Assessment of Safety and Tissue Integration of PEGDE-Based Hyaluronic Acid Filler for Severe Nasolabial Folds: A Prospective Observational Study with Biophysical and Ultrasound Evaluation
Abstract
1. Introduction
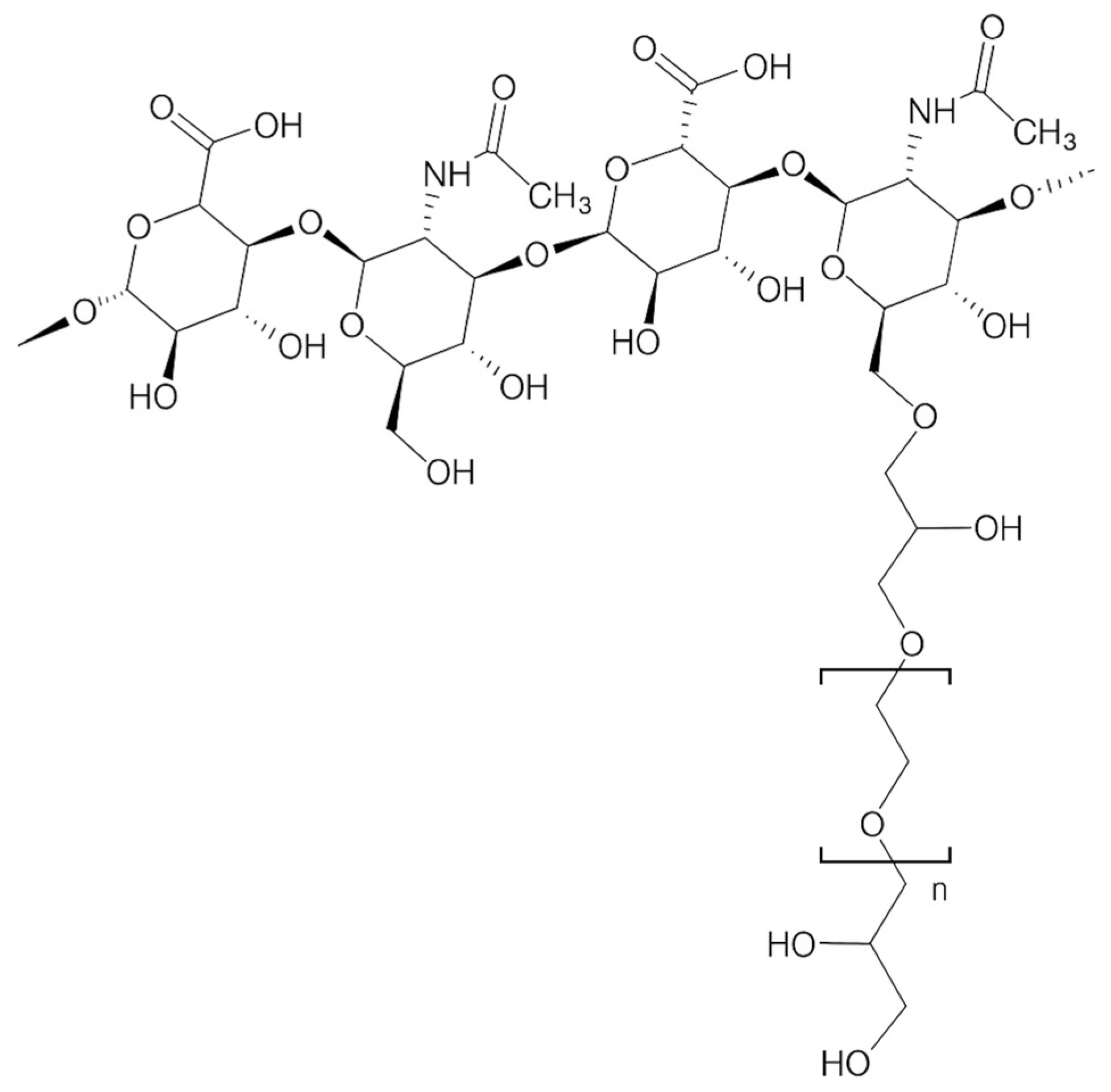
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Treatment Protocol
2.4. Safety Monitoring and Assessments
2.5. Biophysical Measurements
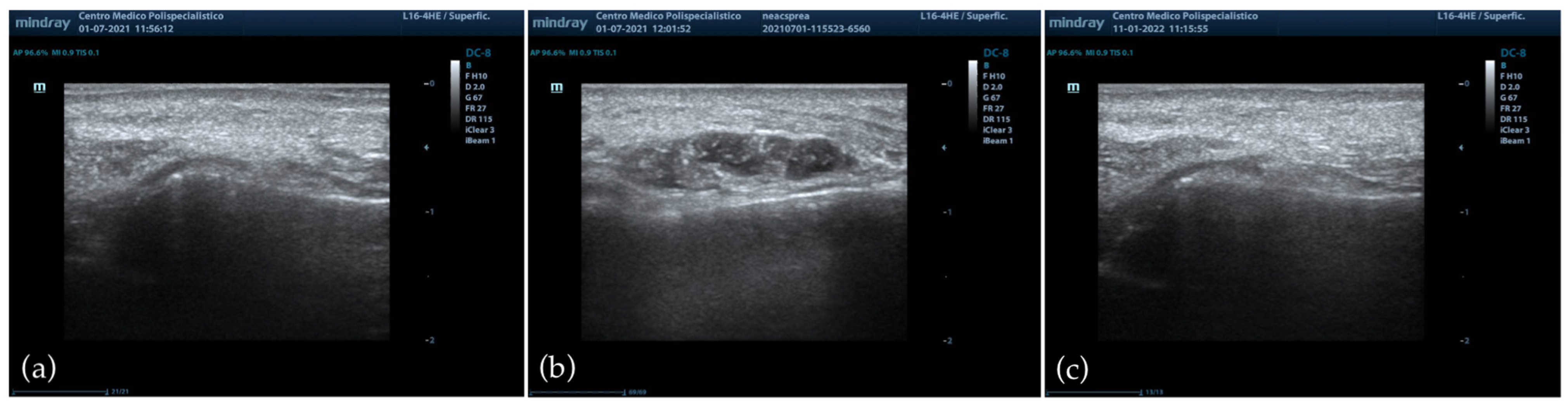
2.6. Ultrasound Evaluation
2.7. Statistical Analysis
3. Results
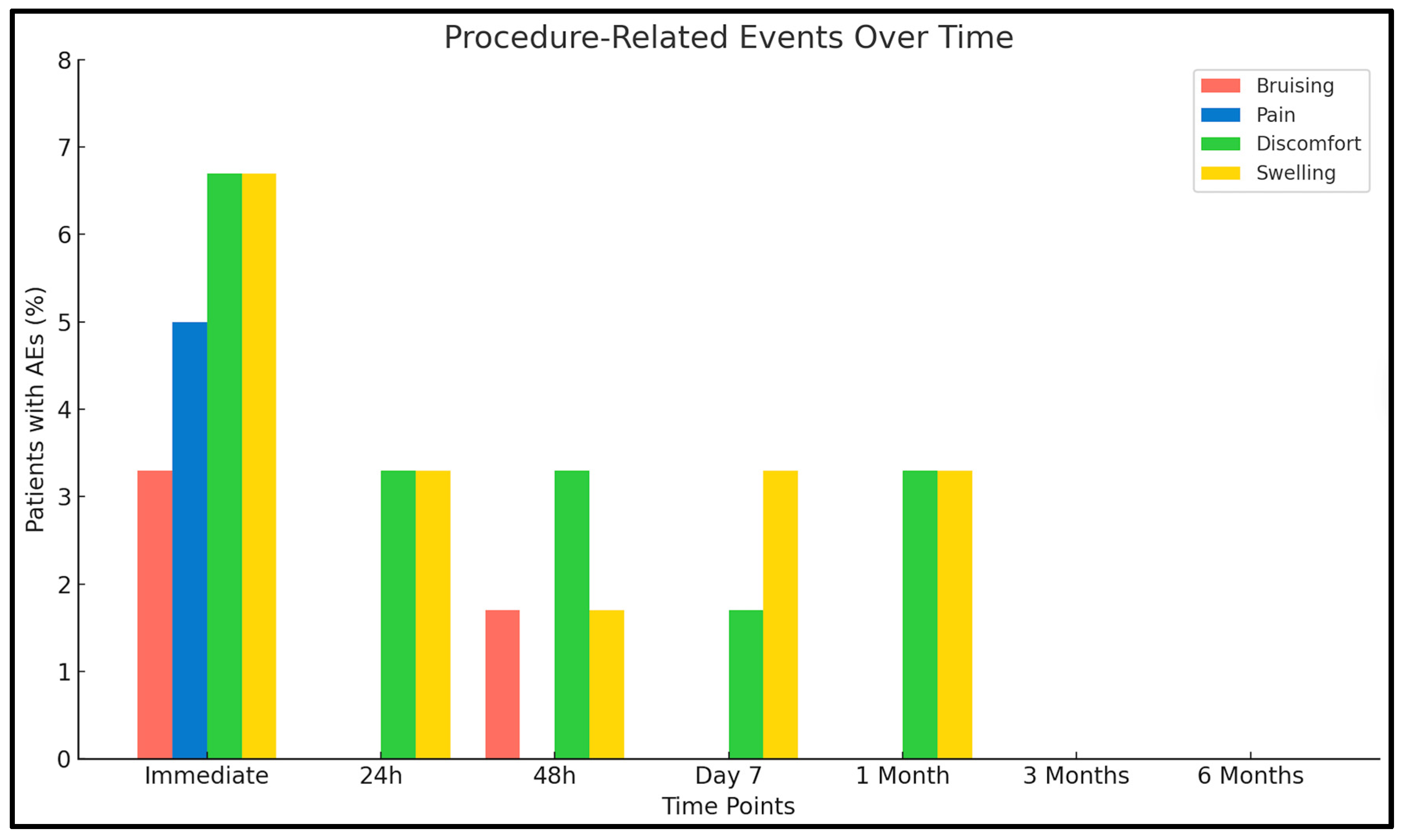
3.1. Adverse Events
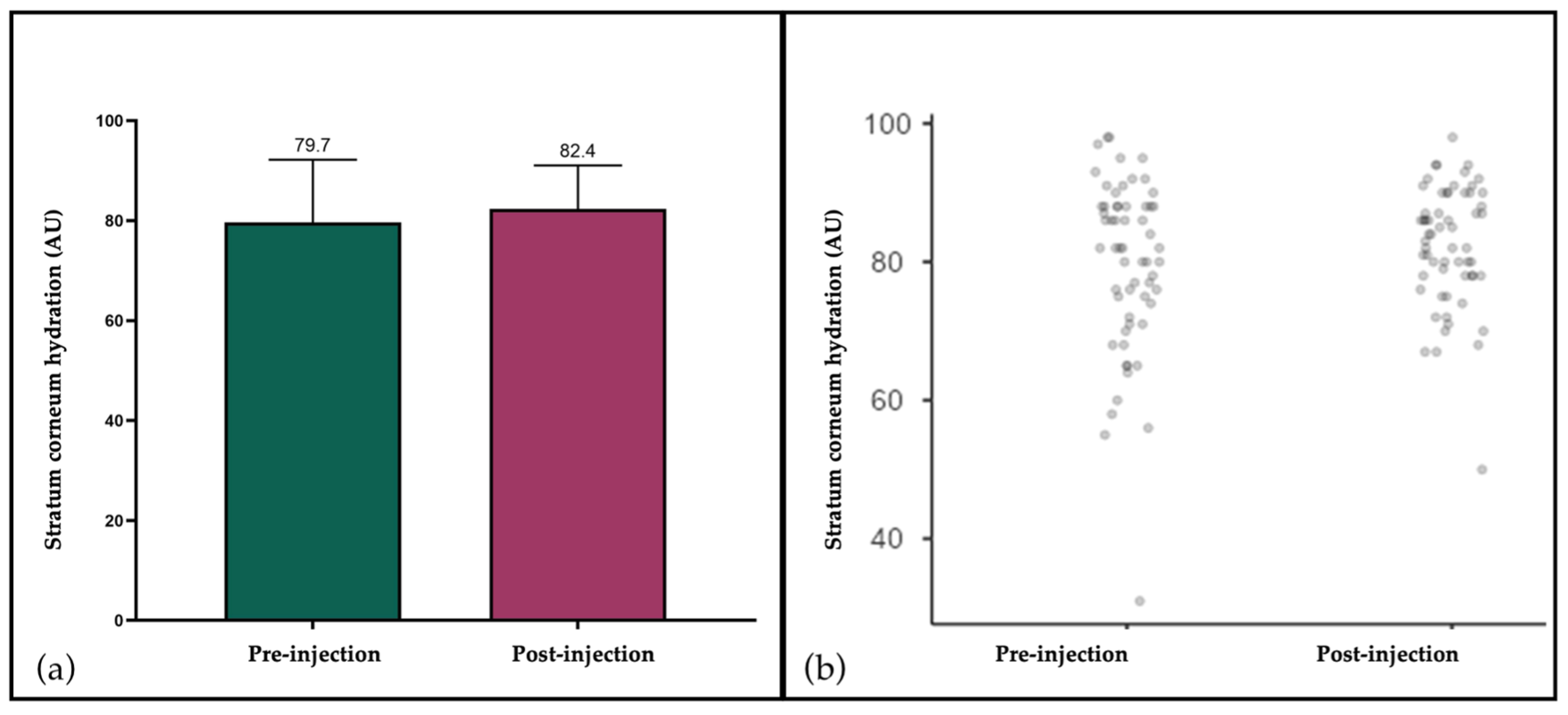
3.2. Corneometry
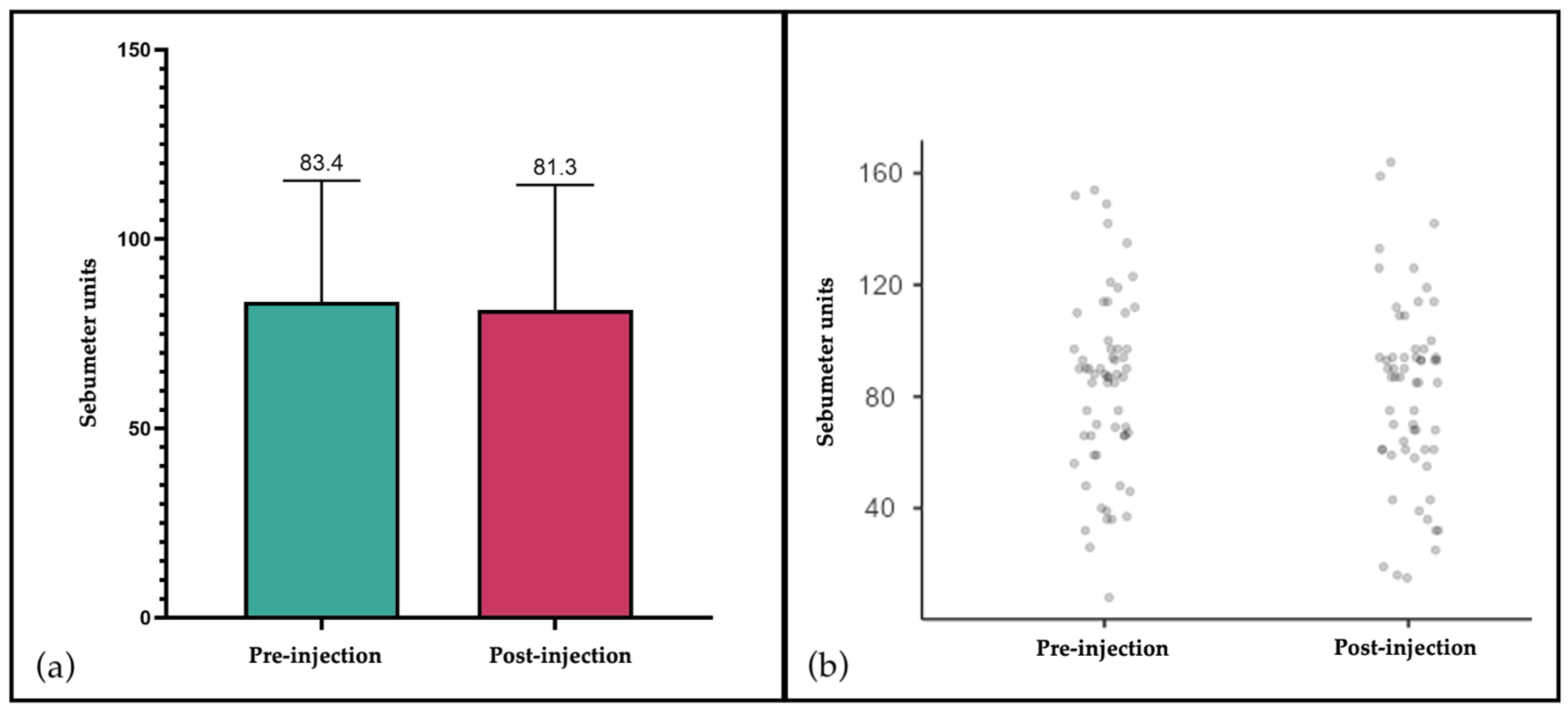
3.3. Sebumetry
3.4. Ultrasound Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE(s) | Adverse Event(s) |
| SAE(s) | Serious Adverse Event(s) |
| HA | Hyaluronic Acid |
| NLF(s) | Nasolabial Fold(s) |
| BDDE | 1,4-Butanediol Diglycidyl Ether |
| PEGDE | Polyethylene Glycol Diglycidyl Ether |
| PEG | Polyethylene Glycol |
| HFUS | High-Frequency Ultrasound |
| AU(s) | Arbitrary Unit(s) |
| SD | Standard Deviation |
| CI | Confidence Interval |
| ICH | International Conference on Harmonization |
| GCP | Good Clinical Practice |
References
- Fallacara, A.; Manfredini, S.; Durini, E.; Vertuani, S. Hyaluronic Acid Fillers in Soft Tissue Regeneration. Facial Plast. Surg. 2017, 33, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Bogdan Allemann, I.; Baumann, L. Hyaluronic acid gel (Juvéderm) preparations in the treatment of facial wrinkles and folds. Clin. Interv. Aging 2008, 3, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Funt, D.; Pavicic, T. Dermal fillers in aesthetics: An overview of adverse events and treatment approaches. Clin. Cosmet. Investig. Dermatol. 2013, 6, 295–316. [Google Scholar] [CrossRef] [PubMed]
- Fagien, S.; Monheit, G.; Jones, D.; Bank, D.; Sadick, N.; Nogueira, A.; Mashburn, J.H. Hyaluronic Acid Gel With (HARRL) and Without Lidocaine (HAJU) for the Treatment of Moderate-to-Severe Nasolabial Folds: A Randomized, Evaluator-Blinded, Phase III Study. Dermatol. Surg. 2018, 44, 549–556. [Google Scholar] [CrossRef]
- Sattler, G.; Philipp-Dormston, W.G.; Van Den Elzen, H.; Van Der Walt, C.; Nathan, M.; Kolodziejczyk, J.; Kerson, G.; Dhillon, B. A Prospective, Open-Label, Observational, Postmarket Study Evaluating VYC-17.5L for the Correction of Moderate to Severe Nasolabial Folds Over 12 Months. Dermatol. Surg. 2017, 43, 238–245. [Google Scholar] [CrossRef]
- Monheit, G.; Beer, K.; Hardas, B.; Grimes, P.E.; Weichman, B.M.; Lin, V.; Murphy, D.K. Safety and Effectiveness of the Hyaluronic Acid Dermal Filler VYC-17.5L for Nasolabial Folds: Results of a Randomized, Controlled Study. Dermatol. Surg. 2018, 44, 670–678. [Google Scholar] [CrossRef]
- Philipp-Dormston, W.G.; Eccleston, D.; De Boulle, K.; Hilton, S.; van den Elzen, H.; Nathan, M. A prospective, observational study of the volumizing effect of open-label aesthetic use of Juvéderm® VOLUMA® with Lidocaine in mid-face area. J. Cosmet. Laser Ther. 2014, 16, 171–179. [Google Scholar] [CrossRef]
- Dayan, S.; Maas, C.S.; Grimes, P.E.; Beer, K.; Monheit, G.; Snow, S.; Murphy, D.K.; Lin, V. Safety and Effectiveness of VYC-17.5L for Long-Term Correction of Nasolabial Folds. Aesthet. Surg. J. 2020, 40, 767–777. [Google Scholar] [CrossRef]
- Liu, M.H.; Beynet, D.P.; Gharavi, N.M. Overview of Deep Dermal Fillers. Facial Plast. Surg. 2019, 35, 224–229. [Google Scholar] [CrossRef]
- Sobanko, J.F.; Dai, J.; Gelfand, J.M.; Sarwer, D.B.; Percec, I. Prospective Cohort Study Investigating Changes in Body Image, Quality of Life, and Self-Esteem Following Minimally Invasive Cosmetic Procedures. Dermatol. Surg. 2018, 44, 1121–1128. [Google Scholar] [CrossRef]
- Doyon, V.C.; Liu, C.; Fitzgerald, R.; Humphrey, S.; Jones, D.; Carruthers, J.D.A.; Beleznay, K. Update on Blindness from Filler: Review of Prognostic Factors, Management Approaches, and a Century of Published Cases. Aesthet. Surg. J. 2024, 44, 1091–1104. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.E.; Ahluwalia, J.; Song, S.S.; Avram, M.M. Analysis of U.S. Food and Drug Administration Data on Soft-Tissue Filler Complications. Dermatol. Surg. 2020, 46, 958–961. [Google Scholar] [CrossRef] [PubMed]
- DeLorenzi, C. New High Dose Pulsed Hyaluronidase Protocol for Hyaluronic Acid Filler Vascular Adverse Events. Aesthet. Surg. J. 2017, 37, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Kroumpouzos, G.; Treacy, P. Hyaluronidase for Dermal Filler Complications: Review of Applications and Dosage Recommendations. JMIR Dermatol. 2024, 7, e50403. [Google Scholar] [CrossRef] [PubMed]
- Žádníková, P.; Šínová, R.; Pavlík, V.; Šimek, M.; Šafránková, B.; Hermannová, M.; Nešporová, K.; Velebný, V. The Degradation of Hyaluronan in the Skin. Biomolecules 2022, 12, 251. [Google Scholar] [CrossRef]
- Chylińska, N.; Maciejczyk, M. Hyaluronic Acid and Skin: Its Role in Aging and Wound-Healing Processes. Gels 2025, 11, 281. [Google Scholar] [CrossRef]
- Fruijtier-Pölloth, C. Safety assessment on polyethylene glycols (PEGs) and their derivatives as used in cosmetic products. Toxicology 2005, 214, 1–38. [Google Scholar] [CrossRef]
- Tripodo, G.; Trapani, A.; Torre, M.L.; Giammona, G.; Trapani, G.; Mandracchia, D. Hyaluronic acid and its derivatives in drug delivery and imaging: Recent advances and challenges. Eur. J. Pharm. Biopharm. 2015, 97, 400–416. [Google Scholar] [CrossRef]
- Lam, J.; Truong, N.F.; Segura, T. Design of cell-matrix interactions in hyaluronic acid hydrogel scaffolds. Acta Biomater. 2014, 10, 1571–1580. [Google Scholar] [CrossRef]
- Faivre, J.; Pigweh, A.I.; Iehl, J.; Maffert, P.; Goekjian, P.; Bourdon, F. Crosslinking hyaluronic acid soft-tissue fillers: Current status and perspectives from an industrial point of view. Expert Rev. Med. Devices 2021, 18, 1175–1187. [Google Scholar] [CrossRef]
- Faivre, J.; Gallet, M.; Tremblais, E.; Trévidic, P.; Bourdon, F. Advanced Concepts in Rheology for the Evaluation of Hyaluronic Acid-Based Soft Tissue Fillers. Dermatol. Surg. 2021, 47, e159–e167. [Google Scholar] [CrossRef] [PubMed]
- Puljic, A.; Frank, K.; Cohen, J.; Otto, K.; Mayr, J.; Hugh-Bloch, A.; Kuroki-Hasenöhrl, D. A Scientific Framework for Comparing Hyaluronic Acid Filler Crosslinking Technologies. Gels 2025, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Marino, F.; Cosentino, M.; Legnaro, M.; Luini, A.; Sigova, J.; Mocchi, R.; Lotti, T.; Zerbinati, N. Immune profile of hyaluronic acid hydrogel polyethylene glycol crosslinked: An in vitro evaluation in human polymorphonuclear leukocytes. Dermatol. Ther. 2020, 33, e13388. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.G.; Lin, P.; Schmidt, C.E. Biodegradable hydrogels composed of oxime crosslinked poly(ethylene glycol), hyaluronic acid and collagen: A tunable platform for soft tissue engineering. J. Biomater. Sci. Polym. Ed. 2015, 26, 143–161. [Google Scholar] [CrossRef]
- Kim, D.H.; Jeong, C.H.; Han, J.H.; Lim, S.J.; Kwon, H.C.; Kim, Y.J.; Keum, D.H.; Lee, K.H.; Han, S.G. Comparative toxicity study of hyaluronic acid fillers crosslinked with 1,4-butanediol diglycidyl ether or poly (ethylene glycol) diglycidyl ether. Int. J. Biol. Macromol. 2025, 296, 139620. [Google Scholar] [CrossRef]
- Zerbinati, N.; Lotti, T.; Monticelli, D.; Rauso, R.; González-Isaza, P.; D’Este, E.; Calligaro, A.; Sommatis, S.; Maccario, C.; Mocchi, R.; et al. In Vitro Evaluation of the Biosafety of Hyaluronic Acid PEG Cross-Linked with Micromolecules of Calcium Hydroxyapatite in Low Concentration. Open Access Maced. J. Med. Sci. 2018, 6, 15–19. [Google Scholar] [CrossRef]
- Smith, L.; Cockerham, K. Hyaluronic acid dermal fillers: Can adjunctive lidocaine improve patient satisfaction without decreasing efficacy or duration? Patient Prefer. Adherence 2011, 5, 133–139. [Google Scholar] [CrossRef][Green Version]
- Rattanawiwatpong, P.; Wanitphakdeedecha, R.; Bumrungpert, A.; Maiprasert, M. Anti-aging and brightening effects of a topical treatment containing vitamin C, vitamin E, and raspberry leaf cell culture extract: A split-face, randomized controlled trial. J. Cosmet. Dermatol. 2020, 19, 671–676. [Google Scholar] [CrossRef]
- Hsu, C.K.; Cheng, N.Y.; Yang, C.C.; Yen, Y.Y.; Tseng, S.H. Investigating the clinical implication of corneometer and mexameter readings towards objective, efficient evaluation of psoriasis vulgaris severity. Sci. Rep. 2022, 12, 7469. [Google Scholar] [CrossRef]
- Ezerskaia, A.; Pereira, S.F.; Urbach, H.P.; Verhagen, R.; Varghese, B. Quantitative and simultaneous non-invasive measurement of skin hydration and sebum levels. Biomed. Opt. Express 2016, 7, 2311–2320. [Google Scholar] [CrossRef]
- Kim, S.A.; Kim, B.R.; Chun, M.Y.; Youn, S.W. Relation between pH in the Trunk and Face: Truncal pH Can Be Easily Predicted from Facial pH. Ann. Dermatol. 2016, 28, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Léger, D.; Gauriau, C.; Etzi, C.; Ralambondrainy, S.; Heusèle, C.; Schnebert, S.; Dubois, A.; Gomez-Merino, D.; Dumas, M. “You look sleepy…” The impact of sleep restriction on skin parameters and facial appearance of 24 women. Sleep. Med. 2022, 89, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Jia, Q.N.; Jin, H.Z.; Li, F.; He, C.; Yang, J.; Zuo, Y.; Fu, L. Long-Term Follow-Up of Longevity and Diffusion Pattern of Hyaluronic Acid in Nasolabial Fold Correction through High-Frequency Ultrasound. Plast. Reconstr. Surg. 2019, 144, 189e–196e. [Google Scholar] [CrossRef] [PubMed]
- Merola, F.; Scrima, M.; Melito, C.; Iorio, A.; Pisano, C.; Giori, A.M.; Ferravante, A. A novel animal model for residence time evaluation of injectable hyaluronic acid-based fillers using high-frequency ultrasound-based approach. Clin. Cosmet. Investig. Dermatol. 2018, 11, 339–346. [Google Scholar] [CrossRef]
- Mlosek, R.K.; Migda, B.; Skrzypek, E.; Słoboda, K.; Migda, M. The use of high-frequency ultrasonography for the diagnosis of palpable nodules after the administration of dermal fillers. J. Ultrason. 2021, 20, e248–e253. [Google Scholar] [CrossRef]
- Guida, S.; Arginelli, F.; Farnetani, F.; Ciardo, S.; Bertoni, L.; Manfredini, M.; Zerbinati, N.; Longo, C.; Pellacani, G. Clinical Applications of In Vivo and Ex Vivo Confocal Microscopy. Appl. Sci. 2021, 11, 1979. [Google Scholar] [CrossRef]
- Zerbinati, N.; Rauso, R.; Protasoni, M.; D’Este, E.; Esposito, C.; Lotti, T.; Tirant, M.; Van Thuong, N.; Mocchi, R.; Zerbinati, U.; et al. Pegylated hyaluronic acid filler enriched with calcium hydroxyapatite treatment of human skin: Collagen renewal demonstrated through morphometric computerized analysis. J. Biol. Regul. Homeost. Agents 2019, 33, 1967–1971. [Google Scholar] [CrossRef]
- Liang, M.; Dong, L.; Guo, Z.; Liu, L.; Fan, Z.; Wei, C.; Mi, S.; Sun, W. Collagen-Hyaluronic Acid Composite Hydrogels with Applications for Chronic Diabetic Wound Repair. ACS Biomater. Sci. Eng. 2023, 9, 5376–5388. [Google Scholar] [CrossRef]
- Karagaiah, P.; Valle, Y.; Sigova, J.; Zerbinati, N.; Vojvodic, P.; Parsad, D.; Schwartz, R.A.; Grabbe, S.; Goldust, M.; Lotti, T. Emerging drugs for the treatment of vitiligo. Expert. Opin. Emerg. Drugs 2020, 25, 7–24. [Google Scholar] [CrossRef]
- The Jamovi Project. Jamovi, Version 2.2; Computer Software; The Jamovi Project: Sydney, Australia, 2021. Available online: https://www.jamovi.org (accessed on 13 January 2025).
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.0; R Foundation for Statistical Computing: Vienna, Austria, 2020. Available online: https://cran.r-project.org (accessed on 30 January 2025).
- Zerbinati, N.; Płatkowska, A.; Guida, S.; Stabile, G.; Mocchi, R.; Barlusconi, C.; Sommatis, S.; Garutti, L.; Rauso, R.; Cipolla, G.; et al. Efficacy and Safety of Neauvia Intense in Correcting Moderate-to-Severe Nasolabial Folds: A Post-Market, Prospective, Open-Label, Single-Centre Study. Clin. Cosmet. Investig. Dermatol. 2024, 17, 1351–1363. [Google Scholar] [CrossRef]
- Fundarò, S.P.; Salti, G.; Malgapo, D.M.H.; Innocenti, S. The Rheology and Physicochemical Characteristics of Hyaluronic Acid Fillers: Their Clinical Implications. Int. J. Mol. Sci. 2022, 23, 10518. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, G.; Galasso, G.; Capillo, M.C.; Alonci, G.; Bighetti, S.; Bettolini, L.; Sommatis, S.; Mocchi, R.; Carugno, A.; Zerbinati, N. Rheology as a Tool to Investigate the Degradability of Hyaluronic Acid Dermal Fillers. Clin. Cosmet. Investig. Dermatol. 2025, 18, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Kubik, P.; Bighetti, S.; Bettolini, L.; Gruszczyński, W.; Łukasik, B.; Guida, S.; Stabile, G.; Murillo Herrera, E.M.; Carugno, A.; D’Este, E.; et al. Effectiveness and Safety of the Use of 1470 nm Laser Therapy in Patients Suffering from Acne Scarring of the Facial Skin. Clin. Cosmet. Investig. Dermatol. 2025, 18, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Kubik, P.; Bighetti, S.; Bettolini, L.; Gruszczyński, W.; Łukasik, B.; Guida, S.; Stabile, G.; Paolino, G.; Murillo Herrera, E.M.; Carugno, A.; et al. The Effectiveness and Safety of 1470 nm Non-Ablative Laser Therapy for the Treatment of Striae Distensae: A Pilot Study. Cosmetics 2025, 12, 148. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Z.; Hu, J. Progress in Neck Rejuvenation Injection Therapy. Aesthetic Plast. Surg. 2025, 49, 5266–5274. [Google Scholar] [CrossRef]
- Scarano, A.; Qorri, E.; Sbarbati, A.; Gehrke, S.A.; Marchetti, M.; Desiderio, V.; Amuso, D.; Tari, S. Mesotherapy with hyaluronic acid solutions enriched by amino acids in the neck area: Open-label uncontrolled, monocentric study. J. Cosmet. Laser Ther. 2025, 27, 121–126. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zerbinati, N.; Carugno, A.; Guida, S.; Mocchi, R.; Sommatis, S.; Cipolla, G.; Rauso, R.; Galadari, H.; Fratton, Z.; Errichetti, E.; et al. Assessment of Safety and Tissue Integration of PEGDE-Based Hyaluronic Acid Filler for Severe Nasolabial Folds: A Prospective Observational Study with Biophysical and Ultrasound Evaluation. Cosmetics 2025, 12, 275. https://doi.org/10.3390/cosmetics12060275
Zerbinati N, Carugno A, Guida S, Mocchi R, Sommatis S, Cipolla G, Rauso R, Galadari H, Fratton Z, Errichetti E, et al. Assessment of Safety and Tissue Integration of PEGDE-Based Hyaluronic Acid Filler for Severe Nasolabial Folds: A Prospective Observational Study with Biophysical and Ultrasound Evaluation. Cosmetics. 2025; 12(6):275. https://doi.org/10.3390/cosmetics12060275
Chicago/Turabian StyleZerbinati, Nicola, Andrea Carugno, Stefania Guida, Roberto Mocchi, Sabrina Sommatis, Giovanna Cipolla, Raffaele Rauso, Hassan Galadari, Zeno Fratton, Enzo Errichetti, and et al. 2025. "Assessment of Safety and Tissue Integration of PEGDE-Based Hyaluronic Acid Filler for Severe Nasolabial Folds: A Prospective Observational Study with Biophysical and Ultrasound Evaluation" Cosmetics 12, no. 6: 275. https://doi.org/10.3390/cosmetics12060275
APA StyleZerbinati, N., Carugno, A., Guida, S., Mocchi, R., Sommatis, S., Cipolla, G., Rauso, R., Galadari, H., Fratton, Z., Errichetti, E., Maronese, C. A., Rossi, M., Bettolini, L., & Bighetti, S. (2025). Assessment of Safety and Tissue Integration of PEGDE-Based Hyaluronic Acid Filler for Severe Nasolabial Folds: A Prospective Observational Study with Biophysical and Ultrasound Evaluation. Cosmetics, 12(6), 275. https://doi.org/10.3390/cosmetics12060275







