1. Introduction
Facial aging is a complex, multifactorial biological process driven by both intrinsic factors (such as genetic predisposition and the chronological decline of collagen and elastin synthesis) and extrinsic influences (including cumulative photodamage, environmental exposure, and mechanical stress). These processes converge to produce the characteristic features of aging: loss of soft tissue volume, descent of fat pads, remodeling of underlying bone, progressive skin laxity, and the formation of dynamic and static rhytides. Together, these changes alter facial contours, reduce structural support, and degrade skin quality, resulting in an aged, fatigued appearance. Injectable dermal fillers have become central to contemporary facial rejuvenation strategies because they offer a minimally invasive means to restore lost volume, redefine contours, and soften wrinkles, often with immediate esthetic benefit and limited downtime. Among the available filler materials, calcium hydroxyapatite (CaHA) and hyaluronic acid (HA) stand out for their complementary biological and mechanical profiles, each with distinct mechanisms of action, durability characteristics, and clinical indications [
1].
Despite the widespread use of each agent individually, there remains a relative paucity of standardized, systematic protocols that integrate their unique properties to jointly address both volume loss and skin quality degradation in a cohesive treatment paradigm. In particular, the concept of combining CaHA’s longer-term biostimulatory effects with HA’s immediate volumizing and hydrating properties—especially in hyper-diluted formulations designed to maximize diffusion and tissue remodeling—has been suggested in preliminary reports but lacks rigorous, standardized evaluation. This study was therefore designed to fill that gap by developing and testing a reproducible combined injection approach that leverages the synergistic potential of CaHA and HA in patients presenting simultaneously with soft tissue volume depletion and cutaneous laxity.
1.1. Calcium Hydroxyapatite
Calcium hydroxyapatite (CaHA) is a semi-solid, biocompatible dermal filler composed of smooth, spherical CaHA microspheres suspended in a carboxymethylcellulose-based carrier gel with water and glycerin as excipients. The formulation is biodegradable and resorbable, being gradually remodeled by the host biology while eliciting a controlled tissue response. CaHA has accrued a substantial clinical track record and an established safety profile in esthetic medicine. In 2006, the United States Food and Drug Administration (FDA) approved CaHA for the correction of moderate-to-severe facial wrinkles and folds and for the restoration of soft tissue volume in both the face and hands [
2,
3,
4]. Mechanistically, the microspheres serve initially as a scaffold and subsequently as a stimulus for neocollagenesis: they induce fibroblast activation and promote the deposition of type I collagen, leading to gradual and sustained tissue augmentation. The carrier gel provides immediate lift and volumization, which diminishes as it is absorbed, leaving the microspheres in place to support and stimulate new connective tissue formation. Over time, this biological remodeling translates into a durable increase in tissue volume as well as improvements in skin texture and quality [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11]. Clinically, CaHA is indicated for the correction of moderate-to-severe dynamic and static wrinkles and folds—such as nasolabial folds, marionette lines, and forehead furrows—as well as volume replenishment in areas like the cheeks, temples, and dorsal hands. Its utility extends to the management of skin laxity across multiple body regions, including the face, neck, décolleté, arms, and abdomen, where its biostimulatory effect can mitigate tissue sagging and improve cutaneous firmness [
1,
2,
3,
4]. Injection techniques for CaHA vary based on indication and anatomical region. It may be delivered via needles or blunt-tip cannulas into the deep dermis or subcutaneous compartment using linear threading, fanning, or serial puncture. For the treatment of skin laxity—where a more diffuse and less volumetric effect is desired—CaHA is frequently diluted with saline and/or lidocaine to reduce viscosity, enhance spread, and create a softer, more natural tissue response [
2,
3]. Dilution of CaHA has become an established strategy to maximize its effect on skin quality, particularly in early-stage laxity, by enabling broader tissue distribution while tempering overt projection. Typical dilution ratios range from 1:1 up to 1:5 (CaHA to diluent), with higher dilutions lowering viscosity and improving diffusion—suitable for more superficial or extensive areas—at the expense of some longevity. Consensus-based recommendations suggest lower dilutions (e.g., 1:1 to 1:2) for facial and cervical applications where control and moderate lift are needed, and higher dilutions (1:2 to 1:5) for larger or more lax body regions such as the arms, legs, and abdomen [
2,
3,
4,
12,
13]. CaHA’s safety profile is well characterized: most adverse events are mild and transient, including injection-site pain, swelling, erythema, and ecchymosis. Rare but more serious complications such as nodules, granulomatous reactions, infection, and tissue necrosis can occur, particularly when standard injection principles are violated. Therefore, meticulous technique, comprehensive anatomical knowledge, and judicious patient selection are essential to minimize risks [
14,
15,
16].
1.2. Hyaluronic Acid
Hyaluronic acid (HA) is a naturally occurring glycosaminoglycan abundant in the dermal extracellular matrix, where it is pivotal for tissue hydration, viscoelasticity, and volume maintenance. HA-based fillers consist of cross-linked HA molecules whose chemical modification confers increased resistance to enzymatic degradation, prolonging persistence in tissues. The resulting viscoelastic gels are injectable and can immediately replenish lost volume, smooth facial rhytides, and contour anatomical structures [
17,
18,
19]. The primary mechanism of action for HA fillers is physical space occupation—“filling in” depressions and restoring projection—while their intrinsic hydrophilic nature attracts and retains water, enhancing tissue turgor and surface quality. Some formulations have also been associated with secondary biological effects, including stimulation of fibroblasts and modest improvements in dermal matrix composition, contributing to longer-term enhancement of skin quality beyond the immediate volumetric effect [
17,
18,
19]. HA fillers are widely indicated for the correction of fine to severe wrinkles and folds—such as nasolabial and marionette lines, glabellar furrows, and periorbital rhytides—as well as for restoring volume in the lips, cheeks, temples, and the dorsum of the hands. They also play a role in addressing early skin laxity in areas like the neck and décolleté where subtle volumization and hydration can yield a rejuvenated appearance [
19]. Injection can be performed with either needles or cannulas, chosen according to the target depth, product rheology, and regional anatomy. Techniques such as linear threading, fanning, serial puncture, and bolus placement are tailored per indication and operator preference to optimize the esthetic effect while minimizing trauma [
17,
18,
19]. While the dilution of HA is less routinely employed compared to CaHA, selective dilution with saline or lidocaine is sometimes utilized to achieve specific goals such as more subtle tissue augmentation, improved spread for fine lines, or enhanced hydration with a less volumetric appearance. Such manipulation decreases viscosity and may shorten duration, so the decision to dilute must balance the desired esthetic refinement against longevity [
20,
21]. HA fillers are generally well tolerated; common immediate reactions include mild swelling, redness, bruising, and discomfort. Rare but important complications include nodule formation, granulomatous reactions, infection, vascular occlusion leading to tissue ischemia or, in very rare instances, visual compromise. As with CaHA, the appropriate technique, an understanding of vascular anatomy, and careful patient selection are vital to risk mitigation [
22,
23,
24].
1.3. CaHA and HA in Combination
Combining CaHA and HA within a unified treatment strategy offers a synergistic paradigm for facial rejuvenation by coupling immediate volumetric correction and hydration (provided by HA) with longer-term biostimulatory remodeling (driven by CaHA). Such a dual mechanism can be particularly advantageous in patients who simultaneously exhibit both soft tissue volume loss and cutaneous laxity, effectively addressing the structural and quality-related components of aging [
5,
25,
26,
27,
28,
29,
30,
31]. Several technical approaches to combination exist. In the simultaneous (mixed) injection technique, CaHA and HA are blended—either within the same syringe or carefully combined immediately prior to administration—to achieve a homogeneous composite that delivers both immediate and progressive benefits in a single pass, potentially enhancing integration and natural appearance. Ensuring compatibility, preventing phase separation or clumping, and standardizing the mixing procedure are critical for consistency. In the layered injection technique, the two products are delivered in discrete planes: CaHA is placed deeper to provide structural support and stimulate collagen over time, whereas HA is positioned more superficially to refine contours and supply early lift and hydration. This stratified approach allows the injector to leverage the distinct spatial and temporal properties of each filler for tailored esthetic correction [
5,
25,
26,
27,
30,
32]. Early reports suggest that the combined use does not inherently increase the risk profile beyond that of the individual agents, provided that each is used with the appropriate technique and awareness of potential interactions. Nevertheless, meticulous attention to product handling, injection depth, volume, and anatomical considerations remains essential to preserve safety and optimize outcomes [
25,
27,
30].
1.4. Objectives
The primary objective of this study was to rigorously evaluate the efficacy and safety of a hyper-diluted combination of calcium hydroxyapatite (CaHA) and hyaluronic acid (HA) fillers for comprehensive facial rejuvenation in subjects manifesting both soft tissue volume loss and skin laxity. The investigation targeted both the immediate esthetic effects—namely volumization and lifting—and the long-term dermal quality improvements attributable to collagen stimulation and matrix remodeling. Secondary aims included quantifying patient-reported satisfaction with regard to the naturalness, durability, and overall perceived improvement of outcomes, utilizing validated clinical scoring systems and standardized satisfaction instruments. Additionally, the study sought to codify a systematic, minimally invasive injection protocol that integrates the dual biological advantages of CaHA and HA, thereby offering a balanced treatment framework capable of delivering prompt visual enhancement while fostering sustained skin improvement.
2. Materials and Methods
This prospective pilot study enrolled nine consecutive adult patients, aged 30 to 65 years, who presented with both soft tissue volume loss and mild-to-moderate skin laxity in the facial region. Patients were recruited from a private practice outpatient clinic within the province of Rome using a convenience consecutive sampling approach; eligibility screening and enrollment procedures were documented to minimize selection bias. All the participants provided written informed consent prior to any intervention. Any deviations or protocol amendments were recorded. The inclusion criteria comprised the following: age between 30 and 65 years, clinically visible soft tissue volume loss in the midface and/or lower face, and mild-to-moderate skin laxity affecting facial contours. The exclusion criteria included active cutaneous infections, known autoimmune or connective tissue disorders, history of hypersensitivity or prior adverse reactions to dermal fillers, recent (within 6 months) facial esthetic procedures in the treatment zones, pregnancy or breastfeeding, coagulopathy or use of anticoagulant therapy with a resulting predisposition to bleeding, and any other condition that, in the investigator’s opinion, could confound efficacy or safety assessments.
The treatment used a hybrid injectable formulation consisting of calcium hydroxyapatite (CaHA) microspheres suspended within a hyaluronic acid (HA) carrier gel. To enhance diffusion for addressing laxity while retaining structural and biostimulatory properties, the composite was hyper-diluted in a 1:1 ratio with sterile normal saline immediately prior to injection. The dilution process was standardized: the contents of the CaHA-HA syringe were aspirated and mixed with an equal volume of sterile saline using a double-syringe connector, performing at least 20 back-and-forth passes to ensure homogenous dispersion while avoiding the introduction of air. The resulting low-viscosity mixture was used within a defined time window (e.g., within 10 min of preparation) to maintain consistency across patients. The rationale for the 1:1 dilution was to balance enhanced tissue spread (for laxity and skin quality) with the preservation of sufficient particulate content to sustain collagen stimulation.
Treatments were performed by experienced injectors using a combination of blunt-tip cannulas and fine-gauge needles, selected based on the anatomical region, target depth, and desired tissue effect. The diluted combination was delivered to predetermined facial zones exhibiting both volume deficit and cutaneous laxity: cheeks, jawline, nasolabial folds, and marionette lines. For deeper structural support—notably in the midface—the product was deposited in the subcutaneous plane (and, when appropriate, at the deep subcutaneous/preperiosteal interface) using fanning or linear threading techniques to re-establish projection and lift. In areas predominantly affected by skin laxity (e.g., lower face and jawline), the same diluted mixture was placed more superficially with a focus on broad, even distribution to optimize diffusion and stimulate skin quality improvement, while preserving natural contours and avoiding overcorrection. The total injected volume per session ranged from 3 to 6 mL, individualized according to the baseline deficit severity, regional response, and real-time clinical judgment. Injection sites, depth, and volumes were recorded per patient to allow transparency and reproducibility.
Efficacy and safety evaluations combined standardized clinical photography, blinded observer scoring, and patient-reported outcomes. Clinical photographs were taken at the baseline (pre-treatment), immediately post-procedure, and at scheduled follow-up visits at 1, 3, and 6 months. To ensure consistency, imaging was performed using the same camera system with fixed settings, uniform lighting, a neutral background, and standardized patient positioning and facial expression protocols. Photographs were de-identified and independently assessed by at least two blinded evaluators (physicians not involved in the treatment) to reduce observer bias; discrepancies were resolved via consensus. Both treating physician and patient provided subjective assessments using validated scales.
The primary efficacy measures included objective and subjective improvements as quantified by the following:
Merz Aesthetic Scale (MAS) to evaluate volume deficits, scored from 0 (no deficit) to 4 (very severe deficit);
Wrinkle Severity Rating Scale (WSRS) to rate the depth/severity of folds and rhytides on a five-point scale;
Global Aesthetic Improvement Scale (GAIS) to capture overall perceived esthetic change from both the investigator’s and patient’s perspectives (typically ranging from “very much improved” to “worsened”) [
33,
34,
35,
36,
37,
38,
39].
Patient-reported outcomes were collected using a structured Patient Satisfaction Questionnaire employing a five-point Likert scale (1 = very dissatisfied, 5 = very satisfied), assessing dimensions such as perceived overall improvement, naturalness of appearance, durability of results, comfort during and after treatment, and willingness to undergo retreatment. Adherence to follow-up and completeness of questionnaire data were tracked.
Safety evaluation encompassed both immediate and delayed adverse events. At each visit, clinical examination targeted common expected reactions (pain, erythema, swelling, bruising) as well as less frequent complications (nodules, granulomatous responses, infection, vascular compromise, and signs of tissue necrosis). Events were categorized by onset time, severity (using a predefined grading rubric), duration, and relationship to treatment. Investigators also queried patients for subjective symptoms and documented any interventions required. All adverse events were tabulated per patient.
Descriptive statistics were used to summarize demographic and clinical data; continuous variables are reported as means ± standard deviations (SD). Changes in MAS, WSRS, and GAIS scores between the baseline (pre-treatment) and each post-treatment follow-up were evaluated with paired t-tests. A two-tailed significance threshold of p < 0.05 was adopted. Effect sizes for these within-subject comparisons were quantified using Cohen’s d for dependent samples (calculated as the mean difference divided by the standard deviation of the differences), with conventional benchmarks for interpretation (small ≈ 0.2, medium ≈ 0.5, large ≥ 0.8). Ninety-five percent confidence intervals (CIs) for the mean differences were also computed to convey precision around the estimates. All statistical analyses were conducted using SPSS software version 29.0 (IBM Corp., Armonk, NY, USA).
3. Results
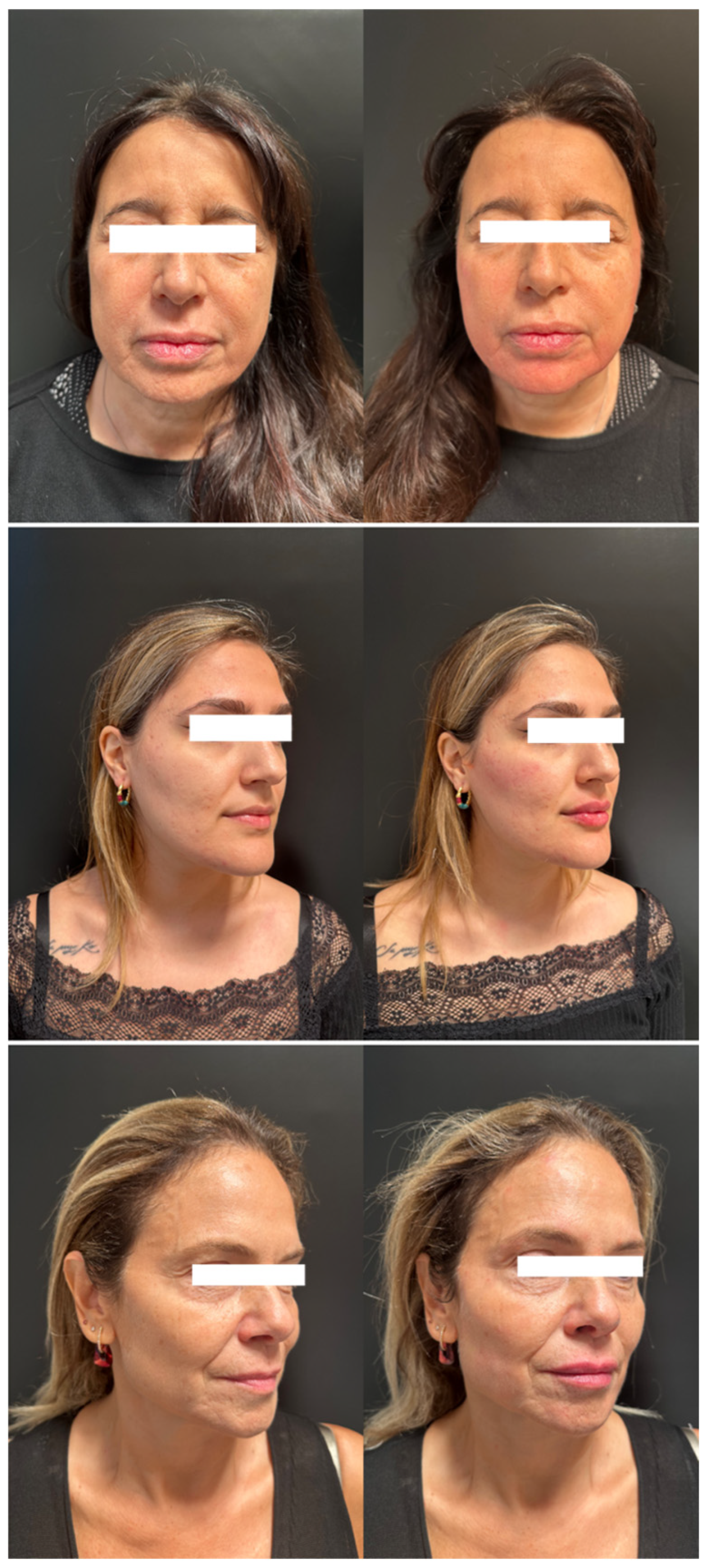
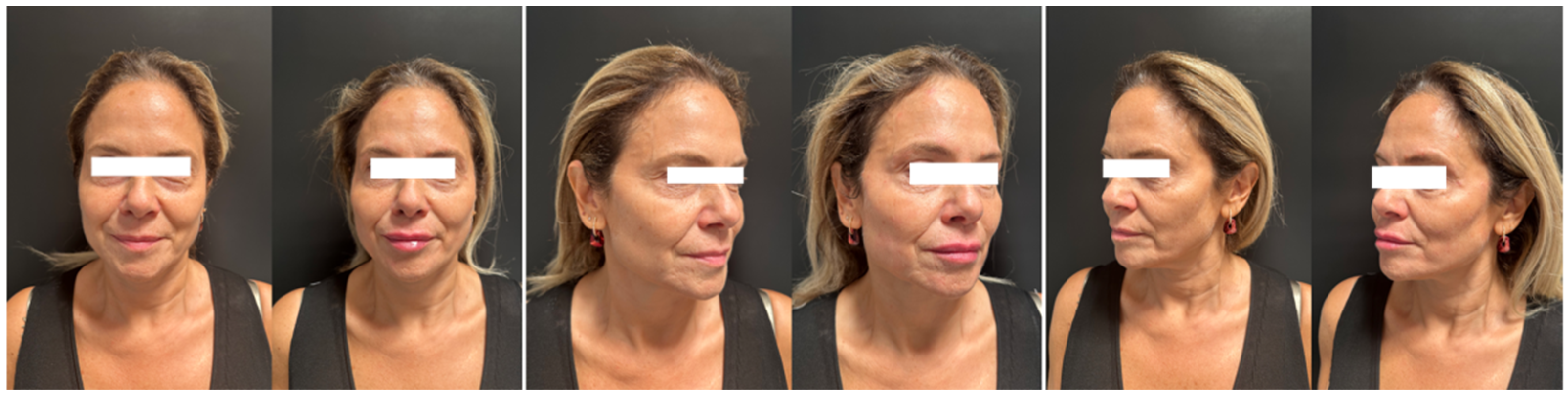
A total of nine patients underwent treatment with a diluted combination of CaHA and HA for facial rejuvenation, with pre- and post-treatment images analyzed to evaluate volume improvement, wrinkle correction, overall esthetic improvement, and safety (
Figure 1 and
Figure 2). The MAS scale was used to assess changes in upper cheek fullness, lower cheek fullness, jawline contour, and nasolabial folds. The WSRS scale measured the reduction in wrinkle severity, while the GAIS scale provided an overall evaluation of improvement (
Table 1).
3.1. Volume Improvement (MAS)
Pre-treatment scores showed a baseline mean of 2.17 ± 0.18 for upper cheek fullness, 2.06 ± 0.14 for lower cheek fullness, 2.00 ± 0.00 for jawline contour, and 3.00 ± 0.00 for nasolabial folds. Post-treatment, the scores significantly improved to 3.11 ± 0.20, 3.00 ± 0.18, 2.89 ± 0.33, and 2.50 ± 0.00, respectively. The mean differences were statistically significant for all MAS parameters (
p < 0.0001 for upper cheek, lower cheek, and nasolabial folds;
p = 0.0003 for jawline contour) (
Table 2). Effect size calculations demonstrated large effects for all areas (Cohen’s d: upper cheek = 5.28, lower cheek = 6.60, jawline = 4.10, nasolabial folds = 6.60), confirming the clinical relevance of these improvements (
Table 3).
3.2. Wrinkle Severity (WSRS)
The baseline mean WSRS score was 3.28 ± 0.18, indicating moderate to severe wrinkles, particularly in the nasolabial folds and midface. Post-treatment, the mean score decreased to 2.50 ± 0.00, representing mild to moderate wrinkles. The improvement was statistically significant (
p < 0.0001) (
Table 2) with a large effect size (Cohen’s d = 4.77) (
Table 3).
3.3. Overall Improvement (GAIS)
The GAIS evaluations demonstrated a consistent improvement across all patients, with all nine participants scoring “2” (very improved) post-treatment. This indicates significant rejuvenation while maintaining a natural esthetic appearance.
3.4. Safety Assessment
No immediate or delayed adverse events were reported during the treatment or follow-up periods. Specifically, there were no occurrences of pain, erythema, bruising, nodules, or other complications. This highlights the excellent safety profile of the treatment protocol, reinforcing its reliability and tolerability.
3.5. Statistical Overview
Paired
t-tests confirmed the significance of improvements for all metrics, with 95% confidence intervals (CI) for mean differences that did not cross zero. For instance, the CI for upper cheek improvement was [0.85, 1.02], and for WSRS, it was [−0.86, −0.70]. These findings further validate the efficacy, safety, and reliability of the treatment protocol (
Table 4).
4. Discussion
The results of this study support the potential of a diluted combination of calcium hydroxyapatite (CaHA) and hyaluronic acid (HA) fillers as an effective and well-tolerated strategy for facial rejuvenation, achieving both immediate volumetric enhancement and longer-term skin quality improvement. While the use of diluted CaHA to improve diffusion and to harness its biostimulatory effects is established in the literature [
3,
4,
12], combining it with diluted HA in a unified protocol remains relatively underexplored, and this work contributes preliminary evidence toward that integration. The rationale—pairing HA’s immediate hydrodynamic and volumizing properties with CaHA’s capacity to induce collagen deposition—offers a biologically plausible synergy that can address the dual components of aging: structural volume loss and cutaneous laxity. The dilution approach further facilitates broader tissue spread and smoother integration, aiming for a natural, harmonious result rather than overt projection [
8,
25,
26,
27,
28,
29,
32].
Quantitative outcomes reinforce this conceptual framework. Improvements in the Merz Aesthetic Scale (MAS) for upper and lower cheek fullness and jawline contour indicate that the combination not only restored deeper soft tissue volume but also had a measurable impact on more superficial contour and perceived laxity. The observed changes (e.g., mean increases in fullness approximating +0.9 points) suggest clinically meaningful modification of facial topography. Reported effect sizes (Cohen’s d) were very large, reflecting substantial within-subject shifts; however, these results must be interpreted with caution because the small sample size inflates the apparent magnitude of statistical effects and increases the risk of type I error. With only nine patients, the variability across individuals cannot be adequately captured, and even consistent improvements should be considered exploratory rather than definitive. Such large estimates can be amplified in limited cohorts, and the possibility of scale ceiling/floor effects or restricted variance affecting standardized metrics cannot be excluded. Therefore, replication in larger and more heterogeneous cohorts is essential to determine whether the observed magnitude of benefit is reproducible and generalizable.
The Wrinkle Severity Rating Scale (WSRS) results, particularly the reduction in nasolabial fold scores, demonstrate a statistically significant attenuation of rhytides, aligning with the dual mechanism of action hypothesized. HA’s immediate volumizing and hydrating influence likely contributed to the initial softening of lines, while CaHA’s ongoing stimulation of fibroblasts and new collagen synthesis may support durability and improvement in skin texture over time. This temporal layering of effects—early visible improvement complemented by gradual matrix remodeling—fits with prior mechanistic insights into combined filler use and biostimulatory strategies in esthetic practice [
25,
26,
27,
28,
29].
The safety findings in this cohort were favorable, with no observed immediate or delayed adverse events such as nodules, significant erythema, infection, or vascular compromise. The dilution strategy may have contributed to reduced tissue trauma and more predictable diffusion, potentially minimizing focal overcorrection or high-pressure deposition that can underlie complications. Nevertheless, the absence of complications in a small sample does not preclude their occurrence in broader clinical application; rare events may only emerge with larger numbers or longer follow-up. Thus, while the preliminary safety profile is reassuring, continued vigilance and systematic reporting in subsequent studies are warranted.
Overall esthetic improvement as captured by the Global Aesthetic Improvement Scale (GAIS), with uniform ratings in the “very improved” category, suggests high concordance between clinical change and perceived outcome. The consistency in patient-perceived naturalness and satisfaction underscores that the protocol may strike a balance between visible rejuvenation and preservation of individualized, non-“overfilled” appearance—an increasingly valued goal in contemporary esthetic medicine.
Despite these promising observations, the study design imposes severe limitations. The lack of a control or comparator group prevents any definitive attribution of clinical changes to the combined diluted protocol itself, since it remains unclear how outcomes might differ if CaHA or HA were used alone, or if alternative strategies were employed. In addition, the very small sample size restricts the external validity, reduces the ability to detect less common adverse events, and limits the statistical robustness of the analyses. These two factors—absence of control and limited cohort size—together mean that the present findings should be viewed as hypothesis generating rather than confirmatory evidence. Follow-up was limited to six months, leaving longer-term durability and the trajectory of biostimulatory changes incompletely characterized. Moreover, while validated scales were employed, they are partially subjective and ordinal, making subtle gradations and inter-rater variability important considerations; future work could be strengthened by incorporating objective imaging modalities (e.g., high-resolution ultrasound, MRI, or skin elasticity measurements) to quantify tissue remodeling and volumetric change more precisely.
In sum, the combined hyper-diluted CaHA–HA protocol represents a promising evolution in minimally invasive facial rejuvenation, offering a single-session approach that targets both volumetric deficits and skin quality degradation. The interplay of immediate and delayed effects, coupled with an apparently favorable tolerability profile, suggests applicability for patients with mild-to-moderate aging changes who seek natural, durable enhancement. However, rigorous validation through randomized controlled trials with larger cohorts, longer follow-up, and appropriate comparators will be essential to establish reproducibility, confirm durability, and delineate the true risk–benefit profile of this approach.
5. Conclusions
This prospective pilot study provides exploratory evidence that a hyper-diluted combination of calcium hydroxyapatite (CaHA) and hyaluronic acid (HA) fillers may represent a safe and effective modality for comprehensive facial rejuvenation. The protocol yielded statistically significant and clinically meaningful improvements in mid- and lower-facial volume, as reflected by Merz Aesthetic Scale enhancements, as well as robust reductions in wrinkle severity on the Wrinkle Severity Rating Scale. Jawline definition also improved markedly, underscoring the technique’s capacity to address both deep structural deficits and more superficial contour irregularities in a single treatment session. Importantly, no immediate or delayed adverse events were observed, suggesting that the dilution method may mitigate tissue trauma and optimize filler integration. Nevertheless, these results should be regarded as preliminary and hypothesis generating, given the very small sample size and absence of a control group. Therefore, although the combined dilution strategy appears to offer an innovative, minimally invasive solution for both immediate esthetic enhancement and longer-term skin quality improvement, the present findings cannot establish definitive efficacy or safety, but rather serve to inform future study design. Accordingly, the contribution of this work lies in providing initial clinical observations that support the rationale for larger, randomized, controlled trials with extended follow-up. Such studies will be essential to confirm reproducibility, refine injection parameters, and more comprehensively delineate the risk–benefit profile of this approach.
Author Contributions
Conceptualization, L.A., C.C., and A.C.; methodology, L.A., C.C., and A.C.; formal analysis, L.A. and C.C.; investigation, C.C. and A.C.; resources, A.C.; data curation, L.A.; writing—original draft preparation, L.A.; writing—review and editing, L.A., C.C., A.C., and G.P.; validation, G.P. and C.C.; visualization, L.A.; supervision, G.P.; project administration, L.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Unit of Dermatology, Department of Medical-Surgical Science and Biotechnologies, Sapienza University of Rome, A. Fiorini Hospital, Terracina, Latina, Italy (protocol code 23/1647 and date of approval 12 January 2022).
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The author, Alessandra Cecchini, works as a physician in private practice. The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Van Loghem, J.; Yutskovskaya, Y.A.; Philip Werschler, W. Calcium hydroxylapatite: Over a decade of clinical experience. J. Clin. Aesthet. Dermatol. 2015, 8, 38–49. [Google Scholar]
- Guida, S.; Galadari, H. A systematic review of Radiesse/calcium hydroxylapatite and carboxymethylcellulose: Evidence and recommendations for treatment of the face. Int. J. Dermatol. 2024, 63, 150–160. [Google Scholar] [CrossRef]
- Goldie, K.; Peeters, W.; Alghoul, M.; Butterwick, K.; Casabona, G. Global Consensus Guidelines for the Injection of Diluted and Hyperdiluted Calcium Hydroxylapatite for Skin Tightening. Dermatol. Surg. 2018, 44 (Suppl. S1), S32–S41. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.T.; Figueredo, V.; da Cunha, A.L.G. Consensus Recommendations for the Use of Hyperdiluted Calcium Hydroxyapatite (Radiesse) as a Face and Body Biostimulatory Agent. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2160. [Google Scholar] [CrossRef]
- Fisher, S.M.; Borab, Z.; Weir, D.; Rohrich, R.J. The Emerging Role of Biostimulators as an Adjunct in Facial Rejuvenation: A Systematic Review. J. Plast. Reconstr. Aesthetic Surg. 2024, 92, 118–129. [Google Scholar] [CrossRef]
- Galadari, H.; Guida, S. A systematic review of Radiesse® (calcium hydroxylapatite): Evidence and recommendations for the body. Int. J. Dermatol. 2024, 63, 881–889. [Google Scholar] [CrossRef]
- Coleman, K.M.; Voigts, R.; Devore, D.P.; Termin, P.; Coleman, I.W.P. Neocollagenesis after Injection of Calcium Hydroxylapatite Composition in a Canine Model. Dermatol. Surg. 2008, 34 (Suppl. S1), S53–S55. [Google Scholar]
- Yutskovskaya, Y.; Kogan, E.; Leshunov, E. A randomized, split-face, histomorphologic study comparing a volumetric calcium hydroxylapatite and a hyaluronic acid-based dermal filler. J. Drugs Dermatol. 2014, 13, 1047–1052. [Google Scholar] [PubMed]
- Berlin, A.L.; Hussain, M.; Goldberg, D.J. Calcium hydroxylapatite filler for facial rejuvenation: A histologic and immunohistochemical analysis. Dermatol. Surg. 2008, 34 (Suppl. S1), S64–S67. [Google Scholar] [CrossRef]
- Nowag, B.; Casabona, G.; Kippenberger, S.; Zöller, N.; Hengl, T. Calcium hydroxylapatite microspheres activate fibroblasts through direct contact to stimulate neocollagenesis. J. Cosmet. Dermatol. 2023, 22, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Meçani, R.; Niehot, C.D.; Phillips, T.; Kolb, J.; Daughtry, H.; Muka, T. Skin Regeneration-Related Mechanisms of Calcium Hydroxylapatite (CaHA): A Systematic Review. Front. Med. 2023, 10, 1195934. [Google Scholar] [CrossRef]
- Halepas, S.; Christiansen, C.; Ferneini, E.M. Diluted and Hyperdiluted Calcium Hydroxylapatite for Skin Tightening. J. Oral Maxillofac. Surg. 2022, 80, S67–S68. [Google Scholar] [CrossRef]
- Lorenc, Z.P.; Black, J.M.; Cheung, J.S.; Chiu, A.; Del Campo, R.; Durkin, A.J.; Graivier, M.; Green, J.B.; Kwok, G.P.; Marcus, K.; et al. Skin Tightening with Hyperdilute CaHA: Dilution Practices and Practical Guidance for Clinical Practice. Aesthet. Surg. J. 2022, 42, NP29–NP37. [Google Scholar] [CrossRef]
- Pavicic, T. Calcium hydroxylapatite filler: An overview of safety and tolerability. J. Drugs Dermatol. 2013, 12, 996–1002. [Google Scholar]
- Sadick, N.S.; Katz, B.E.; Roy, D. A multicenter, 47-month study of safety and efficacy of calcium hydroxylapatite for soft tissue augmentation of nasolabial folds and other areas of the face. Dermatol. Surg. 2007, 33 (Suppl. S2), S122–S126, discussion S126-S127. [Google Scholar] [PubMed]
- Kadouch, J.A. Calcium hydroxylapatite: A review on safety and complications. J. Cosmet. Dermatol. 2017, 16, 152–161. [Google Scholar] [CrossRef]
- Tezel, A.; Fredrickson, G.H. The science of hyaluronic acid dermal fillers. J. Cosmet. Laser Ther. 2008, 10, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Beasley, K.L.; Weiss, M.A.; Weiss, R.A. Hyaluronic acid fillers: A comprehensive review. Facial Plast. Surg. 2009, 25, 86–94. [Google Scholar] [CrossRef]
- Monheit, G.D.; Coleman, K.M. Hyaluronic acid fillers. Dermatol. Ther. 2006, 19, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J. Should Hyaluronic Acid Fillers Be Diluted? J. Drugs Dermatol. 2014, 13, 1437–1438. [Google Scholar]
- Lambros, V. A Technique for Filling the Temples with Highly Diluted Hyaluronic Acid: The “Dilution Solution”. Aesthet. Surg. J. 2011, 31, 89–94. [Google Scholar] [CrossRef]
- Rohrich, R.J.; Bartlett, E.L.; Dayan, E. Practical Approach and Safety of Hyaluronic Acid Fillers. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2172. [Google Scholar] [CrossRef] [PubMed]
- Stojanovič, L.; Majdič, N. Effectiveness and safety of hyaluronic acid fillers used to enhance overall lip fullness: A systematic review of clinical studies. J. Cosmet. Dermatol. 2019, 18, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Master, M. Hyaluronic Acid Filler Longevity and Localization: Magnetic Resonance Imaging Evidence. Plast. Reconstr. Surg. 2021, 147, 50e–53e. [Google Scholar] [CrossRef]
- Chang, J.W.; Koo, W.Y.; Kim, E.K.; Lee, S.W.; Lee, J.H. Facial Rejuvenation Using a Mixture of Calcium Hydroxylapatite Filler and Hyaluronic Acid Filler. J. Craniofacial Surg. 2019, 31, e18–e21. [Google Scholar] [CrossRef]
- Yag-Howard, C.; DeNigris, J. Novel Filler Technique: Hyaluronic acid and Calcium hydroxylappetite mixture resulting in favorable esthetic and longevity outcomes. Int. J. Womens Dermatol. 2021, 7, 817–819. [Google Scholar] [CrossRef]
- Fakih-Gomez, N.; Kadouch, J. Combining Calcium Hydroxylapatite and Hyaluronic Acid Fillers for Aesthetic Indications: Efficacy of an Innovative Hybrid Filler. Aesthetic Plast. Surg. 2022, 46, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Bravo, B.; Carvalho, R.; Bravo, L.; Penedo, L.; Elias, M. Blending Hyaluronic Acid and Calcium Hydroxylapatite for Injectable Facial Dermal Fillers: A Clinical and Ultrasonography Assessment. Cosmetics 2024, 11, 61. [Google Scholar] [CrossRef]
- Bravo, B.S.F.; de Almeida, T.S.C.; de Melo Carvalho, R.; Machado, C.J.; Bravo, L.G.; Elias, M.C. Dermal Thickness Increase and Aesthetic Improvement with Hybrid Product Combining Hyaluronic Acid and Calcium Hydroxyapatite: A Clinical and Sonographic Analysis. Plast. Reconstr. Surg. Glob. Open 2023, 11, e5055. [Google Scholar] [CrossRef]
- Urdiales-Gálvez, F.; Braz, A.; Cavallini, M. Facial rejuvenation with the new hybrid filler HArmonyCaTM: Clinical and aesthetic outcomes assessed by 2D and 3D photographs, ultrasound, and elastography. J. Cosmet. Dermatol. 2023, 22, 2186–2197. [Google Scholar] [CrossRef]
- Barone, M.; Salzillo, R.; Persichetti, P. Minimally Invasive Facial Rejuvenation with the Hybrid Filler HArmonyCaTM. Aesthetic Plast. Surg. 2023, 48, 5251–5253. [Google Scholar] [CrossRef]
- Proietti, I.; Spagnoli, A.; Favaroni, A.; Gritti, A.; Dal Canton, M.; Quartucci, S.; Sciuto, C.; Bertossi, D.; Patalano, M.; Cavallini, M.; et al. HArmonyCaTM hybrid filler to restore connective tissue: An Italian real-life retrospective study. J. Cosmet. Dermatol. 2024, 23, 3883–3892. [Google Scholar] [CrossRef]
- Carruthers, J.; Flynn, T.C.; Geister, T.L.; Görtelmeyer, R.; Hardas, B.; Himmrich, S.; Jones, D.; Kerscher, M.; De Maio, M.; Mohrmann, C.; et al. Validated assessment scales for the mid face. Dermatol. Surg. 2012, 38, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Kefalas, N.; Ruiz Del Cueto, S.; Urdiales-Gálvez, F.; Barry, L.; Gritti, A.; Marchac, A.; Lim, M.; de la Guardia, C.; Kerson, G.; Silberberg, M. Development and Validation of a Photonumeric Scale to Assess Marionette Lines. Dermatol. Surg. 2024, 50, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Pooth, R.; Prinz, V.; Cajkovsky, M.; Green, J.B.; Hernandez, C.A.; Pavicic, T.; Mueller, D.S.; Sattler, S.; Klepetko, H.; Fabi, S.G.; et al. Validated 5-point photonumeric scales for the assessment of the jowls and chin. J. Cosmet. Dermatol. 2022, 21, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Moradi, A.; Bloom, J.; Verma, A.; Cheng, N.; Duncan, A. Development and Validation of a Photonumeric Scale for Evaluation of Jawline Contour. J. Drugs Dermatol. 2023, 22, 203–209. [Google Scholar] [CrossRef]
- Carruthers, A.; Carruthers, J. A validated facial grading scale: The future of facial ageing measurement tools? J. Cosmet. Laser Ther. 2010, 12, 235–241. [Google Scholar] [CrossRef]
- Day, D.J.; Littler, C.M.; Swift, R.W.; Gottlieb, S. The wrinkle severity rating scale: A validation study. Am. J. Clin. Dermatol. 2004, 5, 49–52. [Google Scholar] [CrossRef]
- Callan, P.; Goodman, G.J.; Carlisle, I.; Liew, S.; Muzikants, P.; Scamp, T.; Halstead, M.B.; Rogers, J.D. Efficacy and safety of a hyaluronic acid filler in subjects treated for correction of midface volume deficiency: A 24 month study. Clin. Cosmet. Investig. Dermatol. 2013, 6, 81–89. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).