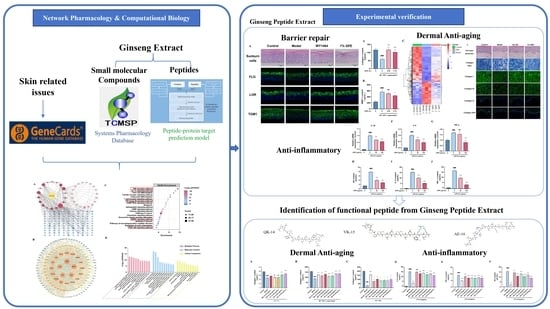
Systematic Evaluation and Identification of Anti-Inflammatory and Anti-Aging Ginseng Peptides for Skincare Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Network Pharmacology
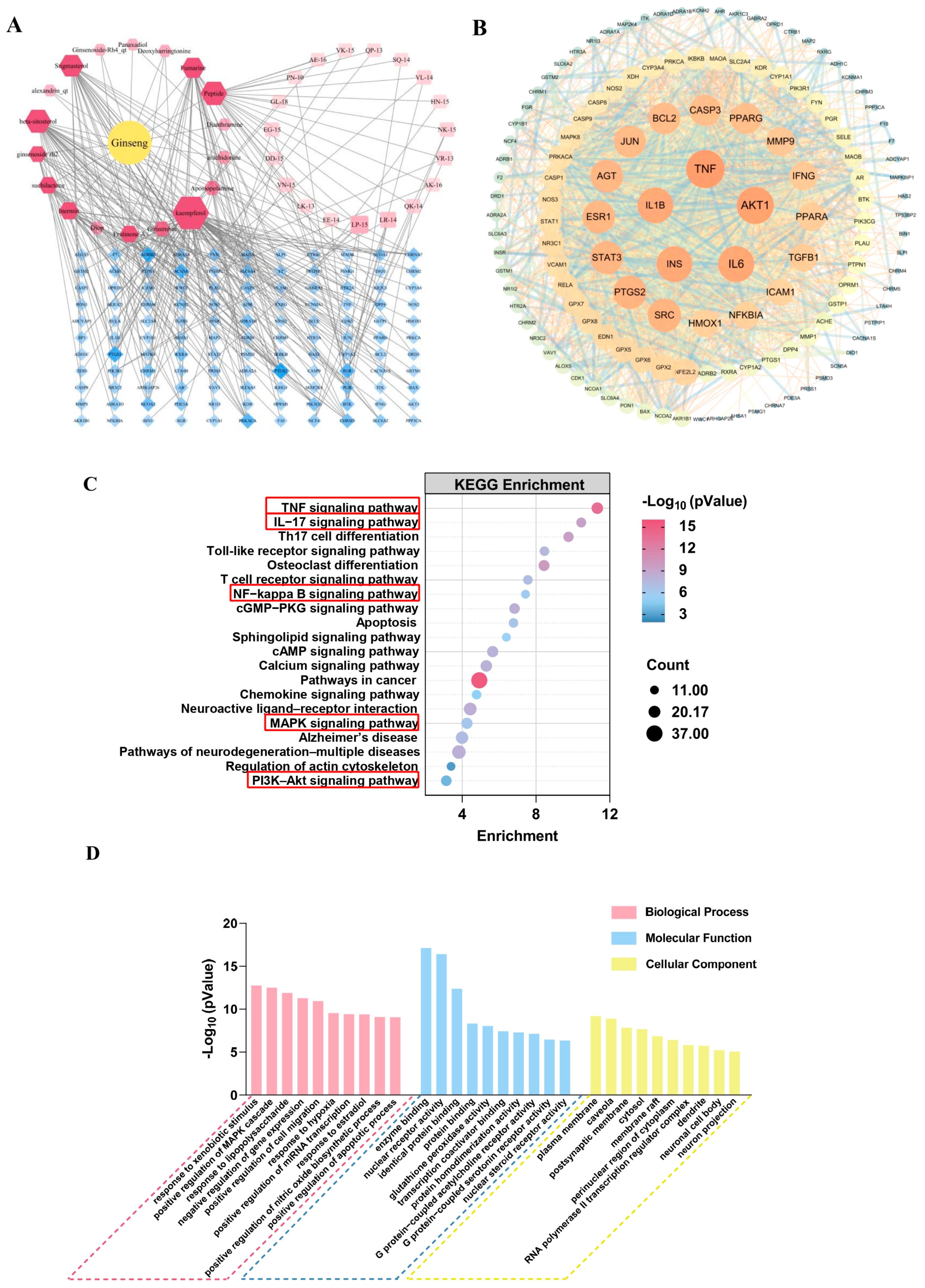
2.1.1. Screening Targets of Ginseng Compounds
2.1.2. Screening of GPs
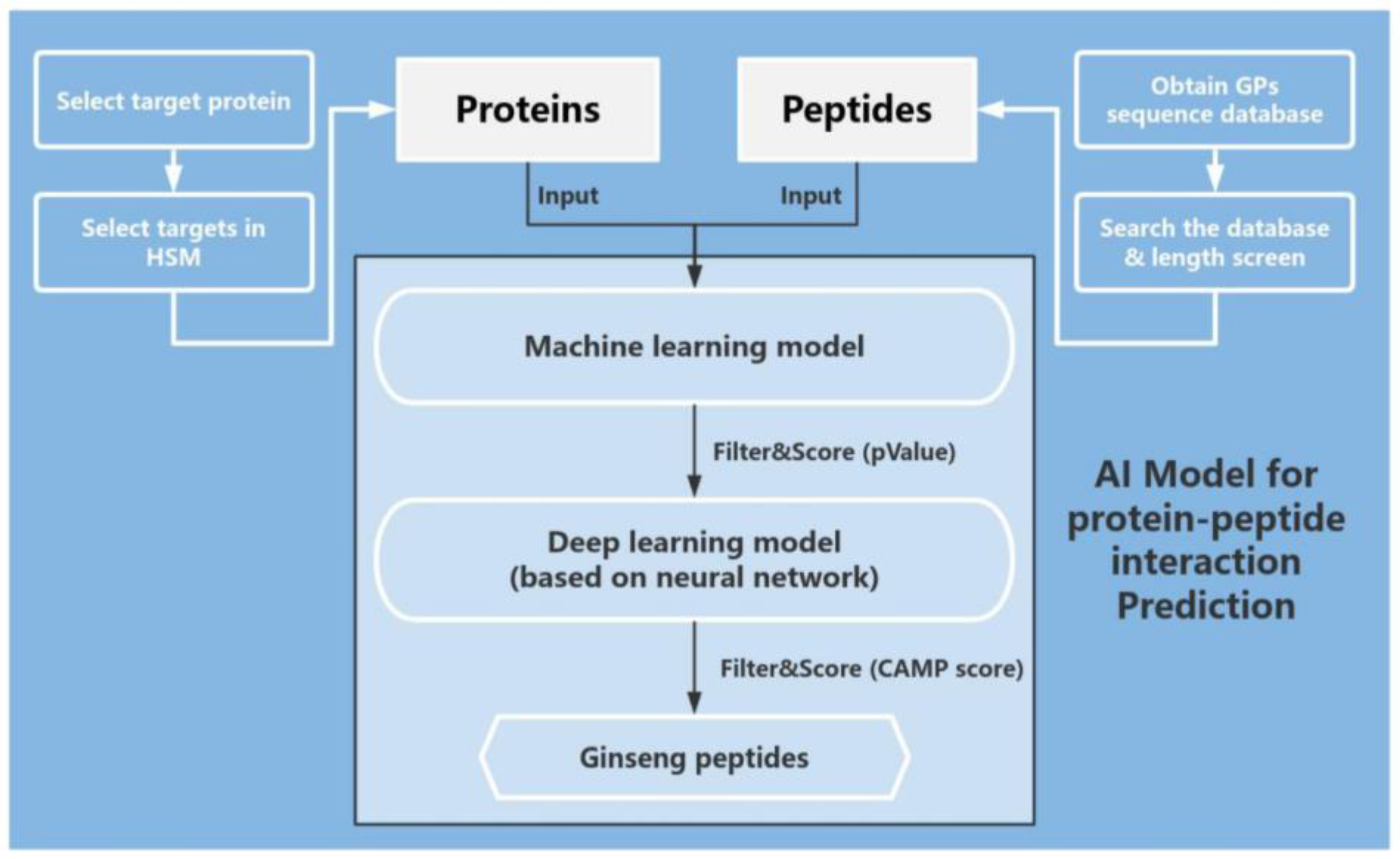
2.1.3. Disease Targets Screened and Targeted with Network Pharmacological Analysis
2.2. Preparation of the Ginseng Peptide Extract
2.3. Free Radical Scavenging Assessment
2.3.1. DPPH Assay
2.3.2. ABTS Assay
2.4. Cell Inflammation Model Based on RAW264.7
2.4.1. Cell Culture of RAW264.7
2.4.2. Cell Viability Assay
2.4.3. Nitric Oxide (NO) Production Assay
2.4.4. Reactive Oxygen Species (ROS) Measurement
2.4.5. RNA Extraction and RT-qPCR
2.5. Histomorphology and Collagen Change Detection Based on In Vitro Skin Model
2.5.1. H&E Staining
2.5.2. Masson Staining
2.5.3. Immunofluorescence
2.6. Tissue Morphological Changes, Detection of Filaggrin (FLG), Loricrin (LOR) and Transglutaminase-1 (TGM1) Content Based on 3D Epidermal Skin Model
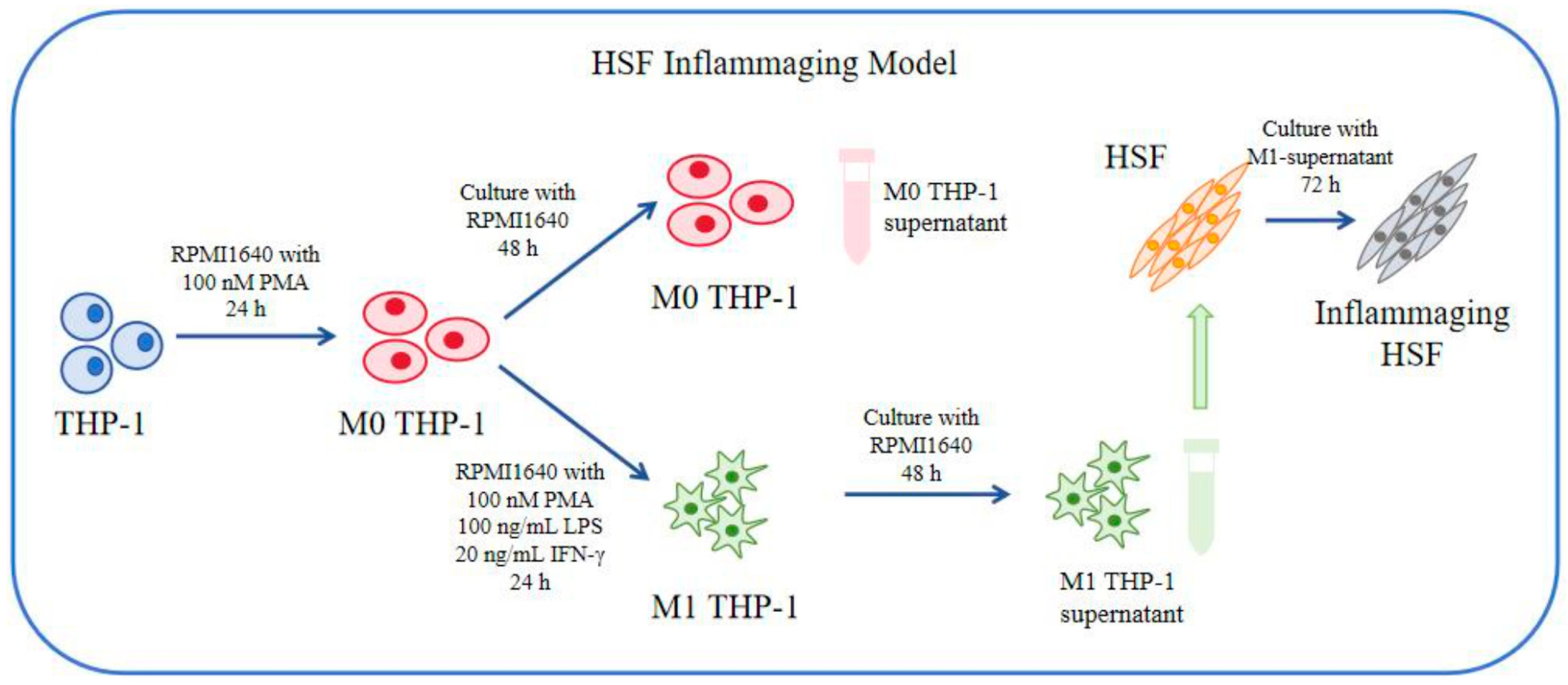
2.7. Biological Assay and Method Based on HSF Cell Inflammaging Model and UV-Induced Senescence Model
2.7.1. Cell Culture of THP-1 and HSF
2.7.2. Induction of Aging to HSF
2.8. Determination of Inflammatory Cytokines (IL-6, TNF-α) and Collagen I by ELISA
2.9. Statistical Analysis
3. Results
3.1. Network Pharmacology Predicts the Anti-Aging and Anti-Inflammatory Efficacy of Ginseng
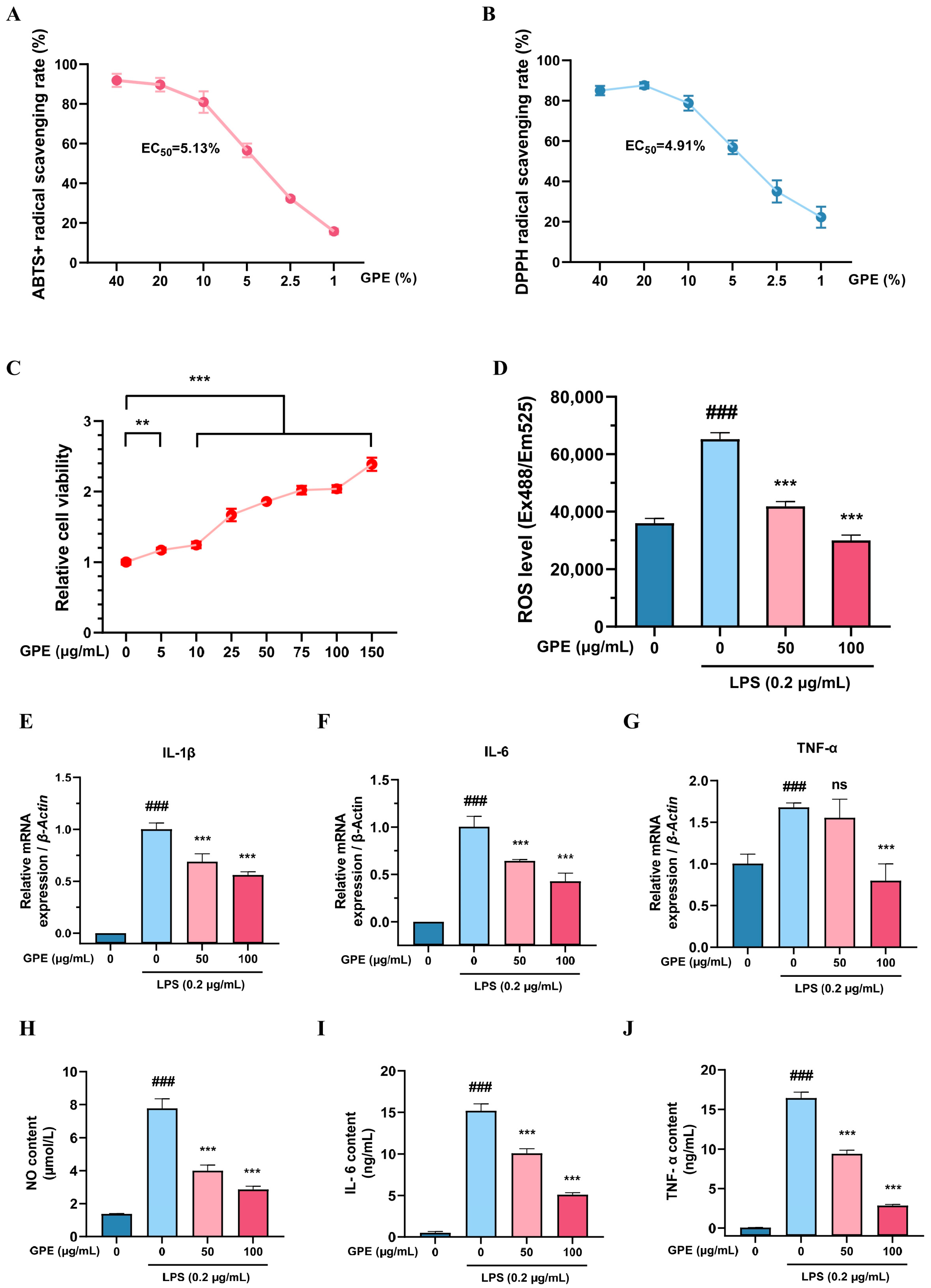
3.2. In Vitro Evaluation of the Antioxidative and Anti-Inflammatory Effects of GPE
3.3. Systematic Evaluation of the Anti-Inflammaging Effects of GPE
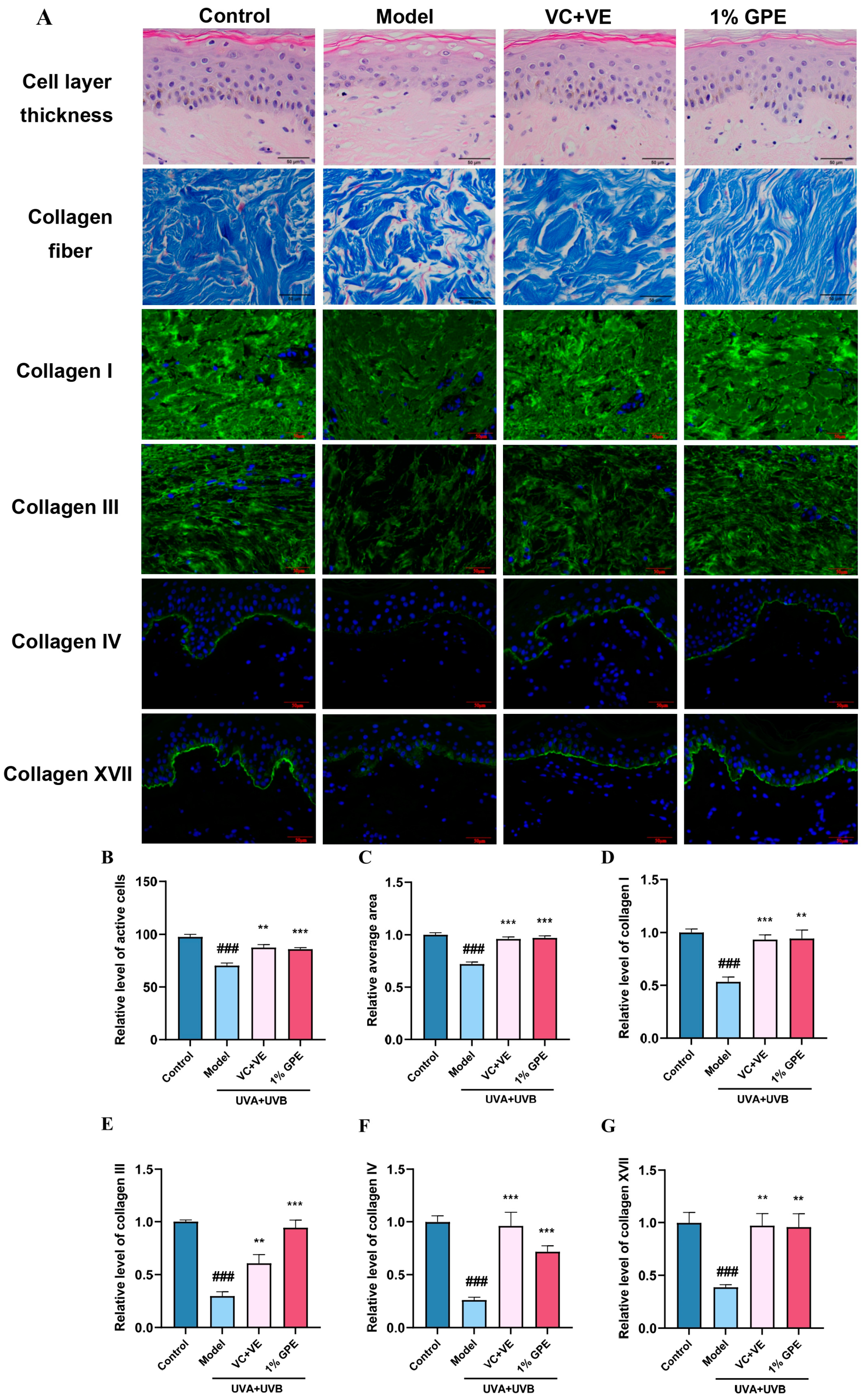
3.4. Systematic Evaluation of Anti-Inflammatory Aging in Dermatological Application Using Multi-Layer Skin Models
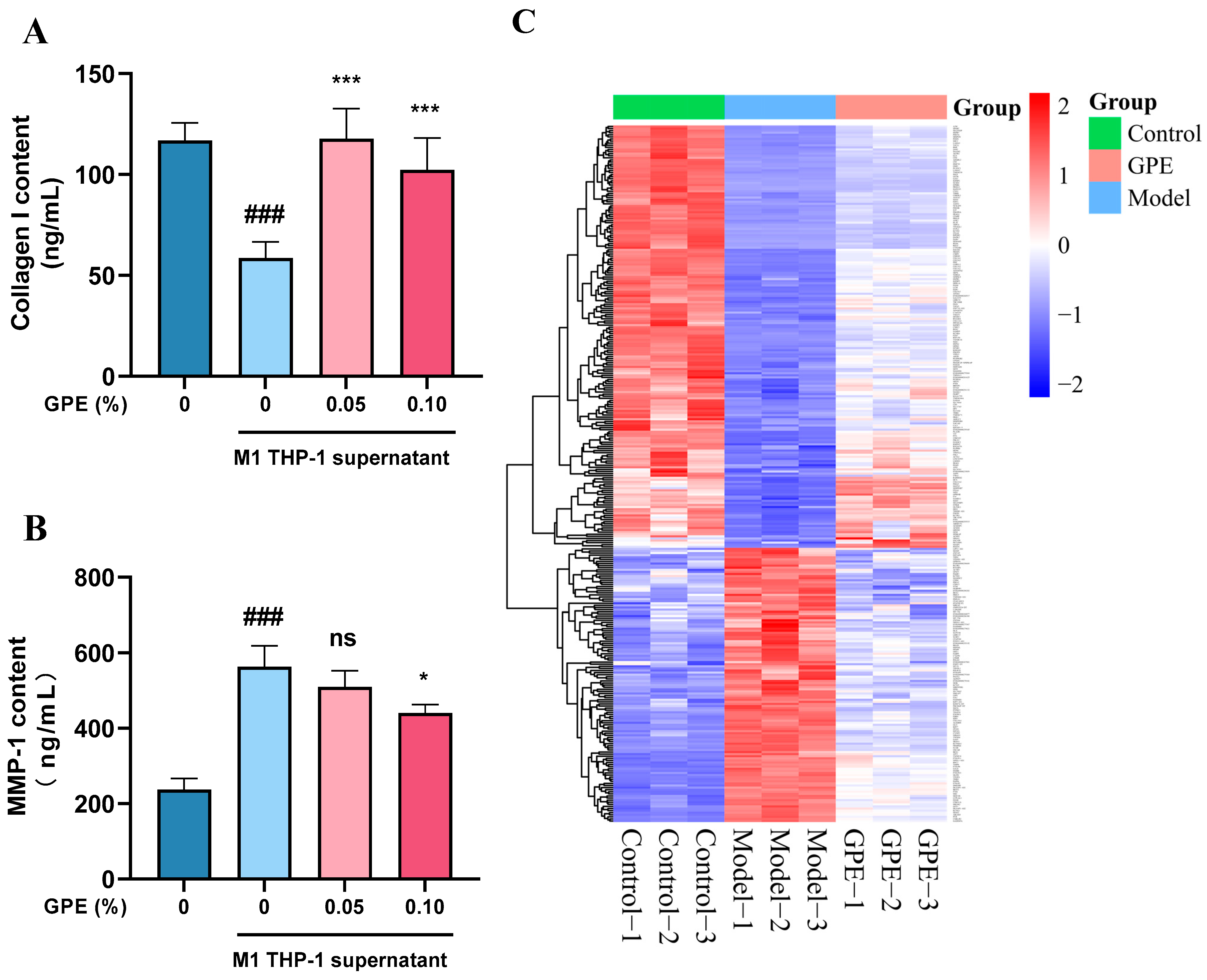
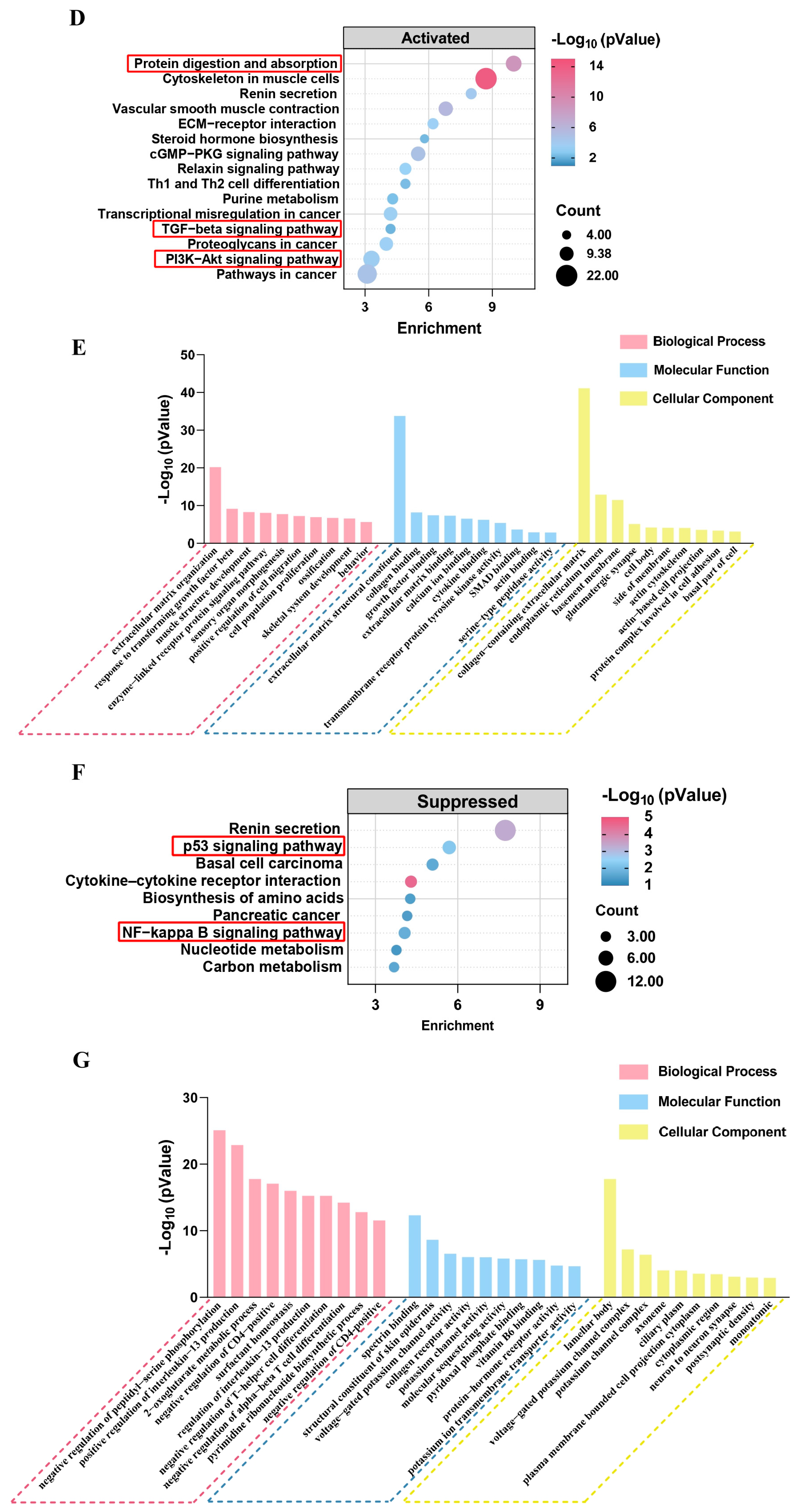
3.5. Prediction of GPs Based on Protein Interaction Model
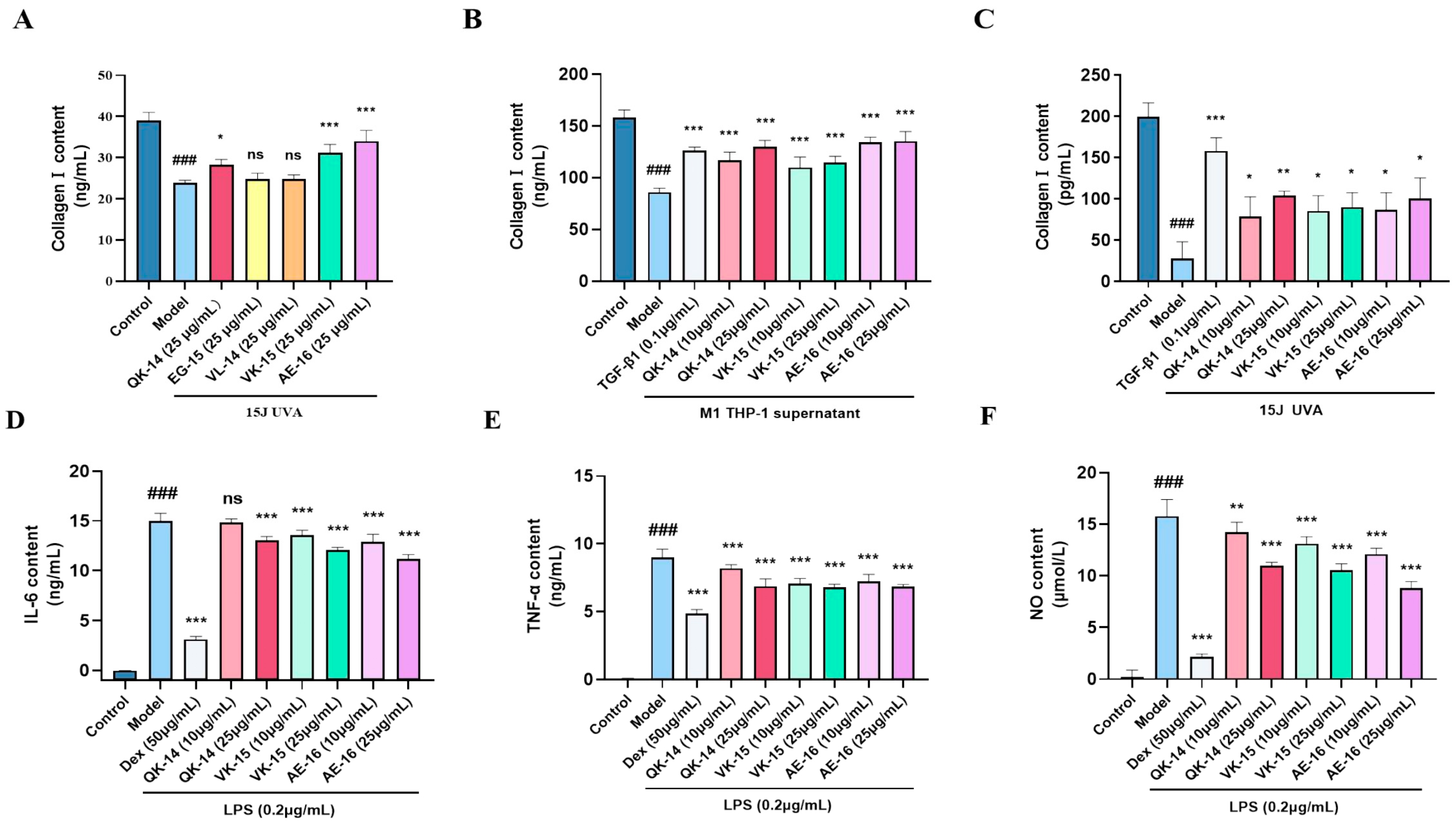
3.6. Evaluation of Anti-Inflammatory and Anti-Aging Effects of GPs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS+ | 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonate) diammonium salt |
| BP | biological processes |
| CC | cellular components |
| COL1A1 | Collagen type I alpha 1 |
| Dex | Dexamethasone |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| ELISA | Enzyme-linked immunosorbent assay |
| FLG | Filaggrin |
| GO | Gene ontology |
| GP | Ginseng peptides |
| GPE | Ginseng peptide extract |
| HSM | Hierarchical statistical mechanical modeling |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LOR | Loricrin |
| MF | molecular functions |
| NO | Nitric oxide |
| OMIM | Online Mendelian inheritance in man |
| PPI | Protein–protein interaction |
| ROS | Reactive oxygen species |
| RT-qPCR | Quantitative real-time polymerase chain reaction |
| SASP | senescence-associated secretory phenotype |
| STRING | Search Tool for the Retrieval of Interacting Genes/Proteins |
| TGM1 | Transglutaminase-1 |
| TCMSP | Traditional Chinese Medicine Systems Pharmacology |
Appendix A
| Peptide_Sequence | Keywords | Protein | HSM Score (p_Value) | CAMP Score |
|---|---|---|---|---|
| LNQEGIYPNNDLYRP | proliferation | BIN1 | 0.000222856 | 0.99666637 |
| LNQEGIYPNNDLYR | Inflammation, proliferation | FGR | 0.000571771 | 0.9769991 |
| QEGIYPNNDLYRPK | Proliferation, proliferation | FGR | 0.0013086 | 0.97212684 |
| LNQEGIYPNNDLYRP | inflammation | PSTPIP1 | 0.000271744 | 0.94917411 |
| ANLLHKLEETLGMNDK | Inflammation, proliferation, oxidat | TP53BP2 | 0.0000043 | 0.908577681 |
| LNQEGIYPNNDLYRP | inflammation | BTK | 0.000251922 | 0.89676905 |
| VDCPTDDATDDYR | proliferation | FYN | 0.000111128 | 0.8797974 |
| NQEGIYPNNDLYRPK | Inflammation, proliferation | FGR | 0.00017643 | 0.86943609 |
| HLNAVPEIDFTKNEN | inflammation | PSTPIP1 | 0.000113509 | 0.86510551 |
| LNQEGIYPNNDLYRP | Inflammation, oxidat | PIK3R1 | 0.000212876 | 0.84001458 |
| VDCPTDDATDDYRL | proliferation | FYN | 0.000110712 | 0.83449197 |
| LNQEGIYPNNDLYR | inflammation | BTK | 0.00057089 | 0.8144055 |
| LNQEGIYPNNDLYR | oxidat | NCF4 | 0.000371933 | 0.79551464 |
| SEYVLTDINVCVNQ | inflammation | BTK | 0.000883059 | 0.79394358 |
| VDCPTDDATDDYR | Inflammation, proliferation | FGR | 0.00016603 | 0.7798081 |
| LNQEGIYPNNDLYR | Inflammation, oxidat | PIK3R1 | 0.000545315 | 0.77899241 |
| QEGIYPNNDLYRP | collagen | WWC1 | 0.000382538 | 0.76215649 |
| VDCPTDDATDDYRLK | proliferation | FYN | 0.0000267 | 0.73349589 |
| ADEVVHHPLDKSSEVE | Inflammation, proliferation, oxidat | JIP1 | 0.0000021 | 0.730936706 |
| PEIDFTKNEN | inflammation | BTK | 0.000421379 | 0.72908759 |
| VDCPTDDATDDYRL | Inflammation, proliferation | FGR | 0.000133978 | 0.72363502 |
| NQEGIYPNNDLYRPK | oxidat | NCF4 | 0.000182778 | 0.71487856 |
| GVQKTEVEATSTVPAQKL | Inflammation, proliferation, oxidat | JIP1 | 0.0000027 | 0.708695173 |
| EGLYPNKTAPYTPVG | Inflammation, proliferation | FGR | 0.000300853 | 0.69255173 |
| DKSSEVETTDRGLFD | proliferation | VAV1 | 0.0000597 | 0.685672045 |
| VQVLEGNGGVGTIKN | proliferation | ARHGAP26 | 0.0000169 | 0.6738382 |
| LNQEGIYPNNDLYRP | oxidat | NCF4 | 0.000162998 | 0.64596671 |
| QEGIYPNNDLYRPK | Inflammation, proliferation | ITK | 0.001193209 | 0.62135625 |
| LTVTPEEPVVVEK | Inflammation, proliferation | FGR | 0.000264268 | 0.59293336 |
| VQVLEGNGGVGTIKN | Inflammation, proliferation | FGR | 0.0000117 | 0.5275951 |
| EGLYPNKTAPYTPVG | oxidat | SGR | 0.000258785 | 0.52582133 |
| EHTNTEDKQFWEHE | inflammation | BTK | 0.0000917 | 0.50152653 |
References
- Hofseth, L.J.; Ying, L. Identifying and Defusing Weapons of Mass Inflammation in Carcinogenesis. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2006, 1765, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Barton, G.M. A Calculated Response: Control of Inflammation by the Innate Immune System. J. Clin. Investig. 2008, 118, 413–420. [Google Scholar] [CrossRef]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-Resident Macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, N.; Kollie, L.; Yang, D.; Zhang, X.; Zhang, H.; Liang, Z. Sasanquasaponin from Camellia Oleifera Abel Exerts an Anti-Inflammatory Effect in RAW 264.7 Cells via Inhibition of the NF-κB/MAPK Signaling Pathways. Int. J. Mol. Sci. 2024, 25, 2149. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, J.; Geng, M.; Tang, S.-J.; Zhang, W.; Shu, J. Nucleoside Reverse Transcriptase Inhibitors (NRTIs) Induce Proinflammatory Cytokines in the CNS via Wnt5a Signaling. Sci. Rep. 2017, 7, 4117. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Miao, X.; Jiang, Y.; Wu, Z.; Zhu, X.; Liu, H.; Wu, X.; Cai, J.; Ding, X.; Gong, W. The Synergistic Antitumor Effect of IL-6 Neutralization with NVP-BEZ235 in Hepatocellular Carcinoma. Cell Death Dis. 2022, 13, 146. [Google Scholar] [CrossRef]
- Ross, E.A.; Devitt, A.; Johnson, J.R. Macrophages: The Good, the Bad, and the Gluttony. Front. Immunol. 2021, 12, 708186. [Google Scholar] [CrossRef]
- van Furth, R.; Cohn, Z.A.; Hirsch, J.G.; Humphrey, J.H.; Spector, W.G.; Langevoort, H.L. The Mononuclear Phagocyte System: A New Classification of Macrophages, Monocytes, and Their Precursor Cells. Bull. World Health Organ. 1972, 46, 845–852. [Google Scholar]
- Fu, J.; Lu, Z.; Wu, G.; Yang, Z.; Wu, X.; Wang, D.; Nie, Z.; Sheng, Q. Gastrodia elata Specific miRNA Attenuates Neuroinflammation via Modulating NF-κB Signaling Pathway. Int. J. Neurosci. 2024, 134, 1652–1662. [Google Scholar] [CrossRef]
- Chen, Q.; Kang, J.; Fu, C. The Independence of and Associations among Apoptosis, Autophagy, and Necrosis. Signal Transduct. Target. Ther. 2018, 3, 18. [Google Scholar] [CrossRef]
- Acosta, J.C.; Banito, A.; Wuestefeld, T.; Georgilis, A.; Janich, P.; Morton, J.P.; Athineos, D.; Kang, T.-W.; Lasitschka, F.; Andrulis, M.; et al. A Complex Secretory Program Orchestrated by the Inflammasome Controls Paracrine Senescence. Nat. Cell Biol. 2013, 15, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Barbé-Tuana, F.; Funchal, G.; Schmitz, C.R.R.; Maurmann, R.M.; Bauer, M.E. The Interplay between Immunosenescence and Age-Related Diseases. Semin. Immunopathol. 2020, 42, 545–557. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Zhang, W.; Wang, Y.; Qian, P.; Huang, H. Inflammation and Aging: Signaling Pathways and Intervention Therapies. Signal Transduct. Target. Ther. 2023, 8, 239. [Google Scholar] [CrossRef]
- Li, Y.; Tian, X.; Luo, J.; Bao, T.; Wang, S.; Wu, X. Molecular Mechanisms of Aging and Anti-Aging Strategies. Cell Commun. Signal. 2024, 22, 285. [Google Scholar] [CrossRef] [PubMed]
- Attele, A.S.; Wu, J.A.; Yuan, C.-S. Ginseng Pharmacology. Biochem. Pharmacol. 1999, 58, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Yun, T.K. Brief Introduction of Panax Ginseng CA Meyer. J. Korean Med. Sci. 2001, 16, S3. [Google Scholar] [CrossRef]
- Xia, P.; Mao, Y.; Liang, Z. Two Important Ginseng Plants in Southeast Asia: A Systematic Review of Their Traditional Uses, Botany, Phytochemistry, and Pharmacology. Acta Physiol. Plant. 2022, 44, 105. [Google Scholar] [CrossRef]
- Lu, G.; Liu, Z.; Wang, X.; Wang, C. Recent Advances in Panax Ginseng C.A. Meyer as a Herb for Anti-Fatigue: An Effects and Mechanisms Review. Foods 2021, 10, 1030. [Google Scholar] [CrossRef]
- Hernández-García, D.; Granado-Serrano, A.B.; Martín-Gari, M.; Naudí, A.; Serrano, J.C. Efficacy of Panax Ginseng Supplementation on Blood Lipid Profile. A Meta-Analysis and Systematic Review of Clinical Randomized Trials. J. Ethnopharmacol. 2019, 243, 112090. [Google Scholar] [CrossRef]
- Fan, W.; Huang, Y.; Zheng, H.; Li, S.; Li, Z.; Yuan, L.; Cheng, X.; He, C.; Sun, J. Ginsenosides for the Treatment of Metabolic Syndrome and Cardiovascular Diseases: Pharmacology and Mechanisms. Biomed. Pharmacother. 2020, 132, 110915. [Google Scholar] [CrossRef]
- Li, J.; Li, F.; Jin, D. Ginsenosides Are Promising Medicine for Tumor and Inflammation: A Review. Am. J. Chin. Med. 2023, 51, 883–908. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biosynthesis and Biotechnological Production of Ginsenosides. Biotechnol. Adv. 2015, 33, 717–735. [Google Scholar] [CrossRef]
- Truong, V.-L.; Keum, Y.-S.; Jeong, W.-S. Red Ginseng Oil Promotes Hair Growth and Protects Skin against UVC Radiation. J. Ginseng Res. 2021, 45, 498–509. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A Database of Systems Pharmacology for Drug Discovery from Herbal Medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1–30. [Google Scholar] [CrossRef]
- Hamosh, A.; Amberger, J.S.; Bocchini, C.; Scott, A.F.; Rasmussen, S.A. Online Mendelian Inheritance in Man (OMIM®): Victor McKusick’s Magnum Opus. Am. J. Med. Genet. Part A 2021, 185, 3259–3265. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING Database in 2023: Protein–Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape Provides a Biologist-Oriented Resource for the Analysis of Systems-Level Datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Lu, M.; Xu, X.; Xi, B.; Dai, Q.; Li, C.; Su, L.; Zhou, X.; Tang, M.; Yao, Y.; Yang, J. Molecular Network-Based Identification of Competing Endogenous RNAs in Thyroid Carcinoma. Genes 2018, 9, 44. [Google Scholar] [CrossRef]
- Ye, X.; Zhao, N.; Yu, X.; Han, X.; Gao, H.; Zhang, X. Extensive Characterization of Peptides from Panax ginseng C.A. Meyer Using Mass Spectrometric Approach. Proteomics 2016, 16, 2788–2791. [Google Scholar] [CrossRef]
- Horiba, S.; Kami, R.; Tsutsui, T.; Hosoi, J. IL-34 Downregulation—Associated M1/M2 Macrophage Imbalance Is Related to Inflammaging in Sun-Exposed Human Skin. JID Innov. 2022, 2, 100112. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, Y.; Li, J.; Li, Y.; Xu, L.; Dong, K.; Lin, X.; Zhang, T. Unveiling a Novel In-Vitro Model of Skin Inflammaging. Front. Med. 2025, 12, 1556680. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-H.; Su, W.-R.; Wang, F.-F.; Li, K.; Zhu, J.-Y.; Zhu, S.-Y.; Kang, S.-N.; He, C.-F.; Li, J.-X.; Lin, X. Computational Biology in Topical Bioactive Peptide Discovery for Cosmeceutical Application: A Concise Review. Biomed. Eng. Commun. 2023, 2, 14. [Google Scholar] [CrossRef]
- Cunningham, J.M.; Koytiger, G.; Sorger, P.K.; AlQuraishi, M. Biophysical Prediction of Protein–Peptide Interactions and Signaling Networks Using Machine Learning. Nat. Methods 2020, 17, 175–183. [Google Scholar] [CrossRef]
- Lei, Y.; Li, S.; Liu, Z.; Wan, F.; Tian, T.; Li, S.; Zhao, D.; Zeng, J. A Deep-Learning Framework for Multi-Level Peptide–Protein Interaction Prediction. Nat. Commun. 2021, 12, 5465. [Google Scholar] [CrossRef]
- Zheng, Y.; Shao, R.; Xia, P.; Liang, Z.; Yan, K. Activity and Function Studies of the Promoter Cis-Acting Elements of the Key Enzymes in Saponins Biosynthesis of DS from Panax Notoginseng. Protoplasma 2022, 259, 163–171. [Google Scholar] [CrossRef]
- Su, J.; Su, Q.; Hu, S.; Ruan, X.; Ouyang, S. Research Progress on the Anti-Aging Potential of the Active Components of Ginseng. Nutrients 2023, 15, 3286. [Google Scholar] [CrossRef]
- Fan, W.; Fan, L.; Wang, Z.; Mei, Y.; Liu, L.; Li, L.; Yang, L.; Wang, Z. Rare Ginsenosides: A Unique Perspective of Ginseng Research. J. Adv. Res. 2024, 66, 303–328. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, C.-Z.; Mohammadi, S.; Sawadogo, W.R.; Ma, Q.; Yuan, C.-S. Pharmacological Effects of Ginseng: Multiple Constituents and Multiple Actions on Humans. Am. J. Chin. Med. 2023, 51, 1085–1104. [Google Scholar] [CrossRef]
- Xie, W.-Y.; Ji, Z.-H.; Ren, W.-Z.; Zhao, P.-S.; Wei, F.-H.; Hu, J.; Yuan, B.; Gao, W. Wheat Peptide Alleviates DSS-Induced Colitis by Activating the Keap1–Nrf2 Signaling Pathway and Maintaining the Integrity of the Gut Barrier. Food Funct. 2024, 15, 5466–5484. [Google Scholar] [CrossRef]
- Wang, W.; Lin, H.; Shen, W.; Qin, X.; Gao, J.; Cao, W.; Zheng, H.; Chen, Z.; Zhang, Z. Optimization of a Novel Tyrosinase Inhibitory Peptide from Atrina Pectinata Mantle and Its Molecular Inhibitory Mechanism. Foods 2023, 12, 3884. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhang, S.; Yue, H.; Wang, M.; Zeng, J.; Wu, W.; Wang, J.; Zheng, H.; Xue, C.; Zhao, Y.-T. Isolation, Identification and in Silico Analysis of Two Novel Cytoprotective Peptides from Tilapia Skin against Oxidative Stress-Induced Ovarian Granulosa Cell Damage. J. Funct. Foods 2023, 107, 105629. [Google Scholar] [CrossRef]
- Zhu, Q.; Xue, J.; Wang, P.; Wang, X.; Zhang, J.; Fang, X.; He, Z.; Wu, F. Identification of a Novel ACE Inhibitory Hexapeptide from Camellia Seed Cake and Evaluation of Its Stability. Foods 2023, 12, 501. [Google Scholar] [CrossRef]
- Yuxiu, Z.; Haisheng, L.; Lei, D.; Jialong, G.; Wenhong, C.; Xiaoming, Q.; Zhongqin, C.; Huina, Z.; Saiyi, Z. A Novel Tyrosinase Inhibitory Peptide Obtained from Sipunculus Nudus Gelatin Hydrolysate: Preparation, Identification, and Action Mechanism. LWT 2024, 202, 116293. [Google Scholar] [CrossRef]
- Feifei, W.; Wenrou, S.; Sining, K.; Siyu, Z.; Xiaolei, F.; Junxiang, L.; Congfen, H.; Xuhui, L. A Novel Functional Peptide, Named EQ-9 (ESETRILLQ), Identified by Virtual Screening from Regenerative Cell Secretome and Its Potential Anti-Aging and Restoration Effects in Topical Applications. Peptides 2023, 169, 171078. [Google Scholar] [CrossRef]
- Baik, I.-H.; Kim, K.-H.; Lee, K.-A. Antioxidant, Anti-Inflammatory and Antithrombotic Effects of Ginsenoside Compound K Enriched Extract Derived from Ginseng Sprouts. Molecules 2021, 26, 4102. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Haidere, M.F.; Hong, Y.H.; Park, S.H.; Lee, J.-O.; Lee, J.; Cho, J.Y. Pharmacological Potential of Ginseng and Its Major Component Ginsenosides. J. Ginseng Res. 2021, 45, 199–210. [Google Scholar] [CrossRef]
- Wu, H.; Pei, H.; Liu, J.; Zeng, J.; Liu, S.; Chen, W.; He, Z.; Du, R. Protective Effect of Total Saponins of Ginseng Stems and Leaves (GSLS) on Chlorpyrifos-Induced Brain Toxicity in Mice through the PTEN/PI3K/AKT Axis. Aging 2022, 14, 8982–8999. [Google Scholar] [CrossRef]
- Yang, J.-E.; Ngo, H.T.T.; Hwang, E.; Seo, S.A.; Park, S.W.; Yi, T.-H. Dietary Enzyme-Treated Hibiscus Syriacus L. Protects Skin against Chronic UVB-Induced Photoaging via Enhancement of Skin Hydration and Collagen Synthesis. Arch. Biochem. Biophys. 2019, 662, 190–200. [Google Scholar] [CrossRef]
- Bai, D.; Wang, Z.; Xiao, Y.; Liu, T.; Pu, Y.; Sun, H.; Wang, M.; Guo, C.; Zhang, J. Transdermal Delivery of Elastin Peptide Assisted by Betaine-Based Deep Eutectic Solvent to Ameliorate Skin Photoaging. Biomater. Adv. 2024, 163, 213965. [Google Scholar] [CrossRef]
- Aursuwanna, T.; Noitang, S.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Puthong, S.; Reamtong, O.; Karnchanatat, A. Investigating the Cellular Antioxidant and Anti-Inflammatory Effects of the Novel Peptides in Lingzhi Mushrooms. Heliyon 2022, 8, e11067. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Wang, L.; Yao, Y.; Lin, D.; Wang, C.; Yao, J.; Sun, H.; Liu, M. Ganoderma Lucidum Polysaccharide Peptide (GLPP) Attenuates Rheumatic Arthritis in Rats through Inactivating NF-κB and MAPK Signaling Pathways. Phytomedicine 2023, 119, 155010. [Google Scholar] [CrossRef]
- Zhu, N.; Xu, M.-H.; Li, Y. Bioactive Oligopeptides from Ginseng (Panax Ginseng Meyer) Suppress Oxidative Stress-Induced Senescence in Fibroblasts via NAD+/SIRT1/PGC-1α Signaling Pathway. Nutrients 2022, 14, 5289. [Google Scholar] [CrossRef]
- Wen, L.; Bi, H.; Zhou, X.; Jiang, Y.; Zhu, H.; Fu, X.; Yang, B. Structure Characterization of Soybean Peptides and Their Protective Activity against Intestinal Inflammation. Food Chem. 2022, 387, 132868. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Khongkow, M.; Klinngam, W.; Chaikul, P.; Lourith, N.; Chueamchaitrakun, P. Recent Insights into Catechins-Rich Assam Tea Extract for Photoaging and Senescent Ageing. Sci. Rep. 2024, 14, 2253. [Google Scholar] [CrossRef] [PubMed]
- Mo, Q.; You, S.; Fu, H.; Wang, D.; Zhang, J.; Wang, C.; Li, M. Purification and Identification of Antioxidant Peptides from Rice Fermentation of Lactobacillus Plantarum and Their Protective Effects on UVA−Induced Oxidative Stress in Skin. Antioxidants 2022, 11, 2333. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Saravanan, K.M.; Wei, Y.; Jiao, Y.; Yang, Y.; Pan, Y.; Wu, X.; Zhang, J.Z.H. Deep Learning-Based Bioactive Therapeutic Peptide Generation and Screening. J. Chem. Inf. Model. 2023, 63, 835–845. [Google Scholar] [CrossRef]
- Feifei, W.; Wenrou, S.; Jinyue, S.; Qiaochu, D.; Jingjing, L.; Jin, L.; Junxiang, L.; Xuhui, L.; Xiao, L.; Congfen, H. Anti-ageing Mechanism of Topical Bioactive Ingredient Composition on Skin Based on Network Pharmacology. Int. J. Cosmet. Sci. 2025, 47, 134–154. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, J.; Wang, X.; Wang, X.; Ge, F. DeepBP: Ensemble Deep Learning Strategy for Bioactive Peptide Prediction. BMC Bioinform. 2024, 25, 352. [Google Scholar] [CrossRef]
- Jing, C.; Guo, J.; Li, Z.; Xu, X.; Wang, J.; Zhai, L.; Liu, J.; Sun, G.; Wang, F.; Xu, Y.; et al. Screening and Research on Skin Barrier Damage Protective Efficacy of Different Mannosylerythritol Lipids. Molecules 2022, 27, 4648. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, R.; Hwang, S.-H.; Choi, S.-H.; Kim, J.-H.; Cho, I.-H.; Lee, J.I.; Nah, S.-Y. Ginseng and Ginseng Byproducts for Skincare and Skin Health. J. Ginseng Res. 2024, 48, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Srivastava, D.; Sahu, M.; Tiwari, S.; Ambasta, R.K.; Kumar, P. Artificial Intelligence to Deep Learning: Machine Intelligence Approach for Drug Discovery. Mol. Divers. 2021, 25, 1315–1360. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic Peptides: Current Applications and Future Directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Yan, Z.-B.; Meng, Y.-M.; Hong, X.-Y.; Shao, G.; Ma, J.-J.; Cheng, X.-R.; Liu, J.; Kang, J.; Fu, C.-Y. Antimicrobial Peptides: Mechanism of Action, Activity and Clinical Potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Song, G.; Sun, Y.; Liu, T.; Zhang, X.; Zeng, Z.; Wang, R.; Li, P.; Li, C.; Jiang, G. Transdermal Delivery of Cu-Doped Polydopamine Using Microneedles for Photothermal and Chemodynamic Synergistic Therapy against Skin Melanoma. Chem. Eng. J. 2021, 426, 130790. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Z.; Liu, W.; Zeng, F.; Kang, S.; Li, J.; Xu, W.; Tang, P.; Zheng, X.; Li, D.; Yang, X.; et al. Systematic Evaluation and Identification of Anti-Inflammatory and Anti-Aging Ginseng Peptides for Skincare Applications. Cosmetics 2025, 12, 85. https://doi.org/10.3390/cosmetics12020085
Xia Z, Liu W, Zeng F, Kang S, Li J, Xu W, Tang P, Zheng X, Li D, Yang X, et al. Systematic Evaluation and Identification of Anti-Inflammatory and Anti-Aging Ginseng Peptides for Skincare Applications. Cosmetics. 2025; 12(2):85. https://doi.org/10.3390/cosmetics12020085
Chicago/Turabian StyleXia, Ze, Wei Liu, Fanmo Zeng, Sining Kang, Junxiang Li, Wenfei Xu, Pingxiang Tang, Xinyi Zheng, Dandan Li, Xuebin Yang, and et al. 2025. "Systematic Evaluation and Identification of Anti-Inflammatory and Anti-Aging Ginseng Peptides for Skincare Applications" Cosmetics 12, no. 2: 85. https://doi.org/10.3390/cosmetics12020085
APA StyleXia, Z., Liu, W., Zeng, F., Kang, S., Li, J., Xu, W., Tang, P., Zheng, X., Li, D., Yang, X., Sheng, Q., & Li, X. (2025). Systematic Evaluation and Identification of Anti-Inflammatory and Anti-Aging Ginseng Peptides for Skincare Applications. Cosmetics, 12(2), 85. https://doi.org/10.3390/cosmetics12020085