Abstract
Silicon (Si) plays a crucial role in maintaining the structural integrity of nails, skin, and hair by supporting collagen synthesis and keratin stability. Despite its recognized benefits, effective topical delivery methods remain underexplored. This study investigates a novel approach using a calcium silicate-based formulation designed to enhance silicon bioavailability. The research comprises two key components: an in vitro assessment of calcium silicate dissolution and ion release, and a 28-day, single-arm, clinical evaluation of its effects on nail thickness and strength. Ion release studies demonstrated stable dissolution with significant silicon and calcium release. In the clinical study, the mean nail thickness score increased from 1.50 ± 0.51 to 2.09 ± 0.53, corresponding to a 39% mean improvement and nail strength scores improved from 1.50 ± 0.51 to 2.45 ± 0.67, reflecting a 64% average increase over 28 days of application (p < 0.001). The findings support the potential of targeted silicon delivery systems in cosmetic applications, offering an alternative to traditional oral supplementation.
1. Introduction
The fields of hair, nail, and skin care encompass vital aspects of human well-being and self-expression, with a profound impact on our physical appearance and self-confidence throughout various stages of life. While advancements in research, technology, and product development have revolutionized these domains, there persist numerous unmet needs, particularly concerning the effects of aging, stress and diseases on the quality of skin, hair, and nails [1]. The pursuit of effective solutions to strengthen and improve these elements has been an ongoing endeavor. Among the many avenues explored, the use of silicon has emerged as a promising approach [2]. Silicon, a naturally occurring mineral, has garnered attention for its potential to enhance the strength and vitality of hair, nails, and skin. By examining the existing research and exploring the potential applications of silicon-based interventions, we can gain insights into the opportunities and challenges associated with this approach. Ultimately, this exploration aims to contribute to the advancement of hair, nail, and skin care practices by shedding light on the role of silicon in promoting their strength and overall well-being.
Silicon (Si), the second-most abundant element on Earth and a significant trace element in the human body, plays a crucial role in maintaining the health of various tissues, including the skin, hair, and nails [3,4]. Its presence is not limited to these external components but extends to vital organs and connective tissues throughout the body. Research suggests that silicon is instrumental in optimizing collagen synthesis, leading to improved skin strength, elasticity, and a reduction in hair loss and nail fragility [2,5]. Studies have demonstrated that food supplements with silicon can enhance the synthesis of collagen type I, primarily by activating enzymes and facilitating cross-linking in connective tissues while increasing the density of elastic fibers [6]. Furthermore, the application of silicon treatments has shown potential in enhancing the keratin structure of hair and nails, resulting in reduced brittleness [2,5]. Additional benefits documented in the literature include increased resistance and thickness of hair fibers, maintenance of blood vessel elasticity, stimulation of elastin synthesis, and promotion of nail hardness [3]. These findings underscore the significance of silicon in promoting the overall health and quality of hair, nails, and skin.
Silicon is a trace mineral that is essential for the health and maintenance of connective tissues, including skin, hair, nails, bones, and blood vessels. It is crucial for the structural integrity and elasticity of these tissues, contributing significantly to collagen synthesis and overall tissue health [7,8]. In recent years, silicon’s role has attracted increased attention, particularly in the health and beauty industries, due to its potential in anti-aging and tissue-strengthening applications [9].
Naturally present in the human body in small but essential amounts, silicon levels range from 1 to 7 g, with the highest concentrations found in connective tissues such as skin, hair, nails, bones, and arteries [4]. Despite being a trace element, silicon contributes to the development and maintenance of these tissues. As people age, silicon levels tend to decline, which has spurred interest in both dietary supplementation and topical applications to combat age-related tissue degradation, especially in the context of anti-aging and bone health treatments [7].
Though a trace mineral, silicon has a significant impact on several biological processes, maintaining the structural and functional integrity of various tissues. It supports bone formation and mineralization by stimulating osteoblast activity and enhancing the incorporation of calcium and phosphorus into the bone matrix, which improves both density and flexibility, reducing fracture risk [4,7,8]. It also contributes to joint and cartilage health by promoting glycosaminoglycan synthesis, which supports elasticity and cushioning, potentially reducing degenerative changes such as those seen in osteoarthritis [6,10]. Furthermore, silicon helps maintain the integrity and elasticity of arterial walls by supporting elastin and collagen structure, with emerging evidence suggesting a protective effect against atherosclerosis [10,11].
Silicon plays a key role in collagen formation by facilitating several biochemical processes essential for collagen stabilization and functionality [12].
Collagen is important for maintaining the quality of our hair, skin and nails, since hair, nails, and skin all share a common structural component: collagen, which is a fibrous protein that provides strength and resilience. In hair, collagen is present in the connective tissues surrounding the hair follicles, contributing to its overall structure and elasticity [1,13]. Collagen is fundamental for maintaining the integrity of the skin by providing structural support to the dermis, the middle layer of the skin. It helps to maintain skin firmness, smoothness, and elasticity, making it an essential component in maintaining a youthful appearance [14]. Similarly, nails also rely on collagen for their strength and durability. Collagen is present in the connective tissues and blood vessels surrounding the nail bed, helping to maintain the structure and prevent brittleness, promoting healthy nail growth [2].
In summary, collagen is a critical factor in the structure and health of hair, skin, and nails, contributing to their strength, elasticity, and overall well-being.
Of the 28 different types of collagens, type I collagen accounts for approximately 90% of the collagen present in the human body. Collagen synthesis primarily occurs within specialized cells called fibroblasts and involves a combination of intracellular processes including transcription, translation, and post-translational modifications, as well as extracellular steps such as peptide cleavage and collagen fibril assembly [6,12,13]. Notably, silicon (Si) has been found to contribute to the activation of hydroxylation enzymes involved in both intracellular and extracellular stages of collagen synthesis, ultimately stimulating the production of collagen [15]. From the diet, concentrations of silicon detected in plasma can be measured to 5–20 µM [6]. The silicon is then transported and distributed throughout the body. As described, silicon levels in red blood cells and plasma are relatively low, whereas silicon concentrations in nails are significantly higher than those in blood. Silicon levels in nails can reach up to and exceed 1500 µg/g, i.e., about 0.15 wt% [16].
Silicon (Si) is commonly found in the form of silicon dioxide (SiO2) or silicate. It is naturally present in various foods and primarily absorbed through our diet. However, it is worth noting that significant amounts of silicon in certain foods may be in an insoluble form, rendering it unable to be absorbed in the gastrointestinal tract. To make it bioavailable, orally administered silicon needs to undergo a conversion process, transforming into orthosilicic acid (OSA) that can cross the intestinal barrier. This conversion relies on the low-pH environment in the human gastrointestinal tract. Nevertheless, the precise absorption profile of silicon is still not fully understood. It is crucial to note that while silicon can be absorbed in the form of orthosilicic acid, concentrations exceeding 10 mg/L without stabilizers to prevent self-association can result in the formation of silicon dioxide, which has limited bioavailability [17].
Topical administration offers an alternative to oral administration, presenting the advantage of bypassing first-pass metabolism, which can result in lower systemic circulation concentrations. By applying substances directly to the target area, topical administration allows for achieving higher concentrations locally. This localized approach facilitates an increasingly pronounced effect on the optimal synthesis of collagen, potentially enhancing its benefits within the specific area of application.
In order to facilitate topical administration, silicon (Si) needs to be delivered to the target area in a soluble form. As previously mentioned, silicon dioxide (SiO2) exhibits limited solubility at neutral pH. To overcome this challenge, one strategy involves delivering a soluble salt of silicon to the target area. By binding silicon with calcium, a family of calcium silicate salts can be formed. These salts, including various forms of calcium silicate, are significantly more soluble in water compared to SiO2. This approach allows for higher concentrations of silicon to be achieved and enhances the diffusion profile through the skin barrier, making it a more favorable option for topical applications. Although the human nail plate is a dense keratin structure, studies have shown it to be permeable to small hydrophilic molecules and ions, enabling transungual delivery. In this context, the release of silicon ions from soluble calcium silicate could allow their penetration through the nail plate to the underlying nail bed, where they may stimulate collagen synthesis and improve nail strength and quality.
The solubility of calcium silicate 0.01% [18]. To determine the molarity of calcium silicate (CaSiO3) in a solution, we start with its solubility, which is 0.1 g/L. To determine the corresponding molarity, the mass of calcium silicate must be established. Considering the atomic masses of silicon (28.0855 g/mol), calcium (40.078 g/mol), and oxygen (15.999 g/mol), the molar mass of CaSiO3 is calculated to be 116.16 g/mol. Based on this value, the number of moles (n) can be determined using the standard relation:
where: n is the number of moles, m is the mass of the solute (in grams), and M is the molar mass (in g/mol).
The molar mass of calcium silicate is 116.16 g/mol. Substituting the values into the formula gives:
Thus approximately 0.000861 moles of calcium silicate can be dissolved into 1 L of solution.
Molarity (M) is defined as the number of moles of solute per liter of solution.
To express this value in micromoles per liter (µmol/L), we multiply by 1,000,000 (since 1 mol = 1,000,000 µmol):
When comparing the 861 µmol/L from calcium silicate dissolution to the 10 µmol/L found in human blood, it becomes evident that calcium silicate could theoretically lead to local concentrations of silicon at the surface that are approximately about 80 times higher than those typically observed in blood.
In this paper, a specific topical administration method involving calcium silicate is described for nail care. When the nail serum enriched with calcium silicate is applied, silicon ions are released, permitting their passage through the nail plate to the underlying nail bed. Within the nail bed, these silicon ions can stimulate collagen synthesis, a pivotal process for maintaining nail structure and strength. Enhanced collagen production contributes to improved nail quality, reducing brittleness and promoting healthier and less prone-to-breakage nails.
Beyond the effects on the nail plate, the application of the nail serum around the nail cuticle area serves an additional purpose. By nourishing the cuticles, the serum promotes the strength and quality of forthcoming nail growth. This comprehensive approach ensures that the entire nail structure, from the nail bed to the surrounding cuticle area, receives support for optimal growth and resilience.
The study experimentally investigates the release of calcium (Ca) and silicon (Si) from the formulation using inductively coupled plasma (ICP) analysis, along with monitoring changes in pH. Furthermore, the impact of this treatment on nail thickness has been assessed in a clinical study involving nail thickness and strength after usage for 28 days.
2. Materials and Methods
2.1. Nail Serum Formulation
A standard oil base was prepared as carrier of the calcium silicate material; see Table 1. One weight percent (1 wt%) of calcium silicate, average particle size 7.0–10.0 µm (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) was added to the base oil. The base nail oil formulation was designed to optimise both functional performance and sensorial properties. Each component was selected based on its contribution to nail and cuticle care, product stability, and user experience.

Table 1.
Composition of the base nail oil, detailing the various ingredients in the formulation.
Caprylic/Capric Triglyceride, a lightweight emollient derived from coconut and glycerin. Chosen for its rapid absorption, excellent skin compatibility, and ability to evenly disperse other components without leaving a greasy residue. Coco-Caprylate/Caprate—A natural-based ester with a dry, silky afterfeel, improving spreadability and enhancing the product’s sensory profile. Dicaprylyl Ether was included as a fast-spreading emollient and solvent that facilitates even distribution of conditioning agents and contributes to smooth application.
To ensure nourishment and barrier support, Canola Oil was incorporated for its high content of omega-3 and omega-6 fatty acids, while Lipex shealight was added to provide long-lasting moisturization and protection to the nail area, all while maintaining a light, non-greasy feel. Tocopherol (Vitamin E) acts as an antioxidant, protecting both the nail and the formulation from oxidative damage. In addition, parfum adds a pleasant fragrance, enhancing the overall user experience and sensory appeal. Finally, Silica was included to control viscosity and maintain particle suspension, while providing a stable, uniform texture.
The combination of lightweight, fast-spreading emollients with nourishing natural oils ensures deep hydration and conditioning of nails and cuticles, while maintaining a pleasant, non-greasy feel.
2.2. Ion Release from the Calcium Silicate Serum
To evaluate the ion concentration of calcium and silicon from the nail serum upon exposure to water, 1 mL of the nail serum was mixed with 9 mL of deionized water in a 10-mL test tube. The test tubes were then incubated at 37 °C for 24 h and tested in triplicate. The test tubes were removed from the oven, and using a pipette, the oil layer was carefully extracted from the water. The remaining water in each test tube was then filtered, and the filtrate was analysed using Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES); see Table 2. The pH of the samples was also determined using a pH meter.

Table 2.
ICP-OES settings.
2.3. Clinical Study Design
A 28-day, open-label, single-arm clinical study was conducted under Good Clinical Practice guidelines at Zurko Research S.L., Madrid. A total of 35 individuals were screened, and 22 completed the study. Participants applied BAM Nail serum twice daily to fingernails and cuticles using a supplied brush. The overall structure of the clinical study is summarized in a flowchart; see Figure 1.
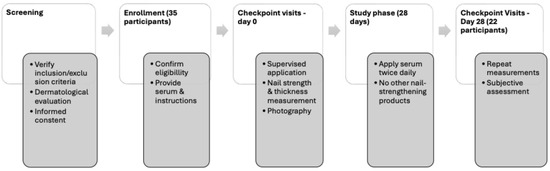
Figure 1.
Flowchart of the study design.
2.3.1. Inclusion Criteria
Participants were eligible for inclusion if they met the following criteria:
- Aged 18 to 70 years.
- Male or female.
- In self-reported good health.
- Availability to complete all study visits and procedures.
- Provided written informed consent.
- Documented weak or thin nails, verified by dermatological evaluation.
- All body skin types and tones (including sensitive skin) were accepted.
- No topical or systemic treatments for nail or skin disorders within 30 days before the study.
- No use of other nail-strengthening products during the study.
2.3.2. Exclusion Criteria
Participants were excluded if they had:
- Active dermatological conditions (e.g., psoriasis, eczema, fungal infection).
- Immunodeficiencies or systemic illnesses (e.g., endocrine, cardiovascular, psychiatric).
- Pregnancy or lactation.
- Allergy or hypersensitivity to cosmetic products or study ingredients.
- Recent vaccinations or planned vaccinations during the study period.
- Use of corticosteroids, antibiotics, retinoids, or similar agents within the previous month.
- Planned intense UV exposure or sunbathing.
- Participation in another clinical study.
2.3.3. Checkpoint Visits
- D0 (Baseline): Product application under supervision; photographed, nail strength and thickness evaluated.
- D28 (Final): Repeat measurements and subjective assessment.
2.4. Characterization and Evaluation Methods
The characterization and evaluation of the test product, BAM Oil, were conducted through a combination of clinical grading, imaging techniques, and subjective assessment instruments. All evaluations were carried out under standardized environmental and procedural conditions to ensure consistency and reproducibility.
2.4.1. Dermatological Assessment of Nail Thickness and Strength
Clinical evaluation of nail thickness and strength was performed at two time points: baseline (Day 0) and post-treatment (Day 28). A board-certified dermatologist, blinded to time points, performed all assessments. Nail thickness was graded visually based on a 5-point ordinal scale ranging from 0 (absence of thickening) to 4 (severe thickening). The clinical definition of thickness referred to the relative expansion of the nail plate as assessed by its contour and opacity under standard lighting conditions.
Nail strength was evaluated by manually testing the resistance of the nail plate to gentle bending or breakage using a standardized qualitative scale from 0 (no resistance) to 4 (high resistance). Each score was determined based on both visual cues and tactile feedback during examination, and care was taken to apply consistent manual force across subjects.
2.4.2. Dermatoscopic Imaging for Visual Documentation
To support visual assessment, nail images were captured using the Dermlite HÜD 2 dermatoscope, an advanced device offering both magnification and polarized light illumination. This tool enabled high-resolution visualization of nail surface topography and microstructural changes. Dermatoscopic imaging was conducted at both baseline and study endpoint, with images taken under identical lighting, magnification, and positioning conditions. A computer-guided alignment system was employed to ensure reproducibility by displaying the Day 0 reference image during Day 28 image acquisition.
Photographic documentation was obtained for 10 representative subjects who exhibited typical clinical changes, providing qualitative evidence of treatment effects. These images were later reviewed alongside clinical grading data for validation.
2.4.3. Environmental Standardization
All biometric and dermatological evaluations were carried out in a controlled environment to minimize variability due to external factors. Prior to measurements, subjects were allowed to acclimate for a minimum of 20 min in a room maintained at a temperature of 18–23 °C and relative humidity between 40% and 60%. This preconditioning phase ensured a stable state of nail hydration and minimized temporary fluctuations in nail pliability or appearance.
2.4.4. Subjective Assessment of Efficacy and Acceptability
Participants completed a structured self-assessment questionnaire at Day 28 to evaluate their perception of the product’s efficacy and cosmetic acceptability. The questionnaire employed a 5-point Likert-type scale (1 = very dissatisfied to 5 = very satisfied) across multiple domains: general satisfaction, perceived nail strength and thickness, resistance to breakage, visual improvement (e.g., cracks or irregularities), odor acceptability, and purchase intent. Example questions included: “Do you feel that your nails look healthier thanks to the thickening obtained? And “Do you find that your nails are less prone to breakage after using the product?”.
The subjective data were analyzed to assess congruence with objective findings and to capture user satisfaction—an important component in cosmetic product evaluation.
2.4.5. Statistical Analysis
For all ordinal clinical variables, the Wilcoxon signed-rank test for paired samples was applied to assess the significance of changes between baseline and Day 28. This non-parametric method was selected due to the ordinal nature of the scoring scales and the relatively small sample size. A p-value of less than 0.05 was considered statistically significant. Where appropriate, cumulative link mixed models (CLMM) were explored but not reported due to convergence limitations in this dataset. Descriptive statistics including mean, median, standard deviation, and percentage variation from baseline were computed for each parameter.
3. Results
3.1. Release Studies
In the control formulation, which lacked silica-gel, the concentrations of calcium (Ca) and silicon (Si) were observed to be lower than the calculated theoretical concentrations, measuring 12.1 mg/L for Ca and 7.7 mg/L for Si. However, when 1% silica-gel was added to the serum, a significant increase in both mineral concentrations was noted. The Ca concentration rose to 41.3 mg/L, while Si was around 131.8 mg/L. The pH remained stable at 10.
3.2. Clinical Outcomes
3.2.1. Dermatological Evaluation of Nail Thickness
Following 28 days of twice-daily application of BAM Oil, clinical grading revealed a statistically significant improvement in nail thickness among the participants. At baseline, the mean nail thickness score was 1.50 ± 0.51, with 50% of subjects graded at level 1 and the remaining 50% at level 2 on a 5-point scale. By Day 28, the average score had increased to 2.09 ± 0.53, indicating an approximate 39% mean increase in nail thickness relative to baseline; see Table 3 and Table 4. The median score also rose from 1.50 to 2.00, corresponding to a 33% median improvement.

Table 3.
Scoring system for the self-assessment questionnaire completed by participants at Day 28.

Table 4.
Descriptive analysis of nail thickness evaluation (n = 22).
Analysis using the Wilcoxon signed-rank test confirmed the statistical significance of these changes (V = 91.00; p = 3.63 × 10−4), affirming that the observed increases were unlikely due to random variation. Furthermore, 59% of participants demonstrated individual improvement in thickness grading, supporting the overall consistency of the treatment effect; see Table 5. These findings are consistent with earlier reports that silicon supplementation can enhance nail thickness and hardness, highlighting its role in supporting nail structure [2,5,6].

Table 5.
Frequencies for the evaluation of nail thickness (n = 22).
3.2.2. Dermatological Evaluation of Nail Strength
Clinical grading of nail strength also showed marked enhancement after the treatment period. The baseline mean strength score was 1.50 ± 0.51. By Day 28, this value had risen to 2.45 ± 0.67, corresponding to a 64% increase in average strength scores. The distribution of grading shifted markedly: whereas 50% of subjects were initially rated at grade 1, none remained at this level by Day 28. Instead, 64% reached grade 2, 27% reached grade 3, and 9% achieved the maximum score of 4; see Table 6 and Table 7.

Table 6.
Descriptive analysis of nail strength evaluation (n = 22).

Table 7.
Frequencies for the evaluation of nail strength (n = 22).
The Wilcoxon signed-rank test yielded a test statistic of V = 190.00 with a p-value of 3.15 × 10−5, indicating a highly significant increase in nail strength. Notably, 86% of participants exhibited measurable improvement in their strength scores, reflecting the robustness of the observed response to the product. These results align with earlier findings that silicon contributes to keratin stability and reduces nail brittleness, thereby supporting stronger and healthier nails [2,5].
3.2.3. Imaging of Nails at Baseline and D28
Representative images from showed a marked improvement in nail health after the 28 days period; see Figure 2. At baseline, several nails displayed visible signs of fragility, including surface roughness, peeling layers, longitudinal ridges, and irregular texture. After 28 days of treatment, the nail surface appeared smoother and more uniform, with reduced flaking and fewer ridges. In addition, the nail plate showed improved translucency and compactness, and the cuticle region appeared healthier; see Figure 3. These visual observations align with the measured improvements in nail thickness and strength [2,5].

Figure 2.
Proposed mechanism of action for calcium silicate in nail care. Upon topical application, calcium silicate releases soluble silicon ions that penetrate the nail plate, reach the underlying nail bed, and stimulate fibroblasts. Dashed arrows indicate the movement of silicon ions.
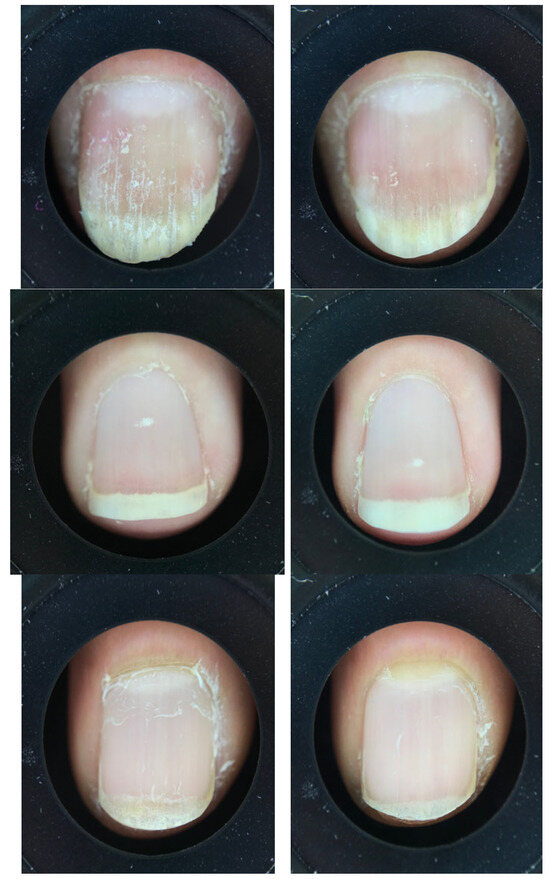
Figure 3.
Representative images of nails at baseline (D0) on the left and after 28 days (D28) on the right.
3.2.4. Subjective Assessment of Efficacy and Sensory Acceptance
Subjective evaluations, gathered through structured questionnaires completed at the end of the 28-day treatment period, corroborated the objective clinical findings. A majority of participants (90.91%) expressed overall satisfaction with the product (defined as selecting “very satisfied” or “somewhat satisfied”), and 86.36% indicated they would choose BAM Oil over their usual product if the price were appropriate.
The product’s sensory characteristics were also favorably rated, with 100% of participants expressing satisfaction with its odor. In terms of specific efficacy parameters, 63.64% reported that their nails appeared healthier due to increased thickness, while 59.09% noticed reduced nail breakage. Furthermore, 68.18% of the panelists agreed that the product was effective in addressing weak or thin nails.
When asked about the perceived price category, 36.36% considered the product “affordable,” 27.27% “mid-range,” 18.18% “premium,” and 13.64% “budget-friendly.” Only 4.55% identified the product as belonging to a luxury price category.
No adverse effects or safety concerns were reported throughout the study duration, and no dropouts occurred due to tolerability issues. The treatment was well accepted, and compliance appeared high across the panel.
4. Discussion
The experimental results show several important trends that align with theoretical expectations and provide new insights into the effectiveness of the serum. By delivering calcium and silicon ions in a controlled manner, the nail serum can enhance various physiological processes in the nail bed. For instance, calcium ions are known to play a key role in the synthesis of collagen, a protein that provides structural integrity and strength to nails. Meanwhile, silicon contributes to the formation of collagen and keratin, another fundamental protein that helps build strong and resilient nails [2,5].
The results of this study support the hypothesis that topical administration of silicon in a calcium silicate-based formulation has a beneficial effect on nail thickness and strength. The combined use of in vitro ion release testing and a clinical study conducted under Good Clinical Practice (GCP) standards strengthens the validity of the findings and provides initial evidence that topical silicon delivery may represent a viable alternative to traditional oral supplementation, particularly for individuals with weak or brittle nails.
A major strength of this investigation lies in its multi-level approach. It combines elemental release analysis through ICP-OES with clinical and dermatological evaluations, as well as subjective assessments of efficacy and user satisfaction. After 28 days of twice-daily application, nail thickness increased by approximately 39% and nail strength by about 64%. These statistically significant improvements suggest that the formulation elicits not only cosmetic benefits but also likely physiological effects on nail tissue. Nevertheless, several limitations must be acknowledged. The clinical study was based on a relatively small sample size (n = 22), which limits the generalizability of the results. While the improvements were statistically significant, larger randomized controlled trials are required to confirm efficacy and rule out potential placebo effects. The study was single-arm and lacked a placebo or control group, making it difficult to distinguish between treatment effects and natural variations in nail growth or seasonal influences.
The 28-day intervention period may also be considered a limitation, given the slow growth rate of nails. Longer-term studies are warranted to assess the durability of treatment benefits and to observe whether effects persist or improve with extended application. Furthermore, potential confounding variables—such as participants’ nutritional status, hormonal balance, and environmental exposures—were not controlled for and could have influenced nail health independently of the intervention.
An unexpected yet noteworthy observation was the presence of calcium in the nail samples post-treatment. While silicon is known to accumulate in keratinized tissues, calcium is not typically emphasized in nail composition studies. Its detection here suggests that the formulation may enhance calcium incorporation or retention, possibly through synergistic interactions with silicon or serum constituents [4,7]. This finding opens the door to further investigations into calcium’s potential role in nail mineralization or keratin structure stabilization.
Future studies should also investigate the diffusion kinetics of silicon and calcium through the nail plate, possibly through confocal microscopy or tracer studies. Elucidating the mechanism of ion transport and uptake could optimize formulation design and enhance bioavailability at the target site.
5. Conclusions
This study provides compelling preliminary evidence that a calcium silicate-based topical serum can enhance nail health by significantly increasing nail thickness and strength. The integration of elemental ion release studies with a short-term clinical evaluation underlines the feasibility of transungual silicon delivery as an alternative to oral supplementation. Despite the study’s limitations—including small sample size, short duration, and lack of a control group—the results are promising and warrant further research. Future studies should aim to validate these findings in larger, placebo-controlled trials, explore the mechanistic roles of both silicon and calcium in nail physiology, and evaluate long-term outcomes. Overall, this research highlights the potential of bioactive mineral-based cosmetic formulations to provide targeted, non-invasive support for improving nail integrity and resilience.
This calcium silicate-based serum may be incorporated into routine nail care for individuals with weak, thin, or brittle nails, applied twice daily to nails and cuticles for optimal results. It can also be used as part of professional treatments in hand and foot care clinics to support nail strength and overall appearance.
Author Contributions
Conceptualization, V.E. and H.E.; methodology, V.E.; formal analysis, V.E. and H.E.; writing—original draft preparation, V.E.; writing—review and editing, H.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding and was fully funded by Lea Cares AB.
Institutional Review Board Statement
The study was conducted in compliance with the ethical principles outlined in the latest version of the Declaration of Helsinki and the principles of Good Clinical Practice (GCP). According to European legislation on cosmetic products (Regulation (EC) No 1223/2009 of the European Parliament and of the Council), ethics committee approval is not required for this type of cosmetic product trial [19].
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The research data is available on request from the authors.
Acknowledgments
Zurko Research S.I. is gratefully acknowledged for taking part in the evaluation of the subjects.
Conflicts of Interest
Both Viktoria Engqvist and Håkan Engqvist are inventors of the studied technology and have shares in Lea Cares AB, and Viktoria Engqvist is employed by Lea Cares AB. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Michalak, M.; Pierzak, M.; Kręcisz, B.; Kręcisz, B.; Suliga, M. Bioactive Compounds for Skin Health: A Review. Nutrients 2021, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, L.A.; Addor, F.; Campos, P.M.B.G.M. Use of silicon for skin and hair care: An approach of chemical forms available and efficacy. An. Bras. Dermatol. 2016, 91, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.O.; Freire, É.S.; Polonini, H.C.; Da Silva, P.J.L.C.; Brandão, M.A.F.; Raposo, N.R.B. Anti-Aging Effects of Monomethylsilanetriol and Maltodextrin-Stabilized Orthosilicic Acid on Nails, Skin and Hair. Cosmetics 2018, 5, 41. [Google Scholar] [CrossRef]
- Jugdaohsingh, R. Silicon and Bone Health. J. Nutr. Health Aging 2007, 11, 99–110. [Google Scholar] [PubMed]
- Barel, A.O.; Calomme, M.; Timchenko, A.; De Paepe, K.; Demeester, N.; Rogiers, V.; Clarys, P.; Vanden Berghe, D. Effect of Oral Intake of Silicon on Skin, Hair, and Nails in Women with Photodamaged Skin: A Randomized, Placebo-Controlled Trial. Arch. Dermatol. Res. 2015, 307, 431–438. [Google Scholar]
- Reffitt, D.M.; Ogston, N.; Jugdaohsingh, R.; Cheung, H.F.J.; Evans, B.A.J.; Thompson, R.P.H.; Powell, J.J.; Hampson, G.N. Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone 2003, 32, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.H. Update on the Possible Nutritional Importance of Silicon. J. Trace Elem. Med. Biol. 2014, 28, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Kalil, C.L.P.V.; Campos, V.; Cignachi, S.; Izidoro, J.F.; Reinehr, C.P.H.; Chaves, C. Evaluation of cutaneous rejuvenation associated with the use of ortho-silicic acid stabilized by hydrolyzed marine collagen. J. Cosmet. Dermatol. 2018, 17, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Spector, T.D.; Calomme, M.R.; Anderson, S.H.; Clement, G.; Bevan, L.; Demeester, N.; Swaminathan, R.; Jugdaohsing, R.; Berghe, D.A.; Powell, J.J. Choline-stabilized orthosilicic acid supplementation as an adjunct to calcium/vitamin D3 stimulates markers of bone formation in osteopenic females: A randomized, placebo-controlled trial. BMC Musculoskelet. Disord. 2008, 9, 85. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schwarz, K. A bound form of silicon in glycosaminoglycans and polyuronides. Proc. Natl. Acad. Sci. USA 1973, 70, 1608–1612. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dudek, Ł.; Kochman, W.; Dziedzic, E. Silicon in prevention of atherosclerosis and other age-related diseases. Front. Cardiovasc. Med. 2024, 11, 1370536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Minisha, S.; Gopinath, A.; Mukherjee, S.; Srinivasan, P.; Madhan, B.; Shanmugam, G. Impact of SiO2 nanoparticles on the structure and property of type I collagen in three different forms. J. Mech. Behav. Biomed. Mater. 2020, 109, 103806. [Google Scholar] [CrossRef]
- Lai-Cheong, J.E.; McGrath, J.A. Structure and function of skin, hair and nails. Medicine 2009, 37, 223–226. [Google Scholar] [CrossRef]
- Reilly, D.M.; Lozano, J. Skin collagen through the life stages: Importance for skin health and beauty. J. Cosmet. Dermatol. 2020, 19, 737–746. [Google Scholar] [CrossRef]
- Riccardi, B.; Resta, S.; Storelli, R. Preliminary Study on the Homeostatic Role of Organic Silicon and Trace Elements for Osteoarticular Well-Being. J. Biotechnol. Bioinform. Res. 2021, 132, 3. [Google Scholar] [CrossRef]
- Austin, J.H. Silicon Levels in Human Tissues. In Biochemistry of Silicon and Related Problems; Bendz, G., Lindqvist, I., Runnström-Reio, V., Eds.; Nobel Foundation Symposia; Springer: Boston, MA, USA, 1978; Volume 40. [Google Scholar] [CrossRef]
- Jurkić, L.M.; Cepanec, I.; Pavelić, S.K.; Pavelić, K. Biological and therapeutic effects of ortho-silicic acid and some ortho-silicic acid-releasing compounds: New perspectives for therapy. Nutr. Metab. 2013, 10, 2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Department of Health and Human Services. NIOSH Pocket Guide to Chemical Hazards U.S. Available online: https://www.cdc.gov/niosh/npg/npgd0094.html (accessed on 25 June 2025).
- European Parliament and Council. Regulation (EC) No 1223/2009 of 30 November 2009 on cosmetic products. Off. J. Eur. Union 2009, L342, 59–209. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).