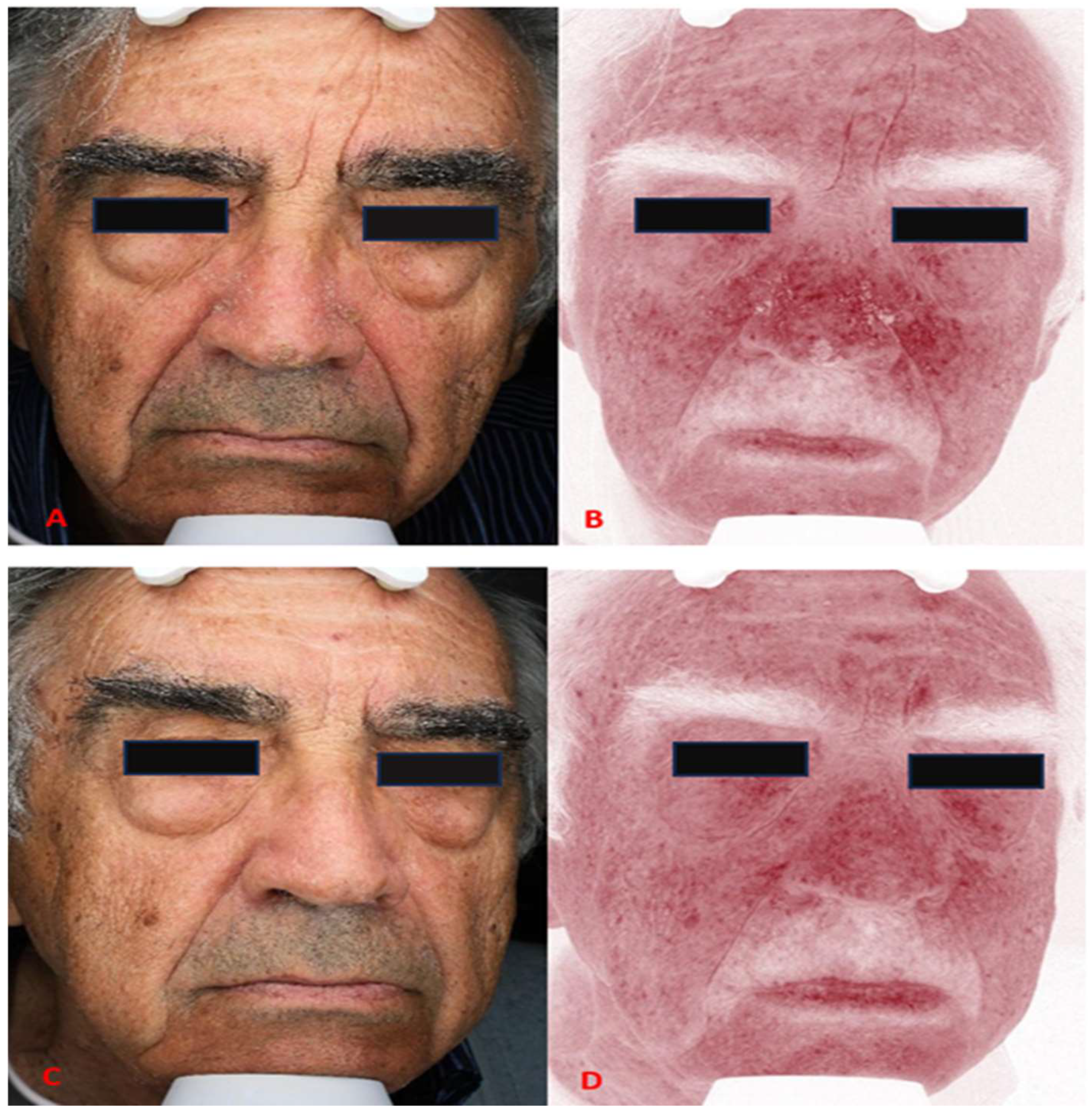
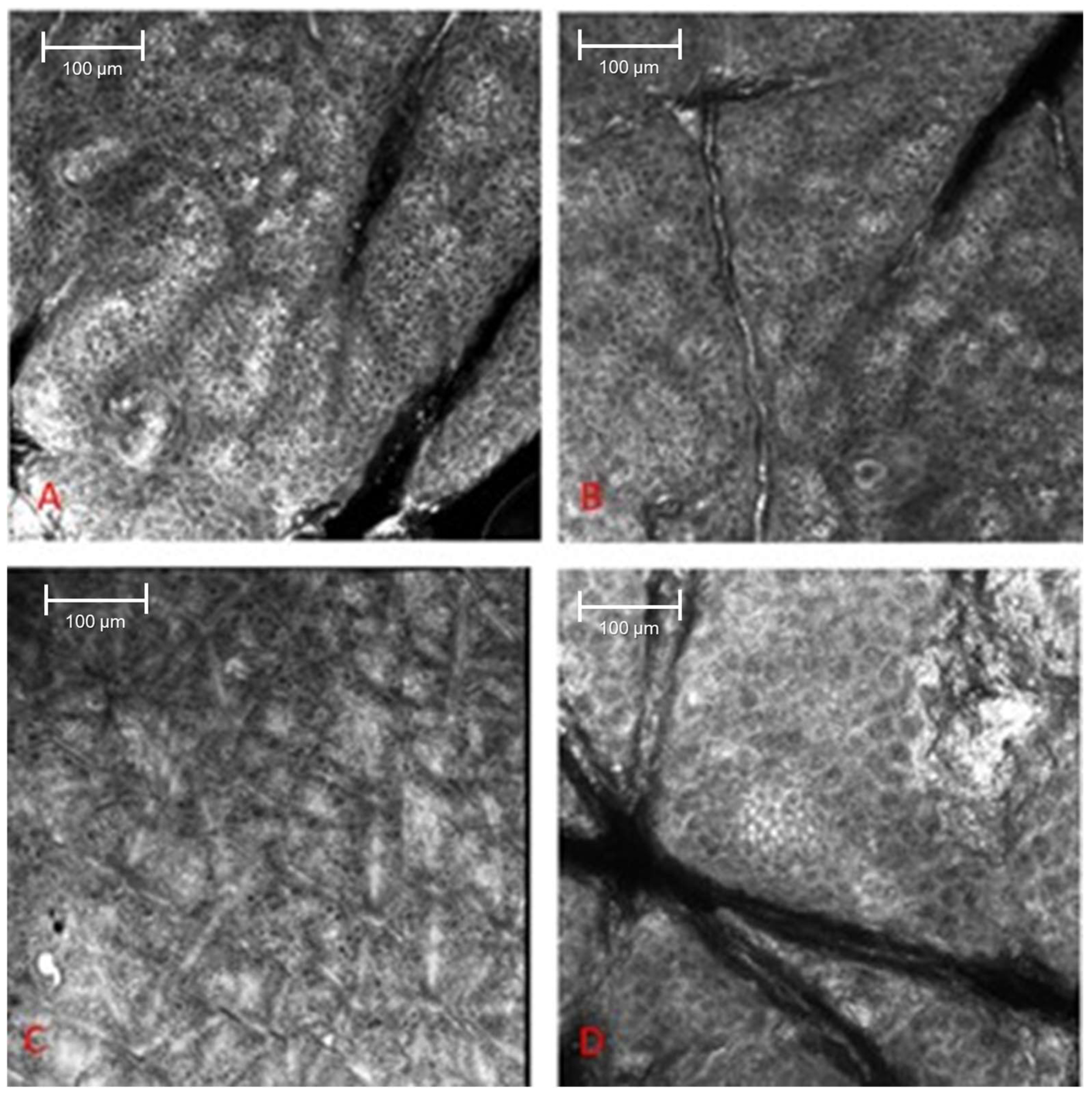
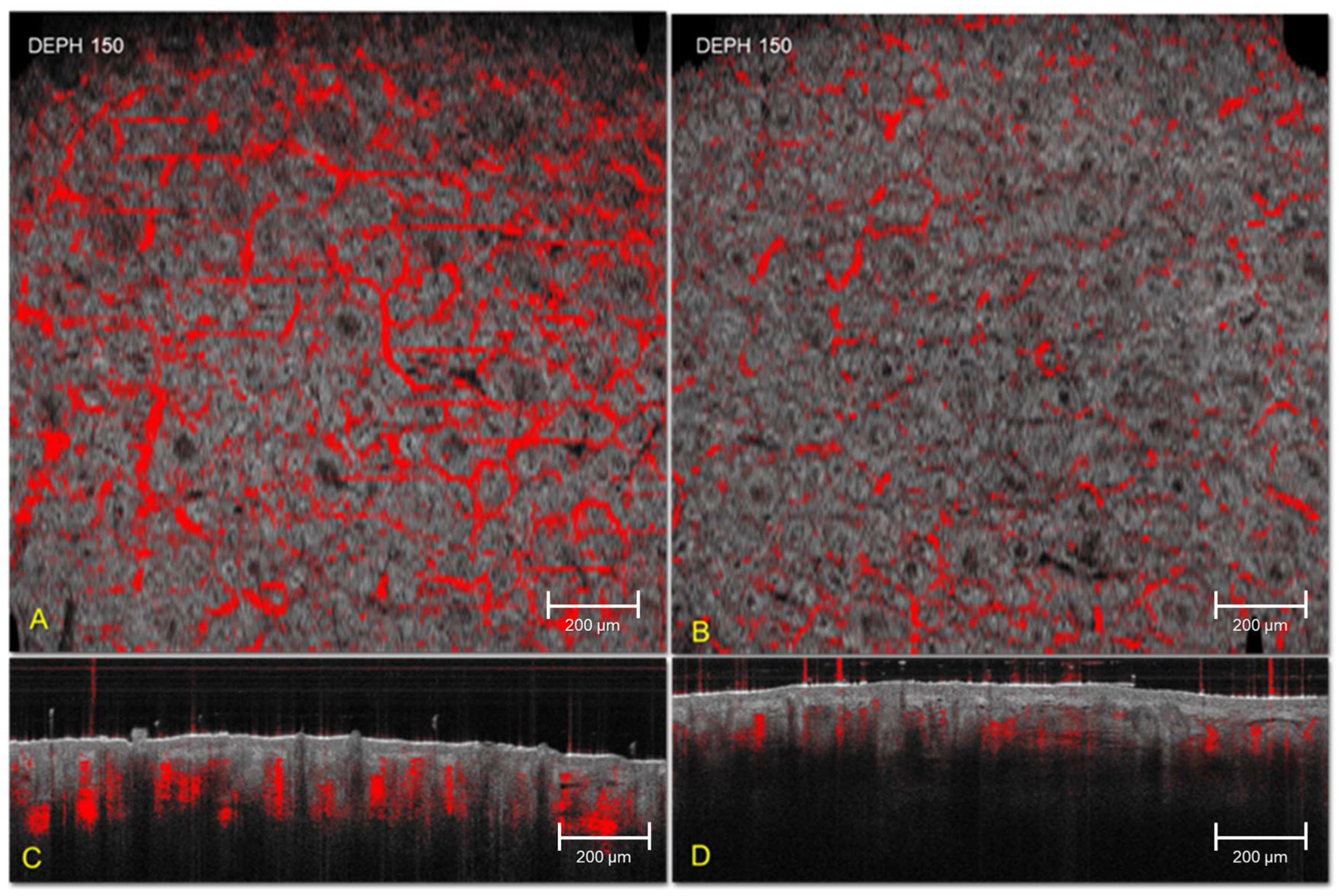
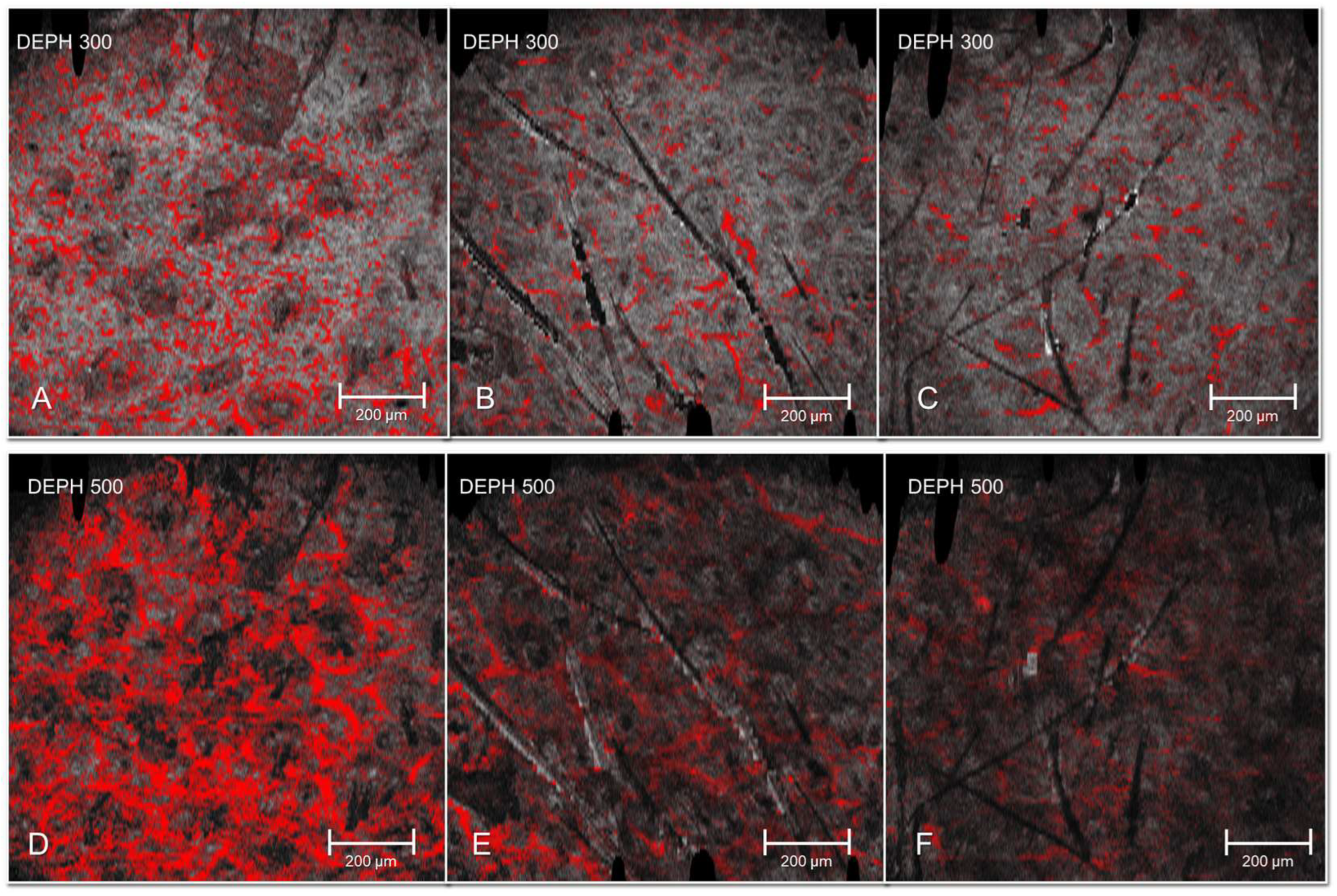
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, H.-J.; Kim, M. Skin Barrier Function and the Microbiome. Int. J. Mol. Sci. 2022, 23, 13071. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811. [Google Scholar] [CrossRef]
- Garzorz-Stark, N.; Weidinger, S.; Sticherling, M.; Ghoreschi, K.; Enk, A.; Eyerich, K. Inflammatory Skin Diseases: The Importance of Immunological Signatures. Dtsch. Ärzteblatt Int. 2025, 122, 277–282. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferček, I.; Lugović-Mihić, L.; Tambić-Andrašević, A.; Ćesić, D.; Grginić, A.G.; Bešlić, I.; Mravak-Stipetić, M.; Mihatov-Štefanović, I.; Buntić, A.M.; Čivljak, R. Features of the Skin Microbiota in Common Inflammatory Skin Diseases. Life 2021, 11, 962. [Google Scholar] [CrossRef]
- Sun, N.; Ogulur, I.; Mitamura, Y.; Yazici, D.; Pat, Y.; Bu, X.; Li, M.; Zhu, X.; Babayev, H.; Ardicli, S.; et al. The epithelial barrier theory and its associated diseases. Allergy 2024, 79, 3192–3237. [Google Scholar] [CrossRef]
- Navarro Triviño, F.J.; Velasco Amador, J.P.; Rivera Ruiz, I. Seborrheic Dermatitis Revisited: Pathophysiology, Diagnosis, and Emerging Therapies—A Narrative Review. Biomedicines 2025, 13, 2458. [Google Scholar] [CrossRef]
- Qi, X.; Yun, C.; Pang, Y.; Qiao, J. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes 2021, 13, 1894070. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, J.; Feng, Y.; Liu, C.; Yang, Z.; de Hoog, S.; Qu, Y.; Chen, B.; Li, D.; Xiong, H.; Shi, D. Presence of Malassezia Hyphae Is Correlated with Pathogenesis of Seborrheic Dermatitis. Microbiol Spectr. 2022, 10, e0116921. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wikramanayake, T.C.; Borda, L.J.; Miteva, M.; Paus, R. Seborrheic dermatitis-Looking beyond Malassezia. Exp. Dermatol. 2019, 28, 991–1001. [Google Scholar] [CrossRef] [PubMed]
- Martin-Loeches, I.; David, S.; Pène, F.; Kreitmann, L.; Russel, L.; Puxty, K.; Silva, J.S.; Mata, A.V.; Creagh-Brown, B.; Castro, P.; et al. Advocating for the Recognition of Underlying Immunosuppression in Critical Illness. EClinicalMedicine 2025, 85, 103300. [Google Scholar] [CrossRef]
- Bieber, T. Atopic dermatitis. N. Engl. J. Med. 2008, 358, 1483–1494. [Google Scholar] [CrossRef]
- Spergel, J.M. Immunology and treatment of atopic dermatitis. Am. J. Clin. Dermatol. 2008, 9, 233–244. [Google Scholar] [CrossRef]
- Li, Y.; Li, L. Contact Dermatitis: Classifications and Management. Clin. Rev. Allergy Immunol. 2021, 61, 245–281. [Google Scholar] [CrossRef]
- Abramovits, W.; Hebert, A.A.; Boguniewicz, M.; Kempers, S.E.; Tschen, E.; Jarratt, M.T.; Lucky, A.W.; Cornelison, R.L.; Swinyer, L.J.; Jones, T.M. Patient-reported outcomes from a multicenter, randomized, vehicle-controlled clinical study of MAS063DP (Atopiclair) in the management of mild-to-moderate atopic dermatitis in adults. J. Dermatolog. Treat. 2008, 19, 327–332. [Google Scholar] [CrossRef]
- Hebert, A.A. Oxidative stress as a treatment target in atopic dermatitis: The role of furfuryl palmitate in mild-to-moderate atopic dermatitis. Int. J. Womens Dermatol. 2020, 6, 331–333. [Google Scholar] [CrossRef]
- Di Guardo, A.; Solito, C.; Cantisani, V.; Rega, F.; Gargano, L.; Rossi, G.; Musolff, N.; Azzella, G.; Paolino, G.; Losco, L.; et al. Clinical and Ultrasound Efficacy of Topical Hypertonic Cream (Jovita Osmocell®) in the Treatment of Cellulite: A Prospective, Monocentric, Double-Blind, Placebo-Controlled Study. Medicina 2024, 60, 781. [Google Scholar] [CrossRef]
- Pigatto, P.D.; Diani, M. Beneficial Effects of Antioxidant Furfuryl Palmitate in Non-pharmacologic Treatments (Prescription Emollient Devices, PEDs) for Atopic Dermatitis and Related Skin Disorders. Dermatol. Ther. 2018, 8, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Li, X.K. Oxidative Stress in Atopic Dermatitis. Oxid. Med. Cell Longev. 2016, 2016, 2721469. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Qin, C.; Wu, J.; Jiang, H.; Xu, Q.; Chen, J.; Xu, X.; Zhang, X.; Guan, M.; Deng, X. The crosstalk between glutathione metabolism and non-coding RNAs in cancer progression and treatment resistance. Redox Biol. 2025, 84, 103689. [Google Scholar] [CrossRef]
- Limongi, D.; Baldelli, S.; Checconi, P.; Marcocci, M.E.; De Chiara, G.; Fraternale, A.; Magnani, M.; Ciriolo, M.R.; Palamara, A.T. GSH-C4 Acts as Anti-inflammatory Drug in Different Models of Canonical and Cell Autonomous Inflammation Through NFκB Inhibition. Front. Immunol. 2019, 10, 155. [Google Scholar]
- Agozzino, M.; Gonzalez, S.; Ardigò, M. Reflectance Confocal Microscopy for Inflammatory Skin Diseases. Actas Dermo-Sifiliográficas 2016, 107, 631–639. [Google Scholar] [CrossRef]
- Schuh, S.; Holmes, J.; Ulrich, M.; Themstrup, L.; Jemec, G.B.E.; De Carvalho, N.; Pellacani, G.; Welzel, J. Imaging Blood Vessel Morphology in Skin: Dynamic Optical Coherence Tomography as a Novel Potential Diagnostic Tool in Dermatology. Dermatol. Ther. 2017, 7, 187–202. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Bissonnette, R.; Paller, A.S.; King, B.; Silverberg, J.I.; Reich, K.; Thyssen, J.P.; Doll, H.; Sun, L.; DeLozier, A.M.; et al. The Validated Investigator Global Assessment for Atopic Dermatitis (vIGA-AD™): A clinical outcome measure for the severity of atopic dermatitis. Br. J. Dermatol. 2022, 187, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Dall’Oglio, F.; Nasca, M.R.; Gerbino, C.; Micali, G. An Overview of the Diagnosis and Management of Seborrheic Dermatitis. Clin. Cosmet. Investig. Dermatol. 2022, 15, 1537–1548. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.H.; Kim, S.M.; Eun, D.H.; Park, K.D.; Park, G.H.; Kim, B.S.; Li, K.; Park, C.O.; Kim, H.O.; Kim, H.S.; et al. Validity and reliability of itch assessment scales for chronic pruritus in adults: A prospective multicenter study. J. Am. Acad. Dermatol. 2020, 82, 80–86. [Google Scholar] [CrossRef]
- Zolotas, M.; Schleusener, J.; Lademann, J.; Meinke, M.C.; Kokolakis, G.; Darvin, M.E. Atopic Dermatitis: Molecular Alterations between Lesional and Non-Lesional Skin Determined Noninvasively by In Vivo Confocal Raman Microspectroscopy. Int. J. Mol. Sci. 2023, 24, 14636. [Google Scholar] [CrossRef]
- Maarouf, M.; Costello, C.M.; Gonzalez, S.; Angulo, I.; Curiel-Lewandrowski, C.N.; Shi, Y.V. In Vivo Reflectance Confocal Microscopy: Emerging Role in Noninvasive Diagnosis and Monitoring of Eczematous Dermatoses. Actas. Dermo.-Sifiliográficas 2019, 110, 626–636. [Google Scholar] [CrossRef]
- Campione, E.; Mazzilli, S.; Di Prete, M.; Dattola, A.; Cosio, T.; Lettieri Barbato, D.; Costanza, G.; Lanna, C.; Manfreda, V.; Gaeta Schumak, R.; et al. The Role of Glutathione-S Transferase in Psoriasis and Associated Comorbidities and the Effect of Dimethyl Fumarate in This Pathway. Front. Med. 2022, 9, 9760852. [Google Scholar] [CrossRef]
- Campione, E.; Mazzilli, S.; Lanna, C.; Cosio, T.; Palumbo, V.; Cesaroni, G.; Lozzi, F.; Diluvio, L.; Bianchi, L. The Effectiveness of a New Topical Formulation Containing GSH-C4 and Hyaluronic Acid in Seborrheic Dermatitis: Preliminary Results of an Exploratory Pilot Study. Clin. Cosmet. Investig. Dermatol. 2019, 12, 881–885. [Google Scholar] [CrossRef]
- Dall’Oglio, F.; Puviani, M.; Milani, M.; Micali, G. Efficacy and tolerability of a cream containing modified glutathione (GSH-C4), beta-Glycyrrhetic, and azelaic acids in mild-to-moderate rosacea: A pilot, assessor-blinded, VISIA and ANTERA 3-D analysis, two-center study (The “Rosazel” Trial). J. Cosmet. Dermatol. 2021, 20, 1197–1203. [Google Scholar] [CrossRef]
- Gur, T.F.; Erdemir, A.V.; Gurel, M.S.; Kocyigit, A.; Guler, E.M.; Erdil, D. The investigation of the relationships of demodex density with inflammatory response and oxidative stress in rosacea. Arch. Dermatol. Res. 2018, 310, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Rams, A.; Baldasaro, J.; Bunod, L.; Delbecque, L.; Strzok, S.; Meunier, J.; ElMaraghy, H.; Sun, L.; Pierce, E. Assessing Itch Severity: Content Validity and Psychometric Properties of a Patient-Reported Pruritus Numeric Rating Scale in Atopic Dermatitis. Adv. Ther. 2024, 41, 1512–1525. [Google Scholar] [CrossRef] [PubMed]
- Bratu, D.; Boda, D.; Caruntu, C. Reflectance Confocal Microscopy in Monitoring Atopic Dermatitis Treated with Topical Calcineurin Inhibitors. Healthcare 2023, 11, 152. [Google Scholar] [CrossRef]
- Alessandrello, C.; Sanfilippo, S.; Minciullo, P.L.; Gangemi, S. An Overview on Atopic Dermatitis, Oxidative Stress, and Psychological Stress: Possible Role of Nutraceuticals as an Additional Therapeutic Strategy. Int. J. Mol. Sci. 2024, 25, 5020. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.; Lax, S.J.; Lowe, A.; Santer, M.; Lawton, S.; Langan, S.M.; Roberts, A.; Stuart, B.; Williams, H.C.; Thomas, K.S. The long-term safety of topical corticosteroids in atopic dermatitis: A systematic review. Skin Health Dis. 2023, 3, e268. [Google Scholar] [CrossRef]
- Fraternale, A.; Brundu, S.; Magnani, M. Glutathione and glutathione derivatives in immunotherapy. Biol. Chem. 2017, 398, 261–275. [Google Scholar] [CrossRef]
- Ong, P.Y.; Leung, D.Y. Bacterial and Viral Infections in Atopic Dermatitis: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 51, 329–337. [Google Scholar] [CrossRef]
| T0 | T1 | T2 | Delta T1-T0 | Delta T2-T0 | |
|---|---|---|---|---|---|
| Parameters | Mean (St. dev.) | Mean (St. dev.) | Mean (St. dev.) | Mean (St. dev.) | Delta St. Dev |
| IGA (0–4) | 2.48 (0.63) | 1.23 * (0.62) | 0.18 * (0.39) | −1.26 * (0.77) | −2.36 * (0.73) |
| VAS (0–10) | 4.52 (2.34) | 2.10 * (2.18) | 0.32 * (1.33) | −2.42 * (2.06) | −4.25 * (2.15) |
| IDL (0–10) | 4.86 (3.13) | 3.10 * (2.66) | 0.79 * (1.45) | −1.45 * (2.00) | −3.75 * (3.04) |
| T0 | T1 | T2 | Delta T1-T0 | Delta T2-T0 | |
|---|---|---|---|---|---|
| Parameter | Mean (St. dev) | Mean (St. dev) | Mean (St. dev) | Mean (St. dev.) | Mean (St. dev.) |
| RCM exocytosis (0–3) | 1.50 (0.68) | 1.10 * (0.62) | 0.50 ** (0.51) | −0.43 * (0.63) | −1.04 ** (0.71) |
| RCM Spongiosis (0–3) | 1.40 (0.67) | 0.90 ** (0.77) | 0.32 ** (0.48) | −0.46 ** (0.58) | −1.04 ** (0.65) |
| RCM vesiculation (0–3) | 0.27 (0.58) | 0.10 * (0.31) | 0.04 * (0.19) | −0.21 * (0.50) | −0.15 * (0.36) |
| RCM inflammation (0–3) | 1.70 (0.47) | 1.10 ** (0.49) | 0.46 ** (0.51) | −0.57 ** (0.57) | −1.22 ** (0.70) |
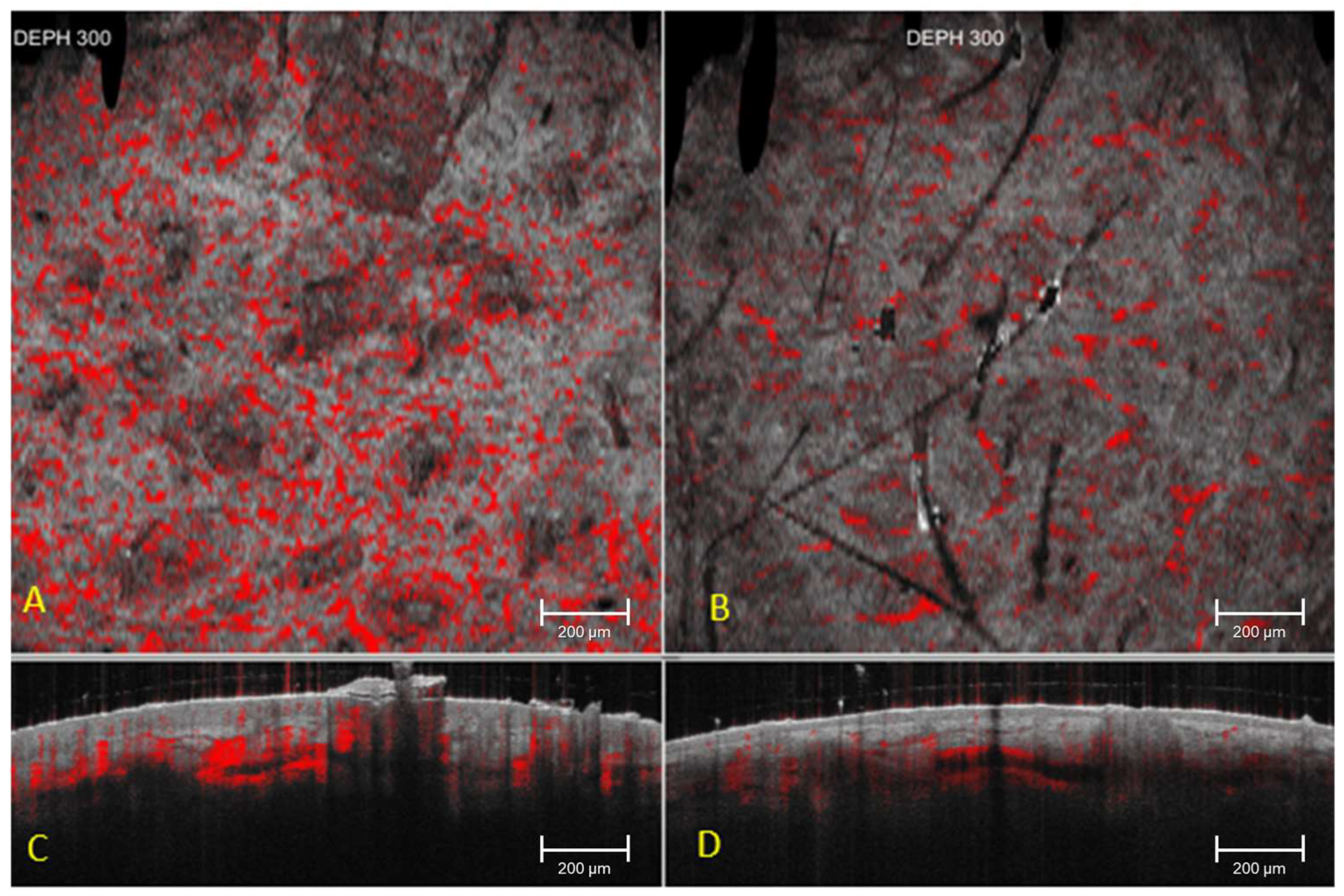
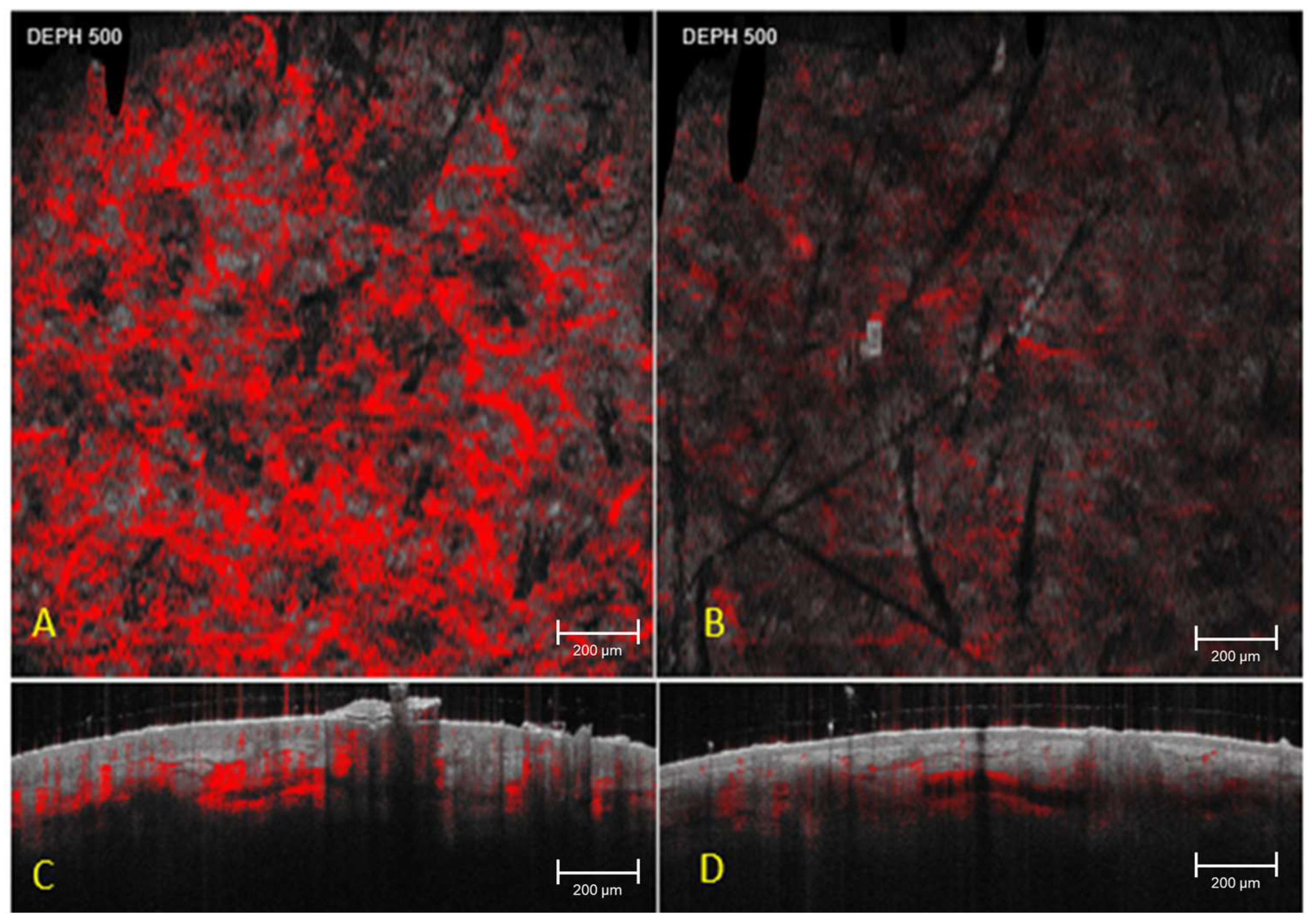
| Parameter | Depth (µm) | T0 Mean ± SD | T1 Mean ± SD | T2 Mean ± SD | p Value T0 vs. T2 |
|---|---|---|---|---|---|
| Vascular network area (pixels) | 150 | 3237 ± 4419 | 3105 ± 4280 | 1630 ± 2500 * | 0.003 * |
| Vascular network area (pixels) | 300 | 2780 ± 3650 | 2710 ± 3520 | 2640 ± 3480 | 0.24 |
| Vascular network area (pixels) | 500 | 2050 ± 3050 | 2020 ± 3010 | 1990 ± 2980 | 0.31 |
| Stromal density index (a.u., 0–100) | 300 | 58.4 ± 12.1 | 58.9 ± 12.0 | 59.2 ± 11.8 | 0.27 |
| Stromal attenuation index (a.u., 0–100) | 300 | 61.2 ± 11.5 | 60.8 ± 11.3 | 60.5 ± 11.1 | 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, M.E.; Di Guardo, A.; Dattola, A.; Ciardo, S.; Campione, E.; Marrapodi, D.; Chello, C.; Cantisani, C.; Michelini, S.; Cosio, T.; et al. Non-Invasive Imaging to Detect the Effects of Topical N-Butanoyl Glutathione (GSH-C4) and Hyaluronic Acid in Inflammatory Eczematous Dermatitis. Cosmetics 2025, 12, 280. https://doi.org/10.3390/cosmetics12060280
Greco ME, Di Guardo A, Dattola A, Ciardo S, Campione E, Marrapodi D, Chello C, Cantisani C, Michelini S, Cosio T, et al. Non-Invasive Imaging to Detect the Effects of Topical N-Butanoyl Glutathione (GSH-C4) and Hyaluronic Acid in Inflammatory Eczematous Dermatitis. Cosmetics. 2025; 12(6):280. https://doi.org/10.3390/cosmetics12060280
Chicago/Turabian StyleGreco, Maria Elisabetta, Antonio Di Guardo, Annunziata Dattola, Silvana Ciardo, Elena Campione, Domenico Marrapodi, Camilla Chello, Carmen Cantisani, Simone Michelini, Terenzio Cosio, and et al. 2025. "Non-Invasive Imaging to Detect the Effects of Topical N-Butanoyl Glutathione (GSH-C4) and Hyaluronic Acid in Inflammatory Eczematous Dermatitis" Cosmetics 12, no. 6: 280. https://doi.org/10.3390/cosmetics12060280
APA StyleGreco, M. E., Di Guardo, A., Dattola, A., Ciardo, S., Campione, E., Marrapodi, D., Chello, C., Cantisani, C., Michelini, S., Cosio, T., Amato, S., Garaci, E., Crimi, R., Nisticò, S. P., & Pellacani, G. (2025). Non-Invasive Imaging to Detect the Effects of Topical N-Butanoyl Glutathione (GSH-C4) and Hyaluronic Acid in Inflammatory Eczematous Dermatitis. Cosmetics, 12(6), 280. https://doi.org/10.3390/cosmetics12060280










