Genetic Insights into Acne, Androgenetic Alopecia, and Alopecia Areata: Implications for Mechanisms and Precision Dermatology
Abstract
1. Why Use Genetics to Approach Dermatology?
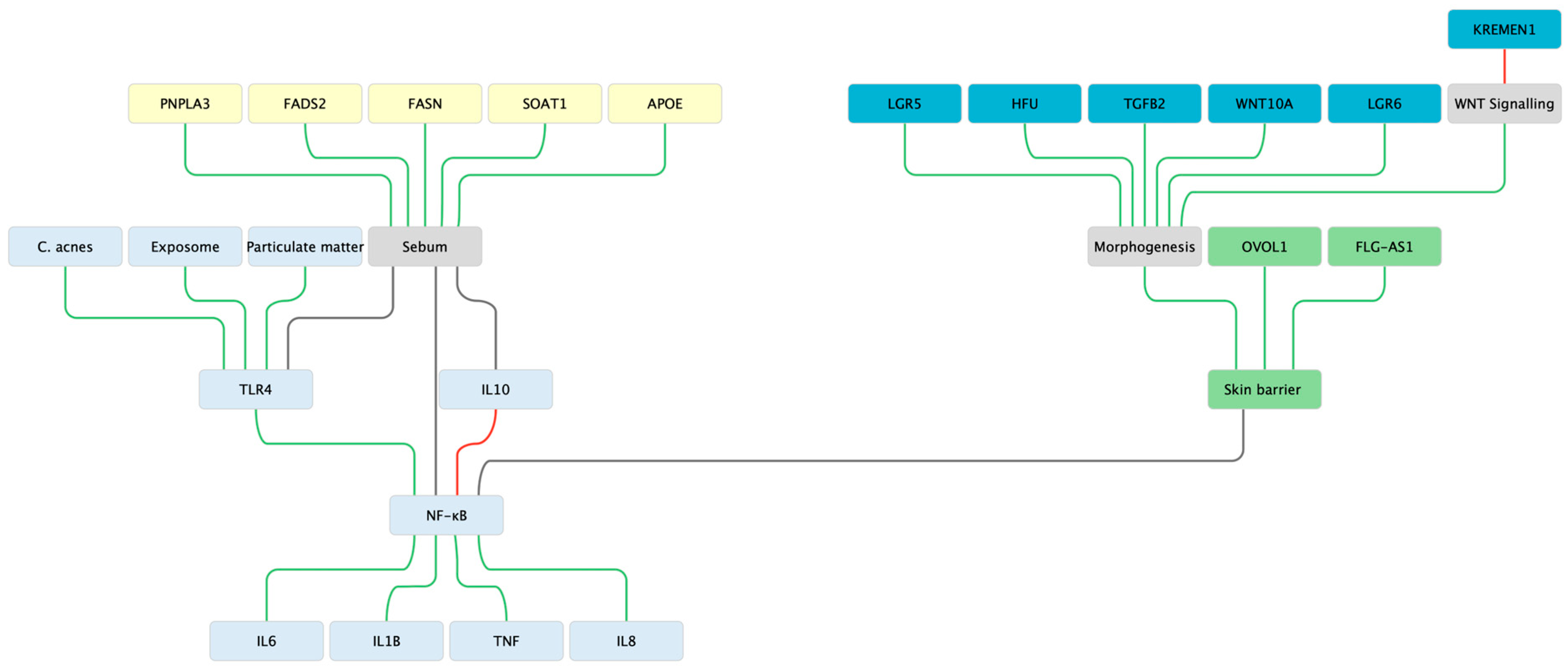
2. Acne Vulgaris—Immune—Morphogenesis—Lipid Triad
2.1. Innate Sensing & Inflammatory Tone
2.2. Morphogenesis & Stem-Cell
2.3. Lipid Biosynthesis & Sebocyte Biology
2.4. Insights from Large Studies
2.5. Barrier as a Modulator
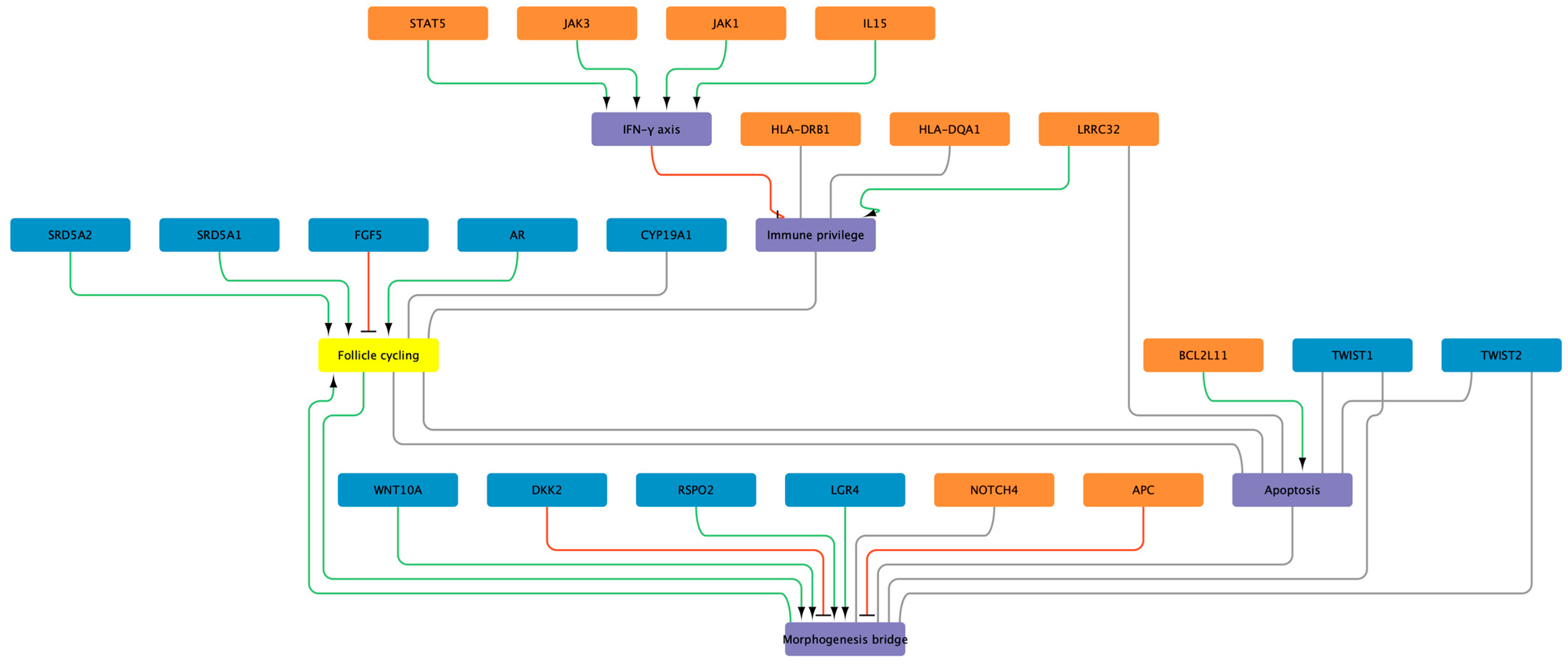
3. Alopecia Areata—Antigen Presentation and Immune Privilege Collapse
3.1. HLA Class II as the Primary Hub
3.2. Non-HLA Immune Regulators
3.3. East-Asian Cohorts and the Immune Theme
3.4. Immune-Privilege Loss: Integrating Genetics and Follicular Biology
3.5. Clinical Implications and Translational Outlook
4. Androgenetic Alopecia—AR–WNT Cross-Talk and Polygenic Prediction
4.1. Genetic Architecture
4.2. Translation Snapshot
5. Cross-Disease Synthesis: Where Networks Meet
6. Conclusions—from Genes to Precision Dermatology & Cosmetics
Funding
Data Availability Statement
Conflicts of Interest
References
- Yakupu, A.; Aimaier, R.; Yuan, B.; Chen, B.; Cheng, J.; Zhao, Y.; Peng, Y.; Dong, J.; Lu, S. The Burden of Skin and Subcutaneous Diseases: Findings from the Global Burden of Disease Study 2019. Front. Public Health 2023, 11, 1145513. [Google Scholar] [CrossRef]
- Szeto, M.D.; Alhanshali, L.; Rundle, C.W.; Adelman, M.; Hook Sobotka, M.; Woolhiser, E.; Wu, J.; Presley, C.L.; Maghfour, J.; Meisenheimer, J.; et al. Dermatologic Data From the Global Burden of Disease Study 2019 and the PatientsLikeMe Online Support Community: Comparative Analysis. JMIR Dermatol. 2024, 7, e50449. [Google Scholar] [CrossRef] [PubMed]
- Pirastu, N.; Joshi, P.K.; de Vries, P.S.; Cornelis, M.C.; McKeigue, P.M.; Keum, N.; Franceschini, N.; Colombo, M.; Giovannucci, E.L.; Spiliopoulou, A.; et al. GWAS for Male-Pattern Baldness Identifies 71 Susceptibility Loci Explaining 38% of the Risk. Nat. Commun. 2017, 8, 1584. [Google Scholar] [CrossRef] [PubMed]
- Prodi, D.A.; Pirastu, N.; Maninchedda, G.; Sassu, A.; Picciau, A.; Palmas, M.A.; Mossa, A.; Persico, I.; Adamo, M.; Angius, A.; et al. EDA2R Is Associated with Androgenetic Alopecia. J. Investig. Dermatol. 2008, 128, 2268–2270. [Google Scholar] [CrossRef]
- Betz, R.C.; Petukhova, L.; Ripke, S.; Huang, H.; Menelaou, A.; Redler, S.; Becker, T.; Heilmann, S.; Yamany, T.; Duvic, M.; et al. Genome-Wide Meta-Analysis in Alopecia Areata Resolves HLA Associations and Reveals Two New Susceptibility Loci. Nat. Commun. 2015, 6, 5966. [Google Scholar] [CrossRef]
- Yang, J.-S.; Liu, T.-Y.; Chen, Y.-C.; Tsai, S.-C.; Chiu, Y.-J.; Liao, C.-C.; Tsai, F.-J. Genome-Wide Association Study of Alopecia Areata in Taiwan: The Conflict Between Individuals and Hair Follicles. Clin. Cosmet. Investig. Dermatol. 2023, 16, 2597–2612. [Google Scholar] [CrossRef]
- Mitchell, B.L.; Saklatvala, J.R.; Dand, N.; Hagenbeek, F.A.; Li, X.; Min, J.L.; Thomas, L.; Bartels, M.; Jan Hottenga, J.; Lupton, M.K.; et al. Genome-Wide Association Meta-Analysis Identifies 29 New Acne Susceptibility Loci. Nat. Commun. 2022, 13, 702. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hysi, P.; Maj, C.; Heilmann-Heimbach, S.; Spector, T.D.; Liu, F.; Kayser, M. Genetic Prediction of Male Pattern Baldness Based on Large Independent Datasets. Eur. J. Human. Genet. 2023, 31, 321–328. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.E.; Lew, B.-L.; Huh, C.H.; Kim, J.; Kwon, O.; Kim, M.B.; Lee, Y.W.; Lee, Y.; Park, J.; et al. Efficacy and Safety of Low-Dose (0.2 Mg) Dutasteride for Male Androgenic Alopecia: A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Phase III Clinical Trial. Ann. Dermatol. 2025, 37, 183. [Google Scholar] [CrossRef]
- Estill, M.C.; Ford, A.; Omeira, R.; Rodman, M. Finasteride and Dutasteride for the Treatment of Male Androgenetic Alopecia: A Review of Efficacy and Reproductive Adverse Effects. Georget. Med. Rev. 2023, 7. [Google Scholar] [CrossRef]
- Choi, G.-S.; Sim, W.-Y.; Kang, H.; Huh, C.H.; Lee, Y.W.; Shantakumar, S.; Ho, Y.-F.; Oh, E.-J.; Duh, M.S.; Cheng, W.Y.; et al. Long-Term Effectiveness and Safety of Dutasteride versus Finasteride in Patients with Male Androgenic Alopecia in South Korea: A Multicentre Chart Review Study. Ann. Dermatol. 2022, 34, 349. [Google Scholar] [CrossRef]
- Egeberg, A.; Linsell, L.; Johansson, E.; Durand, F.; Yu, G.; Vañó-Galván, S. Treatments for Moderate-to-Severe Alopecia Areata: A Systematic Narrative Review. Dermatol. Ther. 2023, 13, 2951–2991. [Google Scholar] [CrossRef] [PubMed]
- Sardana, K.; Bathula, S.; Khurana, A. Which Is the Ideal JAK Inhibitor for Alopecia Areata—Baricitinib, Tofacitinib, Ritlecitinib or Ifidancitinib—Revisiting the Immunomechanisms of the JAK Pathway. Indian Dermatol. Online J. 2023, 14, 465. [Google Scholar] [CrossRef]
- Henne, S.K.; Aldisi, R.; Sivalingam, S.; Hochfeld, L.M.; Borisov, O.; Krawitz, P.M.; Maj, C.; Nöthen, M.M.; Heilmann-Heimbach, S. Analysis of 72,469 UK Biobank Exomes Links Rare Variants to Male-Pattern Hair Loss. Nat. Commun. 2023, 14, 5492. [Google Scholar] [CrossRef]
- Oliva, M.; Sarkar, M.K.; March, M.E.; Saeidian, A.H.; Mentch, F.D.; Hsieh, C.-L.; Tang, F.; Uppala, R.; Patrick, M.T.; Li, Q.; et al. Multi-Ancestry Genome-Wide Association Meta-Analysis Identifies Novel Loci in Atopic Dermatitis. medRxiv 2024. [Google Scholar] [CrossRef]
- Sroka-Tomaszewska, J.; Trzeciak, M. Molecular Mechanisms of Atopic Dermatitis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 4130. [Google Scholar] [CrossRef]
- Facheris, P.; Jeffery, J.; Del Duca, E.; Guttman-Yassky, E. The Translational Revolution in Atopic Dermatitis: The Paradigm Shift from Pathogenesis to Treatment. Cell Mol. Immunol. 2023, 20, 448–474. [Google Scholar] [CrossRef] [PubMed]
- Ramos, Y.Á.L.; Pietrobon, A.J.; Teixeira, F.M.E.; Aoki, V.; Sato, M.N.; Orfali, R.L. Inflammasome Pathways in Atopic Dermatitis: Insights into Inflammatory Mechanisms and Therapeutic Targets. An. Bras. Dermatol. 2025, 100, 501136. [Google Scholar] [CrossRef]
- Törőcsik, D.; Kovács, D.; Póliska, S.; Szentkereszty-Kovács, Z.; Lovászi, M.; Hegyi, K.; Szegedi, A.; Zouboulis, C.C.; Ståhle, M. Genome Wide Analysis of TLR1/2- and TLR4-Activated SZ95 Sebocytes Reveals a Complex Immune-Competence and Identifies Serum Amyloid A as a Marker for Activated Sebaceous Glands. PLoS ONE 2018, 13, e0198323. [Google Scholar] [CrossRef]
- Noh, H.H.; Shin, S.H.; Roh, Y.J.; Moon, N.J.; Seo, S.J.; Park, K.Y. Particulate Matter Increases Cutibacterium Acnes-Induced Inflammation in Human Epidermal Keratinocytes via the TLR4/NF-ΚB Pathway. PLoS ONE 2022, 17, e0268595. [Google Scholar] [CrossRef]
- Firlej, E.; Kowalska, W.; Szymaszek, K.; Roliński, J.; Bartosińska, J. The Role of Skin Immune System in Acne. J. Clin. Med. 2022, 11, 1579. [Google Scholar] [CrossRef]
- Selway, J.L.; Kurczab, T.; Kealey, T.; Langlands, K. Toll-like Receptor 2 Activation and Comedogenesis: Implications for the Pathogenesis of Acne. BMC Dermatol. 2013, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Cho, S.; Chung, J.H.; Hammerberg, C.; Fisher, G.J.; Voorhees, J.J. Inflammation and Extracellular Matrix Degradation Mediated by Activated Transcription Factors Nuclear Factor-ΚB and Activator Protein-1 in Inflammatory Acne Lesions in Vivo. Am. J. Pathol. 2005, 166, 1691–1699. [Google Scholar] [CrossRef]
- Navarini, A.A.; Simpson, M.A.; Weale, M.; Knight, J.; Carlavan, I.; Reiniche, P.; Burden, D.A.; Layton, A.; Bataille, V.; Allen, M.; et al. Genome-Wide Association Study Identifies Three Novel Susceptibility Loci for Severe Acne Vulgaris. Nat. Commun. 2014, 5, 4020. [Google Scholar] [CrossRef]
- Teder-Laving, M.; Kals, M.; Reigo, A.; Ehin, R.; Objärtel, T.; Vaht, M.; Nikopensius, T.; Metspalu, A.; Kingo, K. Genome-Wide Meta-Analysis Identifies Novel Loci Conferring Risk of Acne Vulgaris. Eur. J. Human. Genet. 2024, 32, 1136–1143. [Google Scholar] [CrossRef]
- Petridis, C.; Navarini, A.A.; Dand, N.; Saklatvala, J.; Baudry, D.; Duckworth, M.; Allen, M.H.; Curtis, C.J.; Lee, S.H.; Burden, A.D.; et al. Genome-Wide Meta-Analysis Implicates Mediators of Hair Follicle Development and Morphogenesis in Risk for Severe Acne. Nat. Commun. 2018, 9, 5075. [Google Scholar] [CrossRef]
- Esler, W.P.; Tesz, G.J.; Hellerstein, M.K.; Beysen, C.; Sivamani, R.; Turner, S.M.; Watkins, S.M.; Amor, P.A.; Carvajal-Gonzalez, S.; Geoly, F.J.; et al. Human Sebum Requires de novo Lipogenesis, Which Is Increased in Acne Vulgaris and Suppressed by Acetyl-CoA Carboxylase Inhibition. Sci. Transl. Med. 2019, 11, eaau8465. [Google Scholar] [CrossRef] [PubMed]
- Potter, C.S.; Kern, M.J.; Baybo, M.A.; Pruett, N.D.; Godwin, A.R.; Sundberg, J.P.; Awgulewitsch, A. Dysregulated Expression of Sterol O-Acyltransferase 1 (Soat1) in the Hair Shaft of Hoxc13 Null Mice. Exp. Mol. Pathol. 2015, 99, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Potter, C.S.; Silva, K.A.; Liang, Y.; Reinholdt, L.G.; Alley, L.M.; Rowe, L.B.; Roopenian, D.C.; Awgulewitsch, A.; Sundberg, J.P. Mutations in Sterol O-Acyltransferase 1 (Soat1) Result in Hair Interior Defects in AKR/J Mice. J. Investig. Dermatol. 2010, 130, 2666–2668. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Zhao, P.; Wang, X.; Wu, W.; Yang, J. The Causal Relationship between Serum Metabolites and Acne Vulgaris: A Mendelian Randomization Study. Sci. Rep. 2024, 14, 11045. [Google Scholar] [CrossRef]
- Ju, R.; Ying, Y.; Zhou, Q.; Cao, Y. Exploring Genetic Drug Targets in Acne Vulgaris: A Comprehensive Proteome—Wide Mendelian Randomization Study. J. Cosmet. Dermatol. 2024, 23, 4223–4229. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-Y.; Yamada, S.; Izumi, H.; Tsukamoto, M.; Nakashima, T.; Tasaki, T.; Guo, X.; Uramoto, H.; Sasaguri, Y.; Kohno, K. Critical in Vivo Roles of WNT10A in Wound Healing by Regulating Collagen Expression/Synthesis in WNT10A-Deficient Mice. PLoS ONE 2018, 13, e0195156. [Google Scholar] [CrossRef]
- Benard, E.L.; Hammerschmidt, M. The Fundamentals of WNT10A. Differentiation 2025, 142, 100838. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C. Endocrinology and Immunology of Acne: Two Sides of the Same Coin. Exp. Dermatol. 2020, 29, 840–859. [Google Scholar] [CrossRef] [PubMed]
- Zouboulis, C.C.; Jourdan, E.; Picardo, M. Acne Is an Inflammatory Disease and Alterations of Sebum Composition Initiate Acne Lesions. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 527–532. [Google Scholar] [CrossRef]
- Liu, M.; Diaz-Torres, S.; Mitchell, B.L.; Toledo-Flores, D.; Gharhakhani, P.; Simpson, M.A.; Zhang, H.; Ong, J.-S.; Li, J.; Rentería, M.E. The Role of Lipid Metabolism in Acne Risk: Integrating Blood Metabolite and Genetic Insights. Skin. Health Dis. 2025, 5, 124–129. [Google Scholar] [CrossRef]
- Li, L.; Hajam, I.; McGee, J.S.; Tang, Z.; Zhang, Y.; Badey, N.; Mintzer, E.; Zhang, Z.; Liu, G.Y.; Church, G.M.; et al. Comparative Transcriptome Analysis of Acne Vulgaris, Rosacea, and Hidradenitis Suppurativa Supports High—dose Dietary Zinc as a Therapeutic Agent. Exp. Dermatol. 2024, 33, e15145. [Google Scholar] [CrossRef]
- Furue, M.; Ulzii, D.; Nakahara, T.; Tsuji, G.; Furue, K.; Hashimoto-Hachiya, A.; Kido-Nakahara, M. Implications of IL-13Rα2 in Atopic Skin Inflammation. Allergol. Int. 2020, 69, 412–416. [Google Scholar] [CrossRef]
- Furue, M.; Chiba, T.; Tsuji, G.; Ulzii, D.; Kido-Nakahara, M.; Nakahara, T.; Kadono, T. Atopic Dermatitis: Immune Deviation, Barrier Dysfunction, IgE Autoreactivity and New Therapies. Allergol. Int. 2017, 66, 398–403. [Google Scholar] [CrossRef]
- Bolla, B.S.; Erdei, L.; Urbán, E.; Burián, K.; Kemény, L.; Szabó, K.C. C. acnes Regulates the Epidermal Barrier Properties of HPV-KER Human Immortalized Keratinocyte Cultures. Sci. Rep. 2020, 10, 12815. [Google Scholar] [CrossRef]
- Dębińska, A. New Treatments for Atopic Dermatitis Targeting Skin Barrier Repair via the Regulation of FLG Expression. J. Clin. Med. 2021, 10, 2506. [Google Scholar] [CrossRef]
- Hughes, A.J.; Barbosa, E.; Cernova, J.; Thomas, B.R.; O’Shaughnessy, R.F.L.; O’Toole, E.A. Loss-of-Function FLG Mutations Are Associated with Reduced History of Acne Vulgaris in a Cohort of Patients with Atopic Eczema of Bangladeshi Ancestry in East London. Clin. Exp. Dermatol. 2024, 49, 1547–1553. [Google Scholar] [CrossRef]
- Draelos, Z.D.; Matsubara, A.; Smiles, K. The Effect of 2% Niacinamide on Facial Sebum Production. J. Cosmet. Laser Ther. 2006, 8, 96–101. [Google Scholar] [CrossRef]
- Lee, A.-Y. Molecular Mechanism of Epidermal Barrier Dysfunction as Primary Abnormalities. Int. J. Mol. Sci. 2020, 21, 1194. [Google Scholar] [CrossRef]
- Madnani, N.; Deo, J.; Dalal, K.; Benjamin, B.; Murthy, V.V.; Hegde, R.; Shetty, T. Revitalizing the Skin: Exploring the Role of Barrier Repair Moisturizers. J. Cosmet. Dermatol. 2024, 23, 1533–1540. [Google Scholar] [CrossRef]
- Sandilands, A.; Sutherland, C.; Irvine, A.D.; McLean, W.H.I. Filaggrin in the Frontline: Role in Skin Barrier Function and Disease. J. Cell Sci. 2009, 122, 1285–1294. [Google Scholar] [CrossRef]
- Draelos, Z.D.; Baalbaki, N.; Colon, G.; Dreno, B. Ceramide-Containing Adjunctive Skin Care for Skin Barrier Restoration During Acne Vulgaris Treatment. J. Drugs Dermatol. 2023, 22, 554–558. [Google Scholar] [CrossRef]
- Sun, P.; Vu, R.; Dragan, M.; Haensel, D.; Gutierrez, G.; Nguyen, Q.; Greenberg, E.; Chen, Z.; Wu, J.; Atwood, S.; et al. OVOL1 Regulates Psoriasis-Like Skin Inflammation and Epidermal Hyperplasia. J. Investig. Dermatol. 2021, 141, 1542–1552. [Google Scholar] [CrossRef] [PubMed]
- Dragan, M.; Sun, P.; Chen, Z.; Ma, X.; Vu, R.; Shi, Y.; Villalta, S.A.; Dai, X. Epidermis-Intrinsic Transcription Factor Ovol1 Coordinately Regulates Barrier Maintenance and Neutrophil Accumulation in Psoriasis-Like Inflammation. J. Investig. Dermatol. 2022, 142, 583–593.e5. [Google Scholar] [CrossRef] [PubMed]
- Pratt, C.H.; King, L.E.; Messenger, A.G.; Christiano, A.M.; Sundberg, J.P. Alopecia Areata. Nat. Rev. Dis. Primers 2017, 3, 17011. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Zhang, T.; Miao, F.; Liu, J.; Zhu, Q.; Chen, Z.; Tai, Z.; He, Z. Alopecia Areata: Pathogenesis, Diagnosis, and Therapies. MedComm 2025, 6, e70182. [Google Scholar] [CrossRef]
- Petukhova, L.; Duvic, M.; Hordinsky, M.; Norris, D.; Price, V.; Shimomura, Y.; Kim, H.; Singh, P.; Lee, A.; Chen, W.V.; et al. Genome-Wide Association Study in Alopecia Areata Implicates Both Innate and Adaptive Immunity. Nature 2010, 466, 113–117. [Google Scholar] [CrossRef]
- Paus, R.; Bulfone-Paus, S.; Bertolini, M. Hair Follicle Immune Privilege Revisited: The Key to Alopecia Areata Management. J. Investig. Dermatol. Symp. Proc. 2018, 19, S12–S17. [Google Scholar] [CrossRef] [PubMed]
- Lensing, M.; Jabbari, A. An Overview of JAK/STAT Pathways and JAK Inhibition in Alopecia Areata. Front. Immunol. 2022, 13, 955035. [Google Scholar] [CrossRef]
- Passeron, T.; King, B.; Seneschal, J.; Steinhoff, M.; Jabbari, A.; Ohyama, M.; Tobin, D.J.; Randhawa, S.; Winkler, A.; Telliez, J.-B.; et al. Inhibition of T-Cell Activity in Alopecia Areata: Recent Developments and New Directions. Front. Immunol. 2023, 14, 1243556. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Liu, S.; Zhu, K.; Luo, H.; Li, Q.; Zhang, Y.; Huang, S.; Chen, Q.; Cao, Y. HLA-DRB1 Polymorphisms and Alopecia Areata Disease Risk. Medicine 2018, 97, e11790. [Google Scholar] [CrossRef] [PubMed]
- Oka, A.; Takagi, A.; Komiyama, E.; Yoshihara, N.; Mano, S.; Hosomichi, K.; Suzuki, S.; Haida, Y.; Motosugi, N.; Hatanaka, T.; et al. Alopecia Areata Susceptibility Variant in MHC Region Impacts Expressions of Genes Contributing to Hair Keratinization and Is Involved in Hair Loss. EBioMedicine 2020, 57, 102810. [Google Scholar] [CrossRef]
- Šutić Udović, I.; Hlača, N.; Massari, L.P.; Brajac, I.; Kaštelan, M.; Vičić, M. Deciphering the Complex Immunopathogenesis of Alopecia Areata. Int. J. Mol. Sci. 2024, 25, 5652. [Google Scholar] [CrossRef]
- AL-Eitan, L.N.; Alasmar, M.K.; Aljamal, H.A.; Mihyar, A.H.; Alghamdi, M.A. Investigating the Genetic Association of Selected Candidate Loci with Alopecia Areata Susceptibility in Jordanian Patients. Medicina 2025, 61, 409. [Google Scholar] [CrossRef]
- Nasrallah, R.; Imianowski, C.J.; Bossini-Castillo, L.; Grant, F.M.; Dogan, M.; Placek, L.; Kozhaya, L.; Kuo, P.; Sadiyah, F.; Whiteside, S.K.; et al. A Distal Enhancer at Risk Locus 11q13.5 Promotes Suppression of Colitis by Treg Cells. Nature 2020, 583, 447–452. [Google Scholar] [CrossRef]
- Wing, K.; Onishi, Y.; Prieto-Martin, P.; Yamaguchi, T.; Miyara, M.; Fehervari, Z.; Nomura, T.; Sakaguchi, S. CTLA-4 Control over Foxp3 + Regulatory T Cell Function. Science (1979) 2008, 322, 271–275. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT Signaling Pathway: From Bench to Clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Dai, Z.; Chen, J.; Chang, Y.; Christiano, A.M. Selective Inhibition of JAK3 Signaling Is Sufficient to Reverse Alopecia Areata. JCI Insight 2021, 6, e142205. [Google Scholar] [CrossRef]
- Hawkshaw, N.J.; Hardman, J.A.; Haslam, I.S.; Shahmalak, A.; Gilhar, A.; Lim, X.; Paus, R. Identifying Novel Strategies for Treating Human Hair Loss Disorders: Cyclosporine A Suppresses the Wnt Inhibitor, SFRP1, in the Dermal Papilla of Human Scalp Hair Follicles. PLoS Biol. 2018, 16, e2003705. [Google Scholar] [CrossRef]
- Suzuki, T.; Chéret, J.; Scala, F.D.; Rajabi-Estarabadi, A.; Akhundlu, A.; Demetrius, D.-L.; Gherardini, J.; Keren, A.; Harries, M.; Rodriguez-Feliz, J.; et al. Interleukin-15 Is a Hair Follicle Immune Privilege Guardian. J. Autoimmun. 2024, 145, 103217. [Google Scholar] [CrossRef]
- Ebrahim, A.; Salem, R.; El Fallah, A.; Younis, E. Serum Interleukin-15 Is a Marker of Alopecia Areata Severity. Int. J. Trichol 2019, 11, 26. [Google Scholar] [CrossRef]
- Sanchez, K.; Englander, H.; Salloum, L.; Gregoire, S.; Biba, U.; Ershadi, S.; Mostaghimi, A. Evaluating Current and Emergent JAK Inhibitors for Alopecia Areata: A Narrative Review. Dermatol. Ther. 2025, 15, 2749–2764. [Google Scholar] [CrossRef]
- Sadasivam, I.P.; Sambandam, R.; Kaliyaperumal, D.; Dileep, J.E. Androgenetic Alopecia in Men: An Update On Genetics. Indian J. Dermatol. 2024, 69, 282. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y. Targeting Wnt/β-Catenin Pathway for Developing Therapies for Hair Loss. Int. J. Mol. Sci. 2020, 21, 4915. [Google Scholar] [CrossRef] [PubMed]
- Francès, M.P.; Vila-Vecilla, L.; Russo, V.; Caetano Polonini, H.; de Souza, G.T. Utilising SNP Association Analysis as a Prospective Approach for Personalising Androgenetic Alopecia Treatment. Dermatol. Ther. 2024, 14, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.X.; Sidorenko, J.; Wu, Y.; Kemper, K.E.; Yang, J.; Wray, N.R.; Robinson, M.R.; Visscher, P.M. Dissection of Genetic Variation and Evidence for Pleiotropy in Male Pattern Baldness. Nat. Commun. 2018, 9, 5407. [Google Scholar] [CrossRef]
- Henne, S.K.; Nöthen, M.M.; Heilmann-Heimbach, S. Male-Pattern Hair Loss: Comprehensive Identification of the Associated Genes as a Basis for Understanding Pathophysiology. Med. Genet. 2023, 35, 3–14. [Google Scholar] [CrossRef]
- Lu, G.-Q.; Wu, Z.-B.; Chu, X.-Y.; Bi, Z.-G.; Fan, W.-X. An Investigation of Crosstalk between Wnt/β-Catenin and Transforming Growth Factor-β Signaling in Androgenetic Alopecia. Medicine 2016, 95, e4297. [Google Scholar] [CrossRef]
- Yip, L.; Zaloumis, S.; Irwin, D.; Severi, G.; Hopper, J.; Giles, G.; Harrap, S.; Sinclair, R.; Ellis, J. Gene-Wide Association Study between the Aromatase Gene (CYP19A1) and Female Pattern Hair Loss. Br. J. Dermatol. 2009, 161, 289–294. [Google Scholar] [CrossRef]
- Hagenaars, S.P.; Hill, W.D.; Harris, S.E.; Ritchie, S.J.; Davies, G.; Liewald, D.C.; Gale, C.R.; Porteous, D.J.; Deary, I.J.; Marioni, R.E. Genetic Prediction of Male Pattern Baldness. PLoS Genet. 2017, 13, e1006594. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, S.; Brockschmidt, F.F.; Hillmer, A.M.; Hanneken, S.; Eigelshoven, S.; Ludwig, K.U.; Herold, C.; Mangold, E.; Becker, T.; Kruse, R.; et al. Evidence for a Polygenic Contribution to Androgenetic Alopecia. Br. J. Dermatol. 2013, 169, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Motavaf, M.; Raza, D.; McLure, A.J.; Osei-Opare, K.D.; Bordone, L.A.; Gru, A.A. Revolutionary Approaches to Hair Regrowth: Follicle Neogenesis, Wnt/ß-Catenin Signaling, and Emerging Therapies. Cells 2025, 14, 779. [Google Scholar] [CrossRef]
- Jin, H.; Zou, Z.; Chang, H.; Shen, Q.; Liu, L.; Xing, D. Photobiomodulation Therapy for Hair Regeneration: A Synergetic Activation of β-CATENIN in Hair Follicle Stem Cells by ROS and Paracrine WNTs. Stem Cell Rep. 2021, 16, 1568–1583. [Google Scholar] [CrossRef]
- Yan, W.; Liu, J.; Xie, X.; Jin, Q.; Yang, Y.; Pan, Y.; Zhang, Y.; Zhang, F.; Wang, Y.; Liu, J.; et al. Restoration of Follicular β-Catenin Signaling by Mesenchymal Stem Cells Promotes Hair Growth in Mice with Androgenetic Alopecia. Stem Cell Res. Ther. 2024, 15, 439. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; He, G.; Liu, M.; Sun, Y.; Ma, S.; Sun, Z.; Wang, Y. Pilose Antler Extracts Promotes Hair Growth in Androgenetic Alopecia Mice by Activating Hair Follicle Stem Cells via the AKT and Wnt Pathways. Front. Pharmacol. 2024, 15, 1410810. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, S.; Xu, R.; Xiao, J.; Yi, R.; Mao, J.; Duan, Z.; Fan, D. Recombinant Type XVII Collagen Promotes Hair Growth by Activating the Wnt/β-Catenin and SHH/GLI Signaling Pathways. Cosmetics 2025, 12, 156. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S. Advances in Regenerative Stem Cell Therapy in Androgenic Alopecia and Hair Loss: Wnt Pathway, Growth-Factor, and Mesenchymal Stem Cell Signaling Impact Analysis on Cell Growth and Hair Follicle Development. Cells 2019, 8, 466. [Google Scholar] [CrossRef]
- Hile, G.A.; Gudjonsson, J.E.; Kahlenberg, J.M. The Influence of Interferon on Healthy and Diseased Skin. Cytokine 2020, 132, 154605. [Google Scholar] [CrossRef]
- Mayslich, C.; Grange, P.A.; Castela, M.; Marcelin, A.G.; Calvez, V.; Dupin, N. Characterization of a Cutibacterium Acnes Camp Factor 1-Related Peptide as a New TLR-2 Modulator in In Vitro and Ex Vivo Models of Inflammation. Int. J. Mol. Sci. 2022, 23, 5065. [Google Scholar] [CrossRef] [PubMed]
- Romics, L.; Dolganiuc, A.; Kodys, K.; Drechsler, Y.; Oak, S.; Velayudham, A.; Mandrekar, P.; Szabo, G. Selective Priming to Toll-like Receptor 4 (TLR4), Not TLR2, Ligands by P. Acnes Involves up-Regulation of MD-2 in Mice. Hepatology 2004, 40, 555–564. [Google Scholar] [CrossRef]
- Cros, M.P.; Mir-Pedrol, J.; Toloza, L.; Knödlseder, N.; Maruotti, J.; Zouboulis, C.C.; Güell, M.; Fábrega, M.-J. New Insights into the Role of Cutibacterium Acnes-Derived Extracellular Vesicles in Inflammatory Skin Disorders. Sci. Rep. 2023, 13, 16058. [Google Scholar] [CrossRef] [PubMed]
- Jugeau, S.; Tenaud, I.; Knol, A.C.; Jarrousse, V.; Quereux, G.; Khammari, A.; Dreno, B. Induction of Toll-like Receptors by Propionibacterium Acnes. Br. J. Dermatol. 2005, 153, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, M.; Flori, E.; Mastrofrancesco, A.; Briganti, S.; Lora, V.; Capitanio, B.; Zouboulis, C.C.; Picardo, M. Sebocyte Differentiation as a New Target for Acne Therapy: An in Vivo Experience. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1803–1814. [Google Scholar] [CrossRef]
- Choi, C.W.; Kim, Y.; Kim, J.E.; Seo, E.Y.; Zouboulis, C.C.; Kang, J.S.; Youn, S.W.; Chung, J.H. Enhancement of Lipid Content and Inflammatory Cytokine Secretion in SZ95 Sebocytes by Palmitic Acid Suggests a Potential Link between Free Fatty Acids and Acne Aggravation. Exp. Dermatol. 2019, 28, 207–210. [Google Scholar] [CrossRef]
- Olayinka, J.T.; Richmond, J.M. Immunopathogenesis of Alopecia Areata. Curr. Res. Immunol. 2021, 2, 7–11. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Pavel, A.B.; Diaz, A.; Zhang, N.; Del Duca, E.; Estrada, Y.; King, B.; Banerjee, A.; Banfield, C.; Cox, L.A.; et al. Ritlecitinib and Brepocitinib Demonstrate Significant Improvement in Scalp Alopecia Areata Biomarkers. J. Allergy Clin. Immunol. 2022, 149, 1318–1328. [Google Scholar] [CrossRef]
- Dillon, K.-A.L. A Comprehensive Literature Review of JAK Inhibitors in Treatment of Alopecia Areata. Clin. Cosmet. Investig. Dermatol. 2021, 14, 691–714. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.N.A.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.D.; et al. Common Loss-of-Function Variants of the Epidermal Barrier Protein Filaggrin Are a Major Predisposing Factor for Atopic Dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Ellinghaus, D.; Baurecht, H.; Esparza-Gordillo, J.; Rodríguez, E.; Matanovic, A.; Marenholz, I.; Hübner, N.; Schaarschmidt, H.; Novak, N.; Michel, S.; et al. High-Density Genotyping Study Identifies Four New Susceptibility Loci for Atopic Dermatitis. Nat. Genet. 2013, 45, 808–812. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, G.T. Genetic Insights into Acne, Androgenetic Alopecia, and Alopecia Areata: Implications for Mechanisms and Precision Dermatology. Cosmetics 2025, 12, 228. https://doi.org/10.3390/cosmetics12050228
de Souza GT. Genetic Insights into Acne, Androgenetic Alopecia, and Alopecia Areata: Implications for Mechanisms and Precision Dermatology. Cosmetics. 2025; 12(5):228. https://doi.org/10.3390/cosmetics12050228
Chicago/Turabian Stylede Souza, Gustavo Torres. 2025. "Genetic Insights into Acne, Androgenetic Alopecia, and Alopecia Areata: Implications for Mechanisms and Precision Dermatology" Cosmetics 12, no. 5: 228. https://doi.org/10.3390/cosmetics12050228
APA Stylede Souza, G. T. (2025). Genetic Insights into Acne, Androgenetic Alopecia, and Alopecia Areata: Implications for Mechanisms and Precision Dermatology. Cosmetics, 12(5), 228. https://doi.org/10.3390/cosmetics12050228




