Enzymes DNA Repair in Skin Photoprotection: Strategies Counteracting Skin Cancer Development and Photoaging Strategies
Abstract
1. Introduction to DNA in the Skin
2. Types of DNA Damage in the Skin
3. Active Photoprotection with DNA Repair Enzymes
3.1. Photolyases
3.2. T4-bacteriophage Endonuclease V (T4 Endonuclease V)
3.3. 8-Oxoguanine Glycosylase
3.4. Base and Nucleotide Excision Repair Mechanisms in UV-Induced Damage
3.5. Synergistic Use of DNA Repair Enzymes and Antioxidants
4. Clinical and Cosmetic Relevance
5. Ethical and Regulatory Barriers in the Use of DNA Repair Enzymes in Cosmetics
6. Barriers to the Use of DNA Repair Enzymes in Sunscreens
7. Future Directions and Emerging Technologies
8. Perspectives of Personalization
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nascimento, N.S.; Torres-Obreque, K.M.; Oliveira, C.A.; Rabelo, J.; Baby, A.R.; Long, P.F.; Young, A.R.; Rangel-Yagui, C.d.O. Enzymes for dermatological use. Exp. Dermatol. 2024, 33, 15008. [Google Scholar] [CrossRef]
- Passeron, T.; Krutmann, J.; Andersen, M.; Katta, R.; Zouboulis, C. Clinical and biological impact of the exposome on the skin. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 4–25. [Google Scholar] [CrossRef]
- Garcia-Mouronte, E.; Pérez-González, L.A.; Naharro-Rodriguez, J.; Fernández Guarino, M. Understanding Active Photoprotection: DNA-Repair Enzymes and Antioxidants. Life 2024, 14, 822. [Google Scholar] [CrossRef]
- Luze, H.; Nischwitz, S.P.; Zalaudek, I.; Müllegger, R.; Kamolz, L.P. DNA repair enzymes in sunscreens and their impact onphotoageing-A systematic review. Photodermatol. Photoimmunol. Photomed. 2020, 36, 424–432. [Google Scholar] [CrossRef]
- Qian, M.; Kalbina, I.; Rosenqvist, E.; Jansen, M.A.K.; Strid, A. Supplementary UV-A and UV-B radiation differentially regulate morphology in Ocimum basilicum. Photochem. Photobiol. Sci. 2023, 22, 2219–2230. [Google Scholar] [CrossRef]
- Pfeifer, G.P. Mechanisms of UV-induced mutations and skin cancer. Genome Instab. Dis. 2020, 1, 99–113. [Google Scholar] [CrossRef]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive Oxygen Species Signaling and Oxidative Stress: Transcriptional Regulation and Evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Yang, T.; Yu, D.; Xiong, H.; Zhang, S. Current insights and future perspectives of ultraviolet radiation (UV) exposure: Friends and foes to the skin and beyond the skin. Environ. Int. 2024, 185, 160–4120. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Ratnakumar, K.; Hung, K.F.; Rokunohe, D.; Kawasumi, M. Deciphering UV-induced DNA Damage Responses to Prevent and Treat Skin Cancer. Photochem. Photobiol. 2020, 96, 478–499. [Google Scholar] [CrossRef]
- Lv, X.; Han, Z.; Huo, H.; Liu, X.; Zhang, J.; Chi, J.; Han, B.; Jiang, Z. Anti-inflammatory and antioxidant succinyl-chitosan oligosaccharide protects human epidermal cell and mouse skin against ultraviolet B-induced photodamage. Carbohydr. Polym. 2025, 351, 123102. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.G.; Padron, F.; Gerd, P.; Pfeifer, G.P. UVA Radiation, DNA Damage, and Melanoma. ACS Omega 2022, 7, 32936–32948. [Google Scholar] [CrossRef] [PubMed]
- Kciuk, M.; Marciniak, B.; Mojzych, M.; Kontek, R. Focus on UV-Induced DNA Damage and Repair—Disease Relevance and Protective Strategies. Int. J. Mol. Sci. 2020, 21, 7264. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.F.; Sidorova, J.M.; Nghiem, P.; Kawasumi, M. The 6-4 photoproduct is the trigger of UV-induced replication blockage and ATR activation. Proc. Natl. Acad. Sci. USA 2020, 117, 12806–12816. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Tsuruta, D. What Are Reactive Oxygen Species, Free Radicals, and Oxidative Stress in Skin Diseases? Int. J. Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Herr, L.M.; Schaffer, E.D.; Fuchs, K.F.; Datta, A.; Brosh, R.M. Replication stress as a driver of cellular senescence and aging. Commun. Biol. 2024, 7, 616. [Google Scholar] [CrossRef]
- Provasek, V.E.; Mitra, J.; Malojirao, V.H.; Hegde, M.L. DNA Double-Strand Breaks as Pathogenic Lesions in Neurological Disorders. Int. J. Mol. Sci. 2022, 23, 4653. [Google Scholar] [CrossRef]
- Rai, S.; Rai, G.; Kumar, A. Eco-evolutionary impact of ultraviolet radiation (UVR) exposure on microorganisms, with a specialfocus on our skin microbiome. Microbiol. Res. 2022, 260, 127044. [Google Scholar] [CrossRef]
- Milićević, S.M.; Mijatović, Z.; Stanojević, G.; Radovanović, M.M.; Popović, V. Health risks of extended exposure to low-level UV radiation—An analysis of ground-based and satellite-derived data. Sci. Total Environ. 2022, 831, 154899. [Google Scholar] [CrossRef]
- Available online: https://www.cdc.gov/radiation-health/features/uv-radiation.html (accessed on 18 May 2025).
- Thawabteh, A.M.; Jibreen, A.; Karaman, D.; Thawabteh, A.; Karaman, R. Skin Pigmentation Types, Causes and Treatment-A Review. Molecules 2023, 28, 4839. [Google Scholar] [CrossRef]
- Shin, S.H.; Lee, Y.H.; Rho, N.K.; Park, K.Y. Skin aging from mechanisms to interventions: Focusing on dermal aging. Front. Physiol. 2023, 14, 1195272. [Google Scholar] [CrossRef]
- He, X.; Wan, F.; Su, W.; Xie, W. Research Progress on Skin Aging and Active Ingredients. Molecules 2023, 28, 5556. [Google Scholar] [CrossRef]
- Milanowska, M.; Grudzińska, A.; Jarosz, D.; Tsitko, H.; Dudzińska, P. Skin cancer’s prevention in the light of current medical knowledge. J. Educ. Health Sport 2023, 23, 35–39. [Google Scholar] [CrossRef]
- Subhadarshani, S.; Athar, M.; Elmets, C.A. Photocarcinogenesis. Curr. Derm. Rep. 2020, 9, 189–199. [Google Scholar] [CrossRef]
- Kaltchenko, M.V.; Chien, A.L. Photoaging: Current Concepts on Molecular Mechanisms, Prevention, and Treatment. Am. J. Clin. Dermatol. 2025, 26, 321–344. [Google Scholar] [CrossRef]
- Douki, T.; Caillat, S.; Bacqueville, D.; Géniès, C.; Huyghe, C.; Duplan, H.; Le Digabel, J.; Lauze, C.; Filiol, J.; Marinescu, R.; et al. Nuclear and Urinary Measurements Show the Efficacy of Sun-Protection Factor 50+ Sunscreen against DNA Photoproducts upon Real-Life Recreational Exposure. JID Innov. 2023, 3, 100227. [Google Scholar] [CrossRef]
- Grand View Research. Enzymes Market Size, Share & Trends Analysis Report, 2030. 2020. Available online: https://www.grandviewresearch.com/industry-analysis/specialty-enzymes-market (accessed on 9 May 2025).
- D’Augustin, O.; Huet, S.; Campalans, A.; Radicella, J.P. Lost in the Crowd: How Does Human 8-Oxoguanine DNA Glycosylase 1 (OGG1) Find 8-Oxoguanine in the Genome? Int. J. Mol. Sci. 2020, 21, 8360. [Google Scholar] [CrossRef]
- Nitulescu, G.; Lupuliasa, D.; Adam-Dima, I.; Nitulescu, G.M. Ultraviolet Filters for Cosmetic Applications. Cosmetics 2023, 10, 101. [Google Scholar] [CrossRef]
- Hosokawa, Y.; Maestre-Reyna, M. Light-driven DNA repair at atomic and picosecond resolution revealed via time-resolved serial femtosecond crystallography. Clin. Transl. Med. 2024, 14, 1566. [Google Scholar] [CrossRef]
- Ramírez-Gamboa, D.; Díaz-Zamorano, A.L.; Meléndez-Sánchez, E.R.; Reyes-Pardo, H.; Villaseñor-Zepeda, K.R.; López-Arellanes, M.E.; Sosa-Hernández, J.E.; Coronado-Apodaca, K.G.; Gámez-Méndez, A.; Afewerki, S.; et al. Photolyase Production and Current Applications: A Review. Molecules 2022, 27, 5998. [Google Scholar] [CrossRef]
- Haney, A.M.; Sanfilippo, J.E.; Garczarek, L.; Partensky, F.; Kehoe, D.M. Multiple photolyases protect the marine cyanobacterium Synechococcus from ultraviolet radiation. Mbio 2022, 13, 1511–1522. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Lei, Y.; Liu, Y.; Feng, Y.; Chen, D.; Ma, S.; Xiao, Z.; Hu, M.; Deng, J.; et al. Recombinant Photolyase-Thymine Alleviated UVB-Induced Photodamage in Mice by Repairing CPD Photoproducts and Ameliorating Oxidative Stress. Antioxidants 2022, 11, 2312. [Google Scholar] [CrossRef]
- Wang, P.-H.; Hosokawa, Y.; Soares, J.C.; Emmerich, H.-J.; Fuchs, V.; Caramello, N.; Engilberge, S.; Bologna, A.; Rosner, C.J.; Nakamura, M.; et al. Redox-State-Dependent Structural Changes within a Prokaryotic 6–4 Photolyase. J. Am. Chem. Soc. 2025, 147, 16084–16098. [Google Scholar] [CrossRef]
- Ramírez, N.; Serey, M.; Illanes, A.; Piumetti, M.; Ottone, C. Immobilization strategies of photolyases: Challenges and perspectives for DNA repairing application. J. Photochem. Photobiol B Biol. 2021, 215, 112113. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.; Trullàs, C.; Bauza, G.; Garre, A. 17895 Anti-photoaging effect of a novel tinted facial sunscreen with high sun protection, peptide complex, and encapsulated photolyase after 1 month of use. J. Am. Acad. Dermatol. 2020, 83, AB202. [Google Scholar] [CrossRef]
- Patel, V.A.; Arron, S.T.; Berman, B.; Chapman, M.S.; Jambusaria-Pahlajani, A.; Martin, G.; Rossi, A.M.; Schlesinger, T.; Zeitouni, N.C.; Bhatia, N. Expert consensus-based recommendations on the use of photodynamic therapy in actinic keratosis patients. JAAD Int. 2025, 20, 62–73. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, A.P.; Singh, V.K.; Singh, P.R.; Jaiswal, J.; Kumari, N.; Upadhye, V.; Singh, S.C.; Sinha, R.P. Natural Sun-Screening Compounds and DNA-Repair Enzymes: Photoprotection and Photoaging. Catalysts 2023, 13, 745. [Google Scholar] [CrossRef]
- Ivanova, I.; Svilenska, T.; Maisch, T.; Karrer, S.; Niebel, D.; Berneburg, M.; Kurz, B. The role of UV-induced cutaneous matrix metalloproteinases and mi-RNAs in the pathogenesis of lupus erythematosus. J. Trans. Autoim. 2025, 10, 100265. [Google Scholar] [CrossRef]
- De Rosa, M.; Johnson, S.A.; Opresko, P.L. Roles for the 8-Oxoguanine DNA Repair System in Protecting Telomeres From Oxidative Stress. Front. Cell Dev. Biol. 2021, 9, 758402. [Google Scholar] [CrossRef]
- De Rosa, M.; Barnes, R.P.; Detwiler, A.C.; Nyalapatla, P.R.; Wipf, P.; Opresko, P.L. OGG1 and MUTYH repair activities promote telomeric 8-oxoguanine induced senescence in human fibroblasts. Nat. Commun. 2025, 16, 893. [Google Scholar] [CrossRef]
- Wang, J.; Li, C.; Han, J.; Xue, Y.; Zheng, X.; Wang, R.; Radak, Z.; Nakabeppu, Y.; Boldogh, I.; Ba, X. Reassessing the roles of oxidative DNA base lesion 8-oxoGua and repair enzyme OGG1 in tumorigenesis. J. Biomed. Sci. 2025, 32, 1. [Google Scholar] [CrossRef] [PubMed]
- Caldecott, K.W. Mammalian DNA Base Excision Repair: Dancing in the Moonlight. DNA Repair 2020, 93, 102921. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, E.; Osuch, B.; Młotkowska, P.; Kowalczyk, P.; Roszkowicz-Ostrowska, K.; Misztal, T. Gene Expression and Activity of Selected Antioxidant and DNA Repair Enzymes in the Prefrontal Cortex of Sheep as Affected by Kynurenic Acid. Int. J. Mol. Sci. 2025, 26, 2381. [Google Scholar] [CrossRef] [PubMed]
- Abril, S.P.; Rincón-Díaz, N.; Puyana, M.; Castellanos, L.; Ramos, F.A. Photoprotective activity from Colombian Caribbean brown algae using HPLC-DAD metabolic profiling by MCR-ALS data analysis. Sci. Rep. 2025, 15, 16204. [Google Scholar] [CrossRef]
- Patwa, H.; Babcock, N.; Kurian, P. Quantum-enhanced photoprotection in neuroprotein architectures emerges from collective light-matter interactions. Front. Phys. 2024, 12, 1387271. [Google Scholar] [CrossRef]
- Erber, L.; Groehler, A.S.; Cyuzuzo, C.I.; Baker-Wainwright, J.; Maskey, R.S.; Li, L.; Machida, Y.J.; Tretyakova, N. SPRTN metalloprotease participates in repair of ROS-mediated DNA-protein crosslinks. Sci. Rep. 2024, 14, 30919. [Google Scholar] [CrossRef]
- Neacșu, S.M.; Mititelu, M.; Ozon, E.A.; Musuc, A.M.; Iuga, I.D.M.; Manolescu, B.N.; Petrescu, S.; Cusu, J.P.; Rusu, A.; Surdu, V.-A.; et al. Comprehensive Analysis of Novel Synergistic Antioxidant Formulations: Insights into Pharmacotechnical, Physical, Chemical, and Antioxidant Properties. Pharmaceuticals 2024, 17, 690. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, X.; Zhou, M. DNA damage mediated by UV radiation and relative repair mechanisms in mammals. Genome Instab. Dis. 2022, 3, 331–337. [Google Scholar] [CrossRef]
- Minoretti, P.; Emanuele, E.; García Martín, Á.; Liaño Riera, M.; Gómez Serrano, M.; Santiago Sáez, A. Exploring the Protective Efficacy of Topical Products for Actinic Keratosis Against Ultraviolet-Induced DNA and Protein Damage: An Experimental, Double-Blind Irradiation Study. Cureus 2023, 15, 44065. [Google Scholar] [CrossRef]
- Cao, X.; Luyao Yan, L.; Yang, C.; Wang, L.; Zhang, M.; Zhong, D. Dynamics of DNA Repair by Class-II Photolyases via a Unified Electron-Transfer Bifurcating Mechanism. J. Am. Chem. Soc. 2025, 147, 11291–11300. [Google Scholar] [CrossRef]
- Kong, X.Y.; Vik, E.S.; Nawaz, M.S.; Berges, N.; Dahl, T.B.; Vågbø, C.; Suganthan, R.; Segers, F.; Holm, S.; Quiles-Jiménez, A.; et al. Deletion of Endonuclease V suppresses chemically induced hepatocellular carcinoma. Nucleic Acids Res. 2020, 48, 4463–4479. [Google Scholar] [CrossRef]
- Pan, L.; Hao, W.; Xue, Y.; Wang, K.; Zheng, X.; Luo, J.; Ba, X.; Xiang, Y.; Qin, X.; Bergwik, J.; et al. 8-Oxoguanine targeted by 8-oxoguanine DNA glycosylase 1 (OGG1) is central to fibrogenic gene activation upon lung injury. Nucleic Acids Res. 2023, 51, 1087–1102. [Google Scholar] [CrossRef]
- Chaudhary, M.; Khan, A.; Gupta, M. Skin Ageing: Pathophysiology and Current Market Treatment Approaches. Curr. Aging Sci. 2020, 13, 22–30. [Google Scholar] [CrossRef]
- Wolf, P.; Müllegger, R.R.; Soyer, H.P.; Hofer, A.; Smolle, J.; Horn, M.; Cerroni, L.; Hofmann-Wellenhof, R.; Kerl, H.; Maier, H.; et al. Topical Treatment with Liposomes Containing T4 Endonuclease V Protects Human Skin In Vivo from Ultraviolet-Induced Upregulation of Interleukin-10 and Tumor Necrosis Factor-α. J. Investig. Dermatol. 2000, 114, 149–156. [Google Scholar] [CrossRef]
- Puviani, M.; Barcella, A.; Milani, M. Efficacy of a photolyase-based device in the treatment of cancerization field in patients with actinic keratosis and non-melanoma skin cancer. G. Ital. Dermatol. Venereol. Organo Uff. Soc. Ital. Dermatol. Sifilogr. 2013, 148, 693–698. [Google Scholar]
- Stege, H.; Roza, L.; Vink, A.A.; Grewe, M.; Ruzicka, T.; Krutmann, J. Enzyme plus light therapy to repair DNA damage in ultraviolet-B-irradiated human skin. Proc. Natl. Acad. Sci. USA 2000, 97, 1790–1795. [Google Scholar] [CrossRef]
- Krutmann, J.; Hansen, P.M. Algenenzym Photolyase verbessert Schutz vor UVB-Schäden. Pharm Ztg. 2004, 149, 50–53. [Google Scholar]
- Wu, R.; Li, W.; Yang, P.; Shen, N.; Yang, A.; Liu, X.; Ju, Y.; Lei, L.; Fang, B. DNA hydrogels and their derivatives in biomedical engineering applications. J. Nanobiotechnol. 2024, 22, 518. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://eur-lex.europa.eu/eli/reg/2009/1223/oj (accessed on 18 May 2025).
- Alnuqaydan, A.M. The dark side of beauty: An in-depth analysis of the health hazards and toxicological impact of synthetic cosmetics and personal care products. Front. Public Health 2024, 12, 1439027. [Google Scholar] [CrossRef]
- Liang, J.; Liu, W.; Zhang, T.; Guo, D.; Gong, J.; Yang, Z. Utilization of natural products in diverse pathogeneses of diseases associated with single or double DNA strand damage repair. Chin. Med. 2025, 20, 46. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Zhou, P.K. DNA damage repair: Historical perspectives, mechanistic pathways and clinical translation for targeted cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 254. [Google Scholar] [CrossRef]
- Chavda, V.P.; Acharya, D.; Hala, V.; Daware, S.; Vora, L.K. Sunscreens: A comprehensive review with the application of nanotechnology. J. Drug Deliv. Sci. Technol. 2023, 86, 104720. [Google Scholar] [CrossRef]
- Stahl-Rommel, S.; Li, D.; Sung, M.; Li, R.; Vijayakumar, A.; Atabay, K.D.; Bushkin, G.G.; Castro, C.L.; Foley, K.D.; Copeland, D.S.; et al. A CRISPR-based assay for the study of eukaryotic DNA repair onboard the International Space Station. PLoS ONE 2021, 16, 0253403. [Google Scholar] [CrossRef]
- Zeballos, C.M.; Gaj, T. Next-Generation CRISPR Technologies and Their Applications in Gene and Cell Therapy. Trends Biotechnol. 2021, 39, 692–705. [Google Scholar] [CrossRef]
- Nakamura, Y.; Mochida, A.; Chono, S. Nanocarrier technologies in dermatology: Recent advances and future perspectives. Adv. Drug Deliv. Rev. 2022, 187, 114383. [Google Scholar]
- Villarreal-Gómez, L.J.; Origel-Lucio, S.; Hernández-Hernández, D.A.; Pérez-González, G.L. Use of Exosomes for Cosmetics Applications. Cosmetics 2025, 12, 9. [Google Scholar] [CrossRef]
- Haque, T.; Crowther, J.M.; Lane, M.E. Polymeric nanoparticles in topical drug delivery: A review of recent advancements. J. Control Release 2021, 336, 171–187. [Google Scholar]
- Tran, T.T.; Wright, D.W.; Hoa, N.T. Stimuli-responsive materials for controlled release of bioactives in skin. J. Mater. Chem. B 2020, 8, 10021–10034. [Google Scholar]
- Kang, H.; Park, H.J.; Kang, J.; Hwang, Y.; Lee, Y.; Park, S.-U.; Kim, J.; Lee, K.H.; Jang, S. Advances in DNA damage detection: Current progress, challenges, and future directions. TrAC Trends Anal. Chem. 2025, 189, 118246. [Google Scholar] [CrossRef]
- Chen, J.; Potlapalli, R.; Quan, H.; Chen, L.; Xie, Y.; Pouriyeh, S.; Sakib, N.; Liu, L.; Xie, Y. Exploring DNA Damage and Repair Mechanisms: A Review with Computational Insights. BioTech 2024, 13, 3. [Google Scholar] [CrossRef]
- Slominski, R.M.; Kim, T.-K.; Janjetovic, Z.; Brożyna, A.A.; Podgorska, E.; Dixon, K.M.; Mason, R.S.; Tuckey, R.C.; Sharma, R.; Crossman, D.K.; et al. Malignant Melanoma: An Overview, New Perspectives, and Vitamin D Signaling. Cancers 2024, 16, 2262. [Google Scholar] [CrossRef]
- Gracia-Cazaña, T.; Aguilera, J.; Navarro-Bielsa, A.; González, S.; Lim, H.W.; Gilaberte, Y. New trends on personalized sunscreens. Photodermatol. Photoimmunol. Photomed. 2024, 40, 12967. [Google Scholar] [CrossRef]
- Sullivan, M.; Gonzalez Obezo, C.; Lipsky, Z.; Panchal, A.; Jensen, J. Frontiers in Topical Photoprotection. Cosmetics 2025, 12, 96. [Google Scholar] [CrossRef]
- Eg, R.; Tønnesen, Ö.D.; Tennfjord, M.K. A scoping review of personalized use experiences on social media: The interplay between algorithms and human factors. Comput. Hum. Behav. Rep. 2023, 9, 100253. [Google Scholar] [CrossRef]
- Peng, S.; Long, M.; Chen, Q.; Yin, Z.; Zeng, C.; Zhang, W.; Wen, Q.; Zhang, X.; Ke, W.; Wu, Y. Perspectives on cancer therapy—Synthetic lethal precision medicine strategies, molecular mechanisms, therapeutic targets and current technical challenges. Cell Death Discov. 2025, 11, 179. [Google Scholar] [CrossRef]



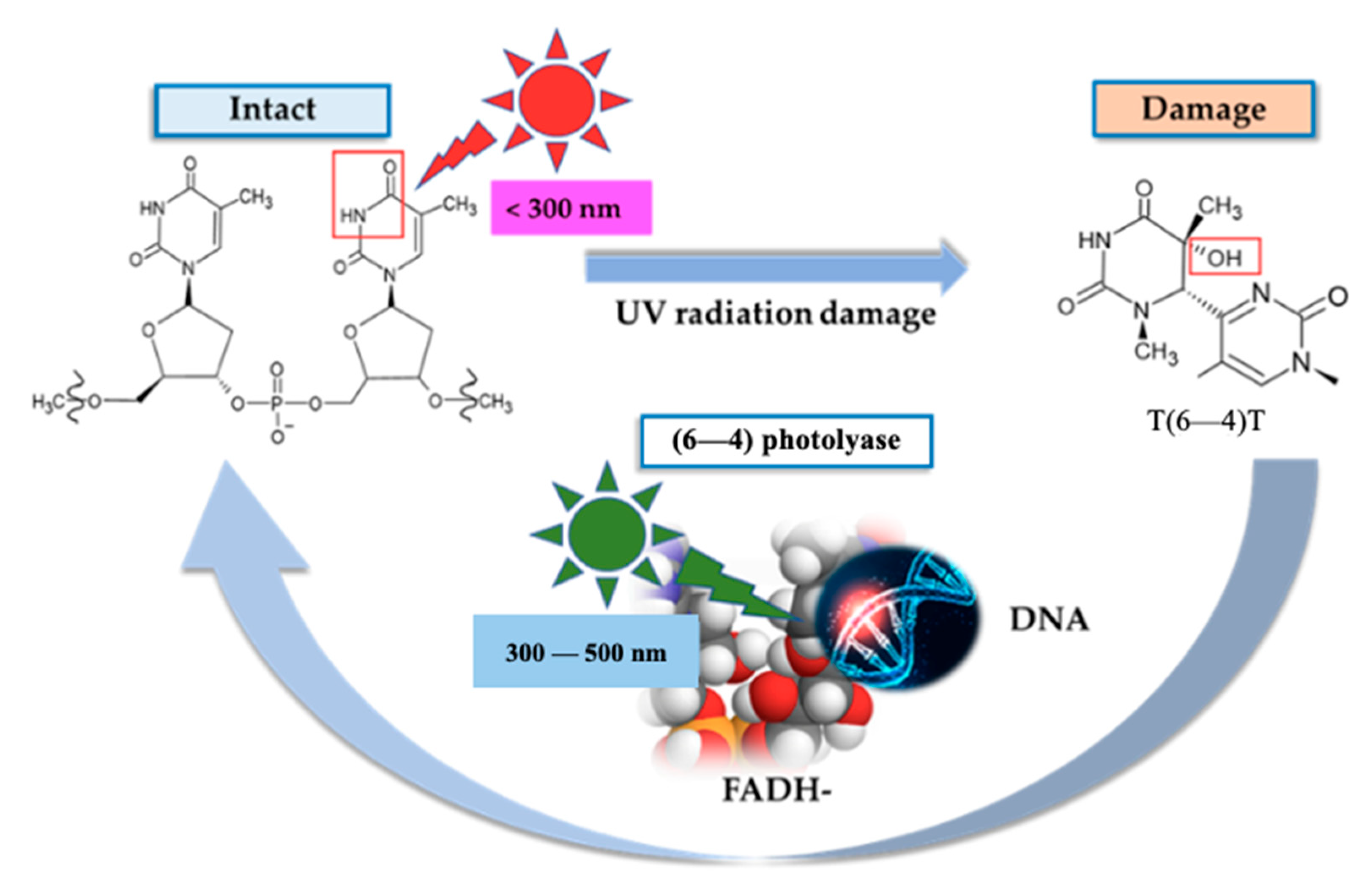
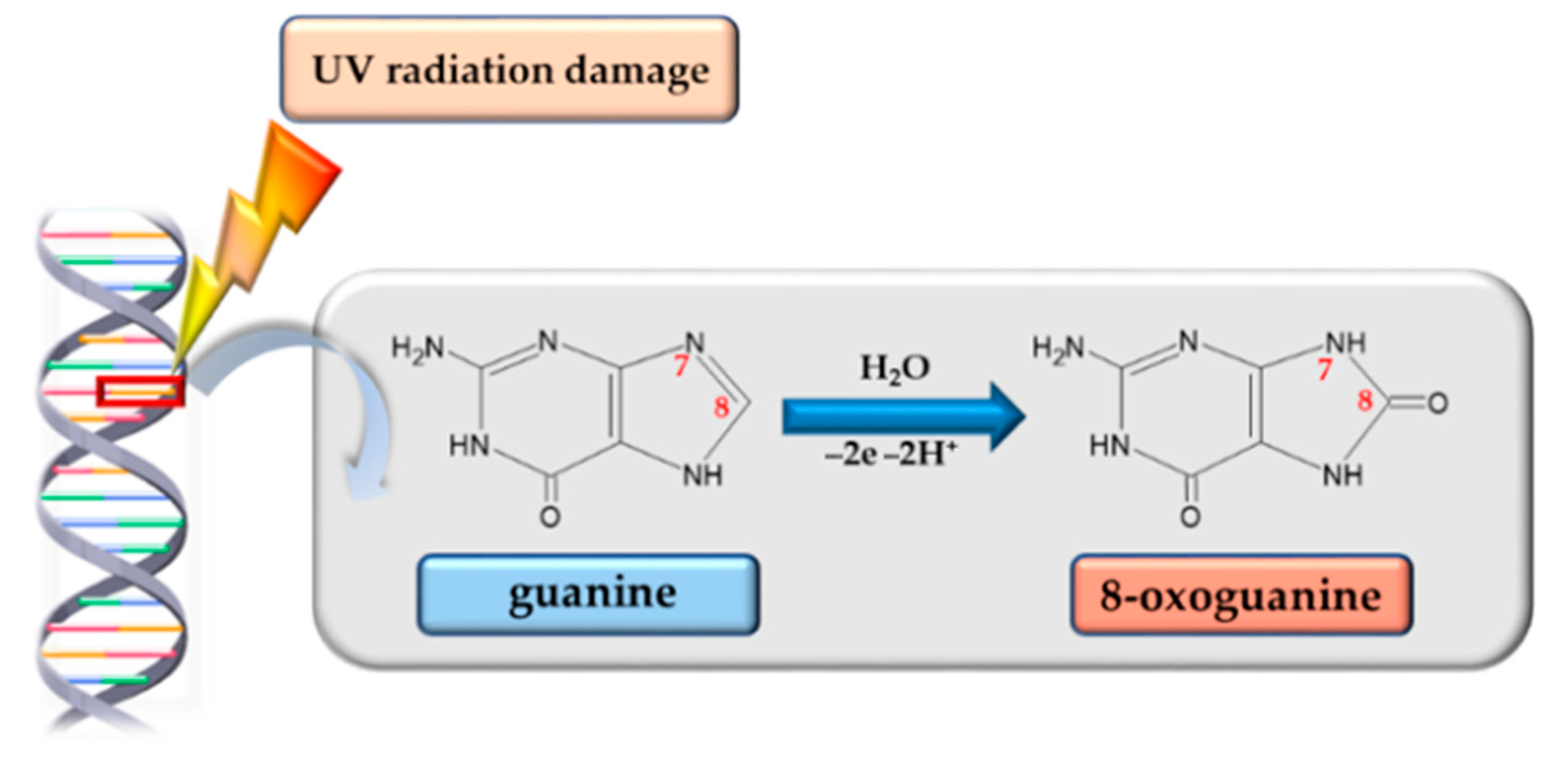

| Skin Damage | Characteristic | Prevention | Reduction of Symptoms | Ref. |
|---|---|---|---|---|
| Hyperpigmentation | Darkening of certain skin areas due to overproduction and accumulation of melanin pigment, resulting in uneven skin tone. |
|
| [22] |
| Skin aging | Premature aging (photoaging), which includes thin, dry, translucent skin, fine lines, loss of subcutaneous fat, reduced sweating, and sunken features. |
|
| [23,24] |
| Photocarcinogenesis | UV-induced processes lead to skin cancer via DNA damage and mutations caused by direct or indirect UV radiation exposure. |
|
| [25,26] |
| Type Enzyme | Mechanism of Action | Clinical Applications | Formulation Barriers | Ref. |
|---|---|---|---|---|
| Photolyase | Repairs CPDs via blue-light photoreactivation | Post-UV skin recovery; prevention of photoaging; adjunct in actinic keratosis management | Limited stability without encapsulation; requires light activation | [52] |
| T4 endonuclease | Initiates NER by recognizing and excising CPDs | Reduces UV-induced lesion counts; supports high-risk patient skin protection | Allergenicity potential; encapsulation needed for delivery | [53] |
| 8-Oxoguanine glycosylase | Removes oxidative lesions (8-oxoG) via BER | Removes oxidative DNA mutations; supports anti-photaging interventions | Large molecular size limits penetration; stability challenges | [54] |
| Product Name | Company | SPF | Key Bioactive Components | Type of DNA Repair Enzyme(s) | Mechanism/Claimed Benefit | Clinical Study Type | Outcome Assessed | Ref. |
|---|---|---|---|---|---|---|---|---|
| Eryfotona® AK-NMSC | ISDIN, Barcelona, Spain | 100+ | DNA Repairsomes®, vitamin E, panthenol | Phytolyase | Enhances CPD repair; supports actinic keratosis regression | Randomized clinical trial (RCT) | AK lesion reduction | [56] |
| Heliocare 360◦AK Fluid | Cantabria Labs, Madrid, Spain | 100+ | Fernblock®+, Genorepair® Complex, sulforaphane | Phytolyase, T4 Endonuclease V, OGG1 | Reduces UV damage; protects DNA; antioxidant synergy | Not available | AK lesion reduction; protective treatment for AK and NMSC | [57] |
| Ateia® | Kwizda Pharma, Vienna, Austria | 25–50+ | Nopasome®, cactus extract, jojoba oil, vitamin E | Phytolyase, T4 Endonuclease V | Targets CPDs and 6—4PPs; supports extracellular matrix integrity | Randomized clinical trial (RCT) | AK lesion reduction; sun allergy prevention | [58] |
| Ladival® med | TADA, Bad Vilbel, Germany | 15–20 | Vitis vinifera seed extract, titanium dioxide | Phytolyase | Broad-spectrum UV filter with enzyme-assisted DNA repair | Not available | Protection against sunburn and sun-induced skin aging | [59] |
| Neova® DNA Damage Control | Pharma Cosmetics, New York, NY, USA | 40–44 | Photolysome, cooper peptides, antioxidants | Phytolyase, T4 Endonuclease V | Reduces DNA mutation burden; supports collagen preservation | Open-label human study | Reduced CPD levels | [56] |
| Priori Tetra® | PRIORI Skincare, Richmond, VA, USA | 50 | Titanium dioxide, melanin, carnostine, mustard seed extracts | Phytolyase, T4 Endonuclease V, OGG1 | Combines ROS neutralization with DNA repair | Not available | Reduced pigmentation wrinkles | [57] |
| Aspect | Details |
|---|---|
| Strengths | Directs DNA repair; complements UV filters; clinically supported for lesion reduction: potential anti-photoaging benefits. |
| Weaknesses | Limited skin penetration; stability challenges; potential allergenicity; high production costs. |
| Opportunities | Integration into multifunctional cosmeceuticals; combination with antioxidants; use in high-risk and personalized skincare. |
| Threats | Regulatory challenges; consumer cost sensitivity; lack of standardized testing models; market saturation with unproven claims. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musielak, E.; Krajka-Kuźniak, V. Enzymes DNA Repair in Skin Photoprotection: Strategies Counteracting Skin Cancer Development and Photoaging Strategies. Cosmetics 2025, 12, 172. https://doi.org/10.3390/cosmetics12040172
Musielak E, Krajka-Kuźniak V. Enzymes DNA Repair in Skin Photoprotection: Strategies Counteracting Skin Cancer Development and Photoaging Strategies. Cosmetics. 2025; 12(4):172. https://doi.org/10.3390/cosmetics12040172
Chicago/Turabian StyleMusielak, Ewelina, and Violetta Krajka-Kuźniak. 2025. "Enzymes DNA Repair in Skin Photoprotection: Strategies Counteracting Skin Cancer Development and Photoaging Strategies" Cosmetics 12, no. 4: 172. https://doi.org/10.3390/cosmetics12040172
APA StyleMusielak, E., & Krajka-Kuźniak, V. (2025). Enzymes DNA Repair in Skin Photoprotection: Strategies Counteracting Skin Cancer Development and Photoaging Strategies. Cosmetics, 12(4), 172. https://doi.org/10.3390/cosmetics12040172







