Abstract
Skin hyperpigmentation is primarily regulated by melanogenesis, in which tyrosinase and related enzymes play pivotal roles. Probiotics have recently been attracting attention as a cosmetic ingredient due to their skin-friendly and eco-friendly properties. In particular, microbial metabolites, known as postbiotics, are gaining attention for their superior safety, stability, and efficacy compared with probiotics. In this study, we investigated the whitening effect and molecular mechanisms of phenyllactic acid (PLA), a metabolite derived from Limosilactobacillus reuteri (L. reuteri) culture broth. In B16F10 melanoma cells, the effects of PLA were evaluated by measuring melanin content, cellular tyrosinase activity, enzyme kinetics, and the expression of melanogenesis-related proteins. PLA significantly inhibited melanin production and cellular tyrosinase activity in α-MSH–stimulated B16F10 melanoma cells without inducing cytotoxicity. PLA downregulated tyrosinase-related proteins such as TRP-1 and TRP-2, and competitively inhibited tyrosinase. The inhibition constants (Ki) for L-tyrosine and L-DOPA were 12.63 mM and 0.68 mM, respectively. These findings suggest that PLA, a postbiotic derived from lactic acid bacteria, may serve as a safe and effective whitening ingredient, providing a scientific basis for the development of functional skin-whitening cosmetics.
1. Introduction
Skin pigmentation is primarily determined by melanin, a pigment synthesized in melanocytes through the process of melanogenesis. Melanin plays a protective role against ultraviolet (UV) radiation by absorbing harmful rays; however, its excessive production can lead to hyperpigmentation disorders, including melasma, freckles, and age spots [1,2]. The rate-limiting step of melanogenesis is catalyzed by tyrosinase, while tyrosinase-related proteins (TRP-1 and TRP-2) also contribute to melanin biosynthesis. Tyrosinase is a copper-containing oxidase that functions as the rate-limiting enzyme in melanin synthesis, catalyzing both the hydroxylation of L-tyrosine to L-DOPA (monophenolase activity) and the oxidation of L-DOPA to dopaquinone (diphenolase activity) [3,4,5].
Because of their pivotal role, tyrosinase and its associated proteins have been recognized as primary targets for the development of skin-whitening agents. Accordingly, inhibition of tyrosinase activity and TRP expression represents a central strategy in whitening research, with several compounds such as arbutin, kojic acid, and L-ascorbic acid derivatives already commercialized based on this mechanism [6,7,8]. However, their application is often limited by side effects such as cytotoxicity, instability, and safety concerns during prolonged use. These drawbacks underscore the need for alternative whitening agents that exhibit both high efficacy and improved safety profiles.
In recent years, probiotics have attracted increasing interest in dermatological and cosmetic applications due to their skin-friendly and eco-friendly properties [9,10,11]. Limosilactobacillus reuteri is a probiotic bacterium capable of colonizing various hosts and has been isolated from multiple human body sites, including the gastrointestinal tract, urinary tract, skin, and breast milk [12]. It has also been reported to exert anti-inflammatory, antimicrobial, and immunomodulatory effects, as well as to regulate gut function and influence body weight and obesity [13,14].
Given these beneficial properties, increasing attention is being paid not only to live probiotics but also to their metabolites, known as postbiotics [15,16]. Postbiotics offer several advantages over probiotics, including consistent safety profiles, improved physicochemical stability, and enhanced functional efficacy [17,18]. In particular, postbiotics derived from lactic acid bacteria have been reported to exhibit diverse biological activities, including antioxidant, anti-inflammatory, and barrier protective effects [19,20,21]. Research on their applications in functional materials and cosmetics has been steadily increasing, highlighting their potential as novel functional ingredients [22].
Phenyllactic acid (PLA) is a representative metabolite produced by various probiotic bacteria, particularly species formerly classified under the genus Lactobacillus [23]. In our previous study, we demonstrated that the culture broth of L. reuteri strain 0333 exhibited antioxidant, anti-aging, whitening, and anti-inflammatory effects, and that PLA is one of its key metabolites [24]. PLA is an aromatic organic acid produced via the metabolic pathway of phenylalanine, an aromatic amino acid. Structurally, it consists of a benzene ring substituted with a hydroxyl group (-OH) and a carboxyl group (-COOH) [25,26]. This amphiphilic property enables PLA to interact effectively in both aqueous environments and lipid rich membranes, providing the structural basis for its diverse biological activities [27].
Furthermore, PLA shares structural similarities with aromatic α-hydroxy acids (AHAs), enabling it to partially replicate the keratolytic and complexion enhancing effects observed in conventional AHAs such as lactic acid and glycolic acid [26]. In addition, due to the presence of a phenyl ring, PLA may structurally resemble the aromatic moieties of tyrosinase substrates, such as L-tyrosine and L-DOPA [25,28]. This resemblance suggests a potential for PLA to act as a competitive or mixed type inhibitor of tyrosinase through interactions at the enzyme’s active site.
However, despite its known antimicrobial properties, the roles of PLA in skin physiology, including its involvement in skin whitening and melanogenesis, remain largely unexamined. In particular, its potential role in skin whitening and its involvement in the regulation of melanogenesis remain poorly understood. Considering the limitations of currently used whitening agents, such as cytotoxicity, instability, and safety concerns, PLA, which is derived from probiotics and structurally resembles tyrosinase substrates, may represent a promising alternative for cosmetic applications. Therefore, we investigated the whitening effects and underlying molecular mechanisms of PLA, a metabolite obtained from L. reuteri, to assess its potential as a functional ingredient for cosmetic use.
2. Materials and Methods
2.1. Chemicals and Reagents
We used commercially available PLA purchased from Sigma-Aldrich to ensure chemical purity, consistent dosing, and experimental reproducibility. The PLA used in this study, along with DPPH, ABTS, MTT, DMSO, α-MSH, L-DOPA, and arbutin, was purchased from Sigma-Aldrich (St. Louis, MO, USA). Tyrosine was obtained from Amresco (Solon, OH, USA). Cell culture reagents, including phosphate-buffered saline (PBS), Dulbecco’s Modified Eagle Medium (DMEM), penicillin/streptomycin (P/S), and fetal bovine serum (FBS), were purchased from Lonza (Walkersville, MD, USA) and Gibco (Thermo Fisher Scientific, Waltham, MA, USA). Antibodies against GAPDH, MITF, TRP-1, and TRP-2 were obtained from Cell Signaling Technology (Danvers, MA, USA), Santa Cruz Biotechnology (Dallas, TX, USA), and EnoGene (Nanjing, China). The instruments used included a CO2 incubator (MCO-18AC-PK, Panasonic, Tokyo, Japan), a microplate reader (Multiskan Sky, Thermo Fisher Scientific, Waltham, MA, USA), and an inverted microscope (ECLIPSE TS100, Nikon, Tokyo, Japan). The cell line used was B16F10 melanoma cells (CRL-6475, ATCC, Manassas, VA, USA), and the bacterial strain used was L. reuteri DS0333 (KCTC15296BP, KCTC, Daejeon, Republic of Korea).
2.2. HPLC Analysis
Quantification and identification of PLA were performed using high performance liquid chromatography (HPLC). The analysis was conducted with a Waters HPLC system (Waters, Milford, MA, USA) equipped with a Waters 1525 binary pump and a Waters 2487 dual wavelength absorbance detector. Separation was carried out on an Agilent Zorbax C18 column (4.6 × 250 mm, 5 µm particle size, Santa Clara, CA, USA) at room temperature. PLA was identified by comparing the retention time of the sample with that of the authentic standard, and detection was performed at 210 nm. The detailed analytical conditions, including mobile phase composition, flow rate, injection volume, and detection wavelength, are summarized in Table 1.

Table 1.
HPLC analysis condition.
2.3. Molecular Docking
Molecular docking of PLA with mushroom tyrosinase was performed using the SwissDock web server (http://www.swissdock.ch/, accessed on 21 October 2025) with the AutoDock Vina (version 1.2.0; The Scripps Research Institute, La Jolla, CA, USA) engine option. SwissDock has an interface and functionality that allows you to directly input protein and ligand structures from the database, modify docking parameters, and visualize the most favorable clusters online [29]. The three-dimensional (3D) structure of mushroom tyrosinase was retrieved from the Protein Data Bank (PDB ID: 2Y9X). Protein ATOM coordinates were retained, and the binuclear Cu2+ ions in the active site were preserved. Water molecules (HOH) and the co-inhibitor tropolone (residue codes TPL and OTR) were removed to avoid interference. The 3D structure of PLA, PubChem CID: 444718) was obtained as an SDF file from PubChem and converted to MOL2 with Open Babel. During conversion, the ligand was protonated at pH 7.0–7.4 to yield the carboxylate form (−1 charge), explicit hydrogens were added, Gasteiger partial charges were assigned, 3D coordinates were generated, and the structure was energy-minimized (MMFF94). Binding affinities (ΔG, kcal/mol) were calculated by Vina scoring, and the top-ranked poses were analyzed for potential hydrogen bonding and hydrophobic interactions [29,30]. Hydrogen bond distances and molecular interactions were visualized and measured using UCSF Chimera version 1.19.
2.4. Mushroom Tyrosinase Inhibition Activity
The assay was performed in a 96-well microplate format with a final reaction volume of 200 μL per well. Each well contained 67 mM sodium phosphate buffer (pH 6.8), PLA at concentrations of 1, 2.5, 5, 10, and 20 mM, and either L-tyrosine or L-DOPA as substrate. The final concentrations of substrates were set at 2 mM for both L-tyrosine and L-DOPA. The final mushroom tyrosinase concentrations were 200 U/mL for the L-tyrosine (monophenolase) assay and 50 U/mL for the L-DOPA (diphenolase) assay. The mixtures were incubated at room temperature for 30 min without shaking. L-ascorbic acid was used as a positive control for comparison of mushroom tyrosinase inhibition activity. The formation of dopachrome was monitored by measuring the absorbance at 475 nm using a microplate reader (Multiskan Sky, Thermo Fisher Scientific, Waltham, MA, USA). Tyrosinase inhibitory activity was calculated as a percentage compared to the control wells containing enzyme and substrate without PLA.
2.5. Enzyme Kinetics Analysis
Monophenolase and diphenolase activities of tyrosinase were determined using a modified version of previously reported methods by measuring the rate of dopachrome formation at 475 nm (ε = 3700 M−1·cm−1) [31]. L-tyrosine was used as the substrate for monophenolase activity, and L-DOPA was used as the substrate for diphenolase activity. The reaction mixtures were prepared in 67 mM sodium phosphate buffer (pH 6.8), and the final substrate concentrations were set at 0.1, 0.2, 0.3, and 0.4 mM. The final concentration of tyrosinase was adjusted to 150 U/mL for the L-tyrosine assays and 75 U/mL for the L-DOPA assays, with a total reaction volume of 150 µL. Substrate and PLA were added to the reaction mixtures, and absorbance was measured at 30 °C at 30-s intervals using a microplate spectrophotometer (Multiskan Sky, Thermo Fisher Scientific, Waltham, MA, USA) to calculate the initial reaction rates. To elucidate the inhibitory mechanism of PLA against tyrosinase, the initial velocity data were analyzed using Lineweaver–Burk double reciprocal plots. The inhibition constant (Ki) was determined from secondary plots of the slope or intercept values against varying concentrations of PLA [32,33].
2.6. Cell Culture
B16F10 melanoma cells were obtained from ATCC (Manassas, VA, USA) and cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. Cells were maintained in an incubator at 37 °C with 5% CO2. When cell confluence reached approximately 90%, they were subcultured using trypsin-EDTA treatment and maintained continuously.
2.7. MTT Assay
Cell viability was evaluated using a modified MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. B16F10 melanoma cells were seeded in 96-well plates at a density of 1 × 104 cells per well and allowed to stabilize for 24 h. The cells were then treated with the indicated concentrations of each sample and incubated for 72 h at 37 °C in a humidified atmosphere containing 5% CO2. Following incubation, MTT solution was added to each well to achieve a final concentration of 0.5 mg/mL, and the cells were further incubated for 3 h at 37 °C. After removing the medium, 200 μL of dimethyl sulfoxide (DMSO) was added to each well to dissolve the resulting formazan crystals. The plates were gently shaken at room temperature for 10 min to ensure complete solubilization, and absorbance was measured at 570 nm using a microplate reader.
2.8. Melanin Contents
Intracellular melanin production was measured using B16F10 melanoma cells. Cells were seeded in 6-well plates at a density of 2 × 105 cells per well and allowed to attach and stabilize for 24 h in a humidified incubator at 37 °C with 5% CO2. The medium was then replaced with treatment medium containing the test samples and α-MSH (100 nM). Arbutin (100 μg/mL) was used as a positive control. After treatment, the cells were further incubated for 48 h. Following incubation, washed with phosphate-buffered saline (PBS) and harvested by centrifugation at 12,000 rpm for 15 min. The collected cell pellets were resuspended in 1 N NaOH containing 10% DMSO and incubated at 80 °C for 1 h to dissolve melanin. The absorbance of the resulting solution was then measured at 475 nm using a microplate spectrophotometer. Melanin content was quantitatively determined based on the absorbance of the sample-treated groups.
2.9. Intracellular Tyrosinase Inhibition Activity
Intracellular tyrosinase inhibitory activity was evaluated using B16F10 melanoma cells. Cells were seeded in 6-well plates at a density of 2 × 105 cells per well and incubated for 24 h at 37 °C in a humidified atmosphere containing 5% CO2. The medium was then replaced with treatment medium containing the test samples and α-MSH (100 nM), while arbutin (100 μg/mL) was used as a positive control. The treated cells were further incubated for 48 h. After incubation, the cells were washed with cold PBS and lysed using Pro-Prep lysis solution (iNtRON Biotechnology, Seongnam, Republic of Korea). The lysates were centrifuged at 13,000 rpm for 5 min at 4 °C to remove cellular debris, and the supernatants were collected for analysis. The cell extracts were then mixed with 0.1 M sodium phosphate buffer (pH 6.8), followed by the addition of L-DOPA (1 mg/mL) as the substrate. The mixtures were dispensed into 96-well plates and incubated at 37 °C for 1 h. After incubation, absorbance was measured at 475 nm to quantify tyrosinase activity.
2.10. Western Blot Analysis
B16F10 melanoma cells were seeded in 6-well plates at a density of 1.5 × 105 cells per well. After treatment with the indicated concentrations of each sample, the cells were incubated for 72 h. The cells were washed with cold PBS and lysed using Pro-Prep lysis solution (iNtRON Biotechnology, Seongnam, Republic of Korea). The lysates were kept on ice for 10 min to facilitate protein extraction and centrifuged at 13,000 rpm for 5 min at 4 °C. The resulting supernatants were collected, and total protein concentrations were determined using the Bradford assay. The quantified proteins were mixed with sterile distilled water and SDS, separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and subsequently transferred onto polyvinylidene difluoride (PVDF) membranes. The transferred membranes were blocked at room temperature for 1 h with 5% skim milk solution prepared in TBS (Tris-buffered saline) containing 0.1% Tween-20. After blocking, the membranes were incubated with the primary antibodies for 1 h at room temperature, with GAPDH serving as the internal control. The membranes were then washed four times with TBS containing 0.1% Tween-20 and incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 h. Protein expression was detected using WesternBright™ enhanced chemiluminescence (ECL) kit (Advansta, San Jose, CA, USA), and band intensities were quantified using the Davinch-Western™ Imaging System (Davinch-K, Seoul, Republic of Korea).
2.11. Statistical Analysis
Results were expressed as the 27.0, IBM, Armonk, NY, USA). Statistical significance was set at p < 0.mean ± standard deviation (SD) of three independent experiments. Statistical significance was determined using Student’s t-test and ANOVA and Duncan’s multiple range test using SPSS software (version 05).
3. Results
3.1. HPLC Analysis of PLA
In our previous study, the culture broth of L. reuteri DS0333 exhibited multiple skin-beneficial activities, including antioxidant, anti-photoaging, and whitening effects [26]. To further identify the active ingredient responsible for these effects, the DS0333 culture broth was analyzed by HPLC to determine whether PLA was the major metabolite. HPLC analysis revealed a distinct peak in the DS0333 culture broth that corresponded to the retention time of the PLA standard, whereas no peak was detected in the control medium (Figure 1). The confirmation of PLA production provides an important biochemical basis for linking the DS0333 culture broth to whitening activity, supporting our subsequent evaluation of PLA as a potential skin-whitening ingredient.
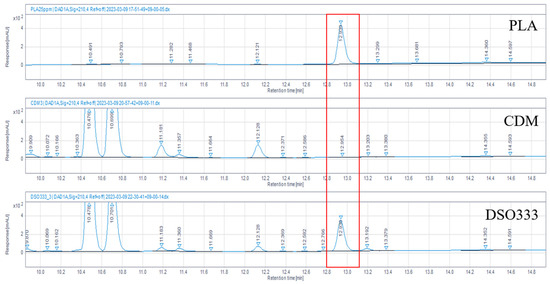
Figure 1.
HPLC chromatograms of PLA standard (150 μM), chemically defined medium (CDM), and L. reuteri DS0333 culture supernatant. Red box indicates the peak corresponding to PLA.
3.2. Molecular Docking Analysis
To determine whether PLA can interact with the active site of mushroom tyrosinase, we performed molecular docking using the SwissDock web server. Using the crystal structure of mushroom tyrosinase as the receptor, we calculated the binding affinities (kcal/mol) of the top 20 docked structures (Table 2). Lower binding energy values indicate more stable predicted complexes. PLA was predicted to bind within the catalytic pocket with a minimum binding free energy of −4.420 kcal/mol (Model 1), while other conformations exhibited similar values ranging from −4.420 to −3.096 kcal/mol.

Table 2.
Calculated binding affinities (kcal/mol) of the top 20 docking conformations of PLA with mushroom tyrosinase, as predicted by SwissDock. Lower binding energy values indicate more stable predicted complexes.
The best binding pose revealed potential hydrogen bonding and hydrophobic contacts with active site residues (Figure 2a). The best binding pose (Model 1) revealed potential hydrogen bonding interactions between PLA and residues GLN48 and ASP10, with distances of 4.826 Å and 4.712 Å, respectively (Figure 2b). These interactions likely contribute to the binding stabilization within the substrate-binding site. Although the predicted binding affinity was weaker than those typically observed for commercial inhibitors, the docking results suggest that PLA is capable of occupying the substrate-binding site, thereby supporting its potential role as a competitive tyrosinase inhibitor, which was later confirmed by in vitro enzyme kinetics and cellular assays.
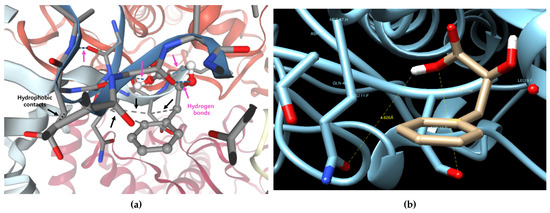
Figure 2.
Predicting the binding form of phenyllactic acid (PLA) and mushroom tyrosinase (PDB ID: 2Y9X). PLA (shown in stick model, gray) is located within the catalytic pocket of tyrosinase. Dashed blue lines indicate predicted hydrogen bonds between PLA and the surrounding amino acid residues. Yellow highlights denote ionic interactions. (a) Visualization of predicted hydrogen bonds (blue dashed lines) and hydrophobic contacts (gray dashed lines) using the SwissDock server. (b) Measurement of key interactions at the top-ranked docking pose using the Chimera tool (Model 1).
3.3. Mushroom Tyrosinase Activity
To experimentally validate the docking results, the inhibitory effect of PLA on mushroom tyrosinase was examined using two different substrates. PLA showed weaker tyrosinase inhibitory activity than L-ascorbic acid at the same concentration, but inhibited both monophenolase and diphenolase activities in a concentration-dependent manner (Figure 3). As shown in Figure 3a, PLA significantly inhibited enzyme activity when L-tyrosine was used as the substrate, reducing activity to 83.8% and 80.9% of control at 15 and 20 mM, respectively. A more pronounced inhibitory effect was observed with L-DOPA (Figure 3b), where PLA treatment at 15 and 20 mM reduced the activity to 54.1% and 40.1% of the control, respectively. These findings confirm that PLA is capable of inhibiting mushroom tyrosinase activity, consistent with the binding prediction from the docking analysis.
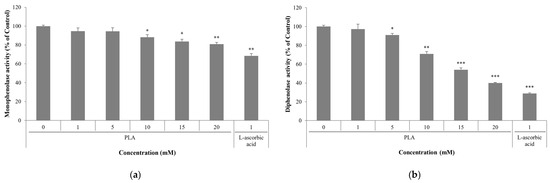
Figure 3.
Effect of PLA mushroom tyrosinase activity. Inhibitory activity of PLA using L-tyrosine (a) and L-DOPA (b) as the substrate. Data are expressed as mean ± SD of three independent experiments. * p < 0.05, ** p < 0.01, *** p < 0.001 compared with control group (0 mM PLA).
3.4. Enzyme Kinetic Analysis of PLA
Lineweaver–Burk analysis showed sets of straight lines with a common y-intercept and in-creasing slopes as PLA concentration increased for both monophenolase (L-tyrosine) and diphenolase (L-DOPA) reactions (Figure 4a,c). The constant y-intercepts (1/Vmax) together with increased slopes (Km/Vmax) indicate that PLA exhibits a competitive inhibition pattern. Secondary plots of the Lineweaver–Burk slopes against [PLA] were linear (Figure 4b,d) and yielded inhibition constants (Ki) of 12.63 mM for monophenolase and 0.68 mM for diphenolase. The markedly smaller Ki for the diphenolase reaction suggests that PLA binds more strongly with the catalytic site that processes L-DOPA. Overall, these kinetic data demonstrate that PLA competitively interferes with substrate binding to tyrosinase and, consistent with the mushroom tyrosinase activity results, exhibits a stronger inhibitory effect on diphenolase activity than on monophenolase activity.
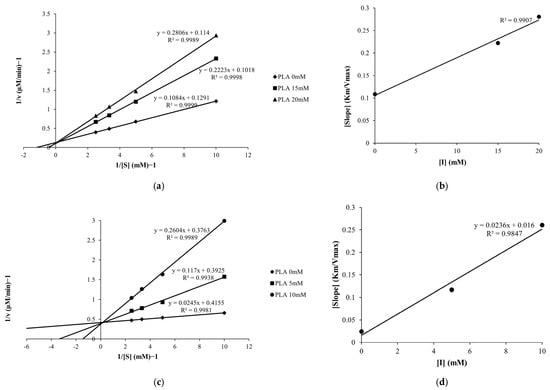
Figure 4.
Lineweaver–Burk plots of tyrosinase monophenolase (a) and diphenolase (c) activity with PLA. Secondary plots of the slope values for monophenolase (b) and diphenolase (d) activity were used to determine the inhibition constants (Ki).
3.5. Effect of PLA on Melanin Synthesis and Tyrosinase Activity in B16F10 Melanoma Cell
MTT assay results showed that PLA at concentrations of 1, 2.5, and 5 mM did not significantly affect cell viability compared to the control group, with viability remaining above 90% in all cases (Figure 5a). To evaluate melanin biosynthesis, B16F10 cells were stimulated with α-MSH and then treated with PLA at concentrations of 1, 2.5, and 5 mM. Arbutin was used as a positive control and is commonly used to measure melanin content and tyrosinase inhibitory activity [34,35]. PLA inhibited melanin production in a concentration-dependent manner (Figure 5b). PLA at a concentration of 5 mM exhibited a more potent inhibitory effect than the positive control, arbutin, by reducing melanin content by approximately 48.01% compared to the α-MSH-treated group. Tyrosinase inhibitory activity was also evaluated under the same conditions as those used for melanin content analysis. PLA significantly suppressed tyrosinase activity in a concentration-dependent manner (Figure 5c). At 5 mM, PLA reduced tyrosinase activity by approximately 102.2% compared to the α-MSH–treated group, exhibiting an inhibitory effect comparable to that of arbutin.
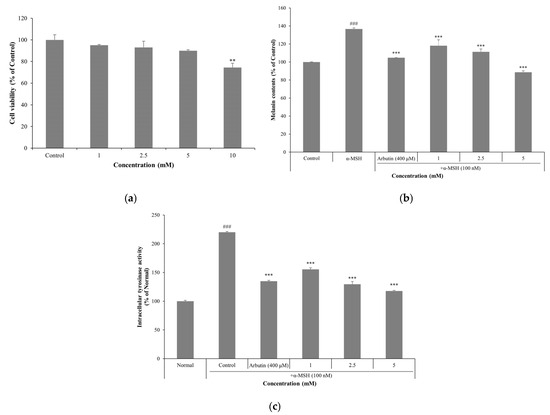
Figure 5.
Effect of PLA on cell viability (a) in B16F10 melanoma cells. Effect of PLA on melanin contents (b) and intracellular tyrosinase inhibition activity (c) in B16F10 mouse melanoma cells. Cells were stimulated with α-MSH (100 nM) and treated with PLA or arbutin (200 μM) for 72 h. Data are expressed as mean ± SD of three independent experiments. ### p < 0.001 compared with untreated normal control, ** p < 0.01, *** p < 0.001 compared with control group.
3.6. Effect of PLA on the Protein Levels MITF, TRP-1, TRP-2 in B16F10 Melanoma Cell
Western blot analysis showed that PLA treatment differentially regulated the expression of melanogenesis-related proteins in α-MSH–induced B16F10 cells (Figure 6a). Specifically, PLA increased the expression of MITF, a key transcription factor in melanogenesis, in a concentration-dependent manner. In contrast, the protein levels of its downstream targets TRP-1 and TRP-2 were significantly suppressed by PLA, with the most pronounced inhibition observed at 2.5 and 5 mM. Densitometric quantification confirmed that PLA markedly attenuated the α-MSH–induced upregulation of TRP-1 and TRP-2 while sustaining or enhancing MITF expression (Figure 6b). These findings suggest that PLA does not directly downregulate MITF but rather disrupts the melanogenic pathway of melanogenesis by selectively inhibiting MITF-regulated enzymes. Consequently, PLA may interfere with melanin biosynthesis at the enzymatic level despite maintaining transcriptional activation, indicating a unique regulatory mechanism distinct from conventional tyrosinase inhibitors.
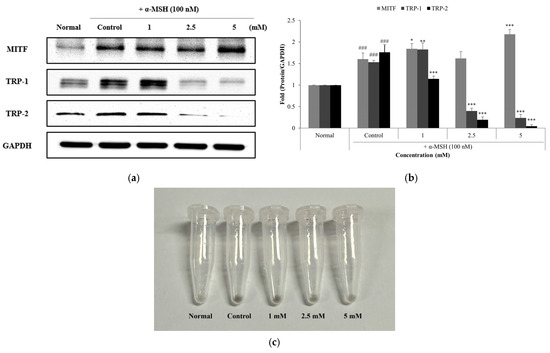
Figure 6.
Effect of PLA on the protein levels of MITF, TRP-1 and TRP-2 (a). Western blot analysis of melanin synthesis-related proteins in α-MSH-induced B16F10 cells treated with PLA. GAPDH was used as a loading control. protein levels were normalized by each unphosphorylated protein and GAPDH (b). Image of cell pellet collected after PLA treatment (c). ### p < 0.001 compared with normal control, * p < 0.05, ** p < 0.01, *** p < 0.001 compared with control group.
4. Discussion
In this study, we demonstrated that phenyllactic acid (PLA), a representative postbiotic metabolite derived from L. reuteri DS0333, exerts inhibitory effects on melanogenesis through multiple mechanisms. HPLC analysis confirmed the presence of PLA as a major metabolite in the culture broth, providing a biochemical link to the whitening effects observed in our previous study.
Molecular docking predicted that PLA could bind to the active site of tyrosinase, with binding affinities in the range of −4.420 to −3.096 kcal/mol. Although these values were weaker than those typically reported for commercial inhibitors, the docking results indicated that PLA is structurally capable of interacting with active-site residues, supporting its potential as a competitive inhibitor.
Functionally, PLA significantly inhibited mushroom tyrosinase activity in vitro. Enzyme kinetic analysis further revealed a competitive inhibition pattern, with unchanged Vmax and increased Km, suggesting that PLA likely binds at the substrate-binding site. Interestingly, the inhibition constant (Ki) was markedly lower for diphenolase activity (0.68 mM) than for monophenolase activity (12.63 mM), indicating that PLA exhibits stronger binding affinity to the active site when L-DOPA is the substrate. The preferential inhibition of diphenolase activity is likely due to the structural features of PLA. While PLA does not contain a catechol moiety, its phenyl ring could mimic the aromatic region of tyrosine and L-DOPA.
At the cellular level, PLA dose-dependently inhibited melanin biosynthesis in α-MSH-stimulated B16F10 melanoma cells without cytotoxicity, and exhibited whitening effects equivalent to or greater than arbutin at high concentrations. Western blot analysis revealed a unique regulatory pattern, as PLA treatment increased the expression of MITF while markedly reducing the expression of its downstream targets, TRP-1 and TRP-2. This finding deviates from the classical mechanism of whitening agents, which typically downregulate MITF to suppress downstream melanogenic enzymes such as TRP-1 and TRP-2 [36]. The paradoxical increase in MITF expression accompanied by decreased TRP-1 and TRP-2 levels suggests that PLA does not primarily act through the classical mechanism of suppressing MITF gene expression. Instead, PLA may induce downregulation of MITF downstream targets by impairing the functional activity of MITF despite its increased protein levels. This effect may occur through post-translational modifications of MITF, such as ERK-mediated phosphorylation, which functions as an upstream signaling pathway of MITF. Some studies have reported that ERK-mediated phosphorylation can destabilize MITF or reduce its transcriptional activity despite elevated protein levels [37,38].
Taken together, these findings indicate that PLA suppresses melanogenesis through multiple and complementary mechanisms: (i) direct competitive inhibition of tyrosinase, particularly against diphenolase activity; and (ii) functional impairment of MITF, leading to the downregulation of its downstream melanogenic enzymes TRP-1 and TRP-2. This dual action distinguishes PLA from conventional whitening agents, which typically act through either direct enzyme inhibition or transcriptional repression of MITF.
Nevertheless, this study has several limitations. To clarify the unique regulatory effects observed in this study, mechanistic studies addressing the precise signaling pathways involved, particularly the role of ERK/MAPK-mediated phosphorylation of MITF, are needed. Finally, although the whitening efficacy of PLA is well supported by in vitro assays and cell-based models, further validation using animal models or reconstituted human skin equivalents may be considered to confirm its efficacy and safety under physiologically relevant conditions.
5. Conclusions
In conclusion, our results demonstrate that PLA, a representative postbiotic metabolite derived from L. reuteri DS0333, effectively suppresses melanogenesis through both enzymatic and transcriptional mechanisms. PLA exhibits whitening effects equivalent to or greater than arbutin without cytotoxicity by competitively inhibiting tyrosinase and selectively inhibiting the expression of genes related to melanin production. These results suggest that PLA, with its low toxicity and probiotic origin, is a promising candidate for development as a safe and effective skin whitening ingredient in functional cosmetics. Furthermore, as an aromatic organic acid with high water solubility and postbiotic properties, PLA is expected to remain stable in mildly acidic environments (pH 3–5). Therefore, it may be suitable for use in aqueous cosmetic formulations such as toners or essences for topical application, although further studies are needed to optimize formulation stability.
Author Contributions
Writing—original draft preparation, project administration, methodology, formal analysis, K.-M.K. and S.-Y.S.; visualization, K.-M.K., S.-Y.S. and N.-R.S.; investigation, J.-H.B. and S.-J.K.; supervision, writing—review and editing, S.O.K. and K.-M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Regional Innovation System & Education (RISE) program through the Jeollanamdo RISE center, funded by the Ministry of Education (MOE) and the Jeollanamdo, Republic of Korea (2025-RISE-14-004). The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon request due to restrictions.
Acknowledgments
During the preparation of this manuscript, the authors used ChatGPT 4o for the purposes of preparing the manuscript for general editing. The authors have re-viewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
S.O.K. is affiliated with a private company (Central R&D Center, B & Tech Co., Ltd., Naju 58205, Republic of Korea); The author declares the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- Burger, P.; Landreau, A.; Azoulay, S.; Michel, T.; Fernandez, X. Skin whitening cosmetics: Feedback and challenges in the development of natural skin lighteners. Cosmetics 2016, 3, 36. [Google Scholar] [CrossRef]
- Ito, S. A chemist’s view of melanogenesis. Pigment Cell Res. 2003, 16, 230–236. [Google Scholar] [CrossRef]
- Rodríguez-López, J.N.; Tudela, J.; Varón, R.; García-Carmona, F.; García-Cánovas, F. Analysis of a kinetic model for melanin biosynthesis pathway. J. Biol. Chem. 1992, 267, 3801–3810. [Google Scholar] [CrossRef]
- Vachtenheim, J.; Borovanský, J. “Transcription physiology” of pigment formation in melanocytes: Central role of MITF. Exp. Dermatol. 2010, 19, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Arbutin as a skin depigmenting agent with antimelanogenic and antioxidant properties. Antioxidants 2021, 10, 1129. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, R.; Mohamed, M.S.; Suhaili, N.; Salleh, M.M.; Ariff, A.B. Kojic acid: Applications and development of fermentation process for production. Biotechnol. Mol. Biol. Rev. 2010, 5, 24–37. [Google Scholar]
- Ravetti, S.; Clemente, C.; Brignone, S.; Hergert, L.; Allemandi, D.; Palma, S. Ascorbic acid in skin health. Cosmetics 2019, 6, 58. [Google Scholar] [CrossRef]
- Lukic, J.; Chen, V.; Strahinic, I.; Begovic, J.; Lev-Tov, H.; Davis, S.C.; Tomic-Canic, M.; Pastar, I. Probiotics or pro-healers: The role of beneficial bacteria in tissue repair. Wound Repair Regen. 2017, 25, 911–922. [Google Scholar] [CrossRef] [PubMed]
- La Fata, G.; Weber, P.; Mohajeri, M.H. Probiotics and the gut immune system: Indirect regulation. Probiotics Antimicrob. Proteins 2018, 10, 11–21. [Google Scholar] [CrossRef]
- Lolou, V.; Panayiotidis, M.I. Functional role of probiotics and prebiotics on skin health and disease. Fermentation 2019, 5, 41. [Google Scholar] [CrossRef]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in human health and diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef]
- Abuqwider, J.; Altamimi, M.; Mauriello, G. Limosilactobacillus reuteri in Health and Disease. Microorganisms 2022, 10, 522. [Google Scholar] [CrossRef]
- Wang, Q.; He, Y.; Li, X.; Zhang, T.; Liang, M.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus reuteri CCFM8631 Alleviates Hypercholesterolaemia Caused by the Paigen Atherogenic Diet by Regulating the Gut Microbiota. Nutrients 2022, 14, 1272. [Google Scholar] [CrossRef]
- Ma, Y.; Zhong, Y.; Tang, W.; Valencak, T.G.; Liu, J.; Deng, Z.; Mao, J.; Liu, D.; Wang, S.; Wang, Y.; et al. Lactobacillus reuteri ZJ617 Attenuates Metabolic Syndrome via Microbiota-Derived Spermidine. Nat. Commun. 2025, 16, 877. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.M.; Hong, T.; van Pijkeren, J.P.; Hemarajata, P.; Trinh, D.V.; Hu, W.; Britton, R.A.; Kalkum, M.; Versalovic, J. Histamine Derived from Probiotic Lactobacillus reuteri Suppresses TNF via Modulation of PKA and ERK Signaling. PLoS ONE 2012, 7, e31951. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Sil, D.; Sharma, R.; Kumar, D.; Komal, K.; Kumar, S.; Mahajan, H.S.; Sharma, M.; Kumar, M. Investigating Postbiotics as Innovative Adjuvants: Deciphering the Gut-Breast Connection in Breast Cancer Therapy, from Gut Microbiome to Personalized Medicine. Mol. Biol. Rep. 2025, 52, 547. [Google Scholar] [CrossRef]
- Rafique, N.; Jan, S.Y.; Dar, A.H.; Dash, K.K.; Sarkar, A.; Shams, R.; Pandey, V.K.; Khan, S.A.; Amin, Q.A.; Hussain, S.Z. Promising Bioactivities of Postbiotics: A Comprehensive Review. J. Agric. Food Res. 2023, 14, 100708. [Google Scholar] [CrossRef]
- Lee, H.W.; Lee, Y.R.; Park, K.M.; Lee, N.K.; Paik, H.D. Antimelanogenic and Antioxidant Effects of Postbiotics of Lactobacillus Strains in α-MSH-Induced B16F10 Melanoma Cells via CREB/MITF and MAPKs Signaling Pathway. J. Microbiol. Biotechnol. 2024, 34, 2279–2289. [Google Scholar] [CrossRef]
- Rawal, S.; Ali, S.A. Probiotics and Postbiotics Play a Role in Maintaining Dermal Health. Food Funct. 2023, 14, 3966–3981. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Cho, M.; Kang, D.J. Anti-Inflammatory Response of New Postbiotics in TNF-α/IFN-γ-Induced Atopic Dermatitis-like HaCaT Keratinocytes. Curr. Issues Mol. Biol. 2024, 46, 6100–6111. [Google Scholar] [CrossRef]
- Jin, X.; Nguyen, T.T.M.; Yi, E.-J.; Zheng, Q.; Park, S.-J.; Yi, G.-S.; Yang, S.-J.; Kim, M.-J.; Yi, T.-H. Emerging Trends in Skin Anti-Photoaging by Lactic Acid Bacteria: A Focus on Postbiotics. Chemistry 2024, 6, 1495–1508. [Google Scholar] [CrossRef]
- Liang, F.; Que, Y.; Liu, Y.; Inam, M.; Yang, Y.; Zhang, Y.; Liu, J.; Lin, Q. Metabolic Profiles of Flammulina velutipes Residues during Lactiplantibacillus plantarum Fermentation. Environ. Technol. Innov. 2025, 37, 103931. [Google Scholar] [CrossRef]
- Song, N.R.; Shin, S.Y.; Kim, K.M.; Choi, S.R.; Park, D.S.; Kim, S.O.; Jung, D.H.; Park, K.M. Anti-Photoaging Activities of Limosilactobacillus reuteri Culture Broth. Biocell 2025, 49, 1291–1310. [Google Scholar] [CrossRef]
- Mu, W.; Yu, S.; Zhu, L.; Zhang, T.; Jiang, B. Recent Research on 3-Phenyllactic Acid, a Broad-Spectrum Antimicrobial Compound. Appl. Microbiol. Biotechnol. 2012, 95, 1155–1163. [Google Scholar] [CrossRef]
- Ruey, J.Y.; Van Scott, E.J. Organic Acids with Novel Functions: α-Hydroxy, Aldobionic, N-Acetylamino Acids, and Related Compounds. In Textbook of Cosmetic Dermatology, 5th ed.; Informa Healthcare: London, UK, 2017; pp. 91–108. [Google Scholar]
- Rajanikar, R.V.; Nataraj, B.H.; Naithani, H.; Ali, S.A.; Panjagari, N.R.; Behare, P.V. Phenyllactic Acid: A Green Compound for Food Biopreservation. Food Control 2021, 128, 108184. [Google Scholar] [CrossRef]
- Ha, J.H.; Park, S.N. Mechanism underlying inhibitory effect of six dicaffeoylquinic acid isomers on melanogenesis and the computational molecular modeling studies. Bioorg. Med. Chem. 2018, 26, 4201–4208. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Bugnon, M.; Röhrig, U.F.; Goullieux, M.; Perez, M.A.S.; Daina, A.; Michielin, O.; Zoete, V. SwissDock 2024: Major Enhancements for Small-Molecule Docking with Attracting Cavities and AutoDock Vina. Nucleic Acids Res. 2024, 52, W324–W332. [Google Scholar] [CrossRef]
- Supino, R. MTT assays. Methods Mol. Biol. 1995, 43, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Chen, Q.-H.; Zhuang, J.-X.; Zhong, X.; Zhou, J.-J.; Guo, Y.-J.; Chen, Q.-X. Inhibitory Effects of α-Cyano-4-Hydroxycinnamic Acid on the Activity of Mushroom Tyrosinase. Food Chem. 2009, 112, 609–613. [Google Scholar] [CrossRef]
- Li, Z.C.; Chen, L.H.; Yu, X.J.; Hu, Y.H.; Song, K.K.; Zhou, X.W.; Chen, Q.X. Inhibition Kinetics of Chlorobenzaldehyde Thiosemicarbazones on Mushroom Tyrosinase. J. Agric. Food Chem. 2010, 58, 12537–12540. [Google Scholar] [CrossRef] [PubMed]
- Sakeh, N.M.; Md Razip, N.N.; Mohd Ma’in, F.I.; Abdul Bahari, M.N.; Latif, N.; Akhtar, M.N.; Balia Yusof, Z.N.; Ahmad, S. Melanogenic Inhibition and Toxicity Assessment of Flavokawain A and B on B16/F10 Melanoma Cells and Zebrafish (Danio rerio). Molecules 2020, 25, 3403. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Lee, E.-H.; Lee, S.-Y.; Lee, Y.; Shin, K.-O.; Park, K.; Kang, I.-J. Morus alba L. root decreases melanin synthesis via sphingosine-1-phosphate signaling in B16F10 cells. J. Ethnopharmacol. 2023, 301, 115848. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M. MITF: A stream flowing for pigment cells. Pigment Cell Res. 2000, 13, 230–240. [Google Scholar] [CrossRef]
- Lee, C.S.; Park, M.; Han, J.; Lee, J.-H.; Bae, I.-H.; Choi, H.; Son, E.D.; Park, Y.-H.; Lim, K.-M. Liver X receptor activation inhibits melanogenesis through the acceleration of ERK-mediated MITF degradation. J. Invest. Dermatol. 2013, 133, 1063–1071. [Google Scholar] [CrossRef]
- Wellbrock, C.; Arozarena, I. Microphthalmia-associated transcription factor in melanoma development and MAP-kinase pathway targeted therapy. Pigment Cell Melanoma Res. 2015, 28, 390–406. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).