Abstract
Sisal (Agave sisalana) is a plant widely cultivated in Brazil for fiber extraction, generating large volumes of underutilized residues with pharmacological potential. In this study, an aqueous extract and its alcoholic fraction were obtained from the plant’s residual mucilage, aiming to investigate their antifungal activity, antioxidant capacity, and cellular safety. Phytochemical screening revealed high levels of phenols, flavonoids, and saponins, particularly in the aqueous fraction. The extracts were evaluated for antioxidant activity using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay and for antifungal activity against the yeasts Candida albicans and Malassezia pachydermatis, as determined by the Minimum Inhibitory Concentration (MIC). The aqueous extract exhibited strong antioxidant activity and inhibited 99% of C. albicans and M. pachydermatis growth at concentrations of 87 μg/mL and 1400 μg/mL, respectively. Furthermore, it showed low cytotoxicity in NIH/3T3 fibroblasts. These findings indicate that sisal residue contains promising bioactive compounds with relevant pharmacological properties, reinforcing its potential as a sustainable source for the development of antifungal and antioxidant phytotherapeutics.
1. Introduction
Fungal infections caused by species of the genera Candida and Malassezia represent emerging and significant challenges to global public health, particularly among immunocompromised populations and in hospital settings. Candidiasis, which ranges from superficial infections to severe systemic conditions, is frequently associated with prolonged use of corticosteroids, invasive medical devices, and immunosuppression [1]. Candida albicans is the most prevalent species in such infections, although non-albicans species are becoming increasingly common and resistant to conventional antifungal agents [2]. In parallel, Malassezia infections, traditionally regarded as opportunistic cutaneous diseases, have emerged as potential pathogens in systemic contexts, especially in neonates and critically ill patients, being associated with conditions such as seborrheic dermatitis, pityriasis versicolor, and catheter-related fungemia [3].
There is a global rise in resistance to conventional antifungal agents [4], largely due to their indiscriminate use in human and veterinary medicine, as well as in agriculture. As a result, the search for new therapeutic alternatives has become a public health priority [4]. Infections caused by opportunistic fungi, such as Candida spp. and Malassezia spp., have proven particularly challenging, often requiring prolonged treatments that favor the selection of resistant strains and increase the therapeutic failure rates [5]. Thus, the growing antifungal resistance, combined with the scarcity of effective therapies and surveillance strategies, further underscores the need to intensify complementary and/or alternative therapeutic approaches [6].
Particular attention is given to the secondary metabolites present in plant extracts, such as phenolic compounds, saponins, alkaloids, and terpenoids, due to their antifungal activity associated with antioxidant properties. These compounds act not only by damaging fungal cellular structures, such as membranes and organelles, but also by modulating the redox balance within the infectious environment [7]. Oxidative stress is a key factor in the virulence of pathogenic fungi such as Candida spp. and Malassezia spp., being involved in biofilm formation, antifungal resistance, and evasion of the host immune response. By exerting antioxidant activity, plant metabolites reduce the levels of reactive oxygen species (ROS), thereby interfering with their cellular signaling and diminishing the fungi’s ability to adapt and proliferate [8]. Moreover, by mitigating oxidative stress, these compounds may help protect host tissues from inflammatory damage, fostering a more favorable environment for infection resolution. These synergistic mechanisms strengthen the therapeutic potential of plant extracts as alternatives or adjuvants in the treatment of resistant mycoses, particularly in cases where conventional antifungal agents present limitations [9].
Given these premises, plant extracts have emerged as promising sources of new antifungal agents due to the structural diversity of their secondary metabolites. Among the species of interest, Agave sisalana, commonly known as sisal, stands out, as the residue from its industrial processing concentrates bioactive compounds with potential antimicrobial activity [10]. In Brazil, sisal holds historical and socioeconomic relevance, particularly in the semi-arid Northeast, where it was introduced in the 1903s to meet global demand for natural fibers [11]. Today, the country is the world’s largest producer, with the state of Bahia accounting for approximately 95% of national production, followed by Paraíba and Rio Grande do Norte [12]. However, sisal processing annually generates millions of tons of residue, equivalent to approximately 95% of the plant’s biomass, which is discarded after fiber extraction [13]. This unused volume, combined with the high concentration of saponins in the liquid fraction of this residue, as reported in some studies, underscores the urgency of developing sustainable strategies for the full valorization of the biomass, focusing on secondary metabolites capable of inhibiting resistant pathogens [14].
Thus, the present study initially aimed to develop an aqueous extract and its alcoholic fraction from the juice obtained by the mechanical pressing of sisal residue, which is commonly discarded during the leaf defibrillation process. Subsequently, with the long-term goal of developing a new phytotherapeutic agent, the following analyses were conducted: (1) evaluation of the antifungal activity of the aqueous extract and its alcoholic fraction against Malassezia pachydermatis ATCC 14522 and Candida albicans ATCC 1023; (2) determination of the antioxidant activity of the aqueous extract and its alcoholic fraction using the DPPH assay; (3) quantification of phenolic compounds, flavonoids, and saponins in the aqueous extract and its alcoholic fraction, in order to establish a relationship between the observed effects and their phytochemical composition; and (4) in vitro cytotoxicity assessment of the aqueous extract using the NIH/3T3 cell line. The methodologies developed were intended to promote the valorization of this agro-industrial residue and contribute to the inclusion of sustainable therapeutic strategies that add value to underutilized plant waste.
2. Materials and Methods
2.1. Plant Material Source
Agave sisalana Perrine, commonly known as sisal, is a perennial monocotyledonous plant of the family Asparagaceae, widely cultivated in Brazil. It has a short, thick stem and a rosette of rigid, sword-shaped leaves, typically 1.0–1.5 m long and 10–15 cm wide, which mature over 3–5 years. Harvested leaves are mechanically decorticated to isolate the hard fibers, which may then be washed and sun-dried to remove residual tissue and moisture. No chemical pre-treatment is usually applied in the leaf residue, used in the preparation of extracts.
The crude sisal juice, derived from the mechanical pressing of sisal residue, was collected from rural producers in Valente, Bahia, Brazil. The species under study was identified as Agave sisalana at the Assis Herbarium of São Paulo State University (UNESP), Assis campus, São Paulo, where a voucher specimen was deposited under number 2597.
2.2. Preparation of the Aqueous Extract and Its Alcoholic Fraction from Sisal Juice
Due to the high water solubility of saponins, the aqueous extract was initially prepared from the sisal juice. For the extract preparation, the sisal juice was centrifuged (FANEM FR 22, Garulhos, São Paulo, Brazil) at 4000 rpm for 20 min at 20 °C, applying a relative centrifugal force of approximately 52,580 N. Subsequently, the supernatant was dried in an oven (FANEM LTDA, model 002CB) at 50 °C until a constant weight was achieved, yielding the aqueous extract of sisal.
In a second step, aiming to enhance the observed activities, the aqueous extract was subjected to alcoholic fractionation. For this, 10 g of each dried extract was added to 1000 mL of a 70% hydroalcoholic solution. The mixture was stirred for 90 min and then left to stand in the dark under static conditions for 3 days. Subsequently, the solution was filtered and the filtrate was concentrated using a rotary evaporator to remove the alcohol content. The resulting alcoholic extract was then dried in an oven at 40 °C.
2.3. Evaluation of the Antifungal Activity of the Sisal Aqueous Extract and Its Alcoholic Fraction
2.3.1. Microorganisms Used
The experiments utilized the fungi Malassezia pachydermatis ATCC 14522 and Candida albicans ATCC 1023.
2.3.2. Isolation and Preservation of Microorganisms
For the isolation of Candida albicans, the liquid YM culture medium was used, containing: yeast extract (0.3%), malt extract (0.3%), peptone (0.5%), dextrose (1%), and chloramphenicol (0.018%), all dissolved in distilled water at pH 6.5. For Malassezia pachydermatis, the MM medium was employed, composed of malt extract (1.8%), peptone (1.8%), Tween 40 or 80 (0.5%), glycerol (0.25%), and chloramphenicol (0.009%), dissolved in water at pH 6.5. Prior to yeast inoculation, all culture media were sterilized in an autoclave (Fabbe-Primar, model 103, São Paulo, Brazil) for 20 min at 1 atm pressure and 121 °C.
After inoculation, the cultures were incubated at 32 °C for 72 h to promote yeast growth (FANEM LTDA incubator). Once isolated, the cultures were preserved in sterile Eppendorf tubes containing approximately 1 mL of 10% glyceraldehyde solution, previously sterilized by autoclaving at 121 °C for 40 min (FABBE-PRIMAR, São Paulo, Brazil). Subsequently, the tubes were frozen in liquid nitrogen containers (Nitropec, Garça, São Paulo, Brazil) and stored in a freezer (Bosch, model Ecoplus 430, São Paulo, Brazil) at −20 °C.
2.3.3. Microorganism Activation
The isolated and preserved fungi were aseptically activated using a platinum loop by transferring each cryopreserved fungal isolate into test tubes containing 9 mL of sterilized liquid medium (sterilized for 15 min at 121 °C). The medium had the following composition: sucrose 2%, yeast extract 0.5%, ammonium sulfate 0.10%, potassium phosphate 0.087%, magnesium sulfate heptahydrate 0.024%, manganese sulfate monohydrate 0.0012%, zinc sulfate heptahydrate 0.0028%, and distilled water, adjusted to pH 5.5. The tubes containing the fungi were incubated for 120 h at 28 °C in an oven (FANEM LTDA, model 002CB—São Paulo, SP, Brazil). After initial growth, two additional subcultures were performed under the same conditions to ensure complete activation of the microorganisms.
2.3.4. Standardization of Microorganisms
In this new phase of the study, inoculum standardization was performed to ensure homogeneity and that equal portions of the microorganisms were introduced into the different test tubes. Initially, yeast isolation was carried out using the streak plate method to obtain a single colony, ensuring that all inoculated yeasts originated from a single organism. Subsequently, microorganism concentration was determined using a Neubauer counting chamber, allowing dilution to the desired concentration.
2.3.5. Minimum Inhibitory Concentration (MIC)
The percentage of inhibition was determined using the methodology described by Jones et al. [15], with some modifications. This method involves preparing different concentrations of the test substance and performing microorganism counts at time zero and after 24 h. The Minimum Inhibitory Concentration (MIC) was evaluated based on the percentage of inhibition, with the initial count taken immediately prior to inoculation. Subsequently, the microorganisms were incubated at 32 °C with shaking at 180 rpm at the different dilution concentrations. After 24 h, a second count was performed. Each concentration was then compared to the control group. Positive controls consisted of the antifungal agents nystatin for Candida albicans and ketoconazole for Malassezia pachydermatis, both commonly used in clinical practice. The extract concentrations tested ranged from 43 to 1400 μg/mL. The MIC was defined as the lowest concentration that exhibited the highest percentage of inhibition.
2.4. Evaluation of the Antioxidant Activity of the Aqueous Extract and Its Alcoholic Fraction
The experiments were performed in triplicate for statistical purposes, using a mixture composed of 1 mL of acetate buffer (pH 5.5, 100 mM), 1.25 mL of P.A. ethanol, 250 μL of DPPH solution (500 μM), and 50 to 100 μL of the samples. DPPH exhibits maximum absorbance at 517 nm, which decreases in the presence of hydrogen-donating molecules, indicated by a color change from purple to yellow. The extracts were allowed to react with the DPPH radical for 30 min in low-light conditions and were subsequently analyzed using a UV–Vis spectrophotometer at a wavelength of 517 nm [16]. The antioxidant activity was calculated according to the following equation:
where Asample is the absorbance of the samples after 30 min and Acontrol is the absorbance of the DPPH solution, both measured at 517 nm.
Antioxidant Activity (%) = [(Acontrol − Asample)/Acontrol] × 100
2.5. Phytochemical Analysis of the Sisal Aqueous Extract and Its Alcoholic Fraction
2.5.1. Total Phenolic Quantification
The Folin–Ciocâlteu method was performed according to the procedure described by Khodaie et al. [17] to determine the total phenolic compounds using gallic acid as the reference standard. For each extracted sample (0.5 mL at various concentrations), 5 mL of distilled water and 0.25 mL of Folin–Ciocâlteu reagent (containing molybdate, tungstate, and phosphoric acid) were added. After 3 min, 1 mL of a saturated 10% Na2CO3 solution was added, and the mixture was allowed to stand for 1 h at room temperature in the dark. Absorbance was measured at a wavelength of 725 nm using a UV–Vis spectrophotometer.
2.5.2. Total Flavonoid Quantification
Flavonoid content in the extracts was determined using a modified version of the method described by Zhishen et al. [18]. An aliquot of 0.5 mL of the extract solution at various concentrations was mixed with 1.5 mL of absolute ethanol and 0.1 mL of 10% AlCl3 solution. Then, 0.1 mL of CH3COONa was added, and the total volume was adjusted to 5.0 mL by adding 2.8 mL of distilled water. The mixtures were homogenized and incubated in the dark for 30 min. Finally, absorbance was measured at 425 nm using quercetin as the reference standard.
2.5.3. Saponin Quantification
The presence of saponins in the extract was evaluated through spectrophotometric analysis, following the established literature protocols [19,20,21]. The analysis was conducted using a UV–Vis spectrophotometer. An aliquot of 1 mL of the aqueous extract and alcoholic fraction was mixed with 1 mL of 0.2% cobalt chloride chromogenic reagent and 1 mL of concentrated sulfuric acid. Absorbance readings were taken at 284 nm after 20 min from the start of the reaction. Quillaja saponins (Merck, Saint Louis, MO, USA), dissolved in water at a concentration of 0.2 mg/mL, were used as the positive standard.
2.6. Cell Viability of the Sisal Aqueous Extract
Mouse normal fibroblasts (NIH/3T3 cell line) were cultured in tissue culture-treated flasks with high-glucose DMEM® medium (Sigma, Cajamar, São Paulo, SP, Brazil) and incubated at 37 °C with 5% CO2. The cell suspension was counted using a Neubauer chamber and diluted to obtain a concentration of 250,000 cells per mL. Subsequently, 80 μL of the cell suspension was seeded into a 96-well ELISA plate for adherent cultures and incubated overnight. Thereafter, 200 μL of the extract diluted in supplemented culture medium at various concentrations was added to each well. After 24 h, the supernatant was removed from each well, and 100 μL of MTT solution (CAS 298-93-1; Sigma Aldrich, Cajamar, São Paulo, Brazil—5 mg MTT per 1 mL PBS) was added and incubated for 4 h. Following incubation, the supernatant was removed again, and 100 μL of sterile DMSO was added to each well to solubilize the formazan crystals, followed by an additional 10 min incubation. The solution was then homogenized, and absorbance was measured using an ELISA spectrophotometer at 540 nm.
2.7. Data Analysis
Statistical analyses were performed using BioEstat 5.0 software. For all tests, the significance level was set at 5%. Data were subjected to one-way ANOVA, with treatment as the independent factor. When appropriate, multiple comparisons were conducted using Tukey’s test.
3. Results
3.1. Antifungal Activity
The evaluation of the antifungal potential of the sisal aqueous extract against the yeast Candida albicans was conducted by analyzing the inhibition of its growth. In this assay, the aqueous sisal extract was initially prepared at a concentration of 5600 μg/mL. From this initial concentration, serial dilutions were performed, resulting in the following concentrations in the last six test tubes after inoculation: 1400, 700, 350, 175, 87, and 43 μg/mL.
The inhibition percentages are presented in Table 1, demonstrating the antifungal activity of the aqueous extract against Candida albicans. At a concentration of 87 μg/mL, the extract was able to inhibit 99% of the yeast’s growth. Nystatin, used as the reference antifungal, exhibited an MIC of 3.1 μg/mL.

Table 1.
Inhibitory concentrations of the sisal aqueous extract and nystatin (positive control) against the yeast Candida albicans following treatment with nystatin at concentrations ranging from 0.8 to 25 μg/mL—positive control (PC) and sisal aqueous extract at concentrations from 43 to 1400 μg/mL. The asterisk (*) indicates the Minimum Inhibitory Concentration (MIC).
The antifungal activity against the yeast Malassezia pachydermatis was also evaluated using the same concentrations of the aqueous extract. However, the commercial antifungal used for comparison was ketoconazole. Against Malassezia pachydermatis, the sisal aqueous extract exhibited an MIC of 1400 μg/mL. Table 2 illustrates the minimum inhibitory concentrations for Malassezia pachydermatis. Ketoconazole, used as the reference antifungal, showed an MIC of 112 μg/mL.

Table 2.
Inhibitory concentrations of the sisal aqueous extract and ketoconazole (positive control) against the yeast Malassezia pachydermatis following treatment with ketoconazole at concentrations ranging from 56 to 1800 μg/mL—positive control (PC) and sisal aqueous extract at concentrations from 43 to 1400 μg/mL. The asterisk (*) indicates the Minimum Inhibitory Concentration (MIC).
Continuing the antifungal study and aiming to enhance the observed results, the alcoholic fraction of the aqueous extract was evaluated at concentrations below 87 μg/mL against Candida albicans and below 1400 μg/mL against Malassezia pachydermatis. The results showed that for Candida albicans, the alcoholic fraction was less potent than the aqueous extract, achieving only 47% inhibition at the highest concentration tested (87 μg/mL). This inhibition was significantly lower compared to the aqueous extract, which inhibited 99% of the yeast at the same concentration. A similar trend was observed against Malassezia pachydermatis, where the alcoholic fraction was also less potent. Even at the highest concentration tested (1400 μg/mL), it produced only 42% inhibition.
3.2. Antioxidant Activity
Table 3 presents the antioxidant effect of different concentrations of the sisal aqueous extract and its alcoholic fraction. The aqueous extract exhibited a higher antioxidant activity compared to the alcoholic fraction at all concentrations tested. Quercetin at 100 μg/mL was used as the positive control (PC). The alcoholic fraction showed a minimum inhibitory concentration of 921.99 μg/mL and the aqueous extract 114.62 μg/mL.

Table 3.
Antioxidant Activity.
3.3. Phytochemical Analysis
To gain a better understanding of the pharmacological effects exhibited by the aqueous extract and its alcoholic fraction, phytochemical analyses were performed. Table 4 presents the results obtained from the spectrophotometric analyses, expressed as grams of standard equivalents per 100 g of dry extract.

Table 4.
Mean ± standard deviation of secondary metabolites present in the sisal aqueous extract.
3.4. Viability
Since the aqueous extract showed the best results in the previous analyses, its cytotoxicity was evaluated in this phase. The results of the cell viability assay on fibroblasts treated with various concentrations of the aqueous extract are presented in Figure 1. This figure illustrates that the positive control (2% Tween 80) and the aqueous extract concentrations of 400, 800, and 1600 µg/mL exhibited a significant difference (p < 0.05) compared to the negative control. At concentrations of 100 and 200 µg/mL, the aqueous extract demonstrated cell viability percentages of 102% and 100%, respectively. Only the highest concentrations, 800 and 1600 µg/mL, reduced the cell viability by more than 30%.
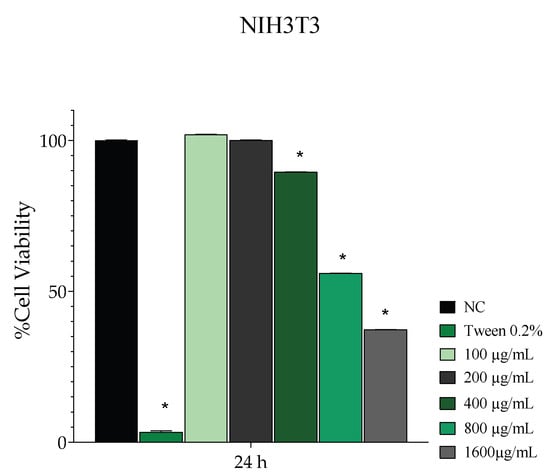
Figure 1.
Mean ± SD of the percentage values corresponding to the cell viability rate of NIH3T3 fibroblasts evaluated 24 h after the following treatments: saline solution—negative control (NC); 2% Tween 80—positive control (PC); sisal aqueous extract at concentrations of 100, 200, 400, 800, and 1600 μg/mL. The asterisk (*) indicates a significant difference (p < 0.05) compared to the NC group.
4. Discussion
The yeasts Candida albicans and Malassezia pachydermatis are opportunistic fungi that can cause severe infections in immunocompromised individuals [22]. Candida albicans is a commensal species of the human gastrointestinal and genitourinary tracts; however, under conditions of microbiota imbalance or immunosuppression, it can become an aggressive pathogen, causing superficial or systemic candidiasis [23]. Malassezia pachydermatis, although more common in animals, can also affect humans, especially hospitalized neonates and critically ill patients, and is associated with fungemia and dermatitis [24]. Both species possess adaptive mechanisms to oxidative stress generated by the immune system, including the production of enzymes such as catalase, superoxide dismutase, and glutathione peroxidase, which neutralize reactive oxygen species (ROS) [25]. This antioxidant capacity contributes to their survival in hostile environments and promotes infection persistence [26].
Furthermore, these yeasts have shown increasing resistance to conventional antifungals, such as azoles, highlighting the need to explore new therapeutic strategies [22,27]. In this context, plant extracts have emerged as promising alternatives, as various plant species are rich in secondary metabolites with antifungal, antioxidant, and anti-inflammatory properties [28,29,30]. These compounds can interfere with essential physiological processes in fungi, such as cell membrane integrity, ergosterol biosynthesis, and oxidative stress response, thereby contributing to both the inhibition of fungal growth and to the strengthening of the host’s defenses [31,32,33,34].
With the aim of evaluating the potential use of the sisal residue extract in the future development of a new antifungal agent, its activity was initially assessed against the pathogenic yeasts Candida albicans and Malassezia pachydermatis. Overall, the results were promising, indicating that the active components present in the sisal aqueous extract exerted a significant antifungal effect. Against Candida albicans, a minimum inhibitory concentration (MIC) of 87 μg/mL was observed, while for Malassezia pachydermatis, the MIC was 1400 μg/mL. According to the criteria proposed by Aligiannis et al. [35], the antimicrobial activity of plant extracts can be classified based on MIC values as follows: products with MIC ≤ 0.5 mg/mL are considered potent inhibitors; those with MIC between 0.6 and 1.5 mg/mL are moderate inhibitors; and those with MIC > 1.6 mg/mL are weak inhibitors.
Based on this classification, the sisal aqueous extract would be categorized as a potent inhibitor against Candida albicans and a moderate inhibitor against Malassezia pachydermatis. Additionally, according to Pelczar et al. [36], variations in antifungal responses observed across studies may also be related to the intrinsic characteristics of each strain. In 2009, Santos and colleagues [37] reported that the hydroalcoholic extract of sisal leaves exhibited an MIC of 390 μg/mL against Candida albicans. Hammuel et al. [38] investigated the methanolic and aqueous extracts of Agave sisalana and observed an MIC ranging from 10 to 20 mg/mL against the yeast Candida albicans. These values indicate a weak antifungal activity. On the other hand, other studies, such as the dissertation by Almeida (2013), reported a significantly lower (and thus more potent) MIC of 98 µg/mL for the liquid residue/mucilage of sisal [39]. These findings suggest that the aqueous extract of sisal may exhibit superior antifungal activity, particularly when compared to leaf extracts or less efficient extraction methods.
To evaluate the antioxidant activity of the sisal aqueous extract and its alcoholic fraction, the DPPH method was employed. This method is based on the electron transfer from an antioxidant compound to an oxidant [40]. DPPH, an oxidizing agent, is reduced to form diphenylpicrylhydrazine, which has a yellow color accompanied by a decrease in absorbance. It is a stable free radical that in the presence of a hydrogen-donating antioxidant (AH) can be reduced in an alcoholic medium, forming diphenylpicrylhydrazine. This reduction can be monitored by spectrophotometry at 515 nm through a decrease in absorbance, with a simultaneous color change from the original dark violet to light yellow [41]. In other words, the greater the reduction of DPPH, the less intense the violet color, indicating higher antioxidant activity of the tested solution.
The results obtained from the DPPH assay revealed that the sisal aqueous extract exhibited superior antioxidant activity compared to its alcoholic fraction. The antioxidant activity of plants is correlated with their phenolic compound content [42], as these compounds have the ability to inhibit xanthine oxidase, an enzyme responsible for tissue oxidation. Consequently, they can inhibit free radical formation and prevent oxidative stress processes that lead to cell death [43]. In this regard, the phytochemical analysis of the sisal aqueous extract revealed a high concentration of total phenolic compounds, measuring 28.38 ± 0.12 g/100 g GAE. Rawat et al. [44] analyzed the tuber of Agave sisalana Perrine and found a total phenolic content of 310.79 mg/g GAE. Daher et al. [45], using 50% hydroalcoholic extraction of sisal residue, obtained a lower concentration of phenolic compounds, measuring 77.93 mg/g GAE. These results illustrate the high content of phenolic compounds present in the sisal residue that is currently being discarded.
The higher concentration of phenolic compounds observed in the aqueous extract compared to its alcoholic fraction may be related to the chemical nature of these metabolites. Low-molecular-weight phenolic compounds, such as phenolic acids and some simple flavonoids, are highly polar and water-soluble, which favors their retention in the aqueous phase during hydroalcoholic fractionation [46]. Conversely, the alcoholic fraction tends to solubilize less polar compounds, which may explain the lower concentration of phenolics detected.
Furthermore, it is possible that structurally more complex compounds, such as lignin, a macromolecule composed of intertwined phenolic units, are present in the aqueous extract. Lignin can react with the Folin–Ciocâlteu reagent, interfering with the quantification of total phenolics [47]. Studies indicate that plant residues rich in lignin, such as those from Agave, may exhibit elevated total phenolic values that do not directly reflect the presence of low molecular weight compounds with biological activity [48].
Another relevant factor is the synergism among different classes of secondary metabolites. Phenolic compounds, flavonoids, and saponins can exert combined effects, enhancing biological activities such as antifungal and antioxidant actions [49]. This synergism may result from their activity on different molecular targets or the mutual regeneration of active compounds, as observed in various plant matrices [50]. Thus, the superior performance of the aqueous extract may be associated with the simultaneous and interactive presence of these compounds.
Saponins, in turn, exhibit a broad spectrum of bioactivities, including antifungal, antioxidant, anti-inflammatory, and immunomodulatory effects [14]. Their ability to interact with lipid membranes by forming complexes with sterols is one of the most widely accepted mechanisms underlying their antifungal action, particularly against yeasts such as Candida albicans and Malassezia pachydermatis [51]. Studies have also demonstrated that saponins can modulate oxidative responses by reducing the reactive oxygen species (ROS) levels [52].
Therefore, the phytochemical complexity of the aqueous extract, even without fractionation, may represent a therapeutic advantage, especially when aiming for a formulation with multiple synergistic mechanisms of action and a reduced likelihood of resistance development.
In the final stage of this study, to assess the potential use of the aqueous extract of sisal, its effect on the viability of murine NIH/3T3 fibroblasts was evaluated using the MTT assay. This widely used methodology assesses the cytotoxic effects of plant extracts on cell lines. The aqueous sisal extract was tested at concentrations ranging from 100 to 1600 µg/mL. Results demonstrated no cytotoxicity at concentrations between 100 and 400 µg/mL, with cell viability exceeding 70%, indicating safety for use [53]. In contrast, higher concentrations caused a reduction in cell viability, suggesting dose-dependent cytotoxic effects potentially linked to the saturation of cellular defense mechanisms or interactions with the plasma membrane. These findings align with previous studies demonstrating low toxicity of extracts derived from Agave sisalana residues in non-tumorigenic cells. For instance, an in vitro assay evaluated the antioxidant and cytotoxic potential of three extracts obtained via acid hydrolysis (AHAS), dry precipitation (DPAS), and hexane extraction (HAS) from the plant in Vero cells, human lymphocytes, and mouse erythrocytes, reporting no significant genotoxicity in the extracts, particularly at concentrations relevant for pharmacological applications [54]. Mazo et al. [55], evaluating the effect of the hydroalcoholic extract from sisal root on NIH/3T3 cells using the MTT assay, found that all tested concentrations, ranging from 100 to 1600 µg/mL, did not reduce cell viability, indicating an absence of cytotoxicity. Additionally, a mucoadhesive gel formulation containing sisal and pomegranate residues, tested on NIH/3T3 cells, showed cell viability values exceeding 70% even at high extract concentrations, demonstrating safety for topical application. The combination of low cytotoxicity and high biological activity reinforces the potential of the aqueous sisal extract as a safe ingredient for pharmaceutical, dermocosmetic, and phytotherapeutic formulations.
Although these results are suggestive, it is essential to acknowledge the inherent limitations of in vitro assays, which do not fully replicate the complexity of human skin and systemic interactions. Nevertheless, the observed low cytotoxicity indicates that Agave sisalana extracts are likely safe for incorporation into cosmetic and dermocosmetic formulations. These in vitro analyses provide preliminary safety data supporting the further development of topical products; however, comprehensive studies, including skin irritation, sensitization, and clinical evaluations, are required to fully assess their suitability and establish effective concentrations for cosmetic applications.
5. Conclusions
Based on the results obtained, we can conclude that the aqueous extract of sisal exhibited potent antifungal activity and a broad inhibition spectrum, effectively inhibiting both the yeast Candida albicans, MIC of 87 μg/mL, responsible for vaginal and cutaneous candidiasis, and the yeast Malassezia pachydermatis, MIC of 1400 μg/mL, which causes mycoses on the scalp and body surface. Additionally, the aqueous sisal extract, IC50 114.62 μg/mL, demonstrated higher antioxidant activity compared to the alcoholic fraction, IC50 921.99 μg/mL.
The phytochemical results suggest that the high concentration of saponins, as well as the synergism among secondary metabolites present in the aqueous extract of sisal residue is likely responsible for its enhanced antifungal and antioxidant effects.
Thus, as the aqueous extract of sisal residue presents therapeutic potential, and represents an important alternative to be explored for the treatment of resistant mycoses, new analyses will be carried out with this extract isolated and inserted into pharmaceutical formulations.
Finally, it is worth highlighting that the results obtained herein not only open up a new line of research but also enable the economic utilization of sisal residue, a byproduct of leaf defibration that is currently underutilized. Adding value to this residue can improve the living conditions, health, and education of the population in the sisal-producing region of Bahia, one of the poorest areas in Brazil.
Author Contributions
Conceptualization, L.T.S.d.C., J.A.R.F., P.d.O.N. and L.d.S.; Data curation, B.d.C.S.; Formal analysis, J.A.R.F.; Methodology, W.R.P.M., F.A.A.D.O., E.B.C., G.O.B. and K.A.A.M.; Project administration, L.d.S.; Software, L.T.S.d.C., J.P.G. and B.d.C.S.; Writing—original draft, R.P.G., W.R.P.M. and L.d.S.; Writing—review and editing, N.A.Z., D.B.B., V.F.X. and L.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the São Paulo Research Foundation (FAPESP), grant number 12/01751-7. J.A.R.F. was financially supported by the Coordination for the Improvement of Higher Education Personnel (CAPES—Brazil—888 87.827298/2023-00), L.T.S.d.C. was financially supported by the Coordination for the Improvement of Higher Education Personnel (CAPES—Brazil—88887.817409-2023-00). L.d.S. and D.B.B. were financially supported by the CAPES Print Unesp Project: Exploring Multidisciplinary Approaches for the Development of Phytotherapeutic Products (Grant—A1266B4).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Soriano, A.; Honore, P.M.; Puerta-Alcalde, P.; Garcia-Vidal, C.; Pagotto, A.; Gonçalves-Bradley, D.C.; Verweij, P.E. Invasive candidiasis: Current clinical challenges and unmet needs in adult populations. J. Antimicrob. Chemother. 2023, 78, 1569–1585. [Google Scholar] [CrossRef]
- Rhimi, W.; Theelen, B.; Boekhout, T.; Otranto, D.; Cafarchia, C. Malassezia spp. yeasts of emerging concern in fungemia. Front. Cell. Infect. Microbiol. 2020, 10, 370. [Google Scholar] [CrossRef]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef]
- Osset-Trénor, P.; Pascual-Ahuir, A.; Proft, M. Fungal drug response and antimicrobial resistance. J. Fungi 2023, 9, 565. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial resistance: A growing serious threat for global public health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.F.; Yap, V.L.; Rajagopal, M.; Wiart, C.; Selvaraja, M.; Leong, M.Y.; Tan, P.L. Plant as an alternative source of antifungals against Aspergillus infections: A review. Plants 2022, 11, 3009. [Google Scholar] [CrossRef]
- Zhou, X.; Zeng, M.; Huang, F.; Qin, G.; Song, Z.; Liu, F. The potential role of plant secondary metabolites on antifungal and immunomodulatory effect. Appl. Microbiol. Biotechnol. 2023, 107, 4471–4482. [Google Scholar] [CrossRef]
- Aboody, M.S.A.; Mickymaray, S. Anti-fungal efficacy and mechanisms of flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, J.S.; Silva, M.C.J.; Santos, E.N.; Lima, F.L.O.; Costa, M.S.F. Atividades biológicas de Agave sisalana com ênfase para a ação antimicrobiana: Uma revisão da literatura. Res. Soc. Dev. 2021, 10, e2510312734. [Google Scholar] [CrossRef]
- Raya, F.T.; Carvalho, L.M.; José, J.; Cruz, L.P.; Almeida, R.L.; Delevatti, H.A.A.; Silveira, N.M.; Silva, S.F.; Pissolato, M.D.; Oliveira, A.B.; et al. Rescuing the Brazilian agave breeding program: Morphophysiological and molecular characterization of a new germplasm. Front. Chem. Eng. 2023, 5, 1218668. [Google Scholar] [CrossRef]
- Duarte, E.A.A.; Damasceno, C.L.; Oliveira, T.A.S.; Barbosa, L.O.; Martins, F.M.; Silva, J.R.Q.; Lima, T.E.F.; Silva, R.M.; Kato, R.B.; Bortolini, D.E.; et al. Putting the mess in order: Aspergillus welwitschiae (and not A. niger) is the etiological agent of sisal bole rot disease in Brazil. Front. Microbiol. 2018, 9, 1227. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.D.; Barreto, D.W.; Coelho, M.A.Z. Use of micellar extraction and cloud point preconcentration for valorization of saponins from sisal (Agave sisalana) waste. Food Bioprod. Process. 2015, 94, 601–609. [Google Scholar] [CrossRef]
- Costa, L.T.S.; Fracasso, J.A.R.; Guarnier, L.P.; Brito, G.R.; Fumis, D.B.; Bittencourt, R.A.C.; Guiotti, A.M.; Barbosa, D.B.; Camargo, I.C.C.; Souza, E.B.; et al. Toxicity and anti-inflammatory effects of Agave sisalana extract derived from agroindustrial residue. Plants 2023, 12, 1523. [Google Scholar] [CrossRef]
- Jones, R.N.; Barry, A.L.; Gavan, T.L.; Washington, J.A., II. Susceptibility tests: Microdilution and macrodilution broth procedures. In Manual of Clinical Microbiology; Lennette, E.H., Balows, A., Hausler, W.J., Jr., Truant, J.P., Eds.; American Society for Microbiology: Washington, DC, USA, 1985; pp. 972–977. [Google Scholar]
- Di Mambro, V.M.; Fonseca, M.J.V. Assays of physical stability and antioxidant activity of a topical formulation added with different plant extracts. J. Pharm. Biomed. Anal. 2005, 37, 287–295. [Google Scholar] [CrossRef]
- Khodaie, L.; Bamdad, S.; Delazar, A.; Nazemiyeh, H. Antioxidant, total phenol and flavonoid contents of two Pedicularis L. species from eastern Azerbaijan, Iran. Bioimpacts 2012, 2, 47–53. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Hiai, S.; Oura, H.; Nakajima, T. Color reaction of some sapogenins and saponins with vanillin and sulfuric acid. Planta Medica 1976, 29, 116–122. [Google Scholar] [CrossRef]
- Fracasso, J.A.R.; Sikina, I.Y.G.; Costa, L.T.S.; Guarnier, L.P.; Ribeiro-Paes, J.T.; Ferreira, F.Y.; Almeida, L.V.C.; Castro Silva, B.; Barbosa, D.B.; Ximenes, V.F.; et al. Toxicological profile and anti-inflammatory effect of mucoadhesive gel from residues of Agave sisalana and Punica granatum. Gels 2023, 9, 942. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.D.; Do, L.T.K.; Dang, T.N.D.; Pham, V.D.; Hoang, V.C. Recovery of saponins, phenolic compounds and antioxidant capacity from Curculigo orchioides Gaertn rhizomes by different extraction methods. Appl. Sci. 2024, 14, 7535. [Google Scholar] [CrossRef]
- Iatta, R.; Cafarchia, C.; Cuna, T.; Montagna, O.; Laforgia, N.; Gentile, O.; Rizzo, A.; Boekhout, T.; Otranto, D.; Montagna, M.T. Bloodstream infections by Malassezia and Candida species in critical care patients. Med. Mycol. 2013, 52, 264–269. [Google Scholar] [CrossRef]
- Lopes, J.P.; Lionakis, M.S. Pathogenesis and virulence of Candida albicans. Virulence 2021, 13, 89–121. [Google Scholar] [CrossRef]
- Chryssanthou, E.; Broberger, U.; Petrini, B. Malassezia pachydermatis fungaemia in a neonatal intensive care unit. Acta Paediatr. 2001, 90, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Chen, H.F.; Yeh, Y.C.; Xue, Y.P.; Lan, C.Y. The transcription factor Sfp1 regulates the oxidative stress response in Candida albicans. Microorganisms 2019, 7, 131. [Google Scholar] [CrossRef] [PubMed]
- Herrero, E.; Ros, J.; Bellí, G.; Cabiscol, E. Redox control and oxidative stress in yeast cells. Biochim. Biophys. Acta Gen. Subj. 2008, 1780, 1217–1235. [Google Scholar] [CrossRef] [PubMed]
- Al-Sweih, N.; Ahmad, S.; Joseph, L.; Khan, S.; Khan, Z. Malassezia pachydermatis fungemia in a preterm neonate resistant to fluconazole and flucytosine. Med. Mycol. Case Rep. 2014, 5, 9–11. [Google Scholar] [CrossRef]
- Rodríguez-Valdovinos, K.Y.; Salgado-Garciglia, R.; Hernández-García, A.; Saavedra-Molina, A.; Río-Torres RENdel López-Meza, J.E.; Monribot-Villanueva, J.L.; Guerrero-Analco, J.A.; Medina-Medrano, J.R. Antioxidant and antifungal activities and characterization of phenolic compounds using UPLC-MS of aqueous extracts and fractions from Verbesina sphaerocephala stems. Plants 2024, 13, 2791. [Google Scholar] [CrossRef]
- Kaur, N.; Bains, A.; Kaushik, R.; Dhull, S.B.; Fogarasi, M.; Chawla, P. A review on antifungal efficiency of plant extracts entrenched polysaccharide-based nanohydrogels. Nutrients 2021, 13, 2055. [Google Scholar] [CrossRef]
- Toiu, A.; Mocan, A.; Vlase, L.; Pârvu, A.E.; Vodnar, D.C.; Gheldiu, A.M.; Moldovan, C.; Oniga, I. Phytochemical composition, antioxidant, antimicrobial and in vivo anti-inflammatory activity of traditionally used Romanian Ajuga laxmannii. Front. Pharmacol. 2018, 9, 7. [Google Scholar] [CrossRef]
- Esmaeili, A.; Saleh, I.; Abu-Dieyeh, M.H. Antifungal potential of plant-based extracts against Candida species: Values, safety concerns, and possible applications. Phytochem. Rev. 2025. ahead of print. [Google Scholar] [CrossRef]
- Liu, R.H.; Shang, Z.C.; Li, T.X.; Yang, M.H.; Kong, L.Y. In vitro antibiofilm activity of eucarobustol E against Candida albicans. Antimicrob. Agents Chemother. 2017, 61, e02707-16. [Google Scholar] [CrossRef]
- Dantas, T.S.; Machado, J.C.B.; Ferreira, M.R.A.; Soares, L.A.L. Bioactive plant compounds as alternatives against antifungal resistance in the Candida strains. Pharmaceutics 2025, 17, 687. [Google Scholar] [CrossRef]
- Hendra, R.; Agustha, A.; Frimayanti, N.; Abdulah, R.; Teruna, H.Y. Antifungal potential of secondary metabolites derived from Arcangelisia flava. Molecules 2024, 29, 2373. [Google Scholar] [CrossRef] [PubMed]
- Aligiannis, N.; Kalpoutzakis, E.; Mitaku, S.; Chinou, I.B. Composition and antimicrobial activity of the essential oils of two Origanum species. J. Agric. Food Chem. 2001, 49, 4168–4170. [Google Scholar] [CrossRef] [PubMed]
- Pelczar, M.J.; Chan, E.C.S.; Krieg, N.R. Microbiologia: Conceitos e Aplicações; McGraw-Hill: Columbus, OH, USA, 2005. [Google Scholar]
- Santos, J.D.G.; Branco, A.; Silva, A.F.; Pinheiro, C.S.R.; Uetanabaro, A.P.T.; Queiroz, S.R.O.D.; Osuna, J.T.A. Antimicrobial activity of Agave sisalana. Afr. J. Biotechnol. 2009, 8, 6181–6184. [Google Scholar] [CrossRef]
- Hammuel, C.; Yebpella, G.G.; Shallangwa, G.A.; Magomya, A.M.; Agbajp, A.S. Phytochemical and antimicrobial screening of methanol and aqueous extracts of Agave sisalana. Acta Pol. Pharm. 2011, 68, 535–539. [Google Scholar] [PubMed]
- Almeida, E.C.S.L. Ação Antifúngica do Resíduo Líquido Integral e Fracionado do Desfibramento da Folha do Sisal (Agave sisalana). Master’s Thesis, UNESP, São Paulo, Brazil, 2013. [Google Scholar]
- Duarte-Almeida, J.M.; Santos, R.J.; Genovese, M.I.; Lajolo, F.M. Evaluation of the antioxidant activity using the β-carotene/linoleic acid system and the DPPH scavenging method. Food Sci. Technol. 2006, 26, 446–452. [Google Scholar] [CrossRef]
- Koleva, I.I.; Van Beek, T.A.; Linssen, J.P.H.; Groot, A.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef]
- Cheung, L.M.; Cheung, P.C.K.; Ooi, V.E.C. Antioxidant activity and total phenolics of edible mushroom extracts. Food Chem. 2003, 81, 249–255. [Google Scholar] [CrossRef]
- Zuanazzi, J.A.S.; Montanha, J.A.; Limberger, R.P. Flavonoides. In Farmacognosia: Da Planta ao Medicamento; Simões, C.M.O., Schenkel, E.P., Gosmann, G., de Mello, J.C.P., Mentz, L.A., Petrovick, P.R., Eds.; UFRGS/UFSC: Farroupilha, Brazil, 2003; pp. 577–614. [Google Scholar]
- Rawat, M.; Varshney, A.; Kandpal, R.; Choudhary, A.; Gupta, A.K.; Pratiksha Naik, B.; Kumar, V.; Kumar, A.; Kheto, A.; Bhatt, S.; et al. Exploration of compositional, functional, nutraceutical, and metabolites of Ram kandmool (Agave sisalana Perrine). Int. J. Biol. Macromol. 2025, 307, 142095. [Google Scholar] [CrossRef]
- Daher, C.C. Uso do Resíduo Industrial do Sisal (Agave sisalana Perrine) em Produtos Cosméticos Sustentáveis com Atividades Antioxidante e Fotoprotetora. Ph.D. Thesis, UFRN, Natal, Brazil, 2022. [Google Scholar]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Martins, C.C.; Rodrigues, R.C.; Mercali, G.D.; Rodrigues, E. New insights into non-extractable phenolic compounds analysis. Food Res. Int. 2022, 157, 111487. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Xu, Q.; Shikov, A.N.; Pozharitskaya, O.N.; Flisyuk, E.V.; Liu, M.; Li, H.; Vargas-Murga, L.; Duez, P. Flavonoids and saponins: What have we got or missed? Phytomedicine 2023, 109, 154580. [Google Scholar] [CrossRef]
- Qin, X.; Lu, Y.; Peng, Z.; Fan, S.; Yao, Y. Systematic chemical analysis approach reveals superior antioxidant capacity via the synergistic effect of flavonoid compounds in red vegetative tissues. Front. Chem. 2018, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Trdá, L.; Janda, M.; Macková, D.; Pospíchalová, R.; Dobrev, P.I.; Burketová, L.; Matułinsky, P. Dual mode of the saponin aescin in plant protection: Antifungal agent and plant defense elicitor Front. Plant Sci. 2019, 10, 1448. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Karima, G.; Khan, M.; Shin, J.; Kim, J. Therapeutic effects of saponins for the prevention and treatment of cancer. Int. J. Mol. Sci. 2022, 23, 10665. [Google Scholar] [CrossRef]
- Barreto, S.M.A.G.; Cadavid, C.O.M.; Moura, R.A.O.; Silva, G.M.M.; Araújo, S.V.F.; Silva Filho, J.A.A.; Rocha, H.A.O.; Oliveira, R.P.; Giordani, R.B.; Ferrari, M. In vitro and in vivo antioxidant activity of Agave sisalana agro-industrial residue. Biomolecules 2020, 10, 1435. [Google Scholar] [CrossRef] [PubMed]
- Araldi, R.P.; Santos, M.O.; Barbon, F.F.; Manjerona, B.A.; Meirelles, B.R.; Oliva Neto, P.; Silva, P.I.; Santos, L.; Camargo, I.C.C.; Souza, E.B. Analysis of antioxidant, cytotoxic and mutagenic potential of Agave sisalana Perrine extracts. Biomed. Pharmacother. 2018, 98, 873–885. [Google Scholar] [CrossRef]
- Mazo, G.S.; Fracasso, J.A.R.; Costa, L.T.S.; Ximenes, V.F.; Zoppe, N.A.; Viel, A.M.; Guarnier, L.P.; Silva, B.C.; Almeida, L.V.C.; Santos, L. Development of an antioxidant, anti-aging, and photoprotective phytocosmetic from discarded Agave sisalana Perrine roots. Cosmetics 2024, 11, 104. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).