Officinal Plants as New Frontiers of Cosmetic Ingredients
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Aesculus hippocastanum L.
3.2. Camellia sinensis L.
3.3. Drosera ramentacea Burch. ex Harv. & Sond.
3.4. Eucalyptus globulus Labill.
3.5. Glycine soja (L.) Merr.
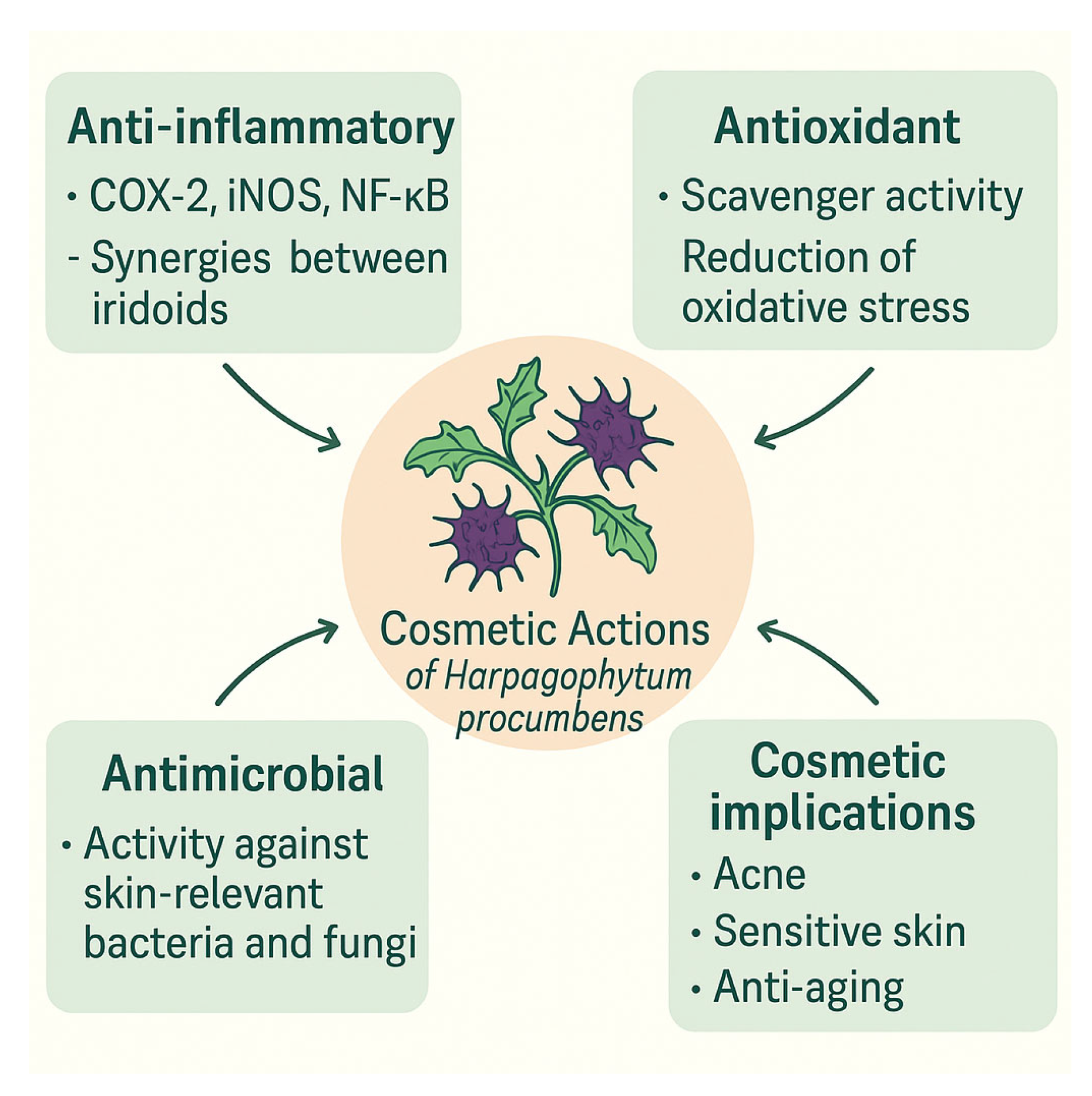
3.6. Harpagophytum procumbens DC.
3.7. Kigelia africana (Lam.) Benth.
3.8. Lycium barbarum L.
3.9. Mentha piperita L.
3.10. Panax ginseng C.A. Meyer
3.11. Prunus amygdalus varietas dulcis (Mill) D.A.Webb
3.12. Ribes nigrum L.
3.13. Ruscus aculeatus L.
3.14. Solanum lycopersicum L.
3.15. Vitis vinifera L.
3.16. Zingiber officinale Rosco
4. Discussion
4.1. Anti-Inflammatory Activity
4.2. Antioxidant and Photoprotective Activities
4.3. Antimicrobial Effect
4.4. Venotonic Effect
4.5. Skin-Brightening Activity
4.6. Regenerative Property
4.7. Photoprotective Effect
4.8. Other
4.9. General Concluding Considerations
4.10. Limitations of the Study
5. Conclusions
Funding
Conflicts of Interest
References
- Barbieri, F.; Tabanelli, G.; Braschi, G.; Bassi, D.; Morandi, S.; Šimat, V.; Čagalj, M.; Gardini, F.; Montanari, C. Mediterranean Plants and Spices as a Source of Bioactive Essential Oils for Food Applications: Chemical Characterisation and In Vitro Activity. Int. J. Mol. Sci. 2025, 26, 3875. [Google Scholar] [CrossRef] [PubMed]
- Bouissane, L.; Elfardi, Y.; Khatib, S.; Fatimi, A.; Pereira, C.; Cruz-Martins, N. Medicinal plants and their derivatives for skin and hair: A Mediterranean perspective of women care. Arch. Dermatol. Res. 2025, 317, 710. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.F.; Magalhães, W.V.; Di Stasi, L.C. Recent Advances in Herbal-Derived Products with Skin Anti-Aging Properties and Cosmetic Applications. Molecules 2022, 27, 7518. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.S.; Magalhães, M.C.; Oliveira, R.; Sousa-Lobo, J.M.; Almeida, I.F. Trends in the Use of Botanicals in Anti-Aging Cosmetics. Molecules 2021, 26, 3584. [Google Scholar] [CrossRef]
- Lianza, M.; Mandrone, M.; Chiocchio, I.; Tomasi, P.; Marincich, L.; Poli, F. Screening of ninety herbal products of commercial interest as potential ingredients for phytocosmetics. J. Enzym. Inhib. Med. Chem. 2020, 35, 1287–1291. [Google Scholar] [CrossRef]
- Plundrich, N.; Grace, M.H.; Raskin, I.; Lila, M.A. Bioactive polyphenols from muscadine grape and blackcurrant stably concentrated onto protein-rich matrices for topical applications. Int. J. Cosmet. Sci. 2013, 35, 394–401. [Google Scholar] [CrossRef]
- Li, L.; Hwang, E.; Ngo, H.T.T.; Seo, S.A.; Lin, P.; Gao, W.; Liu, Y.; Yi, T.H. Ribes nigrum L. prevents UVB-mediated photoaging in human dermal fibroblasts: Potential antioxidant and anti-inflammatory activity. Photochem. Photobiol. 2018, 94, 1032–1039. [Google Scholar] [CrossRef]
- Juhász, M.L.; Levin, M.K.; Marmur, E.S. The use of natural ingredients in innovative Korean cosmeceuticals. J. Cosmet. Dermatol. 2018, 17, 305–312. [Google Scholar] [CrossRef]
- Reuter, J.; Wölfle, U.; Korting, H.C.; Schempp, C. Which plant for which skin disease? Part 2: Dermatophytes, chronic venous insufficiency, photoprotection, actinic keratoses, vitiligo, hair loss, cosmetic indications. J. Dtsch. Dermatol. Ges. 2010, 8, 866–873. [Google Scholar] [CrossRef]
- Owczarek, A.; Kołodziejczyk-Czepas, J.; Marczuk, P.; Siwek, J.; Wąsowicz, K.; Olszewska, M.A. Bioactivity Potential of Aesculus hippocastanum L. Flower: Phytochemical Profile, Antiradical Capacity and Protective Effects on Human Plasma Components under Oxidative/Nitrative Stress In Vitro. Pharmaceuticals 2021, 14, 1301. [Google Scholar] [CrossRef]
- Sharipov, A.; Tursunov, K.; Fazliev, S.; Azimova, B.; Razzokov, J. Hypoglycemic and Anti-Inflammatory Effects of Triterpene Glycoside Fractions from Aeculus hippocastanum Seeds. Molecules 2021, 26, 3784. [Google Scholar] [CrossRef] [PubMed]
- Idris, S.; Mishra, A.; Khushtar, M. Phytochemical, ethanomedicinal and pharmacological applications of escin from Aesculus hippocastanum L. towards future medicine. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 20190115. [Google Scholar] [CrossRef] [PubMed]
- Luzzi, R.; Feragalli, B.; Belcaro, G.; Cesarone, M.R.; Cornelli, U.; Dugall, M.; Hosoi, M. Aescin: Microcirculatory activity. Effects of accessory components on clinical and microcirculatory efficacy. Panminerva Med. 2011, 53 (Suppl. S1), 51–55. [Google Scholar] [PubMed]
- Kapusta, I.; Janda, B.; Szajwaj, B.; Stochmal, A.; Piacente, S.; Pizza, C.; Franceschi, F.; Franz, C.; Oleszek, W. Flavonoids in horse chestnut (Aesculus hippocastanum) seeds and powdered wastewater byproducts. J. Agric. Food Chem. 2007, 55, 8485–8490. [Google Scholar] [CrossRef]
- Gallelli, L. Escin: A review of its anti-edematous, anti-inflammatory, and venotonic properties. Drug. Des. Dev. Ther. 2019, 13, 3425–3437. [Google Scholar] [CrossRef]
- Wilkinson, J.A.; Brown, A.M. Horse chestnut–Aesculus hippocastanum: Potential applications in cosmetic skin-care products. Int. J. Cosmet. Sci. 1999, 21, 437–447. [Google Scholar] [CrossRef]
- Chan, E.W.C. An overview of the chemical constituents, pharmacological properties, and safety evaluation of Camellia sinensis flowers. J. Appl. Pharm. Sci. 2024, 14, 22–29. [Google Scholar] [CrossRef]
- Hsu, S. Green tea and the skin. J. Am. Acad. Dermatol. 2005, 52, 1049–1059. [Google Scholar] [CrossRef]
- Türkoğlu, M.; Uğurlu, T.; Gedik, G.; Yılmaz, A.M.; Yalçin, A.S. In vivo evaluation of black and green tea dermal products against UV radiation. Drug Discov. Ther. 2010, 4, 362–367. [Google Scholar]
- Li, Y.H.; Wu, Y.; Wei, H.C.; Xu, Y.Y.; Jia, L.L.; Chen, J.; Yang, X.S.; Dong, G.H.; Gao, X.H.; Chen, H.D. Protective effects of green tea extracts on photoaging and photoimmunosuppression. Skin Res. Technol. 2009, 15, 338–345. [Google Scholar] [CrossRef]
- Mahmood, T.; Akhtar, N.; Khan, B.A.; Khan, H.M.; Saeed, T. Outcomes of 3% green tea emulsion on skin sebum production in male volunteers. Bosn. J. Basic Med. Sci. 2010, 10, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Waranuch, N.; Phimnuan, P.; Yakaew, S.; Nakyai, W.; Grandmottet, F.; Onlom, C.; Srivilai, J.; Viyoch, J. Antiacne and antiblotch activities of a formulated combination of Aloe barbadensis leaf powder, Garcinia mangostana peel extract, and Camellia sinensis leaf extract. Clin. Cosmet. Investig. Dermatol. 2019, 12, 383–391. [Google Scholar] [CrossRef]
- Gianeti, M.D.; Mercurio, D.G.; Campos, P.M. The use of green tea extract in cosmetic formulations: Not only an antioxidant active ingredient. Dermatol. Ther. 2013, 26, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, T.H.; Kim, W.I.; Park, S.; Kim, J.H.; Cho, M.K. The effects of green tea on acne vulgaris: A systematic review and meta-analysis of randomized clinical trials. Phytother. Res. 2021, 35, 374–383. [Google Scholar] [CrossRef]
- Shin, S.; Kim, M.; Song, N.; Sun, S.; Choi, J.; Park, K. Antioxidant and anti-melanogenesis effects of colloidal gold Camellia sinensis L. extracts. Molecules 2022, 27, 5593. [Google Scholar] [CrossRef]
- Dal Belo, S.E.; Gaspar, L.R.; Maia Campos, P.M.; Marty, J.P. Skin penetration of epigallocatechin-3-gallate and quercetin from green tea and Ginkgo biloba extracts vehiculated in cosmetic formulations. Skin Pharmacol. Physiol. 2009, 22, 299–304. [Google Scholar] [CrossRef]
- Wisuitiprot, W.; Somsiri, A.; Ingkaninan, K.; Waranuch, N. In vitro human skin permeation and cutaneous metabolism of catechins from green tea extract and green tea extract-loaded chitosan microparticles. Int. J. Cosmet. Sci. 2011, 33, 572–579. [Google Scholar] [CrossRef]
- Mahmood, T.; Akhtar, N. Stability of a cosmetic multiple emulsion loaded with green tea extract. Sci. World J. 2013, 2013, 153695. [Google Scholar] [CrossRef]
- Davis, S.L.; Marsh, J.M.; Kelly, C.P.; Li, L.; Tansky, C.S.; Fang, R.; Simmonds, M.S.J. Protection of hair from damage induced by ultraviolet irradiation using tea (Camellia sinensis) extracts. J. Cosmet. Dermatol. 2022, 21, 2246–2254. [Google Scholar] [CrossRef]
- Leite, M.G.A.; Maia Campos, P.M.B.G. Photoprotective effects of a multifunctional hair care formulation containing botanical extracts, vitamins, and UV filters. Photochem. Photobiol. 2018, 94, 1010–1016. [Google Scholar] [CrossRef]
- Bassino, E.; Gasparri, F.; Munaron, L. Protective role of nutritional plants containing flavonoids in hair follicle disruption: A review. Int. J. Mol. Sci. 2020, 21, 523. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Sousa-Baptista, J.; Lenha-Silva, A.F.; Calheiros, D.; Correia, E.; Figueirinha, A.; Salgueiro, L.; Gonçalves, T. Azorean black tea (Camellia sinensis) antidermatophytic and fungicidal properties. Molecules 2023, 28, 7775. [Google Scholar] [CrossRef] [PubMed]
- Luckner, R.; Luckner, M. Naphthochinonderivate aus Drosera ramentacea Burch. ex Harv. et Sond [Naphthoquinone derivatives from Drosera ramentacea Burch. ex Harv. et Sond]. Pharmazie 1970, 25, 261–265. [Google Scholar] [PubMed]
- Krishnamoorthy, V.; Thomson, R.H. A new binaphthaquinone from Drosera ramentacea. Phytochemistry 1969, 8, 1591–1594. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Kuete, V. Review of the chemistry and pharmacology of 7-methyljuglone. Afr. Health Sci. 2014, 14, 201–205. [Google Scholar] [CrossRef]
- Krolicka, A.; Szpitter, A.; Maciag, M.; Biskup, E.; Gilgenast, E.; Wegrzyn, G.; Lojkowska, E. Antibacterial and antioxidant activity of the secondary metabolites from in vitro cultures of the Alice sundew (Drosera aliciae). Biotechnol. Appl. Biochem. 2009, 53, 175–184. [Google Scholar] [CrossRef]
- Oh, T.I.; Yun, J.M.; Park, E.J.; Kim, Y.S.; Lee, Y.M.; Lim, J.H. Plumbagin suppresses α-MSH-induced melanogenesis in B16F10 mouse melanoma cells by inhibiting tyrosinase activity. Int. J. Mol. Sci. 2017, 18, 320. [Google Scholar] [CrossRef]
- Feng, D.; Fang, Z.; Zhang, P. The melanin inhibitory effect of plants and phytochemicals: A systematic review. Phytomedicine 2022, 107, 154449. [Google Scholar] [CrossRef]
- Nair, S.V.; Baranwal, G.; Chatterjee, M.; Sachu, A.; Vasudevan, A.K.; Bose, C.; Banerji, A.; Biswas, R. Antimicrobial activity of plumbagin, a naturally occurring naphthoquinone from Plumbago rosea, against Staphylococcus aureus and Candida albicans. Int. J. Med. Microbiol. 2016, 306, 237–248. [Google Scholar] [CrossRef]
- Luo, P.; Wong, Y.F.; Ge, L.; Zhang, Z.F.; Liu, Y.; Liu, L.; Zhou, H. Anti-inflammatory and analgesic effect of plumbagin through inhibition of nuclear factor-κB activation. J. Pharmacol. Exp. Ther. 2010, 335, 735–742. [Google Scholar] [CrossRef]
- Hayat, U.; Jilani, M.I.; Rehman, R.; Nadeem, F. A review on Eucalyptus globulus: A new perspective in therapeutics. Int. J. Chem. Biochem. Sci. 2015, 8, 85–91. [Google Scholar]
- Sebei, K.; Sakouhi, F.; Herchi, W.; Khouja, M.L.; Boukhchina, S. Chemical composition and antibacterial activities of seven Eucalyptus species essential oils leaves. Biol. Res. 2015, 48, 7. [Google Scholar] [CrossRef] [PubMed]
- Dogan, G.; Kara, N.; Bagci, E.; Gur, S. Chemical composition and biological activities of leaf and fruit essential oils from Eucalyptus camaldulensis. Z. Naturforsch. C J. Biosci. 2017, 72, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Arooj, B.; Asghar, S.; Saleem, M.; Khalid, S.H.; Asif, M.; Chohan, T.; Khan, I.U.; Zubair, H.M.; Yaseen, H.S. Anti-inflammatory mechanisms of eucalyptol-rich Eucalyptus globulus essential oil alone and in combination with flurbiprofen. Inflammopharmacology 2023, 31, 1849–1862. [Google Scholar] [CrossRef]
- Nakamura, T.; Yoshida, N.; Yamanoi, Y.; Honryo, A.; Tomita, H.; Kuwabara, H.; Kojima, Y. Eucalyptus oil reduces allergic reactions and suppresses mast cell degranulation by downregulating IgE-FcεRI signalling. Sci. Rep. 2020, 10, 20940. [Google Scholar] [CrossRef]
- Moreira, P.; Sousa, F.J.; Matos, P.; Brites, G.S.; Gonçalves, M.J.; Cavaleiro, C.; Figueirinha, A.; Salgueiro, L.; Batista, M.T.; Branco, P.C.; et al. Chemical composition and effect against skin alterations of bioactive extracts obtained by the hydrodistillation of Eucalyptus globulus leaves. Pharmaceutics 2022, 14, 561. [Google Scholar] [CrossRef]
- Zonfrillo, M.; Andreola, F.; Krasnowska, E.K.; Sferrazza, G.; Pierimarchi, P.; Serafino, A. Essential oil from Eucalyptus globulus (Labill.) activates complement receptor-mediated phagocytosis and stimulates podosome formation in human monocyte-derived macrophages. Molecules 2022, 27, 3488. [Google Scholar]
- Infante, V.H.P.; Maia Campos, P.M.B.G.; Gaspar, L.R.; Darvin, M.E.; Schleusener, J.; Rangel, K.C.; Meinke, M.C.; Lademann, J. Safety and efficacy of combined essential oils for the skin barrier properties: In vitro, ex vivo and clinical studies. Int. J. Cosmet. Sci. 2022, 44, 118–130. [Google Scholar] [CrossRef]
- Kofsky, J.; Zhang, H.; Song, B.H. The untapped genetic reservoir: The past, current, and future applications of the wild soybean (Glycine soja). Front. Plant Sci. 2018, 9, 949. [Google Scholar] [CrossRef]
- Zeichner, J.; Bussmann, T.; Weise, J.M.; Maass, E.; Krüger, A.; Schade, A.K.; Lain, E.; Mariwalla, K.; Kirchner, F.; Draelos, Z.D. Evaluation of antioxidants’ ability to enhance hyaluronic-acid based topical moisturizers. J. Clin. Aesthet. Dermatol. 2024, 17, 48–51. [Google Scholar]
- Russo, A.; Cardile, V.; Lombardo, L.; Vanella, L.; Acquaviva, R. Genistin inhibits UV light-induced plasmid DNA damage and cell growth in human melanoma cells. J. Nutr. Biochem. 2006, 17, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Grant, L.; McBean, D.E.; Fyfe, L.; Warnock, A.M. A review of the biological and potential therapeutic actions of Harpagophytum procumbens. Phytother. Res. 2007, 21, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Abdelouahab, N.; Heard, C. Effect of the major glycosides of Harpagophytum procumbens (Devil’s Claw) on epidermal cyclooxygenase-2 (COX-2) in vitro. J. Nat. Prod. 2008, 71, 746–749. [Google Scholar] [CrossRef]
- Ouitas, N.A.; Heard, C.M. A novel ex vivo skin model for the assessment of the potential transcutaneous anti-inflammatory effect of topically applied Harpagophytum procumbens extract. Int. J. Pharm. 2009, 376, 63–68. [Google Scholar] [CrossRef]
- Georgiev, M.I.; Alipieva, K.I.; Denev, P. Antioxidant activity and bioactive constituents of the aerial parts of Harpagophytum procumbens plants. Biotechnol. Biotechnol. Equip. 2010, 24 (Suppl. S1), 438–443. [Google Scholar] [CrossRef]
- Weckesser, S.; Engel, K.; Simon-Haarhaus, B.; Wittmer, A.; Pelz, K.; Schempp, C.M. Screening of plant extracts for antimicrobial activity against bacteria and yeasts with dermatological relevance. Phytomedicine 2007, 14, 508–516. [Google Scholar] [CrossRef]
- Lall, N.; Blom van Staden, A.; Rademan, S.; Lambrechts, I.; De Canha, M.N.; Mahore, J.; Winterboer, S.; Twilley, D. Antityrosinase and anti-acne potential of plants traditionally used in the Jongilanga community in Mpumalanga. S. Afr. J. Bot. 2019, 126, 241–249. [Google Scholar] [CrossRef]
- Koycheva, I.K.; Mihaylova, L.V.; Todorova, M.N.; Balcheva-Sivenova, Z.P.; Alipieva, K.; Ferrante, C.; Orlando, G.; Georgiev, M.I. Leucosceptoside A from Devil’s Claw modulates psoriasis-like inflammation via suppression of the PI3K/AKT signaling pathway in keratinocytes. Molecules 2021, 26, 7014. [Google Scholar] [CrossRef]
- Nabatanzi, A.; Nkadimeng, S.M.; Lall, N.; Kabasa, J.D.; McGaw, L.J. Ethnobotany, phytochemistry and pharmacological activity of Kigelia africana (Lam.) Benth. (Bignoniaceae). Plants 2020, 9, 753. [Google Scholar] [CrossRef]
- Picerno, P.; Autore, G.; Marzocco, S.; Meloni, M.; Sanogo, R.; Aquino, R.P. Anti-inflammatory activity of verminoside from Kigelia africana and evaluation of cutaneous irritation in cell cultures and reconstituted human epidermis. J. Nat. Prod. 2005, 68, 1610–1614. [Google Scholar] [CrossRef]
- Nciki, S.; Vuuren, S.; van Eyk, A.; de Wet, H. Plants used to treat skin diseases in northern Maputaland, South Africa: Antimicrobial activity and in vitro permeability studies. Pharm. Biol. 2016, 54, 2420–2436. [Google Scholar] [CrossRef] [PubMed]
- Mabona, U.; Viljoen, A.; Shikanga, E.; Marston, A.; Van Vuuren, S. Antimicrobial activity of southern African medicinal plants with dermatological relevance: From an ethnopharmacological screening approach to combination studies and the isolation of a bioactive compound. J. Ethnopharmacol. 2013, 148, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, S.; Srilakshmi, G. Phytochemical constituents and pharmacological activities of Kigelia africana and Mansoa alliacea—A comprehensive review. Asian, J. Pharm. Clin. Res. 2018, 11 (Suppl. S4), 29–32. [Google Scholar] [CrossRef]
- Makgobole, M.U.; Mpofana, N.; Ajao, A.A. Medicinal plants for dermatological diseases: Ethnopharmacological significance of botanicals from West Africa in skin care. Cosmetics 2023, 10, 167. [Google Scholar] [CrossRef]
- Karatay, K.B.; Muftuler, F.Z.B.; Law, B.; Aras, O. Methanolic extract of Kigelia africana and wound healing: An in vitro study. J. Wound Care 2023, 32, 392–398. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Topically used herbal products for the treatment of psoriasis–Mechanism of action, drug delivery, clinical studies. Planta Med. 2016, 82, 1447–1455. [Google Scholar] [CrossRef]
- Daniyal, M.; Akram, M.; Zainab, R.; Munir, N.; Shah, S.M.A.; Liu, B.; Wang, W.; Riaz, M.; Jabeen, F. Progress and prospects in the management of psoriasis and developments in phyto-therapeutic modalities. Dermatol. Ther. 2019, 32, e12866. [Google Scholar] [CrossRef]
- Oyedeji, F.O.; Bankole, O.S. Quantitative evaluation of the antipsoriatic activity of sausage tree (Kigelia africana). Afr. J. Pure Appl. Chem. 2012, 6, 214–218. [Google Scholar]
- Gao, Y.; Wei, Y.; Wang, Y.; Gao, F.; Chen, Z. Lycium barbarum: A traditional Chinese herb and a promising anti-aging agent. Aging Dis. 2017, 8, 778–791. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Q.; Fang, J.; Wang, C.; Wang, D.; Li, M. The anti-aging activity of Lycium barbarum polysaccharide extracted by yeast fermentation: In vivo and in vitro studies. Int. J. Biol. Macromol. 2022, 209, 2032–2041. [Google Scholar] [CrossRef]
- Zhang, X.X.; Ni, Z.J.; Zhang, F.; Khan, M.R.; Busquets, R.; Wei, Z.J. Physicochemical and antioxidant properties of Lycium barbarum seed dreg polysaccharides prepared by continuous extraction. Food Chem. X 2022, 14, 100282. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Peng, L.; Li, R.; Li, H.; Mo, Z.; Dai, X.; Jiang, N.; Liu, Q.; Zhang, E.; Deng, H.; et al. Lycium barbarum polysaccharide protects HSF cells against ultraviolet-induced damage through the activation of Nrf2. Cell. Mol. Biol. Lett. 2018, 23, 18. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Z.; Peng, L.; Jiang, N.; Liu, Q.; Zhang, E.; Liang, B.; Li, R.; Zhu, H. Lycium barbarum polysaccharide protects human keratinocytes against UVB-induced photo-damage. Free Radic. Res. 2017, 51, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Luan, X.; Jia, Y.; Ma, L.; Wang, Z.; Yang, Y.; Chen, Q.; Cui, X.; Luo, D. Protective effect and mechanism of Lycium barbarum polysaccharide against UVB-induced skin photoaging. Photochem. Photobiol. Sci. 2024, 23, 1931–1943. [Google Scholar] [CrossRef]
- Neves, L.M.G.; Tim, C.R.; Floriano, E.M.; da Silva de Avó, L.R.; Fernandes, J.B.; Parizotto, N.A.; Cominetti, M.R. Lycium barbarum polysaccharide fraction associated with photobiomodulation protects from epithelium thickness and collagen fragmentation in a model of cutaneous photodamage. Lasers Med. Sci. 2021, 36, 863–870. [Google Scholar] [CrossRef]
- Bak, S.G.; Lim, H.J.; Won, Y.S.; Lee, S.; Cheong, S.H.; Lee, S.J.; Bae, E.Y.; Lee, S.W.; Lee, S.J.; Rho, M.C. Regulatory effects of Lycium barbarum extract and isolated scopoletin on atopic dermatitis-like skin inflammation. Biomed. Res. Int. 2022, 2022, 2475699. [Google Scholar] [CrossRef]
- Lee, H.S.; Bae, E.Y.; Ly, S.Y. Protective effect of Lycium barbarum leaf extracts on atopic dermatitis: In vitro and in vivo studies. Nutr. Res. Pract. 2023, 17, 855–869. [Google Scholar] [CrossRef]
- Liu, G.T.; Li, Y.L.; Wang, J.; Dong, C.Z.; Deng, M.; Tai, M.; Deng, L.; Che, B.; Lin, L.; Du, Z.Y.; et al. Improvement of skin barrier dysfunction by phenolic-containing extracts of Lycium barbarum via Nrf2/HO-1 regulation. Photochem. Photobiol. 2022, 98, 262–271. [Google Scholar] [CrossRef]
- Peng, L.; Lu, Y.; Zhong, J.; Ke, Y.; Li, Y.; Liang, B.; Li, H.; Zhu, H.; Li, Z. Lycium barbarum polysaccharide promotes proliferation of human melanocytes via activating the Nrf2/p62 signaling pathway by inducing autophagy in vitro. J. Food Biochem. 2022, 46, e14301. [Google Scholar]
- Zhao, H.; Ren, S.; Yang, H.; Tang, S.; Guo, C.; Liu, M.; Tao , Q.; Ming, T.; Xu, H. Peppermint essential oil: Its phytochemistry, biological activity, pharmacological effect and application. Biomed. Pharmacother. 2022, 154, 113559. [Google Scholar] [CrossRef]
- Diniz do Nascimento, L.; Moraes, A.A.B.; Costa, K.S.D.; Pereira Galúcio, J.M.; Taube, P.S.; Costa, C.M.L.; Neves Cruz, J.; de Aguiar Andrade, E.H.; Faria, L.J.G. Bioactive natural compounds and antioxidant activity of essential oils from spice plants: New findings and potential applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Amended safety assessment of Mentha piperita (Peppermint)-derived ingredients as used in cosmetics. Int. J. Toxicol. 2023, 42, 117S–143S. [Google Scholar] [CrossRef]
- Hudz, N.; Kobylinska, L.; Pokajewicz, K.; Horčinová Sedláčková, V.; Fedin, R.; Voloshyn, M.; Myskiv, I.; Brindza, J.; Wieczorek, P.P.; Lipok, J. Mentha piperita: Essential oil and extracts, their biological activities, and perspectives on the development of new medicinal and cosmetic products. Molecules 2023, 28, 7444. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Park, M.A.; Kim, Y.C. Peppermint oil promotes hair growth without toxic signs. Toxicol. Res. 2014, 30, 297–304. [Google Scholar] [CrossRef] [PubMed]
- European Union Herbal Monograph on Mentha × piperita L.; Aetheroleum. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/european-union-herbal-monograph-mentha-x-piperita-l-aetheroleum-revision-1_en.pdf (accessed on 15 May 2025).
- Razgonova, M.P.; Veselov, V.V.; Zakharenko, A.M.; Golokhvast, K.S.; Nosyrev, A.E.; Cravotto, G.; Tsatsakis, A.; Spandidos, D.A. Panax ginseng components and the pathogenesis of Alzheimer’s disease (Review). Mol. Med. Rep. 2019, 19, 2975–2998. [Google Scholar] [CrossRef]
- Hwang, E.; Lee, T.H.; Park, S.Y.; Yi, T.H.; Kim, S.Y. Enzyme-modified Panax ginseng inhibits UVB-induced skin aging through the regulation of procollagen type I and MMP-1 expression. Food Funct. 2014, 5, 265–274. [Google Scholar] [CrossRef]
- Hwang, E.; Park, S.Y.; Jo, H.; Lee, D.G.; Kim, H.T.; Kim, Y.M.; Yin, C.S.; Yi, T.H. Efficacy and safety of enzyme-modified Panax ginseng for anti-wrinkle therapy in healthy skin: A single-center, randomized, double-blind, placebo-controlled study. Rejuvenation Res. 2015, 18, 449–457. [Google Scholar] [CrossRef]
- Kim, K. Effect of ginseng and ginsenosides on melanogenesis and their mechanism of action. J. Ginseng Res. 2015, 39, 1–6. [Google Scholar] [CrossRef]
- PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004-. PubChem Compound Summary for CID 9852086, Ginsenoside, K. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ginsenoside-K (accessed on 11 June 2025).
- Hou, J.H.; Shin, H.; Jang, K.H.; Park, C.K.; Koo, B.; Shin, H.; Yuk, S.H.; Lee, K.Y. Anti-acne properties of hydrophobic fraction of red ginseng (Panax ginseng C.A. Meyer) and its active components. Phytother. Res. 2019, 33, 584–590. [Google Scholar] [CrossRef]
- Kee, J.Y.; Jeon, Y.D.; Kim, D.S.; Han, Y.H.; Park, J.; Youn, D.H.; Kim, S.J.; Ahn, K.S.; Um, J.Y.; Hong, S.H. Korean red ginseng improves atopic dermatitis-like skin lesions by suppressing expression of proinflammatory cytokines and chemokines in vivo and in vitro. J. Ginseng Res. 2017, 41, 134–143. [Google Scholar] [CrossRef]
- Kim, E.; Kim, D.; Yoo, S.; Hong, Y.H.; Han, S.Y.; Jeong, S.; Jeong, D.; Kim, J.H.; Cho, J.Y.; Park, J. The skin protective effects of compound K, a metabolite of ginsenoside Rb1 from Panax ginseng. J. Ginseng Res. 2018, 42, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Kim, H.G.; Lee, Y.G.; Kim, J.H.; Lee, J.W.; Choi, B.R.; Jang, I.B.; Kim, G.S.; Baek, N.I. Isolation and quantification of ginsenoside Rh23, a new anti-melanogenic compound from the leaves of Panax ginseng. Molecules 2018, 23, 267. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Xiao, Y.K.; Hwang, E.; Haeng, J.J.; Yi, T.H. Antiphotoaging and antimelanogenesis properties of ginsenoside C-Y, a ginsenoside Rb2 metabolite from American ginseng PDD-ginsenoside. Photochem. Photobiol. 2019, 95, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, X.; Jiang, R.; Sun, L.; Zhao, D. Vanillic acid in Panax ginseng root extract inhibits melanogenesis in B16F10 cells via inhibition of the NO/PKG signaling pathway. Biosci. Biotechnol. Biochem. 2019, 83, 1205–1215. [Google Scholar] [CrossRef]
- Lee, H.R.; Jung, J.M.; Seo, J.Y.; Chang, S.E.; Song, Y. Anti-melanogenic property of ginsenoside Rf from Panax ginseng via inhibition of CREB/MITF pathway in melanocytes and ex vivo human skin. J. Ginseng Res. 2021, 45, 555–564. [Google Scholar] [CrossRef]
- Dai, Y.L.; Yang, D.; Song, L.H.; Yang, H.M.; Yu, J.B.; Zheng, F.; Yue, H.; Chen, C.B.; Wang, E.P. Low molecular weight oligosaccharide from Panax ginseng C.A. Meyer against UV-mediated apoptosis and inhibits tyrosinase activity in vitro and in vivo. Evid. Based Complement. Alternat. Med. 2021, 2021, 8879836. [Google Scholar] [CrossRef]
- Kang, M.; Park, S.; Son, S.R.; Noh, Y.; Jang, D.S.; Lee, S. Anti-aging and anti-inflammatory effects of compounds from fresh Panax ginseng roots: A study on TNF-α/IFN-γ-induced skin cell damage. Molecules 2024, 29, 5479. [Google Scholar] [CrossRef]
- Tomishima, H.; Luo, K.; Mitchell, A.E. The almond (Prunus dulcis): Chemical properties, utilization, and valorization of coproducts. Annu. Rev. Food Sci. Technol. 2022, 13, 145–166. [Google Scholar] [CrossRef]
- Blaak, J.; Staib, P. An updated review on efficacy and benefits of sweet almond, evening primrose and jojoba oils in skin care applications. Int. J. Cosmet. Sci. 2022, 44, 1–9. [Google Scholar] [CrossRef]
- Gonçalves, S.; Gaivão, I. Natural ingredients common in the Trás-os-Montes region (Portugal) for use in the cosmetic industry: A review about chemical composition and antigenotoxic properties. Molecules 2021, 26, 5255. [Google Scholar] [CrossRef]
- Musarra-Pizzo, M.; Ginestra, G.; Smeriglio, A.; Pennisi, R.; Sciortino, M.T.; Mandalari, G. The antimicrobial and antiviral activity of polyphenols from almond (Prunus dulcis L.) skin. Nutrients 2019, 11, 2355. [Google Scholar] [CrossRef] [PubMed]
- Arangia, A.; Ragno, A.; Cordaro, M.; D’Amico, R.; Siracusa, R.; Fusco, R.; Marino Merlo, F.; Smeriglio, A.; Impellizzeri, D.; Cuzzocrea, S.; et al. Antioxidant activity of a Sicilian almond skin extract using in vitro and in vivo models. Int. J. Mol. Sci. 2023, 24, 12115. [Google Scholar] [CrossRef] [PubMed]
- Li, J.N.; Henning, S.M.; Thames, G.; Bari, O.; Tran, P.T.; Tseng, C.H.; Heber, D.; Kim, J.; Li, Z. Almond consumption increased UVB resistance in healthy Asian women. J. Cosmet. Dermatol. 2021, 20, 2975–2980. [Google Scholar] [CrossRef]
- Ejaz, A.; Waliat, S.; Afzaal, M.; Saeed, F.; Ahmad, A.; Din, A.; Ateeq, H.; Asghar, A.; Shah, Y.A.; Rafi, A.; et al. Biological activities, therapeutic potential, and pharmacological aspects of blackcurrants (Ribes nigrum L.): A comprehensive review. Food Sci. Nutr. 2023, 11, 5799–5817. [Google Scholar] [CrossRef]
- Ziemlewska, A.; Zagórska-Dziok, M.; Nizioł-Łukaszewska, Z. Assessment of cytotoxicity and antioxidant properties of berry leaves as by-products with potential application in cosmetic and pharmaceutical products. Sci. Rep. 2021, 11, 3240. [Google Scholar] [CrossRef]
- Tabart, J.; Kevers, C.; Evers, D.; Dommes, J. Ascorbic acid, phenolic acid, flavonoid, and carotenoid profiles of selected extracts from Ribes nigrum. J. Agric. Food Chem. 2011, 59, 4763–4770. [Google Scholar] [CrossRef]
- Magnavacca, A.; Piazza, S.; Cammisa, A.; Fumagalli, M.; Martinelli, G.; Giavarini, F.; Sangiovanni, E.; Dell’Agli, M. Ribes nigrum leaf extract preferentially inhibits IFN-γ-mediated inflammation in HaCaT keratinocytes. Molecules 2021, 26, 3044. [Google Scholar] [CrossRef]
- Kendir, G.; Süntar, I.; Çeribaşı, A.O.; Köroğlu, A. Activity evaluation on Ribes species, traditionally used to speed up healing of wounds: With special focus on Ribes nigrum. J. Ethnopharmacol. 2019, 237, 141–148. [Google Scholar] [CrossRef]
- Wyżga, B.; Skóra, M.; Wybraniec, S.; Hąc-Wydro, K. Study on the effect of blackcurrant extract-based preservative on model membranes and pathogenic bacteria. Arch. Biochem. Biophys. 2023, 750, 109806. [Google Scholar] [CrossRef]
- Thomas, P.A.; Mukassabi, T.A. Biological flora of the British Isles: Ruscus aculeatus. J. Ecol. 2014, 102, 1083–1100. [Google Scholar] [CrossRef]
- Ivanova, T.; Dimitrova, D.; Gussev, C.; Bosseva, Y.; Stoeva, T. Ex situ conservation of Ruscus aculeatus L.–ruscogenin biosynthesis, genome-size stability and propagation traits of tissue-cultured clones. Biotechnol. Biotechnol. Equip. 2015, 29, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Lichota, A.; Gwozdzinski, L.; Gwozdzinski, K. Therapeutic potential of natural compounds in inflammation and chronic venous insufficiency. Eur. J. Med. Chem. 2019, 176, 68–91. [Google Scholar] [CrossRef] [PubMed]
- Rauly-Lestienne, I.; Heusler, P.; Cussac, D.; Lantoine-Adam, F.; de Almeida Cyrino, F.Z.G.; Bouskela, E. Contribution of muscarinic receptors to in vitro and in vivo effects of Ruscus extract. Microvasc. Res. 2017, 114, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Kawasaki, A.; Tamura, K.; Minegishi, Y.; Mori, T.; Ota, N. Ruscus aculeatus extract promotes RNase 7 expression through ERK activation following inhibition of late-phase autophagy in primary human keratinocytes. PLoS ONE 2024, 19, e0314873. [Google Scholar] [CrossRef]
- Krishna, R.; Ansari, W.A.; Soumia, P.S.; Yadav, A.; Jaiswal, D.K.; Kumar, S.; Singh, A.K.; Singh, M.; Verma, J.P. Biotechnological interventions in tomato (Solanum lycopersicum) for drought stress tolerance: Achievements and future prospects. Biotechnology 2022, 11, 48. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Q.; Qi, Y.; Deng, J.; Zhang, Y.; Li, R.; Fan, J. The effect of tomato and lycopene on clinical characteristics and molecular markers of UV-induced skin deterioration: A systematic review and meta-analysis of intervention trials. Crit. Rev. Food Sci. Nutr. 2024, 64, 6198–6217. [Google Scholar] [CrossRef]
- Cefali, L.C.; Cazedey, E.C.; Souza-Moreira, T.M.; Correa, M.A.; Salgado, H.R.; Isaac, V.L. Antioxidant activity and validation of quantification method for lycopene extracted from tomato. J. AOAC Int. 2015, 98, 1340–1345. [Google Scholar] [CrossRef]
- Tommonaro, G.; De Prisco, R.; Abbamondi, G.R.; Nicolaus, B. Bioactivity of tomato hybrid powder: Antioxidant compounds and their biological activities. J. Med. Food 2013, 16, 351–356. [Google Scholar] [CrossRef]
- Tarshish, E.; Hermoni, K.; Sharoni, Y.; Wertz, P.W.; Dayan, N. Effects of golden tomato extract on skin appearance—Outlook into gene expression in cultured dermal fibroblasts and on trans-epidermal water loss and skin barrier in human subjects. J. Cosmet. Dermatol. 2022, 21, 3022–3030. [Google Scholar] [CrossRef]
- Calniquer, G.; Khanin, M.; Ovadia, H.; Linnewiel-Hermoni, K.; Stepensky, D.; Trachtenberg, A.; Sedlov, T.; Braverman, O.; Levy, J.; Sharoni, Y. Combined effects of carotenoids and polyphenols in balancing the response of skin cells to UV irradiation. Molecules 2021, 26, 1931. [Google Scholar] [CrossRef]
- Rajkowska, K.; Otlewska, A.; Raczyk, A.; Maciejczyk, E.; Krajewska, A. Valorisation of tomato pomace in anti-pollution and microbiome-balance face cream. Sci. Rep. 2024, 14, 20516. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Miyasaka, K.; Shimoda, H. Lycoperoside H, a steroidal alkaloid saponin in tomato seeds, ameliorates atopic dermatitis-like symptoms in IL-33 transgenic mice. J. Food Biochem. 2021, 45, e13877. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.C.; Greven, M.; Winefield, C.S.; Trought, M.C.; Raw, V. The flowering process of Vitis vinifera: A review. Am. J. Enol. Vitic. 2009, 60, 411–434. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Di Lorenzo, C.; Piazza, S.; Manzoni, Y.; Brunelli, C.; Fumagalli, M.; Magnavacca, A.; Martinelli, G.; Colombo, F.; Casiraghi, A.; et al. Vitis vinifera L. leaf extract inhibits in vitro mediators of inflammation and oxidative stress involved in inflammatory-based skin diseases. Antioxidants 2019, 8, 134. [Google Scholar] [CrossRef]
- Zielonka-Brzezicka, J.; Florkowska, K.; Nowak, A.; Muzykiewicz-Szymańska, A.; Klimowicz, A. The effect of thawing on the antioxidant activity of the leaves and fruit of the grapevine (Vitis vinifera). Pomeranian J. Life Sci. 2021, 67, 63–70. [Google Scholar] [CrossRef]
- Lombardo, G.; Melzi, G.; Indino, S.; Piazza, S.; Sangiovanni, E.; Baruffaldi Preis, F.; Marabini, L.; Donetti, E. Keratin 17 as a marker of UVB-induced stress in human epidermis and modulation by Vitis vinifera extract. Cells Tissues Organs 2022, 211, 611–627. [Google Scholar] [CrossRef]
- Letsiou, S.; Kapazoglou, A.; Tsaftaris, A.; Spanidi, E.; Gardikis, K. Transcriptional and epigenetic effects of Vitis vinifera L. leaf extract on UV-stressed human dermal fibroblasts. Mol. Biol. Rep. 2020, 47, 5763–5772. [Google Scholar] [CrossRef]
- Marabini, L.; Melzi, G.; Lolli, F.; Dell’Agli, M.; Piazza, S.; Sangiovanni, E.; Marinovich, M. Effects of Vitis vinifera L. leaves extract on UV radiation damage in human keratinocytes (HaCaT). J. Photochem. Photobiol. B 2020, 204, 111810. [Google Scholar] [CrossRef]
- Nelson, K.; Lyles, J.T.; Li, T.; Saitta, A.; Addie-Noye, E.; Tyler, P.; Quave, C.L. Anti-acne activity of Italian medicinal plants used for skin infection. Front. Pharmacol. 2016, 7, 425. [Google Scholar] [CrossRef]
- Simonetti, G.; Brasili, E.; Pasqua, G. Antifungal activity of phenolic and polyphenolic compounds from different matrices of Vitis vinifera L. against human pathogens. Molecules 2020, 25, 3748. [Google Scholar] [CrossRef]
- Lin, Y.S.; Chen, H.J.; Huang, J.P.; Lee, P.C.; Tsai, C.R.; Hsu, T.F.; Huang, W.Y. Kinetics of tyrosinase inhibitory activity using Vitis vinifera leaf extracts. Biomed. Res. Int. 2017, 2017, 5232680. [Google Scholar] [CrossRef] [PubMed]
- Naser, W. The cosmetic effects of various natural biofunctional ingredients against skin aging: A review. Int. J. Appl. Pharm. 2021, 13, 10–18. [Google Scholar] [CrossRef]
- Sharafan, M.; Malinowska, M.A.; Ekiert, H.; Kwaśniak, B.; Sikora, E.; Szopa, A. Vitis vinifera (Vine Grape) as a valuable cosmetic raw material. Pharmaceutics 2023, 15, 1372. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H.B. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Banihani, S.A. Effect of ginger (Zingiber officinale) on semen quality. Andrologia 2019, 51, e13296. [Google Scholar] [CrossRef]
- Chen, C.Y.; Cheng, K.C.; Chang, A.Y.; Lin, Y.T.; Hseu, Y.C.; Wang, H.M. 10-Shogaol, an antioxidant from Zingiber officinale, for skin cell proliferation and migration enhancer. Int. J. Mol. Sci. 2012, 13, 1762–1777. [Google Scholar] [CrossRef]
- Kim, S.O.; Chun, K.S.; Kundu, J.K.; Surh, Y.J. Inhibitory effects of [6]-gingerol on PMA-induced COX-2 expression and activation of NF-κB and p38 MAPK in mouse skin. Biofactors 2004, 21, 27–31. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, Y.; Na, K.M.; Surh, Y.J.; Kim, T.Y. [6]-Gingerol prevents UVB-induced ROS production and COX-2 expression in vitro and in vivo. Free Radic. Res. 2007, 41, 603–614. [Google Scholar] [CrossRef]
- Huang, H.C.; Chiu, S.H.; Chang, T.M. Inhibitory effect of [6]-gingerol on melanogenesis in B16F10 melanoma cells and a possible mechanism of action. Biosci. Biotechnol. Biochem. 2011, 75, 1067–1072. [Google Scholar] [CrossRef]
- Oh, T.I.; Jung, H.J.; Lee, Y.M.; Lee, S.; Kim, G.H.; Kan, S.Y.; Kang, H.; Oh, T.; Ko, H.M.; Kwak, K.C.; et al. Zerumbone, a tropical ginger sesquiterpene of Zingiber officinale Roscoe, attenuates α-MSH-induced melanogenesis in B16F10 cells. Int. J. Mol. Sci. 2018, 19, 3149. [Google Scholar] [CrossRef]
- Guahk, G.H.; Ha, S.K.; Jung, H.S.; Kang, C.; Kim, C.H.; Kim, Y.B.; Kim, S.Y. Zingiber officinale protects HaCaT cells and C57BL/6 mice from ultraviolet B-induced inflammation. J. Med. Food 2010, 13, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Thongrakard, V.; Ruangrungsi, N.; Ekkapongpisit, M.; Isidoro, C.; Tencomnao, T. Protection from UVB toxicity in human keratinocytes by Thailand native herbs extracts. Photochem. Photobiol. 2014, 90, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Tang, Y.; Sun, Y.; Veeraraghavan, V.P.; Mohan, S.K.; Cui, C. 6-Shogaol, an active constituent of ginger, prevents UVB radiation-mediated inflammation and oxidative stress through modulating Nrf2 signaling in human epidermal keratinocytes (HaCaT cells). J. Photochem. Photobiol. B 2019, 197, 111518. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G. Recent advances in characterizing biological mechanisms underlying UV-induced wrinkles: A pivotal role of fibroblast-derived elastase. Arch. Dermatol. Res. 2008, 300 (Suppl S1), S7–S20. [Google Scholar] [CrossRef]
- Maleki, H.; Doostan, M.; Khoshnevisan, K.; Baharifar, H.; Maleki, S.A.; Fatahi, M.A. Zingiber officinale and Thymus vulgaris extracts co-loaded polyvinyl alcohol and chitosan electrospun nanofibers for tackling infection and wound healing promotion. Heliyon 2023, 10, e23719. [Google Scholar] [CrossRef]
- Saeed, M.; Naveed, M.; Arif, M.; Kakar, M.U.; Manzoor, R.; Abd El-Hack, M.E.; Alagawany, M.; Tiwari, R.; Khandia, R.; Munjal, A.; et al. Green tea (Camellia sinensis) and l-theanine: Medicinal values and beneficial applications in humans—A comprehensive review. Biomed. Pharmacother. 2017, 95, 1260–1275. [Google Scholar] [CrossRef]


| Plant Species | Plant Part Used | Cosmetic Activities |
|---|---|---|
| Aesculus hippocastanum L. | Seeds | Anti-edematous, AO 1, AI 2, AA 3 [10,11,12,13,14,15,16] |
| Camellia sinensis L. | Leaves | AO, PP 4, AA, AM 5, DP 6 [5,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32] |
| Drosera ramentacea Burch. ex Harv. & Sond. | Whole plant | AM, DP, AA, AO [33,34,35,36,37,38,39,40] |
| Eucalyptus globulus Labill. | Leaves | AM, AI, AO, DP [41,42,43,44,45,46,47,48] |
| Glycine soja (L.) Merr. | Germ, oil | Hydration, ECM support, AO, DP, AI [4,49,50,51] |
| Harpagophytum procumbens DC. | Tubers | AI, AO, AM [52,53,54,55,56,57,58] |
| Kigelia africana (Lam.) Benth. | Fruits, barks | AA, skin firming, AI, WH 7 [59,60,61,62,63,64,65,66,67,68] |
| Lycium barbarum L. | Fruits | AO, anti-photoaging, barrier reinforcement, AI [69,70,71,72,73,74,75,76,77,78,79] |
| Mentha piperita L. | Leaves (essential oil, extract) | AM, AI, AO, soothing [80,81,82,83,84,85] |
| Panax ginseng C.A. Meyer | Roots | AA, moisturizing, AI, PP, skin-whitening [1,86,87,88,89,90,91,92,93,94,95,96,97,98,99] |
| Prunus dulcis (mill.) D.A.Webb | Seeds, skin | Emollient, moisturizing, barrier repair, AM, AO [100,101,102,103,104,105] |
| Ribes nigrum L. | Leaves | AO, AI, AM, WH, DP [5,106,107,108,109,110,111] |
| Ruscus aculeatus L. | Roots, rhizomes | Vasoprotective, AA, skin soothing, antimicrobial peptide modulation [9,112,113,114,115,116] |
| Solanum lycopersicum L. | Fruits, seeds | PP, AO, AI, microbiome balance [117,118,119,120,121,122,123,124] |
| Vitis vinifera L. | Leaves, seeds, skin | AO, AA, PP, AM, DP [125,126,127,128,129,130,131,132,133,134,135] |
| Zingiber officinale Roscoe | Rhizomes | AO, PP, regenerative, skin lightening [136,137,138,139,140,141,142,143,144,145,146,147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitalone, A.; D’Andrea, L.; Di Sotto, A.; Caruso, A.; Parente, R. Officinal Plants as New Frontiers of Cosmetic Ingredients. Cosmetics 2025, 12, 140. https://doi.org/10.3390/cosmetics12040140
Vitalone A, D’Andrea L, Di Sotto A, Caruso A, Parente R. Officinal Plants as New Frontiers of Cosmetic Ingredients. Cosmetics. 2025; 12(4):140. https://doi.org/10.3390/cosmetics12040140
Chicago/Turabian StyleVitalone, Annabella, Lucia D’Andrea, Antonella Di Sotto, Alessandra Caruso, and Rita Parente. 2025. "Officinal Plants as New Frontiers of Cosmetic Ingredients" Cosmetics 12, no. 4: 140. https://doi.org/10.3390/cosmetics12040140
APA StyleVitalone, A., D’Andrea, L., Di Sotto, A., Caruso, A., & Parente, R. (2025). Officinal Plants as New Frontiers of Cosmetic Ingredients. Cosmetics, 12(4), 140. https://doi.org/10.3390/cosmetics12040140








