1. Introduction
Candidiasis is a fungal infection that can occur in different anatomical sites such as the skin, nails, scalp, and vagina. The skin infection, cutaneous candidiasis, appears like a superficial mycosis by the proliferation of
Candida spp., especially in intertriginous areas, causing itching, erosion, inflammation, and pustules.
Candida albicans is the most common pathogen in candidiasis; however, there has been an increase in related cases with non-albicans strains like
Candida glabrata [
1,
2].
C. glabrata is an increasingly important opportunistic pathogen in invasive
Candida infections due to the inherent resistance to azole antifungals. It presents several virulence factors such as the adhesion, biofilm formation, and secretion of hydrolytic enzymes, in addition to not having proteolytic activity, and not forming true hyphae. It is not able to induce several immune responses in a variety of cells, which increases its persistence in the host, causing clinical and microbiological failure, which increases the incidence and antifungal resistance of
C. glabrata [
3,
4].
Just like yeasts, dermatophyte fungi can cause infections in humans. These types of fungi are filamentous and keratinophilic, using keratin hair, skin, and nails as nutrients during infection [
5,
6]. For the transmission of dermatophytosis, contact with humans, animals, or contaminated objects is necessary. Clinical manifestations may vary according to the species and the affected tissue. The immunological condition of the host is responsible for the severity of symptoms; however, invasion of subcutaneous tissues and internal organs is not common [
7]. Several species of dermatophyte fungi causing diseases in humans are already known, with
Trichophyton rubrum considered the main cause of these infections [
8,
9].
The control of these infections caused by fungi results from the immune response of the host and antifungal therapy using fungicide or fungistatic drugs. Such drugs should act in a very specific manner and cause no damage to the host and can be limited by their virulence and pathogenicity factors, as well as their resistance mechanisms [
10].
As an alternative to established antibiotic therapy, the search for new bioactive compounds extracted from plants or produced by microorganisms such as fungi and bacteria has been promoted for some decades, becoming an excellent alternative to overcome the problem of generating multidrug-resistant strains [
11]. World biodiversity serves as an invaluable and remains an underexplored source of compounds of interest to both human and animal health. Surveys carried out show that 25% of this diversity is found in Brazil, which is still little studied [
12]. Searching for new targets to study, it is possible to find, within traditional medicine, great contributions, an example being
Plinia cauliflora.
P. cauliflora is a fruit tree of the Myrtaceae family popularly known as “jabuticabeira”. According to folk medicine, it is indicated for the treatment of diarrhea, skin irritations, asthma, and intestinal inflammation [
13]; studies by our group have shown in vitro activity of its plant extracts against fungal strains and the presence of casuarinin, myricetin, and a glycosylated quercetin [
14,
15]. As highlighted in a literature review, quercetin is a significant phytochemical belonging to the flavonoid class of polyphenols, widely distributed in fruits, vegetables, and beverages, as well as in flowers, leaves, and seeds. The review emphasizes that quercetin exhibits antimicrobial activity against a broad spectrum of Gram-positive and Gram-negative bacteria, fungi, and viruses. Its proposed mechanisms of action include disruption of the cell membrane, alteration in membrane permeability, inhibition of nucleic acid and protein synthesis, suppression of virulence factors, mitochondrial dysfunction, and inhibition of biofilm formation. Furthermore, quercetin has demonstrated effectiveness against drug-resistant strains, underscoring its potential as a broad-spectrum antimicrobial agent [
16]. Myricetin is a dietary flavonoid with notable antimicrobial activity, though its low aqueous solubility limits bioavailability; to enhance efficacy, mediated silver nanoparticles were synthesized, and these spherical nanoparticles showed strong antibacterial activity against
E. coli and
Salmonella, with MICs of 10
−4 and 10
−5 g/L, respectively [
17].
An obstacle in the use of natural products in microbial infections is that they can be very unstable and have their application impaired during pharmaceutical processing, storage, exposure to different pH and temperatures, or during their ingestion [
18]. One way to mitigate these problems is the use of nanotechnology that can improve its functional properties as a site of action, increase the solubility of compounds, and minimize the degradation process [
19]. In addition, nanosystems can substantially reduce the toxicity of substances, especially antimicrobial drugs, since most of them present high toxicity due to the similarity of some cellular components of the fungal pathogen and the host.
A nanostructured system, microemulsions, is used as a vehicle for a wide variety of biologically active substances such as drugs and can be defined as colloidal dispersions of water and oil that are stabilized with the use of surfactants. They are thermodynamically stable, transparent, or semitransparent in appearance and their particles have a size ranging from 10 to 300 nm, enabling their sterilization by filtration [
20,
21]. The surfactant monolayer’s spontaneous curvature and flexibility enable the formation of dispersed oil or water droplets, as well as bicontinuous structures. Surfactant excess can interact with other components, producing complex multiphase formulations. The physicochemical properties and surfactant concentration are key factors influencing the structure and rheological properties of microemulsions. Microemulsions can compartmentalize the drugs in the droplets of their internal phase, which have different physicochemical properties of the dispersing medium, causing an improvement in the solubilization of lipophilic drugs in water, protecting them against enzymatic hydrolysis, in addition to providing an increase in the absorption potential due to the presence of the surfactants [
22,
23].
In this context, the objective of this study is to investigate compounds derived from plant extracts and fractions of P. cauliflora and develop microemulsions incorporating these substances, highlighting their potential significance for antimicrobial therapy. This approach may enhance antifungal activity and support the development of topical products such as creams, ointments, and films for the treatment of skin infections.
To investigate this objective, the following section outlines the materials and methods used in the study.
2. Materials and Methods
2.1. Preparation of Plant Extracts and Fractions
The leaves of P. cauliflora were collected in the Garden of Medicinal and Toxic Plants “Profª. Dr. Célia Cebrian Araújo Reis” from the School of Pharmaceutical Sciences, UNESP, Araraquara, and have been registered and authorized for use by SisGen (National System for Genetic Heritage and Associated Traditional Knowledge Management) under registration number A835CAA. Leaves were dried at 40 °C in a circulating air oven until the moisture content was below 14%, after being crushed in a knife mill. For extraction, 600 g of P. cauliflora powder was placed in 3000 mL of ethanol (LS Chemicals, São Paulo, SP, Brazil) 70%. The mixture was placed in a water bath at 40 °C for 48 h and then the filtration was performed on filter paper. Two more extraction steps were carried out for 24 h, each one using the recovered plant material and the same amount of new solvent. The final proportion of plant material and solvent was 1:15 w/v. The entire filtered volume was rotaevaporated under reduced pressure for the elimination of ethanol followed by freeze-drying. The yield obtained to ethanol extract was 24.35%.
The fractionation of the extract was performed with ethyl acetate (LS Chemicals, São Paulo, SP, Brazil), n-butanol (LS Chemicals, São Paulo, SP, Brazil), and water, by the liquid–liquid partitioning technique generating the fractions FrAcOEt, FrBuOH, and FrAqu, respectively. Firstly, 15 g of the extract was dissolved in 500 mL of water and then three successive partitions with ethyl acetate were performed in the ratio of 1:1, collecting the organic phase at each stage. Subsequently, in the same way, five more partitions were made with the solvent n-butanol. The aqueous phase was recovered, and the three fractions obtained had their solvent removed with the use of a rotaevaporator and were completely dried in an exhaust chapel. The yields obtained to fractions FrAcOEt, FrBuOH, and FrAqu were 11.85%, 13.58%, and 69.03%, respectively.
2.2. Characterization of Extract and Fractions
The extract and fractions
of P. cauliflora were characterized by High-Performance Liquid Chromatography (HPLC) based on a previous methodology [
14], using a Thermo Scientific liquid chromatograph with UV detection. Initially, the samples were cleaned up (10 mg/mL in 95% methanol (JT Baker, Center Valley, PA, USA) in SEP Pak—RP18 cartridges, 500 mg adsorbent, (Waters, Milford, MA, USA), followed by dilution to 3 mg/mL and Thermo Fisher Scientific Inc., Waltham, MA, USA) filtration in a membrane of 0.22 μm. The method used was an exploratory gradient: acetonitrile 5% (Merck, Darmstadt, Germany) to 100%; time: 40 min; flow: 1 mL/min; wavelengths: λ = 220/250/300/350 nm; C18 Thermo Scientific column (250 × 4.6 mm; 5 μm).
2.3. Development of Microemulsion and Incorporation of Samples
The preparation of the microemulsion was based on a previous study [
23]; for the oil phase, cholesterol (10%) (Sigma-Aldrich, St. Louis, MO, USA) and a surfactants system (10%) composed of a mixture of Brij 58
®, polyoxyethylene 20-cetyl ether (Sigma-Aldrich, St. Louis, MO, USA), Epikuron
® 200, soybean phosphatidylcholine (Lipoid GmbH, Ludwigshafen, Germany) in the ratio of 2:1 were used and for the aqueous phase, phosphate buffer 50 mmol. L
−1 pH = 7.4 (80%). The mixture was taken to the sonicator rod (Q700 from QSonica LLC, Newtown, CT, USA), with a power of 700 watts in discontinuous mode, amplitude of 17%, for 10 min with intervals of 30 s every 2 min and sonicated in an ice bath. Ultrasonication was used to facilitate the formation of stable and homogeneous microemulsions. The mechanical energy generated by ultrasonic waves reduces the droplet size and improves the dispersion of the oil and aqueous phases in the presence of surfactants, resulting in better system stability. Additionally, sonication enhances the incorporation and uniform distribution of active compounds, particularly useful for complex materials such as crude extracts. After sonication, the microemulsion was centrifuged at 11,180×
g for 15 min to eliminate titanium residues released by the sonicator rod. The incorporation of the samples in the microemulsion was made with the addition of the extract and fractions at the final concentration of 2500 μg/mL followed by sonication in an ice bath for 3 min, and amplitude of 15% in discontinuous mode, ensuring that there was complete incorporation.
2.4. Characterization of Microemulsion
The characterization of microemulsion was made by the determination of the particle diameter and polydispersity index (PDI) by dynamic light scattering (Zetasizer Nano NS—Malvern Panalytical Ltd., Malvern, UK). The samples were placed in the analysis chamber so that the laser beam would go through the dispersion throughout its length. The temperature of the system was maintained at 20 °C, the laser wavelength was 532 nm, and the refraction index according to the index observed for each sample was analyzed. Ten determinations of the mean diameter and polydispersity index (PDI) of droplets for each sample were performed, with a total duration of 5 min.
2.5. Fungal Strains and Culture Conditions
The microorganisms used were obtained from the “American Type Culture Collection” (ATCC) Trichophyton rubrum ATCC 28,189 and Candida glabrata ATCC 90,030 and a clinical isolated strain of Trichophyton rubrum (T. rubrum 1) was also used. The strain of C. glabrata was inoculated in Sabouraud dextrose broth (Kasvi, Curitiba, PR, Brazil), incubated at 35.5 °C for 48 h, then they were cultivated in Sabouraud dextrose agar (Kasvi, Curitiba, PR, Brazil) Petri dishes (SDA) and incubated for 24 h at 35.5 °C. After, some similar colonies were solubilized in 0.9% saline solution and the inoculum standardized at the concentration of 2 × 104 CFU/mL using Neubauer chamber count. T. rubrum strains were cultivated into potato dextrose agar plates, PDA (Kasvi, Curitiba, PR, Brazil), and after seven-day incubation at 28 °C, the conidia were transferred to 0.9% saline solution and the inoculum standardized with Neubauer chamber count at the concentration of 5 × 103 CFU/mL.
2.6. Determination of Minimum Inhibitory Concentration (MIC) and Determination of Minimum Fungicide Concentration (MFC)
The antimicrobial activity was evaluated by the MIC and MFC determination. The tests were performed following the protocols recommended by the Clinical and Laboratory Standards Institute, CLSI, standard M27-A3 [
24] for the yeast and the M38-A2 standard [
25] for dermatophyte fungi. The MIC was determined by the broth microdilution technique of the samples in 96-well microplates.
The determination of the MIC was performed with the addition of 100 μL of RPMI 1640 medium (Roswell Park Memorial Institute 1640 medium—Sigma-Aldrich, St. Louis, MO, USA) to the microplate wells; in the first wells of each row, 100 μL of the samples was transferred, at the highest concentration to be tested, then serial microdilution was performed followed by the addition of 100 μL of the previously prepared microbial inoculum. Amphotericin B (Sigma-Aldrich, St. Louis, MO, USA) and fluconazole (Sigma-Aldrich, St. Louis, MO, USA) were used as antifungal controls. Also, microbial growth, sample contamination, solvent used in sample dilution, and culture medium were performed. The microplates were incubated at 35.5 °C for 24 h and agitated at 120 rpm for yeast and dermatophytes 28 °C for seven days with agitation of 120 rpm. The MIC verification was performed by verifying the last well in which microbial growth was inhibited by adding 30 μL of 0.01% resazurin (Sigma-Aldrich, St. Louis, MO, USA) aqueous solution that changes from blue to pink in the presence of microbial growth [
26].
The concentration range tested for the extracts was 2500–1.22 μg/mL, while for antibiotics, the concentration ranges were 16.00–0.0078 μg/mL for Amphotericin B and 64.00–0.031 μg/mL for fluconazole. For the MFC determination, before the addition of resazurin, the inoculation of each microplate well samples in Sabouraud agar for C. glabrata and PDA for T. rubrum with incubation under the appropriate conditions was performed. The MFC is defined as the lowest concentration at which microbial growth is inhibited. Evaluating both techniques, it is possible to determine whether the antimicrobial activity is fungistatic (MIC) or fungicidal (MFC). The tests were performed in triplicate, and the result corresponds to the mode of evaluations. For the MIC and MBC values, the mode was used to represent the most frequent result observed across replicates, given the low variability in the data.
2.7. Evaluation of Antioxidant Activity
The evaluation of the antioxidant activity of the extracts and fractions of
P. cauliflora was performed using the capture capacity method of the radical 1,1-diffenil-2-picrilhydrazila, DPPH (Sigma-Aldrich, St. Louis, MO, EUA) [
27]. In 1 mL of the aqueous solution of the samples, at different concentrations (from 0 to 4.57 μg/mL), 2.5 mL of 0.004% DPPH methanolic solution was added. All solutions were kept protected from light for 30 min and then the absorbances were read at 515 nm (Bel Photonics, Osasco, SP, Brazil) [
28,
29]. The average absorbance of the samples was used as the maximum absorbance and the percentage of capture of the DPPH radical was calculated [
30]. With the percentages, an analytical curve was obtained, and the effective concentration required to capture 50% of the DPPH radicals in solution (EC
50) was calculated. Ascorbic acid (Sigma-Aldrich, St. Louis, MO, EUA), gallic acid (Sigma-Aldrich, St. Louis, MO, EUA), rutin (Sigma-Aldrich, St. Louis, MO, EUA), and quercetin (Sigma-Aldrich, St. Louis, MO, EUA) were used as antioxidant activity patterns. The tests were performed in triplicate, and the result corresponds to the mean of the evaluations. The results are reported as mean values, and the significance level for comparisons, where applicable, was set at
p < 0.05.
2.8. Evaluation of In Vivo Toxicity in Galleria mellonella
Groups of 10 larvae weighing between 200 and 250 mg were selected for each sample of the test and controls of the solvent and larvae development. Each group was kept in a Petri dish and remained in food restriction for 24 h at 37 °C in the dark. The proleg region of larvae was sanitized with ethanol 70% and a sterile swab before each application; the 10 μL Hamilton glass syringe to be used was washed with sodium hypochlorite, ethanol 70%, and 0.9% saline solution in this order. Then, 10 μL of the extract samples (2500 μg/mL), fractions (2500 μg/mL), microemulsions, and solvent, DMSO 2.5% (Sigma-Aldrich, St. Louis, MO, EUA) were injected in the last left proleg of each larva. The treated larvae and controls were incubated at 37 °C in the dark and were evaluated after 2, 4, 12, 24 h, and later every 24 h for 7 days for lack of movements, reaction after physical stimulation, and melanization. The tests were performed in triplicate, and the result corresponds to the mean of the evaluations.
The results obtained through these methods are presented below.
4. Discussion
There are few studies in the scientific literature that use leaves of P. cauliflora; the vast majority of studies with this plant species were performed with the fruits that are rich in phenolic compounds, or are agronomic studies aimed at improving fruit production. With the results obtained, it is possible to observe that the hydroethanolic extract 70% of the plant under study, P. cauliflora, has antimicrobial activity. The use of ethanol as an extracting solvent is appropriate because it is a solvent that is less aggressive to the environment, which is in line with the principles of green chemistry, since a greater amount of this solvent is used in the extraction compared to fractionations.
The yield of the EtOH70 extract was like that found previously [
14] with about 5% variation. In the same study, percentages of 28%, 41.69%, and 36.50% were found for the ethyl acetate, n-butanol, and aqueous fractions, respectively, while in the present study, we obtained 11.85%, 13.58%, and 69.03%. This yield difference in fractions may be related to the number of constituents that each solvent can solubilize from the extract, to the technical specificities used during extraction, and the edaphoclimatic conditions of each research. Data on the chemical constituents present in
P. cauliflora leaf extracts are still very limited in the scientific literature, with most studies focusing on the fruit. Previous studies developed by our research group demonstrated the presence of casuarinin [
14], myricetin, and a glycosylated quercetin [
15] in the extracts and fractions of
P. cauliflora leaves. In these available studies, casuarinin was detected in
P. cauliflora leaf extracts with retention times ranging from 25 to 30 min [
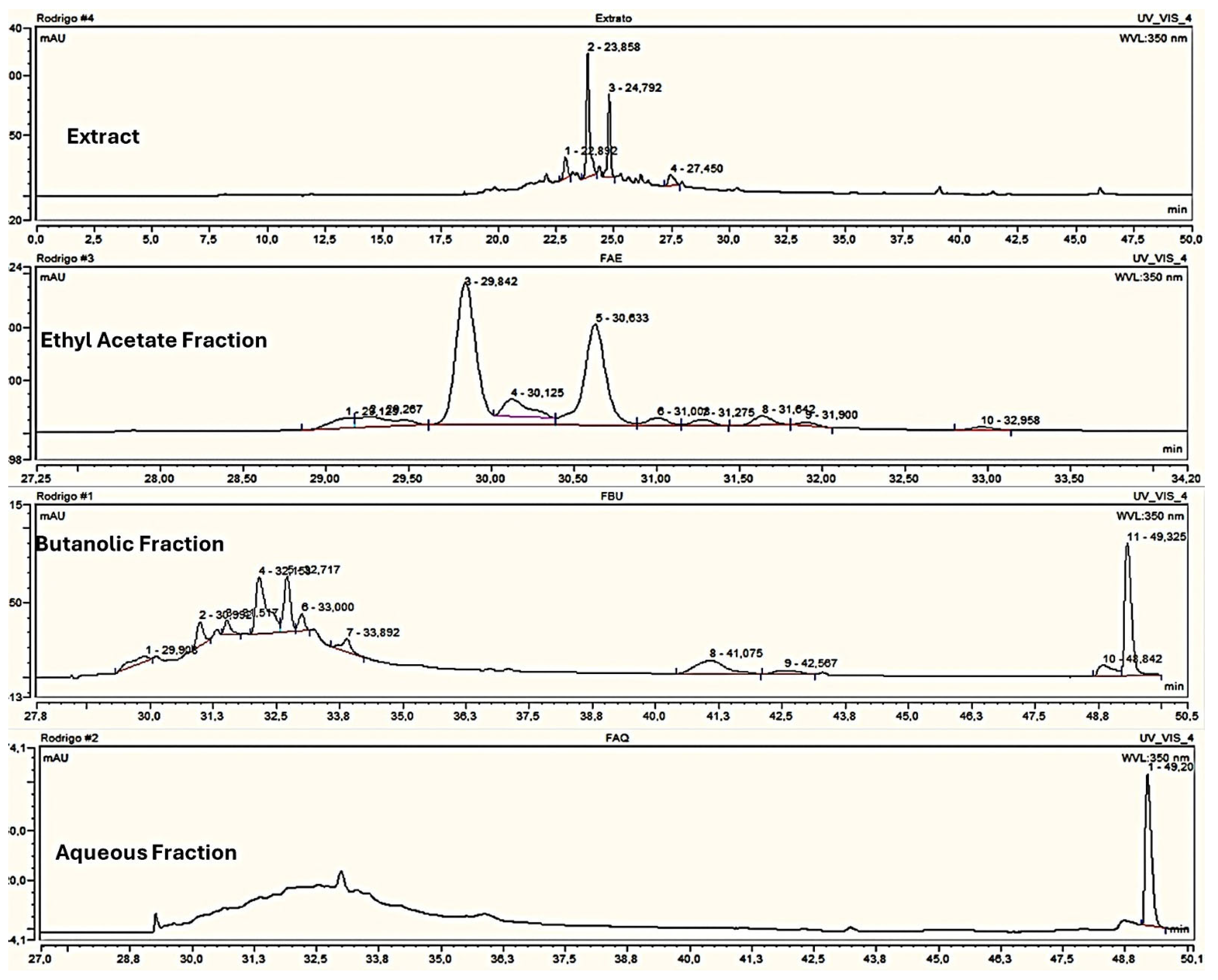
14]. Although the chromatographic conditions used are different from those of the present work, the presence of peaks within a similar retention time range in EtOH70 (peaks 2 and 3), FrAcOH (peaks 3 and 5), and even not being the major compound in FrBuOH (peaks 4 and 5) suggests the possible presence of casuarinin in these samples used in the present investigation.
The evaluation of the antimicrobial activity in this study showed that all strains evaluated for the yeast and the dermatophyte fungi presented some levels of susceptibilities for the extracts and fractions tested. However, the variations in the MICs found between the extracts and fractions were not very marked, which may demonstrate that the separation that occurred in the liquid–liquid partition was not fully efficient for the grouping of the compounds of this plant species. In the HPLC chromatograms, as shown in
Figure 1, it was possible to observe similarities in the retention time of the major peaks, mainly of the butanolic and aqueous fractions.
The trials with
C. glabrata were the ones that showed the best MIC results with 4.88 μg/mL for the extract and fractions, except for the butanolic fraction, which was 9.76 μg/mL. When comparing the data obtained in this study with another [
14], where
P. cauliflora was also used for other
Candida species, it was possible to observe MIC values for
C. albicans of 156 μg/mL, 625 μg/mL, 78 μg/mL, and 312 μg/mL for the ethanol extract, the ethyl acetate fraction, butanolic, and aqueous, respectively [
14]. Taken together, these results clearly demonstrate the capacity of the metabolites of this plant in the treatment of yeast infections.
For the dermatophyte fungus
T. rubrum, the samples tested showed antifungal activity for both the most and the less fluconazole-resistant strain. Another study [
15] using the same plant species demonstrated activity for
T. rubrum strain (78.12 μg/mL) for the ethanol extract and ethyl acetate fraction and for
M. canis (156.25 μg/mL) for the ethanol extract and ethyl acetate fraction. Again, the results show the potential of this plant against fungal species and new strategies can be combined to improve the antifungal activity found.
The extracts and fractions derived from
P. cauliflora exhibit a dark green color, and when diluted and microemulsions were prepared, they appeared light green and were fluid, making them suitable for incorporation into other delivery vehicles. When the incorporation of the extract and fractions occurred in a nanostructured lipid system, the improvement in antimicrobial action was desired and the development of extract microemulsions was able to promote improvement in the activity; for
T. rubrum 1, from 156.25 μg/mL to 78.12 μg/mL, about a two times reduction, and for
C. glabrata, from 4.88 μg/mL to 1.22 μg/mL, about a four times reduction, reaching the proposed objective. Thus, this increase in activity could be related to the presence of surfactants and the composition of the system that can interact with the target cells, enhancing their permeability and consequently, the flow of cations, anions, water, and other substances inside, promoting better capacity for the active compound to reach its target [
22], Also, evidence suggests that the antimicrobial activity of microemulsions arises from the disruption of microbial membranes, driven by energy transfer from the kinetically active microemulsion system and the action of membrane-disruptive surfactants. This interaction leads to membrane rupture, facilitating microemulsion entry and subsequent intracellular damage, ultimately causing cell death [
31].
The results for the EtOH70 extract are superior to other samples, especially when incorporated into the microemulsion; this may be explained by the fact that the extract is crude, without any fractionation, and thus contains all compounds present in the fractions. When this sample is incorporated into microemulsion, compounds that might otherwise have lower solubility may exhibit greater bioavailability, thereby enhancing their antifungal activity. Additionally, a synergistic effect between compounds with initially lower bioavailability may also occur.
The fungal strains used in this study, both
C. glabrata and
T. rubrum, present a large amount of lipids in their outer cell constitution. The nonpolar characteristic of some samples tested, such as the acetate fraction, may be indicative of the biological response obtained, since when in contact with membranes and cell walls, they seem to have the ability to generate channels or pores that destabilize the cells, causing leakage of their internal contents, leading to the death of the microorganism. A previous study also investigating natural products employed the same type of microemulsion for the incorporation of
Myrcia bella extracts and reported improved results against certain
Candida strains. The authors suggested that this enhancement was due to the presence of cholesterol in formulations, which would facilitate the permeability of the extract with a strong interaction with ergosterol present in the fungal plasma membrane. This study shows a fungistatic activity of 3.9 µg/mL for
C. glabrata, whereas in our study, the activity for the non-incorporated extract was slightly higher, at 4.88 µg/mL. In the referenced work, the incorporation into microemulsions did not enhance the antifungal activity, while in the present study, incorporating
P. cauliflora samples into the same type of nanostructured system significantly reduced the MIC and MFC values for both EtOH70 and FrBuOH, demonstrating a clear improvement in antifungal efficacy with the formulation [
32].
The study of antioxidant activity by reducing DPPH radical demonstrated better EC
50 for EtOH70 at the concentration of 25.60 μg/mL, remaining slightly higher for the fractions tested. Among the patterns used, the one with the best sequestering capacity was quercetin EC
50 = 5.94 μg/mL, followed by gallic acid with CE
50 = 6.62 μg/mL. Research work using extracts from twelve Myrtaceae species for their ability to capture DPPH free radicals and for the species
Eugenia klotzschiana found an EC
50 of 6.40 μg/mL, and for
Eugenia dysentherica, an EC
50 = 6.83 μg/mL. For the species
Eugenia bimarginata and
Myrcya splendens, the values were higher: 12.83 μg/mL and 12.48 μg/mL, respectively [
33], which demonstrates great variability in the antioxidant capacity of species of the same family. Thus, the substances under study are able to protect cells against the deleterious effects of free radicals produced by the body by delaying cell aging and can be used in the dermocosmetic sector. Toxicity assays with
G. melonella were chosen due to its increasing application as a reliable, cost-effective, easy-to-perform, and in vivo alternative model. The results showed that at the concentration used (2500 μg/mL), which was the same as the initial antimicrobial activity tests, all larvae remained healthy for seven days, when they turn to pupa, the next stage in the evolutionary cycle. One study, also using a Myrtaceae species called
Eugenia brasiliensis, popularly known as “grumixama”, also reported the same result with the use of this alternative model, failing to find the dose capable of killing 50% of the larvae [
34]. The relevance of the work is demonstrated by this new therapeutic possibility for the treatment of fungal infections, including those caused by fluconazole-resistant strains, which can be evidenced by the tests carried out with this substance against
T. rubrum 1, a clinical strain, and
C. glabrata, in which this resistance is pronounced. The main conclusions drawn from the Results and Discussion are summarized below.