Anti-Aging Potential of Bioactive Peptides Derived from Casein Hydrolyzed with Kiwi Actinidin: Integration of In Silico and In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction and Characterization of Actinidin
2.2.1. Extraction Protocol
2.2.2. SDS-PAGE Electrophoresis
2.3. Enzymatic Hydrolysis
2.3.1. Preparation of Casein Solution
2.3.2. Hydrolysis Procedure
2.3.3. Degree of Hydrolysis
2.4. In Vitro Anti-Aging Activity
2.4.1. Antioxidant Activity (ABTS Assay)
2.4.2. Anti-Collagenase Activity
2.4.3. Anti-Elastase Activity
2.5. Computational Evaluation of Anti-Aging Peptides from Casein Hydrolysates
2.5.1. Protein and Ligand Preparation
2.5.2. Docking Method and Validation
2.5.3. Docking Analysis
2.5.4. Visualization
2.5.5. Web Tools and Software Utilized
3. Results and Discussion
3.1. Electrophoretic and Structural Characterization of Actinidin
3.2. Degree of Hydrolysis Assays
3.3. In Vitro Anti-Aging Activity Assays
| Bioactivity | Source/Compound | % Inhibition | Applied Concentration | Reference |
|---|---|---|---|---|
| Antioxidant (ABTS+•) | Casein-derived peptides (actinidin hydrolysis) | 17.5 ± 4.25% | 0.85 mg/mL | This study |
| Casein-derived peptides (trypsin hydrolysis) | 84.05% | ~ 3 mg/mL | Mokhtari et al. (2023) [46] | |
| Epigallocatechin gallate-EGCG (green tea polyphenol) | 78.5% | 0.1 mg/mL | González-Alfonso et al. (2019) [52] | |
| κ-Casein hydrolysate (ovine, pepsin/trypsin) | ~60% | Not specified | Gómez-Ruiz et al. (2008) [53] | |
| Anti-Collagenase | Casein peptides (actinidin hydrolysis) | 18.55 ± 4.63% | 0.844 mg/mL | This study |
| Protein hydrolysates (PH) derived from Acheta domesticus | 60.23% | Optimized via RSM | Yeerong et al. (2024) [55] | |
| Epigallocatechin gallate-EGCG (green tea polyphenol) | 89.5% | 0.1 mM | Madhan et al. (2007) [56] | |
| Casein peptides (trypsin hydrolysis) | Not quantified | ~3 mg/mL | Mokhtari et al. (2023) [46] | |
| Anti-Elastase | Casein peptides (actinidin hydrolysis) | 28.66 ± 7.2% | 0.844 mg/mL | This study |
| Chia seed peptides (<3 kDa) | 65.32% | 0.43 mg/mL | Aguilar & Liceaga (2020) [57] | |
| Porcine skin peptides (1–3 kDa) | 22.22% | Not specified | Hong et al. (2019) [58] | |
| White tea extract | 89% | Not specified | Thring et al. (2009) [39] |
3.4. Computational Evaluation of Anti-Aging Peptides from Casein Hydrolysates Results
3.4.1. In Silico Hydrolysis of Casein Proteins
3.4.2. Molecular Docking
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdolmaleky, H.M.; Zhou, J. Key Players Inducing Skin Diseases and Skin Aging and Potential Preventive or Therapeutic Strategies Using Phytochemicals. J. Dermatol. Sci. Cosmet. Technol. 2025, 2, 100073. [Google Scholar] [CrossRef]
- Fuchs, E. Skin Stem Cells: Rising to the Surface. J. Cell Biol. 2008, 180, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Amirrah, I.N.; Lokanathan, Y.; Zulkiflee, I.; Wee, M.F.M.R.; Motta, A.; Fauzi, M.B. A Comprehensive Review on Collagen Type I Development of Biomaterials for Tissue Engineering: From Biosynthesis to Bioscaffold. Biomedicines 2022, 10, 2307. [Google Scholar] [CrossRef] [PubMed]
- Van Doren, S.R. Matrix Metalloproteinase Interactions with Collagen and Elastin. Matrix Biol. 2015, 44–46, 224–231. [Google Scholar] [CrossRef]
- Rittie, L.; Fisher, G.J. Natural and Sun-Induced Aging of Human Skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef]
- Madan, K.; Nanda, S. In-Vitro Evaluation of Antioxidant, Anti-Elastase, Anti-Collagenase, Anti-Hyaluronidase Activities of Safranal and Determination of Its Sun Protection Factor in Skin Photoaging. Bioorg. Chem. 2018, 77, 159–167. [Google Scholar] [CrossRef]
- Moita, T.; Pedroso, L.; Santos, I.; Lima, A. Casein and Casein-Derived Peptides: Antibacterial Activities and Applications in Health and Food Systems. Nutrients 2025, 17, 1615. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Aluko, R.E. Structural and functional properties of food protein-derived antioxidant peptides. J. Food Biochem. 2019, 43, e12761. [Google Scholar] [CrossRef]
- Zou, T.-B.; He, T.-P.; Li, H.-B.; Tang, H.-W.; Xia, E.-Q. The Structure-Activity Relationship of the Antioxidant Peptides from Natural Proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef]
- Ketnawa, S.; Wickramathilaka, M.; Liceaga, A.M. Changes on antioxidant activity of microwave-treated protein hydrolysates after simulated gastrointestinal digestion: Purification and identification. Food Chem. 2018, 254, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Dini, I.; Mancusi, A. Food Peptides for the Nutricosmetic Industry. Antioxidants 2023, 12, 788. [Google Scholar] [CrossRef] [PubMed]
- Kurnianto, M.A.; Defri, I.; Syahbanu, F.; Aulia, S.S. Fish-Derived Bioactive Peptide: Bioactivity Potency, Structural Characteristics, and Conventional and Bioinformatics Approaches for Identification. Future Foods 2024, 9, 100386. [Google Scholar] [CrossRef]
- Agrawal, H.; Joshi, R.; Gupta, M. Isolation, Purification and Characterization of Antioxidative Peptide of Pearl Millet (Pennisetum glaucum) Protein Hydrolysate. Food Chem. 2016, 204, 365–372. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Santiago-López, L.; Peres, C.M.; Peres, C.; Garcia, H.S.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Hernández-Mendoza, A. Assessment of Multifunctional Activity of Bioactive Peptides Derived from Fermented Milk by Specific Lactobacillus plantarum Strains. J. Dairy Sci. 2017, 100, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Bieth, J.G.; Spiess, B.; Wermuth, C.G. Selective Loss of Elastase Inhibitory Activity of α1-Proteinase Inhibitor upon Chemical Modification of Its Tyrosyl Residues. J. Biol. Chem. 1981, 256, 6374–6380. [Google Scholar] [CrossRef]
- Takahashi, L.H.; Radhakrishnan, R.; Rosenfield, R.E., Jr.; Meyer, E.F., Jr. Crystallographic Analysis of the Inhibition of Porcine Pancreatic Elastase by a Peptidyl Boronic Acid: Structure of a Reaction Intermediate. Biochemistry 1989, 28, 7610–7617. [Google Scholar] [CrossRef]
- Bam, P.; Bhatta, A.; Krishnamoorthy, G. Design of biostable scaffold based on collagen crosslinked by dialdehyde chitosan with presence of gallic acid. Int. J. Biol. Macromol. 2019, 130, 836–844. [Google Scholar] [CrossRef]
- Kaur, L.; Mao, B.; Bailly, J.; Oladeji, O.; Blatchford, P.; McNabb, W.C. Actinidin in Green and SunGold Kiwifruit Improves Digestion of Alternative Proteins—An In Vitro Investigation. Foods 2022, 11, 2739. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, Q.; Liu, H.J.; Li, S.Z.; Jiang, Z.Q. Characterization of Actinidin from Chinese Kiwifruit Cultivars and Its Applications in Meat Tenderization and Production of Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptides. LWT 2017, 78, 1–7. [Google Scholar] [CrossRef]
- Wang, S.; Qiu, Y.; Zhu, F. Kiwifruit (Actinidia spp.): A Review of Chemical Diversity and Biological Activities. Food Chem. 2021, 350, 128469. [Google Scholar] [CrossRef]
- Morena, F.; Cencini, C.; Calzoni, E.; Martino, S.; Emiliani, C. A Novel Workflow for In Silico Prediction of Bioactive Peptides: An Exploration of Solanum Lycopersicum By-Products. Biomolecules 2024, 14, 930. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Longarela, N.; Paredes Ramos, M.; López Vilariño, J.M. Bioinformatics Tools for the Study of Bioactive Peptides from Vegetal Sources: Evolution and Future Perspectives. Crit. Rev. Food Sci. Nutr. 2025, 65, 3476–3495. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Li, F.; Kang, J.; Xie, S.; Qin, X.; Gao, J.; Chen, Z.; Cao, W.; Zheng, H.; Song, W. In Vitro In Silico Screening Strategy and Mechanism of Novel Tyrosinase Inhibitory Peptides from Nacre of Hyriopsis cumingii. Mar. Drugs 2024, 22, 420. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.; Das, S.; Stanley, A.; Yadav, L.; Sudhakar, A.; Varma, A.K. Optimized Hydrophobic Interactions and Hydrogen Bonding at the Target-Ligand Interface Leads the Pathways of Drug-Designing. PLoS ONE 2010, 5, e12029. [Google Scholar] [CrossRef]
- Wang, P.; Song, X.; Liang, Q. Molecular Docking Studies and In Vitro Activity of Pancreatic Lipase Inhibitors from Yak Milk Cheese. Int. J. Mol. Sci. 2025, 26, 756. [Google Scholar] [CrossRef]
- D’Opazo, V.; Calpe, J.; Escrivá, L.; Melo Nazareth, T.; Meca, G.; Luz, C. Identification and Evaluation of Antioxidant Activity of Bioactive Casein-Derived Peptides during in Vitro Digestion and Transepithelial Transport in Caco-2 Cells. Food Biosci. 2023, 53, 102763. [Google Scholar] [CrossRef]
- Bhandari, D.; Rafiq, S.; Gat, Y.; Gat, P.; Waghmare, R.; Kumar, V. A Review on Bioactive Peptides: Physiological Functions, Bioavailability and Safety. Int. J. Pept. Res. Ther. 2020, 26, 139–150. [Google Scholar] [CrossRef]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef]
- Zhao, C.; Gong, Y.; Zheng, L.; Zhao, M. The Degree of Hydrolysis and Peptide Profile Affect the Anti-Fatigue Activities of Whey Protein Hydrolysates in Promoting Energy Metabolism in Exercise Mice. J. Agric. Food Chem. 2023, 71, 3010–3021. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Jyothirmayi, T.; Diwan, P.V.; Dinesh Kumar, B. Antioxidant Activity and Functional Properties of Enzymatic Protein Hydrolysates from Common Carp (Cyprinus carpio) Roe (Egg). J. Food Sci. Technol. 2015, 52, 5817–5825. [Google Scholar] [CrossRef]
- Sharma, D.; Dhiman, I.; Das, S.; Das, D.K.; Das Pramanik, D.; Dash, S.K.; Pramanik, A. Recent Advances in Therapeutic Peptides: Innovations and Applications in Treating Infections and Diseases. ACS Omega 2025, 10, 17087–17107. [Google Scholar] [CrossRef] [PubMed]
- Puglisi, I.; Petrone, G.; Lo Piero, A.R. Role of Actinidin in the Hydrolysis of the Cream Milk Proteins. Food Bioprod. Process. 2012, 90, 449–452. [Google Scholar] [CrossRef]
- Kaur, S.; Huppertz, T.; Vasiljevic, T. Milk Protein Hydrolysis by Actinidin: Influence of Protein Source and Hydrolysis Conditions. Int. Dairy J. 2021, 118, 105029. [Google Scholar] [CrossRef]
- Katsaros, G.I.; Tavantzis, G.; Taoukis, P.S. Production of Novel Dairy Products Using Actinidin and High Pressure as Enzyme Activity Regulator. Innov. Food Sci. Emerg. Technol. 2010, 11, 47–51. [Google Scholar] [CrossRef]
- Lo Piero, A.R.; Puglisi, I.; Petrone, G. Characterization of the Purified Actinidin as a Plant Coagulant of Bovine Milk. Eur. Food Res. Technol. 2011, 233, 517–524. [Google Scholar] [CrossRef]
- Hoyle, N.T.; Merrltt, J.H. Quality of Fish Protein Hydrolysates from Herring (Clupea harengus). J. Food Sci. 1994, 59, 76–79. [Google Scholar] [CrossRef]
- Wang, L.; Ma, M.; Yu, Z.; Du, S. Preparation and Identification of Antioxidant Peptides from Cottonseed Proteins. Food Chem. 2021, 352, 129399. [Google Scholar] [CrossRef]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-Collagenase, Anti-Elastase and Anti-Oxidant Activities of Extracts from 21 Plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef]
- Nam, K.A.; You, S.G.; Kim, S.M. Molecular and Physical Characteristics of Squid (Todarodes pacificus) Skin Collagens and Biological Properties of Their Enzymatic Hydrolysates. J. Food Sci. 2008, 73, C249–C255. [Google Scholar] [CrossRef]
- Rama, A.R.; Nadeem, M.; Ameer, K.; Choi, M. In silico screening of bioactive peptides from casein with anti-skin aging properties. Mol. Biotechnol. 2024, 66, 2426–2440. [Google Scholar] [CrossRef] [PubMed]
- Albisoru, D.; Radu, N.; Pirvu, L.C.; Stefaniu, A.; Băbeanu, N.; Stoica, R.; Mihai, D.P. Studies Regarding Antimicrobial Properties of Some Microbial Polyketides Derived from Monascus Strains. Antibiotics 2024, 13, 1092. [Google Scholar] [CrossRef] [PubMed]
- Boland, M. Kiwifruit Proteins and Enzymes. Actinidin and Other Significant Proteins. Adv. Food Nutr. Res. 2013, 68, 59–80. [Google Scholar] [CrossRef] [PubMed]
- Baker, E.N. The Structure of Actinidin at 5.5 Å Resolution. J. Mol. Biol. 1976, 101, 185–196. [Google Scholar] [CrossRef]
- Dhiman, V.K.; Chauhan, V.; Kanwar, S.S.; Singh, D.; Pandey, H. Purification and Characterization of Actinidin from Actinidia Deliciosa and Its Utilization in Inactivation of α-Amylase. Bull. Natl. Res. Cent. 2021, 45, 213. [Google Scholar] [CrossRef]
- Mokhtari, R.; Rezaei, M.; Kazemi Fard, M.; Dirandeh, E. Evaluation of Antimicrobial and Antioxidant Activities of Casein-Derived Bioactive Peptides Using Trypsin Enzyme. J. Food Qual. 2023, 2023, 1792917. [Google Scholar] [CrossRef]
- Turgeon, S.L.; Bard, C.; Gauthier, S.F. Comparaison de Trois Méthodes Pour La Mesure Du Degré d’hydrolyse de Protéines Laitières Modifiées Enzymatiquement. Can. Inst. Food Sci. Technol. J. 1991, 24, 14–18. [Google Scholar] [CrossRef]
- Alizadeh, H.; Teymouri, F.; Gilbert, T.I.; Dale, B.E. Pretreatment of Switchgrass by Ammonia Fiber Explosion (AFEX). Appl. Biochem. Biotechnol. 2005, 124, 1133–1142. [Google Scholar] [CrossRef]
- Dale, B.E.; Weaver, J.; Byers, F.M. Extrusion Processing for Ammonia Fiber Explosion (AFEX). In Twentieth Symposium on Biotechnology for Fuels and Chemicals; Humana Press: Totowa, NJ, USA, 1999; pp. 35–45. [Google Scholar]
- Kaur, S.; Huppertz, T.; Vasiljevic, T. Actinidin-Induced Hydrolysis of Milk Proteins: Effect on Antigenicity. LWT 2022, 161, 113294. [Google Scholar] [CrossRef]
- Irshad, I.; Kanekanian, A.; Peters, A.; Masud, T. Antioxidant Activity of Bioactive Peptides Derived from Bovine Casein Hydrolysate Fractions. J. Food Sci. Technol. 2015, 52, 231–239. [Google Scholar] [CrossRef]
- Gonzalez-Alfonso, J.L.; Peñalver, P.; Ballesteros, A.O.; Morales, J.C.; Plou, F.J. Effect of α-Glucosylation on the Stability, Antioxidant Properties, Toxicity, and Neuroprotective Activity of (–)-Epigallocatechin Gallate. Front. Nutr. 2019, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ruiz, J.Á.; López-Expósito, I.; Pihlanto, A.; Ramos, M.; Recio, I. Antioxidant Activity of Ovine Casein Hydrolysates: Identification of Active Peptides by HPLC–MS/MS. Eur. Food Res. Technol. 2008, 227, 1061–1067. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, M.; Zhao, B.; Yang, F. Isolation of Antioxidant Peptides from Yak Casein Hydrolysate. RSC Adv. 2020, 10, 19844–19851. [Google Scholar] [CrossRef] [PubMed]
- Yeerong, K.; Chantawannakul, P.; Anuchapreeda, S.; Wangtueai, S.; Chaiyana, W. Optimization of Hydrolysis Conditions, Isolation, and Identification of Biologically Active Peptides Derived from Acheta Domesticus for Antioxidant and Collagenase Inhibition. Antioxidants 2024, 13, 367. [Google Scholar] [CrossRef]
- Madhan, B.; Krishnamoorthy, G.; Rao, J.R.; Nair, B.U. Role of Green Tea Polyphenols in the Inhibition of Collagenolytic Activity by Collagenase. Int. J. Biol. Macromol. 2007, 41, 16–22. [Google Scholar] [CrossRef]
- Aguilar-Toalá, J.E.; Liceaga, A.M. Identification of Chia Seed (Salvia hispanica L.) Peptides with Enzyme Inhibition Activity towards Skin-Aging Enzymes. Amino Acids 2020, 52, 1149–1159. [Google Scholar] [CrossRef]
- Hong, G.-P.; Min, S.-G.; Jo, Y.-J. Anti-Oxidative and Anti-Aging Activities of Porcine By-Product Collagen Hydrolysates Produced by Commercial Proteases: Effect of Hydrolysis and Ultrafiltration. Molecules 2019, 24, 1104. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Y.; Huang, D.-P.; Bian, Z.; Lu, P.; Fan, D.-M.; Wang, X. Inhibitory mechanism of angiotensin-converting enzyme inhibitory peptides from black tea. J. Zhejiang Univ. Sci. B 2021, 22, 575–589. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Sarkar, R. Site-selective cleavage of peptides and proteins targeting aromatic amino acid residues. RSC Adv. 2025, 15, 9159–9179. [Google Scholar] [CrossRef]
- Kang, Y.-A.; Kim, Y.-J.; Jin, S.-K.; Choi, H.-J. Antioxidant, Collagenase Inhibitory, and Antibacterial Effects of Bioactive Peptides Derived from Enzymatic Hydrolysate of Ulva australis. Mar. Drugs 2023, 21, 469. [Google Scholar] [CrossRef]

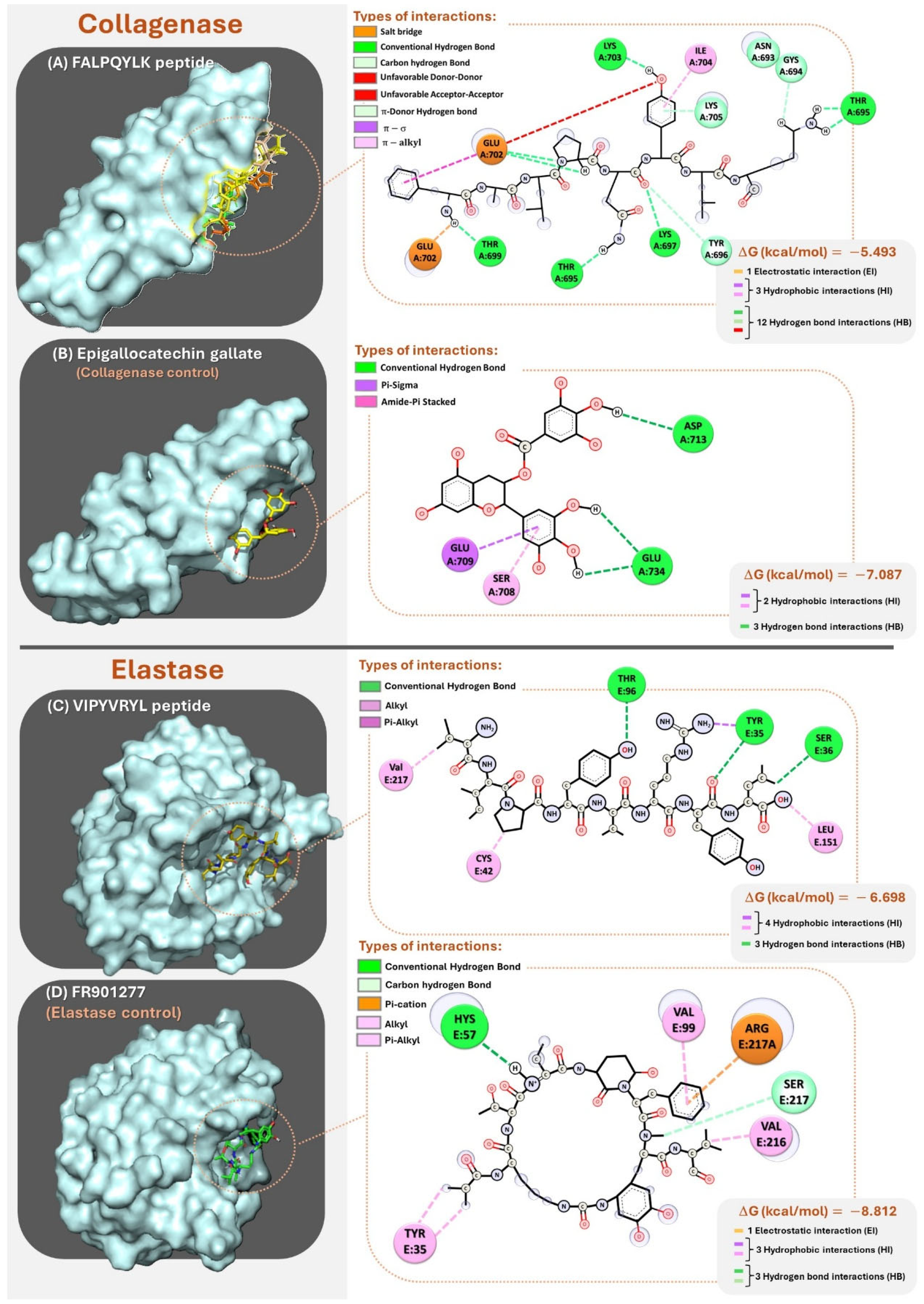
| Protein (UniProt ID) | Total Peptides | Peptides of 4–8 Amino Acids | Theoretical Degree of Hydrolysis (%DHt) |
|---|---|---|---|
| CSN1S1 (P02662) | 16 | 5 | 100% |
| CSN1S2 (P02663) | 26 | 10 | 100% |
| CSN2 (P02666) | 13 | 3 | 100% |
| CSN3 (P02668) | 11 | 2 | 100% |
| Total | 66 | 20 (30.3%) | — |
| Enzyme | Peptide | Hydrogen Bond * | Electrostatic Bond * | Hydrophobic Bond * | ΔG kcal/mol |
|---|---|---|---|---|---|
| Collagenase | FALPQYLK | Ligand:PHE1 – A:GLU702 Ligand:PHE1 – A:THR699 Ligand:GLN5 – A:LYS697 Ligand:GLN5 – A:THR695 Ligand:GLN5 – A:TYR696 Ligand:TYR6 – A:LYS705 Ligand:TYR6 – A:GLU705 Ligand:LYS8 – A:THR695 Ligand:LYS8 – A:GLY694 | Ligand:PHE1 – A:GLU702 | Ligand:PHE1 – A:GLU702 Ligand:TYR6 – A:LYS705 Ligand:TYR6 – A:ILE704 | −5.493 |
| HYQK | Ligand:HIS1 – A:GLU709 Ligand:TYR2 – A:GLU737 Ligand:GLN3 – A:LYS705 Ligand:GLN3 – A:GLU702 Ligand:LYS4 – A:THR695 | Ligand:HIS1 – A:GLU709 | Ligand:LYS4 – A:LYS705 | −5.229 | |
| TTMPLW | Ligand:THR1 – A:GLU737 Ligand:THR1 – A:ASN735 Ligand:THR2 – A:SER707 Ligand:TRP6 – A:GLU702 Ligand:TRP6 – A:LYS703 Ligand:TRP6 – A:LYS697 Ligand:TRP6 – A:LYS705 | Ligand:THR1 – A:GLU737 Ligand:TRP6 – A:LYS705 | Ligand:TRP6 – A:LYS705 | −4.949 | |
| HPIK | Ligand:ILE3 – A:LYS705 Ligand:LYS4 – A:ASN693 Ligand:LYS4 – A:THR695 Ligand:HIS1 – A:PHE706 Ligand:HIS1 – A:ASN735 | Ligand:HIS1 – A:GLU709 Ligand:HIS1 – A:GLU737 | Ligand:HIS1 – A:LYS705 Ligand:ILE3 – A:ILE704 Ligand:ILE3 – A:LYS705 | −4.796 | |
| HIQK | Ligand:HIS1 – A:ASN735 Ligand:HIS1 – A:SER707 Ligand:ILE2 – A:LYS705 Ligand:GLN3 – A:LYS705 Ligand:GLN3 – A:LYS697 Ligand:LYS4 – A:THR695 | - | - | −4.704 | |
| VIPYVRYL | Ligand:TYR4 – A:GLU734 Ligand:TYR6 – A:GLU733 Ligand:TYR6 – A:ASN735 Ligand:LEU7 – A:GLU734 | Ligand:TYR4 – A:GLU734 | Ligand:PRO3 – A:ILE718 | −4.577 | |
| EAMAPK | Ligand:GLU1 – A:GLU734 Ligand:GLU1 – A:SER711 Ligand:GLU1 – A:SER708 Ligand:ALA2 – A:TYR721 Ligand:MET3 – A:TYR721 Ligand:PRO5 – A:VAL719 Ligand:PRO5 – A:GLU734 Ligand:LYS6 – A:VAL719 | Ligand:GLU1 – A:GLU734 | Ligand:ALA2 – A:ILE718 Ligand:MET3 – A:LYS717 Ligand:PRO5 – A:TYR721 Ligand:LYS6 – A:VAL719 | −4.452 | |
| EMPFPK | Ligand:GLU1 – A:GLU709 Ligand:GLU1 – A:SER707 Ligand:MET2 – A:SER707 Ligand:LYS6 – A:LYS705 | Ligand:GLU1 – A:GLU709 | Ligand:MET2 – A:LYS705 Ligand:PHE4 – A:LYS69 | −4.372 | |
| ISQRYQK | Ligand:GLN6 – A:SER707 Ligand:TYR5 – A:ASN693 Ligand:TYR5 – A:LYS705 Ligand:TYR5 – A:TYR696 Ligand:TYR5 – A:SER707 Ligand:GLN6 – A:PHE706 Ligand:GLN6 – A:ASN735 | Ligand:ARG4 – A:GLU709 | Ligand:TYR5 – A:LYS705 Ligand:TYR5 – A:ILE704 Ligand:PRO5 – A:ILE704 | −4.369 | |
| EGIHAQQK | Ligand:GLU1 – A:VAL761 Ligand:ILE3 – A:SER762 Ligand:HIS4 – A:GLY690 Ligand:HIS4 – A:SER760 Ligand:ALA5 – A:VAL761 Ligand:GLN6 – A:VAL761 Ligand:GLN7 – A:LEU687 Ligand:GLN7 – A:PRO688 Ligand:LYS8 – A:TYR689 | - | Ligand:HIS4 – A:SER762 | −4.338 | |
| Elastase | VIPYVRYL | Ligand:TYR4 – A:THR96 Ligand:TYR7 – A:TYR35 L igand:LEU8 – A:SER36 | - | Ligand:VAL1 – A:VAL217 Ligand:PRO3 – A:CYS42 Ligand:ARG6 – A:TYR35 Ligand:LEU8 – A:LEU151 | −6.698 |
| ISQRYQK | Ligand:TYR5 – E:CYS191 Ligand:GLN6 – E:CYS42 Ligand:GLN6 – E:THR41 | Ligand:ARG61 – A:LYS7 | Ligand:ILE1 – E:PHE215 Ligand:TYR5 – E:VAL216 | −6.286 | |
| HYQK | Ligand:GLN3 – E:CYS191 | Ligand:HIS1 – A:TYR2 Ligand:LYS4 – A:LYS4 Ligand:LYS4 – E:HIS57 | Ligand:HIS1 – E:LEU143 | −6.012 | |
| HPIK | Ligand:HIS1 – E:CYS191 Ligand:LYS4 – E:THR41 | - | Ligand:HIS1 – E:VAL216 Ligand:PRO2 – E:HIS57 Ligand:PRO2 – E:VAL99 | −5.772 | |
| FALPQYLK | Ligand:GLN5 – E:CYS191 Ligand:GLN5 – E:VAL216 Ligand:TYR6 – E:ARG217 Ligand:LYS8 – E:CYS58 Ligand:LYS8 – E:ARG61 | - | Ligand:TYR6 – E:VAL99 | −5.759 | |
| HIQK | Ligand:HIS1 – E:HIS57 Ligand:HIS1 – E:SER195 Ligand:ILE2 – E:HIS57 Ligand:ILE2 – E:SER195 Ligand:GLN3 – E:GLY193 | - | Ligand:HIS1 – E:VAL216 Ligand:ILE2 – E:HIS57 Ligand:LYS4 – E:LEU143 Ligand:LYS4 – E:LEU151 | −5.669 | |
| EGIHAQQK | Ligand:GLU1 – E:GLN23 Ligand:GLU1 – E:SER26 Ligand:GLU1 – E:GLN157 Ligand:GLY2 – E:SER26 Ligand:ALA5 – E:TYR207 Ligand:GLN6 – E:SER29 Ligand:GLN6 – E:VAL122 Ligand:GLN7 – E:GLN206 | - | Ligand:ALA5 – E:TYR207 Ligand:LYS8 – E:VAL122 | −5.661 | |
| TTMPLW | Ligand:THR2 – E:TYR101 Ligand:THR2 – E:ALA99 Ligand:MET3 – E:TYR101 Ligand:TRP6 – E:SER232 Ligand:TRP6 – E:SER236 | Ligand:LEU5 – E:HIS91 Ligand:LEU5 – E:TYR93 Ligand:LEU5 – E:TYR101 Ligand:PRO4 – E:ALA233 Ligand:TRP6 – E:ALA126 | −5.531 | ||
| EMPFPK | Ligand:GLU1 – E:ASN25 Ligand:GLU1 – E:GLN119 Ligand:MET2 – E:SER26 Ligand:PHE4 – E:SER26 Ligand:LYS6 – E:ASN25 | - | Ligand:PRO5 – E:TRP27 Ligand:PRO5 – E:TYR137 Ligand:PRO5 – E:TYR207 | −5.143 | |
| EAMAPK | Ligand:GLU1 – E:ARG217 Ligand:ALA4 – E:ARG217 Ligand:LYS6 – E:ARG217 Ligand:LYS6 – E:GLN192 | Ligand:GLU1 – E:LYS177 | Ligand:ALA2 – E:ARG217 Ligand:ALA4 – E:VAL99 Ligand:MET3 – E:VAL99 Ligand:LYS6 – E:ARG217 | −4.950 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caicedo, N.; Gamboa, L.L.; Ciro, Y.; Salamanca, C.H.; Oñate-Garzón, J. Anti-Aging Potential of Bioactive Peptides Derived from Casein Hydrolyzed with Kiwi Actinidin: Integration of In Silico and In Vitro Study. Cosmetics 2025, 12, 189. https://doi.org/10.3390/cosmetics12050189
Caicedo N, Gamboa LL, Ciro Y, Salamanca CH, Oñate-Garzón J. Anti-Aging Potential of Bioactive Peptides Derived from Casein Hydrolyzed with Kiwi Actinidin: Integration of In Silico and In Vitro Study. Cosmetics. 2025; 12(5):189. https://doi.org/10.3390/cosmetics12050189
Chicago/Turabian StyleCaicedo, Nicolas, Lady L. Gamboa, Yhors Ciro, Constain H. Salamanca, and Jose Oñate-Garzón. 2025. "Anti-Aging Potential of Bioactive Peptides Derived from Casein Hydrolyzed with Kiwi Actinidin: Integration of In Silico and In Vitro Study" Cosmetics 12, no. 5: 189. https://doi.org/10.3390/cosmetics12050189
APA StyleCaicedo, N., Gamboa, L. L., Ciro, Y., Salamanca, C. H., & Oñate-Garzón, J. (2025). Anti-Aging Potential of Bioactive Peptides Derived from Casein Hydrolyzed with Kiwi Actinidin: Integration of In Silico and In Vitro Study. Cosmetics, 12(5), 189. https://doi.org/10.3390/cosmetics12050189









