Molecular and Human In Vivo Study of an Innovative Plant-Derived Multifunctional Peptide Signaling the Collagen and Elastin Pathways and Melanin Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. In Vitro Tyrosinase Activity Assay
2.3. Cell Culture Condition
2.4. Cells Viability Assay
2.5. Cellular Melanin Extraction and Quantification
2.6. Enzyme-Linked Immunosorbent Assay (ELISA) Assays
2.7. Western Blot Analysis
2.8. Clinical Study
2.8.1. Chrono Control Penta Formulation
2.8.2. Study Design
2.9. Statistical Analysis
3. Results
3.1. Chrono Control Penta Description
3.2. Chrono Control Penta: Identification of the Mechanism of Action Using Biochemical and Cellular Techniques
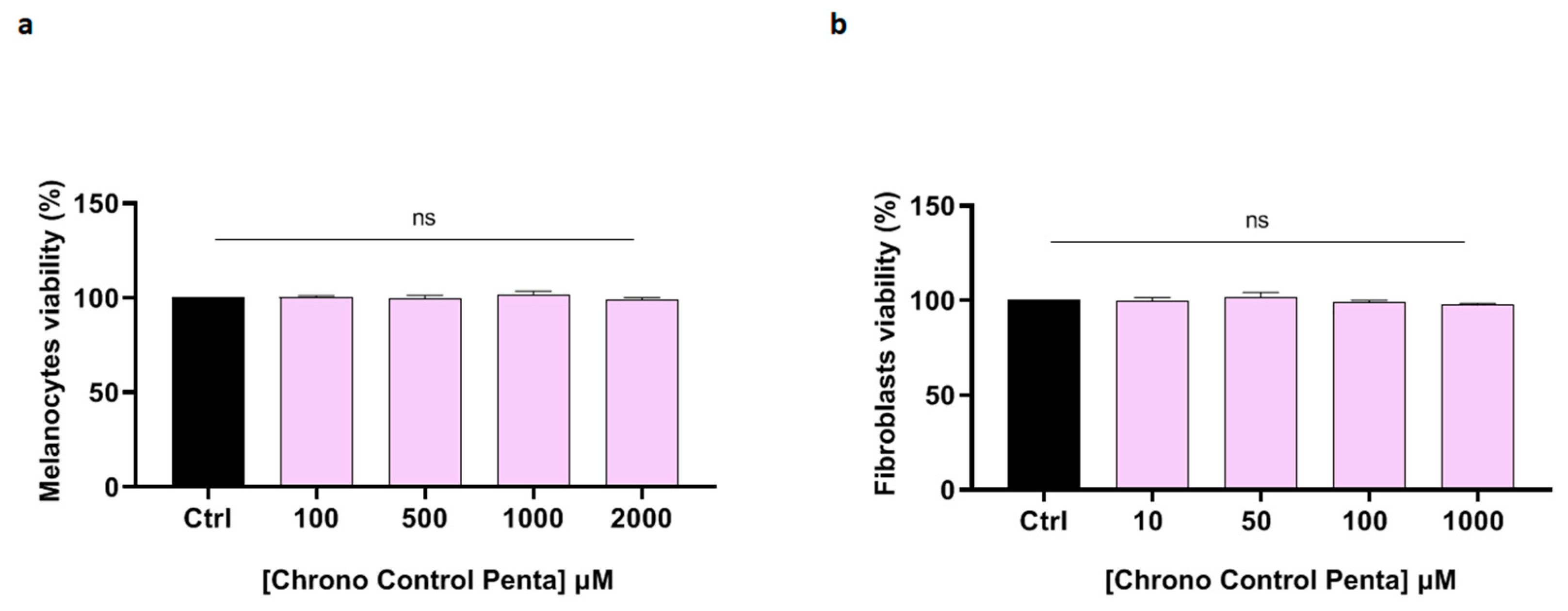
3.2.1. Evaluation of Chrono Control Penta Safety on Human Dermal Melanocytes and Human Dermal Fibroblasts
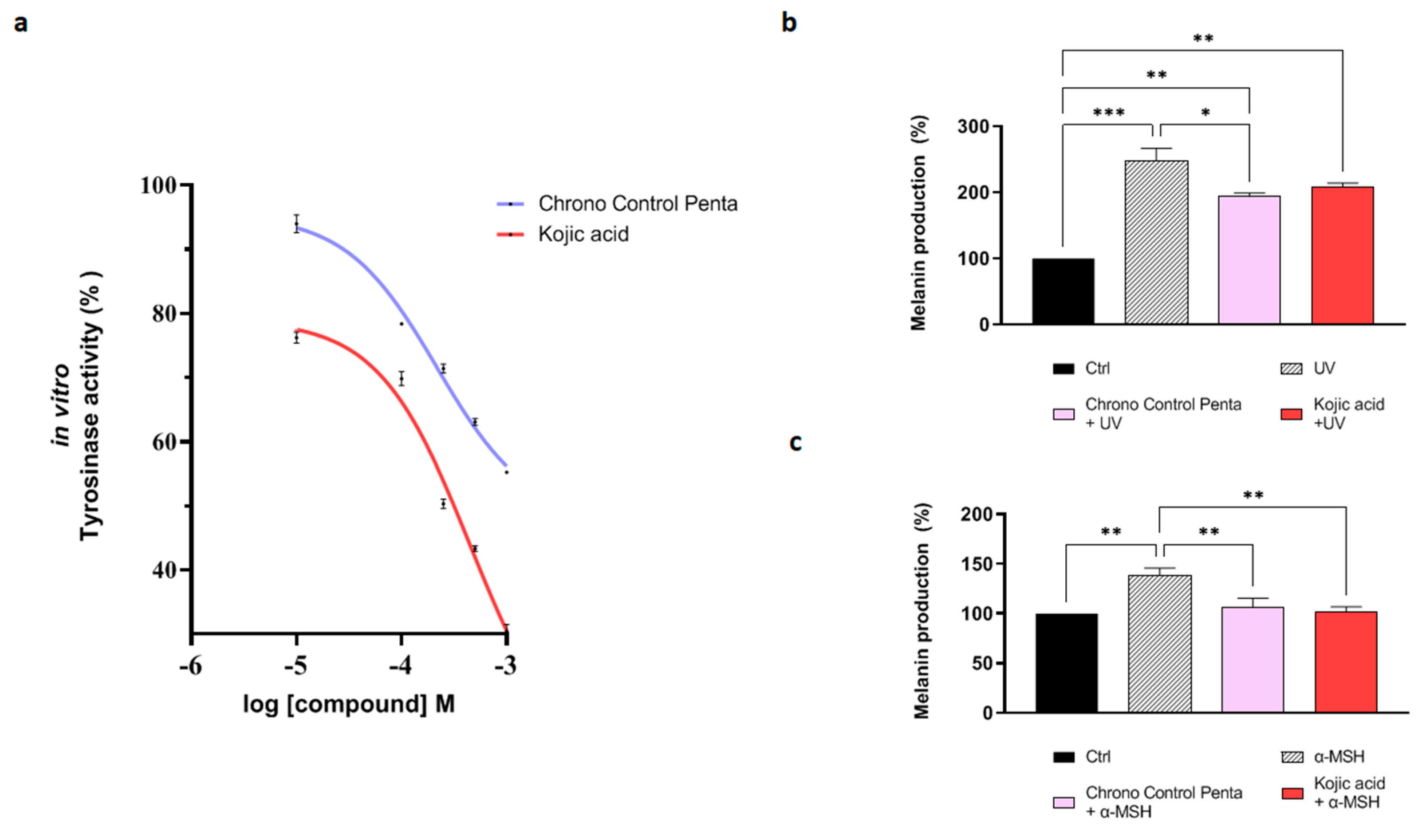
3.2.2. Chrono Control Penta Inhibits Tyrosinase Activity and Reduces Melanin Production in Melanocytes Exposed to UV and α-MSH
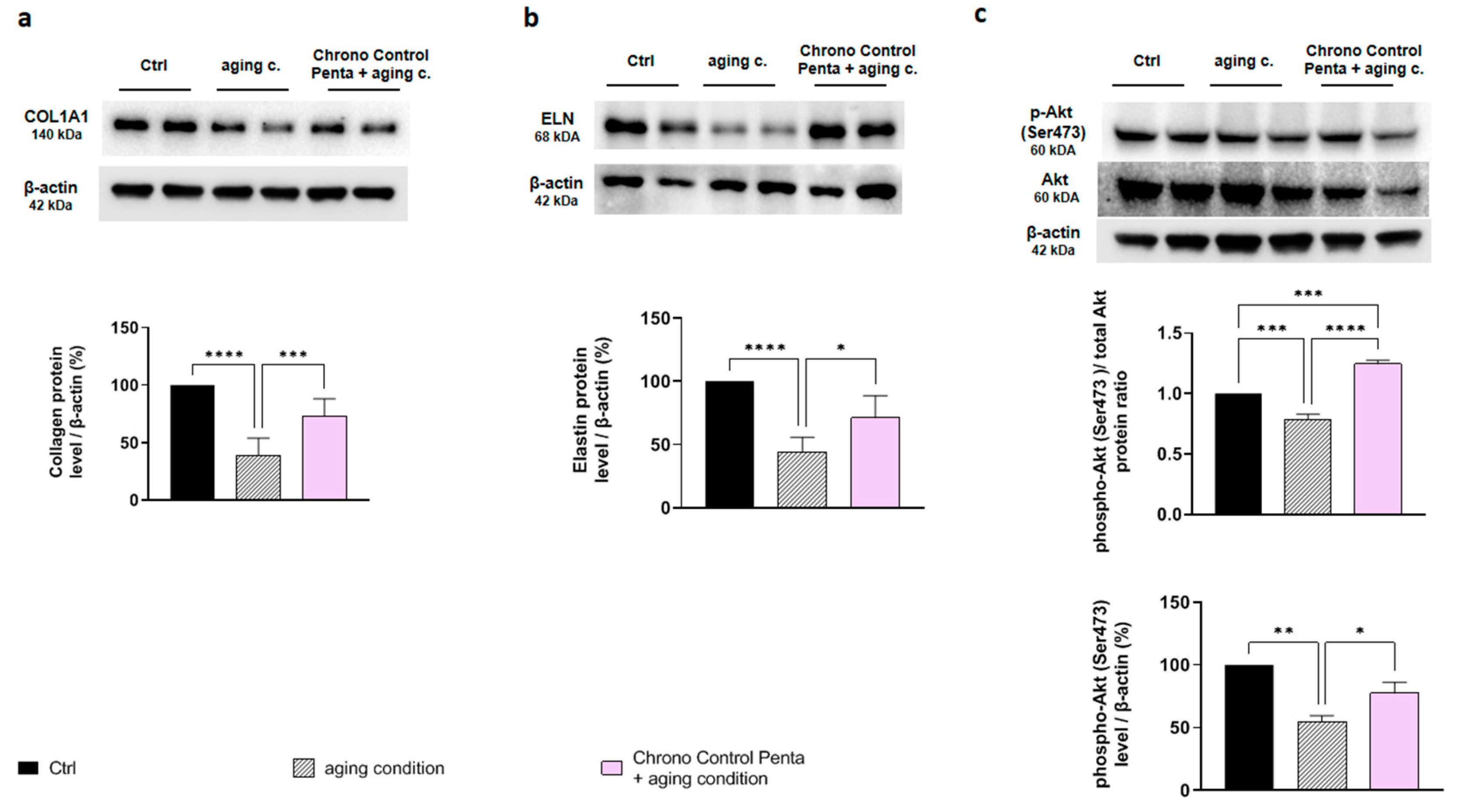
3.2.3. Chrono Control Penta Improves the Synthesis of Collagen and Elastin in Aged Human Dermal Fibroblasts via Akt Signaling Pathway Activation
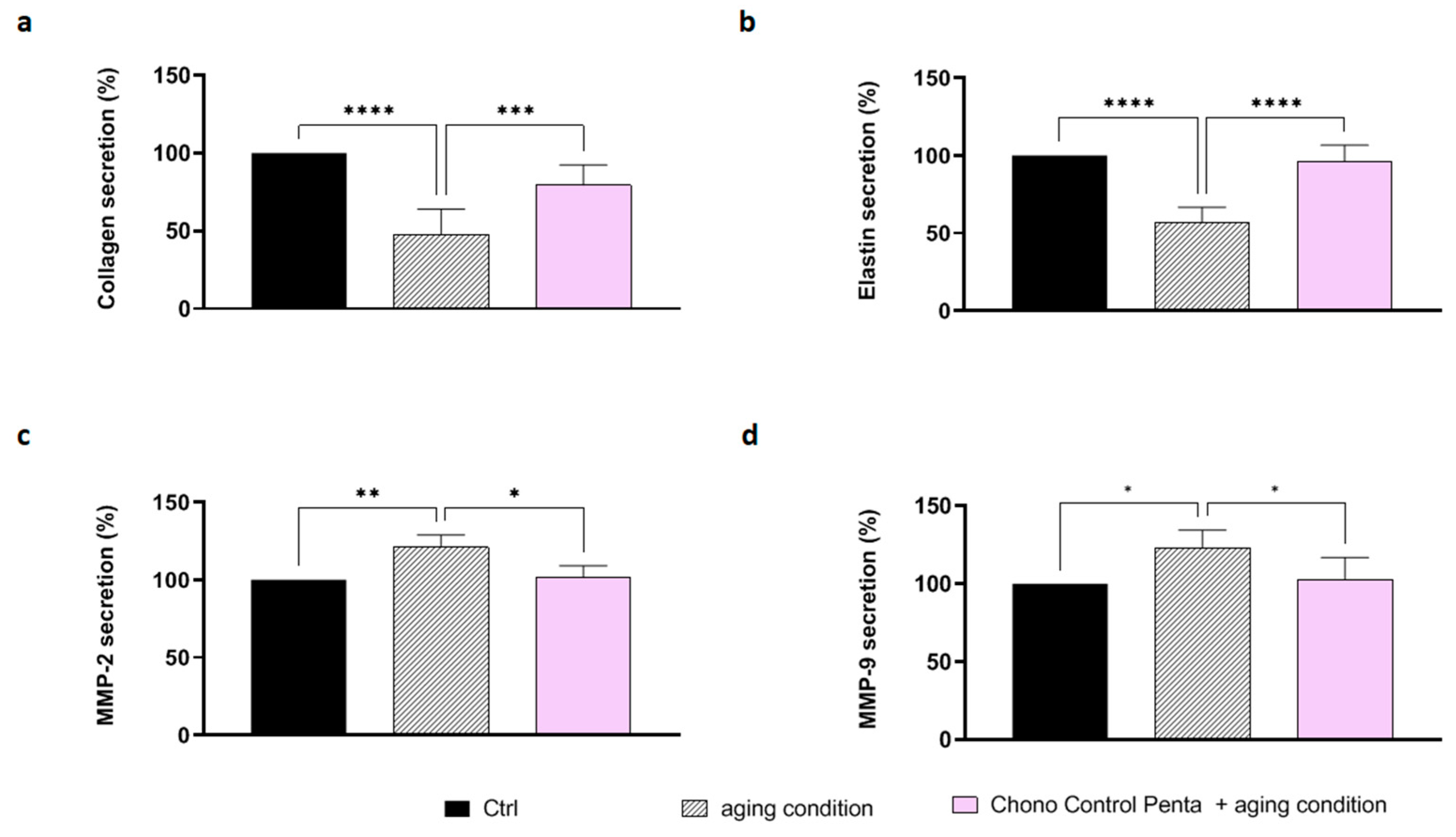
3.2.4. Chrono Control Penta Reduces the Secretion of Collagen, Elastin, and the Matrix Metalloproteinases MMP-2 and MMP-9 in Aged Stimulated Human Dermal Fibroblasts
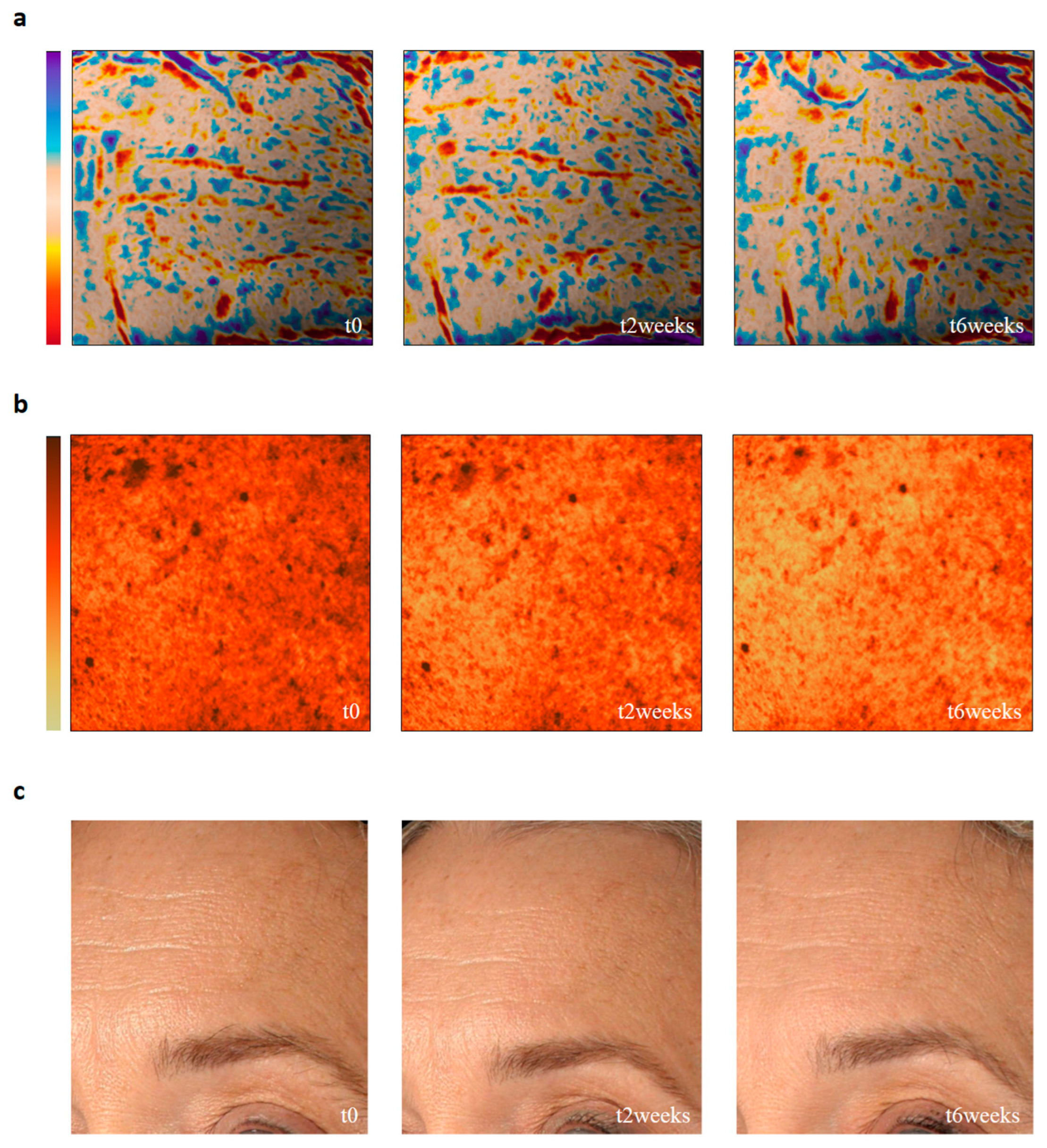
3.3. Clinical Safety and Efficacy Evaluation of Chrono Control Penta
3.4. Subjective Evaluation
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef]
- Purohit, K.; Reddy, N.; Sunna, A. Exploring the Potential of Bioactive Peptides: From Natural Sources to Therapeutics. Int. J. Mol. Sci. 2024, 25, 1391. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.K. Natural Products in Cosmetics. Nat. Prod. Bioprospect. 2022, 12, 40. [Google Scholar] [CrossRef]
- Ngoc, L.T.N.; Moon, J.Y.; Lee, Y.C. Antioxidants for Improved Skin Appearance: Intracellular Mechanism, Challenges and Future Strategies. Int. J. Cosmet. Sci. 2023, 45, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, A.K. Skin Aging & Modern Age Anti-Aging Strategies. Glob. J. Med. Res. 2019, 7, 209–240. [Google Scholar] [CrossRef]
- Schagen, S.K. Topical Peptide Treatments with Effective Anti-Aging Results. Cosmetics 2017, 4, 16. [Google Scholar] [CrossRef]
- Skibska, A.; Perlikowska, R. Signal Peptides—Promising Ingredients in Cosmetics. Curr. Protein Pept. Sci. 2021, 22, 716–728. [Google Scholar] [CrossRef]
- Ngoc, L.T.N.; Moon, J.Y.; Lee, Y.C. Insights into Bioactive Peptides in Cosmetics. Cosmetics 2023, 10, 111. [Google Scholar] [CrossRef]
- Pickart, L.; Vasquez-Soltero, J.M.; Margolina, A. GHK Peptide as a Natural Modulator of Multiple Cellular Pathways in Skin Regeneration. BioMed Res. Int. 2015, 2015, 648108. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Xiao, X.S.; Huo, J.; Zhang, W.D. The Anti-Wrinkle Efficacy of Argireline. J. Cosmet. Laser Ther. 2013, 15, 237–241. [Google Scholar] [CrossRef]
- Liyanaarachchi, G.D.; Samarasekera, J.K.R.R.; Mahanama, K.R.R.; Hemalal, K.D.P. Tyrosinase, Elastase, Hyaluronidase, Inhibitory and Antioxidant Activity of Sri Lankan Medicinal Plants for Novel Cosmeceuticals. Ind. Crops Prod. 2018, 111, 597–605. [Google Scholar] [CrossRef]
- d’Adduzio, L.; Fanzaga, M.; Capriotti, A.L.; Taglioni, E.; Boschin, G.; Laganà, A.; Rueller, L.; Robert, J.; van Gemmern, A.; Bollati, C.; et al. Ultrasonication Coupled to Enzymatic Hydrolysis of Soybean Okara Proteins for Producing Bioactive and Bioavailable Peptides. Curr. Res. Food Sci. 2024, 9, 100919. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.Y.; Kim, K.H.; Cheah, S.H. Inhibitory Effects of Sargassum Polycystum on Tyrosinase Activity and Melanin Formation in B16F10 Murine Melanoma Cells. J. Ethnopharmacol. 2011, 137, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Lammi, C.; Bollati, C.; Lecca, D.; Pia Abbracchio, M.; Arnoldi, A. Lupin Peptide T9 (GQEQSHQDEGVIVR) Modulates the Mutant PCSK9D374Y Pathway: In Vitro Characterization of Its Dual Hypocholesterolemic Behavior. Nutrients 2019, 11, 1665. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, Y.; Liu, J.; Zhang, W.; Sha, Y.; Zhan, Y.; Xiang, M. Mechanism of Akt Regulation of the Expression of Collagens and MMPs in Conjunctivochalasis. Exp. Eye Res. 2023, 226, 109313. [Google Scholar] [CrossRef] [PubMed]
- Żynda, W.; Ruczaj, A.; Galicka, A. Natural Compounds with Beneficial Effects on Skin Collagen Type I and Mechanisms of Their Action. Antioxidants 2025, 14, 389. [Google Scholar] [CrossRef]
- Rodan, K.; Fields, K.; Majewski, G.; Falla, T. Skincare Bootcamp: The Evolving Role of Skincare. Plast. Reconstr. Surg. Glob. Open 2016, 4, e1152. [Google Scholar] [CrossRef]
- Leffell, D.J.; Stetz, M.L.; Milstone, L.M.; Deckelbaum, L.I. In Vivo Fluorescence of Human Skin: A Potential Marker of Photoaging. Arch. Dermatol. 1988, 124, 1514–1518. [Google Scholar] [CrossRef]
- Gilchrest, B.A. A Review of Skin Ageing and Its Medical Therapy. Br. J. Dermatol. 1996, 135, 867–875. [Google Scholar] [CrossRef]
- Lintner, K.; Mas-Chamberlin, C.; Mondon, P.; Peschard, O.; Lamy, L. Cosmeceuticals and Active Ingredients. Clin. Dermatol. 2009, 27, 461–468. [Google Scholar] [CrossRef]
- Apone, F.; Barbulova, A.; Colucci, M.G. Plant and Microalgae Derived Peptides Are Advantageously Employed as Bioactive Compounds in Cosmetics. Front. Plant Sci. 2019, 10, 756. [Google Scholar] [CrossRef] [PubMed]
- Jack, A.R.; Norris, P.L.; Storrs, F.J. Allergic Contact Dermatitis to Plant Extracts in Cosmetics. Semin. Cutan. Med. Surg. 2013, 32, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, F.; Aróstica, M.; Román, T.; Beltrán, D.; Gauna, A.; Albericio, F.; Cárdenas, C. Peptides, Solid-Phase Synthesis and Characterization: Tailor-Made Methodologies. Electron. J. Biotechnol. 2023, 64, 27–33. [Google Scholar] [CrossRef]
- Freeling, F.; Björnsdotter, M.K. Assessing the Environmental Occurrence of the Anthropogenic Contaminant Trifluoroacetic Acid (TFA). Curr. Opin. Green Sustain. Chem. 2023, 41, 100807. [Google Scholar] [CrossRef]
- Arp, H.P.; Gredelj, A.; Glüge, J.; Scheringer, M.; Cousins, I.T. The Global Threat from the Irreversible Accumulation of Trifluoroacetic Acid (TFA). Environ. Sci. Technol. 2024, 58, 19925–19935. [Google Scholar] [CrossRef]
- Liao, Y.; Hung, M.-C. Physiological Regulation of Akt Activity and Stability. Am. J. Transl. Res. 2010, 2, 19. [Google Scholar]
- Liu, S.; Liu, S.; Wang, X.; Zhou, J.; Cao, Y.; Wang, F.; Duan, E. The PI3K-Akt Pathway Inhibits Senescence and Promotes Self-renewal of Human Skin-derived Precursors in Vitro. Aging Cell 2011, 10, 661–674. [Google Scholar] [CrossRef]
- Pratchyapurit, W.O. Combined Use of Two Formulations Containing Diacetyl Boldine, TGF-Β1 Biomimetic Oligopeptide-68 with Other Hypopigmenting/Exfoliating Agents and Sunscreen Provides Effective and Convenient Treatment for Facial Melasma. Either Is Equal to or Is Better Than. J. Cosmet. Dermatol. 2016, 15, 131–144. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Yi, E.-J.; Jin, X.; Zheng, Q.; Park, S.-J.; Yi, G.-S.; Yang, S.-J.; Yi, T.-H. Sustainable Dynamic Wrinkle Efficacy: Non-Invasive Peptides as the Future of Botox Alternatives. Cosmetics 2024, 11, 118. [Google Scholar] [CrossRef]
- Gorouhi, F.; Maibach, H.I. Role of Topical Peptides in Preventing or Treating Aged Skin. Int. J. Cosmet. Sci. 2009, 31, 327–345. [Google Scholar] [CrossRef]
| Time | Hydrating Effect 1 (ti-t0) | Trans- Epidermal Water Loss (tit0) | Skin Elasticity 2 (ti-t0) | Anti-Wrinkle Effect 3 (ti-t0) | Pigmentation Variation (ti-t0) | Purifying Effect (ti-t0) | Dermal Firmness (ti-t0) |
|---|---|---|---|---|---|---|---|
| t0 | - | - | - | - | - | ||
| t2weeks | +9.17% (p < 0.0001) | 0.38% (p > 0.05) | −3.71% (p < 0.05) | −8.38% (p < 0.01) | −4.98% (p < 0.0001) | −9.38% (p < 0.0001) | −3.72% (p < 0.01) |
| t6weeks | +13.05% (p < 0.0001) | 12.33% (p > 0.05) | −9.86% (p < 0.0001) | −11.55% (p < 0.001) | −5.63% (p < 0.0001) | −12.45% (p < 0.0001) | −6.35% (p < 0.0001) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bollati, C.; Fanzaga, M.; d’Adduzio, L.; Lammi, C. Molecular and Human In Vivo Study of an Innovative Plant-Derived Multifunctional Peptide Signaling the Collagen and Elastin Pathways and Melanin Production. Cosmetics 2025, 12, 100. https://doi.org/10.3390/cosmetics12030100
Bollati C, Fanzaga M, d’Adduzio L, Lammi C. Molecular and Human In Vivo Study of an Innovative Plant-Derived Multifunctional Peptide Signaling the Collagen and Elastin Pathways and Melanin Production. Cosmetics. 2025; 12(3):100. https://doi.org/10.3390/cosmetics12030100
Chicago/Turabian StyleBollati, Carlotta, Melissa Fanzaga, Lorenza d’Adduzio, and Carmen Lammi. 2025. "Molecular and Human In Vivo Study of an Innovative Plant-Derived Multifunctional Peptide Signaling the Collagen and Elastin Pathways and Melanin Production" Cosmetics 12, no. 3: 100. https://doi.org/10.3390/cosmetics12030100
APA StyleBollati, C., Fanzaga, M., d’Adduzio, L., & Lammi, C. (2025). Molecular and Human In Vivo Study of an Innovative Plant-Derived Multifunctional Peptide Signaling the Collagen and Elastin Pathways and Melanin Production. Cosmetics, 12(3), 100. https://doi.org/10.3390/cosmetics12030100









