Glutathione Trisulphide Improves Skin Brightness with Anti-Melanogenesis Effects and Maintains Intracellular Persulphide Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
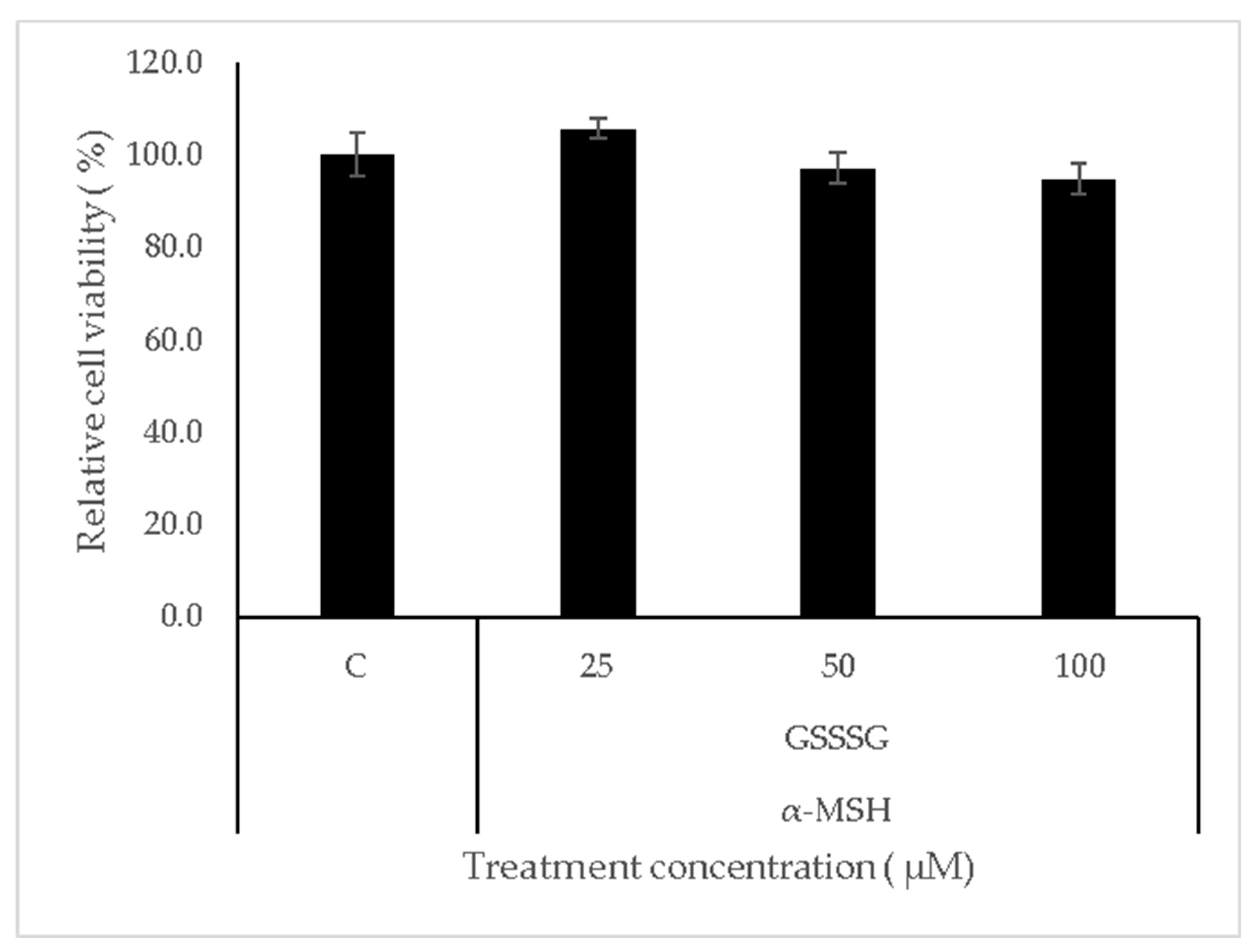
2.2. Cell Viability Assay
2.3. Determination of Melanin Content and Cellular Tyrosinase Activity
2.4. RNA Extraction, cDNA Synthesis, and Quantitative Real-Time PCR (RT-qPCR)
2.5. Detection of Intracellular Persulphide Levels
2.6. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Antioxidant Assay
2.7. GSSSG Preparation
3. Results
3.1. Cell Viability
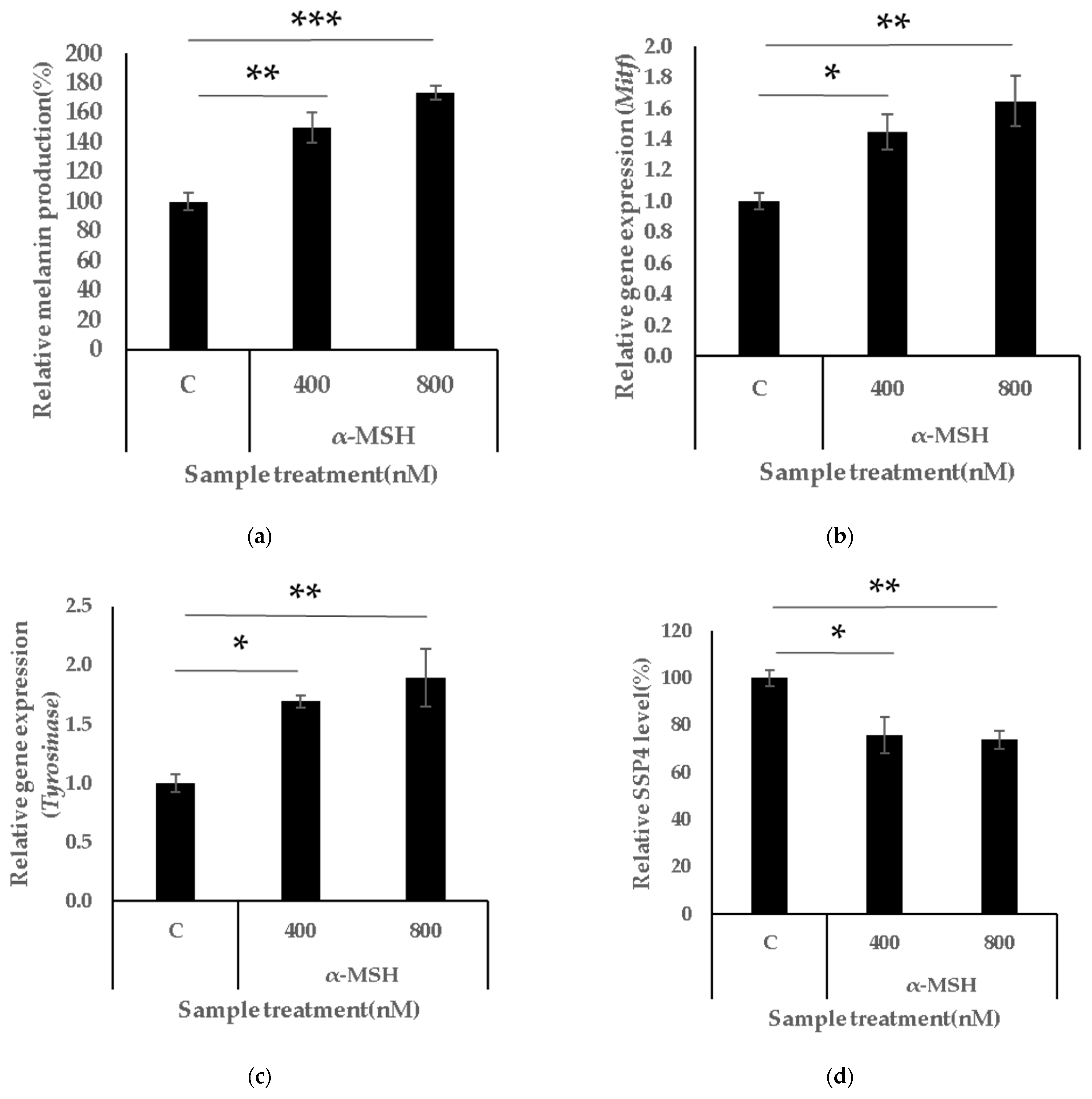
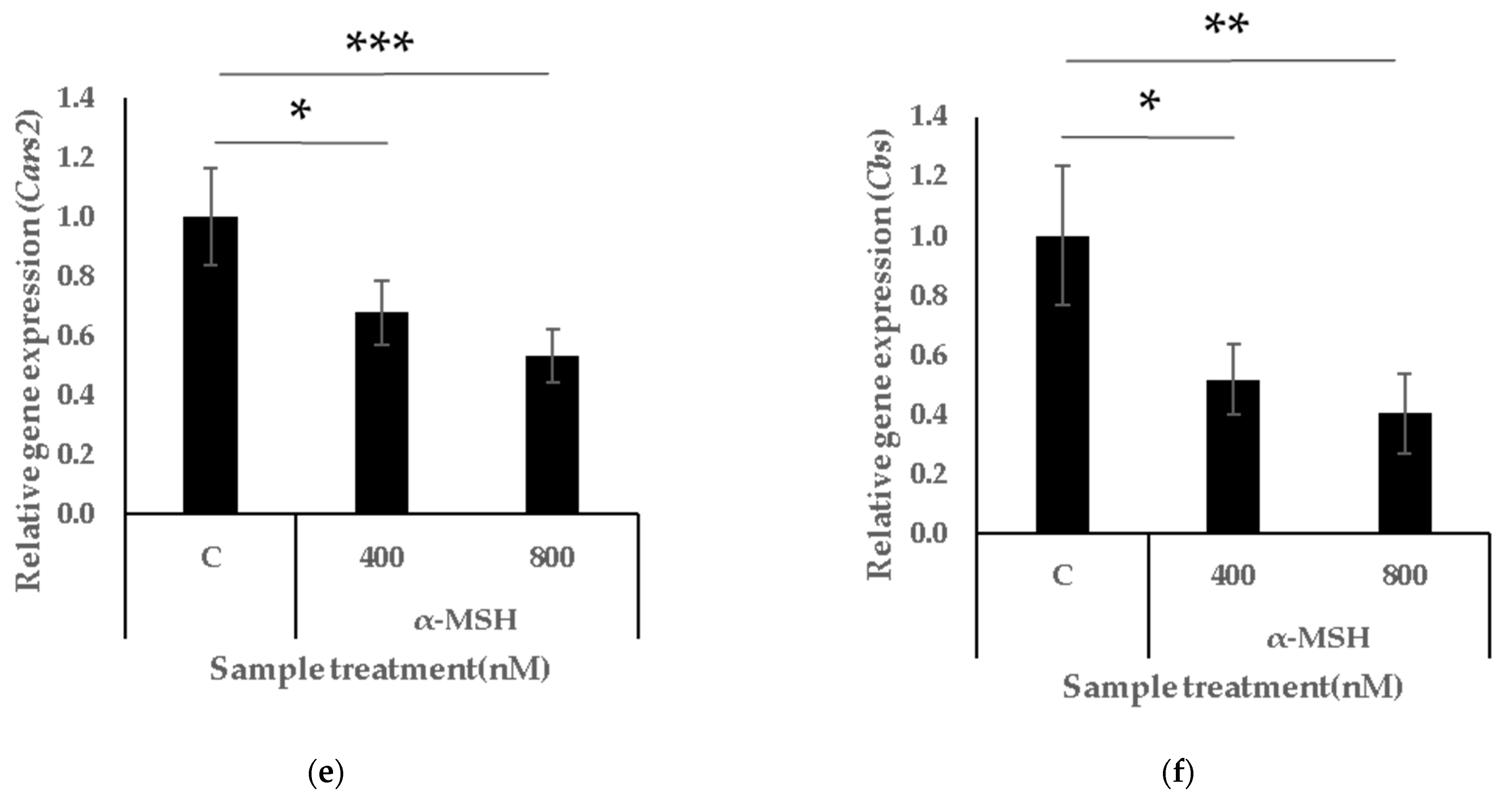
3.2. α-MSH Activates Melanogenesis and Suppresses Intracellular Persulphide Levels and Synthesis-Related Genes
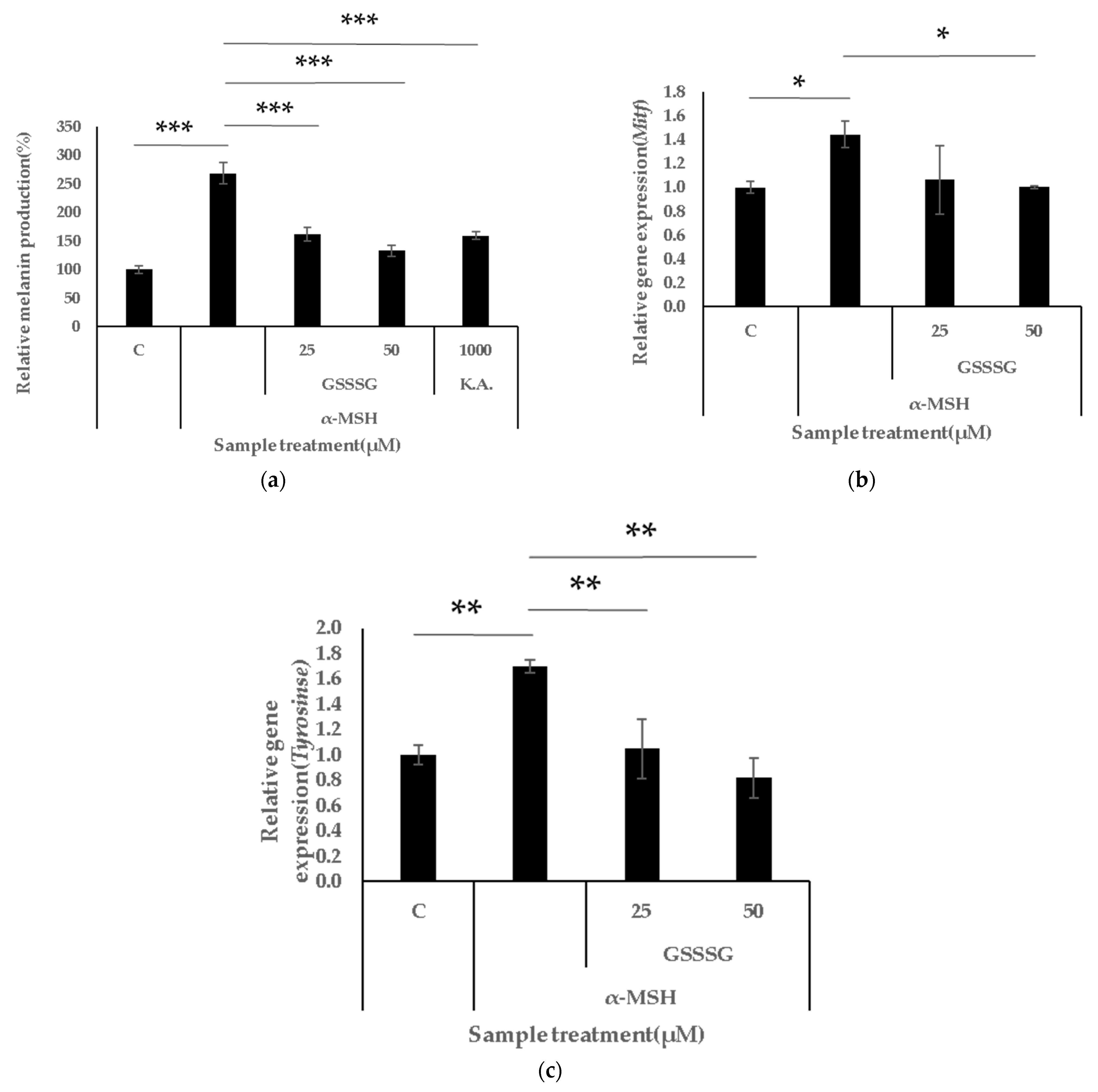
3.3. GSSSG Inhibits α-MSH-Induced Melanogenesis
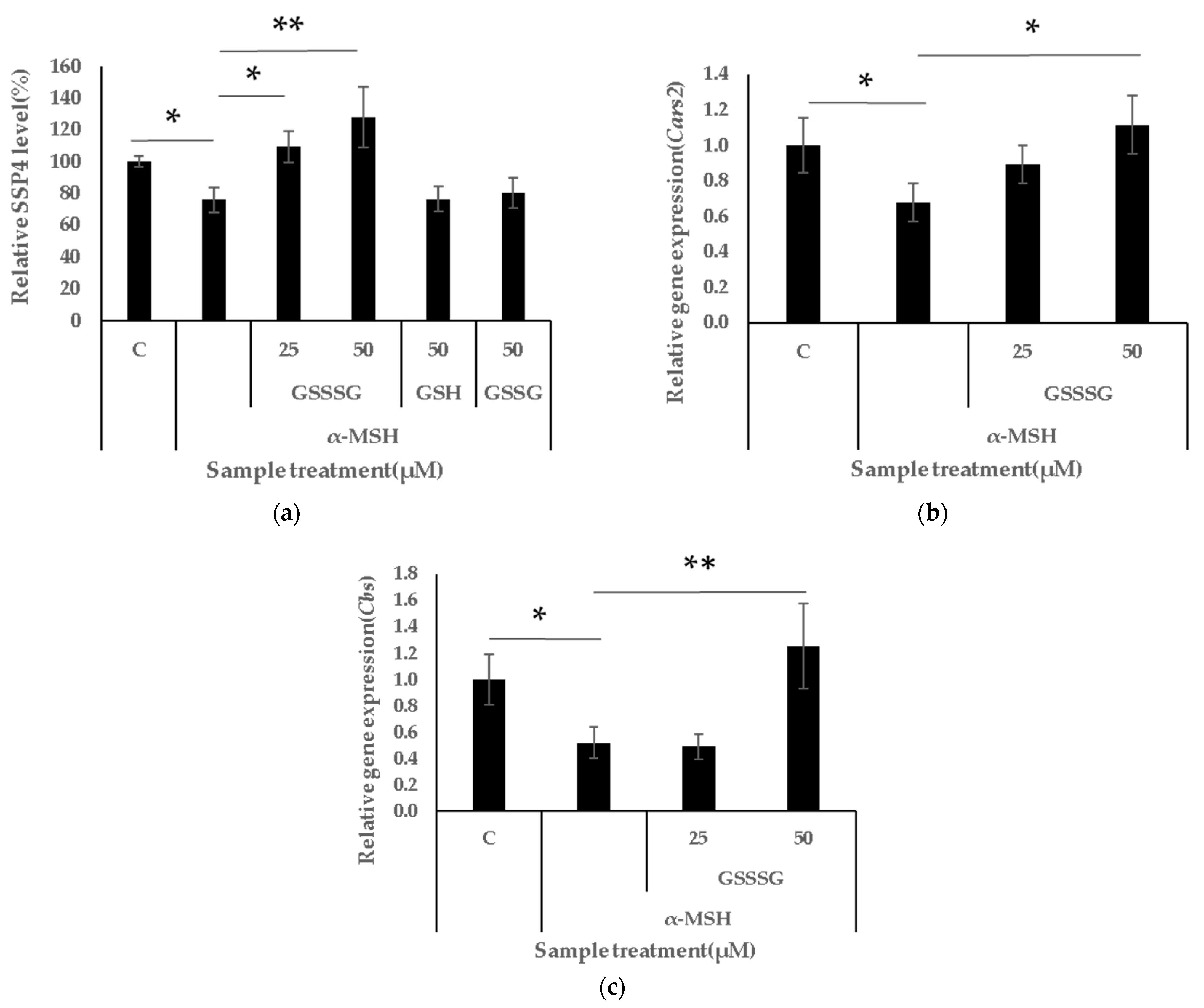
3.4. GSSSG Restores Intracellular Persulphide Levels
3.5. GSSSG Inactivates Intracellular Tyrosinase Activity and Exhibits Antioxidant Effects
3.6. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CARS2 | cysteinyl-tRNA synthetase 2 |
| Cars2 | cysteinyl-tRNA synthetase 2 gene |
| CBS | cystathionine β-synthase |
| Cbs | cystathionine β-synthase gene |
| GSH | glutathione |
| GSSH | glutathione disulphide |
| MITF | microphthalmia-associated transcription factor |
| MITF | microphthalmia-associated transcription factor gene |
| ROS | reactive oxygen species |
| SD | standard deviation |
| TYR | tyrosinase gene |
| α-MSH | alpha-melanocyte-stimulating hormone |
References
- Gromkowska-Kępka, K.J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. The impact of ultraviolet radiation on skin photoaging—review of in vitro studies. J. Cosmet. Dermatol. 2021, 20, 3427–3431. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, A.; Wang, J.; Huang, D.; Deng, Y.; Zhang, X.; Qu, Q.; Ma, W.; Xiong, R.; Zhu, M.; et al. Potential application of natural bioactive compounds as skin-whitening agents: A review. J. Cosmet. Dermatol. 2022, 21, 6669–6687. [Google Scholar] [CrossRef]
- Ferreira, S.M.; Gomes, S.M.; Santos, L. A novel approach in skin care: By-product extracts as natural UV filters and an alternative to synthetic ones. Molecules 2023, 28, 2037. [Google Scholar] [CrossRef]
- Merecz-Sadowska, A.; Sitarek, P.; Kowalczyk, T.; Zajdel, K.; Kucharska, E.; Zajdel, R. The modulation of melanogenesis in B16 cells upon treatment with plant extracts and isolated plant compounds. Molecules 2022, 27, 4360. [Google Scholar] [CrossRef]
- Uchida, Y.; Kaneda, T.; Ono, M.; Matsuoka, M.; Nakamura, U.; Ishida, A.; Yamasaki, Y.; Takeo, H.; Sakurai, T. The effect of cysteine peptide ingestion on skin brightness, a randomized, double-blind, placebo-controlled, parallel-group human clinical trial. Cosmetics 2023, 10, 72. [Google Scholar] [CrossRef]
- Barayeu, U.; Sawa, T.; Nishida, M.; Wei, F.-Y.; Motohashi, H.; Akaike, T. Supersulfide biology and translational medicine for disease control. Br. J. Pharmacol. 2023; early view. [Google Scholar] [CrossRef]
- Akaike, T.; Morita, M.; Ogata, S.; Yoshitake, J.; Jung, M.; Sekine, H.; Motohashi, H.; Barayeu, U.; Matsunaga, T. New aspects of redox signaling mediated by supersulfides in health and disease. Free Radic. Biol. Med. 2024, 222, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.M.; Kishino, A.; Sung, E.; Sekine, H.; Abe, T.; Murakami, S.; Akaike, T.; Motohashi, H. Contribution of NRF2 to sulfur metabolism and mitochondrial activity. Redox Biol. 2023, 60, 102624. [Google Scholar] [CrossRef] [PubMed]
- Maemura, M.; Morita, M.; Ogata, S.; Miyamoto, Y.; Ida, T.; Saito, T.; Yoshitake, J.; Matsunaga, T.; Akaike, T.; Yano, F. Supersulfides contribute to joint homeostasis and bone regeneration. Redox Biol. 2025, 81, 103545. [Google Scholar] [CrossRef]
- Sasama, Y.; Yoshimura, K.; Hoshino, M.; Sasa, K.; Akaike, T.; Morita, M.; Miyamoto, Y. Supersulfides support bone growth by promoting chondrocyte proliferation in the growth plates. J. Oral Biosci. 2024, 66, 76–81. [Google Scholar] [CrossRef]
- Sasaki, Y.; Numakura, T.; Yamada, M.; Sugiura, H.; Matsunaga, T.; Ida, T.; Morita, M.; Suzuki, A.; Matsumoto, S.; Kawaguchi, M.; et al. Glutathione supersulphide regulates T-cell receptor signalling. bioRxiv 2024. bioRxiv: 2024.04.30.591985. [Google Scholar] [CrossRef]
- Ezaka, M.; Marutani, E.; Miyazaki, Y.; Kanemaru, E.; Selig, M.K.; Boerboom, S.L.; Ostrom, K.F.; Stemmer-Rachamimov, A.; Bloch, D.B.; Brenner, G.J.; et al. Oral administration of glutathione trisulfide increases reactive sulfur levels in dorsal root ganglion and ameliorates paclitaxel-induced peripheral neuropathy in mice. Antioxidants 2022, 11, 2122. [Google Scholar] [CrossRef]
- Ikeda, M.; Ishima, Y.; Kinoshita, R.; Chuang, V.T.G.; Tasaka, N.; Matsuo, N.; Watanabe, H.; Shimizu, T.; Ishida, T.; Otagiri, M.; et al. A novel S-sulfhydrated human serum albumin preparation suppresses melanin synthesis. Redox Biol. 2018, 14, 354–360. [Google Scholar] [CrossRef]
- Kienzler, C.; Contreras, M.; Treger, J.; Liau, M.; Owens, C.; Prins, M. Transcriptome analysis of novel B16 melanoma metastatic variants generated by serial intracarotid artery injection. Acta Neuropathol. Commun. 2025, 13, 10. [Google Scholar] [CrossRef]
- Zheng, S.; Deng, R.; Xie, S.; Huang, G.; Ou, Z.; Shen, Z. Typha pollen extract inhibit melanogenesis via α-MSH/MC1R signaling pathway in B16 and melasma mouse model. Arch. Dermatol. Res. 2025, 317, 321. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Ferdousi, F.; Takahashi, S.; Isoda, H. Comprehensive transcriptome profiling of antioxidant activities by glutathione in human HepG2 cells. Molecules 2024, 29, 1090. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Riadh, D.; Sakamoto, K. Grape extract promoted α-MSH-induced melanogenesis in B16F10 melanoma cells, which was inverse to resveratrol. Molecules 2021, 26, 5959. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.Y.; Jung, J.Y.; Yang, J.-K. Camellia japonica essential oil inhibits α -MSH-induced melanin production and tyrosinase activity in B16F10 melanoma cells. Evid. Based Complement. Alternat. Med. 2021, 2021, 6328767. [Google Scholar] [CrossRef]
- Solano, F. Photoprotection and skin pigmentation: Melanin-related molecules and some other new agents obtained from natural sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef]
- Snyman, M.; Walsdorf, R.E.; Wix, S.N.; Gill, J.G. The metabolism of melanin synthesis—From melanocytes to melanoma. Pigment. Cell Melanoma Res. 2024, 37, 438–452. [Google Scholar] [CrossRef]
- Netcharoensirisuk, P.; Umehara, K.; De-Eknamkul, W.; Chaotham, C. Cajanin suppresses melanin synthesis through modulating MITF in human melanin-producing cells. Molecules 2021, 26, 6040. [Google Scholar] [CrossRef]
- Chen, Q.; Tao, W.; Wang, J.; Li, J.; Zheng, M.; Liu, Y.; Fang, Z. Inhibitive mechanism of loquat flower isolate on tyrosinase activity and melanin synthesis in mouse melanoma B16 cells. Biomolecules 2024, 14, 895. [Google Scholar] [CrossRef]
- Nishimura, A.; Yoon, S.; Matsunaga, T.; Ida, T.; Jung, M.; Ogata, S.; Morita, M.; Yoshitake, J.; Unno, Y.; Barayeu, U.; et al. Longevity control by supersulfide-mediated mitochondrial respiration and regulation of protein quality. Redox Biol. 2024, 69, 103018. [Google Scholar] [CrossRef]
- Zhou, L.; Nishimura, A.; Umezawa, K.; Kato, Y.; Mi, X.; Ito, T.; Urano, Y.; Akaike, T.; Nishida, M. Supersulfide catabolism participates in maladaptive remodeling of cardiac cells. J. Pharmacol. Sci. 2024, 155, 121–130. [Google Scholar] [CrossRef]
- Matsunaga, T.; Sano, H.; Takita, K.; Morita, M.; Yamanaka, S.; Ichikawa, T.; Numakura, T.; Ida, T.; Jung, M.; Ogata, S.; et al. Supersulphides provide airway protection in viral and chronic lung diseases. Nat. Commun. 2023, 14, 4476. [Google Scholar] [CrossRef] [PubMed]
- Khodade, V.S.; Toscano, J.P. Reactive sulfur species in biology and medicine. Antioxidants 2023, 12, 1759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Pan, Y.; Sawa, T.; Akaike, T.; Matsunaga, T. Supersulfide donors and their therapeutic targets in inflammatory diseases. Front. Immunol. 2025, 16, 1581385. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Reiner, Ž.; Ruscica, M.; Tedeschi-Reiner, E.; Radbakhsh, S.; Ekta, M.B.; Sahebkar, A. Antioxidant effects of statins by modulating Nrf2 and Nrf2/HO-1 signaling in different diseases. J. Clin. Med. 2022, 11, 1313. [Google Scholar] [CrossRef]
- Iciek, M.; Bilska-Wilkosz, A.; Kozdrowicki, M.; Górny, M. Reactive sulfur species and their significance in health and disease. Biosci. Rep. 2022, 42, BSR20221006. [Google Scholar] [CrossRef]
- Switzer, C.H. How super is supersulfide?: Reconsidering persulfide reactivity in cellular biology. Redox Biol. 2023, 67, 102899. [Google Scholar] [CrossRef]
- Lee, S.-G.; Hwang, J.-W.; Kang, H. Antioxidant and skin-whitening efficacy of a novel decapeptide (DP, KGYSSYICDK) derived from fish by-products. Mar. Drugs 2024, 22, 374. [Google Scholar] [CrossRef]
- Pan, Y.; Matsunaga, T.; Zhang, T.; Akaike, T. The Therapeutic Potential of Supersulfides in Oxidative Stress-Related Diseases. Biomolecules 2025, 15, 172. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kumar, S.; Kim, S.H.; Seong, K.-Y.; Lee, H.; Kim, C.; Jung, Y.-S.; Yang, S.Y. Odorless glutathione microneedle patches for skin whitening. Pharmaceutics 2020, 12, 100. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchida, Y.; Sato, T. Glutathione Trisulphide Improves Skin Brightness with Anti-Melanogenesis Effects and Maintains Intracellular Persulphide Levels. Cosmetics 2025, 12, 234. https://doi.org/10.3390/cosmetics12050234
Uchida Y, Sato T. Glutathione Trisulphide Improves Skin Brightness with Anti-Melanogenesis Effects and Maintains Intracellular Persulphide Levels. Cosmetics. 2025; 12(5):234. https://doi.org/10.3390/cosmetics12050234
Chicago/Turabian StyleUchida, Yoshiaki, and Toshiya Sato. 2025. "Glutathione Trisulphide Improves Skin Brightness with Anti-Melanogenesis Effects and Maintains Intracellular Persulphide Levels" Cosmetics 12, no. 5: 234. https://doi.org/10.3390/cosmetics12050234
APA StyleUchida, Y., & Sato, T. (2025). Glutathione Trisulphide Improves Skin Brightness with Anti-Melanogenesis Effects and Maintains Intracellular Persulphide Levels. Cosmetics, 12(5), 234. https://doi.org/10.3390/cosmetics12050234





