Abstract
Skin dullness contributes to a fatigued and aged appearance, often exceeding one’s biological age. It is a common dermatological concern influenced by aging and poor lifestyle habits, regardless of ethnicity or age. This study aimed to examine advanced glycation end products (AGEs) and their receptor (receptor for AGEs [RAGE]) as contributing factors to skin dullness. AGEs themselves have a yellowish hue, contributing to “yellow dullness.” Additionally, AGE–RAGE signaling promotes melanin production in melanocytes and impairs keratinocyte differentiation as a result of inflammation. Therefore, regulating the AGE–RAGE interaction may help reduce skin dullness. Through screening various natural ingredients, we found that peony root extract (PRE) inhibits AGE formation and blocks AGE–RAGE binding. Furthermore, the presence of PRE leads to the suppression of AGE-induced melanin production in melanocytes and the restoration of impaired keratinocyte differentiation in glycated basement membrane components. In a human clinical study, topical application of a 1% PRE-containing lotion for 2 weeks significantly reduced melanin content, with a trend toward decreased AGE accumulation and visible spots on the cheeks. These findings support the potential of PRE as a multifunctional cosmetic ingredient that comprehensively addresses skin dullness by modulating the AGE–RAGE interaction.
1. Introduction
“Dullness” is a common dermatological concern, often characterized by an uneven skin tone, which negatively affects one’s overall appearance [1]. Despite the development of cosmetic ingredients and final products aimed at reducing skin dullness and enhancing brightness, many individuals continue to struggle with dull skin. This persistence may be attributable to the multifactorial nature of skin dullness, thereby not depending on a single underlying mechanism [2]. Moreover, the contributing factors vary in balance among individuals, making it difficult to accurately identify the cause of their dullness and address it appropriately.
In this study, we focused on the multifaceted role of advanced glycation end products (AGEs) in skin dullness. AGEs are a collective term for products formed through nonenzymatic glycation reactions wherein reducing sugars bind to proteins or lipids [3]. Given that glycation is a nonenzymatic process, factors such as heat, excess sugar, and oxidative stress can accelerate its reaction rate. AGEs that accumulate in the body are broadly classified into those derived from external sources (e.g., food) and those produced endogenously. Approximately 10–30% of AGEs ingested through food are absorbed into the body, although a portion is subsequently excreted [4]. Endogenous AGEs are primarily formed in response to blood glucose levels and can accumulate in vascular endothelium, as well as in the eyes, kidneys, and skin [5]. Oxidative processes occur in the later stages of glycation, and age-related declines in antioxidant capacity contribute to AGE accumulation [6]. Glycated proteins undergo structural alterations that can cause function loss and increased resistance to degradation. In the dermis, long-lived proteins such as collagen and elastin are prone to glycation, leading to wrinkle formation and sagging. In the epidermis, AGEs themselves have a yellowish hue, which causes “yellow dullness” in the skin [7].
Recent studies have confirmed that AGEs contribute not only to yellow dullness but also to various other skin issues. AGEs activate their receptor (receptor for AGEs [RAGE]), by binding to it, thereby triggering various signaling pathways [8]. RAGE is a transmembrane protein expressed on the surface of epidermal keratinocytes, epidermal melanocytes, and dermal fibroblasts. RAGE-mediated responses are diverse. For example, it stimulates melanocytes to produce melanin [9]. Additionally, RAGE signaling induces inflammation [10]. Moreover, glycation in the cellular microenvironment suppresses keratinocyte differentiation [11], leading to impaired skin turnover. Given these backgrounds, both AGEs themselves and the RAGE-mediated signaling can be considered major contributing factors to skin dullness development.
We hypothesized that controlling AGE formation and RAGE-mediated signaling can mitigate skin dullness and promote a more radiant complexion. To this end, we screened natural ingredients capable of inhibiting the AGE–RAGE interaction and successfully identified the peony root extract (PRE) derived from the root of Paeonia albiflora. Although peonies are widely appreciated for their ornamental value because of their striking flowers and numerous cultivars, the root has long been used in traditional medicine for its antispasmodic and anti-inflammatory properties [12]. Paeonia albiflora roots are also rich in bioactive phytochemicals such as paeoniflorin and albiflorin, which have been reported to possess anti-inflammatory, antioxidant, and skin-conditioning activities. However, its potential to inhibit AGE formation or affect RAGE signaling has not yet been reported. In this study, we demonstrate the multifaceted antiglycation activity of PRE, its efficacy in reducing skin dullness, and its potential as a high-value cosmetic ingredient.
2. Materials and Methods
2.1. Preparation of the PRE
The roots of Japanese-grown Paeonia albiflora were purchased from Tochimoto Tenkaido Co., Ltd. (Osaka, Japan), and the roots of Chinese-grown Paeonia albiflora were obtained from Koshiro Co., Ltd. (Kyoto, Japan). Dried peony root was immersed in 15 times its weight of 50% 1,3-butylene glycol (BG) and extracted at room temperature for 1 week. The mixture was then filtered and allowed to stand at 30 °C for another 1 week. After a second filtration, the resulting extract was prepared so that the solid concentration was 2% w/w, and this was used as PRE. BG was selected as the extraction solvent due to its high skin compatibility, moisturizing properties, and widespread use in cosmetic formulations. Its ability to effectively extract glycosidic and polar constituents from peony roots made it a suitable choice for both laboratory extraction and subsequent clinical application.
2.2. Evaluation of the Inhibitory Activity of PRE Against the AGE–RAGE Interaction
Recombinant RAGE protein (BT4127; R&D Systems, Minneapolis, MN, USA) was dissolved in Dulbecco’s phosphate-buffered saline (D-PBS), added to 96-well plates (Nunc, 439454, Thermo Fisher Scientific, Waltham, MA, USA) at 100 ng/50 μL/well concentration, and incubated overnight at 4 °C for coating. After blocking the wells with D-PBS containing 0.1% BSA (Jackson ImmunoResearch, West Grove, PA, USA, 001-000-162), we washed them with D-PBS containing 0.1% Tween 20 (washing buffer). PRE or a solvent control was prepared in blocking buffer and added to the wells, followed by incubation at room temperature for 1 h. After washing the wells with the washing buffer, we added biotin-labeled AGEs (BT4127; R&D Systems) prepared in blocking buffer and incubated them at room temperature for 1 h. Next, the wells were washed again, followed by color development using HRP-conjugated streptavidin (ab7403; Abcam, Cambridge, UK) and 1-Step™ Ultra TMB ELISA Substrate Solution (34028; Thermo Fisher Scientific, Waltham, MA, USA). To detect biotin-labeled AGEs bound to RAGE, we measured the absorbance at 450 nm. The AGE–RAGE binding rate was calculated by defining the absorbance of wells treated with solvent with RAGE coating as 100% binding and that of wells treated with solvent alone (without RAGE coating) as 0% binding. This study used paeoniflorin (CAS: 23180-57-6) (164-21731) and albifolorin (CAS: 39011-90-0) (016-22201), which were purchased from FUJIFILM WAKO (Osaka, Japan).
2.3. Evaluation of the Inhibitory Effect of PRE on Melanin Production by AGEs
Normal human epidermal melanocytes (FC-0030, P5; LIFELINE Cell Technology, Frederick, MD, USA) were seeded into 12-well plates and cultured in an MGM™-4 Melanocyte Growth Medium-4 BulletKit™ (MGM) (CC-3249; Lonza, Walkersville, MD, USA) until reaching 70% confluency. After replacing the culture medium with MGM that lacked fetal bovine serum and incubating it overnight, this medium was replaced with fresh medium containing PRE at 0%, 0.2%, or 0.4%. After 1 h of treatment, AGEs (22968; Cayman, Ann Arbor, MI, USA) were added to a final concentration of 400 μg/mL, and the cells were cultured for 6 days. Cell proliferation was assessed using the water-soluble tetrazolium salt 1 method with Cell Counting Kit-8 (DOJINDO, Kumamoto, Japan, 343-07623). Thereafter, the wells were washed with D-PBS, and the cells were detached and collected using trypsin. Ethanol/ether (3:1 mixture) was added to the collected samples, followed by centrifugation at 2000 rpm to obtain melanin pellets. The pellets were then dried overnight, resuspended in 25 μL of 1 N NaOH/10% dimethyl sulfoxide solution, and heated at 95 °C to disperse the melanin. Melanin content was quantified by measuring the absorbance at 420 nm. Based on preliminary cytotoxicity screening, PRE concentrations were limited to 0.4% or lower in cell-based assays to avoid non-specific effects.
2.4. Restorative Effects of Pretreatment on Keratinocyte Differentiation Defects in the Glycosylated Pseudobasal Layer of PRE
Laminin (iMatrix-332) (892031; Nippi, Tokyo, Japan) was dissolved in D-PBS to a final concentration of 4.2 μg/cm2 and added to 12-well plates. The plates were incubated at 37 °C for 1 h to allow coating. After washing the wells with D-PBS, we added either D-PBS alone or D-PBS containing glucose (100 mg/mL) and incubated the plates at 37 °C for 2 weeks to induce laminin glycation. Normal human epidermal keratinocytes (NHEKs) (KK-4009, P3; KURABO, Osaka, Japan) were precultured in Keratinocyte Growth Medium (00195130; Lonza) until reaching 80% confluence. The cells were then detached and seeded onto glycated or nonglycated laminin-coated plates. Once confluence was achieved, the culture medium was replaced with a differentiation medium containing PRE at final concentrations of 0%, 0.2%, or 0.4% and 0.15 mM calcium and lacking transferrin, hydrocortisone, and epinephrine. After culturing at 37 °C for 6 h, we extracted total RNA by using an RNeasy Kit (74104; QIAGEN, Venlo, The Netherlands). According to the measured total RNA concentrations, cDNA was synthesized using RevaTra Ace qPCR RT Master Mix (FSQ201; TOYOBO, Shiga, Japan). Gene expression was then quantitatively analyzed using THUNDERBIRD Next SYBR qPCR Mix (TOYOBO, QPX201). Target genes included the differentiation markers transglutaminase 1 (TGM1) and keratin 10 (K10). We used the housekeeping gene RPS18 as an internal control. Primers for TGM1 and K10 were obtained from Takara Bio (TGM1: HA171645, K10: HA347462). The primers RPS18 Forward (TTTGCGAGTACTCAACACCAACATC) and RPS18 Reverse (GAGCATATCTTCGGCCCACAC) were used. Based on preliminary cytotoxicity screening, PRE concentrations were limited to 0.4% or lower in cell-based assays to avoid non-specific effects.
2.5. Evaluation of the Inhibitory Effect of PRE on AGE Formation
Albumin and glucose were dissolved in 0.4 M phosphate buffer (pH 7.4) at final concentrations of 1 and 10 mg/mL, respectively. As a control, PRE or 50% BG was added to a final concentration of 1%, and the solution was then filter-sterilized. After incubation at 37 °C for 4 weeks, the autofluorescence derived from AGEs formed by the albumin–glucose reaction was measured (Ex: 370 nm, Em: 440 nm).
2.6. Clinical Studies in Humans
A randomized, single-blinded, split-face study was conducted to evaluate the clinical efficacy of PRE. Seventeen healthy male and female participants (9 males and 8 females; age range: 40–62 years; mean age: 50.5 ± 6.8 years) were enrolled after providing written informed consent. Inclusion criteria included individuals with visible facial dullness or pigmentation but otherwise healthy skin. Exclusion criteria included known allergies to cosmetics, ongoing dermatological treatments, or any acute or chronic skin diseases. They applied a lotion containing 1% PRE to one-half of the face and a placebo lotion to the other half, twice daily (morning and evening) for 2 weeks. At baseline and after 2 weeks, skin glycation levels on the cheeks were measured using the AGE Scanner (DiagnOptics Technologies B.V., Groningen, The Netherlands). Next, melanin content and facial spots were assessed using the Mexameter (Integral, Tokyo, Japan) and the VISIA imaging system (Integral), respectively.
The study was performed at Ichimaru Pharcos Co., Ltd. (Motosu, Japan), and ethical approval was granted by the company’s Institutional Review Board (Approval Code: No. H24-001K, Approval Date: 25 October 2024). The study was conducted in accordance with the principles outlined in the Declaration of Helsinki (1975, revised in 2013).
2.7. Statistical Analysis
The melanocyte cell number, melanin content, and differentiation marker expression were statistically analyzed using Dunnett’s test. For the human clinical trial, comparisons between baseline and two weeks posttreatment in each group were analyzed using the Wilcoxon signed-rank test. A p-value below 0.05 was considered statistically significant.
3. Results
3.1. PRE Inhibits the AGE–RAGE Interaction
Naturally derived ingredients capable of inhibiting the binding between AGEs and RAGE were screened using an ELISA-based binding assay. Among the 380 natural ingredients [13,14] evaluated, PRE showed to be a potent inhibitor. Figure 1 shows that PRE inhibits the binding of AGEs in a concentration-dependent manner. At 1% concentration, PRE derived from Japanese-grown Paeonia albiflora reduced AGE–RAGE binding by approximately 90% compared with the untreated group (Figure 1A). Similarly, PRE derived from Chinese-grown Paeonia albiflora exhibited approximately 70% inhibition at the same concentration (Figure 1B). Furthermore, similar inhibitory effects were confirmed using PREs obtained from different production regions within Japan and China, suggesting that the observed activity is reproducible and not limited to a single source. Since the wells were washed after PRE treatment and AGEs were added afterward in the binding assay, PRE acts on RAGE to inhibit its interaction with AGEs. The PRE contains paeoniflorin and albiflorin as constituent compounds [15], both of which are well-known phytochemicals in Paeonia roots and were thus selected as candidate active components for further evaluation. Therefore, the binding between AGEs and RAGE was evaluated in the presence of paeoniflorin (0.26, 1.3, and 6.5 μg/mL) and albiflorin (0.1, 0.5, 2.5 μg/mL). As a result, neither compound exhibited inhibitory activity even at the highest concentration, which was significantly higher than the levels present in PRE [15]. Thus, paeoniflorin and albiflorin are unlikely to be the active components responsible for the effects of PRE observed in this study.
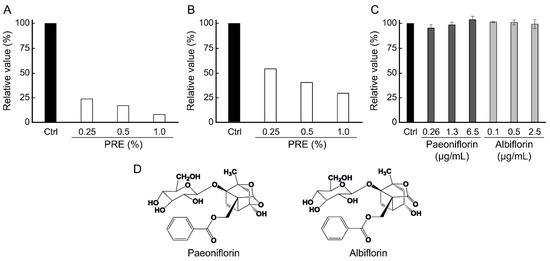
Figure 1.
Inhibitory effect of PRE on advanced glycation end products–Receptor for AGEs interaction. AGE–RAGE interaction rate was evaluated using recombinant protein. The data are presented as percentages (n = 1), with the control (Ctrl) representing 100%. (A) Inhibition effect of PRE, which extracted from Japanese-grown Paeonia albiflora. (B) Inhibition effect of PRE, which extracted from Chinese-grown Paeonia albiflora. (C) Inhibition effect of the compounds (n = 3, mean ± SEM). According to Dunnett’s test, none of the compounds showed statistically significant differences from the control at any tested concentration. (D) Chemical structures of paeoniflorin and albiflorin.
3.2. PRE Suppresses AGE-Induced Melanin Production
The effect of PRE, which inhibits the binding of AGEs to RAGE, on AGE-induced melanin production was evaluated (Figure 2). In normal human epidermal melanocytes (NHEM), the addition of AGEs resulted in a significant 37% increase in melanin production compared with the untreated control group. In the presence of PRE, melanin production of NHEM by AGEs inhibited without cytotoxicity. The effect was concentration-dependent, with 0.4% PRE reducing melanin production by 12%. These results indicate that AGEs promote melanin production in NHEMs, and that the presence of PRE suppresses AGE-induced melanin synthesis.
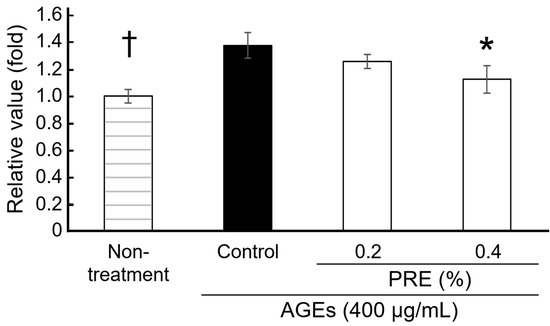
Figure 2.
Effects of PRE on melanin production. Melanin contents of melanocytes were evaluated in the presence or absence of PRE (n = 3, mean ± SEM, * p < 0.05 by Dunnett’s test vs. control, † p < 0.05 by Student t-test vs. control).
3.3. Pretreatment Restores the Differentiation Ability of Keratinocytes on Glycosylated Laminin 332
Laminin 332 is a key component of the epidermal basement membrane that aids in maintaining NHEK homeostasis in the basal layer. When basal layer components become glycated, their protein functions are impaired, negatively affecting keratinocyte differentiation. Suppressed keratinocyte differentiation can lead to abnormal keratinization and uneven skin turnover, ultimately resulting in uneven skin tone. As shown in Figure 3, the effects of glycated laminin 332 and PRE treatment on NHEK differentiation were evaluated using laminin 332–coated wells as a model of basal layer differentiation. In the glycated laminin group, the mRNA expression level of K10, a marker of early to midstage keratinocyte differentiation, was significantly reduced, while that of TGM1, a marker of terminal differentiation, also showed a decreasing trend. Once PRE was added, both the K10 and TGM1 mRNA expression levels showed a dose-dependent recovery, with K10 expression being significantly increased in the 0.4% PRE-treated group compared with that in the glycated group. Therefore, laminin 332 glycation may suppress NHEK differentiation, and PRE treatment may restore it.
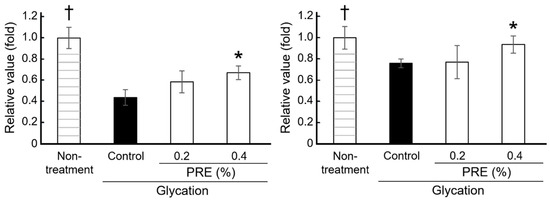
Figure 3.
Effects of PRE on keratinocyte differentiation. The mRNA expression levels of K10 (left) and TGM1 (right) were evaluated in keratinocytes cultured on glycated laminin–coated plates (Ctrl; control, n = 3, mean ± SEM, * p < 0.05 by Dunnett’s test vs. control, † p < 0.05 by Student t-test vs. control).
3.4. PRE Suppresses AGE Formation
The ability of PRE to suppress AGE formation was evaluated. After 4 weeks, the level of AGE-derived autofluorescence in the PRE-treated group was less than half that observed in the 0.5% BG control group (Figure 4). Therefore, PRE may not only suppress melanin production from melanocytes by modulating the AGE–RAGE interaction and restore keratinocyte differentiation (Figure 2 and Figure 3) but also directly inhibit AGE formation.
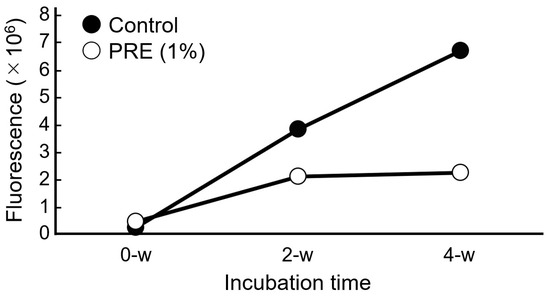
Figure 4.
Anti-glycation effect of PRE in vitro. The AGE production level was measured by the autofluorescence of AGEs (n = 1).
3.5. PRE Exhibits Cosmetic Effects in Human Clinical Trials
In the human clinical trial, facial parameters such as skin glycation levels on the cheeks, melanin content, and skin pigmentation were measured using the AGE Scanner (DiagnOptics Technologies B.V.), Mexameter (Integral), and VISIA (Integral), respectively. The AGE Scanner measures the number of AGEs deposited approximately 1 mm beneath the skin surface by detecting their inherent autofluorescence. The cheek AGE levels in the group treated with the PRE-containing lotion showed a decreasing trend (Figure 5A). In the same group, melanin levels were significantly reduced (Figure 5B). Conversely, these two parameters demonstrated no significant changes in the placebo group. Regarding skin pigmentation, the number of dark spots tended to increase in the placebo group but decrease in the PRE-treated group (Figure 5C). Therefore, PRE may suppress the production of AGEs and melanin in the human skin, thereby reducing pigmentation spots and dullness and subsequently contributing to a more even and radiant complexion. No local irritation or adverse reactions were observed.
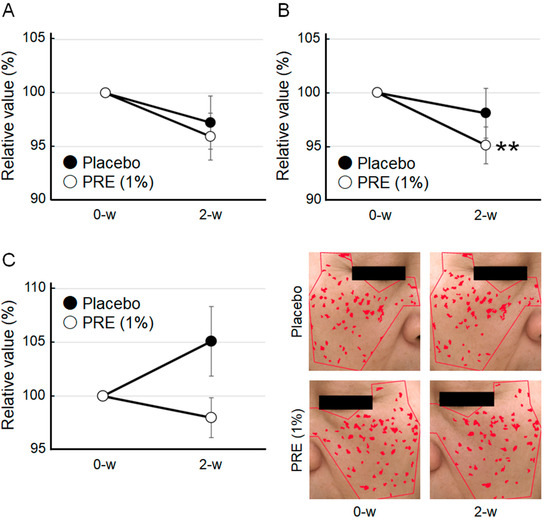
Figure 5.
Cosmetic effects of PRE in vivo. (A) Impact of PRE on AGEs in the human skin. The amount of AGEs was measured using the AGE Scanner. (B) Impact of PRE on melanin production in the human skin. Melanin content was measured using the Mexameter. (C) Impact of PRE on pigmentation spots. The number of pigmentation spots was evaluated using VISIA. Representative pictures are shown. Areas identified as visible spots are indicated in red. Data are expressed as mean ± SEM (n = 17, ** p < 0.01 by Wilcoxon signed-rank test).
4. Discussion
This study identifies PRE, a naturally derived ingredient that blocks the interaction between AGEs and RAGE, as a novel cosmetic ingredient for mitigating skin dullness. PRE is known to exhibit various effects, including wound healing [16], anti-inflammatory activity [17], melanin production inhibition [18], and blood circulation promotion [19]. However, its effect on the AGE–RAGE interaction has not yet been documented. AGE accumulation is accelerated in UV-exposed skin, resulting in a negative loop that further activates RAGE signaling by AGEs and boosts melanin production [20], making it an increasingly important target in cosmetic product development. Currently, PRE’s specific compounds that are responsible for inhibiting the AGE–RAGE interaction remain unknown. We fractionated PRE using a column packed with the synthetic adsorbent HP20 and evaluated the inhibitory activity of the resulting fractions; however, none of the fractions inhibited AGE–RAGE binding. These findings suggest that the active compound(s) may require synergistic interactions among multiple constituents, or that they may have been lost or inactivated during the fractionation process. Although HPLC-based fractionation or mass spectrometry analysis was not performed in the present study, further phytochemical investigation is warranted. Detailed chemical profiling, such as activity-guided HPLC fractionation followed by LC-MS/MS analysis, is considered an important direction for future research. Furthermore, paeoniflorin and albiflorin, which are representative compounds known to be present in PRE, showed no inhibitory activity against AGE–RAGE binding (Figure 1). Although paeoniflorin suppresses RAGE expression [21], our data suggest that neither of these compounds directly acts on RAGE. We also demonstrated that PRE suppresses AGE formation (Figure 4). Natural substances such as quercetin and epigallocatechin gallate reportedly inhibit AGE formation [22,23], but no studies demonstrated that these compounds are present in PRE. Polyphenols have also been reported to inhibit AGE formation and the AGE–RAGE interaction [24]. Given that PRE is rich in polyphenols [25], these compounds might be responsible for the observed inhibitory activities of PRE.
Moreover, we found that PRE suppresses melanin production induced by AGEs (Figure 2). Paeoniflorin inhibits melanin production triggered by α-MSH stimulation [26]. In this mechanism, cAMP response element–binding protein (CREB) activation is suppressed, and tyrosinase and the tyrosinase-related proteins 1 and 2 are downregulated. Conversely, AGEs promote melanin production through a different signaling pathway, triggering downstream ERK phosphorylation via RAGE and subsequently activating CREB [9]. Thus, the entry points of the signaling cascades differ between α-MSH and AGEs. PRE suppresses both the α-MSH and RAGE pathways; thus, it may provide a more robust inhibition of melanin production.
Although RAGE signaling suppression through the application of a lotion containing 1% PRE in human clinical trials has not yet been confirmed, the observed reductions in skin AGE levels and melanin content are nonetheless significant (Figure 5). The reduction in AGE levels might be caused by the inhibitory effect of PRE on AGE formation, whereas the reduction in melanin content might be attributed to the suppression of RAGE signaling by PRE. Given that AGEs are metabolized slowly, their removal involves phagocytosis by anti-inflammatory M2 macrophages [27]. Furthermore, RAGE knockout leads to the polarization of macrophages to the M2 type [28]. Based on these reports and our results, we hypothesize that PRE possibly suppresses RAGE signaling, thereby influencing macrophage polarization and contributing to reduced AGE accumulation. However, it should be emphasized that this remains speculative in the absence of direct experimental evidence, such as analysis of RAGE expression or downstream signaling (e.g., ERK/CREB phosphorylation). Future studies, including in vitro assays, are necessary to directly clarify the effects of PRE on RAGE modulation. This observation represents an important direction for future investigation.
5. Conclusions
This study is the first to identify that PRE possesses the abilities of inhibiting the AGE–RAGE interaction and suppressing AGE formation. Additionally, human clinical trials have demonstrated that PRE has a cosmetic effect of reducing skin dullness. Given that AGEs are waste products associated with lifestyle factors and aging, PRE can potentially improve AGE-induced skin dullness across various ethnicities. Therefore, PRE is a comprehensive cosmetic ingredient that mitigates skin dullness, offering a promising novel approach that addresses the unmet needs of dullness care.
Author Contributions
K.K. conducted data curation, investigation, methodology, writing—original draft; K.B.B. conducted investigation and writing—original draft; A.H. and M.F. conducted data curation; K.T. conducted investigation and supervision; and K.S. conducted investigation, writing—original draft, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Ichimaru Pharcos Co., Ltd. (Approval Code: No. H24-001K, Approval Date: 25 October 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data generated for this study are contained in the manuscript. Raw data are available from the corresponding author upon reasonable request.
Conflicts of Interest
Kyoko Kanai, Kazal Boron Biswas, Asuka Hirasawa, Misaki Futamura, Kiyotaka Tanaka, and Kotaro Sakamoto are employees of Ichimaru Pharcos Co., Ltd. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors affirm that their affiliations did not influence the scientific integrity of the article.
Abbreviations
The following abbreviations are used in this manuscript:
| AGE | advanced glycation end product |
| BG | 1,3-butylene glycol |
| K10 | keratin 10 |
| MGM | MGM™-4 Melanocyte Growth Medium-4 BulletKit™ |
| NHEK | normal human epidermal keratinocytes |
| PRE | peony root extract |
| RAGE | receptor of AGE |
| TGM1 | transglutaminase 1 |
References
- De Rigal, J.; Des Mazis, I.; Diridollou, S.; Querleux, B.; Yang, G.; Leroy, F.; Barbosa, V.H. The effect of age on skin color and color heterogeneity in four ethnic groups. Ski. Res. Technol. 2010, 16, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Nurani, A.M.; Kikuchi, K.; Iino, M.; Shirasugi, Y.; Sonoki, A.; Fujimura, T.; Hasegawa, K.; Shibata, T. Development of a method for evaluating skin dullness: A mathematical model explaining dullness by the color, optical properties, and microtopography of the skin. Ski. Res. Technol. 2023, 29, e13407. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Zhang, J.Q.; Li, L.; Guo, M.M.; He, Y.F.; Dong, Y.M.; Meng, H.; Yi, F. Advanced glycation end products in the skin: Molecular mechanisms, methods of measurement, and inhibitory pathways. Front. Med. 2022, 9, 837222. [Google Scholar] [CrossRef]
- Koschinsky, T.; He, C.J.; Mitsuhashi, T.; Bucala, R.; Liu, C.; Buenting, C.; Heitmann, K.; Vlassara, H. Orally absorbed reactive glycation products (glycotoxins): An environmental risk factor in diabetic nephropathy. Proc. Natl. Acad. Sci. USA 1997, 94, 6474–6479. [Google Scholar] [CrossRef]
- Zheng, D.L.; Wu, Q.R.; Zeng, P.; Li, S.M.; Cai, Y.J.; Chen, S.Z.; Luo, X.S.; Kuang, S.J.; Rao, F.; Lai, Y.Y.; et al. Advanced glycation end products induce senescence of atrial myocytes and increase susceptibility of atrial fibrillation in diabetic mice. Aging Cell 2022, 21, e13734. [Google Scholar] [CrossRef]
- Dyer, D.G.; Dunn, J.A.; Thorpe, S.R.; Bailie, K.E.; Lyons, T.J.; McCance, D.R.; Baynes, J.W. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J. Clin. Investig. 1993, 91, 2463–2469. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Li, L.; Winget, J.; Laughlin, T.; Hakozaki, T. Identification of yellow advanced glycation end products in human skin. Int. J. Mol. Sci. 2024, 25, 5596. [Google Scholar] [CrossRef]
- Stern, D.; Du Yan, S.; Yan, S.F.; Schmidt, A.M. Receptor for advanced glycation endproducts: A multiligand receptor magnifying cell stress in diverse pathologic settings. Adv. Drug Deliv. Rev. 2002, 54, 1615–1625. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, J.Y.; Oh, S.H. Advanced glycation end products (AGEs) promote melanogenesis through receptor for AGEs. Sci. Rep. 2016, 6, 27848. [Google Scholar] [CrossRef]
- Weinhage, T.; Wirth, T.; Schütz, P.; Becker, P.; Lueken, A.; Skryabin, B.V.; Wittkowski, H.; Foell, D. The receptor for advanced glycation endproducts (rage) contributes to severe inflammatory liver injury in mice. Front. Immunol. 2020, 11, 1157. [Google Scholar] [CrossRef]
- Radziszewski, M.; Galus, R.; Łuszczyński, K.; Winiarski, S.; Wąsowski, D.; Malejczyk, J.; Włodarski, P.; Ścieżyńska, A. The RAGE pathway in skin pathology development: A comprehensive review of its role and therapeutic potential. Int. J. Mol. Sci. 2024, 25, 13570. [Google Scholar] [CrossRef]
- He, D.Y.; Dai, S.M. Anti-inflammatory and immunomodulatory effects of Paeonia lactiflora pall., a traditional Chinese herbal medicine. Front. Pharmacol. 2011, 2, 10. [Google Scholar] [CrossRef]
- Sakamoto, K.; Watanabe, C.; Masutani, T.; Hirasawa, A.; Wakamatsu, K.; Iddamalgoda, A.; Kakumu, Y.; Yamauchi, K.; Mitsunaga, T. Arnica montana L. extract containing 6-O-methacryloylhelenalin and 6-O-isobutyrylhelenalin accelerates growth and differentiation of human subcutaneous preadipocytes and leads volumizing of skin. Int. J. Cosmet. Sci. 2023, 45, 1–13. [Google Scholar] [CrossRef]
- Sakamoto, K.; Fujimoto, R.; Nakagawa, S.; Kamiyama, E.; Kanai, K.; Kawai, Y.; Kojima, H.; Hirasawa, A.; Wakamatsu, K.; Masutani, T. Juniper berry extract containing anthricin and yatein suppresses lipofuscin accumulation in human epidermal keratinocytes through proteasome activation, increases brightness and decreases spots in human skin. Int. J. Cosmet. Sci. 2023, 45, 655–671. [Google Scholar] [CrossRef]
- He, C.N.; Peng, Y.; Zhang, Y.C.; Xu, L.J.; Gu, J.; Xiao, P.G. Phytochemical and biological studies of paeoniaceae. Chem. Biodivers. 2010, 7, 805. [Google Scholar] [CrossRef] [PubMed]
- Malviya, N.; Jain, S. Wound healing activity of aqueous extract of Radix paeoniae root. Acta Pol. Pharm. 2009, 66, 543–547. [Google Scholar]
- Lee, B.; Shin, Y.W.; Bae, E.A.; Han, S.J.; Kim, J.S.; Kang, S.S.; Kim, D.H. Antiallergic effect of the root of Paeonia lactiflora and its constituents paeoniflorin and paeonol. Arch. Pharm. Res. 2008, 31, 445–450. [Google Scholar] [CrossRef]
- Lin, D.; Wang, S.H.; Song, T.Y.; Hsieh, C.W.; Tsai, M.S. Safety and efficacy of tyrosinase inhibition of Paeonia suffruticosa Andrews extracts on human melanoma cells. J. Cosmet. Dermatol. 2019, 18, 1921–1929. [Google Scholar] [CrossRef]
- Goto, H.; Shimada, Y.; Akechi, Y.; Kohta, K.; Hattori, M.; Terasawa, K. Endothelium-dependent vasodilator effect of extract prepared from the roots of Paeonia lactiflora on isolated rat aorta. Planta Med. 1996, 62, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Zgutka, K.; Tkacz, M.; Tomasiak, P.; Tarnowski, M. A role for advanced glycation end products in molecular ageing. Int. J. Mol. Sci. 2023, 24, 9881. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Xiao, X.; Guo, D.; Mo, L.; Bu, C.; Ye, W.; Den, Q.; Liu, S.; Yang, X. Protective effects of paeoniflorin against AOPP-induced oxidative injury in HUVECS by blocking the ROS-HIF-1α/VEGF pathway. Phytomedicine 2017, 34, 115–126. [Google Scholar] [CrossRef]
- Li, X.; Zheng, T.; Sang, S.; Lv, L. Quercetin inhibits advanced glycation end product formation by trapping methylglyoxal and glyoxal. J. Agric. Food Chem. 2014, 62, 12152–12158. [Google Scholar] [CrossRef]
- Poojary, M.M.; Zhang, W.; Olesen, S.B.; Rauh, V.; Lund, M.N. Green tea extract decreases Arg-derived advanced glycation endproducts but not Lys-derived AGEs in UHT milk during 1-year storage. J. Agric. Food Chem. 2020, 68, 14261–14273. [Google Scholar] [CrossRef]
- González, I.; Morales, M.A.; Rojas, A. Polyphenols and AGEs/RAGE axis. Trends and challenges. Food Res. Int. 2020, 129, 108843. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yang, J.; Shen, L.; Wu, L.; Wang, C.; Liu, Y. The innovative extraction and purification process of insoluble polyphenols from Paeonia ostii roots: Optimum study and in vitro activities. Process Biochem. 2024, 142, 13–23. [Google Scholar] [CrossRef]
- Wen, S.Y.; Wu, Y.S.; Liu, H.; Ng, S.C.; Padma, V.V.; Huang, C.Y.; Kuo, W.W. Paeoniflorin found in Paeonia lactiflora root extract inhibits melanogenesis by regulating melanin-related signal transduction in B16F10 cells. J. Cosmet. Dermatol. 2023, 22, 2824–2830. [Google Scholar] [CrossRef] [PubMed]
- Madsen, D.H.; Leonard, D.; Masedunskas, A.; Moyer, A.; Jürgensen, H.J.; Peters, D.E.; Amornphimoltham, P.; Selvaraj, A.; Yamada, S.S.; Brenner, D.A.; et al. M2-like macrophages are responsible for collagen degradation through a mannose receptor–mediated pathway. J. Cell Biol. 2013, 202, 951–966. [Google Scholar] [CrossRef]
- Osonoi, S.; Mizukami, H.; Takeuchi, Y.; Sugawa, H.; Ogasawara, S.; Takaku, S.; Sasaki, T.; Kudoh, K.; Ito, K.; Sango, K.; et al. RAGE activation in macrophages and development of experimental diabetic polyneuropathy. JCI Insight 2022, 7, e160555. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).