Abstract
This double-blind, randomized, placebo-controlled clinical study evaluated the efficacy of a shampoo containing Plantago asiatica L. on hair health in adults aged 20–60. Following a 4-week wash-out period, participants used either the test shampoo or a placebo for 12 weeks. Hair measurements using phototrichograms and hair count tests were conducted at baseline and at weeks 4, 8, and 12. The test group demonstrated progressive improvements in hair strand thickness (0.009 mm, 0.017 mm, and 0.020 mm at weeks 4, 8, and 12, respectively) and hair density (0.9 hairs/cm2 at weeks 4 and 8, 1.1 hairs/cm2 at week 12). Additionally, a significant reduction in hair shedding was observed compared to the placebo group. These findings suggest that shampoo containing Plantago asiatica L. effectively improves hair thickness and density while reducing hair loss, offering a natural solution for hair care concerns.
1. Introduction
For a significant proportion of the population, hair is regarded as a symbol of youth and confidence. Androgenetic alopecia, more commonly referred to as male pattern baldness, has been the subject of extensive study, and is primarily influenced by genetic factors, resulting in hereditary hair loss [1,2]. However, in recent years, alopecia has become increasingly prevalent not only among men but among women. This phenomenon is often precipitated by emotional or physical stressors, or significant weight loss [3,4]. Moreover, hair loss can lead to a perception of being less physically and socially attractive, significantly lowering self-esteem. The psychological issues associated with alopecia range from mild to severe symptoms, including anxiety, anger, depression, embarrassment, reduced self-confidence, decreased quality of life, sleep disturbances, social withdrawal, and even suicidal tendencies [4].
Hair growth occurs in three distinct phases: the anagen, catagen, and telogen phases. Normal hair shedding is defined as the natural loss of approximately 100–150 telogen hairs daily [5]. Stable hair density and count are maintained when an optimal equilibrium between the anagen and telogen phases indicates a healthy scalp condition. In instances of hair loss, there is frequently an imbalance in the ratio of hairs in the anagen and telogen phases. The U.S. Food and Drug Administration (FDA) has approved two treatments for alopecia: minoxidil and finasteride. However, these pharmacological agents are associated with adverse effects, including skin irritation, sexual dysfunction, and headaches [6,7]. Consequently, there is an increasing demand for therapeutics derived from natural substances, such as plant extracts, which present fewer side effects than these chemical medications. Accordingly, extensive research is being conducted on natural material-based treatments.
Plantago asiatica L., commonly known as Asian plantain, is a perennial herb widely distributed throughout East Asia, including Korea [8]. This plant has been traditionally used in folk medicine for its various therapeutic properties [9,10]. Recent scientific investigations have corroborated its potential in various biomedical applications, particularly noting its anti-inflammatory, skin-whitening, and antioxidant effects [11,12,13].
While the effects of Plantago asiatica L. on various aspects of health have been studied, its potential impact on hair growth and maintenance has received less attention. However, preliminary research has suggested that certain compounds found in Plantago asiatica L. might influence protection against UV damage in skin and hair cells [14]. The anti-inflammatory and antioxidant properties of the plant could potentially contribute to a healthier scalp environment, which is crucial for optimal hair growth [12]. Therefore, based on preliminary research, we studied the possibility that Plantago asiatica L. could potentially influence maintaining hair growth [15].
Given the increasing consumer interest in natural and plant-based hair care solutions, coupled with the cultural familiarity of Plantago asiatica L. in Korea, there is a compelling rationale to investigate its potential in addressing hair-related concerns. Therefore, this study aims to evaluate the effects of a shampoo containing Plantago asiatica L. extract on hair thickness, density, and hair shedding in adults concerned about their hair condition.
By focusing on these specific hair parameters, we seek to comprehensively assess the potential benefits of Plantago asiatica L. in hair care. This research not only builds upon the traditional uses and known properties of the plant but addresses a significant concern in personal care and aesthetics. The findings from this study could open new avenues for natural hair care solutions and contribute to our understanding of plant-based interventions in hair health.
2. Materials and Methods
2.1. Study Design and Participants
This study was designed as a randomized, double-blind, placebo-controlled clinical trial. It was performed at the ProbeM Skin Research Center in Daejeon, Korea, from December 2023 to April 2024 (IRB number: 1-70094700-AB-N-01-CoR1). This study was conducted over 12 weeks, with a total of five visits for each participant (screening, week 0, week 4, week 8, and week 12). The trial was conducted by the guidelines set forth by the Ministry of Food and Drug Safety of the Republic of Korea for cosmetic human application and efficacy tests (Guideline No. 0333-02), and the internal regulations of the ProbeM Skin Research Center.
The study population consisted of female adults aged 20 to 60 who expressed concern about their hair condition. A total of 40 participants were included in this study (Table 1). A four-week washout period was conducted prior to the commencement of the trial. The trial was conducted with an average age of 45.0 years in the experimental group and 42.2 years in the control group. The trial was completed by all 40 participants, with no dropouts. All participants were female.

Table 1.
Descriptive statistics of the population at baseline.
2.2. Inclusion and Exclusion Criteria
The inclusion criteria were defined as patients aged 20 to 60 who expressed concern about their hair condition. The exclusion criteria were as follows: individuals who do not wish to participate or who have not signed the consent form pregnant or breastfeeding women or those planning to become pregnant within six months; individuals who have used steroid-containing topical treatments for skin conditions for more than one month; individuals who have participated in a similar trial within the past six months; individuals with sensitive or hypersensitive skin; individuals with dermatological abnormalities, such as moles, acne, erythema, or telangiectasia, in the test area; individuals who have used cosmetics or medications with the same or similar effects on the test area within three months prior to this study; individuals with neurological disorders or mental disabilities; any other individuals deemed unsuitable for participation by the principal investigator for any other reason.
2.3. Test Product
The shampoo under investigation was prepared by incorporating 0.03% of the Plantago asiatica L. extract. As we previously reported [15], the extract was prepared via ethanol extraction of dried P. asiatica L. at room temperature. After filtration and solvent removal, the dried extract was dissolved for use in the formulation. Although the specific active or marker compounds within the extract have not yet been identified, the biological activity of each batch was confirmed through functional assays. Specifically, human dermal papilla cells were treated with the extract for 10 min, and the phosphorylation of ERK and CREB proteins was assessed. Only extracts that consistently activated these signaling pathways were selected for use in the shampoo. The placebo shampoo was a scalp shampoo that did not contain the Plantago asiatica L. extract, but did contain all other ingredients in quantities identical to those present in the tested shampoo. The materials were provided to the participants in identical containers, each bearing a corresponding randomization number. The main surfactants in the shampoos are sodium laureth sulfate, cocamidopropyl betaine, and cocamide MEA.
2.4. Measurement of the Clinical Efficacy of the Test Product
To guarantee uniform measurement parameters for all participants, the testing area was maintained in a pristine and dry state for a period of 30 min. This study was conducted under controlled temperature and humidity conditions, with an indoor temperature of 20–24 °C and humidity of 40–60%. These conditions were maintained without air movement or direct sunlight. Subjects were instructed to utilize the product by wetting their scalp and hair with lukewarm water, applying an appropriate amount of the shampoo to create a lather, massaging it in, and then rinsing with lukewarm water once daily for 12 weeks.
2.5. Hair Density and Thickness
High-resolution photographs of the forehead hairline (45°) and crown (90°) were obtained using a Canon EOS 70D camera (Canon, Tokyo, Japan). To minimize participant movement, a custom-designed stage was employed to secure the forehead and chin, thereby ensuring consistent conditions, angles, and positions at each evaluation point. The measurements were taken at the outset of this study (week 0) and at 4-, 8-, and 12-week intervals thereafter. To ensure consistency, the same researcher conducted all the photographic sessions. To assess hair density, the target area was trimmed, and high-resolution images were captured using the KONG Camera (Video Micro Scope, Bomtech, Seoul Korea). This procedure was conducted prior to product administration (week 0) and at 4, 8, and 12 weeks post-product administration. Images were captured at 80× magnification to ascertain the total number of hairs per unit area (per square millimeter). An increase in measurement values indicated an improvement in hair density, with the unit of measurement being “ea/cm2.”
2.6. Number of Shed Hairs
In a designated white test area, participants utilized a hairbrush provided by the center to brush the test area (scalp and hair) 20 times each in the central, left, and right sections, for a total of 60 strokes. Subsequently, the number of shed hairs collected from the brushing was counted. High-resolution images of the shed hairs were captured using a digital single-lens reflex camera (Canon EOS 7D, Tokyo, Japan) prior to product use, and at 2-, 4-, and 12-week intervals thereafter. A reduction in the number of shed hairs was regarded as an improvement, with the unit of measurement being the number of hairs.
2.7. Self-Assessment Questionnaire
A survey was conducted to ascertain the participants’ opinions regarding the usability and preference of the test product after use. The assessment was conducted on a 5-point Likert scale, with 5 indicating strong agreement, 4 indicating agreement, 3 indicating neutrality, 2 indicating disagreement, and 1 indicating strong disagreement. Responses scoring three points or higher were considered indicative of a positive outcome. The questions were as follows:
- Q1: After using the product, I feel my hair has become denser.
- Q2: After using the product, I feel my hair strands have become thicker.
- Q3: After using the product, I notice a reduction in hair shedding.
2.8. Statistical Analysis
Statistical analysis was conducted using the Statistical Program for Social Science (SPSS). After performing tests for normality, the study results were analyzed. Improvement effects due to product use were considered significant at a 95% confidence interval when the significance level (p-value) was less than 0.05. (1) Intra-group comparisons (Experimental group, before vs. after n weeks of product use) (*n: 4 weeks, 8 weeks, 12 weeks): for parametric analysis, paired sample t-tests were used, and for non-parametric analysis, Wilcoxon signed rank tests were employed. (2) Inter-group comparisons (change in the placebo group (Δ) vs. change in the test group (Δ)): for parametric analysis, independent sample t-tests were used, and for non-parametric analysis, Mann–Whitney U tests were employed.
3. Results
3.1. Clinical Efficacy on Hair Thickness and Density
This study enrolled a total of 40 subjects, all of whom were randomized into two groups: a placebo group and a test group, with 20 participants in each group. Both groups completed this study in its entirety, resulting in 20 subjects in the placebo group and 20 subjects in the test group completing the trial as per the study protocol. There were no dropouts or withdrawals from either group during the course of this study (Figure 1).
Figure 1.
Flow chart of the clinical trial.
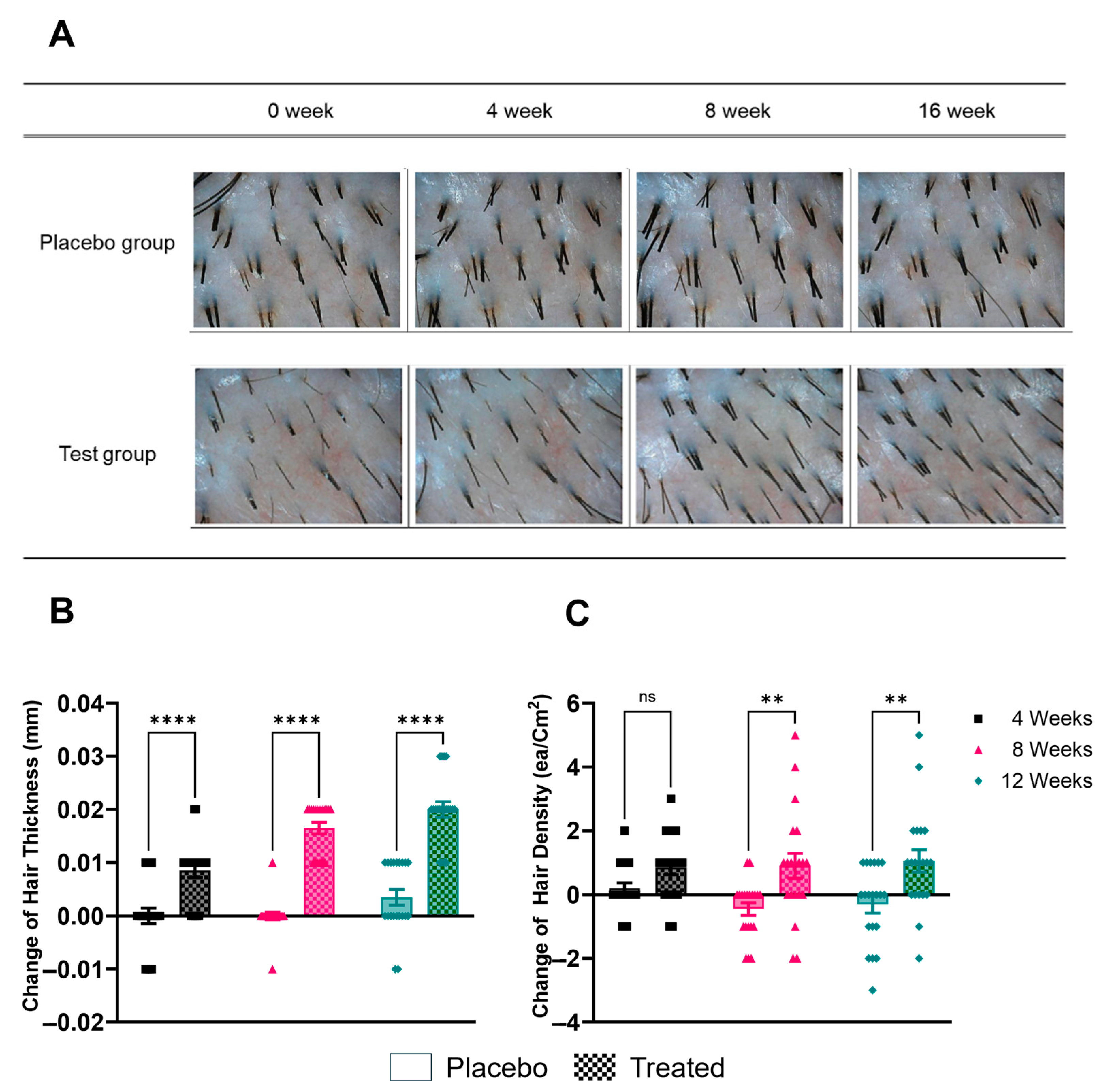
Subjects were instructed to apply the product to the scalp and hair daily with lukewarm water, lathering and massaging it in before rinsing with lukewarm water over a 12-week period. Participants were advised to maintain their usual hair care routines apart from replacing their regular shampoo with the provided product. Hair thickness and density were assessed using a phototrichogram, a non-invasive imaging technique that allows for precise measurement of hair parameters (Figure 2). Measurements were taken at baseline (week 0) and at four, eight, and twelve weeks after the start of the treatment. The placebo control demonstrated a change of 0.004 at the 12-week mark. By contrast, the test group demonstrated changes of 0.009 at four weeks, 0.017 at eight weeks, and 0.020 at 12 weeks. The statistical analysis revealed significant differences between the experimental and placebo groups at the four-week, eight-week, and twelve-week time points (p < 0.05).
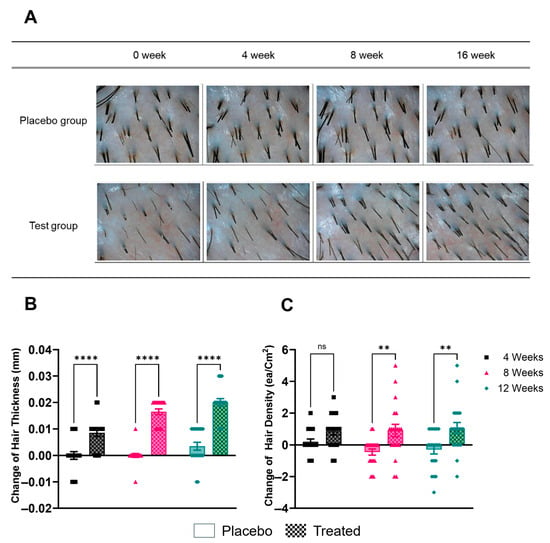
Figure 2.
Photographic and quantitative assessment of hair changes. (A) Representative phototrichogram images showing changes in hair density over time (baseline, 4 weeks, 8 weeks, and 12 weeks) for a participant in each group. (B) Change in hair thickness (mm) in the placebo group after 4, 8, and 12 weeks of use. (C) Change in hair thickness (mm) in the group treated with shampoo containing Plantago asiatica L. extract after 4, 8, and 12 weeks. ** p < 0.01, **** p < 0.001, ns = non-significant.
Hair density was measured in hairs per square centimeter (hairs/cm2) and reported as the change from baseline. The analysis of hair density changes indicated that the placebo group exhibited changes of 0.2 at four weeks, 0.5 at eight weeks, and 0.3 at 12 weeks. By comparison, the test group exhibited changes of 0.9 at 4 weeks, 0.9 at 8 weeks, and 1.1 at 12 weeks. A statistically significant difference was observed between the experimental and placebo groups at four weeks, eight weeks, and twelve weeks (p < 0.05).
These results suggest that the shampoo containing Plantago asiatica L. extract was effective in increasing both hair thickness and density over the 12-week treatment period, with improvements becoming more pronounced over time.
3.2. Measurement of Shed Hairs
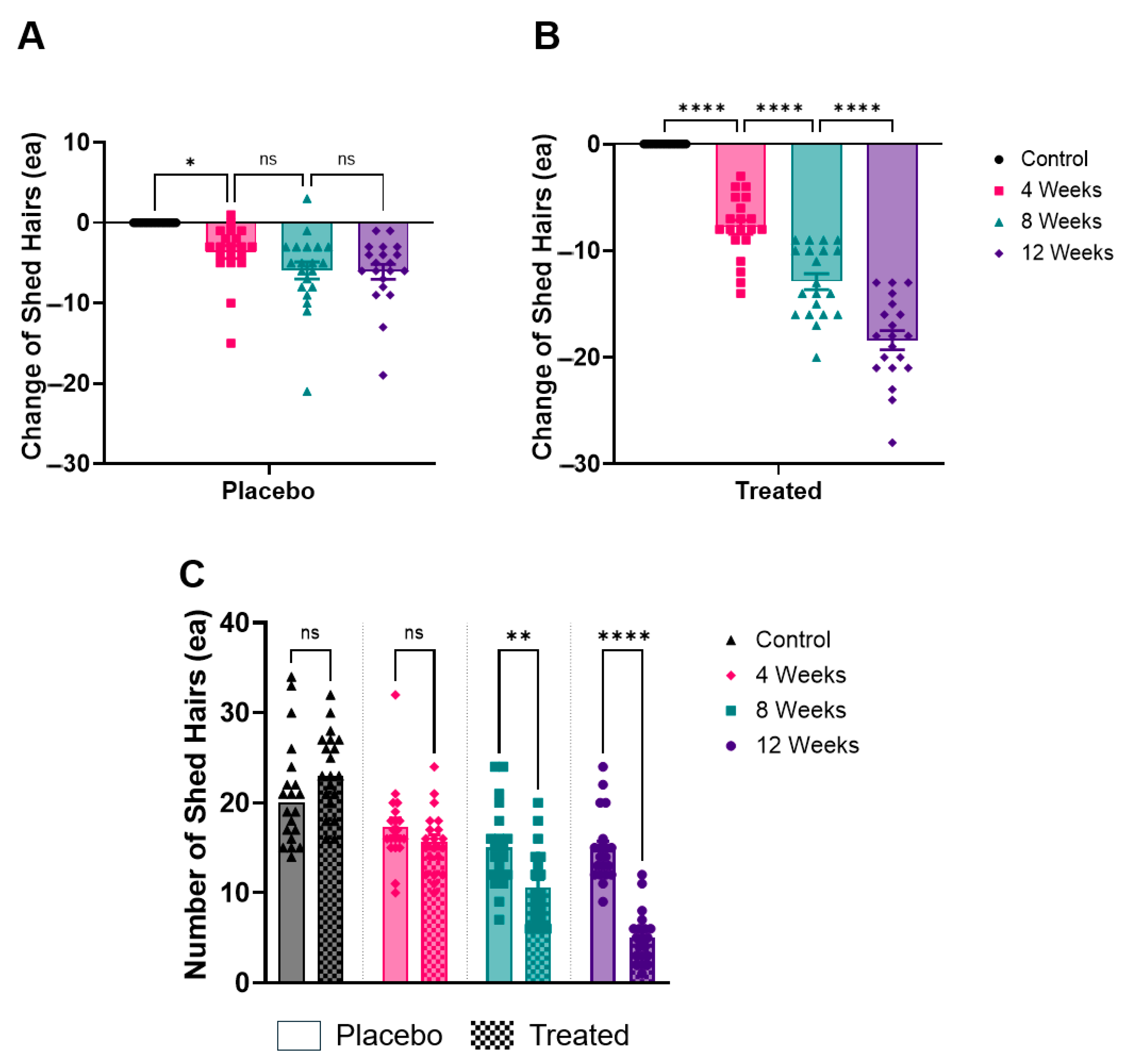
In measuring shed hairs, the placebo group showed a steady decrease over time. The average number of shed hairs in the placebo group decreased from 21.0 hairs before product use to 17.3 hairs at 4 weeks, 15.0 hairs at 8 weeks, and 14.9 hairs at 12 weeks. This indicates a gradual reduction in hair shedding over the 12-week period.
By contrast, the test group exhibited a more pronounced reduction in hair shedding (Figure 3). At baseline, the test group had an average of 23.5 shed hairs, which significantly decreased to 15.7 hairs at 4 weeks, 10.6 hairs at 8 weeks, and 5.1 hairs at 12 weeks. The difference between the test and placebo groups became statistically significant by 12 weeks (p < 0.05).
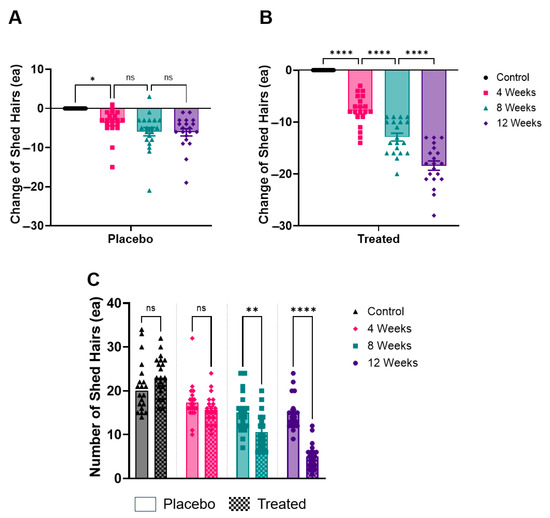
Figure 3.
Analysis of shed hair counts over time. (A) Relative reduction in the number of shed hairs in the placebo group after 4, 8, and 12 weeks. (B) Relative reduction in the number of shed hairs in the Plantago asiatica L. shampoo group over the same time period. (C) Absolute number of shed hairs measured at each time point for both groups. * p < 0.05, ** p < 0.01, **** p < 0.001, ns = non-significant.
This data suggests that, while both groups experienced a reduction in hair shedding, the test group showed a faster and more significant improvement compared to the placebo group.
3.3. Assessment of Self-Assessment Questionnaire
The self-assessments conducted by subjects in the test group revealed statistically significant improvements across several key measures of hair health, particularly when compared to the placebo group. Participants in the test group reported noticeable enhancements in overall hair density, indicating that their hair appeared fuller and thicker as the study progressed.
Most notably, the test group reported a significant reduction in hair fall rate, with many subjects expressing that they experienced less hair shedding during daily activities, such as brushing or washing their hair. The reduction in hair fall was consistent with the objective measurements, which showed a marked decrease in the number of shed hairs over the 12-week period. These findings underscore the efficacy of the test product in promoting hair retention and mitigating hair loss, as perceived by the participants.
By contrast, subjects in the placebo group reported only minimal changes in these same parameters. While some individuals in the placebo group noticed slight improvements in hair health, the degree of change was far less pronounced compared to the test group, with no statistically significant results observed in terms of hair density, thickness, or shedding reduction.
These self-assessments align with the objective data collected during the study and further support the conclusion that the test product provides a significant benefit in improving overall hair quality and reducing hair loss (Table 2).

Table 2.
Self-assessment Questionnaire.
4. Discussion
Hair growth occurs through repetitive stages of anagen, catagen, and telogen phases. However, an imbalance from genetic predisposition, hormonal influences, stress, diet, or other environmental factors can result in the onset or acceleration of hair loss [16]. Among these phases, the anagen phase is the most critical by representing the period of active hair growth. Typically lasting between two and eight years in scalp hair, this phase determines the length and thickness of the hair shaft. Disruption in the regulation of this phase could trigger a premature transition into the catagen or telogen phases, thereby limiting hair growth and contributing to increased shedding [5,17,18].
Therefore, maintaining and prolonging the anagen phase is a primary objective in developing effective hair loss treatments. Strategies to support this phase often include improving scalp microcirculation, reducing oxidative stress, promoting follicular nourishment, and minimizing inflammation that may trigger early entry into the regression or resting phases [19]. Enhancing scalp and follicular health is also essential for creating a conducive environment for continuous hair fiber production.
Indicators such as hair strand thickness, total hair count, and rate of hair shedding serve as key parameters in assessing the progression or improvement of alopecia. A decline in thickness and density usually indicates compromised follicular activity and may represent an early signal of follicle miniaturization—a hallmark of various types of hair loss. Although the normal shedding range for healthy individuals is typically between 50 to 100 hairs per day, consistent values beyond this threshold may reflect a pathological condition, such as telogen effluvium or androgenetic alopecia [20,21].
In our preliminary research, we found that Plantago asiatica L. positively influenced several biological mechanisms related to hair growth [15]. Notably, it enhanced the production of growth factors such as VEGF (vascular endothelial growth factor), KGF (keratinocyte growth factor), and FGF (fibroblast growth factor), which are known to play critical roles in follicular development, cellular proliferation, and angiogenesis. Moreover, it facilitated the phosphorylation of CREB (cAMP response element-binding protein) and increased intracellular accumulation of β-catenin—a key component of the Wnt signaling pathway involved in follicular activation and regeneration. As a result, dermal papilla cell proliferation was significantly enhanced, suggesting a strong mechanistic basis for the observed hair-promoting effects. Additionally, the observed induction of angiogenesis may support hair follicles through enhanced vascularization, allowing for improved nutrient and oxygen delivery during the regeneration process [17]. However, it is important to note that these mechanistic insights were derived from in vitro experiments, and the current clinical study did not include biomarker analysis or molecular assessments using scalp tissue from participants. As such, while the clinical findings are promising, the direct confirmation of these pathways in vivo remains to be elucidated. Future studies incorporating biomarker evaluation in clinical samples or employing ex vivo human scalp models would be valuable to validate the activation of these biological pathways and strengthen the mechanistic understanding of the effects of Plantago asiatica L. in a real-world context.
The primary objective of the present clinical study was to evaluate the practical benefits of Plantago asiatica L. in a real-world setting through a shampoo formulation used by adult participants concerned about hair thinning or shedding. After 12 weeks of consistent use, participants in the test group showed a statistically significant improvement in both hair density and strand thickness relative to the placebo group. Notably, a remarkable 78.80% reduction in hair shedding was recorded, which further supports the hypothesis that Plantago asiatica L. could be beneficial in mitigating excessive hair loss.
These clinical results align with and reinforce the findings of our earlier in vitro studies, demonstrating that Plantago asiatica L. not only promotes dermal papilla activity and angiogenesis but translates these molecular effects into measurable outcomes in human participants. The fact that such results were observed through the use of a rinse-off shampoo formulation highlights the potential for Plantago asiatica L. to exert its effects through relatively brief yet consistent topical exposure. This suggests a promising application for this natural extract in everyday hair care products without the need for complex delivery systems or leave-on treatments.
Taken together, the findings from both the mechanistic and clinical perspectives suggest that Plantago asiatica L. holds considerable potential as a functional ingredient in hair growth-promoting formulations. Its ability to improve key indicators of hair health, such as thickness, density, and shedding, positions it as a compelling candidate for use in non-invasive, cosmetic interventions for individuals experiencing early signs of hair loss or seeking to maintain healthy scalp conditions. Further long-term studies may help determine the duration of these effects and optimize dosage or formulation strategies for enhanced efficacy.
Nevertheless, this study has several limitations. The sample size was relatively small and limited to a single-center setting, which may affect the generalizability of the results. In addition, although no adverse effects were reported during the 12-week period, the long-term safety and sustained efficacy of shampoo containing Plantago asiatica L. remain to be evaluated. Future research should include larger, multi-center studies with extended follow-up periods to confirm and expand upon these findings.
Author Contributions
J.L.: writing—original draft, visualization, validation, methodology, investigation, formal analysis. A.-R.J.: writing—review and editing, methodology, investigation, formal analysis. J.-H.J. and J.-T.B.: writing—review and editing. W.K.: writing—original draft, supervision, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2022R1C1C1007334).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (IRB) of the KOREA BIO RESEARCH CENTER (KBRC) (approval number: 1-7009470-AB-N-01-CoR1, date of approval: 10 May 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Jiyeon Lee, Ah-Reum Jung, Jun-Hwan Jang, and Jun-Tae Bae are employees of J2KBIO Corp., a company engaged in the commercial production of cosmetic raw materials. The company has a vested interest in the microorganisms discussed in this study. Wanil Kim has no conflicts of interest.
References
- Sun, T.T.; Cotsarelis, G.; Lavker, R.M. Hair follicular stem cells: The bulge-activation hypothesis. J. Investig. Dermatol. 1991, 96, S77–S78. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.A.; Harrap, S.B. The genetics of androgenetic alopecia. Clin. Dermatol. 2001, 19, 149–154. [Google Scholar] [CrossRef]
- Minokawa, Y.; Sawada, Y.; Nakamura, M. Lifestyle factors involved in the pathogenesis of alopecia areata. Int. J. Mol. Sci. 2022, 23, 1038. [Google Scholar] [CrossRef]
- Dhami, L. Psychology of hair loss patients and importance of counseling. Indian J. Plast. Surg. 2021, 54, 411–415. [Google Scholar] [CrossRef]
- Natarelli, N.; Gahoonia, N.; Sivamani, R.K. Integrative and mechanistic approach to the hair growth cycle and hair loss. J. Clin. Med. 2023, 12, 893. [Google Scholar] [CrossRef]
- Sattur, S.S.; Sattur, I.S. Pharmacological management of pattern hair loss. Indian J. Plast. Surg. 2021, 422–434. [Google Scholar] [CrossRef]
- Nestor, M.S.; Ablon, G.; Gade, A.; Han, H.; Fischer, D.L. Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. J. Cosmet. Dermatol. 2021, 20, 3759–3781. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Okamoto, K.; Kanazawa, Y.; Hori, Y. Differences in plant-size structure and biomass allocation in plants between exposed and shaded Plantago asiatica populations at a mid-elevated habitat in the cool-temperate region of Japan. Plant Species Biol. 2005, 20, 47–56. [Google Scholar] [CrossRef]
- Dong, Y.; Hou, Q.; Sun, M.; Sun, J.; Zhang, B. Targeted Isolation of Antioxidant Constituents from Plantago asiatica L. and In Vitro Activity Assay. Molecules 2020, 25, 1825. [Google Scholar] [CrossRef]
- Tong, R.-C.; Qi, M.; Yang, Q.-M.; Li, P.-F.; Wang, D.-D.; Lan, J.-P.; Wang, Z.-T.; Yang, L. Extract of Plantago asiatica L. Seeds Ameliorates Hypertension in Spontaneously Hypertensive Rats by Inhibition of Angiotensin Converting Enzyme. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Ye, C.-L.; Hu, W.-L.; Dai, D.-H. Extraction of polysaccharides and the antioxidant activity from the seeds of Plantago asiatica L. Int. J. Biol. Macromol. 2011, 49, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.-Y.; Kim, H.-J.; Lee, S.-J. The effect of antioxidant and whitening action on Plantago asiatica L. leaf ethanol extract for health care. Technol. Health Care 2019, 27, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Huang, D.; Nie, S.; Xie, M. Polysaccharide from the seeds of Plantago asiatica L. protect against lipopolysaccharide-induced liver injury. J. Med. Food 2019, 22, 1058–1066. [Google Scholar] [CrossRef]
- Kim, K.H.; Bae, G.-U.; Kim, Y.K. Plantago asiatica Extracts Inhibit UV-induced Matrix Metalloproteinase-1 in Human Dermal Fibroblasts and Prevent Skin Photoaging in Hairless Mice. Bull. Korean Chem. Soc. 2015, 36, 659–664. [Google Scholar] [CrossRef]
- Lee, J.; Kim, N.; Jung, A.R.; Jang, J.H.; Lee, J.S.; Bae, J.T. Effect of Plantago asiatica L. extract on the anagen phase in human hair follicle dermal papilla cells. J. Cosmet. Dermatol. 2023, 22, 2324–2332. [Google Scholar] [CrossRef]
- Gokce, N.; Basgoz, N.; Kenanoglu, S.; Akalin, H.; Ozkul, Y.; Ergoren, M.C.; Beccari, T.; Bertelli, M.; Dundar, M. An overview of the genetic aspects of hair loss and its connection with nutrition. J. Prev. Med. Hyg. 2022, 63, E228. [Google Scholar]
- Mecklenburg, L.; Tobin, D.J.; Müller-Röver, S.; Handjiski, B.; Wendt, G.; Peters, E.M.; Pohl, S.; Moll, I.; Paus, R. Active hair growth (anagen) is associated with angiogenesis. J. Investig. Dermatol. 2000, 114, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.; Bergfeld, W. Diffuse hair loss: Its triggers and management. Clevel. Clin. J. Med. 2009, 76, 361–367. [Google Scholar] [CrossRef]
- Shin, J.Y.; Choi, Y.-H.; Kim, J.; Park, S.Y.; Nam, Y.J.; Lee, S.Y.; Jeon, J.H.; Jin, M.H.; Lee, S. Polygonum multiflorum extract support hair growth by elongating anagen phase and abrogating the effect of androgen in cultured human dermal papilla cells. BMC Complement. Med. Ther. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Olsen, E.A.; Iorizzo, M. Hair disorders. Harper’s Textb. Pediatr. Dermatol. 2019, 2103–2138. [Google Scholar]
- Tajima, M.; Hamada, C.; Arai, T.; Miyazawa, M.; Shibata, R.; Ishino, A. Characteristic features of Japanese women’s hair with aging and with progressing hair loss. J. Dermatol. Sci. 2007, 45, 93–103. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).