Abstract
This study aims to compare nanoemulsions and conventional emulsions as delivery systems for plant oils. For this reason, the formulation approach was evaluated, followed by an assessment of physicochemical properties and stability. Six different compositions of emulsions and their respective nanoemulsions were prepared using combinations of solid lipids (beeswax or cocoa butter) with liquid lipids (olive, almond, or apricot oil). Their physicochemical characteristics and their colloidal stability over time were assessed using Dynamic Light Scattering or Static Light Scattering. The performance of samples on the skin was assessed by measuring their occlusion effect (F), while their hydrating effect was assessed on healthy volunteers. The nanoemulsions exhibited improved stability compared to the corresponding conventional emulsions of the same composition. However, all samples (emulsions or nanoemulsions) exhibited a satisfactory occlusive effect (F > 10), mainly at 6 h. In addition, all samples caused increased skin hydration by 10–20% one hour post-application. Nanoemulsions containing plant-origin oils showed better physicochemical stability compared to their corresponding emulsions. The in vivo assessment revealed no skin irritation caused by the samples. Nevertheless, subjective evaluations by volunteers unveil a preference for conventional emulsions, which were perceived as providing a more favorable skin texture, regardless of their composition.
1. Introduction
The skin, the largest organ of the body, plays a crucial role in maintaining homeostasis by regulating the bidirectional permeability of water and various molecules [1]. This function, known as the skin barrier, is primarily governed by the structure of lipid lamellae that occupy the extracellular space of the stratum corneum (SC) [2]. Impairments in this barrier, often observed in dermatological conditions such as eczema, psoriasis, and xerosis (skin dryness), have been linked to the depletion of structural lipids from SC lipid lamellae, leading to increased transepidermal water loss and compromised barrier integrity [3]. In response, advanced drug delivery systems have been developed to either restore a dysfunctional skin barrier or facilitate the penetration of active ingredients, making them widely applicable in both medical and cosmetic formulations [4]. A growing consumer preference for natural ingredients and minimal synthetic additives has driven the cosmetic industry to develop personal care products primarily composed of plant-derived components. Plant oils have gained popularity due to their bioactive lipid content, including sterols, phospholipids, triglycerides, and fatty acids, which contribute to their unique skin benefits. For instance, olive oil, rich in oleic acid (C18:1), has been shown to disrupt lipid organization, enhancing skin penetration [5]. Furthermore, studies suggest that plant oils modulate the SC barrier by altering the lateral conformation of intercellular lipids, though they do not significantly affect the deeper SC layers [6,7]. In addition, beeswax and cocoa butter are widely utilized as lipid carriers in cosmetic formulations due to their multifunctional properties and biocompatibility. Beeswax acts as a natural thickening agent and stabilizer, forming a protective occlusive barrier on the skin that reduces transepidermal water loss and enhances moisturization [8]. Furthermore, beeswax has been effectively used in nanostructured lipid carriers (NLCs) for the controlled release of active compounds such as vitamin E, confirming its role in stabilizing lipid-based delivery systems [9,10]. Cocoa butter, on the other hand, is rich in saturated and unsaturated fatty acids and antioxidants such as vitamin E, which support skin hydration, elasticity, and repair. Lipid-based formulations, particularly those incorporating natural butters and oils, have been shown to enhance epidermal regeneration and prevent water loss, highlighting their value in cosmeceuticals. Furthermore, beeswax and cocoa butter of natural origin align with the growing consumer demand for sustainable and clean-label cosmetics. Conventional emulsions have been widely used as delivery systems to enhance the absorption of lipophilic compounds through the skin. However, their application faces challenges such as limited penetration efficiency, stability issues, and potential skin irritation. The stratum corneum, acting as a formidable barrier, further restricts the penetration of many active compounds, prompting the exploration of more advanced systems such as nanoemulsions [11,12,13]. Nanoemulsions, a subcategory of emulsions, consist of oil droplets dispersed in an aqueous external phase with droplet sizes below 200 nm, typically produced by high-shear methods [14,15]. Their nanoscale dimensions impart distinct advantages over conventional emulsions, including enhanced thermodynamic stability, which prolongs shelf life by preventing destabilization phenomena such as flocculation, coalescence, creaming, or sedimentation. Additionally, their transparency, non-greasy texture, and improved spreadability—attributed to their lower viscosity—enhance user compliance. Due to their oil core, nanoemulsions can encapsulate and deliver larger quantities of lipophilic substances compared to liposomes, making them highly effective carriers in pharmaceuticals, cosmetics, and other chemical formulations. In cosmetic applications, nanoemulsions are particularly valued for their ability to reduce transepidermal water loss, reinforce the skin barrier, and regulate the penetration of active ingredients. These attributes make them well suited for skincare products such as sunscreens, moisturizers, and anti-aging formulations [2,3].
The aim of this study is to compare nanoemulsions and conventional emulsions as delivery systems for plant oils. Initially, the formulation approach was evaluated, followed by an assessment of basic physicochemical properties and stability. Furthermore, the in vitro occlusive effects of these formulations were investigated to determine their capacity to minimize transepidermal water loss and enhance skin barrier function. Finally, clinical evaluation and volunteer self-assessment were conducted to determine the efficacy of these formulations in improving skin hydration.
2. Materials and Methods
2.1. Materials
The lipid phase of all preparations consisted of a combination of either beeswax or Theobroma cacao (cocoa) seed butter as solid lipids and olive oil (INCI: Olea europaea fruit oil, Farmalabor, CHEMCO by Syndesmos, Acharnai, Greece), almond oil (INCI: Prunus amygdalus dulcis oil, CHEMCO by Syndesmos, Acharnai, Greece), or apricot kernel oil (INCI: Prunus armeniaca kernel oil, CHEMCO by Syndesmos, Acharnai, Greece) as liquid lipid. Emulmetik™ 900 (Lucas Meyer Cosmetics, Champlan, France, INCI: Lecithin), solutol® HS 15 (BASF, Ludwigshafen, Germany, INCI: 111 Macrogol (15)-hydroxystearate), were used as lipophilic and hydrophilic emulsifiers, respectively. Water for injection (WFI) (Fresenius Kabi Hellas S.A., Athens, Greece) was used as aqueous phase. All materials were of pharmaceutical or cosmetic grade.
2.2. Preparation of Conventional Emulsions (CEs) and Nanoemulsions (NEs)
For the experimental procedure, six samples of conventional emulsions (CEs) were prepared. Specifically, three samples of the CEs contained beeswax and a plant-origin oil (olive oil, almond oil, or apricot oil), while the three other samples contained cocoa butter instead of beeswax. Since the objective of this study was not to develop optimized final formulations but rather to compare the film-forming and occlusive properties of conventional emulsions (CEs) and nanoemulsions (NEs) composed of the same lipid and emulsifier components, formulation development was guided by empirical knowledge, preliminary experiments, and literature precedence rather than by statistical modeling approaches such as Design of Experiment (DoE). The composition of the oil phase of each preparation is shown in Table 1. The CEs were prepared by heating the two phases separately at 65–70 °C. The aqueous phase was added dropwise into the oil phase under stirring on a magnetic stirrer (level 4, for 20 min) at room temperature until a homogenous emulsion was prepared. All samples were kept at room temperature for 24 h to stabilize [16]. Three batches of each sample were prepared. To prepare the nanoemulsions (NEs), the corresponding CEs were subjected to ultrasonication using a VCX130 PB ultrasonic processor (Vibra Cell™, Sonics & Materials Inc., Newtown, CT, USA), operating at a fixed frequency of 20 kHz and amplitude of 85%. Sonication was performed in 3 consecutive 1 min cycles. Between each cycle, the samples were briefly vortexed and allowed to return to room temperature to prevent thermal degradation. After the final cycle, the nanoemulsions were allowed to stabilize for 24 h at RT. Each NE formulation was also prepared in triplicate.

Table 1.
Composition (%w/v) of the conventional emulsions (CEs) and nanoemulsions (NEs).
2.3. Physicochemical Characterization
2.3.1. Optical Microscopy Analysis
The emulsification of CEs was examined using an optical microscope (Leica Microsystems DMLB microscope, equipped with a DI DC300 camera, Wetzlar, Germany). A drop of each sample was diluted with 600 μL of WFI and mixed gently. Subsequently, one drop of the diluted emulsion was placed on a glass slide, covered with a coverslip, and observed under the microscope.
2.3.2. Droplet Size Distribution of Conventional Emulsions (CEs) via Static Light Scattering (SLS)
The mean droplet size of each CE was determined using a Mastersizer S (Malvern Panalytical Ltd., Malvern, UK) based on the principles of Static Light Scattering (SLS). Each sample (1 drop) was diluted with WFI, until the turbidity reached a range of 12–30%. The volume-weighted mean diameter (D[4,3]) and uniformity were recorded for sample characterization.
2.3.3. Hydrodynamic Diameter and Polydispersity of Nanoemulsions (NEs) via Dynamic Light Scattering (DLS)
The hydrodynamic diameter and polydispersity index (PDI) of the dispersed droplets were measured using a Zetasizer Nano-ZS (Malvern Panalytical Ltd., Malvern, UK) at 25 °C. The analysis was performed at a scattering angle of 90° in a folded capillary cell (DTS 1061), which was equilibrated at 293 K, with an accumulation time of 10 s. Each sample was diluted by adding 100 μL of the emulsion to 600 μL of WFI before measurement.
2.3.4. Determination of ζ-Potential of Nanoemulsions (NEs)
The ζ-potentials of the NEs were determined using a Zetasizer Nano-ZS (Malvern Panalytical Ltd., Malvern, UK) at 25 °C. Each sample was diluted with WFI, and the electrophoretic mobility was measured. The ζ-potential and the width of the distribution were recorded for characterization. The obtained values were used to calculate the ζ-potentials according to the Smoluchowski equation.
2.4. Stability Test of Conventional Emulsions (CEs) and Nanoemulsions (NEs)
2.4.1. Centrifugation Stability Test
Following preparation, all samples were subjected to centrifugation using a Hermle Z32HK centrifuge (Hermle Labortechnik GmbH, Wehingen, Germany) at 22 °C, 2000 rpm, for 30 min. After centrifugation, the physical condition of the samples was visually inspected for any signs of phase separation.
2.4.2. Storage Stability Under Various Conditions
To evaluate the effect of storage conditions on sample stability, formulations were stored at 25 °C, 4 °C, and 45 °C for 90 days. At predetermined time points (1, 8, 15, 22, 30, 60, and 90 days post-preparation), the droplet distribution of each sample was assessed. Specifically, the volume-weighted mean diameter (D[4,3]) was measured for CEs, while the mean size, PDI, and ζ-potential for NEs were measured following the previously described methods.
2.4.3. Accelerated Aging
To simulate long-term stability under stress conditions, all samples were subjected to accelerated aging through three consecutive heating–cooling cycles (heating at 45 °C and cooling at 25 °C for 24 h per cycle) using a constant climate chamber (Model: HPP260, Memmert GmbH + Co. KG, Schwabach, Germany). The droplet size distribution was analyzed at the beginning (Day 1) and end (Day 7) of this study. The droplet distribution of each sample was characterized by measuring D [4,3]. For CEs, the volume-weighted mean diameter (D[4,3]) was measured, while, for NEs, the mean size distribution and ζ-potential were determined as previously described.
2.5. Occlusive Effect Assessment
The occlusive effect of CEs and NEs was evaluated using an in vitro water evaporation test [8]. Glass beakers were filled with 40 mL of water and covered with Whatman® filter paper grade 1 (Cytiva, Kent, UK). A 500 μL sample of each CE or NE formulation (6% w/v lipid content) was evenly spread on the filter surface. As a reference, 500 μL of water was applied on the filter paper of a separate baker. Bakers were stored at 32 °C (skin surface temperature) for 48 h. Water loss through the filter paper was determined by weighing each baker at predetermined time points (0, 6, 24, and 48 h). The occlusion factor (F) was calculated using Equation (1):
where R represents the reference water loss and S represents the sample water loss. An F = 0 indicates no occlusive effect, while F = 100 denotes maximum occlusion [17].
F = [(R−S) × 100]/R,
2.6. Film-Forming Capacity Analysis
The film-forming capacity of each sample was assessed using scanning electron microscopy (SEM). A 500 μL sample of each formulation was spread onto Whatman® filter paper grade 1 (Cytiva, Kent, UK) and allowed to dry. The dried samples were sputter-coated with carbon using a JEOL JEE-4B carbon coater (JEOL Ltd., Tokyo, Japan). The coated samples were examined using a scanning electron microscope (JSM5600LV, JEOL Ltd., Tokyo, Japan) at an accelerating voltage of 25 kV to examine the surface morphology of the film [18].
2.7. Clinical Evaluation
A total of four female volunteers (aged 23 to 32 years) participated in this study after providing written informed consent. Volunteers with a history of skin disease were excluded. This study included individuals with all skin types (normal, dry, oily, and combination). Participants were instructed to avoid using any body cream 72 h prior to this study. The study protocol was reviewed by the Ethics Committee board of the University of Patras and complied with the principles of the Declaration of Helsinki. Ethical review and approval were waived for this study due to its noninvasive nature.
2.7.1. Assessment of Skin Moisturization
To ensure acclimatization, volunteers remained in the study room for at least 20 min before measurements, allowing the skin to adapt to the ambient temperature (25 ± 2 °C). Three 9 cm2 test sites were marked on the inner forearm of each participant. Each site was randomly assigned to receive either a CE, NE, or water (control) application. All formulations were applied at a dose of 2 mg/cm2 and gently massaged into the skin using a gloved hand. Prior to each measurement, the hydrolipidic film of the test site was removed using dry tissue paper. Skin hydration was measured using a skin analyzer (DPLITE, Davi & Cia, Barcelona, Spain) at the following time points: baseline (t0, before treatment), 1 h, and 2 h post-application [9,10].
2.7.2. Self-Assessment by Volunteers
To evaluate the subjective perception of the formulations, a self-assessment questionnaire was administered to volunteers following product application. Participants were first asked to evaluate the texture of their skin after applying the product, comparing the effects of the NEs and the CEs. Responses were categorized as good, moderate, or poor. Additionally, volunteers were instructed to indicate whether they observed an improvement in skin hydration, with response options limited to yes or no, ensuring a clear distinction in perceived efficacy. Finally, to assess product tolerance, participants were asked whether they experienced any irritation after use. The severity of irritation was recorded on a four-point scale: none, mild, moderate, or severe. This assessment aimed to provide insights into the perceived effectiveness of the formulations and their acceptability from a consumer perspective [9,10].
2.8. Statistical Analysis
Statistical analysis was performed to determine significant differences among mean values. A t-test (Microsoft Office 365 Excel version 2504, Redmond, WA, USA) was used for comparisons. The significance level was set at p < 0.05.
3. Results and Discussion
3.1. Physicochemical Characterization of Conventional Emulsions (CEs) and Nanoemulsions (NEs)
3.1.1. Assessment of Preparation Efficiency of Conventional Emulsions (CEs) and Nanoemulsions (NEs)
Six CEs and their corresponding NEs were prepared. The emulsification efficiency of CEs was evaluated using optical microscopy, which confirmed the formation of spherical particles in all samples (Figure 1). The successful preparation of NEs was ascertained by their optical properties, specifically their transparency and iridescence when viewed against a light source, indicating nanoscale droplet formation.
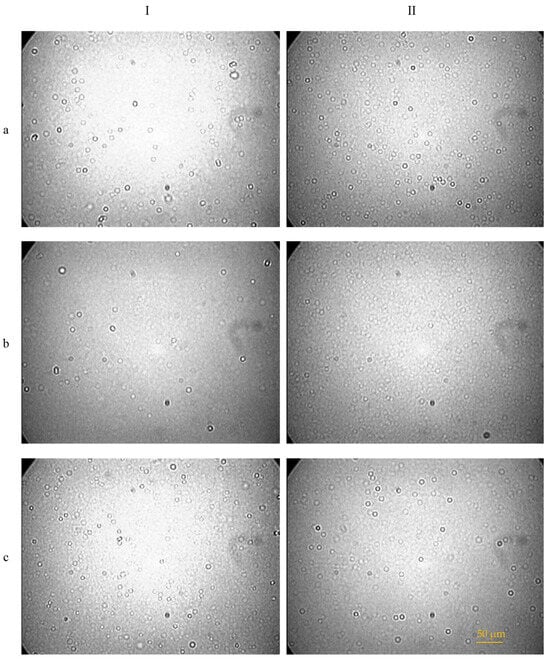
Figure 1.
Droplets of the dispersed phase of conventional emulsions (CEs) observed by optical microscope. Samples prepared with (I) beeswax or (II) cocoa butter and (a) olive oil, (b) almond oil, or (c) apricot oil, respectively.
3.1.2. Characterization of the Dispersed Phase of Conventional Emulsions (CEs)
The particle size of the dispersed phase of the CEs containing beeswax one day after preparation ranged from 8.02 ± 0.15 μm (Uniformity: 0.667 ± 0.098) for eWOL to 10.87 ± 0.85 μm (Uniformity 0.646 ± 0.099) for eWAL, depending on the oil with which they were combined (Figure 2, Table S1). However, when the beeswax was replaced by cocoa butter, the resultant particles had significantly smaller size (p < 0.05), ranging from 2.8 ± 0.4 μm (Uniformity: 0.922 ± 0.182) for eCOL to 4.4 ± 1.1 μm (Uniformity 1.360 ± 0.261) for eCARP (Figure 2, Table S2). The results showed that there were significant differences in droplet size and the PDI (p ≤ 0.05) among emulsions. According to previous reports, the formation of emulsions is primarily influenced by ingredient composition (oil, surfactant, water) [19] and their proportion ratio [20] and, notably, by manufacturing process factors [19], such as energy input and the duration of sonication [21,22]. The PDI values of conventional emulsions exhibited a wide distribution of particle size (more than 0.4), indicating that the samples were of low stability.
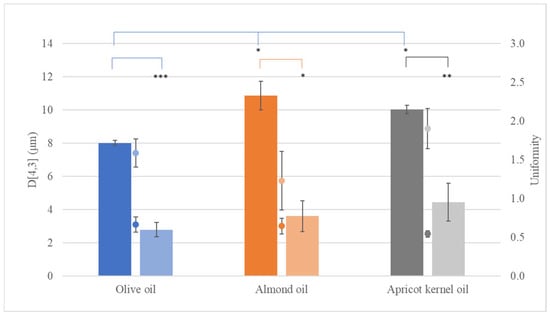
Figure 2.
The volume-weighted mean diameter (D[4,3], bar) and uniformity (dot) of the droplets of conventional emulsions (CEs) containing beeswax (dark hue) or cocoa butter (light hue) in combination with olive oil (blue), almond oil (orange), or apricot kernel oil (grey). *: p < 0.05, **: p < 0.01, ***: p < 0.005.
3.1.3. Characterization of the Dispersed Phase of Nanoemulsions (NEs)
The particle size of the dispersed phase of the NEs containing beeswax (after one day of preparation) ranged from 110 to 136 nm depending on the oil that it was combined with (Figure 3a, Table S3). In the present study, the factor solid lipid did not have a significant effect on droplet size and the PDI. Unlike CEs, when the wax was replaced by cocoa butter, the size of the dispersed phase of the NEs did not change significantly (Figure 3a, Table S4). The PDI of all samples never exceeded 0.3, indicating good stability of the colloid suspension [23], while the ζ-potential of the particles had high absolute values, indicating prolonged stability. The ζ potential refers to the electrokinetic potential difference between the dispersion medium and the stationary fluid layer surrounding a dispersed particle [24]. This parameter is crucial for predicting and managing nanoemulsion stability, as it reflects the extent of repulsion between similarly charged particles in a dispersion [24]. A high ζ-potential value (greater than +30 or less than −30) is associated with the improved stability of dispersed particles [25]. The ζ-potential values for all NE samples ranged around −60 mV (Figure 3b, Tables S3 and S4), indicating that all nanoemulsions prepared here were colloidally stable.
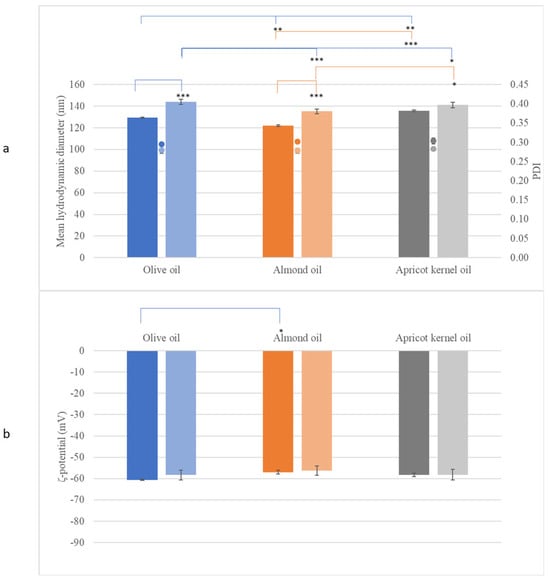
Figure 3.
The characterization of the dispersed phase of the NEs containing beeswax (dark hue) or cocoa butter (light hue) in combination with olive oil, almond oil, or apricot kernel oil, showing (a) the mean hydrodynamic diameter (bar), the PDI (dot), and (b) the ζ-potential. *: p < 0.05, **: p < 0.01, ***: p < 0.005.
3.2. Stability of Conventional Emulsions (CEs) and Nanoemulsions (NEs)
3.2.1. Stability of Conventional Emulsions (CEs)
CEs did not show a phase separation tendency after the centrifugation process, indicating good long-term stability. The stability of CEs was also evaluated by monitoring the droplet size (D[4,3]) and uniformity over time under different storage conditions (Figure 4, Tables S1 and S2). At 4 °C and 25 °C, the droplet size of eWOL significantly decreased from 8.02 ± 0.15 µm at 1 day to 7.48 ± 0.24 µm at 22 days (p < 0.05), while eCAPR showed a reduction from 4.4 ± 1.1 µm at 1 day to 2.2 ± 1.0 µm (p < 0.05) at 90 days (Figure 4Ia,Ib). The formulations containing cocoa butter (eCOL, eCAL, and eCAPR) remained relatively stable (p > 0.05) until 22 days, but, by 30 days, their droplet sizes exhibited significant changes (p < 0.05) (Figure 4IIa,IIb).
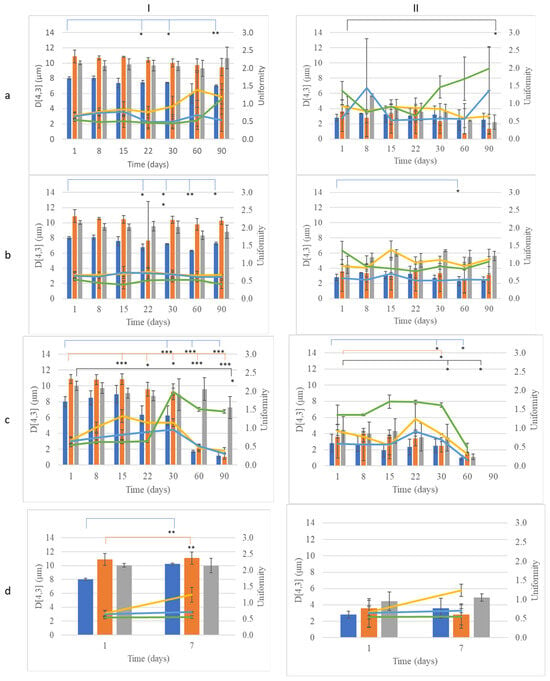
Figure 4.
Monitoring of size (D[4,3], bar) and uniformity (line) of conventional emulsions (CEs) with (I) beeswax or (II) cocoa butter and olive oil (blue bar, blue line), almond oil (orange, yellow line), apricot oil (gray bar, green line) over time during storage at (a) 4 °C, (b) 25 °C, (c) 45 °C, and (d) accelerating aging. *: p < 0.05, **: p < 0.01, ***: p < 0.005.
Under storage at 45 °C, the instability of CEs was more pronounced. The droplet size of eWOL decreased from 8.50 ± 0.87 µm (p < 0.05) at 8 days to 6.24 ± 1.23 µm (p < 0.05) at 30 days, while eWAL exhibited an even earlier reduction, dropping from 10.81 ± 0.63 µm (p < 0.05) at 8 days to 9.27 ± 0.98 µm (p < 0.05) at 30 days. The apricot kernel oil-based formulation (eWAPR) remained stable up to 60 days (p > 0.05) but showed a significant reduction in droplet size by 90 days (p < 0.05) (Figure 4Ic). Similarly, emulsions containing cocoa butter (eCOL, eCAL, and eCAPR) exhibited stable droplet sizes (p > 0.05) up to 22 days but underwent significant reductions by 30 days (p < 0.05) (Figure 4IIc). All CEs showed phase separation at 90 days, indicating that none of the conventional emulsions could maintain stability over prolonged exposure to high temperatures.
Following accelerated aging, the droplet size of nWOL increased from 8.02 ± 0.15 µm to 10.22 ± 0.12 µm (p < 0.05), while nWAL increased from 10.87 ± 0.85 µm to 11.07 ± 0.88 µm (p < 0.05), indicating potential destabilization in these formulations under stress conditions. However, all other CEs did not exhibit significant changes in size after accelerated aging (p > 0.05) (Figure 4Id,IId).
3.2.2. Stability of Nanoemulsions (NEs)
Similarly to CEs, the NE formulations have remained stable without any signs of phase separation following centrifugation, confirming their stability.
The stability of nanoemulsions was assessed based on their mean droplet size, polydispersity index (PDI), and ζ-potential over time (Figure 5 and Figure 6, Tables S3 and S4). At 25 °C, significant size changes were observed by 15 days for nWOL, nWAL, and nWAPR, with nWOL decreasing from 129.4 ± 0.3 nm at 1 day to 120.1 ± 2.6 nm (p < 0.05) at 15 days (Figure 5Ia). Similar reductions were noted for nCOL, nCAL, and nCAPR, with nCOL decreasing from 144.0 ± 2.3 nm at 1 day to 136.0 ± 1.9 nm (p < 0.05) at 15 days (Figure 5IIa). Despite these variations, all nanoemulsions formulated with beeswax (W) maintained a droplet size of approximately 100–110 nm at 90 days, whereas those formulated with cocoa butter (C) ranged from 116 to 125 nm at the same time point. The ζ-potential of all nanoemulsions remained relatively stable (p > 0.05) over 90 days, with nWOL fluctuating between −60.6 ± 1.9 mV and −59.0 ± 2.3 mV and nCAL between −57.4 ± 1.1 mV and −61.0 ± 7.3 mV, indicating no significant reduction in electrostatic stabilization (Figure 5IIa and Figure 6Ia).
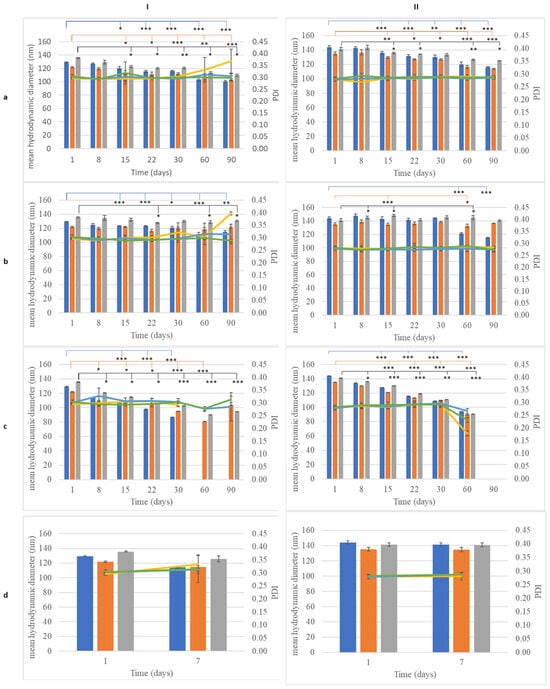
Figure 5.
Monitoring of size (bar) and PdI (line) of nanoemulsions (NEs) with (I) beeswax or (II) cocoa butter and olive oil (blue), almond oil (orange), apricot oil (gray) over time during storage at (a) 4 °C, (b) 25 °C, (c) 45 °C, and (d) accelerating aging. *: p < 0.05, **: p < 0.01, ***: p < 0.005.
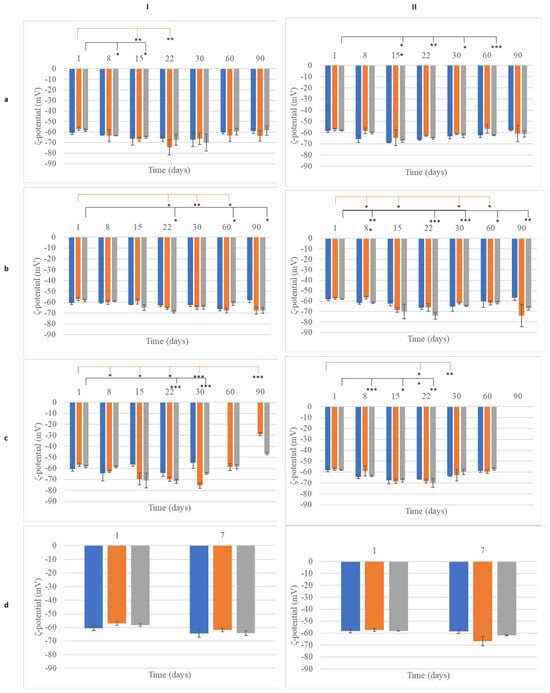
Figure 6.
Monitoring of ζ-potential of nanoemulsions (NEs) with (I) beeswax or (II) cocoa butter and olive oil (blue), almond oil (orange), apricot oil (gray) over time during storage at (a) 4 °C, (b) 25 °C, (c) 45 °C, and (d) accelerating aging. *: p < 0.05, **: p < 0.01, ***: p < 0.005.
At 4 °C, most nanoemulsions exhibited good stability over time. nCOL showed significant size changes (p < 0.05) at 15 days, while nWAL remained unchanged (p > 0.05) throughout the study period. The formulation containing apricot kernel oil, nWAPR, exhibited small but statistically significant (p < 0.05) variations in droplet size over time (Figure 5Ib). Cocoa butter-based nanoemulsions showed varying stability, with nCOL droplet size significantly changing at 60 days, nCAL at 15 days, and nCAPR as early as 8 days (Figure 5IIb). By 90 days, the absolute ζ-potential values of nWAPR and nCAPR were significantly increased (p < 0.05), suggesting enhanced electrostatic stabilization, while all other formulations maintained stable ζ-potential values (p > 0.05, Figure 6b).
At 45 °C, the instability of nanoemulsions became more evident. nWOL and nCOL showed significant droplet size changes (p < 0.05) by 15 days, followed by phase separation at 60 days (Figure 5Ic,IIc). nWAL, nWAPR, and nCAPR exhibited significant size variations (p < 0.05) as early as 8 days, indicating early instability at high temperatures. Notably, all cocoa butter-based nanoemulsions underwent phase separation by 90 days, highlighting the limitations of cocoa butter as a stabilizing component under prolonged thermal stress. Following accelerated aging, all formulations remained stable, maintaining their nanoscale droplet size and electrostatic stability (p > 0.05) (Figure 5Id,IId). Overall, these results suggest that nanoemulsions demonstrated superior stability compared to conventional emulsions, particularly at 4 °C and 25 °C. However, at elevated temperatures (45 °C), both beeswax- and cocoa butter-based nanoemulsions exhibited substantial instability, with phase separation occurring by 90 days. Despite minor fluctuations in droplet size, the ζ-potential values remained relatively stable for most nanoemulsions, indicating that electrostatic stabilization was maintained over time.
Of note, nanoemulsions exhibit greater stability than conventional emulsions at refrigerated and room temperatures primarily due to their small droplet size (typically 20–200 nm), which promotes Brownian motion and minimizes gravitational separation, such as creaming or sedimentation [26,27]. Their high surface area enhances the effectiveness of surfactants, forming strong interfacial layers that prevent coalescence, while additional steric and electrostatic stabilization further maintains droplet separation [28]. However, at elevated temperatures (45 °C), their stability decreases due to increased kinetic energy that intensifies droplet collisions, the degradation or desorption of surfactants from the droplet surface, and accelerated Ostwald ripening—a process where smaller droplets dissolve and larger ones grow, driven by higher solubility and diffusion rates at elevated temperatures [29].
3.3. Evaluation of the Efficacy of the Samples
3.3.1. In Vitro Occlusive Effect and Film-Forming Capacity
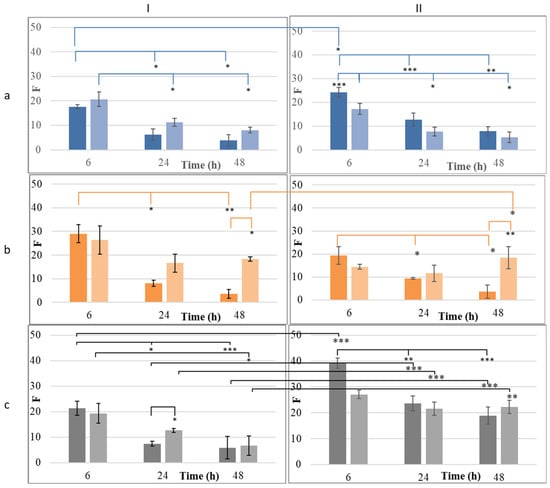
Figure 7.
The occlusive index (F) of conventional emulsions (CE, dark hue) and their corresponding nanoemulsions (NE, light hue) prepared by combining (I) beeswax or (II) cocoa butter with (a) olive oil, (b) almond oil, or (c) apricot kernel oil. *: p < 0.05, **: p < 0.01, ***: p < 0.005.
Olive oil formulations, when combined with beeswax, exhibited moderate occlusion at 6 h (17.64 ± 0.76 for eWOL, 20.64 ± 2.96 for nWOL). However, this effect was not sustained, as F values significantly declined to near or below 10 at 24 and 48 h (p < 0.05) (Figure 7Ia). A similar trend was observed when olive oil was combined with cocoa butter. The CE (eCOL) exhibited significantly higher occlusion at 6 h (24.29 ± 2.03) compared to both its NE counterpart (nCOL: 17.22 ± 2.32, p < 0.05) and the CE formulated with beeswax (eWOL: 17.64 ± 0.76, p < 0.05). However, this occlusive effect also diminished significantly over time, with F values dropping below 10 at 24 and 48 h (p < 0.05) (Figure 7IIa). These findings indicate that, while olive oil provides a moderate occlusive effect influenced by the solid lipid used, it does not ensure long-term occlusion, regardless of whether it is formulated as a CE or NE. The mean particle size of the dispersed phase did not appear to affect the occlusion performance of olive oil-based emulsions.
On the other hand, emulsions containing almond oil, whether formulated with beeswax or cocoa butter, exhibited initial F values ranging from 14.41 to 29.05 at 6 h, with no significant differences between them. However, in CEs, the occlusion index significantly decreased at 24 h (eWAL: 8.03 ± 1.29, eCAL: 9.42 ± 0.35) and further at 48 h (eWAL: 3.64 ± 1.84, eCAL: 3.70 ± 2.89) compared to their initial values (p < 0.05) (Figure 7Ib,IIb). Conversely, the corresponding NEs maintained their initial F values (26.37 ± 5.92 for nWAL and 14.41 ± 1.08 for nCAL) up to 48 h without significant alterations (p > 0.05). At this time point, both NE formulations exhibited significantly higher occlusion indices (18.32 ± 1.00 for nWAL and 18.39 ± 4.76 for nCAL) compared to their respective CEs (p < 0.05). Additionally, at 48 h, the NE formulated with beeswax demonstrated significantly higher occlusion than that formulated with cocoa butter (p < 0.05).
When combined with beeswax, apricot kernel oil showed comparable occlusion for both CEs and NEs at 6 h (eWAPR: 21.33 ± 2.79, nWAPR: 19.34 ± 3.84) (Figure 7Ic). However, the occlusion index for the CE significantly decreased at 24 h (7.44 ± 0.96, p < 0.05), whereas the NE maintained similar values at this time point (12.67 ± 0.81). By 48 h, the occlusion effect of the NE had also significantly declined (6.72 ± 3.85, p < 0.05). In contrast, when apricot kernel oil was combined with cocoa butter, both CEs and NEs initially exhibited high occlusion indices (eCAPR: 39.19 ± 1.94, nCAPR: 27.06 ± 1.64, p > 0.05). However, only the NE formulation maintained its occlusion index unaltered until 48 h (p > 0.05), whereas the CE showed a significant decline over time (Figure 7IIc). The comparison of the influence of plant oil on F showed that, at 6 h, almond oil in a beeswax-based CE (eWAL) exhibited significantly higher occlusion than both olive oil (eWOL) and apricot kernel oil (eCAPR) (p < 0.05). However, this trend was not observed in the corresponding NE formulations. By 48 h, there was no significant difference in the occlusion index among the CEs (p > 0.05). However, for the NEs, the occlusion index of nWAL remained significantly higher than that of nWOL and nWARP (p < 0.05).
These findings suggest that the formation of nanoemulsions enhances the occlusive effect, particularly for formulations containing almond oil and apricot kernel oil. The sustained occlusion observed in these NEs may be attributed to their ability to form a more continuous and uniform lipid film on the skin surface. This conclusion is supported by the TEM images of the films formed on filter paper by eCAPR and nCAPR, which exhibited the highest and most sustained occlusion indices (Figure 8b and Figure 8c, respectively). The film formed by eCAPR shows incomplete coverage of the paper fibers, with visible gaps and irregularities, while the film formed by nCAPR appears denser, more uniform, and structurally intact. Additionally, previous studies demonstrated that nanoemulsions can enhance the occlusive effect, thereby improving skin hydration and facilitating the delivery of active ingredients [30,31,32]. It is worth noting that formulations combining beeswax with plant oils have been shown to exhibit improved structural integrity and functional properties, making them ideal for applications in several cosmetic formulations. Plant oils such as olive oil, almond oil, and apricot kernel oil are utilized in cosmetic formulations due to their rich profiles of mono- and polyunsaturated fatty acids. When plant oils are incorporated into formulations with lipid carriers such as cocoa butter and beeswax, these oils interact at both structural and functional levels, improving spreadability, softening the final product texture, as well as ensuring the controlled release of active ingredients [33,34,35].
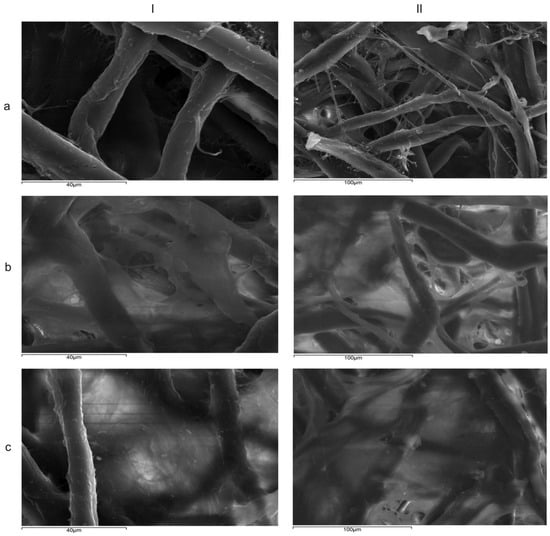
Figure 8.
SEM images showing (a) filter paper without lipids, (b) conventional emulsion with cocoa butter–apricot kernel oil, and with (eCAPR) (c) cocoa butter–apricot kernel oil nanoemulsion (nCAPR) at (I) 1200× and (II) 550×.
3.3.2. Hydration Effect
The skin hydration effect of conventional emulsions (CEs) and nanoemulsions (NEs) was evaluated over a period of 2 h following application. All formulations resulted in an increase in skin hydration at 1 h and 2 h post-application compared to baseline levels (Figure 9, Table S6). However, the hydration change (Δ hydration) at 2 h was significantly reduced for eWAL, eCAL, eCAPR, nCOL, and nCAL, compared to their values at 1 h (p < 0.05). Despite this decline, hydration levels at 2 h remained higher than the initial pre-application values. Notably, eCAPR did not show a significant decrease in Δ hydration at 2 h, as Δ hydration remained stable between 1 h (15.3 ± 6.2%) and 2 h (15.3 ± 5.9%).
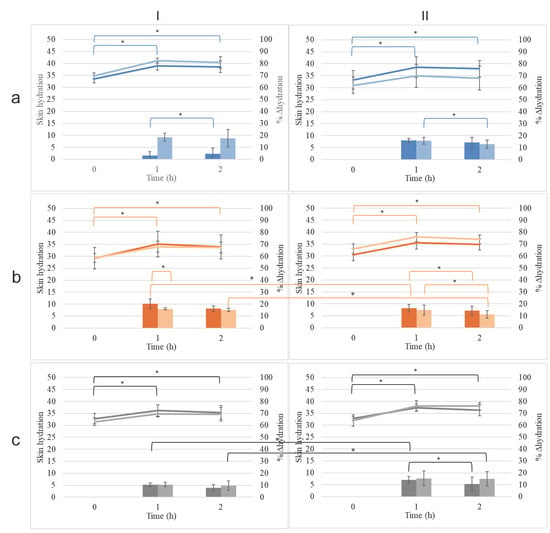
Figure 9.
The skin hydration effect of conventional emulsions (CE, dark hue) and their corresponding nanoemulsions (NE, light hue) prepared by combining (I) beeswax or (II) cocoa butter with (a) olive oil, (b) almond oil, and (c) apricot kernel oil. * p < 0.05.
The comparison between conventional emulsions and nanoemulsions revealed that nWAL exhibited significantly higher hydration at 1 h compared to eWAL (20.3 ± 3.9% vs. 16.0 ± 1.0%, respectively, p < 0.05). However, at 2 h, the Δ hydration levels between nWAL (16.3 ± 2.4%) and eWAL (15.0 ± 1.4%) were not significantly different (p > 0.05). No other nanoemulsion exhibited a statistically significant improvement in hydration compared to its corresponding conventional emulsion at either time point.
The choice of plant oil had a notable impact on skin hydration. At 1 h post-application, nWOL exhibited significantly lower Δ hydration than nWAL (16.5 ± 3.2% vs. 20.3 ± 3.9%, respectively, p < 0.05); however, at 2 h, their hydration levels were comparable (15.5 ± 4.7% vs. 16.3 ± 2.4%, p > 0.05). The opposite trend was observed among conventional emulsions, where eWOL showed higher hydration than eWAL at 1 h (18.5 ± 3.2% vs. 16.0 ± 1.0%, p < 0.05), but, by 2 h, their hydration levels were not significantly different (17.5 ± 7.4% vs. 15.0 ± 1.4%, p > 0.05).
Nanoemulsions and conventional emulsions formulated with olive oil or almond oil (nWOL, nWAL, eWOL, and eWAL) provided significantly higher Δ hydration compared to apricot kernel oil formulations (nWAPR and eWAPR) at both 1 h and 2 h post-application (p < 0.05). Additionally, nCAPR demonstrated significantly better Δ hydration than nCAL at 2 h (15.3 ± 5.9% vs. 10.0 ± 5.8%, respectively, p < 0.05). A similar trend was observed for conventional emulsions, with eCAPR exhibiting higher hydration than eCAL at 2 h (15.3 ± 6.2% vs. 11.8 ± 3.2%, p < 0.05).
The type of solid lipid (beeswax vs. cocoa butter) used in the formulation also influenced hydration retention. nWAL exhibited significantly greater Δ hydration than nCAL at 2 h (16.3 ± 2.4% vs. 14.0 ± 4.5%, p < 0.05), whereas eWAL showed superior Δ hydration compared to eCAL at 1 h (16.0 ± 1.0% vs. 14.8 ± 4.1%, p < 0.05). Furthermore, nCAPR provided significantly better Δ hydration than nWAPR at 2 h (15.3 ± 5.9% vs. 7.4 ± 2.5%, p < 0.05), and the same was observed for conventional emulsions, with eCAPR exhibiting superior Δ hydration compared to eWAPR at 2 h (15.3 ± 6.2% vs. 9.5 ± 3.9%, p < 0.05).
Overall, these results indicate that all formulations effectively increased skin hydration, though the extent and duration of the effect varied based on the type of oil and solid lipid used. Formulations containing olive oil and almond oil generally provided higher Δ hydration compared to apricot kernel oil formulations, aligning with findings from previous studies that demonstrated variations in moisturizing performance depending on oil composition [36]. Nanoemulsions did not consistently outperform conventional emulsions in hydration efficacy, except for nWAL, which exhibited superior hydration compared to eWAL at 1 h. This is consistent with studies highlighting that nanoemulsions, due to their smaller droplet size, can enhance penetration and hydration in certain cases, though their performance depends on formulation specifics [26,37]. Beeswax-based formulations generally exhibited better hydration retention than cocoa butter-based formulations at both 1 h and 2 h post-application, a trend supported by research emphasizing the role of solid lipids in hydration retention and occlusive properties [38]. These findings reinforce the importance of selecting appropriate oils and lipid carriers for optimizing skin hydration and formulation efficacy.
3.3.3. Self-Assessment of the Moisturizing Action on the Skin by Volunteers
The effectiveness of the tested formulations in improving skin hydration and texture was evaluated through a self-assessment questionnaire completed by volunteers. Overall, 8 out of 10 participants provided responses regarding the perceived moisturizing effect and skin texture following application (Figure 10, Table S7).
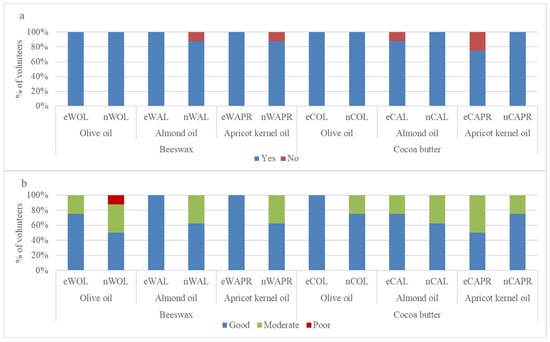
Figure 10.
Self-assessment of the improvement of (a) skin hydration and (b) skin texture after application of the samples.
Regarding improvement of skin hydration, all conventional emulsions (eWOL, eWAL, eWAPR, eCOL, and eCAPR) and most nanoemulsions (nWOL, nCOL, nCAL, and nCAPR) were reported to be 100% effective in improving skin hydration (Figure 9a). However, nWAL and nWAPR exhibited slightly lower ratings, with 88% of volunteers reporting improvement, while 12% did not perceive any change. Similarly, eCAL showed an 88% improvement rate, with 13% of volunteers reporting no effect.
The assessment of skin texture feel revealed differences between formulations (Figure 9b). For emulsions containing olive oil, eWOL was rated as “good” by 75% of volunteers, while only 50% rated nWOL as “good”, with the remaining participants rating it as “moderate” (37.5%) or “poor” (12.5%). Among cocoa butter-based formulations, eCOL received a 100% “good” rating, while nCOL was rated as “good” by 75% and “moderate” by 25% of volunteers.
For emulsions containing almond oil, eWAL was rated as “good” by all volunteers (100%), while nWAL received a lower rating, with only 62.5% describing the skin texture as “good” and 37.5% as “moderate”. A similar pattern was observed for cocoa butter-based formulations, where eCAL received a 75% “good” rating and 25% “moderate”, while nCAL had a lower rating, with 62.5% reporting “good” and 37.5% “moderate”.
For apricot kernel oil-based formulations, eWAPR received a 100% “good” rating, whereas nWAPR had a lower rating, with 62.5% reporting “good” and 37.5% reporting “moderate”. The cocoa butter-based formulation eCAPR received lower ratings compared to other emulsions, with only 50% rating it as “good” and 50% as “moderate”. However, nCAPR showed improved results, with 75% rating it as “good” and 25% as “moderate”.
Notwithstanding the limitation of the small sample size (number of subjects), these findings suggest that, while both conventional emulsions and nanoemulsions were perceived as effective in improving skin hydration, volunteers generally rated conventional emulsions higher in terms of skin texture feel, particularly for almond oil and apricot kernel oil formulations. This aligns with studies indicating that the selection of vegetable oils influences the sensory properties and consumer perception of emulsions [36,39]. Nanoemulsions showed slightly more variation in texture perception, with some formulations receiving ‘moderate’ or ‘poor’ ratings, particularly nWOL (12.5% poor) and nWAL (37.5% moderate). This variability in texture perception may be due to the smaller droplet size and different rheological properties of nanoemulsions, which have been shown to impact spreadability and feel on the skin [26,37]. Cocoa butter-based nanoemulsions, however, appeared to provide a more favorable skin feel compared to their conventional emulsion counterparts, as seen in nCAPR, which received higher ratings than eCAPR. This may be attributed to the enhanced occlusive effect of solid lipids like cocoa butter in nanoemulsions, as previous research has highlighted their role in improving texture perception and hydration retention [38].
4. Conclusions
This study highlights the potential of nanotechnology in formulating stable emulsions, particularly when incorporating beeswax or cocoa butter alongside plant-derived oils. Nanoemulsions containing plant-based oils exhibited superior physicochemical stability compared to their conventional emulsion counterparts, with enhanced stability observed when stored at room or fridge temperature. The occlusive and hydration effects of both conventional emulsions and nanoemulsions were evaluated to assess differences between these carrier systems and the influence of composition (olive oil, almond oil, or apricot oil). According to our results, all formulations demonstrated a comparable occlusive effect (F > 10), particularly at the 6 h mark, attributed to the ability of lipid nanoparticles to form a protective film on the skin. No significant differences were observed between the type of carrier system (CE or NE) or the specific plant oil used, as all formulations contributed to an increase in skin hydration levels ranging from 10% to 20%. However, subjective evaluations by volunteers indicated a preference for conventional emulsions, which were perceived as providing a more favorable skin texture, regardless of their composition.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cosmetics12030102/s1, Table S1: Stability study of conventional emulsions (CEs) formulated combining beeswax (W) and various oils (olive oil (OL), almond oil (AL), or apricot kernel oil (APR)), monitoring mean hydrodynamic diameter (mean size), polydispersity index (PDI), and ζ-potential under different storage conditions and after accelerated aging; Table S2: Stability study of conventional emulsions (CEs) formulated combining cocoa butter (C) and various oils (olive oil (OL), almond oil (AL) or apricot kernel oil (APR)), monitoring mean hydrodynamic diameter (mean size), polydispersity index (PDI), and ζ-potential under different storage conditions and after accelerated aging; Table S3: Stability study of NEs formulated combining beeswax (W) and various oils (olive oil (OL), almond oil (AL), or apricot kernel oil (APR)), monitoring mean hydrodynamic diameter (mean size), polydispersity index (PDI), and ζ-potential under different storage conditions and after accelerated aging; Table S4: Stability study of NEs formulated combining cocoa butter (C) and various oils (olive oil (OL), almond oil (AL), or apricot kernel oil (APR)), monitoring mean hydrodynamic diameter (mean size), polydispersity index (PDI), and ζ-potential under different storage conditions and after accelerated aging; Table S5: The occlusive index (F) of conventional emulsions (e) and their corresponding nanoemulsions (n) prepared by combining beeswax (W) or cocoa butter (C) with olive oil (OL), almond oil (AL), or apricot kernel oil (APR); Table S6: Skin hydration effect of conventional emulsions (e) and their corresponding nanoemulsions (n) prepared combining beeswax (W) or cocoa butter (C) with olive oil (OL), almond oil (AL), or apricot kernel oil (APR); and Table S7: Self-assessment of the improvement of skin hydration and skin texture feel after application of the samples.
Author Contributions
Conceptualization, S.H.; methodology, S.L.; formal analysis, A.L. and S.L.; investigation, A.L.; data curation, A.L., S.L., S.H. and K.A.; writing—original draft preparation, S.H. and S.L.; writing—review and editing, A.L. and K.A.; supervision, S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study protocol was reviewed by the Ethics Committee board of the University of Patras and complied with the principles of the Declaration of Helsinki. Ethical review and approval were waived for this study due to its noninvasive nature (the evaluation of healthy skin by cosmetic ingredients).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
Data available upon request.
Acknowledgments
Authors would like to thank Andreas Seferlis for performing SEM analysis in the Laboratory of Electron Microscopy and Microanalysis, University of Patras.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Walker, M. Human Skin through the Ages. Int. J. Pharm. 2022, 622, 121850. [Google Scholar] [CrossRef] [PubMed]
- van Smeden, J.; Bouwstra, J.A. Stratum Corneum Lipids: Their Role for the Skin Barrier Function in Healthy Subjects and Atopic Dermatitis Patients; Karger: Basel, Switzerland, 2016; Volume 49, pp. 8–26. [Google Scholar]
- Kendall, A.C.; Nicolaou, A. Topical Application of Lipids to Correct Abnormalities in the Epidermal Lipid Barrier. Br. J. Dermatol. 2022, 186, 764–765. [Google Scholar] [CrossRef] [PubMed]
- Raina, N.; Rani, R.; Thakur, V.K.; Gupta, M. New Insights in Topical Drug Delivery for Skin Disorders: From a Nanotechnological Perspective. ACS Omega 2023, 8, 19145–19167. [Google Scholar] [CrossRef] [PubMed]
- Čižinauskas, V.; Elie, N.; Brunelle, A.; Briedis, V. Skin Penetration Enhancement by Natural Oils for Dihydroquercetin Delivery. Molecules 2017, 22, 1536. [Google Scholar] [CrossRef]
- Vaughn, A.R.; Clark, A.K.; Sivamani, R.K.; Shi, V.Y. Natural Oils for Skin-Barrier Repair: Ancient Compounds Now Backed by Modern Science. Am. J. Clin. Dermatol. 2018, 19, 103–117. [Google Scholar] [CrossRef]
- Mack Correa, M.C.; Mao, G.; Saad, P.; Flach, C.R.; Mendelsohn, R.; Walters, R.M. Molecular Interactions of Plant Oil Components with Stratum Corneum Lipids Correlate with Clinical Measures of Skin Barrier Function. Exp. Dermatol. 2014, 23, 39–44. [Google Scholar] [CrossRef]
- Souza, C.; de Freitas, L.A.P.; Maia Campos, P.M.B.G. Topical Formulation Containing Beeswax-Based Nanoparticles Improved In Vivo Skin Barrier Function. AAPS PharmSciTech 2017, 18, 2505–2516. [Google Scholar] [CrossRef]
- Soleimanian, Y.; Goli, S.A.H.; Varshosaz, J.; Sahafi, S.M. Formulation and Characterization of Novel Nanostructured Lipid Carriers Made from Beeswax, Propolis Wax and Pomegranate Seed Oil. Food Chem. 2018, 244, 83–92. [Google Scholar] [CrossRef]
- de Souza, I.D.L.; Saez, V.; de Campos, V.E.B.; Nascimento, M.R.; Mansur, C.R.E. Multiple Response Optimization of Beeswax-Based Nanostructured Lipid Carriers for the Controlled Release of Vitamin E. J. Nanosci. Nanotechnol. 2020, 20, 31–41. [Google Scholar] [CrossRef]
- Otto, A.; du Plessis, J. The Effects of Emulsifiers and Emulsion Formulation Types on Dermal and Transdermal Drug Delivery. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2015; pp. 223–241. [Google Scholar]
- Iliopoulos, F.; Sil, B.C.; Evans, C.L. The Role of Excipients in Promoting Topical and Transdermal Delivery: Current Limitations and Future Perspectives. Front. Drug Deliv. 2022, 2, 1049848. [Google Scholar] [CrossRef]
- Ruela, A.L.M.; Perissinato, A.G.; Lino, M.E.d.S.; Mudrik, P.S.; Pereira, G.R. Evaluation of Skin Absorption of Drugs from Topical and Transdermal Formulations. Braz. J. Pharm. Sci. 2016, 52, 527–544. [Google Scholar] [CrossRef]
- Preeti; Sambhakar, S.; Malik, R.; Bhatia, S.; Al Harrasi, A.; Rani, C.; Saharan, R.; Kumar, S.; Geeta; Sehrawat, R. Nanoemulsion: An Emerging Novel Technology for Improving the Bioavailability of Drugs. Scientifica 2023, 2023, 6640103. [Google Scholar] [CrossRef]
- Mason, T.G.; Wilking, J.N.; Meleson, K.; Chang, C.B.; Graves, S.M. Nanoemulsions: Formation, Structure, and Physical Properties. J. Phys. Condens. Matter 2006, 18, R635–R666. [Google Scholar] [CrossRef]
- Florentino, A.C. Development of Babassu Oil Based Nanoemulsions. Lat. Am. J. Pharm. 2014, 34, 338–343. [Google Scholar]
- López-García, R.; Ganem-Rondero, A. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC): Occlusive Effect and Penetration Enhancement Ability. J. Cosmet. Dermatol. Sci. Appl. 2015, 5, 62–72. [Google Scholar] [CrossRef]
- Deli, G.; Hatziantoniou, S.; Nikas, Y.; Demetzos, C. Solid Lipid Nanoparticles and Nanoemulsions Containing Ceramides: Preparation and Physicochemical Characterization. J. Liposome Res. 2009, 19, 180–188. [Google Scholar] [CrossRef]
- Öztürk, B. Nanoemulsions for Food Fortification with Lipophilic Vitamins: Production Challenges, Stability, and Bioavailability. Eur. J. Lipid Sci. Technol. 2017, 119, 1500539. [Google Scholar] [CrossRef]
- Joung, H.J.; Choi, M.; Kim, J.T.; Park, S.H.; Park, H.J.; Shin, G.H. Development of Food-Grade Curcumin Nanoemulsion and Its Potential Application to Food Beverage System: Antioxidant Property and In Vitro Digestion. J. Food Sci. 2016, 81, N745–N753. [Google Scholar] [CrossRef]
- Mehmood, T.; Ahmad, A.; Ahmed, A.; Ahmed, Z. Optimization of Olive Oil Based O/W Nanoemulsions Prepared through Ultrasonic Homogenization: A Response Surface Methodology Approach. Food Chem. 2017, 229, 790–796. [Google Scholar] [CrossRef]
- Nourbehesht, N.; Shekarchizadeh, H.; Soltanizadeh, N. Investigation of Stability, Consistency, and Oil Oxidation of Emulsion Filled Gel Prepared by Inulin and Rice Bran Oil Using Ultrasonic Radiation. Ultrason. Sonochem. 2018, 42, 585–593. [Google Scholar] [CrossRef]
- Klang, V.; Valenta, C. Lecithin-Based Nanoemulsions. J. Drug Deliv. Sci. Technol. 2011, 21, 55–76. [Google Scholar] [CrossRef]
- Silva, H.D.; Cerqueira, M.Â.; Vicente, A.A. Erratum to: Nanoemulsions for Food Applications: Development and Characterization. Food Bioprocess Tech. 2014, 7, 306. [Google Scholar] [CrossRef]
- Heurtault, B. Physico-Chemical Stability of Colloidal Lipid Particles. Biomaterials 2003, 24, 4283–4300. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, Properties and Applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [PubMed]
- Solans, C.; Solé, I. Nano-Emulsions: Formation by Low-Energy Methods. Curr. Opin. Colloid Interface Sci. 2012, 17, 246–254. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus Microemulsions: Terminology, Differences, and Similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and Stability of Nano-Emulsions. Adv. Colloid. Interface Sci. 2004, 108, 303–318. [Google Scholar] [CrossRef]
- Agostinho, L.; Rocha-Filho, P. Preparation and Characterization of a Topical Delivery System for Nanoemulsions Using a Composite Film of Pectin and Tapioca. Cosmetics 2024, 11, 63. [Google Scholar] [CrossRef]
- Yang, M.; Gu, Y.; Yang, D.; Tang, X.; Liu, J. Development of Triptolide-Nanoemulsion Gels for Percutaneous Administration: Physicochemical, Transport, Pharmacokinetic and Pharmacodynamic Characteristics. J. Nanobiotechnol. 2017, 15, 88. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Potential of Nanoparticles as Permeation Enhancers and Targeted Delivery Options for Skin: Advantages and Disadvantages. Drug Des. Dev. Ther. 2020, 14, 3271–3289. [Google Scholar] [CrossRef]
- Blaak, J.; Staib, P. An Updated Review on Efficacy and Benefits of Sweet Almond, Evening Primrose and Jojoba Oils in Skin Care Applications. Int. J. Cosmet. Sci. 2022, 44, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Jerold, F. Biocosmetics: Technological Advances and Future Outlook. Environ. Sci. Pollut. Res. 2021, 30, 25148–25169. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, M.; Musa, A.; Alnusaire, T.S.; Shalaby, K.; Fouda, M.M.A.; Salama, A.; Al-Sanea, M.M.; Abdelgawad, M.A.; Gamal, M.; Fouad, S.A. Olive Oil/Pluronic Oleogels for Skin Delivery of Quercetin: In Vitro Characterization and Ex Vivo Skin Permeability. Polymers 2021, 13, 1808. [Google Scholar] [CrossRef] [PubMed]
- Sousa, G.; De Souza Dantas, I.; De Santana, D.; Leal, L. New Oils for Cosmetic O/W Emulsions: In Vitro/In Vivo Evaluation. Cosmetics 2018, 5, 6. [Google Scholar] [CrossRef]
- Somwongin, S.; Chaiyana, W. Clinical Efficacy in Skin Hydration and Reducing Wrinkles of Nanoemulsions Containing Macadamia Integrifolia Seed Oil. Nanomaterials 2024, 14, 724. [Google Scholar] [CrossRef]
- Pavlou, P.; Siamidi, A.; Varvaresou, A.; Vlachou, M. Skin Care Formulations and Lipid Carriers as Skin Moisturizing Agents. Cosmetics 2021, 8, 89. [Google Scholar] [CrossRef]
- Dănilă, E.; Moldovan, Z.; Albu Kaya, M.G.; Ghica, M.V. Formulation and Characterization of Some Oil in Water Cosmetic Emulsions Based on Collagen Hydrolysate and Vegetable Oils Mixtures. Pure Appl. Chem. 2019, 91, 1493–1507. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).