Advances in Extraction Technologies of Silybum marianum L. and Its Role in Protecting Against Skin Damage
Abstract
1. Introduction
2. Methodology
3. Flavonolignans from Milk Thistle
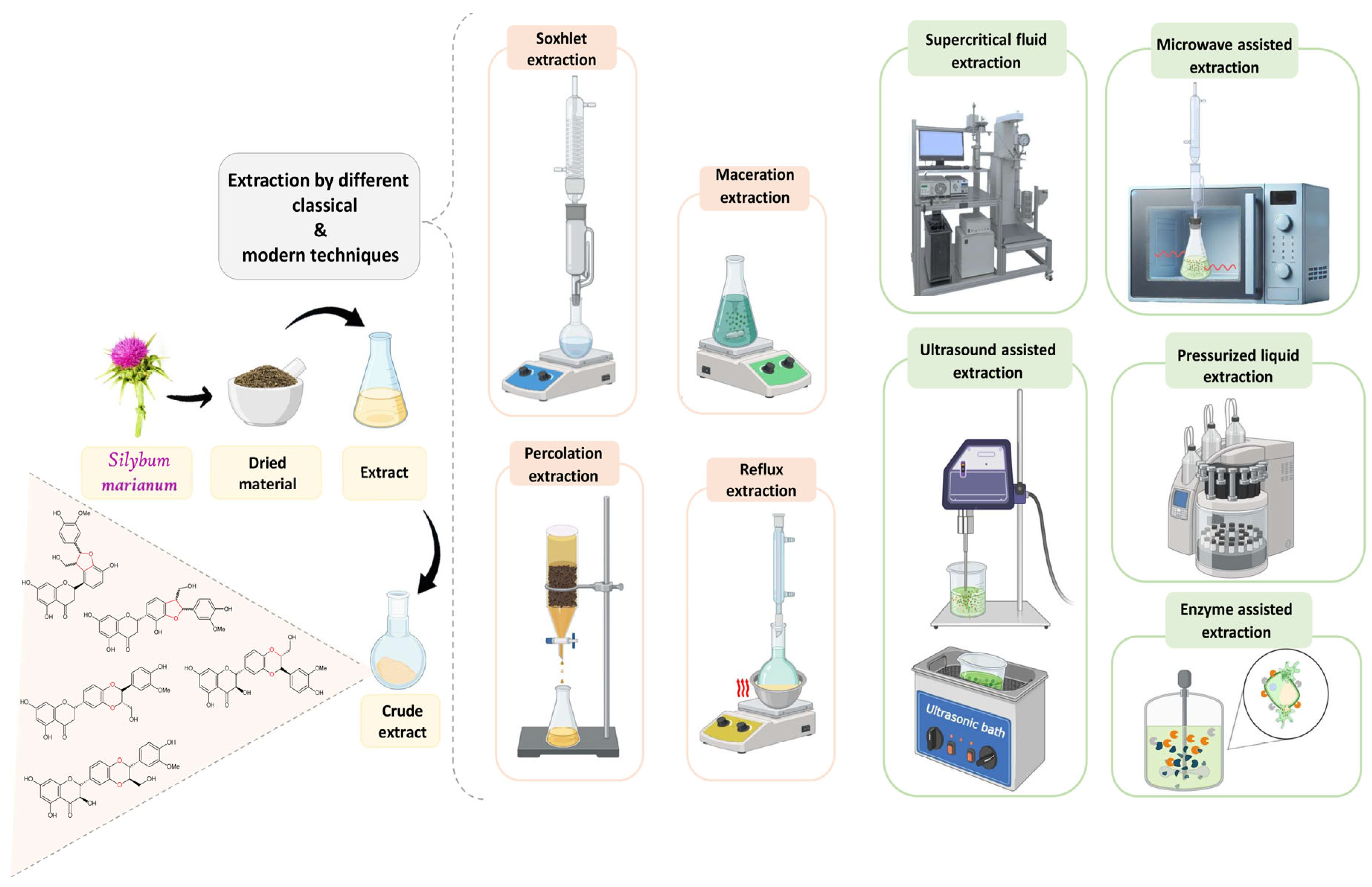
4. Extraction of Bioactive Compounds from Milk Thistle
- –
- Microwave Assisted Extraction (MAE).
- –
- Pressurized Liquid Extraction (PLE)
- –
- Subcritical Water Extraction (SWE)
- –
- Ultrasound-Assisted Extraction (UAE)
- –
- Supercritical Fluid Extraction (SFE)
- –
- Enzyme Assisted Extraction (EAE)
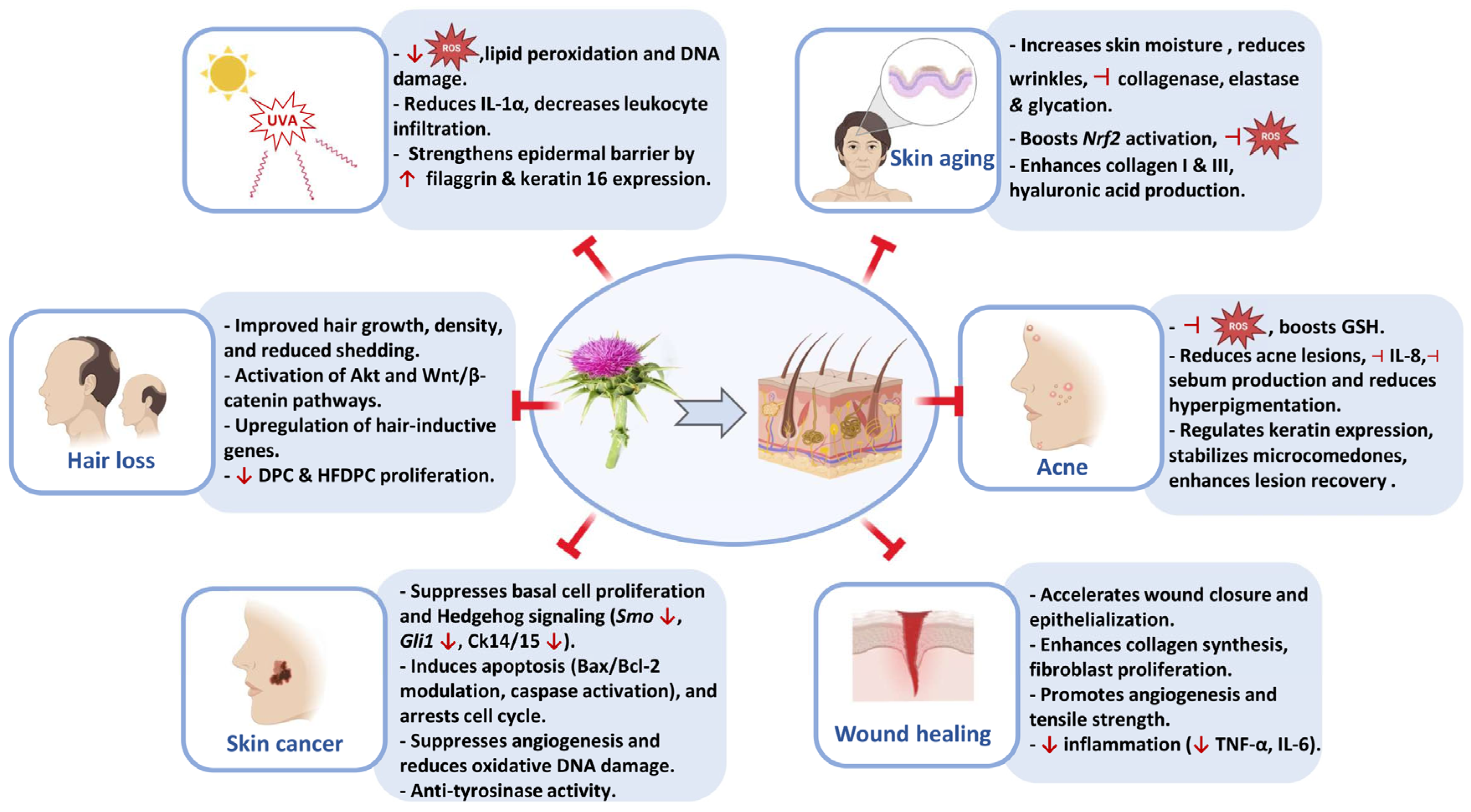
5. Skin-Protective Activity of Silymarin
5.1. Skin Aging
5.2. UVA-Induced Skin Damage
5.3. Acne
5.4. Hair Loss
5.5. Wound Healing
5.6. Skin Cancer
| Skin Disorder/Condition | Model | Active Compound | Concentration/Dose | Biological Effect | Reference |
|---|---|---|---|---|---|
| Skin aging | In vitro cell-free assay | Silybum marianum seed extract | Not disclosed |
| [56] |
| Human clinical study | Silybum marianum seed extract | 4% topical (W/O emulsion) |
| [67] | |
| Human skin explants | Silibinin (from Silybum marianum flower extract) | 1% topical application |
| [77] | |
| D-galactose-induced aging mice | Silybum marianum extract | 50, 100, 200 mg/kg, 2 mg/cm2 (topical) |
| [78] | |
| In vitro enzyme assays | Silymarin Dehydrosilybin Silybin | 0.05–100 mg/L 4.1 mg/L (anti-elastase), 11.2 mg/L (anti-collagenase) 59.1 mg/L (anti-elastase), 25.2 mg/L (anti-collagenase) |
| [79] | |
| Human clinical study | Silybum marianum seed oil | 1% in cream |
| [123] | |
| Human skin explants | Silybum marianum extract | 0.8% topical application |
| [124] | |
| UVA-induced skin damage | Human keratinocytes (HaCaT cell line) | Silymarin | 0.7–34 mg/L |
| [68] |
| C3H/HeN mice | Silymarin | 1 mg/cm2 (topical) |
| [89] | |
| Human Reconstructed Epidermis (RHE) | Silymarin | 1% topical |
| [90] | |
| Human keratinocytes (HaCaT cell line) | Silymarin | 10–250 µg/mL |
| [91] | |
| HaCaT keratinocytes | Silibinin | 75 µM |
| [125] | |
| Primary human dermal fibroblasts | Silymarin and Silybin | 3.013–36.15 mg/L |
| [126] | |
| Acne | Human clinical trial | Silymarin | 210 mg/day orally |
| [69] |
| Human clinical trial | Silymarin | 140 mg oral tablet |
| [96] | |
| Human clinical study | Silymarin | 0.5% topical (in antioxidant serum) |
| [97] | |
| Human clinical trial | Silymarin | 1.4% topical |
| [98] | |
| Human clinical study | Silybum marianum fruit extract (SMFE, patented) | Not disclosed |
| [99] | |
| Human observational study | Silybum marianum fruit extract | 7% topical |
| [127] | |
| Human clinical study | Silybum marianum fruit extract (patented as ComedoclastinTM) | Not disclosed |
| [128] | |
| Hair loss | 3D spheroid-cultured human dermal papilla cells | Silibinin | 10 µM |
| [70] |
| Human follicle dermal papilla cells (DPCs) in vitro | Silymarin | 50 & 100 µM |
| [104] | |
| Human clinical trial | Silybum marianum extract | Part of a serum (exact % not disclosed) |
| [105] | |
| Human dermal papilla cells (HFDPC) in vitro Human clinical trial | Apigenin (from Silybum marianum flower extract) | 2% apigenin (10–100 µg/mL) 0.001% apigenin (0.05% extract in shampoo formulation) |
| [106] | |
| Wound healing | Wistar rats (experimental) | Silibinin | 6 & 12 mg/mL topically |
| [71] |
| Human skin fibroblasts in vitro | Silymarin | 4.5–36 µg/mL |
| [110] | |
| Swiss albino mice | Silibinin | 0.2% hydrogel topically |
| [111] | |
| Wistar rats (abdominal excision) | Silymarin ointment | 2% (in eucerin base) |
| [112] | |
| Wistar rats | Silymarin | 3% topical |
| [113] | |
| Human patients (clinical trial) | Silymarin | 140 mg/day orally |
| [114] | |
| Human clinical trial (primiparous women) | Silybum marianum seeds ointment | 3% topical (in eucerin base) |
| [115] | |
| Wistar rats | Silymarin nanoemulsion | 1% (in loaded chitosan gel) |
| [129] | |
| Skin cancer | Ptch+/− mice, UVB-induced BCC in vivo | Silibinin | 9 mg/200 µL topically |
| [72] |
| Human melanoma cells (A375, Hs294t) Athymic nude mice (A375 xenografts) | Silymarin | 10–80 µg/mL 500 mg/kg orally |
| [119] | |
| B16F10 melanoma cells | Silymarin-loaded in β-cyclodextrin nanosponges | 10–200 µg/mL |
| [120] | |
| B16 murine melanoma cells in vitro Albino mice in vivo | Silymarin-loaded NLC gel Silymarin-NLC gel | 50, 100, 200 μg/mL (applied in gel) 1 mg/cm2 |
| [121] | |
| Balb/c mice in vivo Human lymphocytes in vitro | Silybum marianum leaf extract | 100 mg/kg orally 10–1000 µg/mL |
| [122] | |
| Human melanoma (A2058) and epidermal carcinoma (A431) | Silybum marianum callus extract | 15–125 µg/mL |
| [130] | |
| B16F10 melanoma cell line | Silymarin Inclusion Complex-Based Gel | 10–500 µg/mL |
| [131] |
6. Limitations
7. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abouzid, S.; Ahmed, O.M. Silymarin Flavonolignans: Structure–Activity Relationship and Biosynthesis. Stud. Nat. Prod. Chem. 2013, 40, 469–484. [Google Scholar] [CrossRef]
- Chambers, C.S.; Holečková, V.; Petrásková, L.; Biedermann, D.; Valentová, K.; Buchta, M.; Křen, V. The Silymarin Compositio and Why Does It Matter??? Food Res. Int. 2017, 100, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Batanouny, K.; Hammouda, F.M.; Ismail, S.I.; Abdel-Azim, N.S.; Shams, K.A. A Guide to Medicinal Plants in North Africa; IUCN Centre for Mediterranean Cooperation: Malaga, Spain, 2005. [Google Scholar]
- Karkanis, A.; Bilalis, D.; Efthimiadou, A. Cultivation of Milk Thistle (Silybum marianum L. Gaertn.), a Medicinal Weed. Ind. Crops Prod. 2011, 34, 825–830. [Google Scholar] [CrossRef]
- Montemurro, P.; Fracchiolla, M.; Lonigro, A. Effects of Some Environmental Factors on Seed Germination and Spreading Potentials of Silybum marianum Gaertner. Ital. J. Agron. 2007, 2, 315–320. [Google Scholar] [CrossRef]
- Eita, A.A.B. Milk Thistle (Silybum marianum (L.) Gaertn.): An Overview about Its Pharmacology and Medicinal Uses with an Emphasis on Oral Diseases. J. Oral Biosci. 2022, 64, 71–76. [Google Scholar] [CrossRef]
- Kaur, A.K.; Wahi, A.K.; Kumar, B.; Bhandari, A.; Prasad, N. Milk Thistle (Silybum marianum): A Review. Int. J. Pharm. Res. Dev. 2011, 3, 1–10. [Google Scholar]
- Pepping, J. Milk Thistle: Silybum marianum. Am. J. Heal. Pharm. 1999, 56, 1195. [Google Scholar] [CrossRef]
- Giordano, M.; Luongo, G.; Davinelli, S.; Ladhari, A.; Nappo, G.R.; Giordano, M. Silybum marianum: Not Just Silymarin and Flavonolignans. Rec. Nat. Prod. 2021, 15, 338–350. [Google Scholar] [CrossRef]
- Hlangothia, D.; Abdel Rahman, F.; Nguyen, T. Distribution of Silymarin in the Fruit of Silybum marianum L. Pharm. Anal. Acta 2016, 7, 1000511. [Google Scholar] [CrossRef]
- Karimi, G.; Vahabzadeh, M.; Lari, P.; Rashedinia, M.; Moshiri, M. Silymarin, a Promising Pharmacological Agent for Treatment of Diseases. Iran. J. Basic Med. Sci. 2011, 14, 308–317. [Google Scholar]
- Abenavoli, L.; Capasso, R.; Milic, N.; Capasso, F. Milk Thistle in Liver Diseases: Past, Present, Future. Phytother. Res. 2011, 24, 1423–1432. [Google Scholar] [CrossRef]
- Loguercio, C.; Festi, D. Silybin and the Liver: From Basic Research to Clinical Practice. World J. Gastroenterol. 2011, 17, 2288–2301. [Google Scholar] [CrossRef]
- Biedermann, D.; Vavříková, E.; Cvak, L.; Křen, V. Chemistry of Silybin. Nat. Prod. Rep. 2014, 31, 1138–1157. [Google Scholar] [CrossRef]
- Ung, L.; Le, Q.-U.; Lay, H.-L.; Wu, M.-C.; Kumar Joshi, R.; Ming-Chang Wu, C. Phytoconstituents and Pharmacological Activities of Silybum marianum (Milk Thistle): A Critical Review. Am. J. Essent. Oils Nat. Prod. 2018, 6, 41–47. [Google Scholar]
- Das, S.K.; Mukherjee, S. Biochemical and Immunological Basis of Silymarin Effect, a Milk Thistle (Silybum marianum) against Ethanol-Induced Oxidative Damage. Toxicol. Mech. Methods 2012, 22, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Talbi, A.; Khelili, K.; Remita, F.; Abdennour, C. The Benefit of Silybum marianum in Ethanol-Induced Reprotoxicity of Male Wistar Rat. Braz. J. Pharm. Sci. 2022, 58, 1–10. [Google Scholar] [CrossRef]
- Song, Z.; Deaciuc, I.; Song, M.; Lee, D.Y.W.; Liu, Y.; Ji, X.; McClain, C. Silymarin Protects against Acute Ethanol-Induced Hepatotoxicity in Mice. Alcohol. Clin. Exp. Res. 2006, 30, 407–413. [Google Scholar] [CrossRef]
- Kim, Y.C.; Na, J.D.; Kwon, D.Y.; Park, J.H. Silymarin Prevents Acetaminophen-Induced Hepatotoxicity via up-Regulation of the Glutathione Conjugation Capacity in Mice. J. Funct. Foods 2018, 49, 235–240. [Google Scholar] [CrossRef]
- Okiljević, B.; Martić, N.; Govedarica, S.; Andrejić Višnjić, B.; Bosanac, M.; Baljak, J.; Pavlić, B.; Milanović, I.; Rašković, A. Cardioprotective and Hepatoprotective Potential of Silymarin in Paracetamol-Induced Oxidative Stress. Pharmaceutics 2024, 16, 520. [Google Scholar] [CrossRef]
- Rašković, A.; Stilinović, N.; Kolarović, J.; Vasović, V.; Vukmirović, S.; Mikov, M. The Protective Effects of Silymarin against Doxorubicin-Induced Cardiotoxicity and Hepatotoxicity in Rats. Molecules 2011, 16, 8601–8613. [Google Scholar] [CrossRef] [PubMed]
- Thi, D.; Lien, P.; Thi, C.; Hoang, K.; Hanh, N.T. Hepatoprotective Effect of Silymarin on Chronic Hepatotoxicity in Mice Induced by Carbon Tetrachloride. J. Pharmacogn. Phytochem. 2016, 5, 262–266. [Google Scholar]
- Chtourou, Y.; Garoui, E.M.; Boudawara, T.; Zeghal, N. Therapeutic Efficacy of Silymarin from Milk Thistle in Reducing Manganese-Induced Hepatic Damage and Apoptosis in Rats. Hum. Exp. Toxicol. 2013, 32, 70–81. [Google Scholar] [CrossRef]
- Farjad, E.; Momeni, H.R. Silymarin Ameliorates Oxidative Stress and Enhances Antioxidant Defense System Capacity in Cadmium-Treated Mice. Cell J. 2018, 20, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Oda, S.S.; El-Ashmawy, I.M. Protective Effect of Silymarin on Mercury-Induced Acute Nephro-Hepatotoxicity in Rats. Glob. Vet. 2012, 9, 376–383. [Google Scholar] [CrossRef]
- Emadi, S.A.; Rahbardar, M.G.; Mehri, S.; Hosseinzadeh, H. A Review of Therapeutic Potentials of Milk Thistle (Silybum marianum L.) and Its Main Constituent, Silymarin, on Cancer, and Their Related Patents. Iran. J. Basic Med. Sci. 2022, 25, 1166–1176. [Google Scholar] [CrossRef]
- Koltai, T.; Fliegel, L. Role of Silymarin in Cancer Treatment: Facts, Hypotheses, and Questions. J. Evid. Based Integr. Med. 2022, 27, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Juma’a, K.M.; Ahmed, Z.A.; Numan, I.T.; Hussain, S.A.R. Dose-Dependent Anti-Inflammatory Effect of Silymarin in Experimental Animal Model of Chronic Inflammation. Afr. J. Pharm. Pharmacol. 2009, 3, 242–247. [Google Scholar]
- Lee, D.G.; Kim, H.K.; Park, Y.; Park, S.C.; Woo, E.R.; Jeong, H.G.; Hahm, K.S. Gram-Positive Bacteria Specific Properties of Silybin Derived from Silybum marianum. Arch. Pharm. Res. 2003, 26, 597–600. [Google Scholar] [CrossRef]
- Sobolová, L.; Škottová, N.; Večeřa, R.; Urbánek, K. Effect of Silymarin and Its Polyphenolic Fraction on Cholesterol Absorption in Rats. Pharmacol. Res. 2006, 53, 104–112. [Google Scholar] [CrossRef]
- Nautiyal, B.; Kumar, A.; Malik, J.K. SARS-CoV-2: Silibinin Prospects in Antiviral Drug Development. EAS J. Anaesthesiol. Crit. Care 2021, 3, 55–59. [Google Scholar] [CrossRef]
- Begum, S.A.; Sahai, M.; Ray, A.B. Non-Conventional Lignans: Coumarinolignans, Flavonolignans, and Stilbenolignans. Fortschr. Chem. Org. Naturst. Prog. Chem. Org. Nat. Prod. 2010, 93, 1–70. [Google Scholar]
- Nadeem, M.; Khan, I.T.; Khan, F.; Shah, M.A.; Niaz, K. Lignans and Flavonolignans. Recent Adv. Nat. Prod. Anal. 2020, 98–116. [Google Scholar]
- Křen, V.; Valentová, K. Silybin and Its Congeners: From Traditional Medicine to Molecular Effects. Nat. Prod. Rep. 2022, 39, 1264–1281. [Google Scholar] [CrossRef]
- Abouzid, S. Silymarin, Natural Flavonolignans from Milk Thistle. In Phytochemicals—A Global Perspective of Their Role in Nutrition and Health; InTech: Rijeka, Croatia, 2012; Volume 11, pp. 255–272. [Google Scholar]
- Song, K.; Li, M.; Yang, Y.; Zhang, Z.; Zhu, Q.; Liu, J.; Wang, A. Natural Flavonolignans as Potential Therapeutic Agents against Common Diseases. J. Pharm. Pharmacol. 2022, 74, 337–350. [Google Scholar] [CrossRef]
- Hammad, W.; Sweidan, N.; Zarqa, M.A. A New Flavonolignan from Milk Thistle (Silybum marianum). J. Asian Nat. Prod. Res. 2024, 26, 739–746. [Google Scholar] [CrossRef]
- Zia, S.; Khan, M.R.; Shabbir, M.A.; Aslam, A.; Kashif, M.; Khan, I.; Nadeem, M.; Ahmed, A.; Din, A.; Aadil, R.M. An Inclusive Overview of Advanced Thermal and Nonthermal Extraction Techniques for Bioactive Compounds in Food and Food-Related Matrices An Inclusive Overview of Advanced Thermal and Nonthermal. Food Rev. Int. 2020, 38, 1166–1196. [Google Scholar] [CrossRef]
- AbouZid, S.F.; Chen, S.N.; Pauli, G.F. Silymarin Content in Silybum marianum Populations Growing in Egypt. Ind. Crops Prod. 2016, 83, 729–737. [Google Scholar] [CrossRef]
- Hadolin, M.; Škerget, M.; Knez, Ž.; Bauman, D. High Pressure Extraction of Vitamin E-Rich Oil from Silybum marianum. Food Chem. 2001, 74, 355–364. [Google Scholar] [CrossRef]
- Wallace, S.N.; Carrier, D.J.; Clausen, E.C. Extraction of Nutraceuticals from Milk Thistle: Part II. Extraction with Organic Solvents. Appl. Biochem. Biotechnol. 2003, 108, 891–903. [Google Scholar] [CrossRef]
- Omar, A.A.; Hadad, G.M.; Badr, J.M. First Detailed Quantification of Silymarin Components in the Leaves of Silybum marianum Cultivated in Egypt during Different Growth Stages. Acta Chromatogr. 2012, 24, 463–474. [Google Scholar] [CrossRef]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valéro, J.R. Extraction and Analysis of Polyphenols: Recent Trends. Crit. Rev. Biotechnol. 2011, 31, 227–249. [Google Scholar] [CrossRef]
- Saleh, I.A.; Vinatoru, M.; Mason, T.J.; Abdel-Azim, N.S.; Shams, K.A.; Aboutabl, E.; Hammouda, F.M. Extraction of Silymarin from Milk Thistle (Silybum marianum) Seeds—A Comparison of Conventional and Microwave-Assisted Extraction Methods. J. Microw. Power Electromagn. Energy 2017, 51, 124–133. [Google Scholar] [CrossRef]
- Jahan, N.; Khalil-ur-Rahman; Basra, S.M.A.; Sajid, S.; Afzal, I. Seed Enhancement of Silybum marianum and Optimization of Silymarin Extraction. Int. J. Agric. Biol. 2016, 18, 464–470. [Google Scholar] [CrossRef]
- Mandal, S.C.; Mandal, V.; Das, A.K. Classification of Extraction Methods. In Essentials of Botanical Extraction; Academic Press: Amsterdam, The Netherlands, 2015; pp. 83–136. [Google Scholar] [CrossRef]
- Wianowska, D.; Wisniewski, M. Simplified Procedure of Silymarin Extraction from Silybum marianum L. Gaertner. J. Chromatogr. Sci. 2015, 53, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Wijngaard, H.; Hossain, M.B.; Rai, D.K.; Brunton, N. Techniques to Extract Bioactive Compounds from Food By-Products of Plant Origin. Food Res. Int. 2012, 46, 505–513. [Google Scholar] [CrossRef]
- Plaza, M.; Turner, C. Pressurized Hot Water Extraction of Bioactives. TrAC Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef]
- Teo, C.C.; Tan, S.N.; Yong, J.W.H.; Hew, C.S.; Ong, E.S. Pressurized Hot Water Extraction (PHWE). J. Chromatogr. A 2010, 1217, 2484–2494. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Carrier, D.J.; Clausen, E.C. Silymarin Extraction from Milk Thistle Using Hot Water. Appl. Biochem. Biotechnol. Part A Enzyme Eng. Biotechnol. 2004, 114, 559–568. [Google Scholar] [CrossRef]
- Bunnell, K.A.; Wallace, S.N.; Clausen, E.C.; Penney, W.R.; Carrier, D.J. Comparison of Silymarin Extraction from Silybum marianum Using a Soxhlet Apparatus, Batch Parr, and Countercurrent Pressurized Hot Water Reactors. Trans. ASABE 2010, 53, 1935–1940. [Google Scholar] [CrossRef]
- Saleh, I.; Vinatoru, M.; Mason, T.; Abdel Azim, N.; Aboutabl, E.A.; Hammouda, F. Ultrasonic Assisted Extraction and Conventional Extraction of Silymarin from Silybum marianum Seeds: A Comparison. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 709–717. [Google Scholar]
- Nowak, A.; Florkowska, K.; Zielonka-Brzezicka, J.; Duchnik, W.; Muzykiewicz, A.; Klimowicz, A. The Effects of Extraction Techniques on the Antioxidant Potential of Extracts of Different Parts of Milk Thistle (Silybum marianum L.). Acta Sci. Pol. Technol. Aliment. 2021, 20, 37–46. [Google Scholar] [CrossRef]
- Đorđević, S.; Janković, T.; Mihailović, M. The Influence of the Extraction Method on the Content of Silymarin in Silybi Mariani Fructus. Lek. Sirovine 2018, 38, 5–8. [Google Scholar] [CrossRef]
- Drouet, S.; Leclerc, E.A.; Garros, L.; Tungmunnithum, D.; Kabra, A.; Abbasi, B.H.; Lainé, É.; Hano, C. A Green Ultrasound-Assisted Extraction Optimization of the Natural Antioxidant and Anti-Aging Flavonolignans from Milk Thistle Silybum marianum (L.) Gaertn. Fruits for Cosmetic Applications. Antioxidants 2019, 8, 304. [Google Scholar] [CrossRef]
- Çelik, H.T.; Gürü, M. Extraction of Oil and Silybin Compounds from Milk Thistle Seeds Using Supercritical Carbon Dioxide. J. Supercrit. Fluids 2015, 100, 105–109. [Google Scholar] [CrossRef]
- Milovanovic, S.; Lukic, I.; Kamiński, P.; Dębczak, A.; Klimkowska, K.; Tyśkiewicz, K.; Konkol, M. Green Manufacturing of High-Value Extracts from Milk Thistle Seeds: Parameters That Affect the Supercritical CO2 Extraction Process. J. CO2 Util. 2022, 63, 102134. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme Assisted Extraction of Biomolecules as an Approach to Novel Extraction Technology: A Review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Streimikyte, P.; Viskelis, P.; Viskelis, J. Enzymes-Assisted Extraction of Plants for Sustainable and Functional Applications. Int. J. Mol. Sci. 2022, 23, 2359. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-Assisted Extraction of Bioactives from Plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef]
- Liu, H.; Du, X.; Yuan, Q.; Zhu, L. Optimisation of Enzyme Assisted Extraction of Silybin from the Seeds of Silybum marianum by Box-Behnken Experimental Design. Phytochem. Anal. 2009, 20, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Quan, T. Molecular Insights of Human Skin Epidermal and Dermal Aging. J. Dermatol. Sci. 2023, 112, 48–53. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation Events and Skin Aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Sahawneh, P. Factors Influencing Skin Health from Within. J. Integr. Health 2024, 3, 156–163. [Google Scholar] [CrossRef]
- Bocheva, G.; Slominski, R.M.; Slominski, A.T. Neuroendocrine Aspects of Skin Aging. Int. J. Mol. Sci. 2019, 20, 2798. [Google Scholar] [CrossRef]
- Rasul, A.; Akhtar, N. Anti-Aging Potential of a Cream Containing Milk Thistle Extract: Formulation and In Vivo Evaluation. Afr. J. Biotechnol. 2012, 11, 1509–1515. [Google Scholar] [CrossRef]
- Svobodová, A.; Zdarilová, A.; Malisková, J.; Mikulková, H.; Walterová, D.; Vostalová, J. Attenuation of UVA-Induced Damage to Human Keratinocytes by Silymarin. J. Dermatol. Sci. 2007, 46, 21–30. [Google Scholar] [CrossRef]
- Sahib, A.; Al-Anbari, H.; Abdullah, F. Effects of Oral Antioxidants on Lesion Counts Associated with Oxidative Stress and Inflammation in Patients with Papulopustular Acne. J. Clin. Exp. Dermatol. Res. 2012, 3, 163. [Google Scholar] [CrossRef]
- Cheon, H.I.; Bae, S.; Ahn, K.J. Flavonoid Silibinin Increases Hair-Inductive Property Via Akt and Wnt/β-Catenin Signaling Activation in 3-Dimensional-Spheroid Cultured Human Dermal Papilla Cells. J. Microbiol. Biotechnol. 2019, 29, 321–329. [Google Scholar] [CrossRef]
- Oryan, A.; Tabatabaei Naeini, A.; Moshiri, A.; Mohammadalipour, A.; Tabandeh, M.R. Modulation of Cutaneous Wound Healing by Silymarin in Rats. J. Wound Care 2012, 21, 457–464. [Google Scholar] [CrossRef]
- Paudel, S.; Raina, K.; Tiku, V.R.; Maurya, A.; Orlicky, D.J.; You, Z.; Agarwal, R. Chemopreventive Efficacy of Silibinin against Basal Cell Carcinoma Growth and Progression in UVB-Irradiated Ptch+/– Mice. Carcinogenesis 2022, 43, 557–570. [Google Scholar] [CrossRef]
- George, J.; Sneed, K.; Pathak, Y. The Skin Aging Process and Anti-Aging Strategies. BJSTR 2022, 42, 33377–33386. [Google Scholar] [CrossRef]
- Kang, H.Y.; Lee, J.W.; Papaccio, F.; Bellei, B.; Picardo, M. Alterations of the Pigmentation System in the Aging Process. Pigment Cell Melanoma Res. 2021, 34, 800–813. [Google Scholar] [CrossRef]
- Bonté, F.; Girard, D.; Archambault, J.-C.; Desmoulière, A. Skin Changes During Ageing. Subcell. Biochem. 2019, 91, 249–280. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545. [Google Scholar] [CrossRef]
- Shin, S.; Lee, J.-A.; Kim, M.; Kum, H.; Jung, E.; Park, D. Anti-Glycation Activities of Phenolic Constituents from Silybum marianum (Milk Thistle) Flower in Vitro and on Human Explants. Molecules 2015, 20, 3549–3564. [Google Scholar] [CrossRef]
- He, M.; Fan, L. Evaluation of Anti-Aging Effect of Percutaneous Application of Silybum marianum Extract. China Surfactant Deterg. Cosmet. 2024, 54, 981–987. [Google Scholar] [CrossRef]
- Vostálová, J.; Tinková, E.; Biedermann, D.; Kosina, P.; Ulrichová, J.; Rajnochová Svobodová, A. Skin Protective Activity of Silymarin and Its Flavonolignans. Molecules 2019, 24, 1022. [Google Scholar] [CrossRef]
- Tyrrell, R.M.; Sage, E. The Biology of UVA Radiation. In CRC Handbook of Organic Photochemistry and Photobiology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 1395–1434. [Google Scholar]
- Lee, H.; Hong, Y.; Kim, M. Structural and Functional Changes and Possible Molecular Mechanisms in Aged Skin. Int. J. Mol. Sci. 2021, 22, 12489. [Google Scholar] [CrossRef]
- Liu, C.; Wei, J.; Wang, X.; Zhao, Q.; Lv, J.; Tan, Z.; Xin, Y.; Jiang, X. Radiation-Induced Skin Reactions: Oxidative Damage Mechanism and Antioxidant Protection. Front. Cell Dev. Biol. 2024, 12, 1480571. [Google Scholar] [CrossRef] [PubMed]
- Ciążyńska, M.; Olejniczak-Staruch, I.; Sobolewska-Sztychny, D.; Narbutt, J.; Skibińska, M.; Lesiak, A. Ultraviolet Radiation and Chronic Inflammation-Molecules and Mechanisms Involved in Skin Carcinogenesis: A Narrative Review. Life 2021, 11, 326. [Google Scholar] [CrossRef]
- Dunaway, S.; Odin, R.; Zhou, L.; Ji, L.; Zhang, Y.; Kadekaro, A.L. Natural Antioxidants: Multiple Mechanisms to Protect Skin From Solar Radiation. Front. Pharmacol. 2018, 9, 392. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; He, Y.-Y. Ultraviolet Radiation-Induced Non-Melanoma Skin Cancer: Regulation of DNA Damage Repair and Inflammation. Genes Dis. 2014, 1, 188–198. [Google Scholar] [CrossRef]
- Garmyn, M.; Yarosh, D.B. The Molecular and Genetic Effects of Ultraviolet Radiation Exposure on Skin Cells. In Photodermatology; CRC Press: Boca Raton, FL, USA, 2007; pp. 41–54. [Google Scholar]
- Premi, S.; Wallisch, S.; Mano, C.M.; Weiner, A.B.; Bacchiocchi, A.; Wakamatsu, K.; Bechara, E.J.H.; Halaban, R.; Douki, T.; Brash, D.E. Chemiexcitation of Melanin Derivatives Induces DNA Photoproducts Long after UV Exposure. Science 2015, 347, 842. [Google Scholar] [CrossRef]
- Cui, R.; Widlund, H.R.; Feige, E.; Lin, J.Y.; Wilensky, D.L.; Igras, V.E.; D’Orazio, J.; Fung, C.Y.; Schanbacher, C.F.; Granter, S.R.; et al. Central Role of P53 in the Suntan Response and Pathologic Hyperpigmentation. Cell 2007, 128, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.K.; Meleth, S.; Sharma, S.D. Silymarin, a Flavonoid from Milk Thistle (Silybum marianum L.), Inhibits UV-Induced Oxidative Stress through Targeting Infiltrating CD11b+ Cells in Mouse Skin. Photochem. Photobiol. 2008, 84, 266–271. [Google Scholar] [CrossRef]
- Boira, C.; Chapuis, E.; Scandolera, A.; Reynaud, R. Silymarin Alleviates Oxidative Stress and Inflammation Induced by UV and Air Pollution in Human Epidermis and Activates β-Endorphin Release through Cannabinoid Receptor Type 2. Cosmetics 2024, 11, 30. [Google Scholar] [CrossRef]
- Fidrus, E.; Ujhelyi, Z.; Fehér, P.; Hegedűs, C.; Janka, E.A.; Paragh, G.; Vasas, G.; Bácskay, I.; Remenyik, É. Silymarin: Friend or Foe of UV Exposed Keratinocytes? Molecules 2019, 24, 1652. [Google Scholar] [CrossRef]
- Tan, J.K.L.; Bhate, K. A Global Perspective on the Epidemiology of Acne. Br. J. Dermatol. 2015, 172 (Suppl. 1), 3–12. [Google Scholar] [CrossRef]
- Kurokawa, I.; Danby, F.W.; Ju, Q.; Wang, X.; Xiang, L.F.; Xia, L.; Chen, W.; Nagy, I.; Picardo, M.; Suh, D.H.; et al. New Developments in Our Understanding of Acne Pathogenesis and Treatment. Exp. Dermatol. 2009, 18, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Arican, O.; Kurutas, E.B.; Sasmaz, S. Oxidative Stress in Patients With Acne Vulgaris. Mediat. Inflamm. 2005, 2005, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Zaenglein, A.L.; Pathy, A.L.; Schlosser, B.J.; Alikhan, A.; Baldwin, H.E.; Berson, D.S.; Bhushan, R. Guidelines of Care for the Management of Acne Vulgaris. J. Am. Acad. Dermatol. 2016, 74, 945–973. [Google Scholar] [CrossRef]
- Shie Morteza, M.; Hayati, Z.; Namazi, N.; Abdollahimajd, F. Efficacy and Safety of Oral Silymarin in Comparison with Oral Doxycycline and Their Combination Therapy in the Treatment of Acne Vulgaris. Dermatol. Ther. 2019, 32, e13095. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.N.; Lee, J.; Lee, S.G.; Kim, H.; Choi, Y.S.; Draelos, Z.D.; Kim, J. Efficacy and Safety of Silymarin Containing Antioxidant Serum as an Adjuvant Treatment of Mild-to-Moderate Acne Vulgaris: A Prospective, Open-Label Pilot Study. J. Cosmet. Dermatol. 2023, 22, 561–568. [Google Scholar] [CrossRef]
- Atallah, D.A.-A.; Badran, A.Y.; Makhlouf, A.G.; Mekkawy, M.M. Topical Silymarin Cream as a Novel Therapy Versus Salicylic Acid Peels in Acne Vulgaris: A Split-Face Clinical Trial. J. Cutan. Med. Surg. 2024, 28, 22–28. [Google Scholar] [CrossRef]
- Saurat, J.-H.; Reygagne, P.; Josse, G.; Hamidou, Z.; Bianovici, S.; Ramel, F.; Durbise, E.; Lovati, C.; Bellani, E.; Bystrzanowska, D.; et al. Long-Term Use of Silybum marianum Fruit Extract Contributes to Homeostasis in Acne-Prone Skin-A 12-Month Follow-Up International “Real Life” Cohort Study. J. Pers. Med. 2022, 13, 96. [Google Scholar] [CrossRef]
- Cash, T.F. The Psychological Effects of Androgenetic Alopecia in Men. J. Am. Acad. Dermatol. 1992, 26, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. Molecular Mechanisms of Androgenetic Alopecia. Exp. Gerontol. 2002, 37, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Liang, A.; Fang, Y.; Ye, L.; Meng, J.; Wang, X.; Chen, J.; Xu, X. Signaling Pathways in Hair Aging. Front. Cell Dev. Biol. 2023, 11, 1278278. [Google Scholar] [CrossRef]
- Blumeyer, A.; Tosti, A.; Messenger, A.; Reygagne, P.; Del Marmol, V.; Spuls, P.I.; Blume-Peytavi, U. Evidence-Based (S3) Guideline for the Treatment of Androgenetic Alopecia in Women and in Men. J. Dtsch. Dermatol. Ges. 2011, 9, S1–S57. [Google Scholar] [CrossRef]
- Ashtiani, H.R.A.; Dadgar, A.; Akaberi, M. Improvement of Cell Proliferation and Antioxidant Activity of Silymarin in Hair Follicles Dermal Papillae Isolated from the Human Scalp: Comparison with Vitamin C Effects. Int. J. Trichology 2020, 12, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Turlier, V.; Froliger, M.; Ribet, V.; Mengeaud, V.; Reygagne, P. A Well-Tolerated Hair Serum Containing New Natural Active Ingredients Reduced Hair Loss and Improved Quality of Life in Women with Chronic Telogen Effluvium: A 16-Week Controlled Study. J. Cosmet. Dermatol. 2024, 23 (Suppl. 5), 12–21. [Google Scholar] [CrossRef]
- You, J.; Woo, J.; Roh, K.-B.; Jeon, K.; Jang, Y.; Choi, S.-A.; Ryu, D.; Cho, E.; Park, D.; Lee, J.; et al. Evaluation of Efficacy of Silybum marianum Flower Extract on the Mitigating Hair Loss in Vitro and in Vivo. J. Cosmet. Dermatol. 2024, 23, 529–542. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef]
- Sharifi, R.; Pasalar, P.; Kamalinejad, M.; Dehpour, A.R.; Tavangar, S.M.; Paknejad, M.; Mehrabani Natanzi, M.; Nourbakhsh, M.; Ahmadi Ashtiani, H.R.; Akbari, M.; et al. The Effect of Silymarin (Silybum marianum) on Human Skin Fibroblasts in an in Vitro Wound Healing Model. Pharm. Biol. 2013, 51, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Samanta, R.; Pattnaik, A.K.; Pradhan, K.K.; Mehta, B.K.; Pattanayak, S.P.; Banerjee, S. Wound Healing Activity of Silibinin in Mice. Pharmacogn. Res. 2016, 8, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Tabari, S.A.; Carpi, S.; Polini, B.; Nieri, P.; Esfahani, M.L.; Moghadamnia, A.A.; Ghorbani, H.; Ranaei, M.; Kazemi, S. Topical Application of Silymarin Enhances Cutaneous Wound Healing in Rats. S. Afr. J. Bot. 2019, 124, 494–498. [Google Scholar] [CrossRef]
- Aslan, E.; Aladağ, T.; Demirel, H.H.; Pektaş, M.B. Silymarin Promotes Wound Healing through Regulating Epithelial-Mesenchymal Transition in Rat Model: Histopathological and Immunohistochemical Evidences. Health Sci. Q. 2024, 4, 195–205. [Google Scholar] [CrossRef]
- Mahmoodi-Nesheli, M.; Alizadeh, S.; Solhi, H.; Mohseni, J.; Mahmoodi-Nesheli, M. Adjuvant Effect of Oral Silymarin on Patients’ Wound Healing Process Caused by Thermal Injuries. Casp. J. Intern. Med. 2018, 9, 341–346. [Google Scholar] [CrossRef]
- Toomari, E.; Hajian, S.; Mojab, F.; Omidkhah, T.; Nasiri, M. Evaluation the Effect of Silybum marianum Ointment on Episiotomy Wound Healing and Pain Intensity in Primiparous Women: A Randomized Triple Blind Clinical Trial. BMC Complement. Med. Ther. 2021, 21, 253. [Google Scholar] [CrossRef]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet Radiation and Skin Cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef]
- Wang, S.Q.; Setlow, R.; Berwick, M.; Polsky, D.; Marghoob, A.A.; Kopf, A.W.; Bart, R.S. Ultraviolet A and Melanoma: A Review. J. Am. Acad. Dermatol. 2001, 44, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Flaherty, K.T.; Ribas, A.; Long, G.V. Targeted Agents and Immunotherapies: Optimizing Outcomes in Melanoma. Nat. Rev. Clin. Oncol. 2017, 14, 463–482. [Google Scholar] [CrossRef] [PubMed]
- Vaid, M.; Singh, T.; Prasad, R.; Katiyar, S.K. Silymarin Inhibits Melanoma Cell Growth Both in Vitro and in Vivo by Targeting Cell Cycle Regulators, Angiogenic Biomarkers and Induction of Apoptosis. Mol. Carcinog. 2015, 54, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Dalal, P.; Rao, R. β-Cyclodextrin Nanosponges for Enhanced Anti-Melanoma Potential of Silymarin with Functions of Anti-Oxidant, Anti-Inflammatory and Anti-Tyrosinase. Results Chem. 2023, 6, 101006. [Google Scholar] [CrossRef]
- Iqbal, B.; Ali, J.; Ganguli, M.; Mishra, S.; Baboota, S. Silymarin-Loaded Nanostructured Lipid Carrier Gel for the Treatment of Skin Cancer. Nanomedicine 2019, 14, 1077–1093. [Google Scholar] [CrossRef]
- Alzoubi, K.H.; Khabour, O.F.; Alkofahi, A.S.; Mhaidat, N.M.; Abu-Siniyeh, A.A. Anticancer and Antimutagenic Activity of Silybum marianum L. and Eucalyptus camaldulensis Dehnh. against Skin Cancer Induced by DMBA: In Vitro and In Vivo Models. Pak. J. Pharm. Sci. 2021, 34, 987–993. [Google Scholar] [CrossRef]
- Hahn, H.J.; Jung, H.J.; Schrammek-Drusios, M.C.; Lee, S.N.; Kim, J.-H.; Kwon, S.B.; An, I.-S.; An, S.; Ahn, K.J. Instrumental Evaluation of Anti-Aging Effects of Cosmetic Formulations Containing Palmitoyl Peptides, Silybum marianum Seed Oil, Vitamin E and Other Functional Ingredients on Aged Human Skin. Exp. Ther. Med. 2016, 12, 1171. [Google Scholar] [CrossRef]
- Boira, C.; Chapuis, E.; Lapierre, L.; Tiguemounine, J.; Scandolera, A.; Reynaud, R. Silybum Marianum Extract: A Highly Effective Natural Alternative to Retinoids to Prevent Skin Aging Without Side Effects. J. Cosmet. Dermatol. 2025, 24, e16613. [Google Scholar] [CrossRef]
- Narayanapillai, S.; Agarwal, C.; Tilley, C.; Agarwal, R. Silibinin Is a Potent Sensitizer of UVA Radiation-Induced Oxidative Stress and Apoptosis in Human Keratinocyte HaCaT Cells. Photochem. Photobiol. 2012, 88, 1135–1140. [Google Scholar] [CrossRef]
- Rajnochová Svobodová, A.; Gabrielová, E.; Michaelides, L.; Kosina, P.; Ryšavá, A.; Ulrichová, J.; Zálešák, B.; Vostálová, J. UVA-Photoprotective Potential of Silymarin and Silybin. Arch. Dermatol. Res. 2018, 310, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Fontao, F.; von Engelbrechten, M.; Seilaz, C.; Sorg, O.; Saurat, J.H. Microcomedones in Non-Lesional Acne Prone Skin New Orientations on Comedogenesis and Its Prevention. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Crocco, E.I.; Alves, R.O.; Carvalho, G.S.M.; Silva, R.R.A.; Silva, R.S.B.; Calbucci, K.B.C.V.; Coutinho, A.L.F.; Andrade, C.S.; Braga, J.C.T. Reflectance Confocal Microscopy and Clinical Evaluation of a Product Containing Silybum marianum Fruit Extract in Monotherapy for Acne Vulgaris Treatment: A Prospective Study. JEADV Clin. Pract. 2024, 3, 1085–1094. [Google Scholar] [CrossRef]
- Albarqi, H.A.; Ahmad, M.Z.; Albekairi, T.H.; Alqhamdi, E.M.; Albawardi, S.S.; Ahmad, J.; Alshahrani, R.S.; Algahtani, M.S.; Alqahtani, A.A.; Alasiri, A.; et al. Investigating the Wound Healing Activity of Silymarin Nanoemulsion Loaded in Chitosan Gel. Sci. Adv. Mater. 2024, 16, 357–366. [Google Scholar] [CrossRef]
- Wingren, A.G.; Faik, R.Z.; Holefors, A.; Filecovic, E.; Gustafsson, A. In Vitro Effects of Undifferentiated Callus Extracts from Plantago major L., Rhodiola rosea L. and Silybum marianum L. in Normal and Malignant Human Skin Cells. Heliyon 2023, 9, e16480. [Google Scholar] [CrossRef]
- Mahdi, W.A.; Imam, S.S.; Alotaibi, A.; Alhallaf, S.; Alzhrani, R.F.; Alshehri, S. Formulation and Evaluation of a Silymarin Inclusion Complex-Based Gel for Skin Cancer. ACS Omega 2025, 10, 3006–3017. [Google Scholar] [CrossRef] [PubMed]


| Compounds/ Flavonoid Part | Variety | Linkage | Structure | Molecular Weight (g/mol) |
| Silybin A/taxifolin | Purple flowering | Dioxane ring | 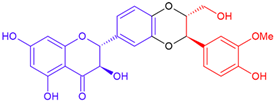 | 482.4 |
| Silybin B/taxifolin | Purple flowering | Dioxane ring | 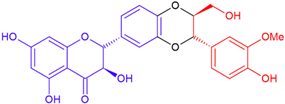 | 482.4 |
| Isosilybin A/taxifolin | Purple flowering | Dioxane ring |  | 482.4 |
| Isosilybin B/taxifolin | Purple flowering | Dioxane ring | 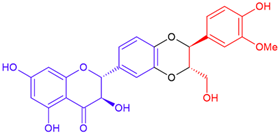 | 482.4 |
| Isosilybin C/taxifolin | Purple flowering | Dioxane ring | 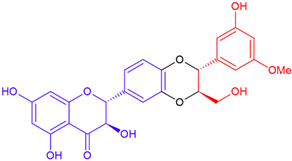 | 482.4 |
| Isosilybin D/taxifolin | Purple flowering | Dioxane ring |  | 482.4 |
| Isosilandrin A/eriodictyol | White flowering | Dioxane ring | 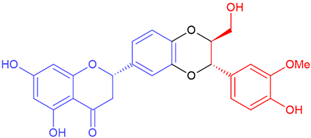 | 466.4 |
| Isosilandrin B/eriodictyol | White flowering | Dioxane ring | 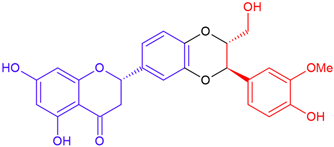 | 466.4 |
| Silandrin A/eriodictyol | White flowering | Dioxane ring | 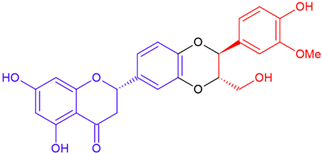 | 466.4 |
| Silandrin B/eriodictyol | White flowering | Dioxane ring | 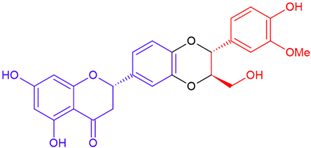 | 466.4 |
| Silyhermin/eriodictyol | White flowering | cyclic ether | 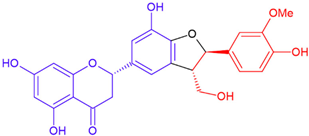 | 466.4 |
| Neosilyhermin A/eriodictyol | White flowering | Cyclic ether | 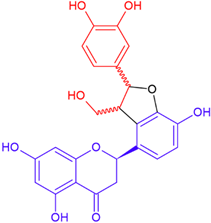 | 466.4 |
| Neosilyhermin B/eriodictyol | White flowering | Cyclic ether | 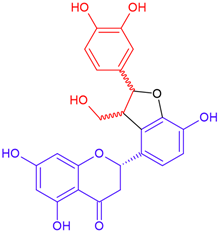 | 466.4 |
| Silyamandin/taxifolin | White flowering | B-ring fission | 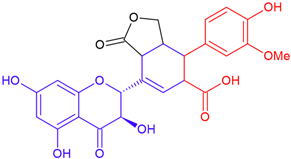 | 498.4 |
| Silydianin/taxifolin | Purple flowering | B-ring fission | 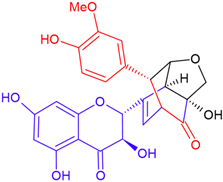 | 482.4 |
| Silychristin A/taxifolin | Purple flowering | Cyclic ether | 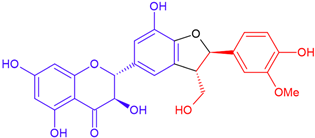 | 482.4 |
| Silychristin B/taxifolin | Purple flowering | Cyclic ether | 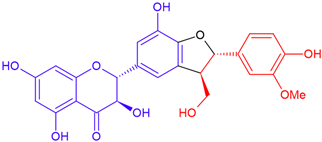 | 482.4 |
| 2,3-Dehydrosilybin A/quercetin | Purple flowering | Dioxane ring | 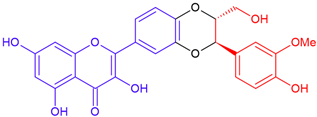 | 480.42 |
| 2,3-Dehydrosilybin B/quercetin | Purple flowering | Dioxane ring |  | 480.42 |
| Neusilychristin/taxifolin | White flowering | Cyclic ether |  | 482.4 |
| 2,3-cis Silybin A/taxifolin | Purple flowering | Dioxane ring | 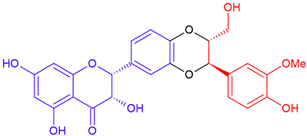 | 482.4 |
| 2,3-cis Silybin A/taxifolin | Purple flowering | Dioxane ring | 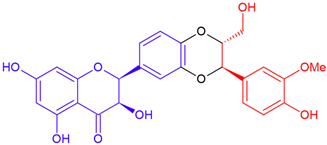 | 482.4 |
| 2,3-cis Silybin B/taxifolin | Purple flowering | Dioxane ring | 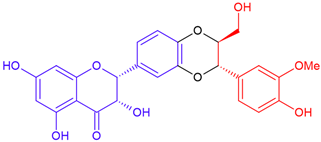 | 482.4 |
| 2,3-cis Silybin B/taxifolin | Purple flowering | Dioxane ring | 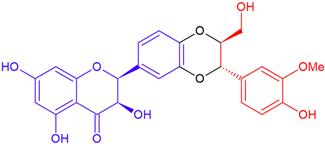 | 482.4 |
| Silymonin/eriodictyol | White flowering | B-ring fission | 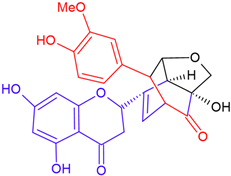 | 482.4 |
| Sonyamandin/taxifolin | Purple flowering | B-ring fission | 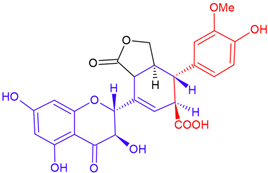 | 498.4 |
| Extraction Methods | Solvent | Compounds/Extracts | Best Method Identified | Key Findings | References |
|---|---|---|---|---|---|
| MAE, Soxhlet, Heat reflux | Methanol 80% | Silybin A, silybin B, taxifolin, silychristin, isosilybin A isosilybin B, silydianin | MAE |
| [44] |
| Reflux, Soxhlet, Maceration, MAE | Methanol, Ethanol | Silymarin mixture | MAE followed by Soxhlet |
| [45] |
| Maceration, Ultrasound direct and indirect sonication | Methanol 80% | Silybin A, silybin B, taxifolin, silychristin, isosilybin A, isosilybin B, silydianin | Ultrasound direct sonication |
| [53] |
| Soxhlet, Shaking, UAE | Ethanol (96%, 70%), methanol, acetone and petroleum ether | Seeds, leaves and flowers extracts | Soxhlet extraction |
| [54] |
| Percolation, Maceration, UAE, Extraction on a water bath | Ethanol 60% | Taxifolin, silychristin, silydianin, silybin A, silybin B, isosilybin | Water bath |
| [55] |
| UAE, Maceration | Ethanol(v/v)% | Taxifolin, silybin A, silybin B, isosilybin A, isosilybin B, silychristin, silydianin | UAE |
| [56] |
| PLE, Soxhlet | Methanol, Acetone, Ethyl acetate | Silybin A, silybin B, silychristin, isosilybin A isosilybin B, silydianin | PLE |
| [47] |
| Supercritical CO2 | _ | Oil, silybin A and silybin B | _ |
| [57] |
| Reflux, EAE | Ethanol | Silybin | EAE using cellulase |
| [62] |
| SWE | Hot water | Taxifolin, silybin A, silybin B, silychristin | _ |
| [51] |
| Soxhlet, Batch PHWE, Countercurrent PHWE | Hot water, ethanol | Silybin A, silybin B, silychristin, silydianin, isosilybin A, isosilybinB | Soxhlet: 0.59 mm seed meal; PHWE & Countercurrent PHWE: 1.48 mm seed meal |
| [52] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iraqi, O.; Jalal, M.; El Mouzazi, I.; Jbene, M.; Taboz, Y.; Habsaoui, A. Advances in Extraction Technologies of Silybum marianum L. and Its Role in Protecting Against Skin Damage. Cosmetics 2025, 12, 211. https://doi.org/10.3390/cosmetics12050211
Iraqi O, Jalal M, El Mouzazi I, Jbene M, Taboz Y, Habsaoui A. Advances in Extraction Technologies of Silybum marianum L. and Its Role in Protecting Against Skin Damage. Cosmetics. 2025; 12(5):211. https://doi.org/10.3390/cosmetics12050211
Chicago/Turabian StyleIraqi, Oumayma, Mariam Jalal, Issam El Mouzazi, Mourad Jbene, Youness Taboz, and Amar Habsaoui. 2025. "Advances in Extraction Technologies of Silybum marianum L. and Its Role in Protecting Against Skin Damage" Cosmetics 12, no. 5: 211. https://doi.org/10.3390/cosmetics12050211
APA StyleIraqi, O., Jalal, M., El Mouzazi, I., Jbene, M., Taboz, Y., & Habsaoui, A. (2025). Advances in Extraction Technologies of Silybum marianum L. and Its Role in Protecting Against Skin Damage. Cosmetics, 12(5), 211. https://doi.org/10.3390/cosmetics12050211







