Abstract
The cashew apple (Anacardium occidentale L.) is rich in antioxidant bioactive constituents that have anti-aging and wound healing properties. The objective of this study is to evaluate the biological activities of cashew apple extract (CAE) and to improve the issue involving the instability of ascorbic acid, the principal active compound, by encapsulating the extract in liposomes in order to enhance its stability and skin permeation for cosmetic applications. CAE was obtained from fresh cashew apple via ethanol maceration, solvent evaporation, and freeze-drying. Ascorbic acid content, total phenolic content (TPC), total flavonoid content (TFC), and total caffeoylquinic acid content (TCQAC) were determined. The ascorbic acid content and its tautomer in the extract were quantified using the LC-MS/MS method. Biological activities, including antioxidant, anti-tyrosinase, fibroblast collagen synthesis, cytoprotection against oxidative stress, wound healing, and cytotoxicity, were assessed. CAE was encapsulated in liposomes to enhance the stability of its inherent ascorbic acid and improve its skin in comparison to free-CAE. The CAE and liposomal-CAE were incorporated and formulated into a solution, and their physicochemical stability was assessed after storage. CAE appeared as a brown, viscous liquid with a characteristic sweet, fruity scent. Each gram of CAE contained 0.90 ± 0.05 mg of ascorbic acid, TPC, 81.40 ± 7.14 mg of gallic acid equivalents (GAE), TFC, 3.73 ± 0.30 mg of rutin equivalents (RE), and TCQAC, 4.48 ± 0.05 mg of chlorogenic acid equivalents (CGAE). CAE exhibited antioxidant properties (IC50 = 282.19 ± 11.16 and 963.66 ± 3.95 µg/mL for DPPH and ABTS assay, respectively) and weak anti-tyrosinase activity (IC50 = 4213.77 ± 138.97 µg/mL). It was non-cytotoxic to fibroblast and monocyte cells at a concentration of less than 1 mg/mL. In vitro wound healing assays demonstrated that CAE stimulated collagen production in a dose-dependent manner at CAE concentrations above 250 µg/mL. Additionally, CAE exhibited cytoprotective effects against H2O2-induced oxidative stress and did not induce inflammatory responses in immune cells. The liposomal formulation containing CAE achieved high encapsulation efficiency (79.75–84.55%) based on ascorbic acid content. In skin permeation studies, CAE-loaded liposomes demonstrated an enhancement ratio approximately two-fold greater than that of free-CAE. Stability testing over 3 months showed that the ascorbic acid content in CAE-loaded liposomes remained significantly higher than that in the free-CAE under both refrigerated and long-term conditions (30 °C/75% RH). CAE demonstrated potential anti-aging properties for improving aging skin. Liposomal incorporation markedly improved ascorbic acid stability and skin permeability.
Keywords:
anti-aging; antioxidant; cashew apple extract; formulation; stability; penetration; wound healing 1. Introduction
The cashew apple (Anacardium occidentale L.), as shown in Figure 1, is a tropical fruit classified in the family Anacardiaceae that is native to Brazil and widely cultivated in Africa, America, and Southeast Asia [1]. The cashew tree is primarily valued for its highly nutritious nuts, which have a good taste, are effective against free radicals, and are in high demand for consumption [2,3,4]. In contrast, the cashew apple, a juicy, astringent pseudo-fruit, is less popular for direct consumption due to its distinct taste and high perishability [5]. Nevertheless, cashew apples are utilized in the food and fermentation industries, including the production of alcoholic beverages [6,7].

Figure 1.
Cashew apple (Anacardium occidentale L.) photographed by the author in Hat Yai District, Songkhla Province, southern Thailand. The cashew apple is a pseudo-fruit (accessory fruit), whereas the cashew nut is the true fruit of the cashew tree.
Cashew apples are a largely underutilized agricultural byproduct. The substantial waste produced by this pseudo-fruit has now led to an investigation into the fruit’s bioactive compounds [5,8]. Several studies have revealed that cashew apple contains high levels of bioactive compounds such as ascorbic acid, carotenoids, vitamins B1, B2, and B3, flavonoids, proanthocyanidins, tannins, and phenolic compounds, which are associated with strong antioxidant and antimicrobial properties [5,6,8,9,10,11,12]. Moreover, studies have demonstrated that cashew apple juice has been shown to possess anti-inflammatory effects and promote wound healing properties [13], which support its potential in pharmaceutical and cosmetic applications.
Antioxidants play a vital role in maintaining skin health by neutralizing free radicals that contribute to oxidative stress, premature aging, and cellular damage. Natural plants with antioxidant activity usually contain the most abundant amounts of polyphenol, phenolic compounds, and flavonoids [14]. Cashew apple exhibits significant antioxidant activity, mainly attributed to its high phenolic content and abundance of ascorbic acid [9,15]. Additionally, the tannins present in cashew apple are associated with antimicrobial efficacy, particularly against Staphylococcus aureus and Staphylococcus epidermidis, two common pathogens associated with skin infections [9,16]. Although there are well-known natural antioxidants derived from plant extracts and vitamin C-rich sources from plants such as acerola cherries, rose hips, citrus fruits, Kakadu plum, and sea buckthorn [17,18], these plants are often quite expensive. Using natural products that are undesirable and inexpensive but potentially useful as ingredients for cosmetic applications is therefore intriguing.
Given these beneficial properties, cashew apple represents a promising active ingredient for protecting the skin against oxidative stress and providing anti-aging benefits in cosmetic formulations [19,20]. Therefore, the bioactive extract from cashew apple has the potential to be developed into novel cosmetic ingredients. Among the major bioactive constituents of cashew apple, ascorbic acid is present in remarkably high concentration, up to 241.13 mg/100 g of fresh weight, which is reported to be nearly five times higher than that of citrus fruits [21,22,23]. However, ascorbic acid is highly susceptible to degradation during processing and storage [24,25,26,27]. Therefore, in this study, ascorbic acid was used as a marker in the formulation development and stability testing.
Liposomes have been explored as an effective strategy in the protection of sensitive bioactive compounds, particularly ascorbic acid, against oxidation and environmental stressors [28,29,30,31,32,33,34,35,36]. Liposomal encapsulation not only enhances the stability of active compounds but also improves their skin penetration, thereby boosting the effectiveness of topical formulations [37]. Hence, the development of a liposomal delivery system for the bioactive compounds derived from cashew apple presents a novel and promising approach, as it has not yet been reported in existing literature. Subsequently, this study aimed to extract bioactive compounds from freshly homogenized cashew apples in order to obtain cashew apple extract (CAE) and investigate its biological activities, including antioxidant potential, tyrosinase inhibition, stimulation of collagen synthesis in fibroblasts, cytoprotection against oxidative stress, wound healing properties, and cytotoxicity. CAE was encapsulated in liposomes and its potential in preserving ascorbic acid content under environmental stress and enhancing skin penetration was investigated. Finally, a solution containing liposomal-loaded CAE was formulated and assessed for its physicochemical properties and stability.
2. Materials and Methods
2.1. Materials
The chemicals purchased from Sigma-Aldrich (St. Louis, MO, USA) consist of 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), cholesterol (CH), 3,4-dihydroxy-L-phenylalanine (levodopa), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT), 2,2-diphenyl-1-picrylhydrazyl (DPPH), gallic acid monohydrate, L-ascorbic acid, lipopolysaccharide from E. coli, L-α-phosphatidylcholine (PC), metaphosphoric acid, mushroom tyrosinase enzyme, Transcutol®, Poloxamer 407, hydrogen peroxide, and sodium nitrite (Griess reagent). The chemical purchased from Loba Chemie (Mumbai, India) is sodium acetate. The chemicals purchased from Gibco® (Carlsbad, CA, USA) consist of Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), penicillin, streptomycin and trypsin-EDTA. Folin & Ciocalteu’s phenol reagent was procured from Merck (Rahway, NJ, USA). Sodium carbonate and sodium hydroxide were obtained from Ajax Finchem Pty Ltd., Taren Point, Australia. HPLC-grade solvents and ethanol (95%) were purchased from RCI Labscan Limited (Bangkok, Thailand). Phenoxyethanol, propylene glycol, and sodium metabisulfite were purchased from S. Tong Chemicals Co., Ltd. (Bangkok, Thailand).
2.2. Cashew Apple Fruit Extraction
Fresh, ripe cashew apples (Anacardium occidentale L.) were identified and selected based on the expertise of local farmers. Samples were harvested from Tha Sala District, Nakhon Si Thammarat Province, and Hat Yai District, Songkhla Province, in southern Thailand. Fruit exhibiting uniform ripeness (orange-red color) and that were free from physical defects were selected for further processing. The unpeeled cashew apples were thoroughly washed with tap water to eliminate surface impurities, rinsed with distilled water, and then chopped into small pieces and homogenized using a blender (Electrolux Ultimate Taste 300, Rayong, Thailand). A 95% ethanol solution was used as the extraction solvent in the maceration process. The homogenized cashew apples were subjected to maceration in the ethanol solution for 24 h in order to extract the bioactive compounds. The mixture was then filtered through muslin cloth to separate the solid residues (bagasse). The filtrate was collected and concentrated using a rotary evaporator (IKA, RV10 digital, Staufen, Germany) at 40 °C. Finally, the obtained concentrated extract was freeze-dried with a pilot freeze dryer (Epsilon model, Martin Christ, Osterode am Harz, Germany) to remove residual moisture. The final CAE was stored in an airtight glass container and kept in the refrigerator (2–8 °C) for further analysis. A schematic overview of the CAE extraction process is illustrated in Figure 2.

Figure 2.
Schematic representation of the extraction process used to obtain cashew apple extract (CAE). The diagram was created using BioRender.com under a publication license, with selected components manually illustrated by the author.
2.3. Physical Properties Evaluation of CAE
The physical characteristics of CAE, including color, odor, and clarity, were evaluated under natural lighting conditions through organoleptic observation to assess the overall appearance. A 10% w/v aqueous solution of CAE was prepared by diluting the extract with distilled water. The pH was measured using a digital pH meter (Mettler Toledo, S400, Greifensee, Switzerland), with readings taken in triplicate in order to determine the average pH value.
2.4. Determination of Ascorbic Acid Content by HPLC
Ascorbic acid content was quantified using high-performance liquid chromatography (HPLC), following the method described by Rizzolo et al. [38]. A stock standard solution of L(+)-ascorbic acid (2000 µg/mL) was prepared and subsequently diluted with 6% metaphosphoric acid to obtain working standards ranging from 1 to 200 µg/mL. Each standard solution was filtered through a syringe membrane filter into vials before HPLC analysis, which was conducted using the DionexTM UltiMate 3000TM HPLC System (Thermo Scientific Inc., Dreieich, Germany), equipped with a stainless steel C18 column (Partisil 10 SAX; pore size 85 Å, 10 µm particle size, dimensions 25 cm × 4.6 mm I.D.). The mobile phase consisted of 0.1 M sodium acetate (pH 4.25), with a flow rate of 1.3 mL/min and an injection volume of 10 µL. Ultraviolet (UV) detection was performed at a wavelength of 250 nm with a run time of 10 min. Chromatographic data were processed using ChromeleonTM 7.3 software. For the sample preparation, CAE and solution containing CAE (with or without liposomes) were diluted with 6% metaphosphoric acid to obtain a final concentration of 10 mg/mL of CAE. The sample solutions were filtered through a 0.45 μm nylon syringe membrane before injection.
2.5. Liquid Chromatograph Mass Spectrometer Ion Trap/Time-of-Flight System (LC-MS/IT-TOF)
The liquid fraction was collected after ascorbic acid analysis by HPLC. The evaluation was performed by direct injection using MeOH as the mobile phase. The flow rate was optimized at 1 mL/min with an injection volume of 0.1 µL, under the detective voltage of 1.72 kV. The mass spectrum was analyzed using positive electrospray ionization (ESI). The acquisition mass range was set to 100–250 m/z with 1 min observation. Data curation and evaluation were performed using ACD/Labs (Version S05S41) and LabSolutions LCMS.
2.6. Determination of Total Phenolic Content (TPC)
TPC was determined using the Folin–Ciocalteu assay, as outlined by Saeed et al. [39] and Sinsuebpol et al. [40]. CAE 20 µL was combined with 10% (v/v) Folin–Ciocalteu reagent 100 µL followed by the addition of 7.5% (w/v) sodium carbonate (Na2CO3) 80 µL. The mixture was incubated at room temperature in the dark for 1 h to allow complete reaction. The microplate reader (VarioskanTM LUX, Thermo Fisher Scientific, Vantaa, Finland) was used to measure the absorbance at 765 nm. TPC was calculated from a gallic acid calibration curve and expressed as milligrams of gallic acid equivalents per gram of CAE (mg GAE/g).
2.7. Determination of Total Flavonoid Content (TFC)
TFC was determined according to the method described by Saeed et al. [39] and Sinsuebpol et al. [40]. Briefly, CAE 300 µL was mixed with 30% ethanol (3.4 mL), 0.5 M sodium nitrite (NaNO2) (100 µL), and 0.3 M aluminum chloride hexahydrate (AlCl3·6H2O) (150 µL). After incubation at room temperature for 5 min, 1 mL of 1 M sodium hydroxide (NaOH) was added and thoroughly mixed. The absorbance of the resulting solution was measured at 506 nm using a UV-VIS spectrophotometer (Jasco, V-630, Tokyo, Japan). A calibration curve was prepared using rutin at concentrations ranging from 0.1 to 1 mg/mL. TFC was expressed as milligrams of rutin equivalents per gram of CAE (mg RE/g).
2.8. Determination of Total Caffeoylquinic Acid Content (TCQAC)
The TCQAC of CAE was evaluated using the protocols described by Sinsuebpol et al. [40] and Chan et al. [41]. The molybdate reagent was prepared by dissolving sodium molybdate (16.5 g), dipotassium hydrogen phosphate (8.0 g), and potassium dihydrogen phosphate (7.9 g) in deionized water with the pH adjusted to 6.5 and the volume adjusted to 1000 mL. To determine CQA content, CAE (200 μL) was mixed with molybdate reagent (10 mL) and incubated for 10 min. The UV-VIS spectrophotometer (Jasco, V-630, Tokyo, Japan) was used to measure the absorbance of the sample at 370 nm. TCQAC was expressed as milligrams of chlorogenic acid equivalents per g (mg CGAE/g) calculated using a calibration curve of chlorogenic acid in the concentration range of 0.1–1 mg/mL.
2.9. Antioxidant Activity Assays
The antioxidant activity of the CAE was determined using the DPPH and ABTS radical scavenging assays, following the protocols of Saeed et al. [39] and Sinsuebpol et al. [40].
DPPH Assay: A 0.25 mM solution of 2,2-diphenyl-1-picrylhydrazyl (DPPH) was freshly prepared in ethanol. Stock solutions of L-ascorbic acid (1 mg/mL), gallic acid (0.1 mg/mL), and CAE (10 mg/mL) were serially diluted to obtain the final concentrations of 6.25–50 µg/mL, 0.78–100 µg/mL, and 0.08–10 mg/mL, respectively. Then, 100 µL of each standard or sample dilution was pipetted into a 96-well plate, followed by the addition of 100 µL of DPPH solution. The plate was gently mixed and incubated in the dark at room temperature for 30 min. The absorbance was measured at 517 nm using a microplate reader (VarioskanTM LUX, Thermo Fisher Scientific, Vantaa, Finland). The percentage of radical scavenging was calculated relative to the control using Equation (1). The IC50 value (concentration required to inhibit 50% of DPPH radicals) was obtained by linear regression analysis of the concentration-response curve.
ABTS Assay: The ABTS•+ stock solution was prepared by mixing 7 mM ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) with an equal volume of 2.45 mM potassium persulfate and incubating the mixture in the dark at room temperature for 12–16 h. Before use, the ABTS•+ stock solution (2 mL) was diluted to 100 mL with distilled water to obtain an absorbance of 0.7 ± 0.05 at 734 nm. For the assay, 20 µL of L-ascorbic acid, standard gallic acid, CAE sample, or purified water (control) was mixed with 180 µL of diluted ABTS•+ solution. After a 6 min incubation at room temperature, absorbance was measured at 734 nm using a microplate reader (VarioskanTM LUX, Thermo Fisher Scientific, Vantaa, Finland). The percentage of ABTS radical scavenging activity was calculated using Equation (1).
2.10. Anti-Tyrosinase Activity
The anti-tyrosinase activity of CAE was assessed using the method described by Sinsuebpol et al. [40] and Whangsomnuek et al. [42], with minor modifications. CAE (0.08–10 mg/mL) or standard kojic acid (0.008–1 mg/mL) or phosphate–buffered solution pH 6.8 (control) of 20 µL was mixed with 200 units/mL of mushroom tyrosinase (40 µL) and 20 mM of phosphate-buffered pH 6.8 (140 µL) in a 96-well plate. The mixture was incubated at room temperature for 10 min. Subsequently, 40 µL of 2.5 mM levodopa was added as the substrate, and the reaction was allowed to proceed for an additional 20 min at room temperature. Absorbance was measured at 470 nm using a microplate reader (VarioskanTM LUX, Thermo Fisher Scientific, Vantaa, Finland). Tyrosinase inhibition (%) was calculated using Equation (1).
2.11. Cell Culture Conditions
All cell culture experiments were carried out with the approval of the Walailak University Institutional Biosafety Committee (WU-IBC), under approval number WU-IBC-68-019, dated 31 March 2025. The L929 murine fibroblast cell line (Chinese Academy of Preventive Medical Sciences, Beijing, China) and the RAW264.7 murine monocyte/macrophage cell line (ATCC TIB-71, Manassas, VA, USA) were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 U/mL streptomycin. Cells were maintained at 37 °C in a humidified incubator with 5% CO2. The culture medium was refreshed every 2 days. When the cells reached confluence, they were harvested using 0.25% trypsin-EDTA (Gibco®, Carlsbad, CA, USA) and resuspended in fresh medium to obtain a single-cell suspension for subsequent experiments.
2.12. Cell Viability Assay
CAE was diluted in culture medium and sterilized through a 0.2 µm syringe membrane filter (Corning®, Kaiserslautern, Germany) before use. L929 or RAW264.7 cells were seeded in a 96-well plate at a density of 1 × 105 cells/mL (100 mL/well) and incubated at 37 °C for 24 h. Cells were treated with CAE at concentrations of 62.5–1000 µg/mL for 24 h. Cell viability was determined using the MTT assay. After treatment, the culture medium was replaced with 80 µL of fresh medium and 20 µL MTT solution (5 mg/mL), followed by incubation at 37 °C for 4 h. The supernatant was removed, and 100 µL of dimethyl sulfoxide (DMSO) was added to dissolve the formazan crystals. Absorbance was measured at 570 nm using a microplate reader (Biohit® 830, Biohit®, Helsinki, Finland). The percentage of cell viability was calculated according to Equation (2):
2.13. Cytoprotective Effect of CAE Against H2O2-Induced Oxidative Stress
This assay was adapted from the methods described by Sinsuebpol et al. [43] and Balekar et al. [44] with modifications. The experiment was conducted in two steps:
Step 1: Determination of H2O2 cytotoxic concentration
L929 murine fibroblast cells were seeded in a 96-well plate at a density of 1 × 105 cells/mL (50 µL/well) in complete DMEM and incubated at 37 °C under 5% CO2 for 24 h. Cells were then treated with H2O2 at concentrations ranging from 62.5 to 1000 µM for 24 h. Cell viability was assessed using the MTT assay, and the concentration of H2O2 that resulted in approximately 80% cell death was selected for oxidative stress induction in the next step.
Step 2: Evaluation of the CAE Protective Effect
L929 cells were seeded under the same conditions and incubated for 24 h. Cells were then treated with various non-cytotoxic concentrations of CAE for an additional 24 h. Following treatment, the medium was replaced with complete media containing hydrogen peroxide (H2O2) at a concentration previously determined to induce 80% cell death. The cells were then incubated for an additional 24 h under identical conditions. Viability was then measured using the MTT assay to determine the protective effect of CAE against ROS-induced oxidative stress.
2.14. Assessment of the Immunological Response to CAE
The ability of CAE to provoke an inflammatory response was evaluated by measuring nitric oxide (NO) production in RAW264.7 murine macrophage cells following the method described by Sudsai et al. [45]. Cells were seeded in 96-well plates at a density of 1 × 105 cells/well and incubated at 37 °C with 5% CO2 for 1 h to allow cell adherence. The medium was then replaced with fresh DMEM containing various concentrations of CAE or lipopolysaccharide (LPS) from E. coli (1 µg/mL) as a positive control. After 24 h of incubation, 100 µL of the culture supernatant was collected and mixed with 100 µL of freshly prepared Griess reagent. The microplate reader (Biohit® 830, Biohit®, Helsinki, Finland) was used to determine the amount of nitrite, a stable end-product of the breakdown of NO, at 570 nm.
2.15. Assessment of CAE-Induced Fibroblast Collagen Type I Production
L929 murine fibroblast cells were cultured under the same conditions as described in the section on the cell viability assay. Based on the cell viability results, CAE was applied at non-toxic concentrations ranging from 62.5 to 1000 µg/mL. After 24 h of incubation in a 5% CO2 incubator, the culture supernatants were collected for quantification of soluble collagen type I. The Sircol® Collagen Assay Kit (Biocolor® Ltd., Carrickfergus, UK) was used to measure the collagen content according to the manufacturer’s instructions. The microplate reader (Biohit® 830, Biohit, Helsinki, Finland) was used to measure the absorbance at 500 nm and calculate collagen concentrations with a standard curve generated from the soluble collagen type 1 provided in the kit. All samples were analyzed in quadruplicate.
2.16. In Vitro Wound Healing Induced by CAE
L929 fibroblast cells were seeded into a 6-well plate at a density of 5 × 104 cells per well and cultured until a confluent monolayer was formed. A sterile pipette tip was then used to create a linear scratch across the monolayer to simulate the wound. Detached cells and debris were removed by washing, and the wells were replenished with 2 mL of DMEM containing CAE at a concentration of 1000 µg/mL. Untreated cells served as the control group. Images of the scratch area were captured under a light microscope at 10× magnification at 0, 24, 48, and 72 h post-treatment. Scratch closure was quantified using ImageJ software (version 1.42q/Java 1.6.0_10), and the percentage of cell migration was subsequently calculated. Data were reported as the mean of four independent replicates [40,44].
2.17. CAE-Loaded Liposomes Preparation
Liposomes were prepared using L-α-phosphatidylcholine (PC) and cholesterol (CH) as lipid components at weight ratios of 4:0 and 4:1. The thin-film hydration method was employed to encapsulate CAE, following the procedure described in previous studies [46,47]. The lipid components were dissolved in 10 mL of chloroform and transferred into a 1000 mL round-bottom flask. Chloroform was removed by a rotary evaporator (Heidolph HED rotary evaporator, Schwabach, Germany) under vacuum at 40 °C and 60 rpm for 20 min, forming a thin lipid film on the inner wall of the flask. The residual chloroform was eliminated by further drying under a vacuum oven (WTC Binder GmbH, Tuttlingen, Germany) for 24 h. To hydrate the lipid film, CAE (250 mg) was dissolved in a 100 mL citrate buffer pH 3.0 and transferred to a round-bottom flask, followed by rotation at 60 rpm until the film was completely detached for 30 min. The resulting liposome dispersion was sonicated in an ultrasonic bath (Elma E300H, Elma Schmidbauer GmbH, Singen, Germany) at an amplitude of 20 W for 30 min to reduce vesicle size. Unencapsulated CAE was removed by dialysis using a membrane with a molecular weight cutoff (MWCO) of 14,000 Da for 24 h, followed by three washes with purified water.
2.18. Physicochemical Characterization of CAE-Loaded Liposomes
A ZetaSizer dynamic light-scattering analyzer (Malvern Panalytical Ltd., Malvern, UK) was used to determine the particle size, polydispersity index (PDI), and zeta potential of the CAE-loaded liposomes. The total ascorbic acid content in the liposome formulations was determined as described in the previous section. The entrapment efficiency (%EE) was calculated following Equation (3). Since the preparation process involves removing unencapsulated CAE through dialysis, the analyzed ascorbic acid content represents the amount of ascorbic acid entrapped within liposomes. Therefore, the %EE calculation was based on the ratio of the entrapped ascorbic acid (measured after dialysis) to the amount of ascorbic acid initially loaded into the formulation.
The liposome dispersion with the desired properties was converted into a dry powder using the freeze-drying technique for use in subsequent experiments.
2.19. In Vitro Skin Permeation Study of CAE vs. CAE-Loaded Liposomes
The skin permeation study was performed using a Franz diffusion cell (MicroettePlus; Hanson Research, Chatsworth, CA, USA) with Strat-M® artificial human skin membrane (Millipore, Billerica, MA, USA) as the diffusion membrane. The membrane was equilibrated in phosphate buffer pH 7.4 (PBS, pH 7.4) and mounted between the donor and receptor compartments of the Franz cell. The receptor chamber (12 mL volume) was filled with PBS containing 20% ethanol to ensure sink condition and maintained at 37 ± 0.5 °C with continuous stirring using a water bath (model 3047, Köttermann, Hänigsen, Germany). The effective diffusion area was approximately 1.77 cm2. CAE and CAE-loaded liposomes (with the amount of samples based on containing equivalent ascorbic acid) were applied separately to the donor compartment. Sampling (1 mL) from the receptor fluid at the time intervals of 0, 1, 2, 4, 6, and 8 h was conducted and then it was immediately replaced with an equal volume of fresh medium. The amount of permeated ascorbic acid in each sample was determined by HPLC, as described in the previous section. The cumulative amount of ascorbic acid permeated per unit area (μg/cm2) was plotted as a function of time.
2.20. Preparation of CAE Solution and CAE-Loaded Liposomes Solution
The components used in the formulation are listed in Table 1. To prepare the solution, poloxamer 407 was first dissolved in distilled water under continuous stirring until fully solubilized. Sodium metabisulfite was then added and mixed until a clear solution was achieved. Next, CAE or CAE-loaded liposomes—containing an equivalent amount of ascorbic acid—were incorporated and thoroughly mixed to ensure uniform distribution. Transcutol® was subsequently added, followed by propylene glycol, with continuous stirring to maintain homogeneity. Finally, phenoxyethanol was added and stirred until evenly dispersed throughout the formulation. The prepared CAE solution was then evaluated for its physical appearance and pH as described earlier.

Table 1.
Formulation of CAE solution and CAE-loaded liposomes in solution.
2.21. Viscosity Evaluation
A rheometer (HAAKE Mars 60, Thermo Fisher Scientific, Bremen, Germany) was used to determine the viscosity of both the CAE solution and the CAE-loaded liposomal solution. The equipment included a 60 mm parallel plate system and a Peltier temperature control unit with a fixed gap of 0.5 mm between the plates. Samples were carefully placed on the lower plate, and the upper plate was lowered to the preset gap. Rheological flow behavior was evaluated over a shear rate of 1 to 1000 s−1 at a constant temperature of 25 °C. Viscosity values (in mPa⋅s or cP) were recorded at a shear rate of 20 s−1. All measurements were performed in triplicate, and the average values were reported.
2.22. Assessment of Ascorbic Acid Stability
To investigate the protective effect of liposomal encapsulation on the stability of CAE, a comparative stability study was conducted. The CAE solution and CAE-loaded liposomal solution were stored in tightly sealed amber containers under two conditions: refrigerated storage at 4 ± 2 °C and long-term storage at 30 ± 2 °C with 75 ± 5% relative humidity, by applying ICH guidelines for climatic zone IV [48]. Samples were collected at 0, 1, 2, and 3 months and analyzed for ascorbic acid content using HPLC as described in the previous section. At each time point, physical appearance, pH, the percentage of remaining ascorbic acid, and viscosity were recorded. All measurements were performed in triplicate.
3. Results and Discussion
3.1. Analytical Validation of Ascorbic Acid in CAE
Several varieties of cashew apples have been reported to be rich in ascorbic acid [9,15,49,50]. Therefore, this study focuses on ascorbic acid as a marker bioactive compound of CAE in order to monitor the stability of the extract and formulation by employing an analytical method described in a previous report, which was simple to perform [38] and has been validated according to the ICH guidelines. Figure 3 displays the chromatogram of the ascorbic acid standard solution and the ascorbic acid in the CAE. Evaluation of specificity showed that the peak of ascorbic acid in the standard and sample had a high resolution at a retention time of approximately 1.963 and 2.340 min for both the standard and the samples, and no signals were shown for any other compounds in the extract.
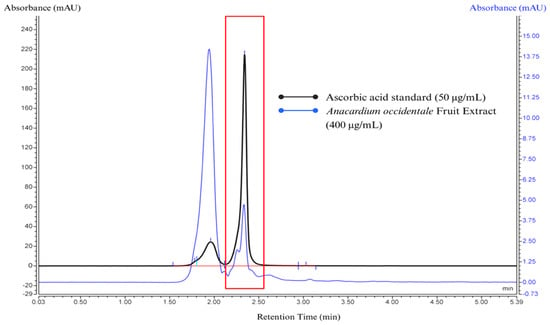
Figure 3.
The HPLC chromatogram represents the overlay of standard ascorbic acid at a concentration of 50 μg/mL (black line) and the cashew apple extract (CAE) sample at a concentration of 400 μg/mL (blue line). The small light blue line is the integrate data from the chromatogram. Standard and sample peaks were eluted at the same retention time at 1.963 min, corresponding to the keto tautomer of ascorbic acid, and at 2.340 min (represented in the red frame), corresponding to ascorbic acid.
The HPLC analysis of CAE revealed two distinct peaks. The first peak (retention time at 1.960 min) corresponds to the keto tautomer of L-ascorbic acid, as indicated by its relatively lower height compared to the main peak. This suggests partial tautomerization under the given analytical conditions. The second peak (retention time at 2.340 min) represents L-ascorbic acid in its enediol form, which is the dominant and more stable form detected. The formation of the keto tautomer is due to the analytical conditions such as mobile phase pH and oxidation. L-ascorbic acid is known to be unstable at an elevated pH and readily converts into the keto tautomer if the environment is not maintained at an acidic pH of 2–3. Additionally, partial oxidation of L-ascorbic acid may contribute to this transformation, leading to structural modifications that facilitate tautomerization.
Ascorbic acid also converts into two unstable diketone tautomers by proton transfer, although it is most stable in the enol form. The proton of the enol is lost and then re-acquired by electrons from the double bond to produce a diketone. However, there are two possible forms of diketone: 1,2-diketone and 1,3-diketone [51]. These findings emphasize the necessity of maintaining proper analytical conditions in order to minimize keto tautomer formation and ensure the accurate quantification of L-ascorbic acid in CAE (Figure 4).

Figure 4.
Keto-enol tautomerism of ascorbic acid, the cause of the two peaks found in the HPLC chromatogram by conversion of L-ascorbic acid in both the standard solution and CAE.
The proposed mechanism of action of ascorbic acid as an antioxidant in mammalian cells is the donation of a hydrogen atom to form monodehydroascorbate, which prefers to react with radicals rather than with non-radical compounds, and the fully oxidized form of ascorbic acid is a bicyclic hemiketal [52]. Electrons from ascorbic acid can react with metals such as copper and iron, leading to the generation of superoxide and hydrogen peroxide, which may later result in the formation of reactive oxygen species (ROS). Consequently, in certain situations, ascorbic acid will give rise to an oxidizing agent by way of its operation as a reducing agent. This reaction occurs in the biological system when different pharmacological doses of ascorbic acid are absorbed in the plasma and extracellular fluid compartment as well as low doses of ascorbic acid in cell culture media containing metals [53].
To determine which compounds the two peaks analyzed by HPLC were, the LC-MS/MS technique was used for further analysis, and the LC-MS analysis was performed in both positive and negative modes (Figure 5), with the results showing the same spectra. In positive ESI mode, m/z values were observed at 136.05, 172.95 and 186.96 m/z, while in negative ESI mode, m/z values were observed at 126.99, 141.01, 205.14, and 225.14 m/z.
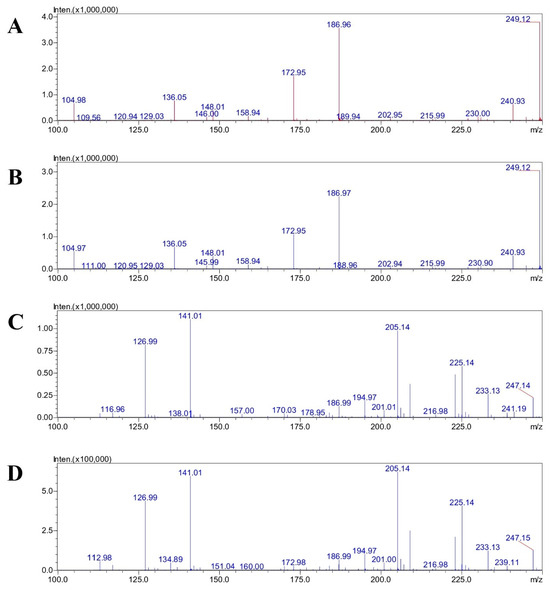
Figure 5.
Mass spectra of standard ascorbic acid at 1000 μg/mL (A) and CAE (B) in centroid analysis using positive ESI mode. Mass spectra of standard ascorbic acid at 1000 μg/mL (C) and CAE (D) in centroid analysis using negative ESI mode.
The negative mode at the mass per charge ratio of 172.98 may relate to the monomer of dehydroascorbic acid. The 225.14 m/z peak was most likely formed from the 227 m/z ion of dehydroascorbic acid trihydrate via a fragmentation process in the mass spectrometer. The loss of a molecule with a mass of 2 Da could represent the loss of two hydrogen atoms. This is a possibility given the complex degradation and fragmentation patterns observed for dehydroascorbic acid in mass spectrometry. It is also possible that the adduct was formed with a different combination of water molecules and ions, such as the sodium adduct of a less hydrated form [54]. The m/z 141 fragment is a result of the ascorbic acid undergoing a specific fragmentation pathway within the mass spectrometer. This process involves the loss of a neutral molecule from the parent ion. One possible explanation for the m/z 141 peak in negative ion mode is the loss of a formyl radical CHO from the deprotonated molecular ion m/z 175. The reaction would be [C6H7O6]– → [C5H6O5]– + [CHO] ([175]– → [141]– + [30]). The mass of the m/z 233 ion is an adduct from acetate and dehydroascorbic acid or, equivalently, from acetic acid and dehydroascorbate [55].
Table A1 in Appendix A displays the parameters of the validation results. The calibration curve of the ascorbic acid standard at concentrations ranging from 1 to 1000 µg/mL showed good linearity with r2 of 0.9999. The accuracy was determined by the %recovery, which was 99.2% ± 0.5%. The intra- and inter-day precisions were evaluated as %RSD, which were 0.15% and 0.10%, respectively. The calculations of LOD and LOQ were 0.1 and 1.4 µg/mL, respectively. These outcomes confirm the suitability of this analytical technique using HPLC for measurement of the ascorbic acid in CAE.
3.2. Physical Appearance of CAE
The CAE appeared as a brownish-yellow liquid (Figure 6) with a sweet, fruity scent characteristic of cashew apples. After undergoing the freeze-drying process, the extract turned into a sticky, viscous, brown-colored substance with a more pronounced sweet, fruity scent. The pH measurement of the CAE solution was found to be 4.98 ± 0.05.

Figure 6.
Photograph illustrating CAE after being freeze-dried, appearing as a brownish-yellow sticky substance with a characteristic sweet odor.
3.3. Quantification of Bioactive Constituents in CAE
Ascorbic acid is widely recognized as a major contributor to the antioxidant capacity found in the cashew apple pseudo-fruit [56]. However, several studies have demonstrated that cashew apple fruits contain various other bioactive secondary metabolites, such as phenolic acids and flavonoids [21,56,57,58,59,60]. These compounds are known to significantly contribute to the biological activities relevant to their application as cosmetics ingredients, particularly those with anti-aging properties [9,15]. This study quantified the bioactive compounds present in CAE in order to better understand their contribution to its multifunctional biological activities. The results are summarized as the levels of ascorbic acid, TPC, TFC, and TCQAC, as shown in Table 2. Ascorbic acid is recognized as the major antioxidant compound in cashew apples [21,56,60] and has been reported to be present at all stages of fruit maturity, with fully ripe cashew apples containing approximately 1.7 times higher levels than unripe fruit [56]. Based on this, fully ripe cashew apples were chosen for extraction in the present study. The ascorbic acid content was found to be 0.9 mg/g CAE, which corresponds to approximately 7.75 mg/100 g of fresh cashew apple when calculated based on the extraction yield. When compared to previous studies, the reported ascorbic acid content in cashew apples varies widely, ranging from 51.8 to 256.0 mg/100 mL or g of fresh cashew apple juice [22,57].

Table 2.
The bioactive compounds content in the CAE (mean ± S.D., n = 3).
This variation is influenced by several factors, including the extraction methods, fruit variety (yellow or red), maturity stage (unripe, medium-ripe, ripe), and sample type (fresh, dried, or juice) [9,21,56,59,61]. Additionally, environmental conditions such as soil type, climate, growing season, and post-harvest storage can significantly affect the composition of bioactive compounds [22]. In this study, the relatively lower ascorbic acid content compared to previous reports may be attributed to the use of 95% ethanol as the extraction solvent. Ethanol is a semi-polar solvent with a dielectric constant of 25.02–26.47 [62,63], whereas ascorbic acid is a highly polar molecule due to the presence of multiple hydroxyl groups [64]. According to the principle of “like dissolves like,” this polarity mismatch may result in an incomplete extraction of ascorbic acid using ethanol [65]. However, the rationale for selection of 95% ethanol as the extraction solvent was determined by the objective to obtain a broad phytochemical profile, rather than to solely maximize the extraction amount of ascorbic acid.
In addition, cashew apple fruit contains approximately 85% moisture [21], with water being the primary component. The extraction process involves macerating the homogenized fresh cashew apples, without separating the cashew apple juice, in 95% ethanol (1:1 by weight). Due to cashew apples’ primary water content, the amount of water obtained from the fresh fruit should be considered as it must be combined with ethanol to form a hydroalcoholic solvent. Therefore, this extraction solvent allows ascorbic acid to be soluble in the system and may also allow other biological compounds that are highly soluble in alcohol to be extracted. Other target compounds such as phenolics and flavonoids are generally more soluble in ethanol than in water and previous reports demonstrated that the use of an alcohol-based solvent can extract high phenolic content from cashew apples [28]. Furthermore, the higher ascorbic acid values reported in other studies are likely due to analyses being performed on fresh juice, when ascorbic acid is mostly retained. In contrast, in this study, ascorbic acid was employed as a marker to monitor and ensure the chemical stability of CAE and CAE-containing formulations, as it is a readily degradable substance. Thus, this research involved extraction and freeze-drying steps that prolonged the processing time, potentially leading to greater degradation and reduced content.
Analysis of the bioactive compounds showed that the TPC of CAE was 81.40 ± 7.14 mg GAE/g, which corresponds to approximately 700.85 mg GAE per 100 g of fresh cashew apple, based on the extraction yield. This value is higher than those reported in previous studies, which ranged from 12.2 to 196.0 mg GAE/100 g or mL of fresh fruit or juice [28,57,58]. However, when compared to a study using a similar extraction solvent (90% ethanol), TPC values as high as 209.58 mg GAE/g of dried extract were reported [66]. These results suggest that the TPC is highly influenced by the sample preparation and the extraction technique employed. The main phenolic compounds found in cashew apples are myricetin 3-O-rhamnoside, quercetin 3-O-galactoside, and gallic acid [56]. Their composition varies with fruit maturity, with unripe cashew apples containing higher levels than medium-ripe or ripe fruit [56]. Tannins represent the predominant class of phenolics in cashew apples and are primarily responsible for their astringent properties [57,67].
Flavonoids are a subclass of compounds and exhibit diverse biological activities, including antioxidant, anti-inflammatory, and anticancer properties [68]. In this study, the TFC in CAE was 3.73 ± 0.30 mg RE/g. However, this value cannot be directly compared with those reported in other studies because a different reference standard was used. Flavonoid content found in cashew apple juice has been reported in the range of 0.122–249.6 mg/g when expressed as quercetin equivalents. Caffeoylquinic, a polyphenolic compound, is a natural antioxidant widely utilized in food, pharmaceuticals, and cosmetics [69]. In the CAE, the TCQAC was determined to be 4.48 ± 0.05 mg CGAE/g of CAE.
3.4. Biological Activity of CAE
The antioxidant activity of CAE, expressed as IC50, for the DPPH and ABTS assays is presented in Table 3. The free radical scavenging capacity of the extract was assessed by comparing its antioxidant activity to that of standard reference compounds—gallic acid and ascorbic acid. IC50 values were calculated using a linear regression equation. In the DPPH assay, gallic acid showed the strongest activity with an IC50 of 2.27 ± 0.02 µg/mL, followed by ascorbic acid at 23.62 ± 0.05 µg/mL, whereas CAE exhibited an IC50 of 282.19 ± 11.16 µg/mL. The ABTS assay revealed a comparable pattern of antioxidant potency.

Table 3.
The biological activities of cashew apple extract (CAE) reported as the IC50 values (mean ± S.D., n = 3).
Considering that 1 g of CAE contains 0.9 mg of ascorbic acid, the amount of ascorbic acid present at the IC50 of CAE (282.19 µg/mL) is approximately 0.25 µg/mL, ~93 times lower than the IC50 of pure ascorbic acid. This suggests that the antioxidant activity of CAE arises not only from ascorbic acid but from the synergistic effects of various bioactive compounds present in the extract. This is consistent with the high levels of TPC, TFC, and CQA detected in the extract (Table 2), all of which are recognized for their strong antioxidant properties.
The aging process is associated with an increased production of ROS and heightened oxidative stress in the skin, leading to a range of structural and functional changes. These include wrinkles, loss of elasticity, uneven pigmentation, discoloration, dryness, and slower regeneration and wound healing [70,71]. Oxidative stress damages cellular components such as lipids, proteins, and DNA, accelerating the intrinsic and extrinsic aging processes.
Antioxidants play a crucial role in mitigating these effects by neutralizing free radicals and preventing oxidative damage. The most effective antioxidants are those capable of interrupting the propagation of free radical reactions. Many of these compounds contain aromatic or phenolic rings, enabling them to donate hydrogen atoms (H•) to free radicals generated during oxidation [71]. Therefore, the antioxidant activity of CAE, which can effectively neutralize free radicals, holds significant potential for delaying or preventing the visible signs of aging and improving skin health.
Tyrosinase is a crucial enzyme involved in melanin production, the pigment that determines skin color. It serves as the rate-limiting factor in this pathway; hence, decreasing tyrosinase activity is a principal method for diminishing melanin synthesis. Natural extracts demonstrate tyrosinase inhibition activity, showing potential for managing hyperpigmentation and are widely explored for cosmetic applications, including skin lightening formulations [72]. The anti-tyrosinase activity of CAE showed an IC50 value of 4213.77 ± 138.97 µg/mL, which is approximately 112 times higher than that of the positive control kojic acid (IC50 = 37.42 ± 1.40 µg/mL), indicating that CAE possesses only weak tyrosinase inhibitory activity. Kubo et al. [73] reported that anacardic acid, methylcardols, and cardols extracted from different parts of the cashew fruit have been found to exhibit tyrosinase inhibitory activity. Tan and Chan [74] demonstrated that a leaf extract at a concentration of 250 µg/mL could inhibit tyrosinase activity by 40% [74]. Similarly, Srisuksomwong et al. [72] reported IC50 values ranging from 111 to 183 µg/mL for cashew leaf extracts prepared using ethanol, ethyl acetate, and distilled water. Based on the results of this study, it is possible that compounds with tyrosinase inhibitory activity may not be present in sufficient quantities in cashew apple fruits. Furthermore, Sinsuebpol et al. [40] found that rutin and CGA, both detected in CAE, did not exhibit tyrosinase inhibition at concentrations below 1000 µg/mL. This suggests that certain bioactive compounds present in cashew leaves, but present in much lower amounts in the fruit, may be primarily responsible for the anti-tyrosinase activity observed in leaf extracts. This may partly explain why CAE shows relatively weak anti-tyrosinase activity.
3.5. Cell Viability
To assess the safety profile of CAE and establish a suitable concentration range for further cell-based bioactivity assays, an MTT assay was conducted using concentrations ranging from 31.25 to 1000 µg/mL. L929 fibroblast was used to evaluate collagen synthesis, wound healing, and the cytoprotective effect of CAE against ROS-induced oxidative stress, while RAW264.7 mouse monocyte/macrophage cells were used to assess the potential to stimulate the immunological response of CAE. The cytotoxicity of CAE and the negative control (culture medium) against L929 fibroblast and RAW264.7 mouse monocyte/macrophage cells are shown in Figure 7A and Figure 7B, respectively. The results indicate that cell viability was approximately 100% for all tested concentrations, suggesting that CAE is non-toxic to both cell lines within the concentration range of 31.25 to 1000 µg/mL.

Figure 7.
Percentage of cell viability of (A) RAW264.7 mouse monocyte/macrophage cells, and (B) L929 fibroblast cells after being treated with CAE at various concentrations (31.25–1000 µg/mL). (C) Cell viability following exposure to H2O2-induced oxidative stress and (D) the cytoprotective effect of CAE against oxidative stress. (E) Nitric oxide (NO) production in RAW264.7 cells after treatment with CAE or stimulation with LPS from E. coli. (F) Collagen type I production in L929 fibroblast cells. The control is the culture medium, LPS is lipopolysaccharide, and CAE is cashew apple extract. Data are expressed as mean ± S.D. (represented as error bar), n = 4. The asterisks (*, **) indicate the significant differences (p-value < 0.05 and p-value < 0.01, respectively) compared to the treated and non-treated samples.
3.6. Cytoprotective Effect of CAE Against Reactive Oxygen Species—Induced Cell Death
Reactive oxygen species include oxygen-containing free radicals, including superoxide anion (O2−), hydroxyl radical (·OH), hydrogen peroxide (H2O2), and singlet oxygen (1O2). ROS can originate from endogenous sources, such as mitochondrial respiratory chain activity and enzymatic systems like NADPH oxidase, xanthine oxidase, cyclooxygenase, and lipoxygenases. Additionally, ROS production can be stimulated by exogenous factors, including ultraviolet (UV) radiation (UVA and UVB), environmental pollutants, cigarette smoke, heavy metals, particulate matter (PM2.5), chemical exposure, high temperature, and psychological stress. The body’s antioxidant defense system, consisting of enzymatic components like superoxide dismutase (SOD), catalase, and glutathione peroxidase, along with non-enzymatic antioxidants such as glutathione, ascorbic acid, and tocopherol, is essential for maintaining redox balance by neutralizing reactive species and preventing oxidative damage. However, when the generation of ROS exceeds the capacity of this system, an imbalance occurs, leading to a state known as oxidative stress. In the skin, oxidative stress induces DNA damage, resulting in mutations and cellular senescence, reducing the proliferative capacity of keratinocytes and fibroblasts. Furthermore, ROS activate matrix metalloproteinases (MMPs), accelerating the degradation of collagen and elastin fibers, leading to wrinkle formation and decreased elasticity [71].
Furthermore, excessive oxidative stress during wound healing disrupts tissue repair by promoting chronic inflammation and inducing extensive cellular damage. Elevated ROS levels oxidize lipids, proteins, and DNA, leading to apoptosis or cellular senescence of fibroblast and endothelial cells, thereby impairing the synthesis of collagen, elastin, and extracellular matrix components. Moreover, excessive ROS suppress key growth factors such as VEGF and TGF-β, resulting in delayed angiogenesis and tissue [75].
Several studies have reported that substances with strong antioxidant activity can mitigate oxidative stress and improve cell survival under conditions induced by ROS, such as H2O2 [40,44]. Based on this evidence, CAE, which possesses strong antioxidant properties, was evaluated for its ability to exert a cytoprotective effect against H2O2-induced oxidative stress. Figure 7C illustrates the viability of L929 fibroblast cells exposed to H2O2 at concentrations ranging from 62.5 to 1000 µM. The results showed that H2O2 concentrations above 250 µM caused more than 80% cell death. Therefore, 250 µM H2O2 was selected as the inducer of oxidative stress in L929 fibroblast cells. Figure 7D demonstrates the cytoprotective effect of CAE under oxidative stress conditions. Pretreatment of cells with CAE at concentrations of 62.5–1000 µg/mL before H2O2 exposure resulted in a significant, dose-dependent increase in cell viability. In the absence of CAE, oxidative stress reduced the cell survival rate to approximately 20%. These findings indicate that CAE provides a cytoprotective effect against oxidative stress in L929 cells.
3.7. Immunostimulatory Potential of CAE
To assess the safety of CAE, this study evaluated the immune response of RAW264.7 mouse monocyte/macrophage cells upon exposure to CAE. Nitric oxide (NO), a key pro-inflammatory mediator, was measured after stimulating the cells with CAE at concentrations ranging from 62.5 to 1000 µg/mL. The results were compared with both a negative control (cells cultured in growth medium under the same conditions) and a positive control (cells stimulated with LPS from E. coli). As shown in Figure 7F, CAE did not elicit an immune response in RAW264.7 cells at all tested concentrations, as evidenced by NO levels that were not significantly different from those of the negative control. In contrast, cells stimulated with LPS from E. coli produced a markedly higher amount of NO, confirming immune activation. These findings indicate that CAE is safe and does not induce an immune response in RAW264.7.
Additionally, this study investigated the anti-inflammatory potential of CAE by stimulating RAW264.7 cells with LPS from E. coli to induce NO production, followed by treatment with CAE at varying concentrations. The results demonstrated that CAE did not exhibit significant anti-inflammatory activity.
3.8. Collagen Fibroblast Synthesis
Fibroblasts are the most abundant cells in connective tissue and are primarily responsible for producing and organizing the extracellular matrix (ECM), including collagen. They provide structural support for tissue and play a critical role in wound healing through the synthesis of ECM components and collagen. In cosmetics, collagen is essential for improving skin quality by enhancing strength and elasticity, reducing wrinkles, and maintaining hydration.
Ascorbic acid is crucial in collagen biosynthesis, acting as a cofactor for the enzymes prolyl hydroxylases and lysyl-hydroxylases. These enzymes are essential for the hydroxylation of proline and lysine residues, which is critical for stabilizing the collagen triple helix [76]. Boyera et al. [77] demonstrated that ascorbic acid induces a dose-dependent increase in type I collagen deposition by fibroblasts. Additionally, flavonoids have been reported to enhance fibroblast collagen synthesis by inhibiting matrix metalloproteinases (MMPs), thereby reducing collagen degradation. Flavonoids can also bind directly to collagen, making it more resistant to collagenase activity. Consequently, treating fibroblasts with flavonoids increases both the rate and amount of collagen synthesis [78].
The collagen production results following CAE treatment are presented in Figure 7E. At CAE concentrations below 125 µg/mL, the detected collagen levels were not significantly different from control. However, when the CAE concentrations exceeded 250 µg/mL, the fibroblast showed a noticeable increase in collagen production. Collagen synthesis increased in a dose-dependent manner with CAE treatment, reaching approximately 150 µg/mL at the highest CAE concentration of 1000 µg/mL. These results confirm the potential of CAE as a valuable starting material for cosmetic applications aimed at anti-aging and skin rejuvenation, improving firmness, and enhancing elasticity. Furthermore, its collagen-stimulating ability suggests additional applications in wound healing and scar formation control.
3.9. Wound Healing Activity by Scratch Assay
The bioactive compound profile shown in Table 2 reveals that CAE is rich in ascorbic acid, phenolic acids, and flavonoids—all of which are known to promote fibroblast activity and stimulate collagen synthesis. Therefore, CAE was evaluated for its potential to stimulate type I collagen production in L929 fibroblast cells. Based on the above finding, CAE demonstrated the ability to promote wound healing, attributed to its capacity to stimulate collagen synthesis and its antioxidant activity in mitigating ROS-induced oxidative stress. Therefore, the wound healing effect of CAE was evaluated using a scratch assay. In the preliminary wound healing assay, CAE was employed at a concentration of 1000 µg/mL, which was based on cytotoxicity testing. This concentration was chosen to promote a wound healing effect, and the results revealed that it effectively enhanced cell migration. However, a lower concentration may also produce a similar effect. Therefore, further studies should be conducted in the lower concentration range to determine the optimal effective concentration range and dose–response relationship.
Figure 8A illustrates the wound closure in L929 fibroblast cells over 3 days, comparing cells treated with CAE and the negative control. Quantitative analysis (Figure 8B) showed that the percentage of cell migration in CAE-treated cells was significantly higher than that in the negative control on days 1 and 2. This effect may be attributed to the collagen-stimulating properties of CAE as well as the presence of flavonoid and phenolic compounds [79,80], which are known to significantly accelerate wound healing. Furthermore, Vasconcelos et al. [13] previously reported that cashew apple juice exhibits potential for wound healing. In the context of cosmetic applications, the wound healing activity of CAE is crucial for promoting skin repair and regeneration, contributing to healthier and more esthetically pleasing skin. CAE can leverage wound healing properties to address various skin concerns, including scars, blemishes, and signs of aging. Additionally, CAE possesses antimicrobial activity [6,20], further supporting its potential therapeutic benefits in wound care.

Figure 8.
Wound healing assessed by scratch assay observed under an inverted light microscope (A) and percentage of cell migration (B) after exposure to CAE for 0, 1, 2 and 3 days. Data is expressed as mean ± S.D. (represented as error bar). The asterisk (*) indicates significant differences (p-value < 0.05) compared to the treated and non-treated samples at the same intervals.
3.10. Formulation Development and Physicochemical Properties of CAE-Loaded Liposomes
Based on the experimental results, CAE demonstrated strong potential as an active ingredient in cosmetic products for anti-aging and wound healing applications. However, a major limitation is the instability of ascorbic acid, a key bioactive component in CAE, which undergoes rapid degradation [81]. To address this challenge, liposome encapsulation was employed as an effective strategy to protect ascorbic acid and improve the stability of CAE. The liposome formulations used L-α phosphatidylcholine (PC) and cholesterol (CH) as lipid components to form a bilayer structure, prepared at weight ratios of 4:0 and 4:1 (PC:CH). These ratios were selected based on several previous studies reporting that a higher proportion of PC relative to CH enhances encapsulation efficiency [34,47,82,83]. However, the entrapment of drugs or extracts within liposomes depends on multiple factors, including the solubility profile (hydrophilic or lipophilic), vesicle size, and the preparation method used [84,85]. Ideally, liposomes within the size range of 50 to 500 nm exhibit superior efficacy in penetrating cellular and skin barriers. Additionally, higher absolute zeta potential (either positive or negative) contributes significantly to the colloidal stability of liposome dispersions [86,87].
The physical properties of CAE-loaded liposomes are presented in Table 4. The liposome formulations were prepared at two PC:CH ratios, 4:0 and 4:1. The mean particle size ranged from 272.17 ± 2.21 nm (PC:CH, 4:0) to 307.00 ± 4.26 nm (PC:CH, 4:1). The observed increase in size with cholesterol incorporation may be attributed to the stabilization of the lipid bilayer structure, which reduced vesicle flexibility and promoted the formation of slightly larger liposomes. Both formulations exhibited a polydispersity index (PDI) of 0.21–0.24, indicating a moderately narrow size distribution [88]. The zeta potential, which reflects colloidal stability, was in the range –39.5 ± 1.10 to –34.83 ± 1.60 mV, which is considered indicative of strong electrostatic stability [89]. Additionally, the negatively charged liposomes have also been reported to enhance skin penetration [90].

Table 4.
Physical properties of CAE-loaded liposomes (mean ± S.D., n = 3).
CAE is rich in hydrophilic bioactive compounds such as ascorbic acid, phenolic compounds, and polar flavonoids, which contain multiple hydroxyl and carboxylic groups. This hydrophilic nature facilitates their entrapment within the aqueous core of liposome vesicles. Encapsulation efficiency (EE) of these hydrophilic bioactive compounds was significantly affected by the presence of cholesterol. The formulation containing cholesterol (PC:CH, 4:1) exhibited a slightly larger particle size and achieved a higher EE (84.55 ± 4.12%) compared to the cholesterol-free formulation (PC:CH, 4:0), which showed an EE of 79.75 ± 2.49%. This finding is consistent with previous studies reporting that cholesterol enhances bilayer packing, reduces membrane permeability, and minimizes drug leakage, thereby improving encapsulation efficiency [91].
Based on the higher EE and acceptable particle size, the CAE-loaded liposomes formulation with PC:CH (4:1) was selected for freeze drying and subsequent experiments. The ascorbic acid content assay of the lyophilized liposome powder revealed that the content was 56 µg of ascorbic acid per gram of powder.
3.11. Skin Permeation Study
The skin permeation profiles of the CAE and CAE-loaded liposome solution across a Strat-M® artificial skin membrane are shown in Figure 9. Ascorbic acid, the marker compound in CAE, was monitored over an 8 h period. The steady-state flux (Jss) of ascorbic acid from the CAE-loaded liposomes was 16.52 ± 3.11 µg/cm2/h, which was approximately 2.1 times higher than that of the CAE (7.77 ± 4.12 µg/cm2/h). The enhancement ratio (ER) was calculated as 2.1, indicating that liposomal encapsulation significantly improved permeation. Statistical analysis revealed that the flux of ascorbic acid from CAE-loaded liposome was considerably higher than that from the CAE at all sampling points (p < 0.01). The calculated permeability coefficient (Pc) was 5.5 × 10−2 cm/h for the CAE-loaded liposome compared to 2.5 × 10−2 cm/h for CAE. These findings are consistent with previous reports demonstrating that liposomes enhance skin permeation by facilitating the transport of substances through the skin barrier [92,93]. In this study, despite the high polarity and water solubility of ascorbic acid, the liposomal formulation still achieved approximately two-fold higher penetration compared to the non-encapsulated CAE. The improved penetration of CAE entrapped within liposomes through the Strat-M® membrane can be attributed to the structural similarity of liposomal bilayers to biological cell membranes and the small particle size of liposomes, which facilitates better permeation compared to free CAE, which is highly polar. Additionally, negatively charged liposomes have been shown to enhance skin permeability compared to neutral or positively charged vesicles. This effect is likely due to electrostatic interaction between the negatively charged liposomal surface and positively charged components of the stratum corneum, which may promote adhesion and facilitate penetration [94].
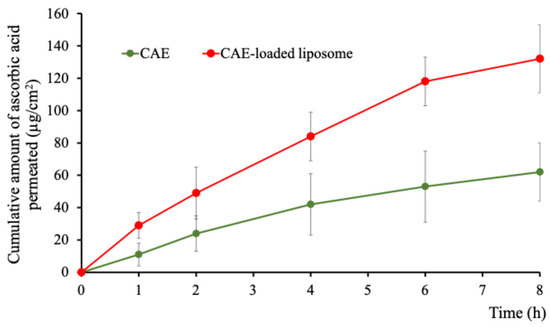
Figure 9.
Cumulative permeation of ascorbic acid per unit area (µg/cm2) of CAE-loaded liposomes compared with CAE. Data expressed as mean ± SD, n = 4.
3.12. Formulation Development and Physical Properties of CAE Solution and CAE-Loaded Liposome Solution
The physical properties of the CAE solution and the CAE-loaded liposome solution are shown in Table 5. The CAE solution exhibited a gel-like consistency with slight viscosity, pale yellow color, and a noticeable sweet scent derived from the CAE. In contrast, the CAE-loaded liposomes appeared as a light yellowish colloidal solution (Figure 10). The pH values of the CAE solution and the CAE-loaded liposome solution were 5.35 ± 0.01 and 5.69 ± 0.01, respectively. A pH range of 4.0–6.0 is generally considered suitable for facial applications [95]. Upon application, the CAE solution and CAE-loaded liposomes flowed smoothly, were easily absorbed, and provided a pleasant sensory feel. Rheological analysis (Figure 11A) illustrated the relationship between viscosity (η) and shear rate, showing that viscosity remained constant above the shear rate of 5 s−1. At the shear rate of 20 s−1, the viscosity of the CAE solution was 102 ± 4 cP (mPa⋅s). The CAE-loaded liposome solution exhibited slightly higher viscosity (140 ± 3 cP). Flow behavior analysis (Figure 11B) revealed that both formulations displayed Newtonian characteristics, as evidenced by the linear relationship between shear stress and shear rate. Despite the products containing poloxamer 407 at a concentration up to 15%, the formulations demonstrated Newtonian behavior, which can be attributed to their relatively low polymer content and low viscosity.

Table 5.
Physical properties of CAE solution and CAE-loaded liposome solution.

Figure 10.
Appearance of CAE solution (top row) and CAE-loaded liposome solution (bottom row). Panels (A,C) show the formulation after preparation and bottling. Panels (B,D) illustrate the application of the respective solutions spread on the forearm skin for demonstration purposes.
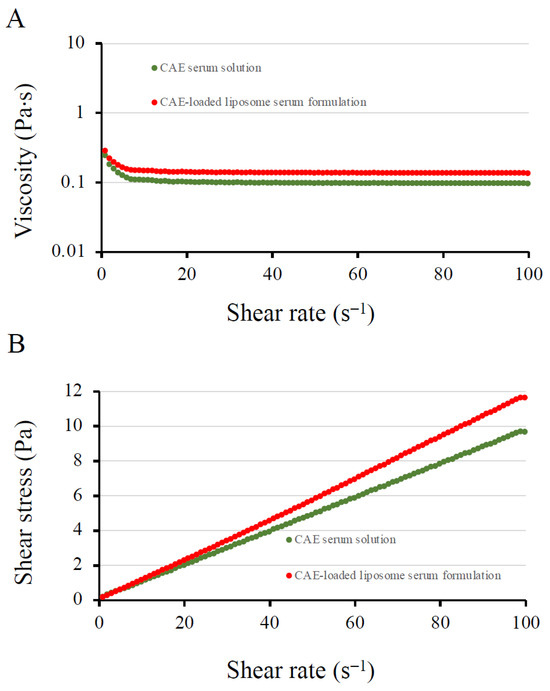
Figure 11.
Rheological behavior of CAE solution and CAE-loaded liposome solution. (A) Viscosity profile as a function of shear rate, showing that viscosity remained constant with an increasing shear rate. (B) Flow curve illustrating Newtonian flow behavior for both formulations.
Several studies have reported the potential of cashew apple juice or extract application as providing cosmetic benefits, such as antioxidant and anti-aging activities [72], controlled sebum secretion [96], whitening efficacy [72], and antimicrobial activity [6,20]. Therefore, the findings of the present study confirm and support the effectiveness of CAE as a functional ingredient in cosmetic applications.
3.13. Stability of CAE and the Formulations
The stability results of CAE and the formulations are summarized in Table 6. CAE stored at 2–8 °C for 3 months showed a minor decrease in ascorbic acid content, approximately 7% from its initial value. In contrast, storage at 30 °C/75% RH resulted in a substantial reduction of 31%, highlighting the significant influence of elevated temperature on ascorbic acid degradation in cashew apples [97]. In addition, other biologically active compounds in CAE, such as TPC and total tannin content (TTC), may also undergo degradation during storage under high temperature and humidity, although these were not monitored in this study [97]. The appearance of the CAE remained unchanged, and the pH of CAE remained stable under refrigerated conditions but showed a slight increase after storage at 30 °C and 75% RH, likely due to the oxidative degradation of ascorbic acid.

Table 6.
Stability of CAE, solution containing CAE, and CAE-loaded liposome solution at 30 °C with 75% relative humidity and refrigerator (2–8°) for 3 months. The data was mean ± S.D., n = 3.
Both the CAE solution and the CAE-loaded liposomal solution retained their physical characteristics under both storage conditions—refrigeration and 30 °C/75% RH—maintaining their pale yellow color, slight viscosity, and characteristic sweet aroma, consistent with their initial appearance. The pH values of the CAE solution and CAE-loaded liposome solution remained within the range of 5.33–5.34, closely aligned with the initial values under both storage conditions. In addition, the rheological properties of both formulations remained unchanged, as demonstrated by the viscosity values.
Encapsulation within liposomes effectively preserved ascorbic acid stability. After 3 months, the ascorbic acid concentration in the CAE-loaded liposome solution was diminished to a lesser extent than that in the CAE solution under both storage conditions. This confirms the role of liposomes in protecting bioactive compounds from environmental stress factors such as heat, light, and oxygen. The incorporation of cholesterol into the liposomal bilayer likely contributed to enhanced membrane rigidity and reduced permeability, thereby minimizing drug leakage. However, the CAE solution exhibited acceptable stability only under refrigerated conditions. Storage at 30 °C/75% RH resulted in significant degradation, with ascorbic acid content declining by over 80%, reflecting the inherent instability observed in the extract itself. These findings indicate that liposomal encapsulation is a suitable strategy to ensure long-term stability of ascorbic acid in CAE, particularly under conditions that accelerate degradation.
4. Conclusions
This study successfully developed CAE, which contains ascorbic acid and other biologically active compounds, including phenolic and flavonoid compounds. The overall findings indicate that CAE possesses free radical scavenging activity, promotes fibroblast collagen synthesis, demonstrates a cytoprotective effect against oxidative stress, and supports wound healing activity. However, CAE exhibited low tyrosinase-inhibitory activity. CAE was confirmed to be safe for use, showing no cytotoxicity toward fibroblast and monocyte cells and no stimulation of immune responses. A prototype solution containing CAE-loaded liposomes was developed and evaluated for its physicochemical properties and stability. Incorporation of CAE into liposomes significantly enhances the stability of ascorbic acid and improves its skin permeation compared to non-encapsulated formulations. The final formulation demonstrated suitable characteristics for skincare applications, supporting its potential as a stable, anti-aging cosmetic product. A summary of the study’s key findings is presented in Figure 12. Future research should focus on long-term stability evaluations and clinical trials that can validate the efficacy and safety of CAE formulations as potential alternatives for anti-aging skincare products.

Figure 12.
The graphical abstract presents a schematic diagram of the current investigation. The bioactive components and bioactivity of CAE were assessed through testing. The encapsulation of CAE in liposomes improved the stability of ascorbic acid within the CAE and enhanced skin permeation. The overall results indicate that CAE possesses strong potential for cosmetic applications in anti-aging products.
Author Contributions
Conceptualization, N.C., A.A., P.M., T.N., T.S. and S.S.; methodology, N.C., A.A., P.M., P.S., T.N., T.S., R.S., W.C., N.B., N.N., M.L. and S.S.; validation, N.C., A.A., P.M., P.S. and S.S.; formal analysis, N.C., A.A., P.M., P.S., T.N., T.S., R.S., W.C., N.B., N.N., M.L. and S.S.; investigation, N.C., A.A., P.M., P.S., T.N., T.S., R.S., W.C., N.B., N.N., M.L. and S.S.; resources, T.S. and S.S.; writing—original draft preparation, N.C., A.A., P.M., P.S., R.S., N.N., M.L. and S.S.; writing—review and editing, N.C., A.A., P.M., P.S., T.S., R.S., W.C., N.B. and S.S.; visualization, N.C., A.A., P.M., P.S., T.N., R.S., N.N., M.L. and S.S.; supervision, S.S.; project administration, S.S.; funding acquisition, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The research methods of this paper do not involve human or animal experiments. The methods only included in vitro cell culture experiments. The study was conducted and approved by the Institutional Review Board of Walailak University Institutional Biosafety Committee (WU-IBC), under approval number WU-IBC-68-019, approved date 31 March 2025.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this study are available within the article. Raw data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to thank the scientists in the Drug Delivery System Excellence Center, Faculty of Pharmaceutical Sciences, Prince of Songkla University for laboratory assistance. The authors would like to declare that ChatGPT (version 4) was used for purposes of improving the readability of the manuscript (AI-assisted translation and AI-assisted writing). In addition, the authors used QuillBot to paraphrase the content. After using these tools, the authors have reviewed and revised the content and take full responsibility for the entire publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ABTS | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| ATCC | American Type Culture Collection |
| CAE | Cashew apple extract |
| CGA | chlorogenic acid |
| CH | cholesterol |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DSPG | 1,2-Disteroyl-sn-glycero-3-phosphoglycerol |
| FALGPA | N-[3-(2-Furyl)acryloyl]-Leu-Gly-Pro-Ala |
| EGCG | epigallocatechin gallate |
| FBS | fetal bovine serum |
| GAE | gallic acid equivalent |
| HPLC | high performance liquid chromatography |
| IC50 | 50% of inhibitory concentration |
| IL-1β | interleukin-1 beta |
| LC-MS/IT-TOF | Liquid Chromatograph Mass Spectrometer Ion trap/Time-of-flight system |
| LPS | lipopolysaccharide |
| MTT | 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| PC | L-α-phosphatidylcholine |
| PDI | polydispersity index |
| TCQAC | total caffeoylquinic acid content |
| TNF-α | tumor necrosis factor—alpha |
| TPC | total phenolic content |
| RE | rutin equivalent |
| ROS | reactive oxygen species |
Appendix A

Table A1.
Validation results of HPLC analytical method of ascorbic acid.
Table A1.
Validation results of HPLC analytical method of ascorbic acid.
| Parameters | Results Obtained |
|---|---|
| Specificity | pass |
| Accuracy (%Recovery) | 99.2% ± 0.5% |
| Intra-day precision (%RSD) | 0.15% |
| Inter-day precision (%RSD) | 0.10% |
| Linearity (r2) | 0.9999 |
| Limit of detection (LOD) | 0.1 µg/mL |
| Limit of quantitative (LOQ) | 1.4 µg/mL |
References
- Dakuyo, R.; Konaté, K.; Bazié, D.; Sanou, A.; Kaboré, K.; Sama, H.; Santara, B.; Konkobo, F.A.; Dicko, M.H. Correlating the morphology of Anacardium occidentale L. fruits from 30 orchards with their physicochemical and nutritional properties. Front. Plant Sci. 2022, 13, 1033577. [Google Scholar] [CrossRef]
- Griffin, L.E.; Dean, L.L. Nutrient composition of raw, dry-roasted, and skin-on cashew nuts. J. Food Res. 2017, 6, 13–28. [Google Scholar] [CrossRef]
- Rico, R.; Bulló, M.; Salas-Salvadó, J. Nutritional composition of raw fresh cashew (Anacardium occidentale L.) kernels from different origin. Food Sci. Nutr. 2016, 4, 329–338. [Google Scholar] [CrossRef]
- Júnior, R.R.S.; Rodrigues, V.I.O.; Carvalho, C.F.M.; Moura, M.M.B.; Feitosa, D.D.M.; Lima, E.K.F.; Andrade, A.M.; Arrais, J.F.A.; Souza, L.N.; Knackfuss, M.I.; et al. Unveiling the impacts of cashew nuts on oxidative stress in rats: A systematic review. Antioxidants 2025, 14, 441. [Google Scholar] [CrossRef] [PubMed]
- Abreu, F.P.; Dornier, M.; Dionisio, A.P.; Carail, M.; Caris-Veyrat, C.; Dhuique-Mayer, C. Cashew apple (Anacardium occidentale L.) extract from by-product of juice processing: A focus on carotenoids. Food Chem. 2013, 138, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Bhagirathi, L.; Asna, U. Phytochemical profile and antimicrobial activity of cashew apple (Anacardium occidentale L.) extract. GCS Biol. Pharm. Sci. 2018, 5, 95–98. [Google Scholar] [CrossRef]
- Bhat, B.; Paliyath, G. Fruits of tropical climate: Biodiversity and dietary importance. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 138–143. [Google Scholar]
- Gutiérrez-Paz, C.; Rodríguez-Moreno, M.; Hernández-Gómez, M.; Fernández-Trujillo, J.P. The cashew pseudofruit (Anacardium occidentale): Composition, processing effects on bioactive compounds and potential benefits for human health. Foods 2024, 13, 2357. [Google Scholar] [CrossRef]
- Lopes, M.M.A.; Miranda, M.R.A.; Moura, C.F.H.; Filho, J.E. Bioactive compounds and total antioxidant capacity of cashew apples (Anacardium occidentale L.) during the ripening of early dwarf cashew clones. Ciênc. Agrotecnologia 2012, 36, 325–332. [Google Scholar] [CrossRef]
- Salehi, B.; Gültekin-Özgüven, M.; Kirkin, C.; Özçelik, B.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Bezerra, C.F.; Silva, T.G.; Coutinho, H.D.M.; Amina, B.; et al. Antioxidant, antimicrobial, and anticancer effects of Anacardium plants: An ethnopharmacological perspective. Front. Endocrinol. 2020, 11, 295. [Google Scholar] [CrossRef]
- Sousa, J.M.S.; Abreu, F.A.P.; Ruiz, A.L.T.; Silva, G.G.; Machado, S.L.; Garcia, C.P.G.; Filho, F.O.; Wurlitzer, N.J.; Figueiredo, E.A.T.; Magalhães, F.E.A.; et al. Cashew apple (Anacardium occidentale L.) extract from a by-product of juice processing: Assessment of its toxicity, antiproliferative and antimicrobial activities. J. Food Sci. Technol. 2021, 58, 764–776. [Google Scholar] [CrossRef] [PubMed]
- Dheeraj; Srivastana, A.; Mishra, A. Mitigation of cashew apple fruits astringency. Environ. Sustain. 2023, 6, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.S.; Gomes-Rochette, N.; Oliveira, M.L.M.; Nunes-Pinheiro, D.C.S.; Tomé, A.R.; Sousa, F.Y.M.; Pinheiro, F.G.M.; Moura, C.F.H.; Miranda, M.R.A.; Mota, E.F.; et al. Anti-inflammatory and wound healing potential of cashew apple juice (Anacardium occidentale L.) in mice. Exp. Biol. Med. 2015, 240, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Jiang, Z.; Lin, X.; Wei, X. Application of plant extracts cosmetics in the field of anti-aging. J. Dermatol. Sci. Cosmet. Technol. 2024, 1, 100014. [Google Scholar] [CrossRef]
- Dias, C.C.Q.; Madruga, M.S.; Pintado, M.M.E.; Almeida, G.H.O.; Alves, A.P.V.; Dantas, F.A.; Bezerra, J.K.G.; Melo, M.F.F.T.; Viera, V.B.; Soares, J.K.B. Cashew nuts (Anacardium occidentale L.) decrease visceral fat, yet augment glucose in dyslipidemic rats. PLoS ONE 2019, 14, e0225736. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.; Moon, J.; Lee, Y. Natural antioxidants from plant extracts in skincare cosmetics: Recent applications, challenges and perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- See, X.Z.; Yeo, W.S.; Saptoro, A. A comprehensive review and recent advances of vitamin C: Overview, function, sources, applications, market survey and processes. Chem. Eng. Res. Des. 2024, 206, 108–129. [Google Scholar] [CrossRef]
- Fenech, M.; Amaya, I.; Valpuesta, V.; Botella, M. Vitamin C content in fruits: Biosynthesis and regulation. Front. Plant Sci. 2019, 9, 2006. [Google Scholar] [CrossRef]
- Mah, E.; Schulz, J.A.; Kaden, V.N.; Lawless, A.L.; Rotor, J.; Mantilla, L.B.; Liska, D.J. Cashew consumption reduces total and LDL cholesterol: A randomized, crossover, controlled-feeding trial. Am. J. Clin. Nutr. 2017, 105, 1070–1078. [Google Scholar] [CrossRef]
- Gonçalves, G.M.S.; Gobbo, J. Antimicrobial effect of Anacardium occidentale extract and cosmetic formulation development. Braz. Arch. Biol. Technol. 2012, 55, 843–850. [Google Scholar] [CrossRef]
- Akyereko, Y.G.; Yeboah, G.B.; Wireko-Manu, F.; Alemawor, F.; Mills-Robertson, F.C.; Odoom, W. Nutritional value and health benefits of cashew apple. JSFA Rep. 2023, 3, 110–118. [Google Scholar] [CrossRef]
- Queiroz, C.; Lopes, M.L.M.; Fialho, E.; Valente-Mesquita, V.L. Changes in bioactive compounds and antioxidant capacity of fresh-cut cashew apple. Food Res. Int. 2011, 44, 1459–1462. [Google Scholar] [CrossRef]
- Budiarto, R.; Mubarok, S.; Sholikin, M.M.; Sari, D.N.; Khalisha, A.; Sari, S.L.; Rahmat, B.P.N.; Ujilestari, T.; Adli, D.N. Vitamin C variation in citrus in response to genotype, storage temperatures, and storage times: A systematic review and meta-analysis. Heliyon 2024, 10, e29125. [Google Scholar] [CrossRef]
- Sapei, L.; Hwa, L. Study on the kinetics of vitamin C degradation in fresh strawberry juice. Procedia Chem. 2014, 9, 62–68. [Google Scholar] [CrossRef]
- Herbig, A.; Renard, M.G.C. Factors that impact the stability of vitamin C at intermediate temperatures in a food matrix. Food Chem. 2017, 220, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Feszterová, M.; Kowalska, M.; Mišiaková, M. Stability of vitamin C content in plant and vegetable juices under different storing conditions. Appl. Sci. 2023, 13, 10640. [Google Scholar] [CrossRef]
- Sheraz, M.A.; Khan, M.F.; Ahmed, S.; Kazi, S.H.; Ahmad, I. Stability and stabilization of ascorbic acid a review. Househ. Pers. Care Today 2015, 10, 22–25. [Google Scholar]
- Andrade, R.A.M.S.; Silva, D.C.; Souza, M.M.B.; Oliveira, R.L.; Maciel, M.I.S.; Porto, A.L.F.; Melo, E.A.; Andrade, L.L.; Arruda, L.; Porto, T.S. Microencapsulation of phenolic compounds from cashew apple (Anacardium occidentale L.) agro-food waste: Physicochemical characterization, antioxidant activity, biodisponibility and stability. Food Chem. Adv. 2023, 3, 100364. [Google Scholar] [CrossRef]
- Kirby, C.J.; Whittle, C.J.; Rigby, N.; Coxon, D.T.; Law, B.A. Stabilization of ascorbic acid by microencapsulation in liposomes. Int. J. Food Sci. Technol. 1991, 26, 437–449. [Google Scholar] [CrossRef]
- Liu, X.; Lin, X.; Wei, Y.; Fei, T.; Hu, X.; Wang, L. Enhancing the stability and bio-accessibility of carotenoids extracted from canistel (Lucuma nervosa) via liposomes encapsulation. LWT-Food Sci. Technol. 2024, 204, 116455. [Google Scholar] [CrossRef]
- Gibis, M.; Zeeb, B.; Weiss, J. Formation, characterization, and stability of encapsulated hibiscus extract in multilayered liposomes. Food Hydrocoll. 2014, 38, 28–39. [Google Scholar] [CrossRef]
- Pamunuwa, G.K.; Karunaratne, D.N. Liposomal delivery of plant bioactives enhances potency in food systems: A review. J. Food Qual. 2022, 2022, 5272592. [Google Scholar] [CrossRef]
- Jahanfar, S.; Gahavami, M.; Khosravi-Darani, K.; Jahadi, M.; Mozafari, M.R. Entrapment of rosemary extract by liposomes formulated by Mozafari method: Physicochemical characterization and optimization. Heliyon 2021, 7, e08632. [Google Scholar] [CrossRef]
- Jahanfar, S.; Gahavami, M.; Khosravi-Darani, K.; Jahadi, M. Liposomal green tea extract: Optimization and physicochemical characterization. J. Appl. Biotechnol. Rep. 2021, 8, 5–12. [Google Scholar] [CrossRef]
- Romano, E.; Palladino, R.; Cannavale, M.; Lamparelli, E.P.; Maglione, B. Enhanced stability of oral vitamin C delivery: A novel large-scale method for liposomes production and encapsulation through dynamic high-pressure microfluidization. Nanomaterials 2024, 14, 516. [Google Scholar] [CrossRef] [PubMed]
- Favarin, F.R.; Gündel, S.S.; Ledur, C.M.; Roggia, I.; Fagan, B.; Gündel, A.; Fogaça, A.O.; Ourique, A.F. Vitamin C as a shelf-life extender in liposomes. Braz. J. Pharm. Sci. 2022, 58, e20492. [Google Scholar] [CrossRef]
- Musielak, E.; Krajka-Kuzniak, V. Liposomes and ethosomes: Comparative potential in enhancing skin permeability for therapeutic and cosmetic applications. Cosmetics 2024, 11, 191. [Google Scholar] [CrossRef]
- Rizzolo, A.; Forni, E.; Polesello, A. HPLC assay of ascorbic acid in fresh and processed fruit and vegetables. Food Chem. 1984, 14, 189–199. [Google Scholar] [CrossRef]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 221. [Google Scholar] [CrossRef]
- Sinsuebpol, C.; Nakpheng, T.; Srichana, T.; Sawatdee, S.; Pipatrattanaseree, W.; Burapapadh, K.; Changsan, N. Assessing the anti-aging and wound healing capabilities of Etlingera elatior inflorescence extract: A comparison of three inflorescence color varieties. Molecules 2023, 28, 7370. [Google Scholar] [CrossRef]
- Chan, E.; Ling, S.; Tan, S.; Lim, K.; Khoo, M. Caffeoylquinic acids from leaves of Etlingera species (Zingiberaceae). LWT-Food Sci. Technol. 2009, 42, 1026–1030. [Google Scholar] [CrossRef]
- Whangsomnuek, N.; Mungmai, L.; Mengamphan, K.; Amornlerdpison, D. Bioactive compounds of aqueous extracts of flower and leaf of Etlingera elatior (Jack) R.M.Sm. for cosmetic application. Maejo Int. J. Sci. Technol. 2019, 13, 196–208. [Google Scholar]
- Sinsuebpol, C.; Burapapadh, K.; Chowjaroen, V.; Changsan, N. The radical scavenging activity of vanillin and its impact on the healing properties of wounds. J. Adv. Pharm. Technol. Res. 2023, 14, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Balekar, N.; Katkam, N.G.; Nakpheng, T.; Jehtae, K.; Srichana, T. Evaluation of the wound healing potential of Wedelia trilobata (L.) leaves. J. Ethnopharmacol. 2012, 141, 817–824. [Google Scholar] [CrossRef]
- Sudsai, T.; Wattanapiromsakul, C.; Nakpheng, T.; Tewtrakul, S. Evaluation of the wound healing property of Boesenbergia longiflora rhizomes. J. Ethnopharmacol. 2013, 150, 223–231. [Google Scholar] [CrossRef]
- Kesharwani, P.; Md, S.; Alhakamy, N.A.; Hosny, K.M.; Haque, A. QbD enabled azacitidine loaded liposomal nanoformulation and its in vitro evaluation. Polymers 2021, 13, 250. [Google Scholar] [CrossRef] [PubMed]
- Changsan, N.; Atiapirin, A.; Sakdiset, P.; Muenraya, P.; Balekar, N.; Srichana, T.; Sritharadol, R.; Phanapithakkun, S.; Sawatdee, S. BrSPR-20-P1 peptide isolated from Brevibacillus sp. developed into liposomal hydrogel as a potential topical antimicrobial agent. RSC Adv. 2024, 14, 27394. [Google Scholar] [CrossRef]
- International Council for Harmonisation. ICH Harmonised Tripartite Guideline: Stability Testing of New Drug Substances and Products Q1A(R2). 2003. Available online: https://www.ich.org (accessed on 12 February 2024).
- Zié, M.; Alabi, T.; Karamoko, G.; Blecker, C. Valorization of cashew apple bagasse in food application: Focus on the use and extraction of nutritional or bioactive compounds. Food Humanit. 2023, 1, 848–863. [Google Scholar] [CrossRef]
- Wanraven, N.; Stark, A.H. From food waste to functional component: Cashew apple pomace. Crit. Rev. Food Sci. Nutr. 2024, 64, 7101–7117. [Google Scholar] [CrossRef]
- Roy, M.N.; Sarkar, K.; Sinha, A. Physico-chemical studies of vitamin-C in aqueous 2-propanol: Manifestation of molecular interactions. J. Solut. Chem. 2014, 43, 2212–2223. [Google Scholar] [CrossRef]
- Njus, D.; Kelley, P.M.; Tu, Y.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef]
- Agwu, E.; Ezihe, C.; Kaigama, G. Chapter 4: Antioxidant roles/functions of ascorbic acid (vitamin C). In Ascorbic Acid—Biochemistry and Functions; Kükürt, A., Gelen, V., Eds.; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Cioffi, N.; Losito, I.; Terzano, R.; Zambonin, C.G. An electrospray ionization ion trap mass spectrometric (ESI-MS-MSn) study of dehydroascorbic acid hydrolysis at neutral pH. Analyst 2000, 25, 2244–2248. [Google Scholar] [CrossRef]
- Violet, P.-C.; Munyan, N.; Luecke, H.F.; Wang, Y.; Lloyd, J.; Patra, K.; Blakeslee, K.; Ebenuwa, I.C.; Levine, M. Dehydroascorbic acid quantification in human plasma: Simultaneous direct measurement of the ascorbic acid/dehydroascorbic acid couple by UPLC/MS-MS. Redox Biol. 2024, 78, 103425. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.; Friedrich, M.; Matta, V.M.; Moura, C.F.H.; Mark, F. Changes in phenolic composition, ascorbic acid and antioxidant capacity in cashew apple (Anacardium occidentale L.) during ripening. Fruits 2012, 67, 267–276. [Google Scholar] [CrossRef]
- Reina, L.J.C.; Durán-Aranguren, D.D.; Forero-Rojas, L.F.; Tarapuez-Viveros, L.F.; Durán-Sequeda, D.; Carazzone, C.; Sierra, R. Chemical composition and bioactive compounds of cashew (Anacardium occidentale) apple juice and bagasse from Colombian varieties. Heliyon 2022, 8, e09528. [Google Scholar] [CrossRef]
- Marc, A.; Ange, K.D.; Achille, T.F.; Georges, A.N. Phenolic profile of cashew apple juice (Anacardium occidentale L.) from Yamoussoukro and Korhogo (Côte d’Ivoire). J. Appl. Biosci. 2012, 49, 3331–3338. [Google Scholar]
- Lagnika, C.; Amoussa, A.M.O.; Sanni, A.; Lagnika, L. Effect of blanching and ultrasound on drying time, physicochemical and bioactive compounds of dried cashew apple. Am. J. Food Sci. Technol. 2019, 7, 227–233. [Google Scholar] [CrossRef]
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The potential health benefits of gallic acid: Therapeutic and food applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef]
- Figueroa-Valencia, F.; Rosalez-Martinez, P.; Santoyo-Tepole, F.; Ramos-Monroy, O.A.; García-Ochoa, F.; Hermández-Botello, M.T.; López-Cortez, M.S. Antioxidant properties of red and yellow varieties of cashew apple, nut and husk (Anacardium occidentale L.) harvested in Mexico. J. Antioxid. Act. 2019, 1, 19–32. [Google Scholar] [CrossRef]
- Mohsen-Nia, M.; Amiri, H.; Jazi, B. Dielectric constants of water, methanol, ethanol, butanol and acetone: Measurement and computational study. J. Solut. Chem. 2010, 39, 701–708. [Google Scholar] [CrossRef]
- Ndayishimiye, A.; Sengul, M.Y.; Sada, T.; Dursun, S.; Bang, S.H.; Grady, Z.A.; Tsuji, K.; Funahashi, S.; Duin, A.C.T.; Randall, C.A. Roadmap for densification in cold sintering: Chemical pathways. Open Ceram. 2020, 2, 100019. [Google Scholar] [CrossRef]
- Hassan, S.; Adam, F.; Baker, M.R.A.; Mudalip, S.K.A. Evaluation of solvents’ effect on solubility, intermolecular interaction energies and habit of ascorbic acid crystals. J. Saudi. Chem. Soc. 2019, 23, 239–248. [Google Scholar] [CrossRef]
- Zhuang, B.; Ramanauskaite, G.; Koa, Z.Y.; Wang, Z. Like dissolves like: A first-principles theory for predicting liquid miscibility and mixture dielectric constant. Sci. Adv. 2021, 7, eabe7275. [Google Scholar] [CrossRef]
- Jhansyrani, T.; Sujatha, D.; Bharathi, K.; Prasad, K.V.S.R.G. Ethanolic extract of cashew apple inhibits lipid metabolism and ameliorates obesity in atherogenic diet-induced obese rats. Asian Pac. J. Trop Biomed. 2019, 9, 405–414. [Google Scholar] [CrossRef]
- Braga, D.C.; Filho, E.G.A.; Ribeiro, P.R.V.; Araújo, Ì.M.S.; Brito, E.S.; Garruti, D.S. Multivariate correlation of the astringency sensory perception with the phenolic profiling of cashew apple genotypes. Food Biosci. 2021, 41, 100931. [Google Scholar] [CrossRef]
- Majía, J.J.; Sierra, L.J.; Ceballos, J.G.; Martínez, J.R.; Stashenko, E.E. Color, antioxidant capacity and flavonoid composition in Hibiscus rosa-sinensis cultivars. Molecules 2023, 28, 1779. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; Xia, F.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Pierzak, M.; Krecisz, B.; Suliga, E. Bioactive compounds for skin health: A review. Nutrients 2021, 12, 203. [Google Scholar] [CrossRef]
- Hussen, N.H.; Abdulla, S.K.; Ali, N.M.; Ahmed, V.A.; Hasan, A.H.; Qadir, E.E. Role of antioxidants in skin aging and the molecular mechanism of ROS: A comprehensive review. Asp. Mol. Med. 2025, 5, 100063. [Google Scholar] [CrossRef]
- Srisuksomwong, P.; Kaenhin, L.; Mungmai, L. Collagenase and tyrosinase inhibitory activities and stability of facial cream formulation containing cashew leaf extract. Cosmetics 2023, 10, 17. [Google Scholar] [CrossRef]
- Kubo, I.; Kinst-Horist, I.; Yokokawa, Y. Tyrosinase inhibitors from Anacardium occidentale fruits. J. Nat. Prod. 1994, 57, 545–551. [Google Scholar] [CrossRef]
- Tan, Y.P.; Chan, E.W.C. Antioxidant, antityrosinase and antibacterial properties of fresh and processed leaves of Anacardium occidentale and Piper betle. Food Biosci. 2014, 6, 17–23. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Z. ROS-scavenging materials for skin wound healing: Advancements and applications. Front. Bioeng. Biotechnol. 2023, 11, 1304835. [Google Scholar] [CrossRef] [PubMed]
- Tsala, D.E.; Amadou, D.; Habtemariam, S. Natural wound healing and bioactive natural products. Phytopharmacology 2013, 4, 532–560. [Google Scholar]
- Boyera, N.; Galey, I.; Bernard, B.A. Effect of vitamin C and its derivatives on collagen synthesis and cross-linking by normal human fibroblasts. Int. J. Cosmet. Sci. 1998, 20, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Stipcevic, T.; Piljac, J.; Berghe, D.V. Effect of different flavonoids on collagen synthesis in human fibroblasts. Plant Foods Hum. Nutr. 2006, 61, 29–34. [Google Scholar] [CrossRef]
- Carvalho, M.T.B.; Araújo-Filho, H.; Barreto, A.; Quintans-Júnior, L.J.; Quintans, J.S.; Barreto, R.S. Wound healing properties of flavonoids: A systematic review highlighting the mechanisms of action. Phytomedicine 2021, 90, 153636. [Google Scholar] [CrossRef]
- Mssillou, I.; Bakour, M.; Slighoua, M.; Laaroussi, H.; Saghrouchni, H.; Amrati, F.E.; Lyoussi, B.; Derwich, E. Investigation on wound healing effect of Mediterranean medicinal plants and some related phenolic compounds: A review. J. Ethnopharmacol. 2022, 298, 115663. [Google Scholar] [CrossRef]
- Popovici, V.; Boldianu, A.B.; Pintea, A.; Caraus, V.; Ghendov-Mosanu, A.; Subotin, I.; Druta, R.; Sturza, R. In vitro antioxidant activity of liposomal formulations of sea buckthorn and grape pomace. Foods 2024, 13, 2478. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Inafuku, K.; Miyagi, T.; Oku, H.; Wada, K.; Imura, T.; Kitamoto, D. Efficient preparation of liposomes encapsulating food materials using lecithins by a mechanochemical method. J. Oleo Sci. 2007, 56, 35–42. [Google Scholar] [CrossRef]
- Khosravi-Darani, K.; Khoosfi, M.E.; Hosseini, H. Encapsulation of Zataria multiflora Boiss. essential oil in liposome: Antibacterial activity against E. Coli O157:H7 in broth media and minced beef. J. Food Saf. 2016, 36, 515–523. [Google Scholar] [CrossRef]
- Eloy, J.O.; Souza, M.C.; Petrilli, R.; Barcellos, J.P.A.; Lee, R.J.; Marchetti, J.M. Liposomes as carriers of hydrophilic small molecule drugs: Strategies to enhance encapsulation and delivery. Colloids Surf. B Biointerfaces 2014, 123, 345–363. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Bawab, A.A.; Alshaer, W. Liposome: Structure, composition, type, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Kiselev, M.A. Methods of liposomes preparation: Formation and control factors of versatile nanocarriers for biomedical and nanomedicine application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Wang, D.; Mei, H.C.; Ren, Y.; Busscher, H.J.; Shi, L. Lipid-based antimicrobial delivery-systems for the Treatment of bacterial infections. Front. Chem. 2020, 7, 872. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Németh, Z.; Csóka, I.; Jazani, R.S.; Sipos, B.; Haspel, H.; Kozma, G.; Kónya, Z.; Dobó, D.G. Quality by design-driven zeta potential optimisation study of liposomes with charge imparting membrane additives. Pharmaceutics 2022, 14, 1798. [Google Scholar] [CrossRef]
- Gillet, A.; Compère, P.; Lecomte, F.; Hubert, P.; Ducat, E.; Evrard, B.; Piel, G. Liposome surface charge influence on skin penetration behaviour. Int. J. Pharm. 2011, 411, 223–231. [Google Scholar] [CrossRef]
- Pavlovic, N.; Mijalković, J.; Balanč, B.; Luković, N.; Knežević-Jugović, Z. Role of cholesterol in modifying the physical and stability properties of liposomes and in vitro release of Vitamin B12. Eng. Proc. 2025, 99, 10. [Google Scholar] [CrossRef]
- Amnuaikit, T.; Rajagopal, R.S.; Nilsuwan, K.; Benjakul, S. Enhancement of physical appearance, skin permeation, and odor reduction using liposome of hydrolyzed salmon collagen for cosmetic products. Scientifica 2024, 2024, 7843660. [Google Scholar] [CrossRef]
- Liu, X.; Falconer, R.A. Liposomal nanocarriers to enhance skin delivery of chemotherapeutics in cancer therapy. Bioengineering 2025, 12, 133. [Google Scholar] [CrossRef]
- Ogiso, T.; Yamaguchi, T.; Iwaki, M.; Tanino, T.; Miyake, Y. Effect of positively and negatively charged liposomes on skin permeation of drugs. J. Drug Target. 2001, 9, 49–59. [Google Scholar] [CrossRef]
- Luki’c, M.; Panteli’c, I.; Savi’c, S. Towards optimal pH of the skin and topical formulations: From the current state of the art to tailored products. Cosmetics 2021, 8, 69. [Google Scholar] [CrossRef]
- Mercurio, D.G.; Wagemaker, T.A.L.; Campos, P.M.B.G. Anacardium occidentale L. extract in cosmetic formulations: Benefits for oily skin. Biomed. Biopharm. Res. 2017, 1, 75–87. [Google Scholar] [CrossRef]
- Vu, N.D.; Tran, T.Y.N.; Nguyen, T.X.T.; Nguyen, T.T.N.H.; Pham, T.T.H.; Long, H.B.; Pham, B.A. Physicochemical and biological properties of cashew apples in the form of juice, concentrated juice, and fresh under different storage conditions. J. Food Qual. 2025, 2025, 4458814. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).