Abstract
The harmful effects of solar radiation on the skin are known and scientifically proven, with recent studies indicating that not only ultraviolet (UV) radiation but also infrared (IR) radiation contributes to skin photoaging and increases the risk of carcinogenesis. Infrared radiation is also responsible for the degradation of protective carotenoids in the skin, the disruption of calcium homeostasis, and the activation of apoptosis pathways. The biological mechanisms underlying these effects include an increased level of reactive oxygen species and increased expression of metalloproteinases in the skin. The aim of this study was to evaluate the photoprotective properties of 10 cold-pressed plant oils in the infrared spectral range from 1000 nm to 2500 nm by assessing their impact on the directional–hemispherical reflectance (DHR) of human skin after their topical application. This study was conducted in vivo on the skin of 12 volunteers, with non-invasive DHR measurements taken before and directly after the application of the oil and 30 min later. Additionally, the correlation between the oil’s compounds (chlorophyll a, chlorophyll b, lycopene, and β-carotene) and antioxidant activity, expressed as the DPPH free radical scavenging capacity, was analyzed in relation to the differences in the skin’s DHR observed. An interesting result was obtained in the context of protecting the skin against IR radiation. A statistically significant increase in the skin’s reflectance after the penetration of the oil (p < 0.05) was observed in the 1700–2500 nm range for the chokeberry, fig, pomegranate, and perilla oils, suggesting their potential as photoprotective agents against IR. These findings indicate that chokeberry, fig, pomegranate, and perilla oils may serve as ingredients in cosmetic formulations designed for broad-spectrum skin photoprotection, complementing traditional UV filters with additional protection against infrared radiation. However, further research is needed to confirm these findings in a larger population.
1. Introduction
Solar radiation is high-energy electromagnetic radiation. Only a limited spectral range of solar radiation reaches the Earth’s surface—mainly visible (VIS) radiation, infrared (IR) radiation, and a small portion of ultraviolet (UV) radiation [1,2].
The harmful biological effects of solar radiation on the skin are well known and scientifically proven [2,3,4]. UV causes skin burns, photoaging, and carcinogenesis [4,5,6]. The mechanisms underlying its adverse effects are the direct absorption of radiation by the skin and the subsequent conversion of the absorbed energy into biochemical signals and photosensitization [7,8]. Photosensitizers present in the skin absorb the energy from UV electromagnetic waves and enter an excited state. This causes the formation of reactive oxygen species (ROS) and reactive nitrogen species, which damage the DNA and cause adverse biological effects in the skin. Long-term and excessive UV exposure ultimately leads to the breakdown of the natural antioxidant mechanisms and autophagy mechanisms in cells [3,5].
For many years, sun protection has been focused on protection against UV radiation. Sunscreen products are designed to absorb, scatter, and reflect UV photons [8]. Other spectral bands, such as VIS and IR, have also been reported by scientists as harmful in recent years [7,8,9,10]. Sunscreens are not designed to reduce the effects of radiation other than UV radiation and therefore do not provide full skin protection. Even if they declare VIS or IR protection, further research into this field of photoprotection is still needed.
Although infrared radiation carries a lower energy than UV, it penetrates deeper into the skin and causes an increase in the skin’s temperature [7,9,10]. Repeated and long-term IR exposure increases the expression of metalloproteinases in the skin, triggers inflammatory mediator pathways, and leads to the formation of ROS, which are responsible for photoaging and an increased risk of carcinogenesis. IR may also contribute to the decomposition of the protective carotenoids in the skin, disrupt calcium homeostasis, and activate apoptosis pathways, including the expression of pro-apoptotic genes and receptors [9,10].
Skin photoprotection is defined as the mechanisms and strategies for protecting the skin from the harmful effects of solar radiation. It includes the body’s natural defense mechanisms (e.g., melanin synthesis, the action of antioxidant enzymes), as well as external methods such as avoiding excessive sun exposure, using sunscreen, topically applying cosmetics containing antioxidant compounds, and wearing protective clothing [11]. Sunscreens may contain two categories of filters: organic (also known as chemical) and inorganic (also known as mineral). UV filters absorb or reflect electromagnetic radiation in specific spectral ranges [2]. The effectiveness of sunscreens can be enhanced by adding plant extracts, herbs, and marine organisms to cosmetic formulations which have anti-inflammatory and antioxidant properties. They contain ascorbate, tocopherols, carotenoids, polyphenols, and flavonoids [6,11,12,13]. The use of synthetic sunscreens is controversial due to issues related to particle stability, interactions with other chemicals, penetration into the cells and into the bloodstream, allergic reactions, and the inhibition of vitamin D synthesis in the skin [2,14].
Plant-based ingredients also provide UV protection through three possible mechanisms: preventing the interaction of radiation with the skin, mitigating the damage caused, and reversing its effects by repairing and regenerating the skin [6,15,16]. Cold-pressed plant oils are among the natural ingredients considered for this purpose. When applied to the skin, they have been proven to strengthen the skin barrier and exhibit anti-inflammatory, antioxidant, wound-healing, antibacterial, anti-aging, and anti-cancer properties [17,18,19]. Plant oils applied to the skin penetrate into the first upper layers of the stratum corneum [20].
The main components of oils include triglycerides, phospholipids, fatty acids (saturated, monounsaturated, polyunsaturated), tocopherols, carotenoids, sterols, and waxes. Other components present in smaller amounts but that also influence the activity of oils include flavonoids (polyphenols, phenolic acids, flavones, procyanidins) and terpenes (e.g., squalene). Individual oils show quantitative and qualitative differences in their composition, which lead to subtle variations in their activity profiles [18]. The chemical composition of oils—particularly the presence of tocopherols, polyphenols, carotenoids, and fatty acids—allows for their broad application in cosmetics as potential protective agents [17,18,19,21,22]. However, studies in the literature highlight the need for further research on the photoprotective properties of plant oils and their use as sun filters. The current sun protection guidelines remain incomplete, as they do not fully consider the impact of IR radiation on the skin, nor do they account for variations in skin type, condition, or clinical status [8,23].
The introduction of effective sunscreens that can block over 95% of ultraviolet B radiation and nearly 90% of ultraviolet A radiation has led to an increase in the risk of IR radiation. The use of these sunscreens, which protect the skin from UV radiation, results in spending more time in the sun, exposing the skin to high doses of radiation in the remaining spectral range. In addition, IR radiation, through its vasodilatory effect, significantly increases the harmfulness of UV radiation. Hence, great emphasis should be placed on protecting the skin from the entire spectrum of solar radiation and not just a selected UV spectral band [8,24].
Electromagnetic radiation interacts with matter in three ways. Radiation can be reflected, transmitted, or absorbed by matter. The relationship between this three parameters is expressed by the equation
where
α + ρ + T = 1
- α = absorbance;
- ρ = reflectance;
- T = transmittance.
The reflection of radiation is a physical phenomenon in which the energy flow of optical radiation incident on a surface is returned without the frequency of its monochromatic components being changed. Radiation can be reflected either in a regular manner (specular reflection) or in a diffuse manner. Traditionally, the total amount of reflected radiation consists of these two types of reflection [25]. It should also be noted that the optical characteristics of human skin are complex, as the skin is a dynamic, variable, and multilayered optical medium with different properties and chromophores. The skin absorbs and reflects electromagnetic radiation on the surface, but there is also the phenomenon of internal reflection and back-scattered radiation [26,27,28]. The Kubelka–Munk equation describes the propagation of light in scattering and absorbing materials, such as biological tissues, in terms of their absorption and scattering coefficients. Scattering in the tissue leads to a change in the direction of light propagation. The Kubelka–Munk theory defines two fluxes: one corresponding to forward-scattered light and the other to back-scattered light. The forward-scattered flux describes the transmission properties of the medium, represented by a transmission coefficient, while the back-scattered flux provides information about the tissue’s reflection properties, described by a reflection coefficient [27,29,30].
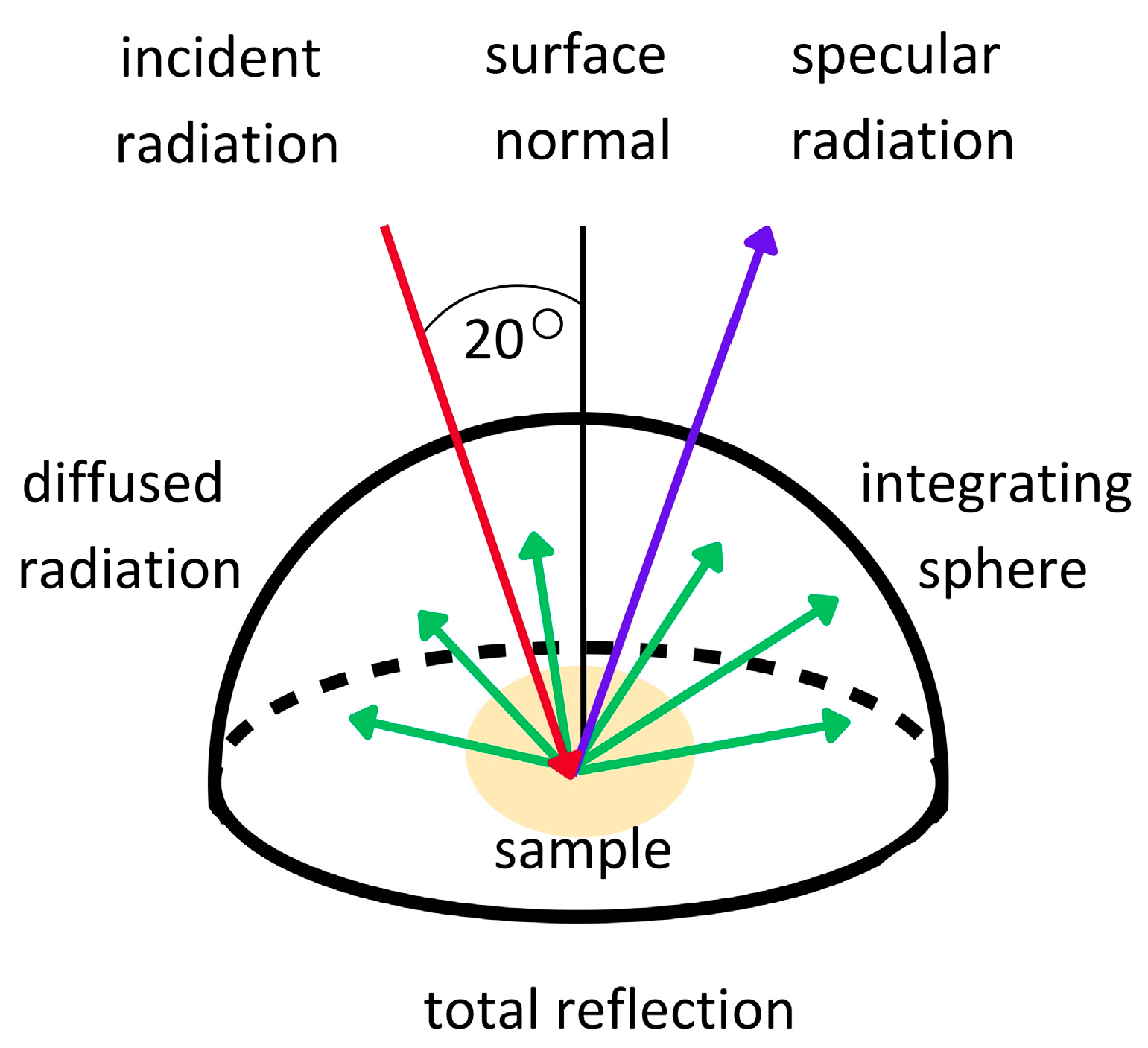
The reflection of radiation from a surface measured in directional–hemispherical geometry is expressed in terms of the directional–hemispherical reflectance (DHR), hereinafter referred to as reflectance [31]. DHR refers to the total fraction of the incident power on a unit area of a surface reflected into the upper hemisphere per the unit area of the surface at a specific wavelength [32]. In contrast, the directional reflectance is the concept of the reflection in a specific direction. DHR is particularly important when it is necessary to measure how radiation is reflected from surfaces in different directions. Therefore, DHR more accurately determines the interaction of electromagnetic waves with a tested surface because all of the diffused reflection into the hemisphere is measured [33,34,35] (Figure 1). For this reason, DHR measurements are a good method for assessing the skin’s reflectance compared to measurements performed in the directional–directional geometry, and it is a potential direct and non-invasive method for assessing the photoprotective properties of ingredients or cosmetic products under in vivo conditions.
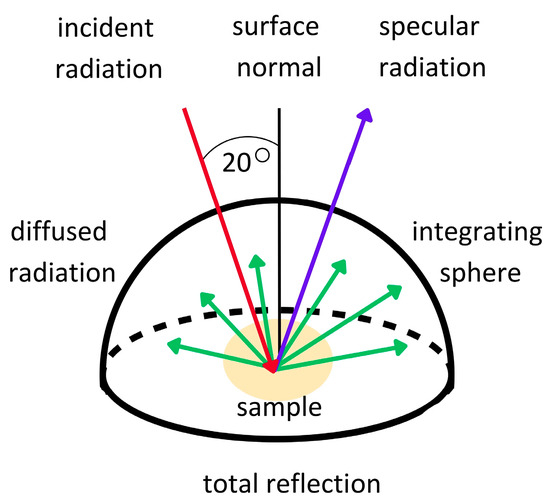
Figure 1.
Diagram illustrating the concept of directional–hemispherical reflectance measurements.
The aim of this study was to evaluate the photoprotective properties of 10 cold-pressed plant oils by determining their effect on the skin’s reflectance using the DHR parameter. Tested oils produced by the company OleoWita (Stawiec, Milicz, Poland) have been the object of several studies [36,37,38,39]. M. Michalak et al. tested bioactive compounds such as carotenoids and chlorophylls using a spectrophotometric method and evaluated the antioxidant properties based on the DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical scavenging capacity using electron paramagnetic resonance spectroscopy (EPR) [36]. The correlation between the data from the aforementioned study in terms of the oils’ compounds (chlorophyll a, chlorophyll b, lycopene, and β-carotene) and the antioxidant activity, expressed based on DPPH, in relation to the observed differences in the skin’s DHR was also analyzed.
2. Materials and Methods
2.1. Materials
In this study, ten cold-pressed plant oils produced by the company OleoWita (Milicz, Poland) were tested: 1—chokeberry (Aronia Melanocarpa Seed Oil); 2—elderberry (Sambucus Nigra Seed Oil); 3—blackcurrant (Ribes Nigrum Seed Oil); 4—rosehip (Rosa Canina Seed Oil); 5—fig (Ficus Carica Seed Oil); 6—pomegranate (Punica Granatum Seed Oil); 7—fenugreek (Trigonella Foenum-Graecum Seed Oil); 8—poppy (Papaver Rhoeas Seed Oil); 9—carrot (Daucus Carota Sativa Seed Oil); 10—perilla (Perilla Ocymoides Seed Oil) (Figure 2).
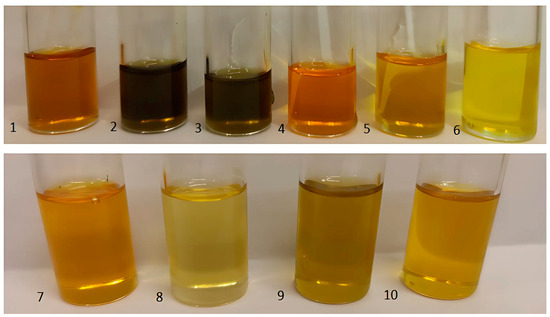
Figure 2.
Oils from kernels—chokeberry (1), elderberry (2), blackcurrant (3), rosehip (4), fig (5), and pomegranate (6)—and seeds—fenugreek (7), poppy (8), carrot (9), and perilla (10)—used in this study [photo by Michalak].
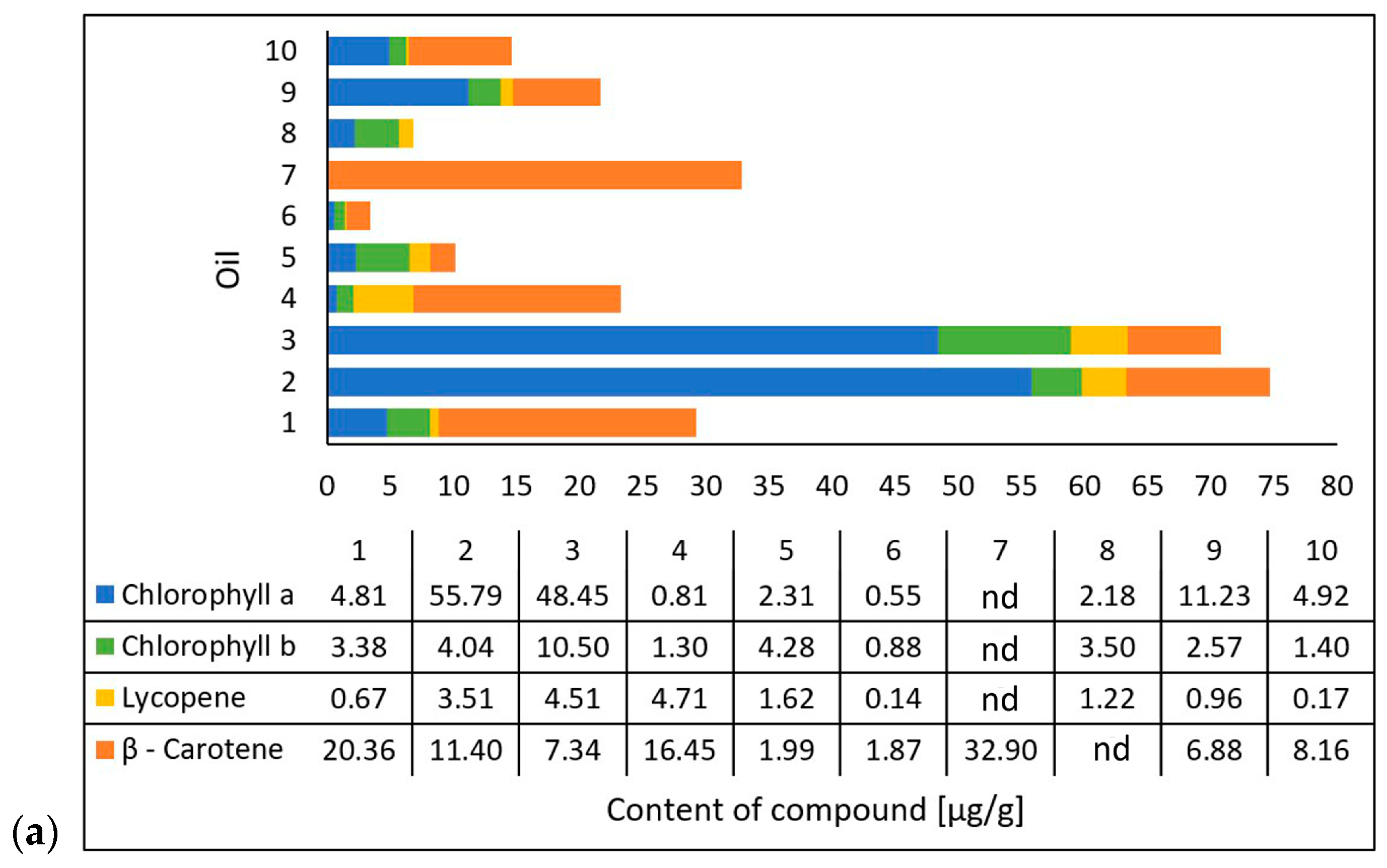
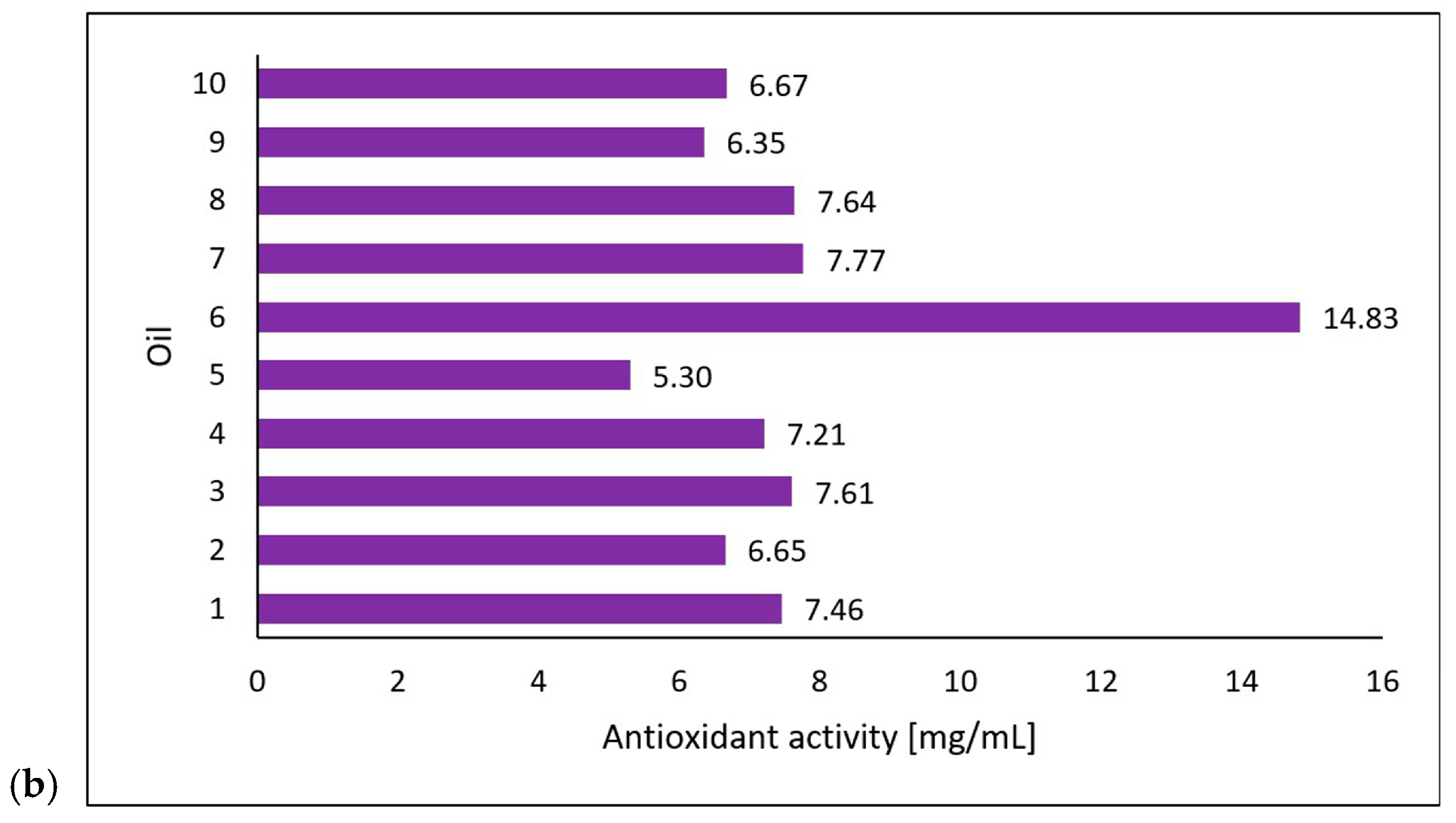
Michalak et al. [36] extracted chlorophyll a, chlorophyll b, lycopene, and β-carotene using an acetone/n-hexane mixture (6:4) at a ratio of 1 g of oil per 15 mL of mixture. They determined the content of the compounds using a UV-VIS spectrophotometric method at 453, 505, 645, and 663 nm and presented the results as micrograms per gram of the oil’s weight [36]. The researchers also evaluated the antioxidant properties of the oils based on their 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging capacity through electron paramagnetic resonance spectroscopy (EPR). They mixed 50 µL of oil and 0.5 mL of DPPH solution and, after incubating it in the dark for 20 min, performed measurements using the EPR method with reference to an oil sample as a positive control and DPPH solution plus ethanol as a negative control [36]. In cooperation with the researchers from the mentioned study, the tested oil samples were obtained. The results (on chlorophyll a, chlorophyll b, lycopene, β-carotene, and DPPH scavenging) (Figure 3) were correlated with those from the study conducted in this paper on the photoprotective properties of the oils on the skin using the DHR for spectral ranges of 1000–1700 nm and 1700–2500 nm.
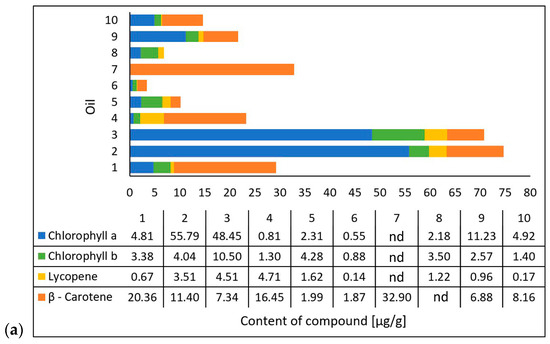
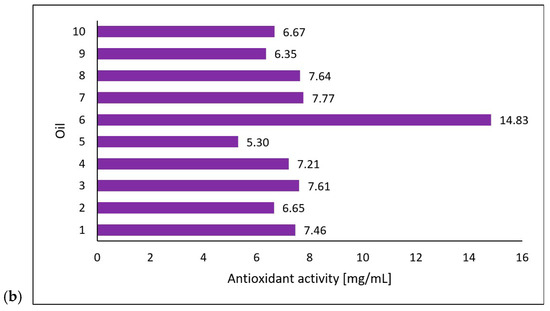
Figure 3.
Carotenoid and chlorophyll content (a) and antioxidant activity (b) of the tested oils (chokeberry (1), elderberry (2), blackcurrant (3), rosehip (4), fig (5), pomegranate (6), fenugreek (7), poppy (8), carrot (9), and perilla (10)) based on data from Michalak et al. [36]. Content of compounds expressed as micrograms per gram of oil weight [µg/g]. Antioxidant activity was measured using electron paramagnetic resonance and expressed as milligrams of the DPPH (2,2-diphenyl-1-picrylhydrazyl) radical neutralized by 1 mL of oil.
2.2. The Course of the Measurements
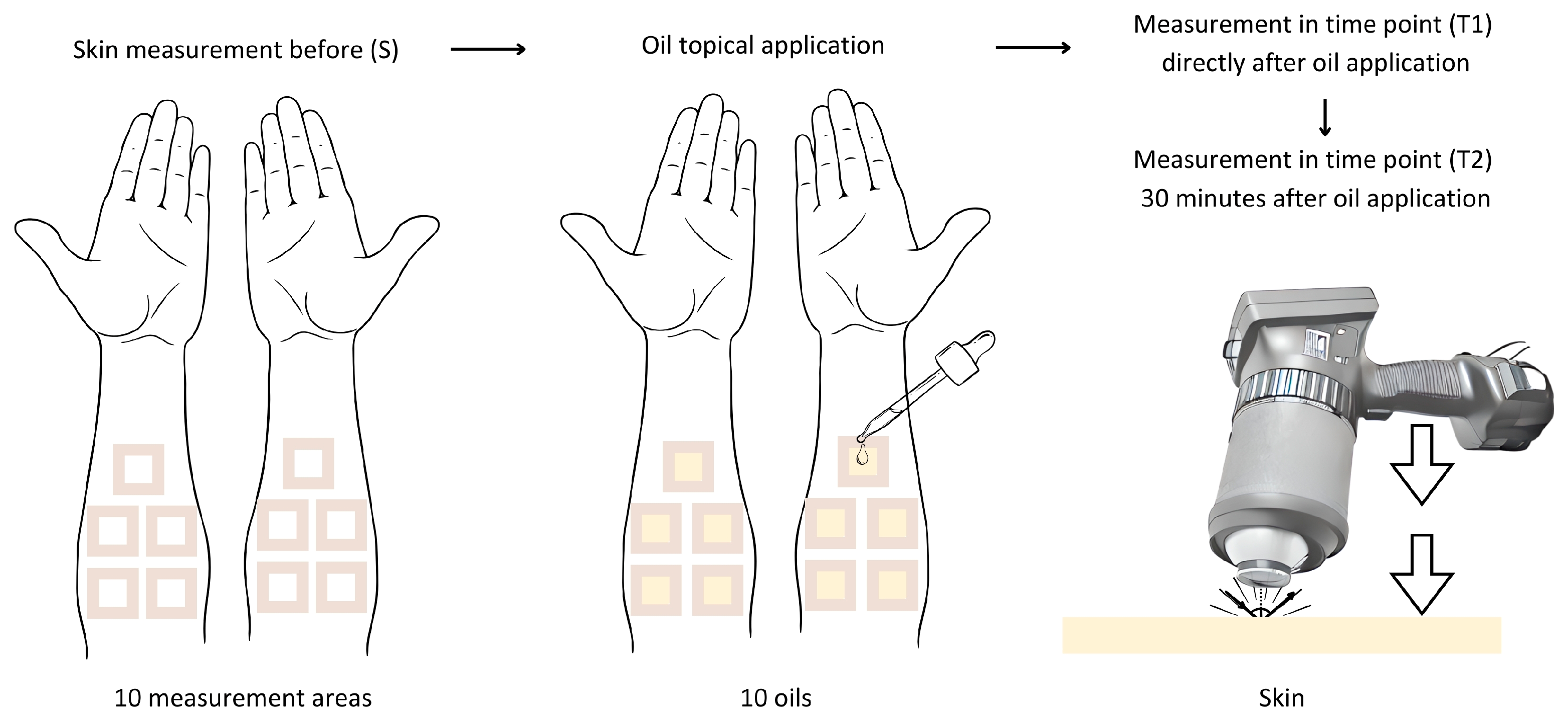
This study was approved by the Bioethics Committee of the Medical University of Silesia, No. PCN/CBN/0052/KB1/62/22. This study was conducted on a group of healthy volunteers—8 women and 4 men, aged between 30 and 45 years, classified as phototypes I-III according to the Fitzpatric scale. Ten oils were tested on each volunteer. Prior to this study, the volunteers completed a consent form and were interviewed using qualifying questions about any general and chronic medical conditions and dermatological or allergic reactions. Measurements were taken on the same day. The measurement area was hairless skin on the inner left and right forearms. The oils were applied to the skin at a rate of approximately 2 mg/cm2 (1 drop) on test areas of 3 cm × 3 cm that had previously been marked and surrounded with plasters (Figure 4). The average mass of a drop of each oil was previously standardized to determine the amount applied. The mass of a drop for all of the oils was in the range of 18–20 mg.
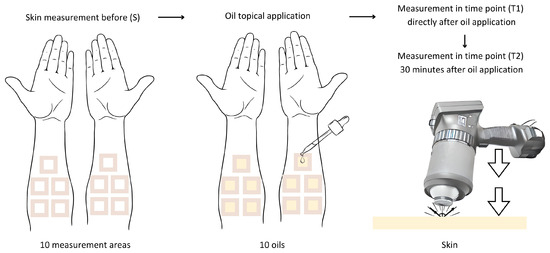
Figure 4.
Diagram illustrating the course of the measurements in this study.
A SOC 410 Solar DHR Reflectometer (Surface Optics Corporation, San Diego, CA, USA) was used to measure the directional–hemispherical reflectance of the skin at an incident angle of 20 degrees for seven spectral bands: 335–380 nm, 400–540 nm, 480–600 nm, 590–720 nm, 700–1100 nm, 1000–1700 nm, and 1700–2500 nm. Only two infrared bands were included in a further analysis: 1000–1700 nm and 1700–2500 nm. The SOC 410 Solar DHR Reflectometer has an integrating sphere that captures the light reflected from the tested material and integrates reflections from all directions.
In the first part of this study, the DHR was measured: before the application of oil (S), immediately after the oils’ application (T1), and 30 min after the oils’ application (T2). The duration of 30 min allowed for the penetration of the oil samples applied onto the skin. After the measurements, the oils were washed off the skin. None of the volunteers reported skin irritation or allergic reactions, which was directly visually checked and questioned. The effect of the oils on the skin’s reflectance was evaluated in the study group. A quantitative comparative analysis of the skin’s reflectance before and after the oils’ application onto the skin in spectral bands of 1000–1700 nm and 1700–2500 nm was used to assess the photoprotective properties of the tested oils. If the skin’s reflectance changes after the oil’s application (increases or decreases), the oil shows photoprotective properties.
A control group was not used due to the lack of a suitable reference standard. There are currently no established standards for IR protection, and there are no verified and widely accepted IR sunscreens that reflect radiation. In addition, a UV sunscreen could not be used as a comparative standard due to the fundamental differences in the characteristics of UV and IR electromagnetic waves. This study is therefore a step towards understanding and exploring IR photoprotection, and further research is needed.
In the second part of this study, the reflectance values of the skin before the oil’s application (S) were subtracted from the results of the reflectance values measured immediately after the oil’s application (T1) and 30 min after the oil’s application (T2), and the calculated differences (T1-S and T2-S) were obtained for each person in two wavelength ranges, 1000–1700 nm and 1700–2500 nm, for each of the oils. The active components of the oils—chlorophyll a, chlorophyll b, lycopene, β-carotene, and antioxidant activity—were selected based on data from the literature [36], and the average value for each component for the ten oils was correlated with the median reflectance based on the calculated differences (T1-S and T2-S) in the study group for each of the oils in the two wavelength ranges, 1000–1700 nm and 1700–2500 nm.
2.3. The Statistical Analysis
The statistical analysis was conducted using the licensed software Statistica 13 (StatSoft, Kraków, Poland) and an Excel spreadsheet. The normal distribution of the reflectance values was tested using the Shapiro–Wilk test. Where the data did not conform to a normal distribution, the nonparametric ANOVA Friedman test with a post hoc Dunn’s test was used to assess intergroup differences. The Spearman’s rank test was used to assess the correlations. Results were considered statistically significant at p < 0.05.
3. Results
3.1. Analysis of the Directional–Hemispherical Reflectance
3.1.1. The Spectral Range of 1000–1700 nm
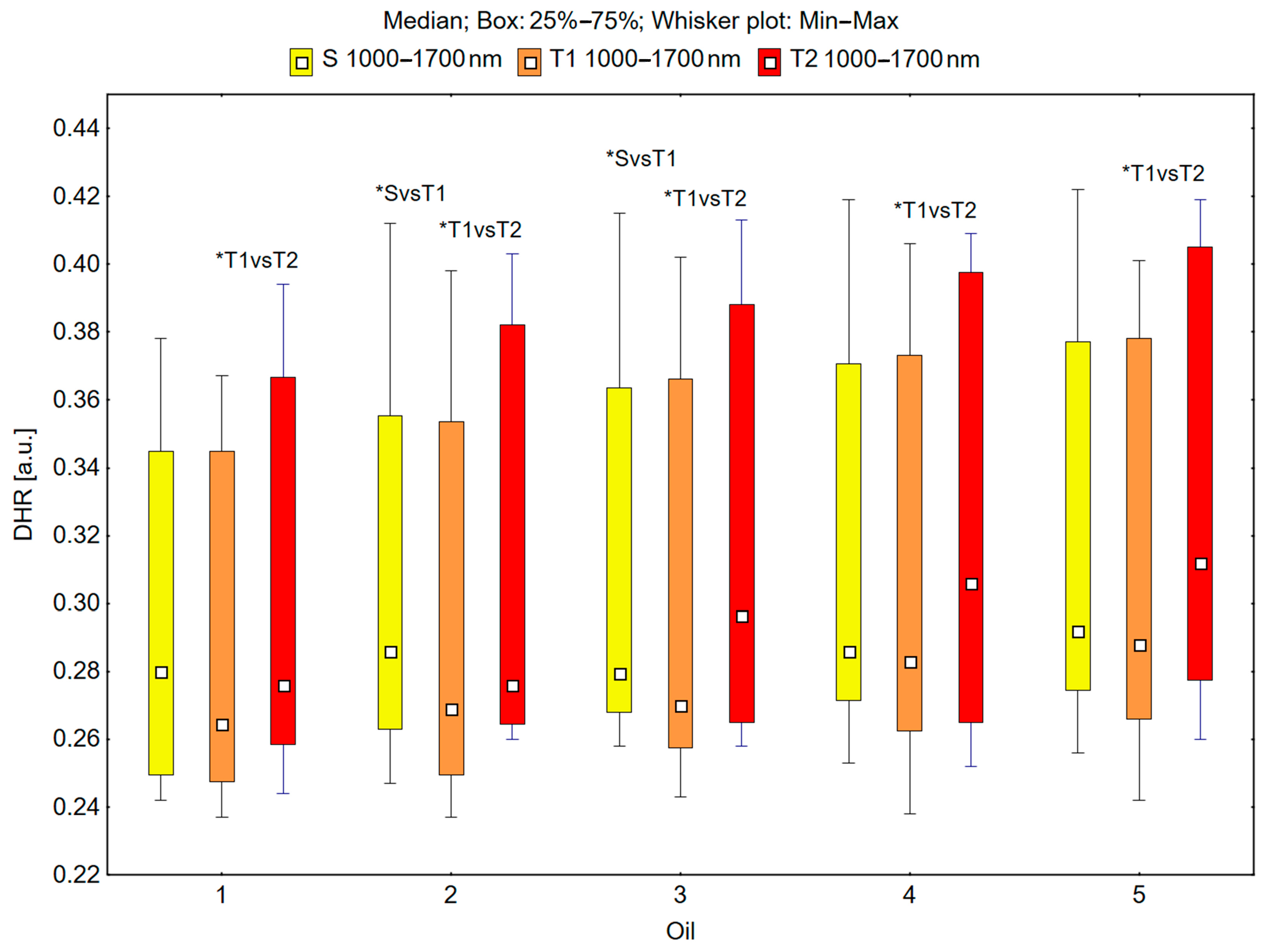
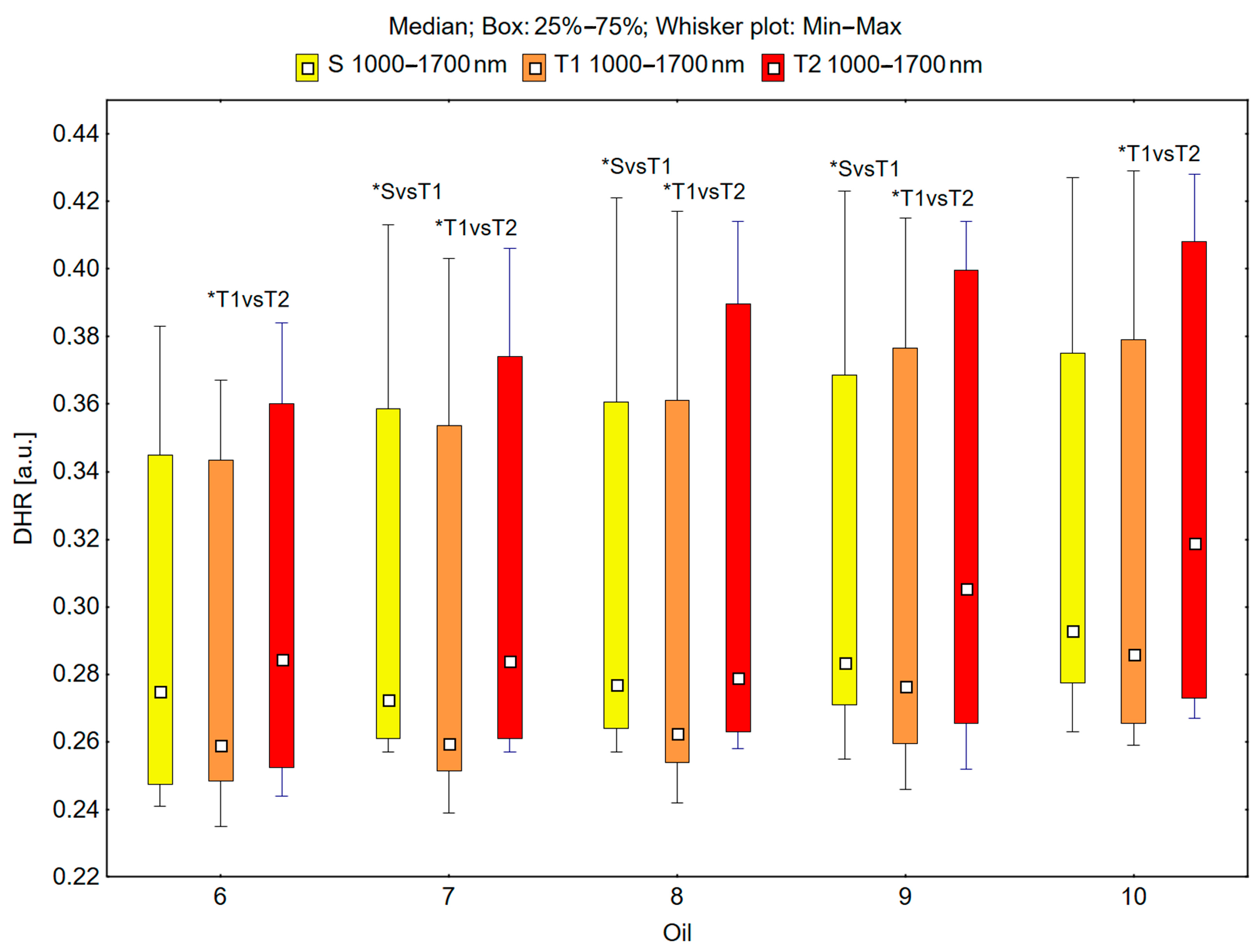
In the spectral range of 1000–1700 nm, the skin’s reflectance measured before oil application (S), immediately after oil application (T1), and 30 min after oil application (T2) differed statistically significantly for the oils: chokeberry (1): p = 0.049; elderberry (2): p = 0.018; blackcurrant (3): p = 0.009; rosehip (4): p = 0.017; fig seeds (5): p = 0.007; pomegranate (6): p = 0.011; fenugreek (7): p = 0.001; poppy (8): p = 0.018; carrot (9): p = 0.018; perilla (10): p = 0.028 (Figure 5 and Figure 6).
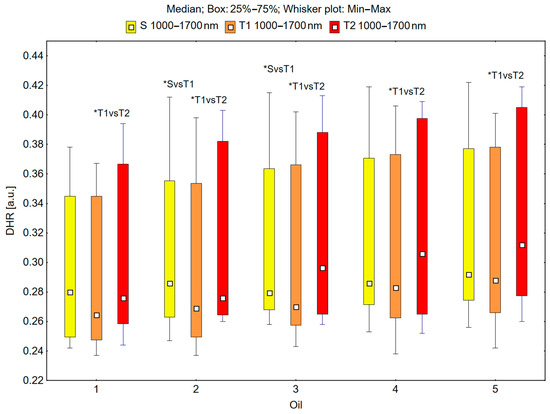
Figure 5.
Directional–hemispherical reflectance (DHR) in the spectral range of 1000–1700 nm of the skin before the oil’s application (S), immediately after the oil’s application (T1), and 30 min after the oil’s application (T2) measured for chokeberry (1), elderberry (2), blackcurrant (3), rosehip (4), and fig (5) oils; * p < 0.05.
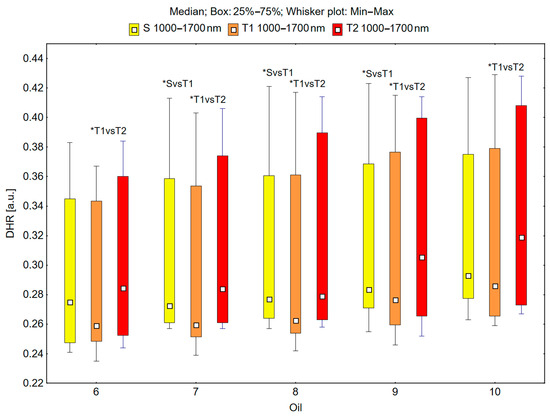
Figure 6.
Directional–hemispherical reflectance (DHR) in the spectral range of 1000–1700 nm of the skin before the oil’s application (S), immediately after the oil’s application (T1), and 30 min after the oil’s application (T2) for pomegranate (6), fenugreek (7), poppy (8), carrot (9), and perilla (10) oils; * p < 0.05.
It could be observed that the median skin reflectance immediately after the application of the oils (T1) was lower than the median reflectance of the skin before the oils’ application (S). A statistically significant result (pS vs. T1 < 0.05) was obtained using the following oils: elderberry (2), blackcurrant (3), fenugreek (7), poppy (8), and carrot (9) (Figure 5 and Figure 6). The percentage changes in these values were as follows: −13.7% for elderberry (2), −13.3% for blackcurrant (3), −13.7% for fenugreek (7), and −12.9% for poppy (8). The skin’s reflectance 30 min after the application of the oils (T2) was statistically significantly higher than the skin’s reflectance immediately after the oils’ application (T1) (pT1 vs. T2 < 0.05) for all ten tested oils (Figure 5 and Figure 6). The percentage changes in these values were as follows: 4.3% for chokeberry (1), 2.6% for elderberry (2), 9.8% for blackcurrant (3), 8.1% for rosehip (4), 8.3% for fig (5), 9.8% for pomegranate (6), 9.4% for fenugreek (7), 6.3% for poppy (8), 10.5% for carrot (9), and 11.5% for perilla (10). There were no statistically significant differences in the skin’s reflectance before the oils’ application (S) and 30 min after the oils’ application (T2) for any of the ten oils (Figure 5 and Figure 6).
3.1.2. The Spectral Range of 1700–2500 nm
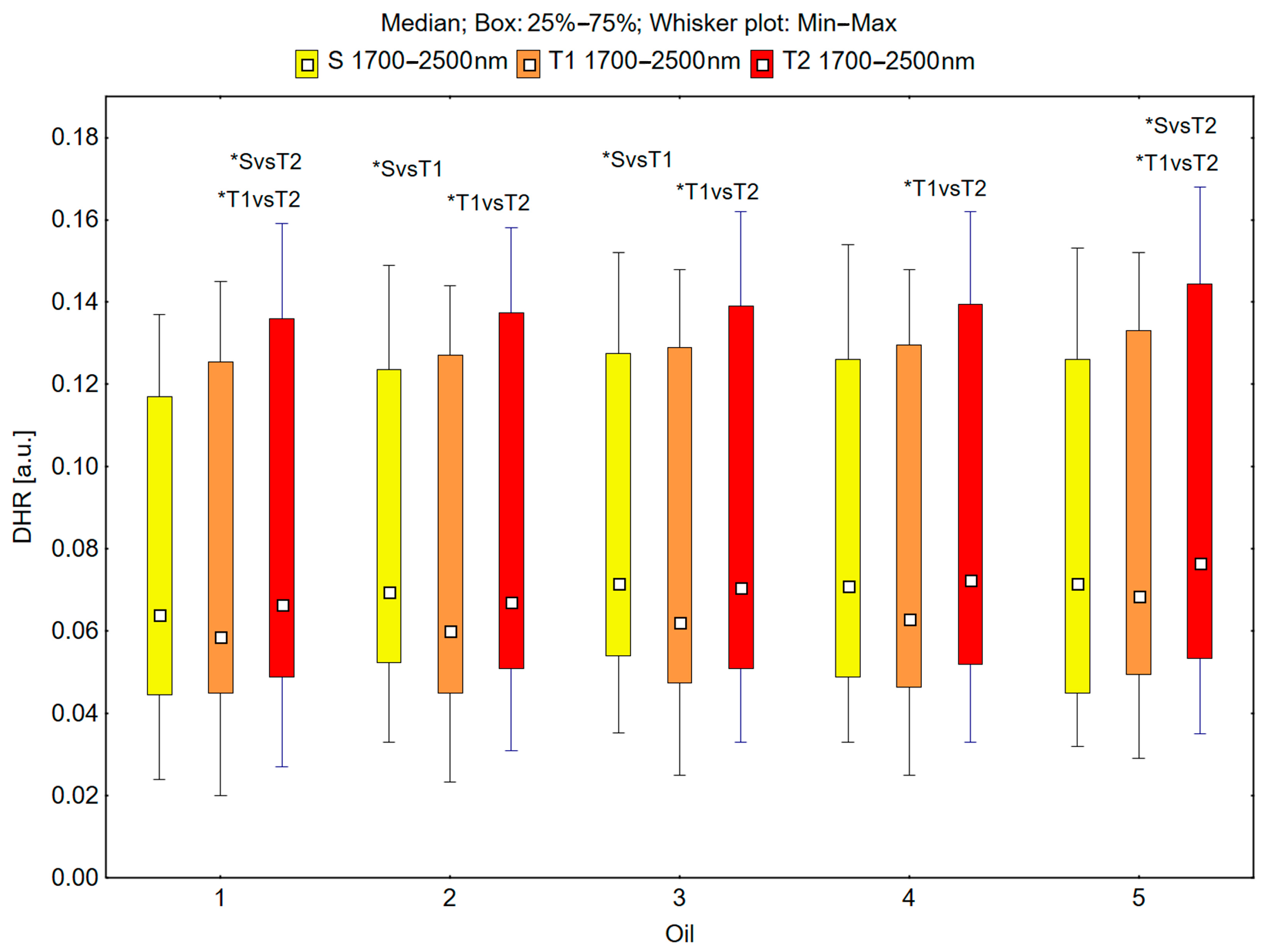
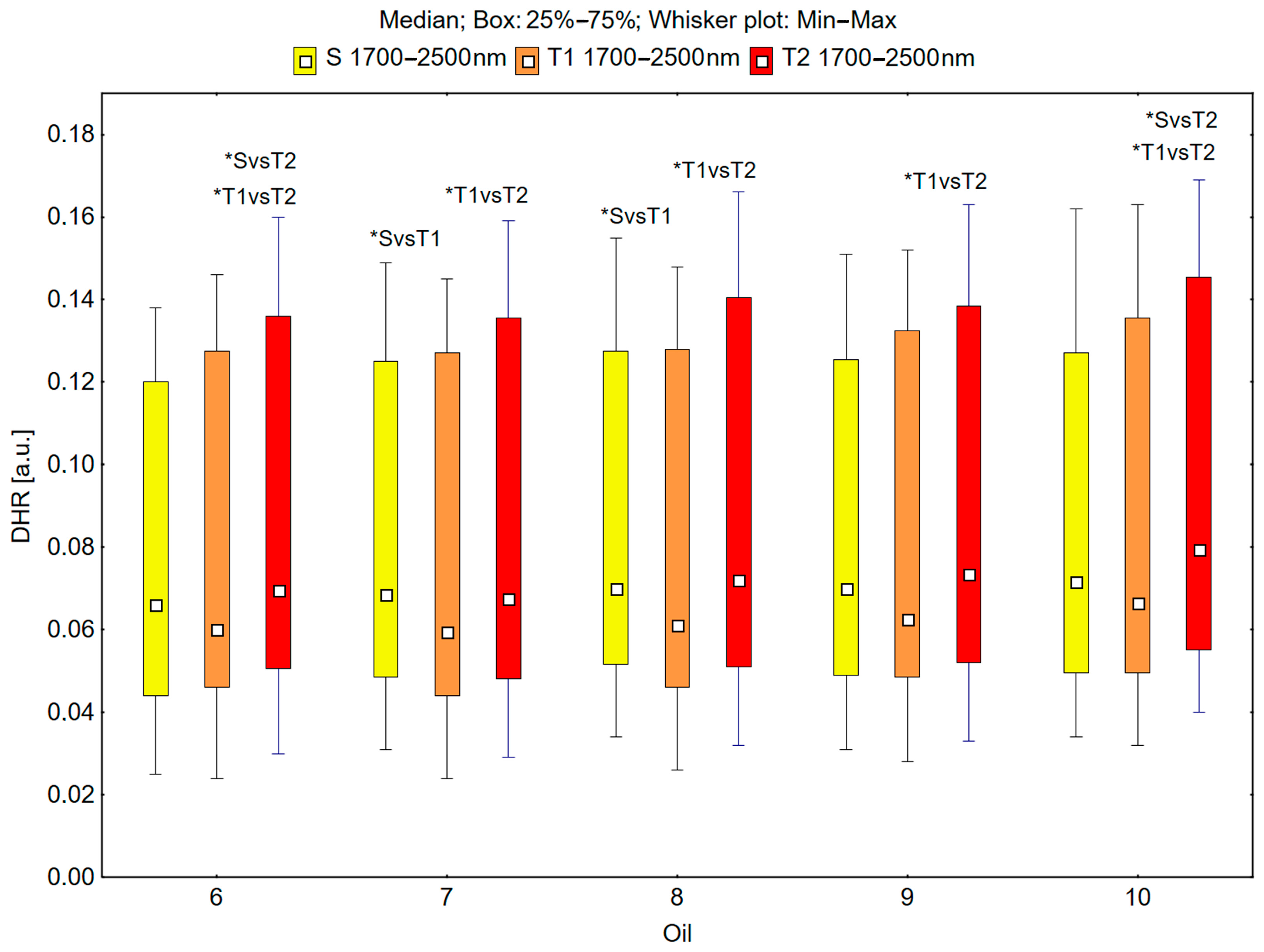
In the spectral range of 1700–2500 nm, the skin’s reflectance measured before the oil’s application (S), immediately after the oil’s application (T1), and 30 min after the oil’s application (T2) differed statistically significantly for the oils: chokeberry (1): p = 0.001; elderberry (2): p = 0.010; blackcurrant (3): p = 0.005; rosehip (4): p = 0.004; fig (5): p = 0.001; pomegranate (6): p = 0.001; fenugreek (7): p = 0.009; poppy (8): p = 0.009; carrot (9): p = 0.002; perilla (10): p = 0.001 (Figure 7 and Figure 8).
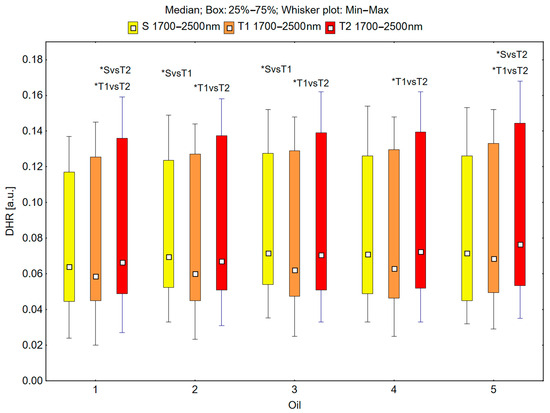
Figure 7.
Directional–hemispherical reflectance (DHR) in the spectral range of 1700–2500 nm of the skin before oil application (S), immediately after oil application (T1), and 30 min after oil application (T2) measured for the chokeberry (1), elderberry (2), blackcurrant (3), rosehip (4), and fig (5) oils; * p < 0.05.
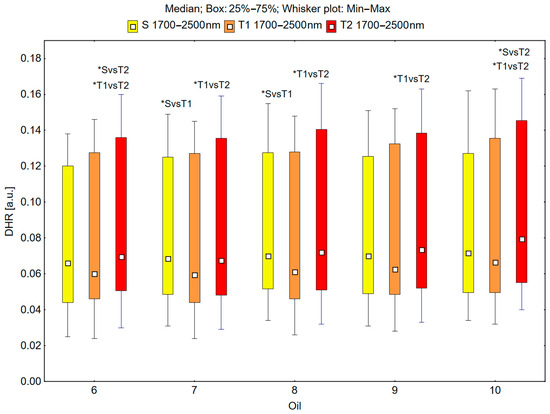
Figure 8.
Directional–hemispherical reflectance (DHR) in the spectral range of 1700–2500 nm of the skin before oil application (S), immediately after oil application (T1), and 30 min after oil application (T2) measured for the pomegranate (6), fenugreek (7), poppy (8), carrot (9), and perilla (10) oils; * p < 0.05.
The median reflectance of the skin immediately after the oil’s application (T1) was lower than the median reflectance of the skin before the oil’s application (S). A statistically significant result (pS vs. T1 < 0.05) was obtained using the following oils: elderberry (2), blackcurrant (3), fenugreek (7), and poppy (8) (Figure 7 and Figure 8). The percentage changes in these values were as follows: −13.7% for elderberry (2), −13.3% for blackcurrant (3), −13.7% for fenugreek (7), and −12.9% for poppy (8). The skin’s reflectance 30 min after the application of the oil (T2) was statistically significantly higher than the skin’s reflectance immediately after the application of the oil (T1) (pT1 vs. T2 < 0.05) for all ten oils (Figure 7 and Figure 8). The percentage changes in these values were as follows: 13.7% for chokeberry (1), 11.7% for elderberry (2), 13.7% for blackcurrant (3), 15.1% for rosehip (4), 11.7% for fig (5), 15.8% for pomegranate (6), 13.4% for fenugreek (7), 18.0% for poppy (8), 17.6% for carrot (9), and 19.5% for perilla (10). There were also statistically significant differences in the skin’s reflectance before the application of the oil (S) and 30 min after the application of the oil (T2) (pS vs. T2 < 0.05) for the chokeberry (1), fig (5), pomegranate (6), and perilla (10) oils, where the median reflectance increased (Figure 7 and Figure 8). The percentage changes in these values were as follows: 3.9% for chokeberry (1), 7.0% for fig (5), 5.3% for pomegranate (6), and 11.2% for perilla (10).
3.2. Analysis of the Correlation Between the Directional–Hemispherical Reflectance of and the Compounds in the Oils
Based on data from the literature [36], it was determined that the oils varied in their compositions of compounds. The oils with the highest chlorophyll a content were elderberry oil (2) and blackcurrant oil (3), while the oil with the lowest chlorophyll a content was the pomegranate oil (6). The oils with the highest lycopene contents were the rosehip oil (4), the blackcurrant oil (3), and the elderberry oil (2), while the oils with the lowest lycopene contents were the pomegranate oil (6) and the perilla oil (10) (Figure 3a).
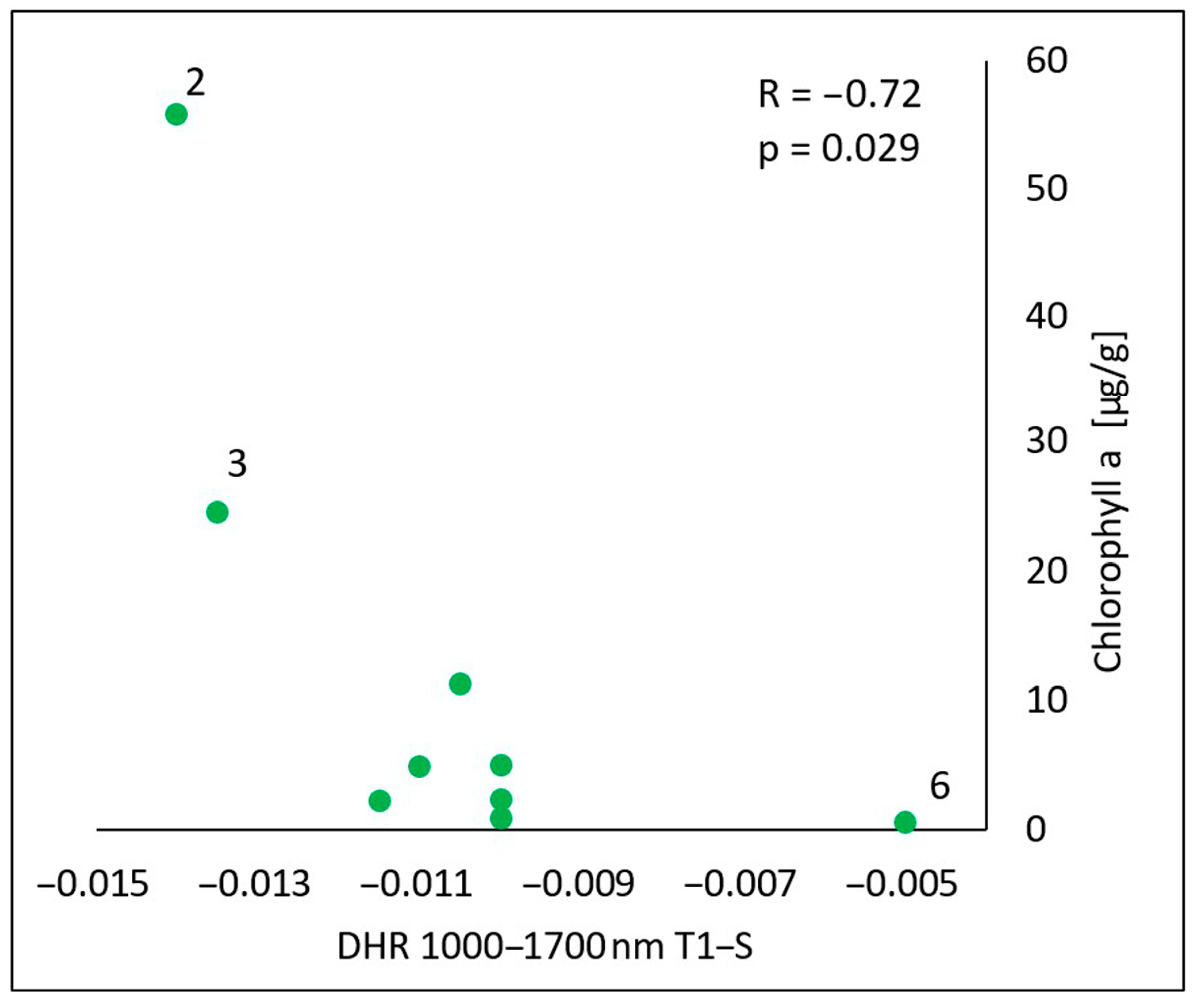
In the spectral range of 1000–1700 nm, there was a statistically significant negative correlation (R = −0.72, p = 0.029) between the content of chlorophyll a in the oils and the difference in the reflectance obtained by subtracting the value measured for the skin before the oil’s application (S) from the value measured immediately after the oil’s application (T1) (T1-S) (Figure 9). The higher the content of chlorophyll a in the oil, the more the skin’s reflectance decreased as a result of the oil’s application.
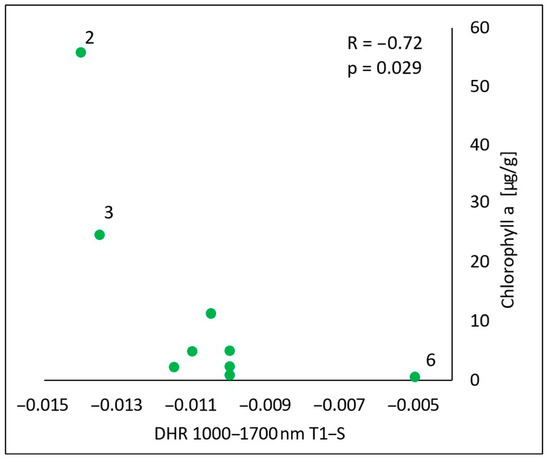
Figure 9.
Correlations between the calculated differences in the skin’s directional–hemispherical reflectance (DHR) measured immediately after oil application and before oil application (T1-S) and the chlorophyll a in the spectral range of 1000–1700 nm: 2—elderberry oil; 3—blackcurrant oil; 6—pomegranate oil.
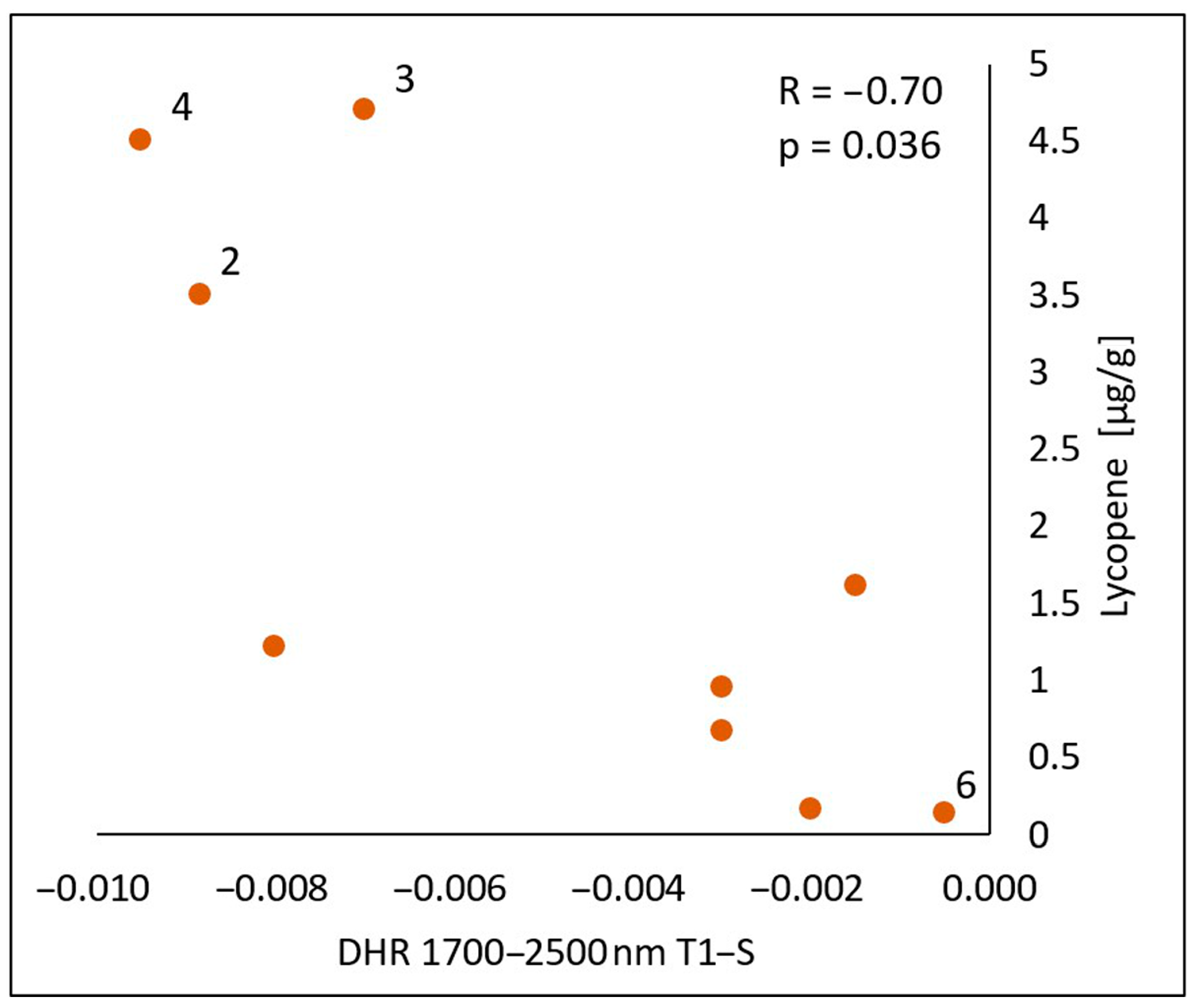
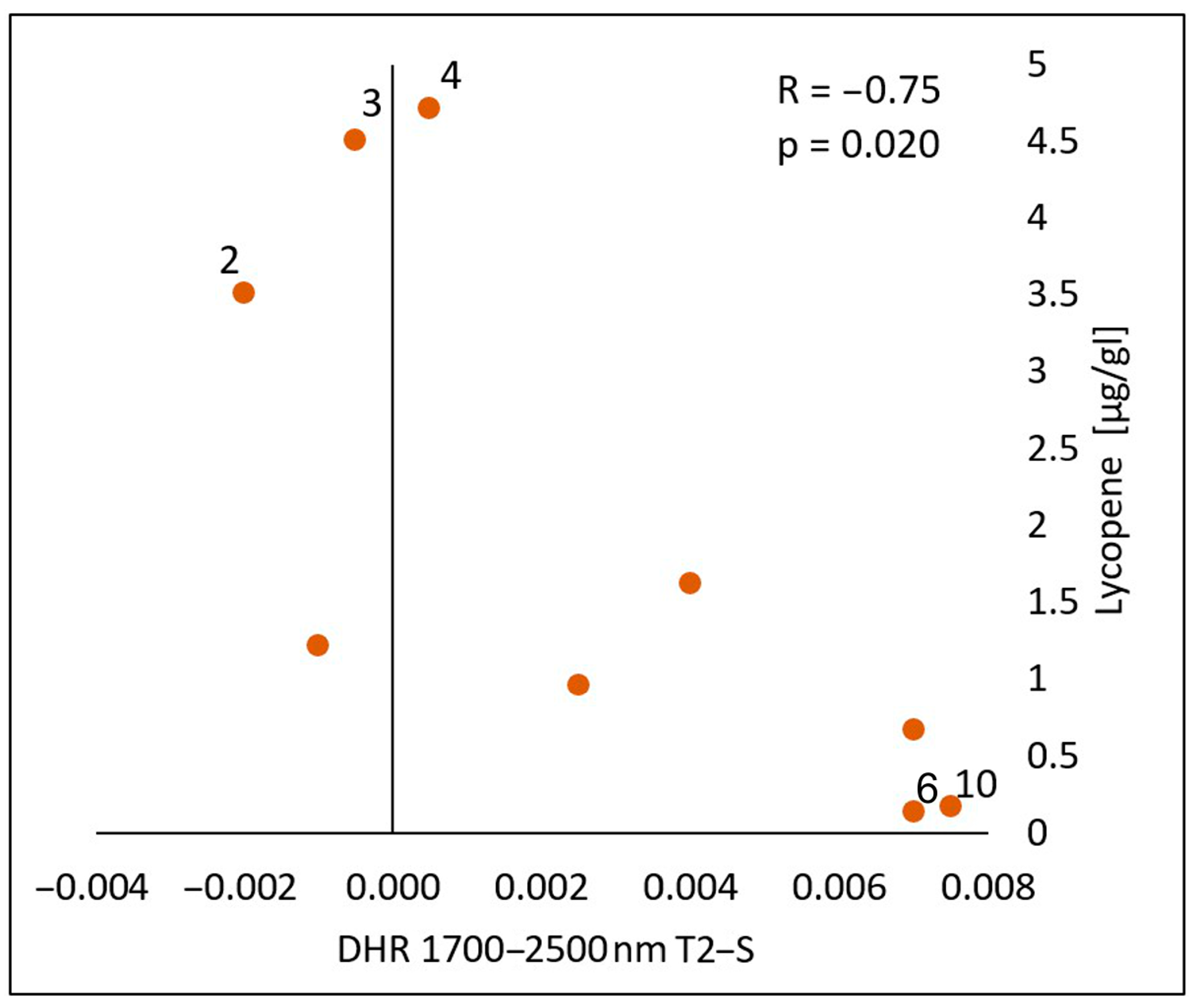
In the spectral range of 1700–2500 nm, a statistically significant negative correlation was found between the lycopene content and the calculated differences in both the reflectance measurements immediately after the oil’s application (T1-S) (Figure 10) and 30 min after the application of the oil onto the skin (T2-S) (Figure 11), respectively (R = −0.70, p = 0.036) (R = −075, p = 0.020). As with chlorophyll a, an increase in the lycopene content was reflected in a decrease in the DHR values.
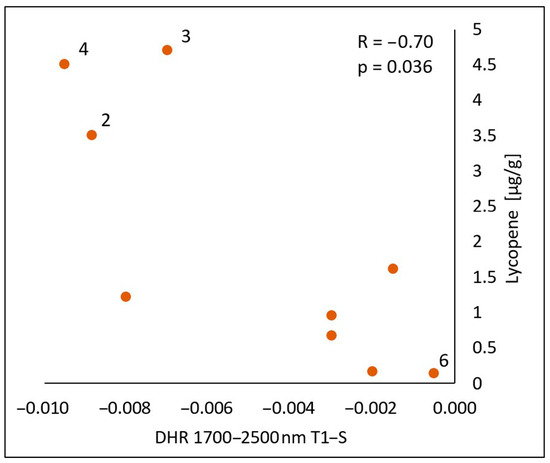
Figure 10.
Correlations between the calculated differences in the skin’s directional–hemispherical reflectance (DHR) measured immediately after oil application and before oil application (T1-S) and the content of lycopene in the spectral range of 1700–2500 nm: 2—elderberry oil; 3—blackcurrant oil; 4—rosehip oil; 6—pomegranate oil.
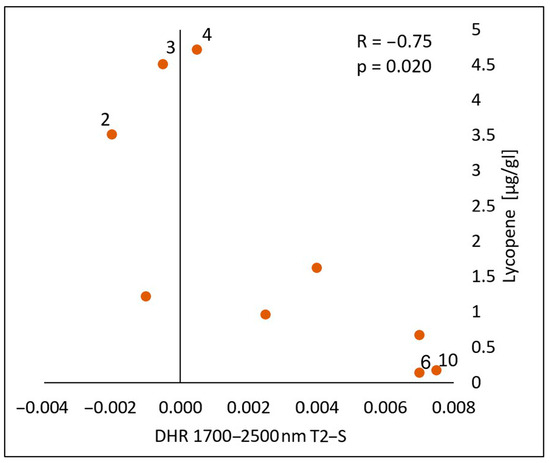
Figure 11.
Correlations between the calculated differences in the skin’s directional–hemispherical reflectance (DHR) measured 30 min after oil application and before oil application (T2-S) and the content of lycopene in the spectral range of 1700–2500 nm: 2—elderberry oil; 3—blackcurrant oil; 4—rosehip oil; 6—pomegranate oil; 10—perilla.
Taking into account the difference in the reflectance between the skin immediately after the oil’s application and before the oil’s application (T1-S), in the spectral range of 1000–1700 nm, the content of β-carotene in the oils and the antioxidant potential based on the DPPH method showed no effect on the skin’s reflectance. The chlorophyll b content showed a borderline value (R= −0.61, p = 0.081) (Table 1).

Table 1.
Correlations between the calculated differences in the skin’s directional–hemispherical reflectance (DHR) measured at different examination points and the content of oil compounds in the two spectral ranges of 1000–1700 nm and 1700–2500 nm.
4. Discussion
The present study hypothesized that plant oils might protect the skin from IR radiation. This study aimed to evaluate the photoprotective properties of ten plant oils by measuring their effect on the skin’s directional–hemispherical reflectance (DHR), a parameter that describes the amount of radiation reflected by the skin. A challenge in the development of IR photoprotection is the lack of standardized in vitro or in vivo tests for validating IR photoprotective properties. Unlike UV-induced erythema and pigmentation, which serve as well-established biological endpoints for UV protection, no such measurable, non-invasive endpoints have been identified for IR protection in human skin [40].
The results of this study provide important information on the potential photoprotective properties of cold-pressed plant oils. It was shown that some oils, such as chokeberry, fig, pomegranate, and perilla oils, have the ability to increase the reflection of radiation in the near-infrared range, 1700–2500 nm, after their penetration into the epidermis. This finding suggests that these oils may protect the skin from the harmful effects of infrared radiation, which is often overlooked in the context of photoprotection. These results are particularly important in light of the growing awareness of the harmful effects of IR on the skin, which can lead to photoaging and cancer risk [41].
Another aspect of this study is the observation of the variability in the skin’s reflectance over time after the application of the oils. The initial decrease in the reflectance (T1-S) after the application of the oils may indicate their ability to absorb IR radiation, suggesting a protective mechanism against its penetration into the deeper layers of the skin. In turn, the later increase in the reflectance (T2–T1) may be related to the penetration of the oils into the epidermis and a change in its optical properties. This result suggest the potential of the directional–hemispherical reflectance method as a tool for assessing the kinetics of cosmetics’ penetration and their protective properties, an area to explore further in the future.
An interesting result of this study is the lack of a clear correlation between the content of β-carotene in the tested oils and changes in the skin’s reflectance. From the perspective of IR photoprotection, a particularly valuable result was the negative correlation observed between the lycopene content and the changes in the reflectance between T2 (after the oil’s absorption) and the baseline measurements (S). This finding suggests that lycopene absorbs IR radiation, which is consistent with fundamental physical principles—higher absorption results in lower reflectance and transmittance values.
Regarding the antioxidant properties of the tested oils, this study sought to determine whether the current approach to IR photoprotection, which relies on the topical application of antioxidants, correlates with changes in the skin’s reflectance. There is scientific evidence that IR induces skin damage via a mechanism that generates ROS in the skin [42,43]. For this reason, IR photoprotection of human skin mainly involves topically applied antioxidants. The results showed no correlation between the antioxidant potential of the oils and changes in skin reflectance. However, the oil with the highest antioxidant activity—pomegranate oil—also had the lowest overall concentration of the tested bioactive compounds but showed a statistically significant increase in the skin’s reflectance at wavelengths of 1700–2500 nm (T2-S, T2-T1) and 1000–1700 nm (T2-T1). This suggests that other components of this oil reported in the research, such as punicic acid [44], may be responsible for its photoprotective effects or other properties. Future studies should investigate the relationship between reflectance and the presence of specific compounds such as punicic acid to elucidate the photoprotective mechanisms of pomegranate oil further.
The results of the present study involve an additional practical aspect beyond photoprotection. The effect of reducing the reflectance of IR radiation through the skin after the application of oil may have a beneficial effect not in the context of radiation protection but in the context of physiotherapy or aesthetic medicine treatments. Infrared radiation is commonly used in physical therapy, especially in treatments aiming to improve circulation, muscle relaxation, and pain relief [45]. Increased penetration of IR into the skin after the application of oils can intensify its therapeutic effect. In aesthetic medicine, IR radiation is used to stimulate the fibroblasts and collagen production, which helps improve the skin’s elasticity and reduce wrinkles [30]. The use of cold-pressed oils before treatments can increase the effectiveness of collagen therapies and improve the heat distribution. With the reflection of the radiation reduced immediately after the application of oils, IR energy can be used more effectively by the tissues. The use of oils before therapy may also affect the patients’ subjective feelings—oils can act as a thermal buffer, evenly distributing heat across the skin’s surface. However, the above-mentioned potential benefits are only speculation and require experimental confirmation.
In summary, this study represents an important step towards a better understanding of the IR photoprotective properties of plant oils and their potential use in cosmetology.
5. Conclusions
This study demonstrated that selected cold-pressed plant oils influence the skin’s reflectance properties in the infrared spectral band. In particular, chokeberry, fig, pomegranate, and perilla oils showed the greatest ability to increase the skin’s reflectance after their penetration into the epidermis in the range of 1700–2500 nm by 3.9%, 7.0%, 5.3%, and 11.2%, respectively. This suggests potential photoprotective properties against IR and harmful effects, such as photoaging or cancer risk. These oils could be an alternative or complement to synthetic sunscreens, especially in the context of protection against IR radiation.
Variability in the optical properties of the skin was observed after the application of the oils—the initial decrease in the reflectance indicated the absorption of IR radiation, whereas the later increase in the reflectance suggested a change in the optical properties of the skin. These results emphasize the potential of directional–hemispherical reflectance measurements as a non-invasive in vivo method for assessing the kinetics of cosmetics’ penetration and their protective effects.
The correlation between the reflectance changes and bioactive compounds, as well as antioxidant activity, suggests that certain chemical components may contribute to these effects. In this study, chlorophyll a and lycopene showed a negative correlation. No correlation was observed for chlorophyll b or β-carotene. Further research is needed to precisely determine the mechanisms responsible for these interactions and identify the compounds that are related to changes in skin reflectance.
Limitations of This Study
The limitations of this study include the small number of participants (12 volunteers) and its limitation to skin phototypes I–III according to the Fitzpatrick scale, which means that the results may not be representative of people with darker skin. In addition, this study evaluated the changes in skin reflectance over a time frame of up to 30 min. Future studies should include a broader range of skin phototypes to determine whether these findings are generalizable to different populations, as well as including an analysis with more frequent time point measurements and longer durations than 30 min. Despite these limitations, these findings provide valuable preliminary data supporting the role of plant oils in IR photoprotection of the skin and related mechanisms.
Author Contributions
Conceptualization: E.M., S.W. and M.M. Methodology: E.M. Validation: R.K. and P.B. Formal analysis: M.H-P. Investigation: E.M. Resources: M.M. Data curation: E.M. and A.B. Writing—original draft preparation: E.M. Writing—review and editing: S.W. Visualization: E.M. and M.H.-P. Supervision: S.W. Project administration: E.M. Funding acquisition: M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Bioethics Committee of the Medical University of Silesia, No. PCN/CBN/0052/KB1/62/22, on 21 June 2022.
Informed Consent Statement
Informed consent was obtained from all of the subjects involved in this study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the sensitive nature of the participants data.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Donya, M.; Radford, M.; ElGuindy, A.; Firmin, D.; Yacoub, M.H. Radiation in Medicine: Origins, Risks and Aspirations. Glob. Cardiol. Sci. Pract. 2014, 2014, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Skotarczak, K.; Osmola-Mańkowska, A.; Lodyga, M.; Polańska, A.; Mazur, M.; Adamski, Z. Photoprotection: Facts and Controversies. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 98–112. [Google Scholar] [PubMed]
- Tyrrell, R.M. Modulation of Gene Expression by the Oxidative Stress Generated in Human Skin Cells by UVA Radiation and the Restoration of Redox Homeostasis. Photochem. Photobiol. Sci. 2012, 11, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Omer, H. Radiobiological Effects and Medical Applications of Non-Ionizing Radiation. Saudi J. Biol. Sci. 2021, 28, 5585–5592. [Google Scholar] [CrossRef]
- Wang, M.; Charareh, P.; Lei, X.; Zhong, J.L. Autophagy: Multiple Mechanisms to Protect Skin from Ultraviolet Radiation-Driven Photoaging. Oxid. Med. Cell Longev. 2019, 2019, 8135985. [Google Scholar] [CrossRef]
- Saewan, N.; Jimtaisong, A. Natural Products as Photoprotection. J. Cosmet. Dermatol. 2015, 14, 47–63. [Google Scholar] [CrossRef]
- Sample, A.; He, Y.Y. Mechanisms and Prevention of UV-Induced Melanoma. Photodermatol. Photoimmunol. Photomed. 2018, 34, 13–24. [Google Scholar] [CrossRef]
- Lyons, A.B.; Trullas, C.; Kohli, I.; Hamzavi, I.H.; Lim, H.W. Photoprotection beyond Ultraviolet Radiation: A Review of Tinted Sunscreens. J. Am. Acad. Dermatol. 2021, 84, 1393–1397. [Google Scholar] [CrossRef]
- Hudson, L.; Rashdan, E.; Bonn, C.A.; Chavan, B.; Rawlings, D.; Birch-Machin, M.A. Individual and Combined Effects of the Infrared, Visible, and Ultraviolet Light Components of Solar Radiation on Damage Biomarkers in Human Skin Cells. FASEB J. 2020, 34, 3874–3883. [Google Scholar] [CrossRef]
- Fernández, E.; Fajarí, L.; Rodríguez, G.; Cócera, M.; Moner, V.; Barbosa-Barros, L.; Kamma-Lorger, C.S.; de la Maza, A.; López, O. Reducing the Harmful Effects of Infrared Radiation on the Skin Using Bicosomes Incorporating β-Carotene. Ski. Pharmacol. Physiol. 2016, 29, 169–177. [Google Scholar] [CrossRef]
- Kalemba-Drożdż, M.; Grzywacz-Kisielewska, A.; Cierniak, A. Surowce polifenolowe. Zastosowania i perspektywy (red). Oficyna Wydawnicza Kraków AFM 2022, 117–139. [Google Scholar]
- Solano, F. Photoprotection and Skin Pigmentation: Melanin-Related Molecules and Some Other New Agents Obtained from Natural Sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef] [PubMed]
- Darvin, M.; Zastrow, L.; Sterry, W.; Lademann, J. Effect of supplemented and topically applied antioxidant substances on human tissue. Ski. Pharmacol. Physiol. 2006, 19, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, V.K.; Sharma, N.; Sharma, V.; Verma, R.; Chandel, M.; Singh, R. Topical Sunscreens: A Narrative Review for Contact Sensitivity, Potential Allergens, Clinical Evaluation, and Management for their Optimal Use in Clinical Practice. Indian Dermatol. Online J. 2024, 15, 920–929. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC11616936/ (accessed on 7 April 2025).
- Kostyuk, V.; Potapovich, A.; Albuhaydar, A.R.; Mayer, W.; De Luca, C.; Korkina, L. Natural Substances for Prevention of Skin Photoaging: Screening Systems in the Development of Sunscreen and Rejuvenation Cosmetics. Rejuvenation Res. 2018, 21, 91–101. [Google Scholar] [CrossRef]
- Korać, R.R.; Khambholja, K.M. Potential of herbs in skin protection from ultraviolet radiation. Pharmacogn. Rev. 2011, 5, 164–173. [Google Scholar] [CrossRef]
- Lin, T.K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef]
- Vaughn, A.R.; Clark, A.K.; Sivamani, R.K.; Shi, V.Y. Natural Oils for Skin-Barrier Repair: Ancient Compounds Now Backed by Modern Science. Am. J. Clin. Dermatol. 2018, 19, 103–117. [Google Scholar] [CrossRef]
- Sarkar, R.; Podder, I.; Gokhale, N.; Jagadeesan, S.; Garg, V.K. Use of Vegetable Oils in Dermatology: An Overview. Int. J. Dermatol. 2017, 56, 1080–1086. [Google Scholar] [CrossRef]
- Patzelt, A.; Lademann, J.; Richter, H.; Darvin, M.E.; Schanzer, S.; Thiede, G.; Sterry, W.; Vergou, T.; Hauser, M. In vivo investigations on the penetration of various oils and their influence on the skin barrier. Ski. Res. Technol. 2012, 18, 364–369. [Google Scholar] [CrossRef]
- Poljšak, N.; Kočevar Glavač, N. Vegetable Butters and Oils as Therapeutically and Cosmetically Active Ingredients for Dermal Use: A Review of Clinical Studies. Front. Pharmacol. 2022, 13, 868461. [Google Scholar] [CrossRef] [PubMed]
- Poljšak, N.; Kreft, S.; Kočevar Glavač, N. Vegetable butters and oils in skin wound healing: Scientific evidence for new opportunities in dermatology. Phytother. Res. 2020, 34, 254–269. [Google Scholar] [CrossRef] [PubMed]
- Passeron, T.; Lim, H.W.; Goh, C.L.; Kang, H.Y.; Ly, F.; Morita, A.; Ocampo Candiani, J.; Puig, S.; Schalka, S.; Wei, L.; et al. Photoprotection according to Skin Phototype and Dermatoses: Practical Recommendations from an Expert Panel. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1460–1469. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Meinke, M.C.; Schanzer, S.; Albrecht, S.; Zastrow, L. Neue Aspekte bei der Entwicklung von Sonnenschutzmitteln. Hautarzt 2017, 68, 349–353. [Google Scholar] [CrossRef]
- Nicodemus, F.E.; Richmond, J.C.; Hsia, J.J.; Ginsberg, I.W.; Limperis, T. Geometrical Considerations and Nomenclature for Reflectance; National Institute of Standards and Technology: Washington, DC, USA, 1977. [Google Scholar]
- Bydlon, T.M.; Nachabé, R.; Ramanujam, N.; Sterenborg, H.J.; Hendriks, B.H. Chromophore Based Analyses of Steady-State Diffuse Reflectance Spectroscopy: Current Status and Perspectives for Clinical Adoption. J. Biophoton. 2015, 8, 9–24. [Google Scholar] [CrossRef]
- Anderson, R.R.; Parrish, J.A. The Optics of Human Skin. J. Investig. Dermatol. 1981, 77, 13–19. [Google Scholar] [CrossRef]
- Pleitez, M.A.; Hertzberg, O.; Bauer, A.; Lieblein, T.; Glasmacher, M.; Tholl, H.; Mäntele, W. Infrared Reflectometry of Skin: Analysis of Backscattered Light from Different Skin Layers. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 184, 220–227. [Google Scholar] [CrossRef]
- Jasiński, M. Modelling of light and human skin interaction using Kubelka-Munk theory. Pr. Nauk. Inst. Mat. I Inform. Politech. Częstochowskiej. 2011, 10, 71–81. [Google Scholar]
- Sliney, D.H.; Trokel, S.L. Laser-Tissue Interactions. In Medical Lasers and Their Safe Use; Springer: New York, NY, USA, 1993. [Google Scholar] [CrossRef]
- Pietrzykowski, J. Geometrie pomiaru stosowane w kolorymetrii i spektrofotometrii odbitego promieniowania optycznego i ich notacje. Pr. Inst. Elektrotechniki 2008, 237, 125–136. [Google Scholar]
- Tan, K.; Cheng, X.; Cheng, X. Modeling hemispherical reflectance for natural surfaces based on terrestrial laser scanning backscattered intensity data. Opt. Express 2016, 24, 22971–22988. [Google Scholar] [CrossRef]
- Wilczyński, S.; Deda, A.; Koprowski, R.; Banyś, A.; Błońska-Fajfrowska, B. The Use of Directional Reflectance Measurement for In Vivo Assessment of Protective Properties of Cosmetics in the Infrared Radiation Range. Photochem. Photobiol. 2017, 93, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Cooksey, C.C.; Allen, D.W.; Tsai, B.K. Reference Data Set of Human Skin Reflectance. J. Res. Natl. Inst. Stand. Technol. 2017, 122, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Owda, A.Y.; Salmon, N.; Casson, A.J.; Owda, M. The Reflectance of Human Skin in the Millimeter-Wave Band. Sensors 2020, 20, 1480. [Google Scholar] [CrossRef]
- Michalak, M.; Błońska-Sikora, E.; Dobros, N.; Spałek, O.; Zielińska, A.; Paradowska, K. Bioactive Compounds, Antioxidant Properties, and Cosmetic Applications of Selected Cold-Pressed Plant Oils from Seeds. Cosmetics 2024, 11, 153. [Google Scholar] [CrossRef]
- Ligęza, M.; Wyglądacz, D.; Tobiasz, A.; Jaworecka, K.; Franiczek, R.; Krzyżanowska, B.; Aniołowska, M.; Reich, A. Assessment of the Composition and Microbiological Purity of Cold-Pressed Oils Manufactured by OleoWita. Forum Dermatol. 2016, 2, 85–89. [Google Scholar]
- Rigano, F.; Vento, F.; Cafarella, C.; Trovato, E.; Trozzi, A.; Dugo, P.; Mondello, L. Determination of Main Lipids and Volatile Compounds in Unconventional Cold-Pressed Seed Oils through Chromatographic Techniques. J. Food Sci. 2025, 90, e17661. [Google Scholar] [CrossRef]
- Ligęza, M.; Wyglądacz, D.; Tobiasz, A.; Jaworecka, K.; Reich, A. Natural Cold Pressed Oils as Cosmetic Products. Fam. Med. Prim. Care Rev. 2016, 18, 443–447. [Google Scholar] [CrossRef]
- Sondenheimer, K.; Krutmann, J. Novel Means for Photoprotection. Front. Med. 2018, 5, 162. [Google Scholar] [CrossRef]
- Schieke, S.M.; Schroeder, P.; Krutmann, J. Cutaneous Effects of Infrared Radiation: From Clinical Observations to Molecular Response Mechanisms. Photodermatol. Photoimmunol. Photomed. 2003, 19, 228–234. [Google Scholar] [CrossRef]
- Schroeder, P.; Lademann, J.; Darvin, M.E.; Stege, H.; Marks, C.; Bruhnke, S.; Krutmann, J. Infrared radiation-induced matrix metalloproteinase in human skin: Implications for protection. J. Investig. Dermatol. 2008, 128, 2491–2497. [Google Scholar] [CrossRef]
- Schroeder, P.; Pohl, C.; Calles, C.; Marks, C.; Wild, S.; Krutmann, J. Cellular response to infrared radiation involves retrograde mitochondrial signaling. Free Radic. Biol. Med. 2007, 43, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Wójcicki, K.; Klensporf-Pawlik, D.; Marzec, M.; Lucarini, M.; Durazzo, A.; Fonseca, J.; Santini, A.; Nowak, I.; Souto, E.B. Cold-Pressed Pomegranate Seed Oil: Study of Punicic Acid Properties by Coupling of GC/FID and FTIR. Molecules 2022, 27, 5863. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.R.; Hamblin, M.R. Biological Effects and Medical Applications of Infrared Radiation. J. Photochem. Photobiol. B 2017, 170, 197–207. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).