Abstract
This study presents the formulation of two natural antioxidant creams based on an oil-in-water emulsion system, incorporating either hydroxycitrate (HCA) from Garcinia cambogia (Gaertn.) or red wine powder (RWP) derived from Aglianico del Vulture red wine (Vitis vinifera L.). HCA, a derivative of citric acid, and RWP, rich in polyphenolic compounds, were chosen for their bioactive properties. The creams underwent a series of in vitro tests to assess their stability, cytocompatibility, and antioxidant properties. Cellular assays using HaCaT keratinocytes showed that both formulations were effective in reducing blue light-induced oxidative damage.
1. Introduction
Ultraviolet radiation from the sun, specifically UVA and UVB, has known physiological benefits; however, overexposure to these rays is linked to various skin and ocular conditions. Prolonged exposure to sunlight poses well-documented risks to human health. Although public understanding of sun-related hazards has grown in recent years, actual protective behaviors often fall short of what is recommended [1].
In particular, UVA rays penetrate deeply into the dermis, initiating the process of photoaging. It also causes an immediate, though temporary, tanning effect—known as the Meirowsky phenomenon—by oxidizing pre-existing melanin, with more lasting pigmentation developing over the following hours [2].
Around 70% of UVB rays, instead, are absorbed by chromophores in the epidermis, leading to acute skin responses such as sunburn (erythema) and activating melanocytes to synthesize melanin. Due to its high energy, UVB can also lead to DNA damage in skin cells that manifests later [3,4].
More recently, attention has shifted toward the effects of high-energy visible (HEV) light, particularly wavelengths between 380 and 500 nm, commonly referred to as blue light. Both researchers and the photoprotection industry are increasingly focused on assessing how this portion of the light spectrum influences the skin [5].
Studies have indicated that light in the 415–465 nm range (violet-blue) can trigger hyperpigmentation, such as melasma. This reaction appears to be mediated by opsin3, a photoreceptor protein that modulates melanin production. Previously, such pigmentary effects were attributed mainly to UVA exposure. Additionally, blue light under 453 nm has been found to generate oxidative stress, although at a level about 25% of that induced by UVA rays. Blue light affects skin cells in ways similar to other wavelengths of visible light, leading to the formation of reactive oxygen species (ROS), DNA damage, reduced cell viability, inflammation, and impaired cell function [6,7,8]. Among these, ROS generation is considered the primary threat, as it likely drives most of the other harmful effects. With increasing exposure to artificial blue light from digital screens and indoor LED lighting, there is growing concern about whether such sources—though weaker than sunlight—might cumulatively contribute to pigmentary changes and other skin disorders [9,10].
Environmental factors, including ultraviolet and visible light (especially blue light) contribute significantly to premature skin aging, and a recent approach to counteract this effect consists of adding skin health ingredients into cosmetic formulations as natural antioxidants for oxidative stress.
Hydroxycitrate (HCA), a citric acid derivative found in Garcinia cambogia peel, exerts its primary biological effects by inhibiting ATP-citrate lyase (ACLY), a metabolic enzyme that converts mitochondrial citrate into cytosolic acetyl-CoA. This step is essential for the de novo synthesis of fatty acids and cholesterol. Inhibition of ACLY by HCA reduces lipogenesis, promotes lipid oxidation, and contributes to fat mass reduction, as demonstrated in both preclinical and clinical studies [11,12]. Beyond its role in energy metabolism, ACLY is also implicated in inflammation, as it supplies acetyl-CoA necessary for epigenetic regulation and serves as a substrate source for the synthesis of inflammatory mediators [13,14]. In vitro studies on M1-like macrophages have shown that HCA-especially in liposomal form-suppresses the production of ROS, nitric oxide, and prostaglandin E2, indicating a strong anti-inflammatory activity [15].
Furthermore, we have previously demonstrated that Aglianico del Vulture red wine powder (RWP), rich in phenolic compounds, which exhibit a range of biological properties, including antioxidant and anti-inflammatory effects, also exhibits anti-inflammatory potential by targeting ACLY [16].
Notably, extracts from Aglianico del Vulture wine, including its pomace, are particularly rich in polyphenolic compounds and exhibit strong antioxidant activity, highlighting their potential for applications in nutraceutical, pharmaceutical, and cosmetic formulations [16,17,18]. Considering that both HCA and RWP demonstrated protective activity against oxidative stress, largely through the suppression of ROS production and modulation of ACLY, their common identified molecular target of inflammatory pathways, the development of topical creams incorporating HCA or RWP could be a promising cosmetic strategy for the prevention of photoaging and skin damage associated with modern light exposure. The efficacy of formulations was evaluated based on their ability to prevent ROS formation, one of the main contributors to skin aging.
2. Materials and Methods
2.1. Preparation of Samples
Hydroxycitric acid tripotassium salt was purchased from Sigma Aldrich (St Louis, MO, USA). For the preparation of HCA, a 500 mM stock solution was prepared using the cell growth medium as a solvent from which, by successive dilutions, the 100 mM and the 50 mM solutions were obtained. The concentrations tested were 50, 100, and 500 μM.
RWP was the same as used in our previous study [16]. Distilled water was used as a solvent, and the tested concentrations were 20, 200, and 400 μg/mL.
For the preparation of the cream samples, stock solutions of 1.7 mg/g (10×) were prepared for HCA and 4 mg/g (10×) for RWP. The concentrations tested were the same as those of the samples not incorporated into the cream.
2.2. Cream Formulation and Preparation
An oil/water (O/W) emulsion was prepared [19] using jojoba oil, shea butter, xanthan gum, glycerin, and emulsifiers as reported in Table 1. Then, HCA and RWP were added in specific concentrations.

Table 1.
Ingredients used for the formulation of the cream.
The preparation of the cream involved first dispersing A3 in A2 and then joining it to A1 by heating to 60 °C. A4 was hydrated in A1 and then A2 and A3 were added.
The components of phase B were joined separately and brought to a temperature of 60 °C.
At this point, phase A was mixed with phase B by shaking (Argo Lab AM20-D, GiorgioBormac, Carpi, MO, Italy), and then cooled before adding phase C.
RWP (20 mg/5 g) and HCA (8.5 mg/5 g) were added to the basic formulation, thus obtaining a cream enriched with HCA (cHCA) or RWP (cRWP).
2.3. Stability Testing of Cream
One of the key concerns when developing a skin formulation is that it be stable without being toxic or irritating to the skin [20]. Numerous tests can be performed on the skin [21]. In this work, determination of the stability of the formulation consists of verifying the chemical-physical and microbiological stability of the product over time. The test consists of an aging period of 1 month, subjected to thermal shock, and includes maintaining the finished product at a temperature of 40 °C and 5 °C, to simulate in vitro the conditions to which the product could be subjected during storage and use [22].
During the test period, at pre-established time intervals, the product is subjected to control of the chemical-physical and microbiological parameters, such as, appearance of the product, variation in color and odor, visible alterations, non-deterioration over time and conservation of its characteristics.
The thermal shock tests, i.e., exposure to alternating thermal cycles, can reveal some types of product inadequacies more rapidly than constant temperature or light exposure tests [23]. Equipment used was an incubator (ICN-16, ARGO LAB, Carpi, Italy) capable of maintaining the temperature at 40 ± 1 °C and a refrigerator capable of maintaining the temperature at 5 ± 1 °C.
The analytical evaluation of the samples included several key tests: pH levels were recorded using a pH meter (Hanna Instruments, Padova, Italy), while viscosity measurements were carried out with a viscometer (PCE-RVI 4, Barcelona, Spain). To evaluate the emulsion’s mechanical stability and its resistance to phase separation, centrifugation was performed with a CN-45 model centrifuge (Orma, Taranto, Italy) [23].
Product stability and microbial content were assessed using ready-to-use Contact Slides 1 and 2 (Liofilchem™, Teramo, Italy). Contact Slide 1 is a ready-to-use, dual-medium device designed for general microbial monitoring on surfaces and in liquids. One medium supports total bacterial counts, while the other selectively detects Enterobacteriaceae, even in the presence of residual disinfectants. Contact Slide 2 also features a dual-medium system on a plastic support, specifically tailored for the isolation and enumeration of yeasts and molds, allowing effective microbial assessment of liquids and surfaces while accommodating disinfectant residues. For both slides, quantitative and qualitative limits were established following the European Standard EN ISO 17516:2014 (Geneva, Switzerland, 2014) Cosmetics-Microbiology-Microbiological limits [24,25], ensuring reliable and standardized microbial evaluation [19].
2.4. Cell Culture and Treatments
The human keratinocyte HaCaT cell line [26], obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA), was used in the study. HaCat cells were cultured in a complete medium constituted of Dulbecco’s modified Eagle’s medium (ThermoFisher Scientific, San Jose, CA, USA) supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin and incubated at standard culture conditions (5% CO2, 37 °C).
Blue light exposure was performed with two different kinds of lamps: a light-emitting diode (Honglitronic, Guangzhou, China), wavelength 450 nm, source LED, 53 mW/cm2 at 9 cm distance, and a blue light-emitting LED (BLE LED) (Philips GmbH Innovative Technologies, Aachen, Germany), wavelength 453 nm, source LED, irradiance 50 mW/cm2.
2.5. Evaluation of Cell Proliferation
The effects on HaCaT cell proliferation were determined using a Millipore Scepter™ handheld automated cell counter (Merck Millipore, Darmstadt, Germany) according to the manufacturer’s instructions. Cells were seeded in 96-well plates (1 × 104 cells/well) and treated with HCA (50–100–500 µM) or RWP (20–200–400 µg/mL) for 72 h. The proliferation of HaCaT cells was also evaluated before and after blue light (BL) irradiation with the two kinds of lamps following pre-treatment for 1 h with HCA (50–100–500 µM), RWP (20–200–400 µg/mL), 500 µM cHCA, or 400 µg/mL cRWP. After incubation, HaCaT cells were collected in 1.5 mL microfuge tubes and counted. Untreated cells represented the negative control [27].
In particular, to reproduce in the cream formulations the same concentrations tested in vitro, 10× RWP and HCA stock solutions were initially prepared (4 mg/mL for RWP and 5 mM for HCA). Assuming a density of 1 g/mL and a total cream weight of 5 g, this corresponded to 20 mg/5 g for RWP (4 mg/g, 10×) and 8.5 mg/5 g for HCA (1.7 mg/g, 10×). Since RWP is water-soluble and HCA is soluble in cell culture medium, these solvents were used as vehicles for the respective emulsions. This approach ensured that the active concentrations in the creams (500 µM cHCA and 400 µg/mL cRWP) matched those employed in the in vitro experiments, thereby guaranteeing methodological consistency between the two experimental settings.
2.6. ROS Assay
HaCaT cells (1 × 105 cells/well) were seeded in 24-well plates and pre-treated with HCA (50–100–500 µM), RWP (20–200–400 µg/mL), cHCA 500 µM, or cRWP 400 µg/mL for 1 h before exposure to blue light for an additional 1 h. Thereafter, ROS levels were measured by using 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA, Thermo Fisher Scientific, San Jose, CA, USA), as previously described [16].
2.7. Statistical Analysis
Data are shown as mean ± SD of three independent experiments. Statistical significance was determined by one-way analysis of variance (ANOVA). When the ANOVA indicated significance (p < 0.05), a Dunnett’s or Tukey’s post hoc test was applied. Specifically, Dunnett’s test was used to compare each treatment with the corresponding untreated control, whereas Tukey’s test was performed to compare all group means with each other. The statistical method employed for each experiment is detailed in the corresponding figure legends. Statistical significance is indicated in the figures as follows: * p < 0.05, ** p < 0.01, *** p < 0.001. For Tukey’s post hoc tests, different letters are used in the figures to indicate significant differences between treatments at p < 0.05.
3. Results
3.1. Quality Check of Cream
The cream demonstrated high physical and microbiological stability under stress conditions. The sensory analysis allowed us to evaluate the main characteristics of the cream, which has a pink color, due to the addition of wine extract to the formulation, the texture is light and the skinfeel is pleasant (Figure 1).
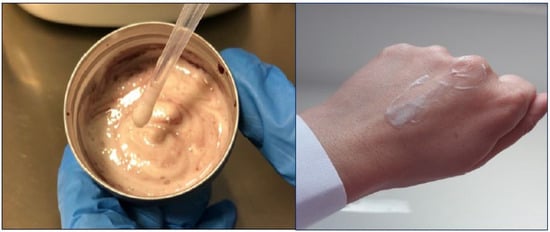
Figure 1.
Evaluation of texture and skin feel of the cream.
According to the technical sheets, listed below, at time 0 (zero), the emulsion has a characteristic milky white color, does not have lumps and has a pH of 6.04.
Considering the following parameters, the stability tests did not show any alteration, either in the appearance or in the chemical-physical characteristics of the sample after 1 month at +5 °C, after 1 month at +40 °C, thermal shock +5 °C/+40 °C alternating (1 week for 28 days).
Furthermore, we can consider the pH values obtained during the controls acceptable, included in the range 5.71–6.04, considering that the optimal pH value of the skin on most of the face and body is between 4.7 and 5.75.
The viscosity values obtained using the rotational viscometer (Figure 2A), can be considered satisfactory, as it guarantees a good spreading of the product. By subjecting the cream to centrifugation cycles at 3000 rpm for 30 min at room temperature, it was possible to visually detect that no phase separation phenomenon occurred (Figure 2B). The microbiological analysis, whose purpose is to qualitatively and quantitatively determine the microorganisms present in the sample, revealed the absence of bacteria, yeasts, molds and pathogens (Figure 2C).
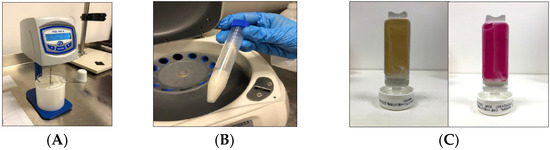
Figure 2.
Quality check of cream: (A) Viscosity values obtained using the rotational viscometer; (B) Separation phenomenon by centrifugation cycles; (C) microbiological analysis.
Below are the control and stability test sheets performed on the cream (Table 2, Table 3, Table 4 and Table 5). As can be seen from the data, the cream, at all time intervals and at all temperatures tested, exhibits the consistency of an emulsion before lumps, creaming, and sedimentation. It has a milk-white color and a characteristic odor that remain unchanged over time. The pH ranges between 5.71 and 6.04, and the viscosity ranges between 73.2 and 74.5%. Separation under mechanical stress, bacteria, yeasts, molds, and pathogens are not present.

Table 2.
Control and stability at time 0 of cream just prepared.

Table 3.
Control and stability at +5 °C of cream.

Table 4.
Control and stability at +40 °C of cream.

Table 5.
Control and stability of thermal shock +5 °C/+40 °C altered (1 week for 28 days) of cream.
3.2. Effect of RWP or HCA on HaCaT Cell Proliferation
To determine whether RWP or HCA affects keratinocyte cell proliferation, HaCaT cells were exposed to 20–200–400 µg/mL RWP or 50–100–500 µM HCA for 72 h. Untreated cells represented the negative control. As shown in Figure 3A,B, neither RWP nor HCA significantly affected cell proliferation at the tested concentrations.
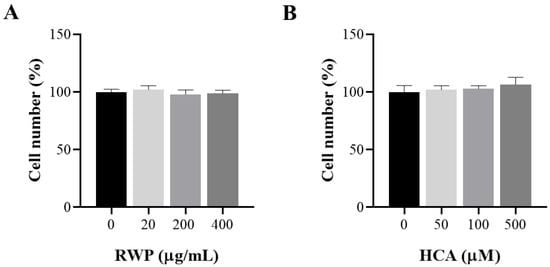
Figure 3.
Effect of RWP or HCA on keratinocyte cell number. HaCat cells were treated with increasing concentrations of RWP (20–400 μg/mL; (A), or HCA (50–500 µM; (B), and cell viability was assessed by cell count after 72 h exposure. The mean values ± SD of three independent experiments with four replicates in each are shown. No statistically significant differences were detected by one-way ANOVA.
To verify whether BL exposure impacted cell proliferation, we treated HaCaT cells for 1 h with 20–200–400 µg/mL RWP or 50–100–500 µM HCA and then irradiated them with blue light. After 1 h of exposure to BL, we evaluated cell proliferation. Compared to unirradiated cells, a slight reduction of approximately 20% in proliferation emerged following exposure to BL (Figure 4). In cells treated with RWP at 20 and 200 µg/mL (Figure 4A) or 50 and 100 µM HCA (Figure 4B) and irradiated, we observed reductions similar to those of cells exposed only to BL. A less marked cytotoxic effect was observed in the cells treated with 400 µg/mL RWP or 500 µM HCA. No relevant differences were observed when a Honglitronic lamp or a BLE LED was used.
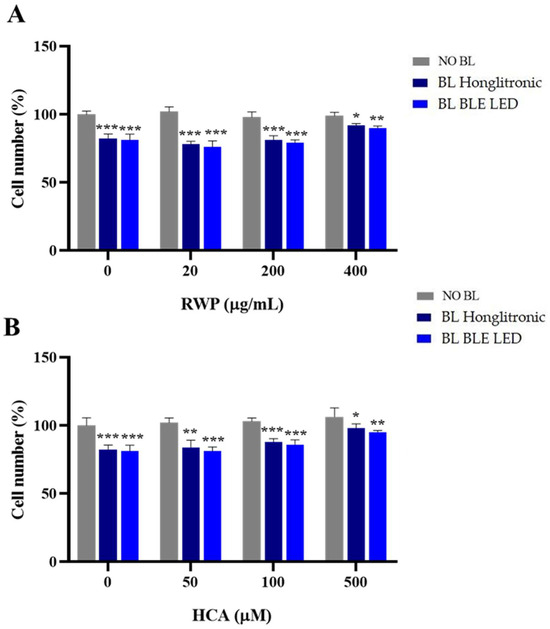
Figure 4.
Effect of RWP or HCA on keratinocyte cell proliferation following exposure to blue light (BL). HaCat cells were treated with increasing concentrations of RWP (20–400 μg/mL; (A), or HCA (50–500 µM; (B), for 1 h and exposed to BL for 1 h. Cell viability was assessed by cell counting. The mean values ± SD of three independent experiments with four replicates in each are shown. Statistical significance was determined by one-way ANOVA followed by Dunnett’s post hoc test, comparing BL Honglitronic/BL BLE LED-irradiated cells with their corresponding NO BL controls (* p < 0.05, ** p < 0.01, *** p < 0.001).
3.3. Effect of RWP or HCA on Blue Light-Induced ROS Production
Since the cytotoxic effects of blue light are closely associated with excessive ROS generation [28], we sought to determine whether RWP or HCA could attenuate blue light-induced cellular ROS production. To this end, HaCaT cells were pretreated with RWP (20, 200, and 400 µg/mL) or HCA (50, 100, and 500 µM) for 1 h before exposure to blue light for an additional hour. As expected, blue light from both the Honglitronic and BLE LED lamps significantly increased intracellular ROS levels compared to their respective non-irradiated controls, confirming the pro-oxidant effects of blue light exposure (Figure 5). Interestingly, in both models, pretreatment with RWP reduced ROS generation in a dose-dependent manner, with 400 µg/mL being the most effective, lowering ROS levels nearly to those of the control group (Figure 5A). In contrast, HCA treatment (Figure 5B) only resulted in a significant reduction in ROS levels at the highest concentration tested of 500 µM, under both light sources. No significant differences were observed at 50 or 100 µM compared to irradiated cells.
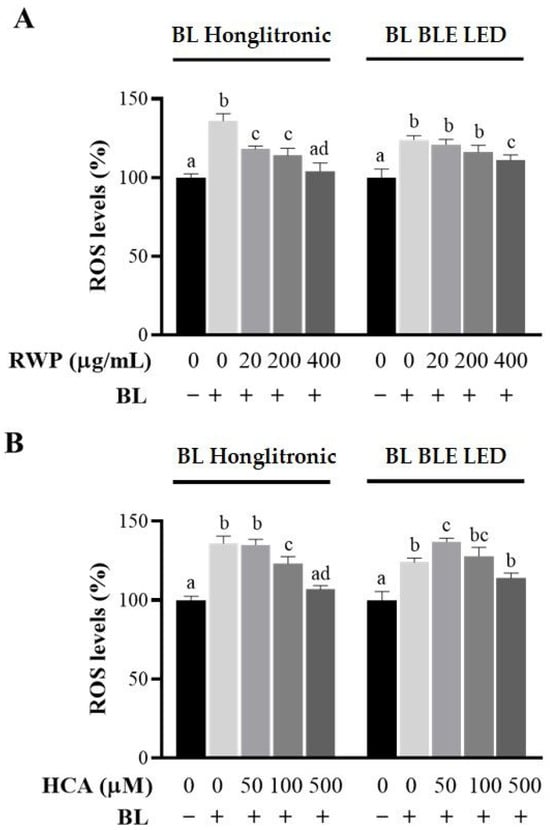
Figure 5.
Effect of RWP or HCA on ROS production in keratinocytes exposed to blue light (BL). HaCat cells were treated with increasing concentrations of RWP (20–400 μg/mL; (A), or HCA (50–500 µM; (B), for 1 h. After 1 h of BL exposure under Honglitronic lamp (BL Honglitronic) or BLE LED lamp (BL BLE LED), ROS levels were quantified. The mean values ± SD of three independent experiments with four replicates in each are shown. Statistical significance was determined by one-way ANOVA followed by Tukey’s post hoc test. Comparisons were performed within each BL exposure group (BL Honglitronic or BL BLE LED). Treatments sharing the same letter are not significantly different, whereas treatments labeled with different letters are significantly different (p < 0.05).
These findings suggest that both RWP and HCA provide substantial antioxidant protection against ROS generated by different blue light sources, and prompt us to select 400 µg/mL RWP and 500 µM HCA as optimal concentrations for inclusion in the cream formulations.
3.4. Effect of Topical Creams Enriched with RWP or HCA on HaCat Cell Proliferation
Similarly, we evaluated the effects of 400 µg/mL cRWP and 500 µM cHCA on keratinocyte proliferation before and after blue light irradiation. Cells were pre-treated for 1 h with topical formulations dissolved in culture medium for cHCA or sterile water for RWP and exposed to blue light for 1 h. Treated but not irradiated cells were used as controls. We found that in the absence of irradiation, the cell proliferation of keratinocytes treated with topical creams enriched with RWP or HCA was comparable to that of untreated keratinocytes at the tested concentrations (Figure 6). Exposure to blue light, however, significantly reduced the number of cells, especially under a BLE LED lamp. However, pre-treatment with cRWP and cHCA partially limited the effect of blue light, as shown in Figure 6.
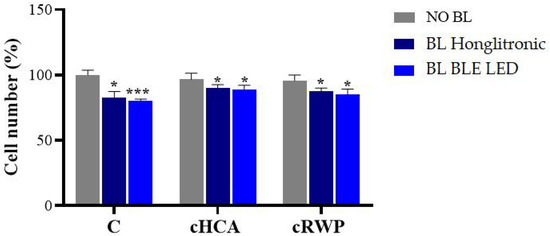
Figure 6.
Effect of topical cream enriched with RWP or HCA on keratinocyte cell number exposed or not to blue light (BL). HaCat cells were treated with 400 μg/mL cRWP or 500 µM cHCA for 1 h and exposed to BL under Honglitronic lamp (BL Honglitronic) or BLE LED lamp (BL BLE LED). Cell viability was assessed by cell count after 1 h of exposure. The mean values ± SD of three independent experiments with four replicates in each are shown. Statistical significance was determined by one-way ANOVA followed by Dunnett’s post hoc test, comparing BL Honglitronic/BL BLE LED-treated cells with their corresponding NO BL controls (* p < 0.05, *** p < 0.001).
3.5. Effect of Topical Creams Enriched with RWP or HCA on Blue Light-Induced ROS Production
To evaluate the antioxidant efficacy of the cosmetic formulations, intracellular ROS levels were assessed in HaCaT cells exposed to blue light irradiation. As illustrated in Figure 7, blue light exposure led to a significant increase in ROS levels compared to untreated cells (p < 0.001). Treatment with 400 µg/mL cRWP or 500 µM cHCA effectively attenuated blue light-induced ROS production, reducing levels to near those of the unirradiated control group. Notably, the protective effect of cRWP was slightly more pronounced under exposure to the Honglitronic lamp, although both formulations demonstrated comparable efficacy in mitigating oxidative stress.
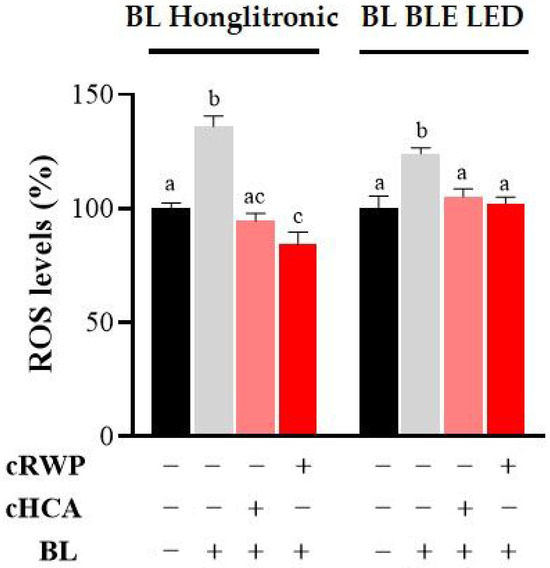
Figure 7.
Effect of topical formulations containing RWP or HCA on blue light-induced ROS production in HaCaT cells. HaCaT cells were treated with 400 μg/mL cRWP or 500 µM cHCA for 1 h. After 1 h of BL exposure under Honglitronic lamp (BL Honglitronic) or BLE LED lamp (BL BLE LED), ROS levels were quantified. The mean values ± SD of three independent experiments with four replicates in each are shown. Statistical significance was determined by one-way ANOVA followed by Tukey’s post hoc test. Comparisons were performed within each BL exposure group (BL Honglitronic or BL BLE LED). Treatments sharing the same letter are not significantly different, whereas treatments labeled with different letters are significantly different (p < 0.05).
4. Discussion
The present work demonstrated that creams enriched with hydroxycitrate (HCA) or Aglianico del Vulture red wine powder (RWP) effectively attenuate blue light-induced oxidative stress in HaCaT keratinocytes, without affecting basal cell proliferation. This outcome aligns with previous studies showing that visible light, particularly in the blue spectrum (380–500 nm), contributes to oxidative stress, DNA damage, and hyperpigmentation through excessive reactive oxygen species (ROS) production [6,9]. Although the observed approximately 20% reduction in cell proliferation may be considered moderate, it could have biologically significant consequences in scenarios of cumulative or repeated blue light exposure, particularly in individuals with impaired skin barrier function or pre-existing dermatological conditions [29]. Accordingly, these results underscore the necessity for comprehensive in vivo investigations to elucidate the clinical relevance of such effects.
Importantly, both formulations maintained high stability under stress conditions and preserved favorable sensorial characteristics, supporting their applicability in cosmetic formulations. The stability and safety profile observed here is in accordance with recent findings highlighting the need for robust formulations when incorporating natural bioactives into cosmetic products [19].
Among the tested bioactives, RWP showed a more pronounced and dose-dependent protective effect, consistent with its high content of polyphenolic compounds, which are known to exert strong antioxidant and anti-inflammatory activities [16]. Indeed, polyphenols have been widely reported to mitigate photo-induced oxidative stress and to modulate signaling pathways associated with NF-κB activation and mitochondrial ROS production [10]. On the other hand, HCA reduced ROS production significantly only at the highest concentration tested, which may reflect its different molecular mechanisms. By inhibiting ATP-citrate lyase, HCA not only modulates lipid metabolism but also exerts an indirect antioxidant and anti-inflammatory activity by limiting acetyl-CoA availability for pro-inflammatory mediator synthesis [13,14,15]. These mechanistic differences suggest that RWP and HCA may complement each other in providing photoprotection through distinct but synergistic pathways.
The topical formulations tested in this study therefore represent promising cosmetic strategies for preventing oxidative damage linked to modern light exposure, including solar and artificial sources. Their efficacy in reducing ROS levels to values comparable with non-irradiated controls underlines the relevance of developing multifunctional creams that not only preserve skin homeostasis but also counteract premature photoaging. These results are in line with current trends in cosmetic science, which emphasize the integration of natural antioxidants into formulations to improve skin barrier defense against environmental aggressors [10,19]. Nevertheless, further investigations are warranted, including in vivo clinical studies and long-term application trials, to confirm the translational potential of these formulations and to assess their role in daily photoprotection routines.
5. Conclusions
This work aimed to formulate creams paying particular attention to natural antioxidant substances, evaluating their intrinsic activity in counteracting possible skin damage caused by prolonged exposure to blue light, emitted by the sun and artificial light sources. The studies carried out have shown that the cause of photoaging is not attributable only to UVA-UVB rays, but that blue light, emitted by the sun, is increasingly involved, characterized by a greater intensity than artificial light sources.
All the analyses performed on the cosmetics produced have shown satisfactory results both from the point of view of chemical-physical stability and microbiological risk. In vitro tests on keratinocyte cell line, aimed at evaluating the intrinsic antioxidant capacity of HCA and RWP, yielded promising results—specifically, a significant reduction in ROS levels. These findings supported the incorporation of HCA and RWP into the topic creams, resulting in finalized skin health products with enhanced antioxidant potential. These results warrant further in vivo investigations and suggest the potential for developing innovative anti-aging and protective skincare products.
Author Contributions
Conceptualization, I.P., I.F. and A.V.; methodology, I.P., I.F., A.S., A.M., P.C. and A.V.; software, I.P., A.S. and P.C.; validation, I.P., I.F., A.S., A.M., P.C. and A.V.; formal analysis, I.P., I.F., A.S., A.M., P.C. and A.V.; investigation, I.P., A.S., P.C. and A.V.; resources, A.V.; data curation, I.P., I.F., A.S., P.C. and A.V.; writing—original draft preparation, I.P., I.F., A.S. and P.C.; writing—review and editing, I.P., I.F., A.S., P.C. and A.V.; visualization, I.P., I.F., A.S., A.M., P.C. and A.V.; supervision, A.V.; project administration, A.V.; funding acquisition, A.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FSC European Funds, grant number C37G22000400001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
Immacolata Faraone is partner of Innovative Startup Farmis s.r.l. Antonio Vassallo is partner of Spinoff TNcKILLERS s.r.l. Alessandra Miraglia is the employee of Laboratori Miracosm di A.M. The remain authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Riedmann, U.; Dibben, C.; de Gruijl, F.R.; Gorman, S.; Hart, P.H.; Hoel, D.G.; Levy, C.; Lindqvist, P.G.; Norval, M.; Parikh, S.S. Beneficial health effects of ultraviolet radiation: Expert review and conference report. Photochem. Photobiol. Sci. 2025, 24, 867–893. [Google Scholar] [CrossRef]
- Maeda, K.; Hatao, M. Involvement of photooxidation of melanogenic precursors in prolonged pigmentation induced by ultraviolet A. J. Investig. Dermatol. 2004, 122, 503–509. [Google Scholar] [CrossRef]
- Battie, C.; Verschoore, M. Cutaneous solar ultraviolet exposure and clinical aspects of photodamage. Indian J. Dermatol. Venereol. Leprol. 2012, 78, 9. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Rokohl, A.C.; Guo, Y.; Chen, H.; Gao, T.; Kakkassery, V.; Heindl, L.M. Narrative review: Mechanism of ultraviolet radiation-induced basal cell carcinoma. Front. Oral Maxillofac. Med. 2023, 5. [Google Scholar] [CrossRef]
- Ramser, A.; Casey, A. Blue Light and Skin Health. J. Drugs Dermatol. JDD 2022, 21, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Pourang, A.; Tisack, A.; Ezekwe, N.; Torres, A.E.; Kohli, I.; Hamzavi, I.H.; Lim, H.W. Effects of visible light on mechanisms of skin photoaging. Photodermatol. Photoimmunol. Photomed. 2022, 38, 191–196. [Google Scholar] [CrossRef]
- Regazzetti, C.; Sormani, L.; Debayle, D.; Bernerd, F.; Tulic, M.K.; De Donatis, G.M.; Chignon-Sicard, B.; Rocchi, S.; Passeron, T. Melanocytes sense blue light and regulate pigmentation through opsin-3. J. Investig. Dermatol. 2018, 138, 171–178. [Google Scholar] [CrossRef]
- Krassovka, J.M.; Suschek, C.V.; Prost, M.; Grotheer, V.; Schiefer, J.L.; Demir, E.; Fuchs, P.C.; Windolf, J.; Stürmer, E.K.; Opländer, C. The impact of non-toxic blue light (453 nm) on cellular antioxidative capacity, TGF-β1 signaling, and myofibrogenesis of human skin fibroblasts. J. Photochem. Photobiol. B Biol. 2020, 209, 111952. [Google Scholar] [CrossRef]
- De Gálvez, E.N.; Aguilera, J.; Solis, A.; de Gálvez, M.V.; de Andrés, J.R.; Herrera-Ceballos, E.; Gago-Calderon, A. The potential role of UV and blue light from the sun, artificial lighting, and electronic devices in melanogenesis and oxidative stress. J. Photochem. Photobiol. B Biol. 2022, 228, 112405. [Google Scholar] [CrossRef]
- Furukawa, J.Y.; Martinez, R.M.; Morocho-Jácome, A.L.; Castillo-Gómez, T.S.; Pereda-Contreras, V.J.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. Skin impacts from exposure to ultraviolet, visible, infrared, and artificial lights—A review. J. Cosmet. Laser Ther. 2021, 23, 1–7. [Google Scholar] [CrossRef]
- Sullivan, C.; Triscari, J. Metabolic regulation as a control for lipid disorders. I. Influence of (—)-hydroxycitrate on experimentally induced obesity in the rodent. Am. J. Clin. Nutr. 1977, 30, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Onakpoya, I.; Hung, S.K.; Perry, R.; Wider, B.; Ernst, E. The use of Garcinia extract (hydroxycitric acid) as a weight loss supplement: A systematic review and meta-analysis of randomised clinical trials. J. Obes. 2011, 2011, 509038. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, M.; Brüne, B.; Namgaladze, D. Exploring the role of ATP-citrate lyase in the immune system. Front. Immunol. 2021, 12, 632526. [Google Scholar] [CrossRef] [PubMed]
- Infantino, V.; Pierri, C.L.; Iacobazzi, V. Metabolic routes in inflammation: The citrate pathway and its potential as therapeutic target. Curr. Med. Chem. 2019, 26, 7104–7116. [Google Scholar] [CrossRef]
- Vassallo, A.; Santoro, V.; Pappalardo, I.; Santarsiero, A.; Convertini, P.; De Luca, M.; Martelli, G.; Infantino, V.; Caddeo, C. Liposome-mediated inhibition of inflammation by hydroxycitrate. Nanomaterials 2020, 10, 2080. [Google Scholar] [CrossRef]
- Santarsiero, A.; Convertini, P.; Vassallo, A.; Santoro, V.; Todisco, S.; Iacobazzi, D.; Fondufe-Mittendorf, Y.; Martelli, G.; de Oliveira, M.R.; Montanaro, R. Phenolic compounds of red wine Aglianico del Vulture modulate the functional activity of macrophages via inhibition of NF-κB and the citrate pathway. Oxidative Med. Cell. Longev. 2021, 2021, 5533793. [Google Scholar] [CrossRef]
- Savino, M.; Basile, T.; Alba, V.; Bolettieri, D.; Paradiso, F.; Tamborra, P.; Suriano, S.; Tarricone, L. Detection of Intra-Varietal Diversity Based on Differences in the Accumulation of Secondary Metabolites for Winemaking Management of High-Quality Red Wines. Beverages 2017, 3, 45. [Google Scholar] [CrossRef]
- Tat, L.; Comuzzo, P.; Battistutta, F.; Zironi, R. Sweet-like off-flavor in Aglianico del Vulture wine: Ethyl phenylacetate as the mainly involved compound. J. Agric. Food Chem. 2007, 55, 5205–5212. [Google Scholar] [CrossRef]
- Bruno, M.R.; Ponticelli, M.; Sinisgalli, C.; Milella, L.; Todaro, L.; Faraone, I. Natural Bioactive Compounds from Orchard Biomass Waste and Cosmetic Applications. Forests 2025, 16, 79. [Google Scholar] [CrossRef]
- Donthi, M.R.; Munnangi, S.R.; Krishna, K.V.; Saha, R.N.; Singhvi, G.; Dubey, S.K. Nanoemulgel: A novel nano carrier as a tool for topical drug delivery. Pharmaceutics 2023, 15, 164. [Google Scholar] [CrossRef]
- Sohail, M.; Baig, M.M.F.A.; Akhtar, N.; Chen, Y.; Xie, B.; Li, B. Topical lycopene emulgel significantly improves biophysical parameters of human skin. Eur. J. Pharm. Biopharm. 2022, 180, 281–288. [Google Scholar] [CrossRef]
- Kamaruzaman, N.; Yusop, S.M. Determination of stability of cosmetic formulations incorporated with water-soluble elastin isolated from poultry. J. King Saud Univ. -Sci. 2021, 33, 101519. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Yeoh, T.; Shah, J.C.; Walsh, T. Assessing and predicting physical stability of emulsion-based topical semisolid products: A review. J. Pharm. Sci. 2023, 112, 1772–1793. [Google Scholar] [CrossRef]
- Tabari, M.R.; Osterwalder, U. Standardization: ISO and Cosmetics. In Handbook of Cosmetic Science and Technology; CRC Press: Abingdon, UK, 2022; pp. 419–425. [Google Scholar]
- Halla, N.; Fernandes, I.P.; Heleno, S.A.; Costa, P.; Boucherit-Otmani, Z.; Boucherit, K.; Rodrigues, A.E.; Ferreira, I.C.; Barreiro, M.F. Cosmetics preservation: A review on present strategies. Molecules 2018, 23, 1571. [Google Scholar] [CrossRef] [PubMed]
- El-Mahdy, M.A.; Zhu, Q.; Wang, Q.E.; Wani, G.; Patnaik, S.; Zhao, Q.; Arafa, E.S.; Barakat, B.; Mir, S.N.; Wani, A.A. Naringenin protects HaCaT human keratinocytes against UVB-induced apoptosis and enhances the removal of cyclobutane pyrimidine dimers from the genome. Photochem. Photobiol. 2008, 84, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Y.; Kim, J.-S. Cherry fruit anthocyanins cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside protect against blue light-induced cytotoxicity in HaCaT cells. Appl. Biol. Chem. 2023, 66, 3. [Google Scholar] [CrossRef]
- Suitthimeathegorn, O.; Yang, C.; Ma, Y.; Liu, W. Direct and indirect effects of blue light exposure on skin: A review of published literature. Ski. Pharmacol. Physiol. 2022, 35, 305–318. [Google Scholar] [CrossRef]
- Kondo, S.; Ozawa, N.; Sakurai, T. The effect of degeneration of elastic fibres on loss of elasticity and wrinkle formation. Int. J. Cosmet. Sci. 2025, 47, 205–212. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).