Total Flavonoid Extraction from Baihao Yinzhen Utilizing Ultrasound-Assisted Deep Eutectic Solvent: Optimization of Conditions, Anti-Inflammatory, and Molecular Docking Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals and Reagents
2.3. Preparation of DESs
2.4. Extraction Procedure
2.5. Determination of Total Flavonoids
2.6. Optimization of SNF Extraction
2.7. Determination of Antioxidant Activity
2.7.1. DPPH Radical Scavenging Activity
2.7.2. ABTS Radical-Scavenging Activity
2.8. Cell Culture
2.9. Cell Viability Assay
2.10. Liquid Chromatography–Mass Spectrometry Analysis
2.11. Enzyme-Linked Immunosorbent Assay (ELISA)
2.12. Molecular Docking
2.13. Statistical Analysis
3. Results
3.1. Screening of DES
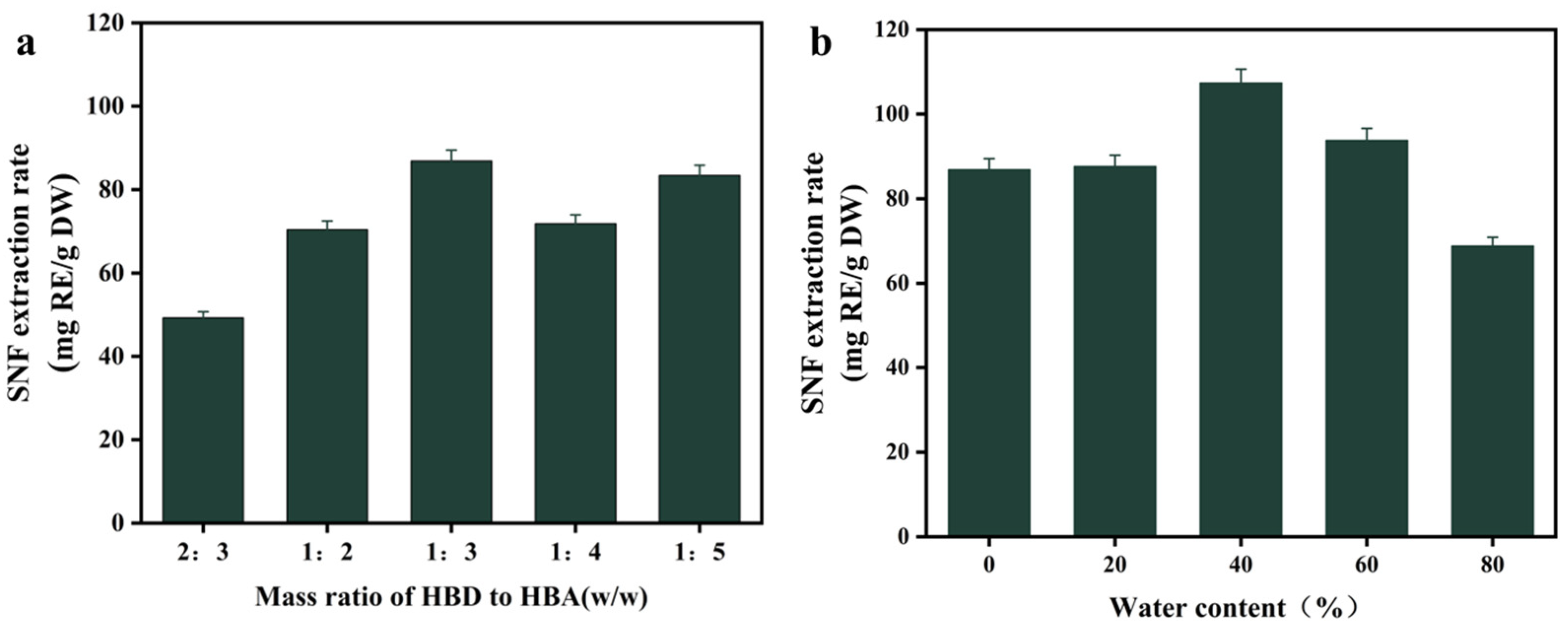
3.2. Effect of Molar Ratio and Water Content in DES on Extraction Efficiency
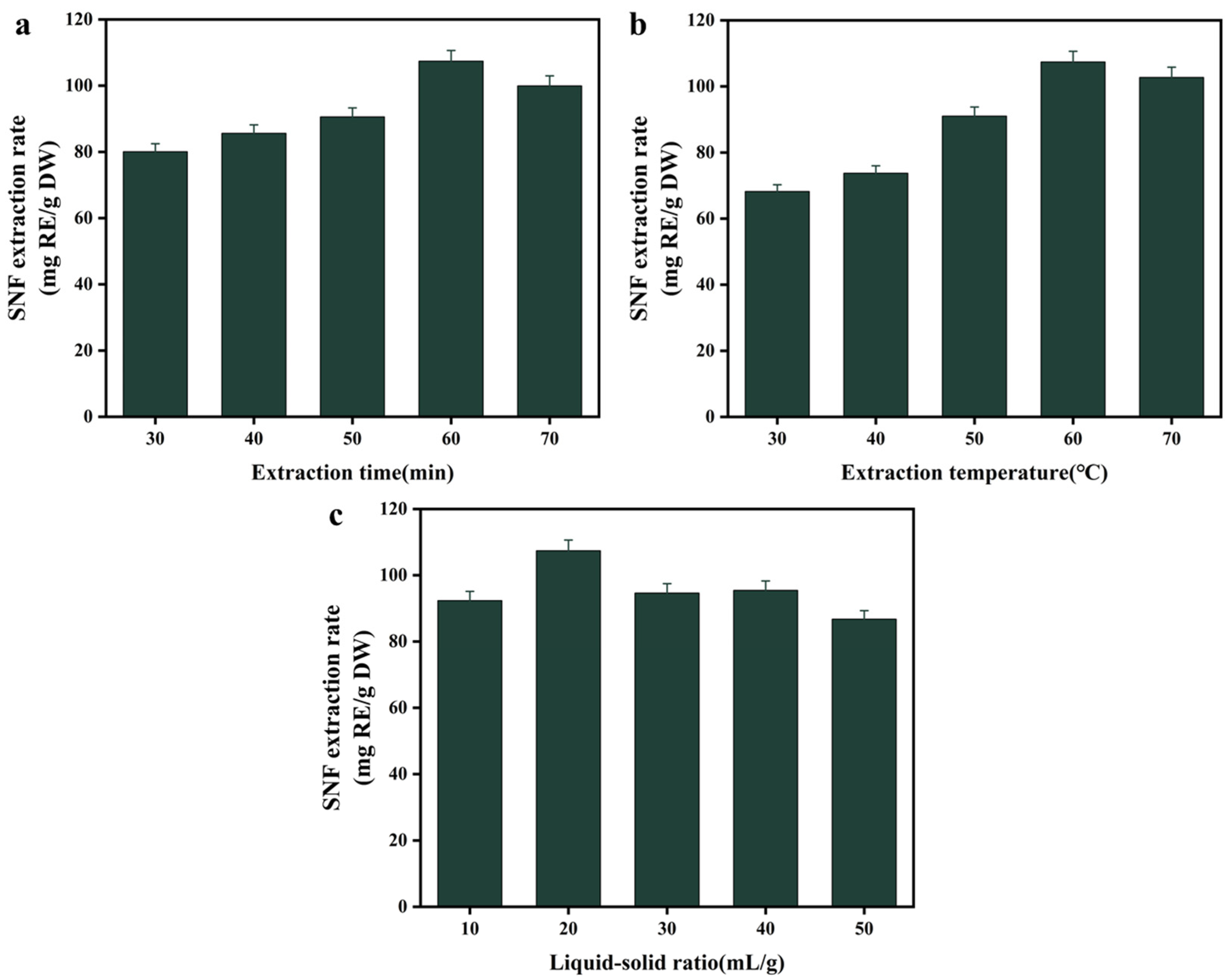
3.3. Single-Factor Experiments
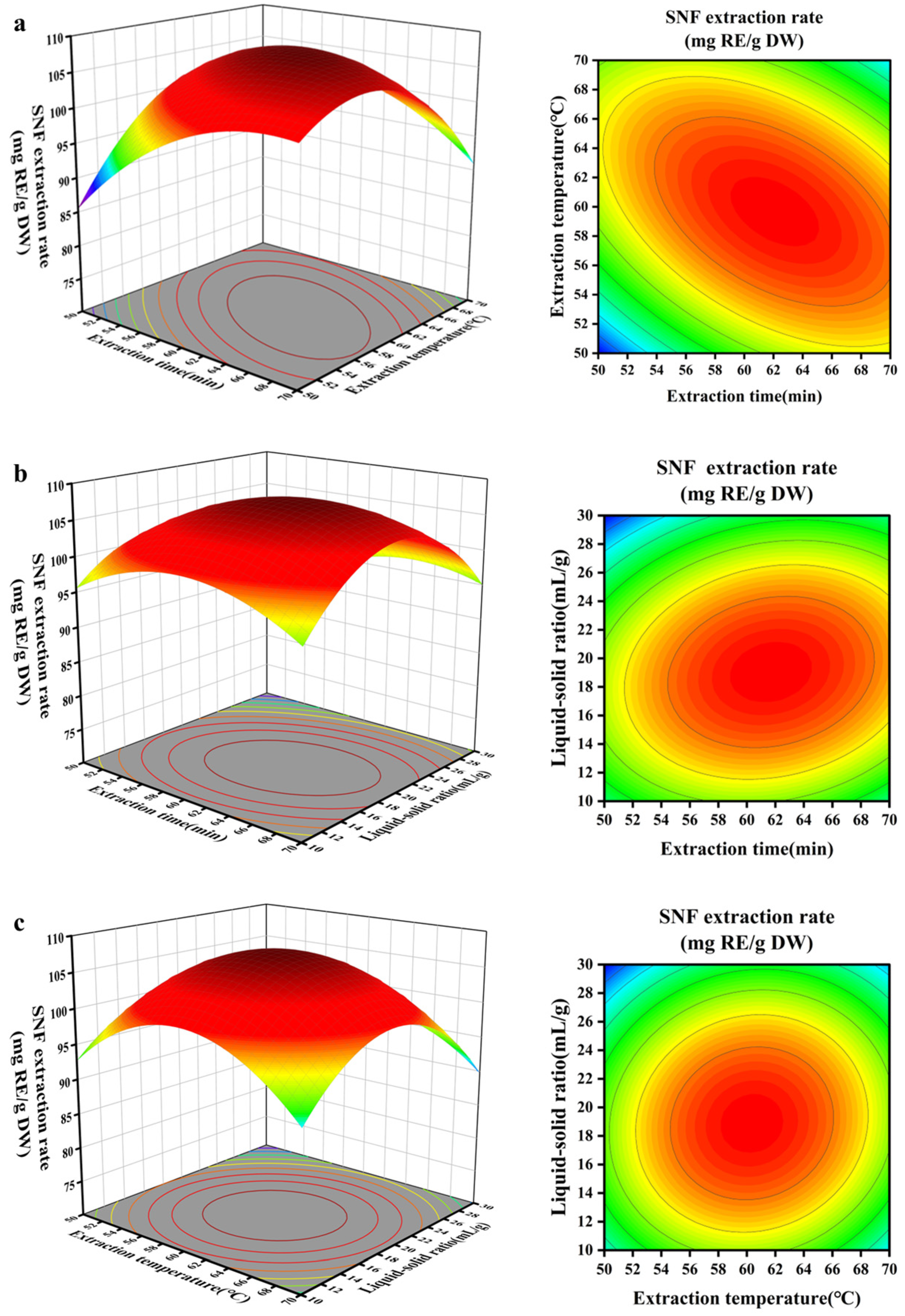
3.4. Optimization of Extraction Conditions by Box–Behnken Design
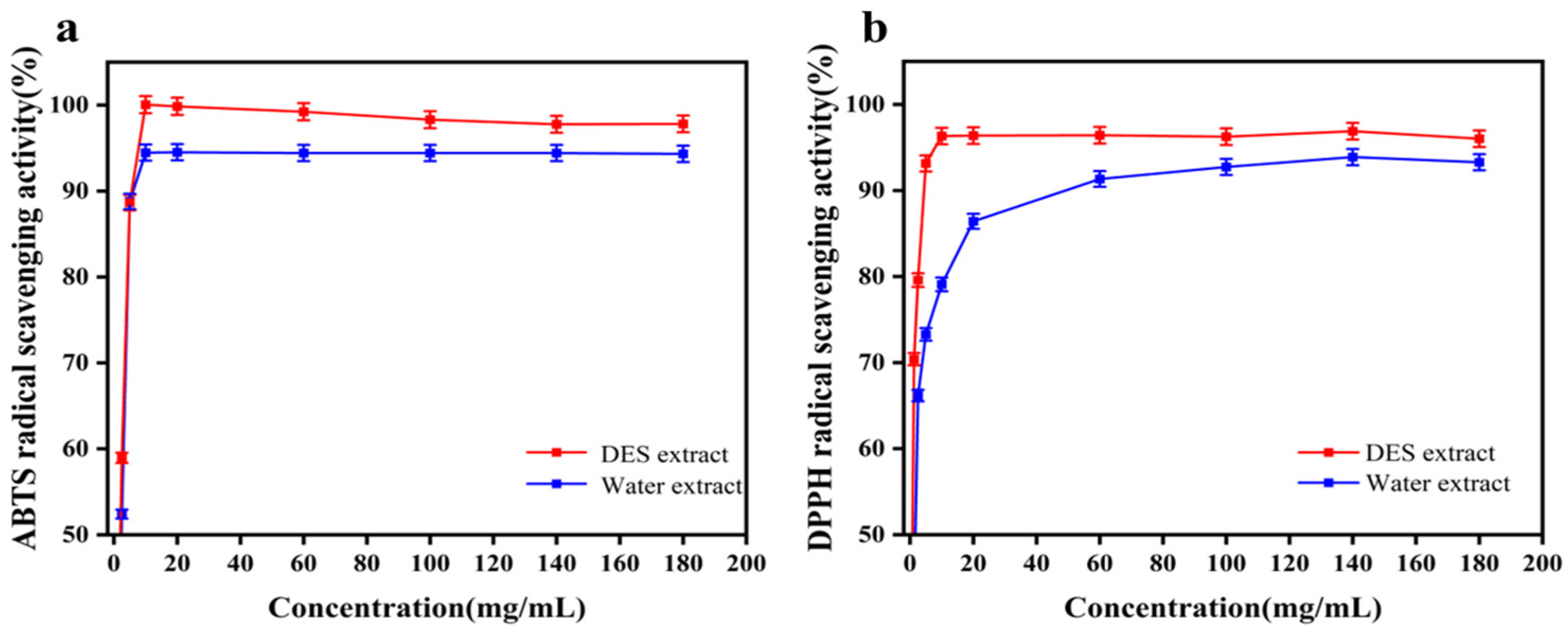
3.5. Antioxidant Activity
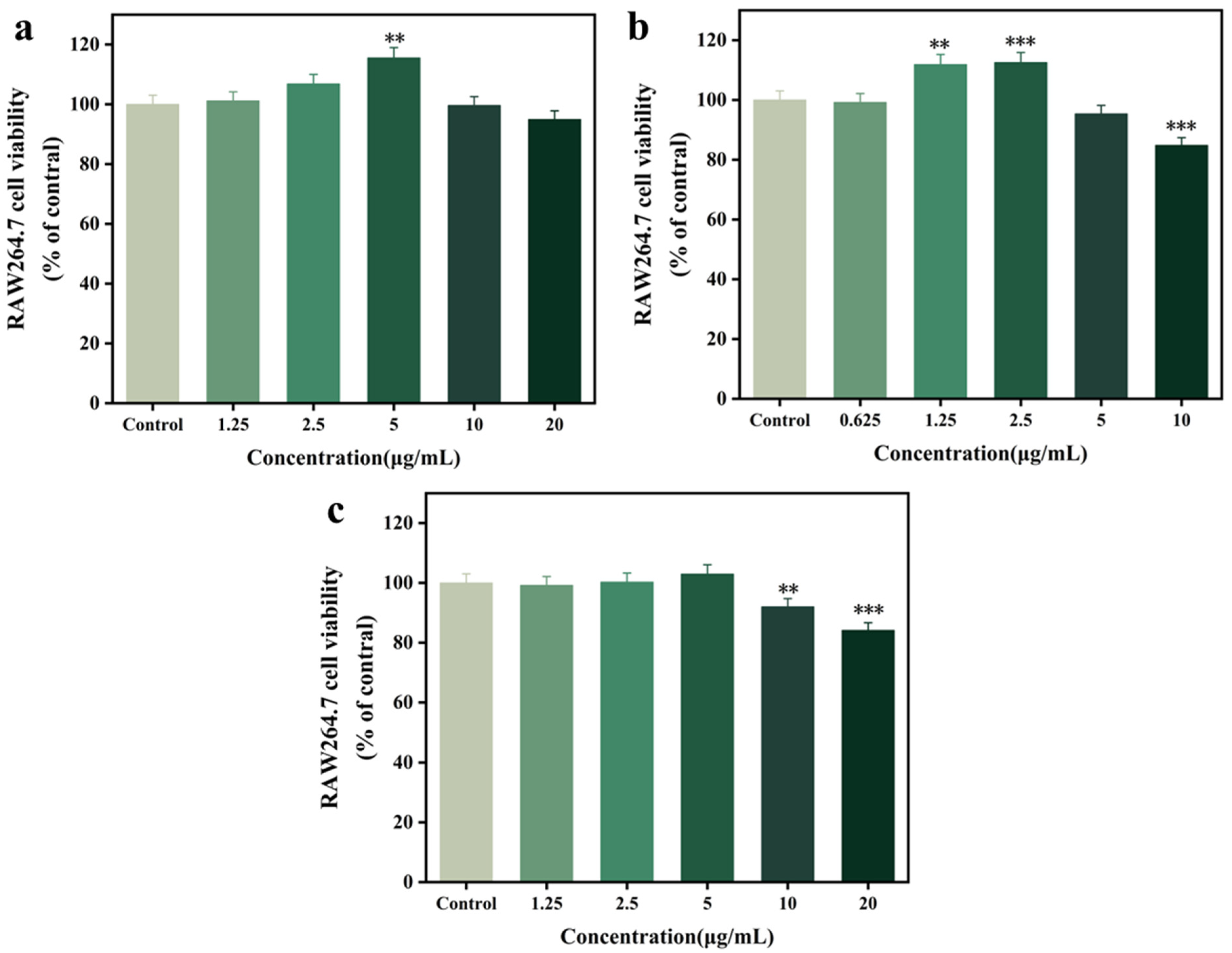
3.6. Cytotoxicity Assay of RAW 264.7 Cells
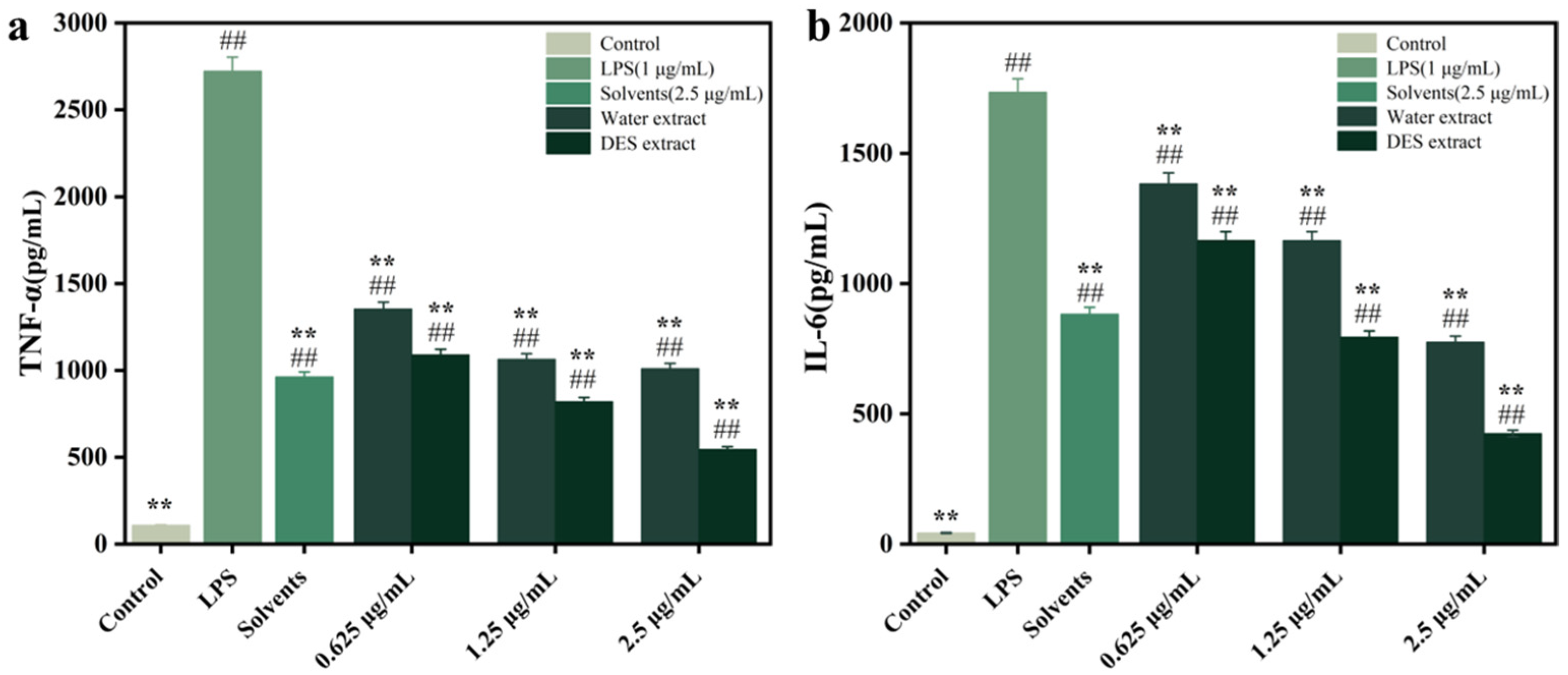
3.7. Inhibiting RAW264.7 Cell Inflammatory Factor Release
3.8. Components Analysis of SNF by UHPLC-Q-TOF-MS Anlysis
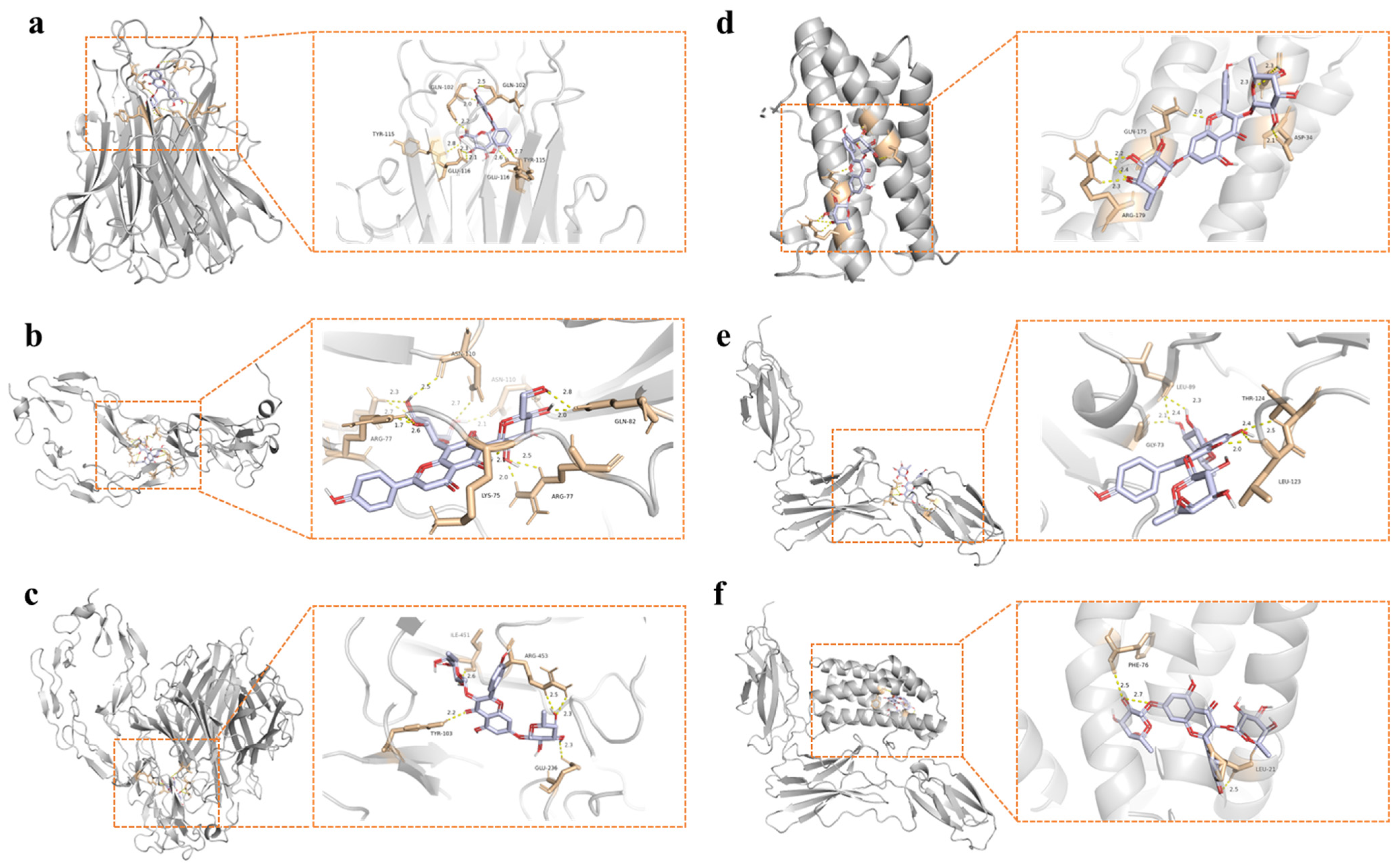
3.9. Molecular Docking Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Somavanshi, D.B.; Kamble, P.R.; Patil, C.D.; Jadhav, S.P.; Sonawane, G.B. A Review on the Antioxidant and Antiaging Properties of White Tea. JPRI 2021, 33, 129–136. [Google Scholar] [CrossRef]
- Gabr, G.A.; Hassan, H.M.M.; Seshadri, V.D.; Hassan, N.M.M. Comparative Study of Phenolic Profile, Antioxidant and Antimicrobial Activities of Aqueous Extract of White and Green Tea. Z. Naturforschung C 2022, 77, 483–492. [Google Scholar] [CrossRef]
- Dai, W.; Xie, D.; Lu, M.; Li, P.; Lv, H.; Yang, C.; Peng, Q.; Zhu, Y.; Guo, L.; Zhang, Y.; et al. Characterization of White Tea Metabolome: Comparison against Green and Black Tea by a Nontargeted Metabolomics Approach. Food Res. Int. 2017, 96, 40–45. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, X.; Liu, H.; Qian, J. In Vitro and in Vivo Anti-Inflammatory Models Demonstrate Oligopeptides Play a Significant Role in Anti-Inflammatory Properties of White Tea. J. Funct. Foods 2024, 112, 105983. [Google Scholar] [CrossRef]
- Gao, J.; Chen, D.; Xie, D.; Peng, J.; Hu, Z.; Lin, Z.; Dai, W. Investigations of the Highly Efficient Processing Technique, Chemical Constituents, and Anti-Inflammatory Effect of N-Ethyl-2-Pyrrolidinone-Substituted Flavan-3-Ol (EPSF)-Enriched White Tea. Food Chem. 2024, 450, 139328. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liang, Q.; Zhang, B.; Zhang, J.; Fan, L.; Kang, J.; Lin, Y.; Huang, Y.; Tan, T.-C.; Ho, L.-H. Adsorption and Desorption Characteristics of Flavonoids from White Tea Using Macroporous Adsorption Resin. J. Chromatogr. A 2024, 1715, 464621. [Google Scholar] [CrossRef]
- Wang, R.; Yang, Z.; Zhang, J.; Mu, J.; Zhou, X.; Zhao, X. Liver Injury Induced by Carbon Tetrachloride in Mice Is Prevented by the Antioxidant Capacity of Anji White Tea Polyphenols. Antioxidants 2019, 8, 64. [Google Scholar] [CrossRef] [PubMed]
- Selim, D.A.; Shawky, E.; Abu El-Khair, R.M. Identification of the Discriminatory Chemical Markers of Different Grades of Sri Lankan White, Green and Black Tea (Camellia sinenesis L.) via Metabolomics Combined to Chemometrics. J. Food Compos. Anal. 2022, 109, 104473. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, F.; Wen, M.; Jiang, S.; Long, P.; Ke, J.-P.; Han, Z.; Zhu, M.; Zhou, Y.; Zhang, L. LC-MS and GC–MS Based Metabolomics Analysis Revealed the Impact of Tea Trichomes on the Chemical and Flavor Characteristics of White Tea. Food Res. Int. 2024, 191, 114740. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, F.; Zhou, H.; Wang, Y.; Bian, J.; Sun, Y.; Du, X. Utilizing Nontargeted Metabolomics Integrated with Quality Component Quantitation via Differential Analysis to Reveal the Taste Differences between and Metabolite Characteristics of Hawk Black Tea and Hawk White Tea. Food Res. Int. 2024, 197, 115216. [Google Scholar] [CrossRef]
- Elkhedir, A.E.; Iqbal, A.; Zogona, D.; Mohammed, H.H.; Murtaza, A.; Xu, X. Apigenin Glycosides from Green Pepper Enhance Longevity and Stress Resistance in Caenorhabditis Elegans. Nutr. Res. 2022, 102, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Bojilov, D.; Manolov, S.; Nacheva, A.; Dagnon, S.; Ivanov, I. Characterization of Polyphenols from Chenopodium Botrys after Fractionation with Different Solvents and Study of Their In Vitro Biological Activity. Molecules 2023, 28, 4816. [Google Scholar] [CrossRef]
- Li, J.; Yang, J.; Xian, Q.; Su, H.; Ni, Y.; Wang, L. Kaempferitrin Attenuates Unilateral Ureteral Obstruction-induced Renal Inflammation and Fibrosis in Mice by Inhibiting NOX4 -mediated Tubular Ferroptosis. Phytother. Res. 2024, 38, 2656–2668. [Google Scholar] [CrossRef]
- Li, J.; Si, C.; Hong, W.; Xia, C.; Yang, Y.; He, Y.; Su, M.; Long, X.; Zhang, H. Identification of the Chemical Components of Ethanol Extract of Chenopodium Ambrosioides and Evaluation of Their in Vitro Antioxidant and Anti Tumor Activities. Trop. J. Pharm. Res. 2022, 21, 1689–1697. [Google Scholar] [CrossRef]
- De Rijke, E.; Out, P.; Niessen, W.M.A.; Ariese, F.; Gooijer, C.; Brinkman, U.A.T. Analytical Separation and Detection Methods for Flavonoids. J. Chromatogr. A 2006, 1112, 31–63. [Google Scholar] [CrossRef]
- Hao, Y.; Pei, F.; Huang, J.; Li, G.; Zhong, C. Application of Deep Eutectic Solvents on Extraction of Flavonoids. J. Sep. Sci. 2024, 47, 2300925. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel Solvent Properties of Choline Chloride/Urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Radojčić Redovniković, I.; Vidović, S.; Radošević, K.; Andreou, T.; Mourtzinos, I.; Cvjetko Bubalo, M. Coupling Deep Eutectic Solvents with Innovative Extraction Techniques towards Plant Derived Bioactive Compositions. RSC Sustain. 2024, 2, 1675–1691. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Wang, X.; Liu, W. Application of Deep Eutectic Solvents in Food Analysis: A Review. Molecules 2019, 24, 4594. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Xiong, Y.; Wei, X.; Zhou, J.; Feng, C. Extraction Efficiency of Loganic Acid and Gentiopicroside from Gentianae Radix et Rhizoma by Deep Eutectic Solvent-Based Ultrasound-Assisted Extraction. Results Chem. 2024, 10, 101740. [Google Scholar] [CrossRef]
- Jiao, P.; He, X.; Ma, S.; Wang, S.; Niu, Q. Ultrasonic-Assisted Extraction of Antioxidants from Perilla Frutescens Leaves Based on Tailor-Made Deep Eutectic Solvents: Optimization and Antioxidant Activity. Molecules 2023, 28, 7554. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Z.; Gul, Z.; Tian, M.; Wang, J.; Zheng, K.; Zhao, C.; Li, C. Advances of Responsive Deep Eutectic Solvents and Application in Extraction and Separation of Bioactive Compounds. J. Sep. Sci. 2023, 46, 2300098. [Google Scholar] [CrossRef]
- Morozova, O.V.; Vasil’eva, I.S.; Shumakovich, G.P.; Zaitseva, E.A.; Yaropolov, A.I. Deep Eutectic Solvents for Biotechnology Applications. Biochem. Mosc. 2023, 88, S150–S175. [Google Scholar] [CrossRef]
- Peng, W.; Wang, X.; Wang, W.; Wang, Y.; Huang, J.; Zhou, R.; Bo, R.; Liu, M.; Yin, S.; Li, J. Comparison, Optimization and Antioxidant Activity of Ultrasound-Assisted Natural Deep Eutectic Solvents Extraction and Traditional Method: A Greener Route for Extraction of Flavonoid from Moringa Oleifera Lam. Leaves. Ultrason. Sonochem. 2024, 109, 107003. [Google Scholar] [CrossRef]
- Huang, D.; Chen, L.; Chen, X.; Huang, X.; Yang, Y.; Liu, J.; Lin, Y.; Liu, Y.; Li, H. Supramolecular Deep Eutectic Solvents as Green Media for Efficient Extraction of Tea Polyphenols and Its Application in Bio-Active Film. Food Chem. 2025, 465, 141904. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, X.; Liu, X.; Wang, N.; An, Q.; Ye, X.M.; Zhao, Z.T.; Zhao, M.; Han, Y.; Ouyang, K.H.; et al. Investigation of Chemical Composition, Antioxidant Activity, and the Effects of Alfalfa Flavonoids on Growth Performance. Oxidative Med. Cell. Longev. 2020, 2020, 8569237. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, D.; Ma, X.; Jiang, F.; He, Q.; Qiu, S.; Li, Y.; Wang, G. Ionic Liquid–Ultrasound-Based Extraction of Biflavonoids from Selaginella Helvetica and Investigation of Their Antioxidant Activity. Molecules 2018, 23, 3284. [Google Scholar] [CrossRef]
- Dong, J.; Dong, Z.; Zhao, L.; Yang, D.; Bo, Y.; Zhang, X.; Wu, G.; An, M. The Evaluation of Five Bioactive Compounds Content and in Vitro Antioxidant of Caryophylli Flos Extracts Obtained by Natural Deep Eutectic Solvents. Sustain. Chem. Pharm. 2022, 30, 100838. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Y.; Lu, B.; Alrosan, M.; Easa, A.M.; Alu’datt, M.H.; Tan, T.C. Flavonoids Extracts from Zhenghe White Tea. Food Res. 2024, 8, 140–147. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Chen, Y.-W.; Hou, C.-Y. Antioxidant and Antibacterial Activity of Seven Predominant Terpenoids. Int. J. Food Prop. 2019, 22, 230–238. [Google Scholar] [CrossRef]
- Li, M.; Rao, C.; Wang, C.; Cui, X.; Xiong, Y. Natural Deep Eutectic Solvents-Based Selective Extraction of Saponins from Panax Notoginseng: Process Optimization, Chemical Profiling, and Bioactivities Evaluation. Sep. Purif. Technol. 2024, 341, 126882. [Google Scholar] [CrossRef]
- Xing, C.; Cui, W.-Q.; Zhang, Y.; Zou, X.-S.; Hao, J.-Y.; Zheng, S.-D.; Wang, T.-T.; Wang, X.-Z.; Wu, T.; Liu, Y.-Y.; et al. Ultrasound-Assisted Deep Eutectic Solvents Extraction of Glabridin and Isoliquiritigenin from Glycyrrhiza Glabra: Optimization, Extraction Mechanism and in Vitro Bioactivities. Ultrason. Sonochem. 2022, 83, 105946. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Qiao, L.; Gao, Q.; Zhang, F.; Zhang, X.; Lei, J.; Ren, M.; Xiao, S.; Kuang, J.; Deng, S.; et al. Total Biflavonoids Extraction from Selaginella Chaetoloma Utilizing Ultrasound-Assisted Deep Eutectic Solvent: Optimization of Conditions, Extraction Mechanism, and Biological Activity in Vitro. Ultrason. Sonochem. 2023, 98, 106491. [Google Scholar] [CrossRef]
- Jiang, Z.-M.; Wang, F.-F.; Liu, J.; Huang, T.-Q.; Zhang, H.; Liu, E.-H. Deep Eutectic Solvents in the Efficient Detoxification and Extraction of Alkaloids of Aconiti Lateralis Radix Praeparata. ACS Sustain. Chem. Eng. 2022, 10, 5674–5682. [Google Scholar] [CrossRef]
- Liu, Y.; Zhe, W.; Zhang, R.; Peng, Z.; Wang, Y.; Gao, H.; Guo, Z.; Xiao, J. Ultrasonic-Assisted Extraction of Polyphenolic Compounds from Paederia scandens (Lour.) Merr. Using Deep Eutectic Solvent: Optimization, Identification, and Comparison with Traditional Methods. Ultrason. Sonochem. 2022, 86, 106005. [Google Scholar] [CrossRef]
- Ma, P.; Li, Z.; Jin, Y.; Zuo, J.; Zhang, Y.; Dong, A.; Xiao, D.; Burenjargal, M. Green and Efficient Extraction Process of Flavonoids from Sea Buckthorn Fruits by Natural Deep Eutectic Solvents Aided with Ultrasound. Microchem. J. 2024, 205, 111265. [Google Scholar] [CrossRef]
- Gao, J.; Xie, L.; Peng, Y.; Li, M.; Li, J.; Ni, Y.; Wen, X. Deep Eutectic Solvents as New Extraction Media for Flavonoids in Mung Bean. Foods 2024, 13, 777. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; He, J.; Yang, Y.; Gou, Y.; Wang, Z.; Chen, H.; Zhao, X. White Tip Silver Needle (Slightly Fermented White Tea) Flavonoids Help Prevent Aging via Antioxidative and Anti-Inflammatory Effects. Drug Des. Dev. Ther. 2021, 15, 1441–1457. [Google Scholar] [CrossRef]
- Wang, Y.; Ye, R.; Fan, L.; Zhao, X.; Li, L.; Zheng, H.; Qiu, Y.; He, X.; Lu, Y. A TNF-α Blocking Peptide That Reduces NF-κB and MAPK Activity for Attenuating Inflammation. Bioorg. Med. Chem. 2023, 92, 117420. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.T.P.; Le, M.D.; Trinh, H.X.; Nong, H.V. In Silico Studies for the Interaction of Tumor Necrosis Factor-Alpha (TNF-α) with Different Saponins from Vietnamese Ginseng (Panax vietnamesis). Biophysics 2016, 13, 173–180. [Google Scholar] [CrossRef]
- Damergi, B.; Essid, R.; Fares, N.; Khadraoui, N.; Ageitos, L.; Ben Alaya, A.; Gharbi, D.; Abid, I.; Rashed Alothman, M.; Limam, F.; et al. Datura stramonium Flowers as a Potential Natural Resource of Bioactive Molecules: Identification of Anti-Inflammatory Agents and Molecular Docking Analysis. Molecules 2023, 28, 5195. [Google Scholar] [CrossRef]
- Kang, S.; Kishimoto, T. Interplay between Interleukin-6 Signaling and the Vascular Endothelium in Cytokine Storms. Exp. Mol. Med. 2021, 53, 1116–1123. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, X.; Fan, Q.; Li, C.; Zhang, M.; Wang, Y.; Wu, Q.; Li, J.; Liu, X.; Wang, S.; et al. Deciphering the Active Compounds and Mechanisms of HSBDF for Treating ALI via Integrating Chemical Bioinformatics Analysis. Front. Pharmacol. 2022, 13, 879268. [Google Scholar] [CrossRef]
- Bakrim, S.; Fessikh, M.E.; Elhrech, H.; Omari, N.E.; Amanullah, M.; Ming, L.C.; Moshawih, S.; Bouyahya, A. Targeting Inflammation in Cancer Therapy: From Mechanistic Insights to Emerging Therapeutic Approaches. J. Transl. Med. 2025, 23, 588. [Google Scholar] [CrossRef]
- Wang, J.; Yang, P.; Yu, T.; Gao, M.; Liu, D.; Zhang, J.; Lu, C.; Chen, X.; Zhang, X.; Liu, Y. Lactylation of PKM2 Suppresses Inflammatory Metabolic Adaptation in Pro-Inflammatory Macrophages. Int. J. Biol. Sci 2022, 18, 6210–6225. [Google Scholar] [CrossRef]
- Theodorou, I.M.; Kapoukranidou, D.; Theodorou, M.; Tsetis, J.K.; Menni, A.E.; Tzikos, G.; Bareka, S.; Shrewsbury, A.; Stavrou, G.; Kotzampassi, K. Cosmeceuticals: A Review of Clinical Studies Claiming to Contain Specific, Well-Characterized Strains of Probiotics or Postbiotics. Nutrients 2024, 16, 2526. [Google Scholar] [CrossRef]
- Sharma, R.; Hastings, C.; Staretz-Chacham, O.; Raiman, J.; Paucar, M.; Spiegel, R.; Murray, B.; Hurst, B.; Liu, B.; Kjems, L.; et al. Long-Term Administration of Intravenous Trappsol® CycloTM (HP-β-CD) Results in Clinical Benefits and Stabilization or Slowing of Disease Progression in Patients with Niemann-Pick Disease Type C1: Results of an International 48-Week Phase I/II Trial. Mol. Genet. Metab. Rep. 2023, 36, 100988. [Google Scholar]
- Sahu, S.; Kumari, D.; Kusam; Kuila, A.; Gurjar, R.S.; Sharma, K.; Verma, R. Deep Eutectic Solvent Extraction of Polyphenol from Plant Materials: Current Status and Future Prospects in Food Applications. Food Chem. 2025, 482, 144125. [Google Scholar] [CrossRef]
- De Souza Mesquita, L.M.; Contieri, L.S.; e Silva, F.A.; Bagini, R.H.; Bragagnolo, F.S.; Strieder, M.M.; Sosa, F.H.B.; Schaeffer, N.; Freire, M.G.; Ventura, S.P.M.; et al. Path2Green: Introducing 12 Green Extraction Principles and a Novel Metric for Assessing Sustainability in Biomass Valorization. Green Chem. 2024, 26, 10087–10106. [Google Scholar] [CrossRef]
- Tagliavento, L.; Nardin, T.; Chini, J.; Vighi, N.; Lovatti, L.; Testai, L.; Meneguzzo, F.; Larcher, R.; Zabini, F. Sustainable Exploitation of Apple By-Products: A Retrospective Analysis of Pilot-Scale Extraction Tests Using Hydrodynamic Cavitation. Foods 2025, 14, 1915. [Google Scholar] [CrossRef]

| Solvent Abbreviation | HBA | HBD | Molar/Mass Ratio |
|---|---|---|---|
| Bet/Lac | Betaine | Lactic acid | 1:2 |
| Bet/MA | Malic acid | 1:2 | |
| Bet/Cit | Citric acid | 1:1 | |
| Bet/Gl | Glycerol | 1:2 | |
| Pro/Gl | Proline | Glycerol | 1:2 |
| Pro/EG | Glycol | 1:2 | |
| Pro/Lac | Lactic acid | 1:2 | |
| Pro/Hac | Levulinic acid | 1:2 | |
| Glu/Lac | Glucose | Lactic acid | 1:6 |
| Glu/Cit | Citric acid | 1:2 | |
| Glu/Gl | Glycerol | 1:2 | |
| Gly/Lac | Glycine | Lactic acid | 1:2 |
| β-CD/Pac | β-cyclodextrin | Pyruvate acid | 1:5 (w/w) |
| HP-β-CD/Lac | Hydroxypropyl-β-cyclodextrin | Lactic acid | 1:5 (w/w) |
| Run | X1: Tim (min) | X2: Temperature (°C) | X3: Liquid–Solid Ratio (mL/g) | Y: Apparent SNF (mg RE/g DW) |
|---|---|---|---|---|
| 1 | 50.00 (−1) | 50.00 (−1) | 20.00 (0) | 85.81 ± 0.37 |
| 2 | 70.00 (+1) | 50.00 (−1) | 20.00 (0) | 101.71 ± 0.50 |
| 3 | 50.00 (−1) | 70.00 (+1) | 20.00 (0) | 99.36 ± 0.23 |
| 4 | 70.00 (+1) | 70.00 (+1) | 20.00 (0) | 90.59 ± 0.64 |
| 5 | 50.00 (−1) | 60.00 (0) | 10.00 (−1) | 94.64 ± 0.38 |
| 6 | 70.00 (+1) | 60.00 (0) | 10.00 (−1) | 94.57 ± 0.25 |
| 7 | 50.00 (−1) | 60.00 (0) | 30.00 (+1) | 87.37 ± 0.25 |
| 8 | 70.00 (+1) | 60.00 (0) | 30.00 (+1) | 95.93 ± 0.44 |
| 9 | 60.00 (0) | 50.00 (−1) | 10.00 (−1) | 93.25 ± 0.77 |
| 10 | 60.00 (0) | 70.00 (+1) | 10.00 (−1) | 92.38 ± 0.70 |
| 11 | 60.00 (0) | 50.00 (−1) | 30.00 (+1) | 84.74 ± 0.21 |
| 12 | 60.00 (0) | 70.00 (+1) | 30.00 (+1) | 89.13 ± 0.19 |
| 13 | 60.00 (0) | 60.00 (0) | 20.00 (0) | 109.46 ± 0.50 |
| 14 | 60.00 (0) | 60.00 (0) | 20.00 (0) | 108.00 ± 0.56 |
| 15 | 60.00 (0) | 60.00 (0) | 20.00 (0) | 108.59 ± 0.66 |
| 16 | 60.00 (0) | 60.00 (0) | 20.00 (0) | 106.10 ± 0.61 |
| 17 | 60.00 (0) | 60.00 (0) | 20.00 (0) | 107.41 ± 0.19 |
| Solvent | Temperature (°C) | Time (min) | Liquid–Solid Ratio (g/mL) | SNF Extraction Rate (mg RE/g DW) | |
|---|---|---|---|---|---|
| DES-UAE | DES | 60 | 62 | 1:19 | 108.72 ± 2.17 |
| Water-UAE | Water | 60 | 62 | 1:19 | 63.677 ± 1.91 |
| Binding Energy (kcal/mol) | ||||||
|---|---|---|---|---|---|---|
| IL-6 | IL-6R | IL-6-IL-6R | TNF-α | TNFR1 | TNF-α-TNFR1 | |
| Quercetin | −7.1 | −7.2 | −8.1 | −9.2 | −8.1 | −8.8 |
| L-Threonine | −3.9 | −3.7 | −4.1 | −5.1 | −3.9 | −4.7 |
| L-Glutamic acid | −4.5 | −4.4 | −4.6 | −5.5 | −4.4 | −5.1 |
| L-Proline | −4.6 | −4.7 | −4.2 | −5.2 | −4.2 | −5.0 |
| L-Valine | −4.4 | −3.9 | −4.0 | −4.8 | −4.0 | −5.0 |
| Quinic acid | −5.7 | −5.1 | −5.5 | −7.5 | −6.3 | −7.1 |
| Theanine | −4.6 | −3.6 | −4.5 | −5.2 | −4.8 | −5.9 |
| L-Leucine | −4.5 | −3.9 | −4.3 | −5.3 | −4.4 | −5.3 |
| L-Tyrosine | −5.2 | −4.8 | −5.5 | −6.0 | −5.0 | −5.9 |
| Phenylalanine | −4.8 | −4.5 | −5.5 | −5.8 | −4.8 | −6.5 |
| Guanine | −5.2 | −5.2 | −5.2 | −6.8 | −5.5 | −6.6 |
| L-Tryptophan | −5.5 | −4.9 | −5.8 | −6.7 | −4.4 | −7.4 |
| Methyl gallate | −5.6 | −5.1 | −5.6 | −6.1 | −5.8 | −6.8 |
| Lysine | −4.1 | −3.4 | −4.1 | −4.8 | −3.9 | −4.7 |
| Kaempferol-3-glucoside | −7.6 | −7.4 | −8.6 | −9.1 | −7.7 | −11.0 |
| Protocatechuic acid | −5.8 | −5.1 | −6.2 | −6.8 | −5.5 | −6.6 |
| Benzaldehyde | −5.1 | −4.3 | −5.8 | −5.8 | −4.5 | −5.8 |
| Protocatechualdehyde | −5.5 | −5.1 | −5.7 | −6.1 | −5.0 | −5.9 |
| Caffeine | −5.2 | −5.0 | −5.7 | −6.2 | −5.8 | −6.5 |
| Quercetin 3-O-β-d-glucuronide | −7.2 | −7.1 | −7.9 | −11.1 | −7.5 | −9.5 |
| Ferulic acid | −5.8 | −4.9 | −5.1 | −6.2 | −5.8 | −6.5 |
| Apigenin-6,8-di-C-glucoside | −6.6 | −7.1 | −7.7 | −8.4 | −10.2 | −9.7 |
| Epicatechin | −6.6 | −6.9 | −8.3 | −9.0 | −8.0 | −8.7 |
| Quercetin | −7.1 | −7.2 | −8.1 | −9.2 | −8.1 | −8.8 |
| L-Threonine | −3.9 | −3.7 | −4.1 | −5.1 | −3.9 | −4.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Chu, Y.; Huang, W.; Chen, H.; Hong, S.; Kong, D.; Du, L. Total Flavonoid Extraction from Baihao Yinzhen Utilizing Ultrasound-Assisted Deep Eutectic Solvent: Optimization of Conditions, Anti-Inflammatory, and Molecular Docking Analysis. Cosmetics 2025, 12, 245. https://doi.org/10.3390/cosmetics12060245
Zhang Z, Chu Y, Huang W, Chen H, Hong S, Kong D, Du L. Total Flavonoid Extraction from Baihao Yinzhen Utilizing Ultrasound-Assisted Deep Eutectic Solvent: Optimization of Conditions, Anti-Inflammatory, and Molecular Docking Analysis. Cosmetics. 2025; 12(6):245. https://doi.org/10.3390/cosmetics12060245
Chicago/Turabian StyleZhang, Ziqi, Yan Chu, Wanting Huang, Huan Chen, Shengbao Hong, Dingfeng Kong, and Liyong Du. 2025. "Total Flavonoid Extraction from Baihao Yinzhen Utilizing Ultrasound-Assisted Deep Eutectic Solvent: Optimization of Conditions, Anti-Inflammatory, and Molecular Docking Analysis" Cosmetics 12, no. 6: 245. https://doi.org/10.3390/cosmetics12060245
APA StyleZhang, Z., Chu, Y., Huang, W., Chen, H., Hong, S., Kong, D., & Du, L. (2025). Total Flavonoid Extraction from Baihao Yinzhen Utilizing Ultrasound-Assisted Deep Eutectic Solvent: Optimization of Conditions, Anti-Inflammatory, and Molecular Docking Analysis. Cosmetics, 12(6), 245. https://doi.org/10.3390/cosmetics12060245





