Abstract
Background: Nicotinamide mononucleotide (NMN) has gained attention as an anti-aging compound due to its ability to replenish NAD+ levels, which typically decline with age and stress. While improvements in skin conditions have been reported, clinical studies on human hair remain lacking. In this study, we evaluated the effects of NMN supplementation on hair conditions in middle-aged women and explored its association with quality-of-life (QOL) factors such as fatigue. Methods: Torula yeast-fermented NMN was evaluated in this clinical trial. A single-arm, pre-post intervention study was conducted involving 15 healthy Japanese women aged between 40 and 50 years who orally consumed NMN for 12 weeks. Hair growth cycles and hair shaft diameters were assessed using TrichoScan (TrichoGrabV3B) analysis and scanning electron microscopy (SEM). Hair metabolites and hormone levels were also measured. Subjective indices, including fatigue and hair texture, were evaluated using a visual analog scale (VAS) questionnaire. Results: Following NMN supplementation, anagen hair elongation density (hairs/cm2) significantly increased from 55.9 to 87.7 (p = 0.03). Hair diameter (µm) also significantly increased from 75.3 to 78.8 (p < 0.01), with improvements in hair cuticle condition. Metabolomic analyses revealed significant changes in amino acids and energy metabolism-related compounds. No marked changes were observed in hair hormone concentrations. The VAS questionnaire indicated improvements in subjective hair characteristics such as elasticity, gloss, and volume, as well as reductions in fatigue and perceived hair loss, suggesting enhanced QOL. Conclusions: Oral supplementation with NMN may be a beneficial strategy for promoting hair growth and improvement in hair cuticle condition in middle-aged women, thus potentially enhancing overall hair care and quality of life.
1. Introduction
Nicotinamide mononucleotide (NMN), a precursor of nicotinamide adenine dinucleotide (NAD+), has attracted attention due to its roles in cellular energy metabolism and aging control. Recently, NMN has gained popularity for its potential benefits in skin health and anti-aging. Orally administered NMN is absorbed in the body and primarily metabolized into NAD+ and nicotinic acid mononucleotide (NAMN) [1,2,3], thus contributing to replenishing NAD+ levels, a crucial coenzyme involved in diverse physiological functions such as energy production, DNA repair, and immune regulation. NAD+ levels typically decrease with aging and stress, which may accelerate cardiovascular diseases, neurodegenerative disorders, and age-related conditions [4,5,6,7]. Therefore, supplementing NAD+ precursors like NMN has been proposed as beneficial [8,9,10]. In the context of a globally aging society, maintaining youthful appearance and quality of life (QOL) has become increasingly significant. Skin and hair are directly associated with external appearance, and NMN’s anti-aging effects have garnered particular attention in cosmetic applications. Although improvements in skin elasticity, hydration, and reduced UV damage following NMN supplementation have been reported [11,12,13], no clinical studies on human hair exist, and animal studies suggesting effects on hair quality and hair follicle health remain limited.
Hair is an appendage of the skin, regulated by a unique growth cycle comprising anagen, catagen, and telogen phases. Disruption of this hair cycle due to aging, stress, or poor nutritional status can lead to thinning hair and reduced hair shaft diameter, contributing to aesthetic concerns. In terms of controlling hair loss and hair follicle apoptosis, conventional medicines such as Minoxidil have been reported to improve blood flow around hair follicles by vasodilatory action and promote the supply of growth factors and hair growth, and Finasteride has been reported to suppress the production of dihydrotestosterone, a substance that causes male pattern baldness by inhibiting 5α-reductase [14]. As a precursor of NAD+, NMN is involved in many metabolic pathways in the body, such as cellular energy metabolism, mitochondrial function, DNA repair, and antioxidant defense, and it is expected to compensate for the decline in systemic function associated with aging. In previous reports on mice and in vitro studies, NMN has been reported to suppress hair follicle atrophy and thinning caused by dihydrotestosterone, and to significantly reduce the release of inflammatory factors induced by DHT in cultured human hair papilla cells [15]. From previous reports, NMN may act in a multifaceted manner through systemic and endogenous metabolic regulation and may be a different therapeutic approach from conventional medicines and herbs. NMN intake is thought to be highly safe for the human body, as it works by supplementing NAD+, which is present in the human body and decreases with age [13,16,17,18].
This study aimed to explore the effects of 12-week oral NMN supplementation on hair conditions and associated energy and amino acid metabolism in women aged 40–50 years, who commonly experience age-related hair concerns. Additionally, subjective changes in fatigue and hair quality were evaluated using a visual analog scale (VAS) questionnaire to assess QOL impacts. The findings of this study may provide practical insights into hair care and anti-aging strategies for middle-aged women and further validate NMN’s applicability.
2. Materials and Methods
2.1. Subjects
Healthy Japanese women aged 40–50 years with concerns about their hair (e.g., thinning hair or loss of hair elasticity and gloss) were recruited and preferentially selected to participate in this study conducted at Clinic F (Tokyo, Japan). Exclusion criteria included individuals with serious current or past illnesses, those under treatment for sleep disorders (e.g., insomnia) or psychiatric conditions (e.g., depression), and those habitually consuming supplements containing NMN or niacin.
2.2. Study Design and NMN Supplementation
This open-label study involved all participants receiving the test supplement. Participants orally ingested an NMN supplement (Mitsubishi Corporation Life Sciences Limited, Tokyo, Japan) at a daily dose of 500 mg NMN (12 tablets) for 12 weeks (from 12 September to 5 December 2024). The clinical trial evaluated torula yeast-fermented NMN produced in Saiki, Japan. The supplement was taken in the morning, primarily around breakfast, before lunch.
Pre-supplementation examinations (2 visits) and post-supplementation examinations (2 visits) were conducted at Clinic F (Table 1). Measurements included the following: (1) hair density using TrichoScan (Tricholog GmbH, Freiburg, Germany); (2) subjective questionnaire responses; (3) SEM imaging analysis; and (4) hair metabolite analysis via metabolomics.

Table 1.
Hair examination schedule.
2.3. Evaluation Measures
2.3.1. Hair Assessment by TrichoScan
Hair was sampled from the back of the head. More than 30 hairs were collected from the selected area as close to the scalp as possible. The hair samples were collected with haircutting scissors on the initial visit. The sampling area (approximately 1.0 cm2) was then shaved using clippers and marked. Participants revisited the clinic three days later to measure hair regrowth using TrichoScan at the same site. The NMN supplement was provided during the second pre-supplementation visit, with daily intake continuing until the end of the study. After 12 weeks of supplementation, hair sampling, shaving, and TrichoScan assessments were repeated at the same scalp location, with an additional follow-up imaging conducted three days later. TrichoScan measurements were performed three to five times per visit at the same site to minimize measurement error, and the average value was used [19].
Outcome variables included total hair density (hairs per cm2), vellus hair density, and terminal hair density, as well as growth indices calculated as the difference between measurements taken on the day of shaving and 3 days later: anagen hair density elongation, vellus hair density elongation, and terminal hair density elongation. Table 2 shows the definitions of hair types as described in the TrichoScan device manual.

Table 2.
Definitions of hair types as described in the TrichoScan device manual.
2.3.2. SEM Observation of Collected Hair
Hair surface conditions and shaft diameter changes pre- and post-NMN supplementation were evaluated by SEM. Hair samples (2–3 cm from the scalp side) were examined using a benchtop scanning electron microscope (SEM; Miniscope TM4000Plus II, Hitachi, Ltd., Tokyo, Japan). Hair shaft diameters were measured using SEM images of the two hairs collected at each time point (two hairs before and two hairs after NMN supplementation).
2.3.3. Hair Metabolomic Analysis (CE-TOFMS)
Hair samples were collected from 15 healthy female volunteers. The hair strands at the posterior vertex were cut as close to the scalp as possible. The collected hair strands were cut at a length of 2 cm from the scalp and used for the measurement. Metabolomic analysis was performed by Human Metabolome Technologies, Inc. (HMT; Yamagata, Japan) using capillary electrophoresis time-of-flight mass spectrometry (CE-TOFMS; Agilent Technologies, Santa Clara, CA, USA), following HMT’s Basic Scan package based on previous studies [20,21]. The mass spectrometer was scanned over an m/z range of 50–1000. Peak extraction for m/z, peak area, and migration time (MT) was conducted using automatic integration software MasterHands (Keio University, Yamagata, Japan).
2.3.4. Hair Hormone Concentration Analysis (LC-MS/MS)
For extracting steroids, hair samples were collected from 15 healthy female volunteers. The collected hair strands were cut at a length of 2 cm from the scalp and used for the measurement. A 2 cm length of hair with fifteen–twenty strands was weighed and washed twice with 2-propanol. Washed hair samples were collected and crushed into powder with a ball mill (ShakeMaster® NEO, Biomedical Science Co., Ltd., Tokyo, Japan), and an internal standard was added. A mixture of 0.5 mL of 0.1 M TFA and 50% acetonitrile solution was added to the pulverized hair and incubated at 40 °C for 1 h. After adding methyl tert-butyl ether to the extract, the mixture was shaken. The organic layer was then separated and dried with a centrifugal evaporator. To the residue, methanol and water were sequentially added to dissolve it, which was applied into the mixed-mode type solid-phase extraction (SPE) column. The SPE column was washed with 1% acetic acid solution, 45% methanol solution, and 1 M sodium hydroxide solution. Androgen, progestogen, and glucocorticoid fraction were then eluted with 1 mL of methanol (Fraction A). The further washing procedure was performed with 1% acetic acid solution and 1% pyridine in 60% methanol. After that, estradiol fraction was eluted with 1% pyridine in 90% methanol (Fraction B). Fractions A and B were dried with a centrifugal evaporator. Fraction A was derivatized with picolinic acid and then purified on the HyperSep SI cartridge, which is a normal-phase SPE column. Fraction B was derivatized sequentially with pentafluoro-pyridine and fusaric acid, and then purified with the HyperSep SI cartridge. The detailed conditions of derivatization were reported elsewhere [22]. After the eluent was evaporated, the residue was dissolved in 80% acetonitrile solution and used for quantification by LC-MS/MS.
2.3.5. Subjective Assessment (VAS Questionnaire)
Self-administered visual analog scale (VAS) questionnaires were administered at baseline and at the end of the intervention. Participants rated the following items by marking a position on a 100 mm horizontal line with anchors representing the best and worst conditions ever experienced: fatigue, hair elasticity, hair manageability, hair gloss, hair volume, perception of gray hair, scalp part visibility, perceived hair loss upon waking, and perceived hair loss during shampooing. The fatigue item was constructed with reference to the method proposed in the Japanese Society of Fatigue Science Clinical Fatigue Evaluation Guidelines. Table S1 shows the VAS questionnaire used in this hair study.
2.4. Statistical Analysis
Hair-related outcomes (density of total, anagen, telogen, vellus, and terminal hairs, hair shaft diameter, and subjective VAS scores) were analyzed pre- and post-NMN supplementation using paired t-tests. Hair metabolite analysis and metabolomic results were compared using Wilcoxon signed-rank tests. Smirnov–Grubbs tests were performed on data suspected of being outliers. Statistical analyses were conducted using R version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria) with significance set at p < 0.05 (two-sided).
3. Results
The basic characteristics of the subjects are presented in Table 3. The mean age ± SD of the participants was 49.7 ± 4.8 years. At baseline, the mean ± SD total hair density (hairs/cm2) was 190.8 ± 34.0, and the anagen hair density (hairs/cm2) was 176.0 ± 29.1, comprising 92.3% of total hair. Vellus hair density (hairs/cm2) was 46.7 ± 10.0, and terminal hair density (hairs/cm2) was 144.1 ± 27.9, with terminal hairs representing 75.5% of total hair.

Table 3.
Baseline characteristics of subjects (n = 15).
3.1. TrichoScan Analysis
Table 4 shows hair density before and after NMN supplementation. The mean ± SD total hair density (hairs/cm2) significantly decreased from 190.8 ± 34.0 pre-supplementation to 167.9 ± 35.8 post-supplementation (p < 0.01). Anagen hair elongation density (3 days after shaving, hairs/cm2) significantly increased from 55.9 ± 43.5 pre-supplementation to 87.7 ± 21.9 post-supplementation (p = 0.03). Vellus hair density significantly decreased from 46.7 ± 10.0 pre-supplementation to 36.7 ± 10.6 post-supplementation (p = 0.03). Terminal hair density also significantly decreased from 144.1 ± 27.9 pre-supplementation to 131.2 ± 30.3 post-supplementation (p = 0.03). No significant change was observed in vellus hair elongation density (pre: 17.3 ± 15.8 vs. post: 21.5 ± 12.9 hairs/cm2). However, terminal hair elongation density significantly increased from 38.6 ± 33.7 pre-supplementation to 66.2 ± 15.1 post-supplementation (p < 0.01). Figure 1 shows representative TrichoScan images before and after NMN intake. Compared to before NMN intake, hair appeared to be thicker after NMN intake.

Table 4.
Hair density before and after NMN supplementation.
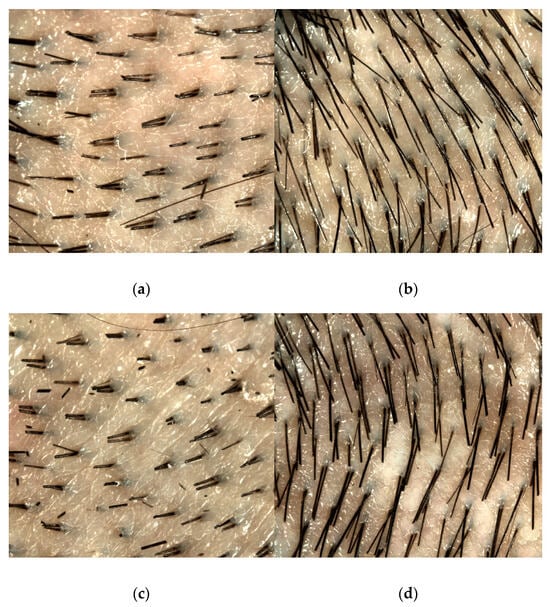
Figure 1.
TrichoScan images (before/after NMN supplementation).(a) Before NMN supplementation, Day 0 (shaving day); (b) Before NMN supplementation, Day 3 after shaving; (c) After NMN supplementation, Day 0 (shaving day); (d) After NMN supplementation, Day 3 after shaving.
3.2. SEM Imaging Analysis
Table 5 summarizes changes in hair diameter before and after NMN supplementation. Two of fifteen subjects were excluded due to significant statistical outliers. The analysis was carried out on 13 of the 15 subjects. The increase in hair diameter was observed in 11 subjects. The mean hair diameter ± SD significantly increased from 75.3 ± 7.6 μm pre-supplementation to 78.8 ± 9.7 μm post-supplementation (p < 0.01).

Table 5.
Hair diameter comparison before and after NMN supplementation (n = 13).
SEM imaging demonstrated improvements in hair cuticle condition and increased diameter after NMN supplementation. Representative examples are shown in Figure 2.
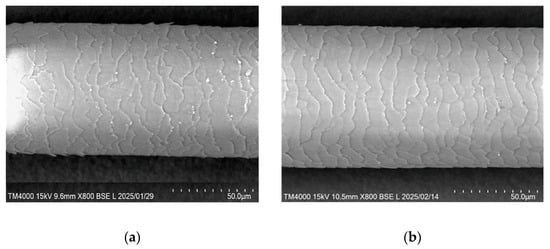
Figure 2.
SEM images showing changes in hair cuticle and diameter pre- and post-NMN supplementation. (a) Before NMN supplementation, Hair diameter 74.7 µm; (b) After NMN supplementation, Hair diameter 84.1 µm.
3.3. Metabolomic Analysis
Table 6 summarizes hair metabolite changes identified by metabolomic analysis pre- and post-NMN supplementation. NMN and NAD+ were below detection limits in all samples. Nicotinamide riboside (NR) and cystine were undetectable pre-supplementation but detected post-supplementation in four and two subjects, respectively. Hydroxyproline and 6-hydroxynicotinic acid were undetectable pre-supplementation but detected post-supplementation in one subject each. Nicotinic acid was predominantly below detection limits and thus excluded from statistical analysis due to insufficient power. Significant increases post-supplementation were observed in 1-methylnicotinamide (p = 0.03), tryptophan, glutamic acid (both p < 0.01), isovalerylcarnitine (p < 0.01), creatine (p = 0.02), creatinine (p = 0.03), carnitine (p = 0.02), isoleucine (p = 0.02), leucine (p = 0.02), lysine (p < 0.01), tyrosine (p = 0.013), aspartic acid (p = 0.02), phenylalanine (p < 0.01), 5-oxoproline (p = 0.05), and uric acid (p < 0.01). Significant decreases were observed in lactic acid (p < 0.01), terephthalic acid (p = 0.02), diethanolamine (p = 0.03), urea (p = 0.04), and trimethylamine N-oxide (p = 0.05). Adenosine, valine, glycine, serine, proline, threonine, and arginine showed no significant changes. Figure S1 shows the chromatograms of the components that exhibited significant changes before and after NMN intake.

Table 6.
Comparison of relative peak areas of hair metabolites before and after NMN supplementation.
3.4. Hormone Concentrations
Table 7 presents changes in hair hormone concentrations pre- and post-NMN supplementation. One outlier each was excluded from cortisol and testosterone analyses. Median (IQR) hormone changes were as follows: cortisol, −0.48 (−4.41 to 3.47) pg/mg; testosterone, −0.05 (−0.16 to 0.14) pg/mg; progesterone, −0.08 (−0.90 to 5.34) pg/mg. No significant changes were observed.

Table 7.
Hormone concentrations in hair pre- and post-NMN supplementation.
3.5. Subjective Assessment
Table 8 shows the results of the change in subjective scores using VAS. Significant improvements post-supplementation were observed for fatigue (4.6 to 3.0), gray hair perception (6.0 to 4.0), hair parting concerns (4.9 to 3.2), and hair loss upon waking (3.6 to 2.4) and shampooing (5.8 to 3.7) (all p < 0.01). Significant increases were reported for hair elasticity (4.1 to 7.2), combing ease (4.5 to 7.2), gloss (4.5 to 7.5), and volume (4.5 to 6.8) (all p < 0.01).

Table 8.
Change in subjective score using VAS pre- and post-NMN supplementation.
4. Discussion
This clinical study investigated the effects of oral NMN supplementation on hair in middle-aged women. Our findings revealed increased anagen hair elongation and hair diameter following NMN supplementation. Metabolomic analysis showed significant alterations in amino acids and metabolites related to energy metabolism. Conversely, no significant changes in hair hormone concentrations were observed. Additionally, subjective improvements in hair elasticity, gloss, volume, and reductions in perceived fatigue and hair loss were noted based on VAS questionnaire responses, suggesting that NMN supplementation may contribute to qualitative hair improvements and enhanced subjective satisfaction.
To our knowledge, this is the first human clinical study examining the impact of oral NMN supplementation on hair. Contrary to our hypothesis, a significant decrease in hair count was observed after taking NMN. Although we cannot deny the possibility that the decrease in hair count is due to taking NMN, we speculate that it is due to seasonal hair loss. Previous studies among Japanese populations have confirmed seasonal variations in hair shedding, peaking in September due to summer UV damage [23]. Consequently, increased hair loss during the September–December study period may explain the observed reduction in overall hair density. However, anagen hair elongation, particularly terminal hair, significantly improved, indicating that NMN supplementation may enhance follicle maturation and nutrient supply to dermal papilla cells, thereby promoting growth of existing terminal hairs rather than stimulating new vellus hair formation. This could lead to improved hair volume and texture through strengthening and thickening existing hair, potentially attributable to enhanced scalp conditions and follicular cell functions.
SEM analysis revealed thicker hair shafts and improved cuticle conditions, consistent with TrichoScan findings. Similar increases in hair diameter and elongation have been observed in NMN-treated mice [15]. Together, these findings suggest NMN stimulates dermal papilla metabolism, improving follicular cell function, thickness, and surface structure. Metabolomic data support this, showing increased amino acids and energy metabolism, potentially linked to antioxidant and sirtuin pathway activation. However, external factors (e.g., hair dye, UV) and study limitations necessitate further double-blind verification.
In metabolomic analyses, NMN and NAD+ remained below detection limits in hair samples, contrasting with previous studies that documented increases in blood NMN metabolites following oral administration [16]. Because NMN-related metabolites have low stability, it is possible that these components are decomposed in the daily hair treatment and UV exposure. The detection of nicotinamide riboside and nicotinic acid derivatives, which were barely detected before NMN intake, suggests that orally ingested NMN may have a direct effect on hair follicles in the form of NMN metabolites. After NMN supplementation, significant increases were observed in tryptophan and glutamine, both of which are key precursors in the NAD+ synthesis pathway. In addition, NMN intake led to elevated levels of metabolites related to energy metabolism, including isovalerylcarnitine, creatine, creatinine, leucine, and isoleucine, together with a reduction in lactate. These metabolic changes suggest an enhancement of mitochondrial ATP production and amino acid metabolism. These findings are consistent with previous reports demonstrating the beneficial effects of NMN supplementation on skin conditions [12,13]. Interestingly, there were also significantly increases in lysine, which has previously been reported to contribute to hair loss [24]. Previous animal studies have demonstrated that elevated NAD+ levels activate hair follicle stem cells (HFSCs) and promote hair regeneration via the sirtuin (SIRT1) pathway [15]. Furthermore, SIRT 7 has been reported to be involved in the progression of the hair cycle and the transition to the anagen phase, playing an important role in hair growth [25]. NMN supplementation has also been reported to reverse age-related hair follicle decline and improve mitochondrial function. Mitochondrial dysfunction has been implicated in the pathogenesis of androgenetic alopecia (AGA) [26]. In line with a previous animal study, the TricoScan analysis in this study demonstrated an increase in terminal hair density following NMN supplementation. These findings indicate that NAD+, through its ability to enhance the sirtuin pathway and mitochondrial function, plays an important role in hair growth and the prevention of hair loss. Therefore, oral NMN supplementation may represent a promising strategy for maintaining healthy hair.
Hormone concentrations such as cortisol, testosterone, and progesterone showed considerable individual variation, and no statistically significant changes were observed before and after NMN oral supplementation. Although hormone levels in hair samples may reflect long-term averages, subtle variations potentially induced by NMN supplementation might not have been detectable due to individual differences, limited sample sizes, and sensitivity limitations of the analytical method. Particularly, female hormones are substantially influenced by age and lifestyle factors, and their interaction with cortisol may further increase variability. Therefore, it is plausible that the short duration and small sample size of this study hindered the detection of significant hormonal changes. Regarding changes in testosterone levels, there was also a large degree of individual variability, and since there was no placebo group, the results of this study did not allow us to understand the effects or specific changes caused by NMN intake.
Changes observed in VAS questionnaire following NMN supplementation suggest improvements in subjective perceptions, implying that the physical and psychological effects associated with NMN intake could positively influence participants’ perceptions of their hair conditions. Specifically, the association between reduced fatigue and improvements in hair condition may be attributed to NMN’s role in enhancing mitochondrial function and basal metabolic activity through the elevation in NAD+ levels, thereby potentially reducing overall physical fatigue. A decrease in fatigue may lead to a reduction in subjective stress and tension, contributing to a more positive perception of hair conditions such as reduced concerns about hair loss and gray hair. Additionally, decreases in stress hormones and oxidative stress could improve scalp health and hair follicle activity, potentially resulting in enhanced hair elasticity, gloss, and overall quality. Perceived conditions such as gray hair, scalp part visibility, and hair loss are typically associated with aging-related hair deterioration. NMN’s anti-aging effects may improve scalp blood flow and hair follicle activity, potentially reducing the visibility of gray hair and hair loss while maintaining the overall quality of hair, including its volume, elasticity, and gloss. Indeed, the subjective improvements observed in this study align well with objective measurements from SEM image analysis and TrichoScan evaluation, indicating that NMN supplementation is likely to have provided noticeable improvements in hair conditions from the participants’ perspectives.
In this clinical trial, an increase in NMN metabolites in hair components was observed after NMN intake. This suggests that orally administered NMN may reach the hair root as metabolites such as NAD+ and act directly on hair follicle cells, as suggested in previous studies. Since increases in ATP production and amino acid levels in hair components were observed after NMN intake, it is also possible that oral NMN may promote basal metabolism and amino acid synthesis throughout the body, thereby increasing the supply of amino acids and ATP to the scalp, which are essential for hair growth. Study limitations include small sample size, single-group design, seasonal influences, and short duration (3 months). Results cannot be generalized beyond middle-aged Japanese women, and placebo-controlled, longer-term studies are required. This study employed systemic administration through oral ingestion; future research should also investigate the potential of topical application as an external agent. Oral administration influences the hair follicle environment via systemic metabolism, whereas topical delivery may exert a more direct and efficient effect. A multifaceted strategy that integrates both systemic and topical approaches could potentially enhance the efficacy of interventions against hair aging.
5. Conclusions
Our findings indicate that oral NMN supplementation positively impacts energy metabolism and protein synthesis, potentially promoting hair growth and diameter improvements in middle-aged women. Despite limitations, including hormonal assessment variability and lack of placebo controls, NMN supplementation appears to offer significant benefits for hair care and quality of life. Further extensive and rigorous studies are necessary to validate these promising results comprehensively.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cosmetics12050204/s1, Figure S1. Chromatographs of the main components that showed significant differences in Table 6; Table S1. Visual Analog Scale (VAS) Questionnaire for this hair study.
Author Contributions
Study planning and IRB approval: T.F., T.C. and S.F.; study physician responsible: T.F.; hair evaluation by TrichoScan and SEM: H.K. and S.F.; study preparation and execution: M.I., H.K., S.F. and T.F.; hair sample analysis: H.K. and S.F.; data curation: T.H. and H.K.; writing—original draft preparation: M.I., T.H., H.K. and S.F.; writing—review and editing: S.F., M.I., T.H., H.K., T.F., T.C. and O.N.; project administration: T.F., T.C., S.F. and H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Mitsubishi Corporation Life Sciences Limited, under the collaborative research agreements with Clinic F (Approval Number LS24002541) and Waseda University (Approval Number LS24003708). No additional external funding received.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki (revised October 2013, Fortaleza) and the Ethical Guidelines for Medical and Biological Research Involving Human Subjects (implemented March 2021). Ethical approval was initially obtained from the C&C Ethics Review Committee (Study No.: 24-MCLS-001, approved on 5 August 2024). Subsequently, the study was registered with UMIN-CTR (Trial ID: UMIN000055442). Following this, additional ethical approval, taking into account the review conducted by the C&C Ethics Review Committee, was obtained from the Ethics Review Committee on Research with Human Subjects, Waseda University (approval No. 2024-348, approved on 9 September 2024), before commencement of the study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Participants were provided with information regarding the purpose, procedures, potential risks, and benefits of the research. They were informed that participation was voluntary and that they could withdraw from the study at any time without penalty. All participants gave their consent prior to inclusion in the study.
Data Availability Statement
The data presented in this study are available from the corresponding author upon reasonable request, due to ethical restrictions on participant confidentiality.
Conflicts of Interest
Fukumoto and Kunitomo are employees of Mitsubishi Corporation Life Sciences Limited (MCLS), which provided funding for this research. Chiba and Ito, affiliated with Waseda University, received research grants from MCLS. At Clinic F, Fujimoto has received speaking fees from MCLS, and Hataoka has contributed as a researcher and expert. Nureki of the University of Tokyo declares no conflicts of interest.
References
- Imai, S.; Guarente, L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014, 24, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Peluso, A.; Damgaard, M.V.; Mori, M.A.S.; Treebak, J.T. Age Dependent Decline of NAD+—Universal Truth or Confounded Consensus? Nutrients 2021, 14, 101. [Google Scholar] [CrossRef]
- She, J.; Sheng, R.; Qin, Z.H. Pharmacology and Potential Implications of Nicotinamide Adenine Dinucleotide Precursors. Aging Dis. 2021, 12, 1879–1897. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Raju, R.P. Regulation of NAD+ Metabolism in Aging and Disease. Metabolism 2022, 126, 154923. [Google Scholar] [CrossRef]
- Fang, E.F.; Lautrup, S.; Hou, Y.; Demarest, T.G.; Croteau, D.L.; Mattson, M.P.; Bohr, V.A. NAD+ in Aging: Molecular Mechanisms and Translational Implications. Trends Mol. Med. 2017, 23, 899–916. [Google Scholar] [CrossRef]
- Radenkovic, D.; Reason; Verdin, E. Clinical Evidence for Targeting NAD Therapeutically. Pharmaceuticals 2020, 13, 247. [Google Scholar] [CrossRef]
- Zhang, M.; Ying, W. NAD+ Deficiency Is a Common Central Pathological Factor of a Number of Diseases and Aging: Mechanisms and Therapeutic Implications. Antioxid. Redox Signal. 2019, 30, 890–905. [Google Scholar] [CrossRef] [PubMed]
- Massudi, H.; Grant, R.; Braidy, N.; Guest, J.; Farnsworth, B.; Guillemin, G. Age Associated Changes in Oxidative Stress and NAD+ Metabolism in Human Tissue. PLoS ONE 2012, 7, e42357. [Google Scholar] [CrossRef]
- Yoshino, J.; Baur, J.A.; Imai, S. NAD+ Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab. 2018, 27, 513–528. [Google Scholar] [CrossRef]
- Campagna, R.; Vignini, A. NAD+ Homeostasis and NAD+-Consuming Enzymes: Implications for Vascular Health. Antioxidants 2023, 12, 376. [Google Scholar] [CrossRef]
- Shimamoto, Y.; Ono, T.; Sawabe, A. Examination of the Effects of a Food Containing Nicotinamide Mononucleotide on Skin Function and Safety. Jpn. Pharmacol. Ther. 2023, 51, 75–87. [Google Scholar]
- Morita, Y.; Izawa, H.; Hirano, A.; Mayumi, E.; Isozaki, S.; Yonei, Y. Clinical Evaluation of Changes in Biomarkers by Oral Intake of NMN. Glycative Stress Res. 2022, 9, 33–41. [Google Scholar]
- Ohashi, Y.; Hirayama, A.; Ishikawa, T.; Nakamura, S.; Shimizu, K.; Ueno, Y.; Tomita, M.; Soga, T. Depiction of Metabolome ChUennges in Histidine Starved Escherichia coli by CE TOFMS. Mol. Biosyst. 2008, 4, 135–147. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Long, Y.; Li, Z.; Li, J. Controlling Hair Loss by Regulating Apoptosis in Hair Follicles: A Comprehensive Overview. Biomolecules 2024, 14, 20. [Google Scholar] [CrossRef]
- Xu, C.; Dai, J.; Ai, H.; Du, W.; Ji, H. β-Nicotinamide Mononucleotide Promotes Cell Proliferation and Hair Growth by Reducing Oxidative Stress. Molecules 2024, 29, 798. [Google Scholar] [CrossRef]
- Okabe, K.; Yaku, K.; Uchida, Y.; Fukamizu, Y.; Sato, T.; Sakurai, T.; Tobe, K.; Nakagawa, T. Oral Administration of Nicotinamide Mononucleotide Is Safe and Efficiently Increases Blood Nicotinamide Adenine Dinucleotide Levels in Healthy Subjects. Front. Nutr. 2022, 9, 868640. [Google Scholar] [CrossRef]
- Mills, K.F.; Yoshida, S.; Stein, L.R.; Grozio, A.; Kubota, S.; Sasaki, Y.; Redpath, P.; Migaud, M.E.; Apte, R.S.; Uchida, K.; et al. Long-term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 2016, 24, 795–806. [Google Scholar] [CrossRef]
- Fukamizu, Y.; Uchida, Y.; Shigekawa, A.; Sato, T.; Kosaka, H.; Sakurai, T. Safety Evaluation of B-Nicotinamide Mononucleotide Oral Administration in Healthy Adult Men and Women. Sci. Rep. 2022, 12, 14442–14452. [Google Scholar] [CrossRef]
- Hoffmann, R. TrichoScan: A Novel Tool for the Analysis of Hair Growth in Vivo. J. Investig. Dermatol. Symp. Proc. 2003, 8, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Ooga, T.; Sato, H.; Nagashima, A.; Sasaki, K.; Tomita, M.; Soga, T.; Ohashi, Y. Metabolomic Anatomy of an Animal Model Revealing Homeostatic Imbalances in Dyslipidaemia. Mol. Biosyst. 2011, 7, 1217–1223. [Google Scholar] [CrossRef]
- Sugimoto, M.; Wong, D.T.; Hirayama, A.; Soga, T.; Tomita, M. Capillary Electrophoresis Mass Spectrometry Based Saliva Metabolomics Identified Oral, Breast and Pancreatic Cancer-Specific Profiles. Metabolomics 2009, 6, 78–95. [Google Scholar] [CrossRef]
- Hobo, Y.; Nishikawa, J.; Miyashiro, Y.; Fujikata, A. Measurement of steroid hormones by liquid chromatography-tandem mass spectrometry with small amounts of hair. Steroids 2020, 164, 108732. [Google Scholar] [CrossRef]
- Kita, F.; Kaneda, N.; Watanabe, Y. Evaluation Method of the Hair Growth Promoter Based on Dynamics of Fall Hairs. Skin Res. 1989, 31, 548–562. [Google Scholar]
- Rushton, D.H. Nutritional factors and hair loss. Clin. Exp. Dermatol. 2002, 27, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Tang, X.; Zhang, S.; Jin, M.; Wang, M.; Deng, Z.; Liu, Z.; Qian, M.; Shi, W.; Wang, Z.; et al. SIRT7 activates quiescent hair follicle stem cells to ensure hair growth in mice. EMBO J. 2020, 39, e104365. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Li, Y.; Jin, S.; Yang, F.; Xiong, R.; Dai, Y.; Song, X.; Guan, C. Progress on mitochondria and hair follicle development in androgenetic alopecia: Relationships and therapeutic perspectives. Stem Cell Res. Ther. 2025, 16, 44. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).