A Novel In Vitro Dry Skin Model Using Minipig and Human Cadaver Skin for Evaluating Moisturizer Efficacy
Abstract
1. Introduction
2. Materials and Methods
2.1. Skin Tissues
2.2. Dry Skin Conditions
2.3. Nile Red Staining
2.4. ImageJ Analysis
2.5. Transepidermal Water Loss (TEWL) Measurement
2.6. Fluorescein Isothiocyanate (FITC)-Dextran Permeability Test
2.7. Statistics
3. Results
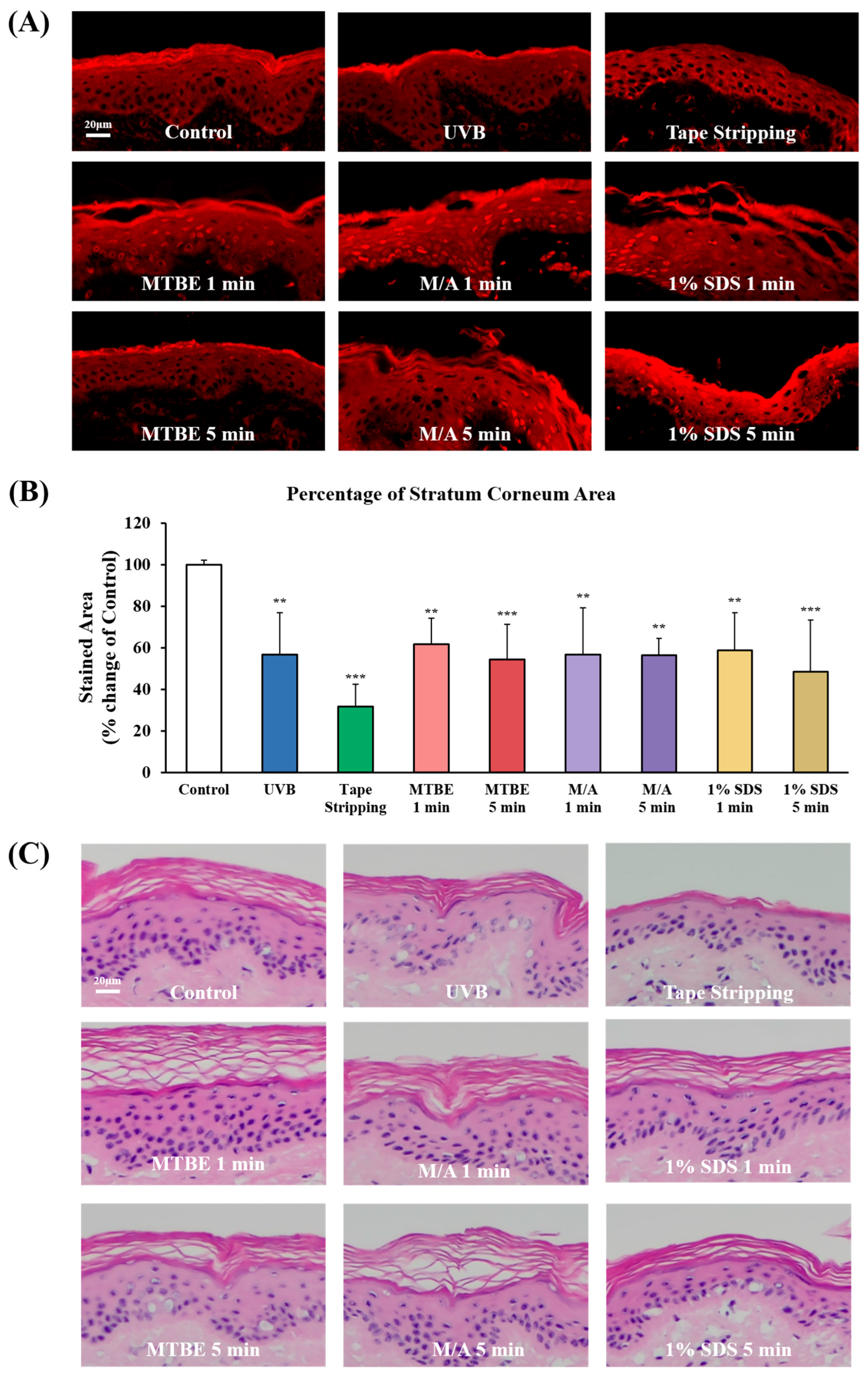
3.1. Development of Dry Skin Models Using Isolated Minipig Epidermis
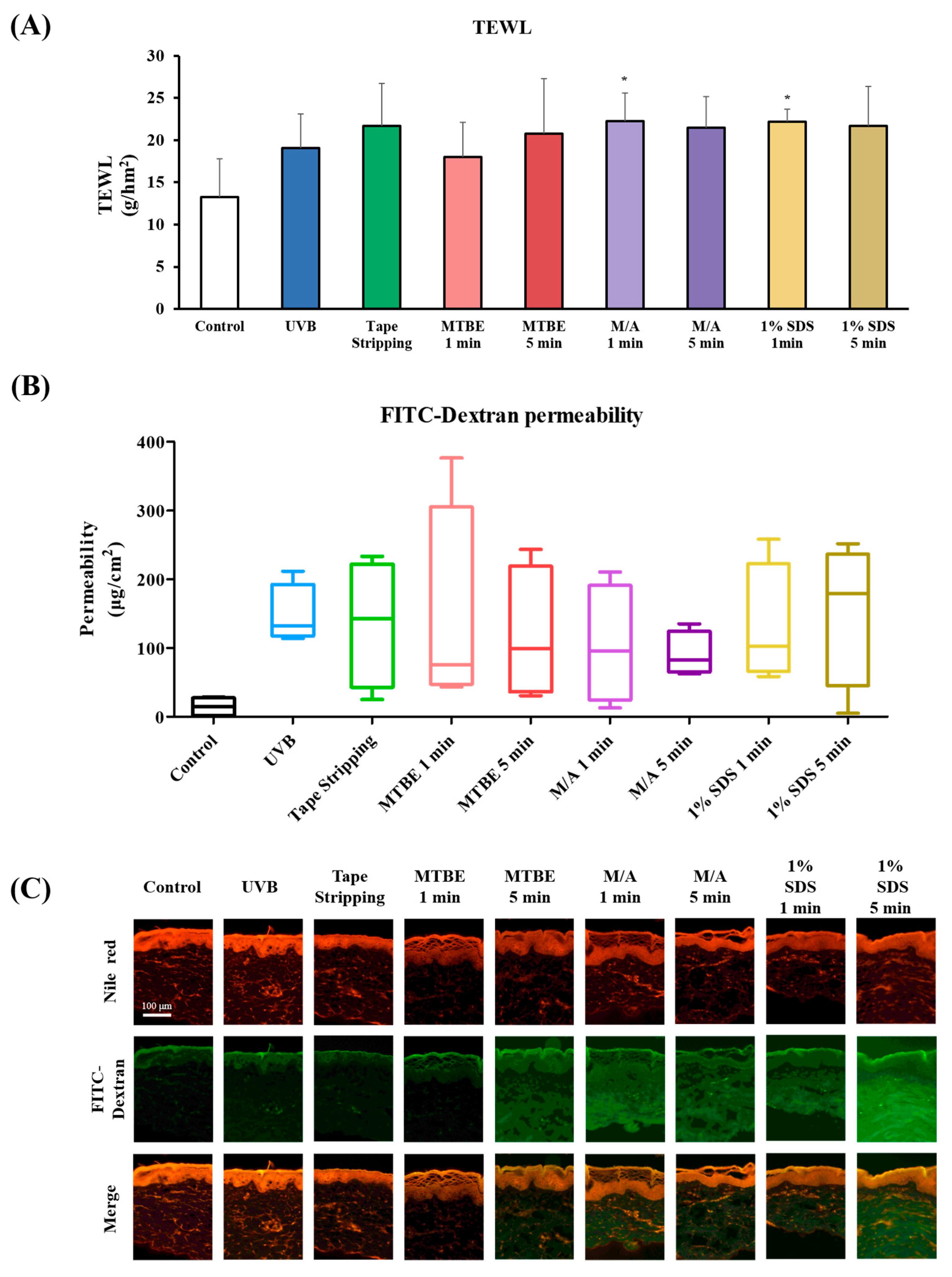
3.2. Evaluation of Skin Barrier Function in Dry Skin Models
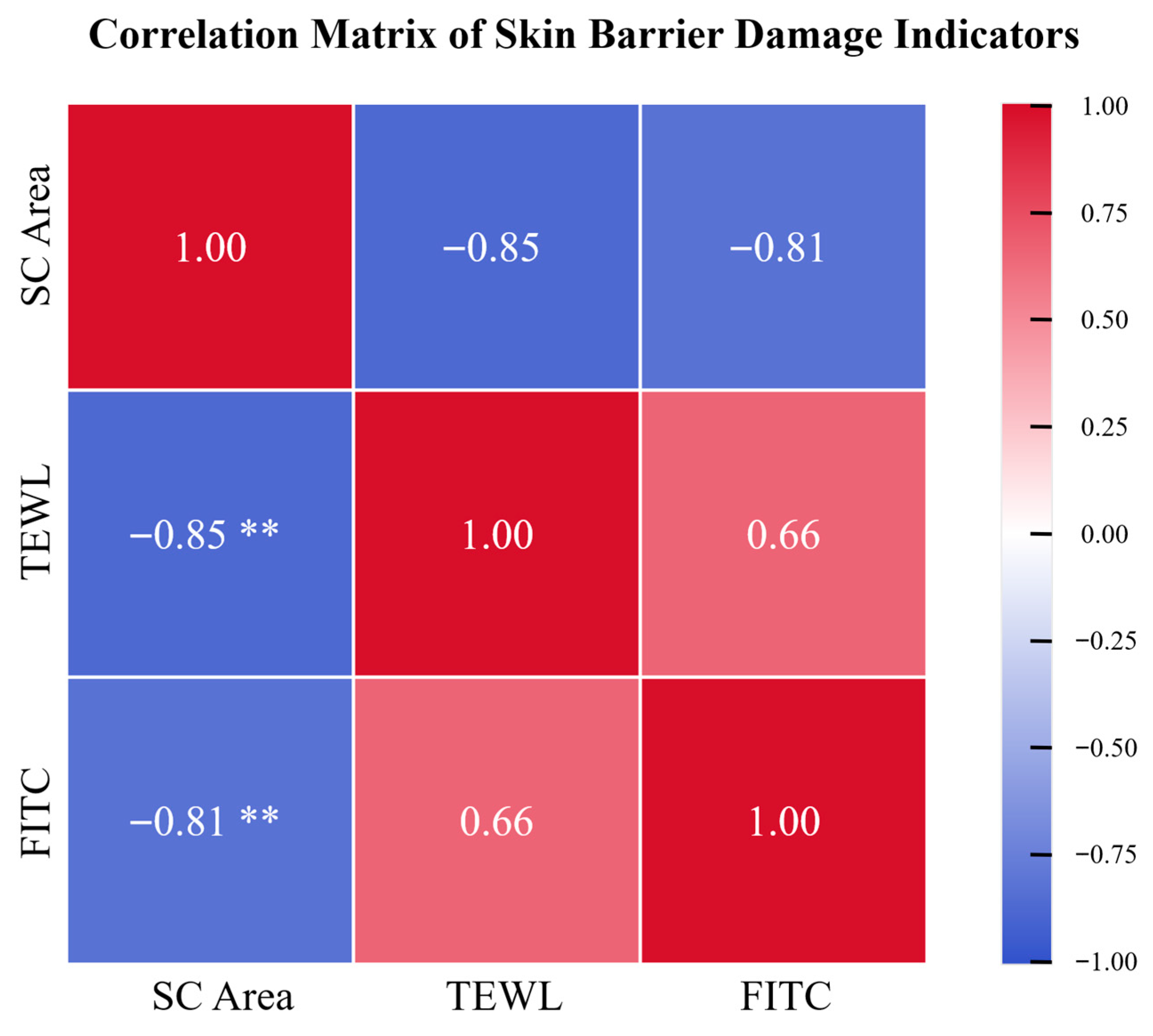
3.3. Correlation of Dry Skin Parameters in the Minipig Epidermis Model
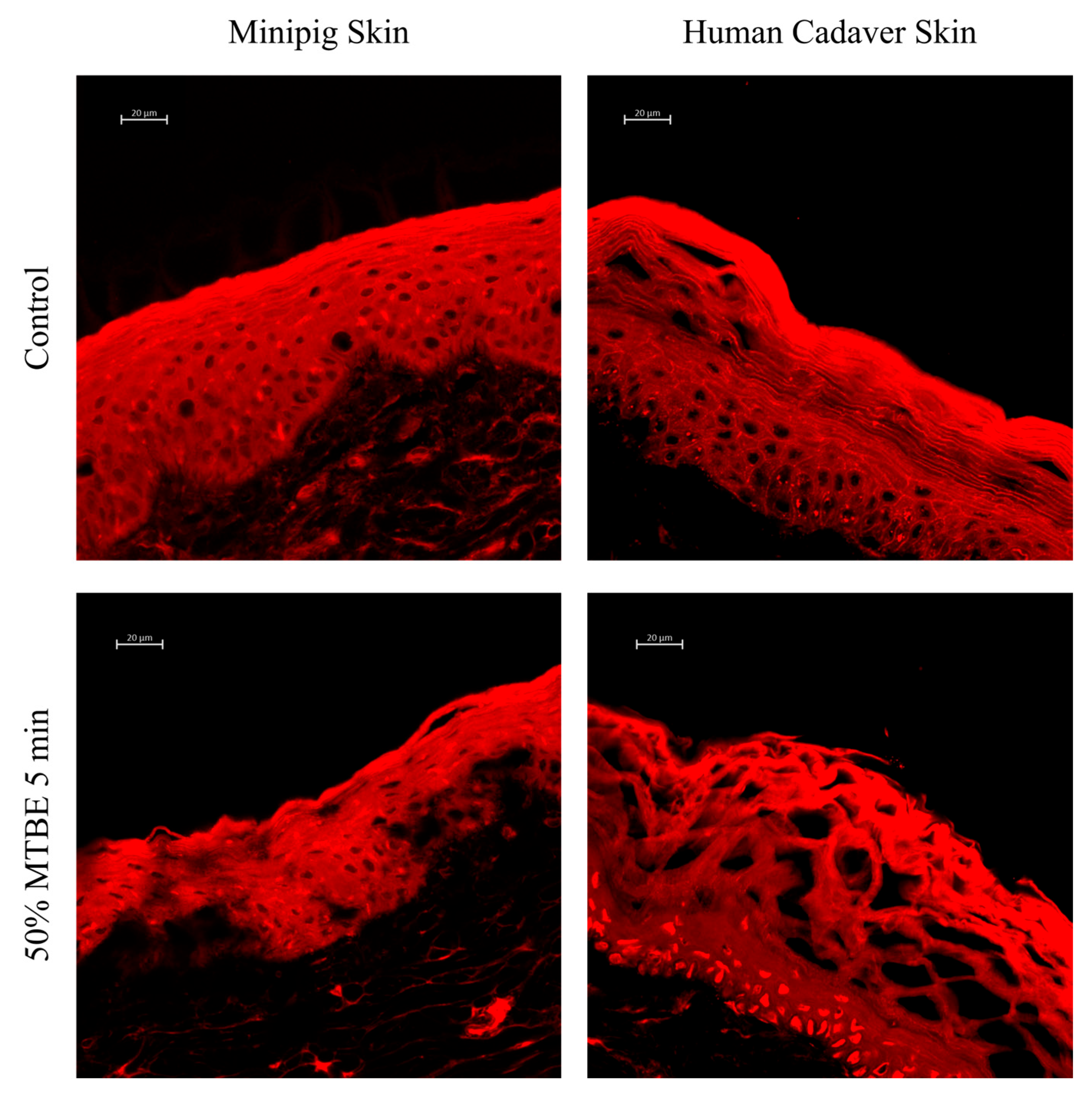
3.4. MTBE/Acetone-Induced Dry Skin Model Using Human Cadaver Skin Tissue
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thakur, A.; Shah, D.; Rai, D.; Parra, D.C.; Pathikonda, S.; Kurilova, S.; Cili, A. Therapeutic values of exosomes in cosmetics, skin care, tissue regeneration, and dermatological diseases. Cosmetics 2023, 10, 65. [Google Scholar] [CrossRef]
- Mawazi, S.M.; Ann, J.; Othman, N.; Khan, J.; Alolayan, S.O.; Al thagfan, S.S.; Kaleemullah, M. A review of moisturizers; history, preparation, characterization and applications. Cosmetics 2022, 9, 61. [Google Scholar] [CrossRef]
- Ueda, Y.; Murakami, Y.; Saya, Y.; Matsunaka, H. Optimal application method of a moisturizer on the basis of skin physiological functions. J. Cosmet. Dermatol. 2022, 21, 3095–3101. [Google Scholar] [CrossRef]
- Liu, J.-K. Natural products in cosmetics. Nat. Prod. Bioprospecting 2022, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Bhat, B.B.; Kamath, P.P.; Chatterjee, S.; Bhattacherjee, R.; Nayak, U.Y. Recent updates on nanocosmeceutical skin care and anti-aging products. Curr. Pharm. Des. 2022, 28, 1258–1271. [Google Scholar] [CrossRef]
- Danby, S.G.; Andrew, P.V.; Taylor, R.N.; Kay, L.J.; Chittock, J.; Pinnock, A.; Ulhaq, I.; Fasth, A.; Carlander, K.; Holm, T. Different types of emollient cream exhibit diverse physiological effects on the skin barrier in adults with atopic dermatitis. Clin. Exp. Dermatol. 2022, 47, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-Y.; Um, J.-Y.; Chung, B.-Y.; Lee, S.-Y.; Park, J.-S.; Kim, J.-C.; Park, C.-W.; Kim, H.-O. Moisturizer in patients with inflammatory skin diseases. Medicina 2022, 58, 888. [Google Scholar] [CrossRef]
- Shindo, S.; Murota, H.; Seki, T.; Mori, K.; Kaizu, K.; Nishizaka, T.; Takagi, Y.; Katayama, I. Effects of a moisturizer containing pseudo-ceramide and a eucalyptus extract on sweating function in adult atopic dermatitis: A double-blind, randomized, controlled left-right comparison clinical trial. J. Cosmet. Dermatol. 2022, 21, 4503–4509. [Google Scholar] [CrossRef]
- Ainurofiq, A.; Haya, A.F.F.D.; Safitri, A.N.; Solihatin, I.Z.; Nusriya, S.B.; Nugroho, T.S. Characterization and application of moisturizer in skin treatment: A review: Moisturizer in Skin Treatment. J. Pak. Assoc. Dermatol. 2023, 33, 1602–1613. [Google Scholar]
- Chandan, N.; Rajkumar, J.R.; Shi, V.Y.; Lio, P.A. A new era of moisturizers. J. Cosmet. Dermatol. 2021, 20, 2425–2430. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Algahtani, M.S.; Ahmad, M.Z.; Ahmad, J. Investigation utilizing the HLB concept for the development of moisturizing cream and lotion: In-vitro characterization and stability evaluation. Cosmetics 2020, 7, 43. [Google Scholar] [CrossRef]
- Pavlou, P.; Siamidi, A.; Varvaresou, A.; Vlachou, M. Skin care formulations and lipid carriers as skin moisturizing agents. Cosmetics 2021, 8, 89. [Google Scholar] [CrossRef]
- Del Rosso, J.; Zeichner, J.; Alexis, A.; Cohen, D.; Berson, D. Understanding the epidermal barrier in healthy and compromised skin: Clinically relevant information for the dermatology practitioner: Proceedings of an expert panel roundtable meeting. J. Clin. Aesthetic Dermatol. 2016, 9, S2. [Google Scholar]
- Harding, C.R. The stratum corneum: Structure and function in health and disease. Dermatol. Ther. 2004, 17, 6–15. [Google Scholar] [CrossRef]
- Misra, M.; Ananthapadmanabhan, K. changes in epidermis and transepidermal water loss. J. Soc. Cosmet. Chem. 1997, 48, 219–234. [Google Scholar]
- Ananthapadmanabhan, K.; Mukherjee, S.; Chandar, P. Stratum corneum fatty acids: Their critical role in preserving barrier integrity during cleansing. Int. J. Cosmet. Sci. 2013, 35, 337–345. [Google Scholar] [CrossRef]
- Rajkumar, J.; Chandan, N.; Lio, P.; Shi, V. The skin barrier and moisturization: Function, disruption, and mechanisms of repair. Ski. Pharmacol. Physiol. 2023, 36, 174–185. [Google Scholar] [CrossRef]
- Biniek, K.; Levi, K.; Dauskardt, R.H. Solar UV radiation reduces the barrier function of human skin. Proc. Natl. Acad. Sci. USA 2012, 109, 17111–17116. [Google Scholar] [CrossRef]
- Panich, U.; Sittithumcharee, G.; Rathviboon, N.; Jirawatnotai, S. Ultraviolet radiation-induced skin aging: The role of DNA damage and oxidative stress in epidermal stem cell damage mediated skin aging. Stem Cells Int. 2016, 2016, 7370642. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, X.; Chen, S.; Li, S.; Liu, X. Acute skin barrier disruption with repeated tape stripping: An in vivo model for damage skin barrier. Ski. Res. Technol. 2013, 19, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Keurentjes, A.J.; Jakasa, I.; Kezic, S. Research techniques made simple: Stratum corneum tape stripping. J. Investig. Dermatol. 2021, 141, 1129–1133.e1. [Google Scholar] [CrossRef]
- Danby, S.G.; Andrew, P.V.; Brown, K.; Chittock, J.; Kay, L.J.; Cork, M.J. An investigation of the skin barrier restoring effects of a cream and lotion containing ceramides in a multi-vesicular emulsion in people with dry, eczema-prone, skin: The RESTORE study phase 1. Dermatol. Ther. 2020, 10, 1031–1041. [Google Scholar] [CrossRef]
- Draelos, Z.D.; Diaz, I.; Namkoong, J.; Wu, J.; Boyd, T. Efficacy evaluation of a topical hyaluronic acid serum in facial photoaging. Dermatol. Ther. 2021, 11, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-Q.; Li, X.; Zhang, R.-Y.; Yuan, C.; Yan, B.; Humbert, P.; Quan, Z.-X. Effects of investigational moisturizers on the skin barrier and microbiome following exposure to environmental aggressors: A randomized clinical trial and ex vivo analysis. J. Clin. Med. 2023, 12, 6078. [Google Scholar] [CrossRef] [PubMed]
- Koseki, K.; Kawasaki, H.; Atsugi, T.; Nakanishi, M.; Mizuno, M.; Naru, E.; Ebihara, T.; Amagai, M.; Kawakami, E. Assessment of skin barrier function using skin images with topological data analysis. npj Syst. Biol. Appl. 2020, 6, 40. [Google Scholar] [CrossRef]
- Qassem, M.; Kyriacou, P. Review of modern techniques for the assessment of skin hydration. Cosmetics 2019, 6, 19. [Google Scholar] [CrossRef]
- Rogiers, V. EEMCO guidance for the assessment of transepidermal water loss in cosmetic sciences. Ski. Pharmacol. Physiol. 2001, 14, 117–128. [Google Scholar] [CrossRef]
- Wu, Y.; Ran, J.; Tanaka, T.; Liu, Q. Quantitative skin surface hydration measurement by visible optical image processing: A pilot study. Ski. Res. Technol. 2024, 30, e13773. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Maitra, P.; Nguyen, A.; Kadoya, K.; Mehta, R.C. Development of an in vitro Functional Assay to Evaluate the Occlusive Properties of Moisturizers on Dry Skin. Ski. Pharmacol. Physiol. 2023, 36, 140–148. [Google Scholar] [CrossRef]
- Uehara, O.; Kusuhara, T.; Nakamura, T. Transepidermal water loss estimation model for evaluating skin barrier function. Adv. Biomed. Eng. 2023, 12, 1–8. [Google Scholar] [CrossRef]
- Gibbsa, M.P.S.; Boelsmaa, G.P.E.; Koertenb, H.; Mommaasa, J.B.M. Barrier function in reconstructed epidermis and its resemblance to native human skin. Ski. Pharmacol. Appl. Ski. Physiol. 2001, 14, 63–71. [Google Scholar]
- Barthe, M.; Clerbaux, L.-A.; Thénot, J.-P.; Braud, V.M.; Osman-Ponchet, H. Systematic characterization of the barrier function of diverse ex vivo models of damaged human skin. Front. Med. 2024, 11, 1481645. [Google Scholar] [CrossRef]
- Hwang, J.-h.; Jeong, H.; Lee, N.; Hur, S.; Lee, N.; Han, J.J.; Jang, H.W.; Choi, W.K.; Nam, K.T.; Lim, K.-M. Ex vivo live full-thickness porcine skin model as a versatile in vitro testing method for skin barrier research. Int. J. Mol. Sci. 2021, 22, 657. [Google Scholar] [CrossRef] [PubMed]
- Mojumdar, E.H.; Madsen, L.B.; Hansson, H.; Taavoniku, I.; Kristensen, K.; Persson, C.; Morén, A.K.; Mokso, R.; Schmidtchen, A.; Ruzgas, T. Probing skin barrier recovery on molecular level following acute wounds: An in vivo/ex vivo study on pigs. Biomedicines 2021, 9, 360. [Google Scholar] [CrossRef]
- Uhm, C.; Jeong, H.; Lee, S.H.; Hwang, J.S.; Lim, K.M.; Nam, K.T. Comparison of structural characteristics and molecular markers of rabbit skin, pig skin, and reconstructed human epidermis for an ex vivo human skin model. Toxicol. Res. 2023, 39, 477–484. [Google Scholar] [CrossRef]
- Hong, J.-h.; Lim, K.-M. Development of skin corrosion and irritation test methods employing ex vivo porcine skin model. Toxicol. Vitr. 2025, 106, 106055. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.O.; Jeong, H.; Cho, Y.; Lee, E.O.; Jang, H.W.; Kim, J.; Nam, K.; Lim, K.M. Lysates of a Probiotic, Lactobacillus rhamnosus, Can Improve Skin Barrier Function in a Reconstructed Human Epidermis Model. Int. J. Mol. Sci. 2019, 20, 4289. [Google Scholar] [CrossRef]
- McDaniel, D.H.; Dover, J.S.; Wortzman, M.; Nelson, D.B. In vitro and in vivo evaluation of a moisture treatment cream containing three critical elements of natural skin moisturization. J. Cosmet. Dermatol. 2020, 19, 1121–1128. [Google Scholar] [CrossRef]
- Tu, Y.; Wang, L.; An, L.; He, L. Hsa-miR-31-3p targets CLDN8 to compromise skin barrier integrity in psoriasis. Biochem. Biophys. Rep. 2025, 42, 101976. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Lee, S.-H. Epidermal permeability barrier defects and barrier repair therapy in atopic dermatitis. Allergy Asthma Immunol. Res. 2014, 6, 276–287. [Google Scholar] [CrossRef]
- Levi, K.; Weber, R.; Do, J.; Dauskardt, R. Drying stress and damage processes in human stratum corneum. Int. J. Cosmet. Sci. 2010, 32, 276–293. [Google Scholar] [CrossRef]
- Sözener, Z.C.; Cevhertas, L.; Nadeau, K.; Akdis, M.; Akdis, C.A. Environmental factors in epithelial barrier dysfunction. J. Allergy Clin. Immunol. 2020, 145, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, H. Skin aging and dry skin. J. Dermatol. 2004, 31, 603–609. [Google Scholar] [CrossRef]
- Boyce, J.M.; Kelliher, S.; Vallande, N. Skin irritation and dryness associated with two hand-hygiene regimens: Soap-and-water hand washing versus hand antisepsis with an alcoholic hand gel. Infect. Control Hosp. Epidemiol. 2000, 21, 442–448. [Google Scholar] [CrossRef]
- Slotosch, C.M.; Kampf, G.; Löffler, H. Effects of disinfectants and detergents on skin irritation. Contact Dermat. 2007, 57, 235–241. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, R.; Wang, M.; Zhai, L.; Liu, J.; Xu, X.; Sun, L.; Zhao, D. Ginsenosides repair UVB-induced skin barrier damage in BALB/c hairless mice and HaCaT keratinocytes. J. Ginseng Res. 2022, 46, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Uchi, H.; Furue, M. Antioxidant cinnamaldehyde attenuates UVB-induced photoaging. J. Dermatol. Sci. 2019, 96, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Furue, K.; Ulzii, D.; Tanaka, Y.; Ito, T.; Tsuji, G.; Kido-Nakahara, M.; Nakahara, T.; Furue, M. Pathogenic implication of epidermal scratch injury in psoriasis and atopic dermatitis. J. Dermatol. 2020, 47, 979–988. [Google Scholar] [CrossRef]
- Held, E.; Lund, H.; Agner, T. Effect of different moisturizers on SLS-irritated human skin. Contact Dermat. 2001, 44, 229–234. [Google Scholar] [CrossRef]
- Hwang, J.H.; Kang, Y.; Park, H.J.; Kim, S.; Lee, S.H.; Kim, H.; Nam, S.J.; Lim, K.M. Skin wound healing effects of (+)-syringaresinol from ginseng berry. J. Ginseng Res. 2023, 47, 654–661. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choe, J.-W.; Kang, B.-G.; Hong, J.-h.; Liu, K.; Lim, K.-M. A Novel In Vitro Dry Skin Model Using Minipig and Human Cadaver Skin for Evaluating Moisturizer Efficacy. Cosmetics 2025, 12, 203. https://doi.org/10.3390/cosmetics12050203
Choe J-W, Kang B-G, Hong J-h, Liu K, Lim K-M. A Novel In Vitro Dry Skin Model Using Minipig and Human Cadaver Skin for Evaluating Moisturizer Efficacy. Cosmetics. 2025; 12(5):203. https://doi.org/10.3390/cosmetics12050203
Chicago/Turabian StyleChoe, Ji-Woo, Bae-Gon Kang, Jeong-hyun Hong, Kwanghyeon Liu, and Kyung-Min Lim. 2025. "A Novel In Vitro Dry Skin Model Using Minipig and Human Cadaver Skin for Evaluating Moisturizer Efficacy" Cosmetics 12, no. 5: 203. https://doi.org/10.3390/cosmetics12050203
APA StyleChoe, J.-W., Kang, B.-G., Hong, J.-h., Liu, K., & Lim, K.-M. (2025). A Novel In Vitro Dry Skin Model Using Minipig and Human Cadaver Skin for Evaluating Moisturizer Efficacy. Cosmetics, 12(5), 203. https://doi.org/10.3390/cosmetics12050203







