Functional Performance and Safety Evaluation of Optimized Plant-Based Dye Mixtures for Intense Hair Coloration
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemical Materials
2.3. Extraction of Natural Dyes
2.4. Characterization of Natural Dye Extracts by Ultraviolet–Visible (UV/VIS) Spectroscopy
2.5. Development of Herbal Mixtures Containing L. inermis Leaves, C. ternatea Flowers, and I. tinctoria Leaves
2.6. Irritation Test by Hen’s Egg Chorioallantoic Membrane (HET-CAM) Test
2.7. Color Staining Performance Test
2.8. Color Stability Test After Washing
2.9. Color Stability Test After Light Exposure
2.10. Hair Morphology by SEM
3. Results and Discussion
3.1. Dried Powder of Natural Dye Materials
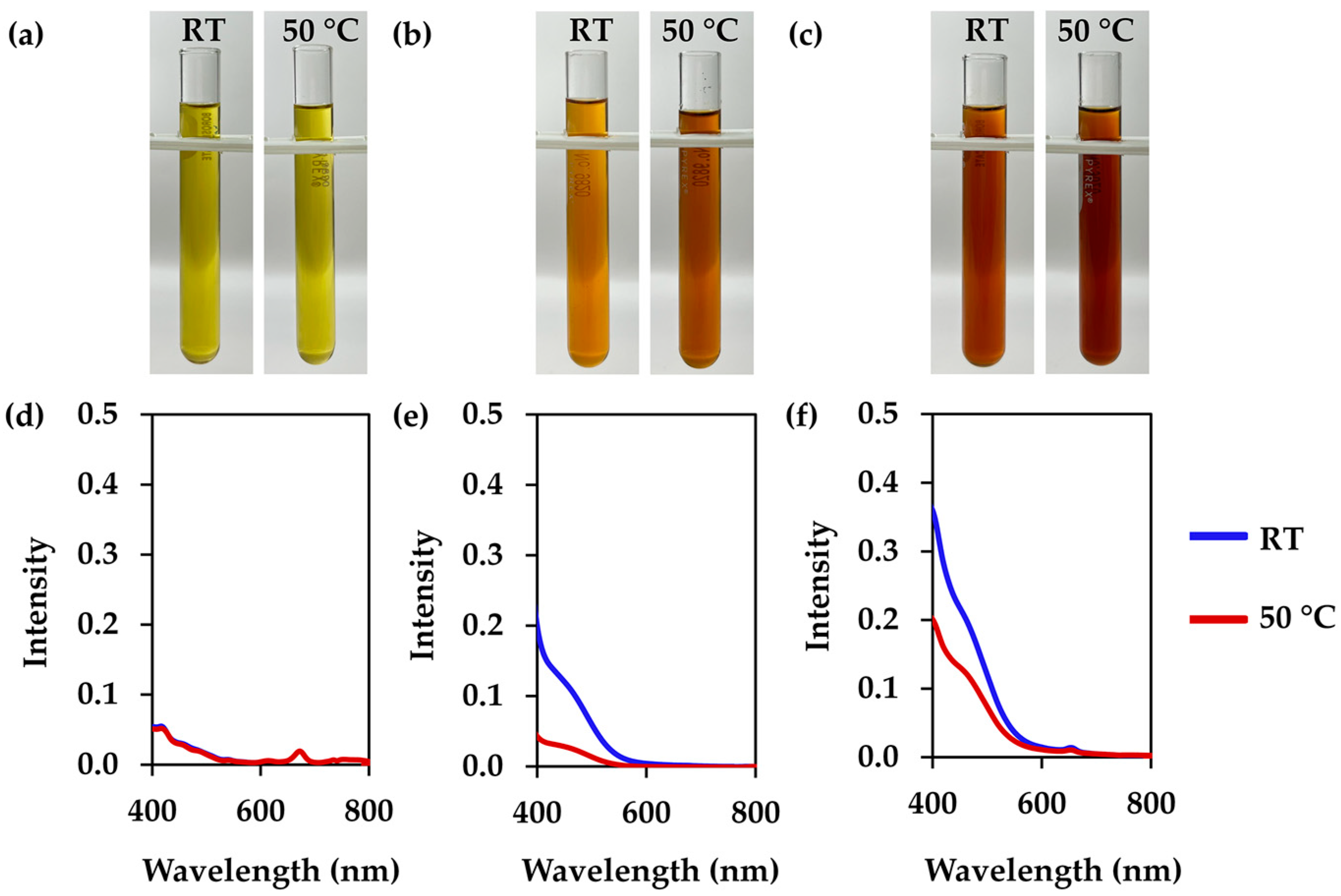
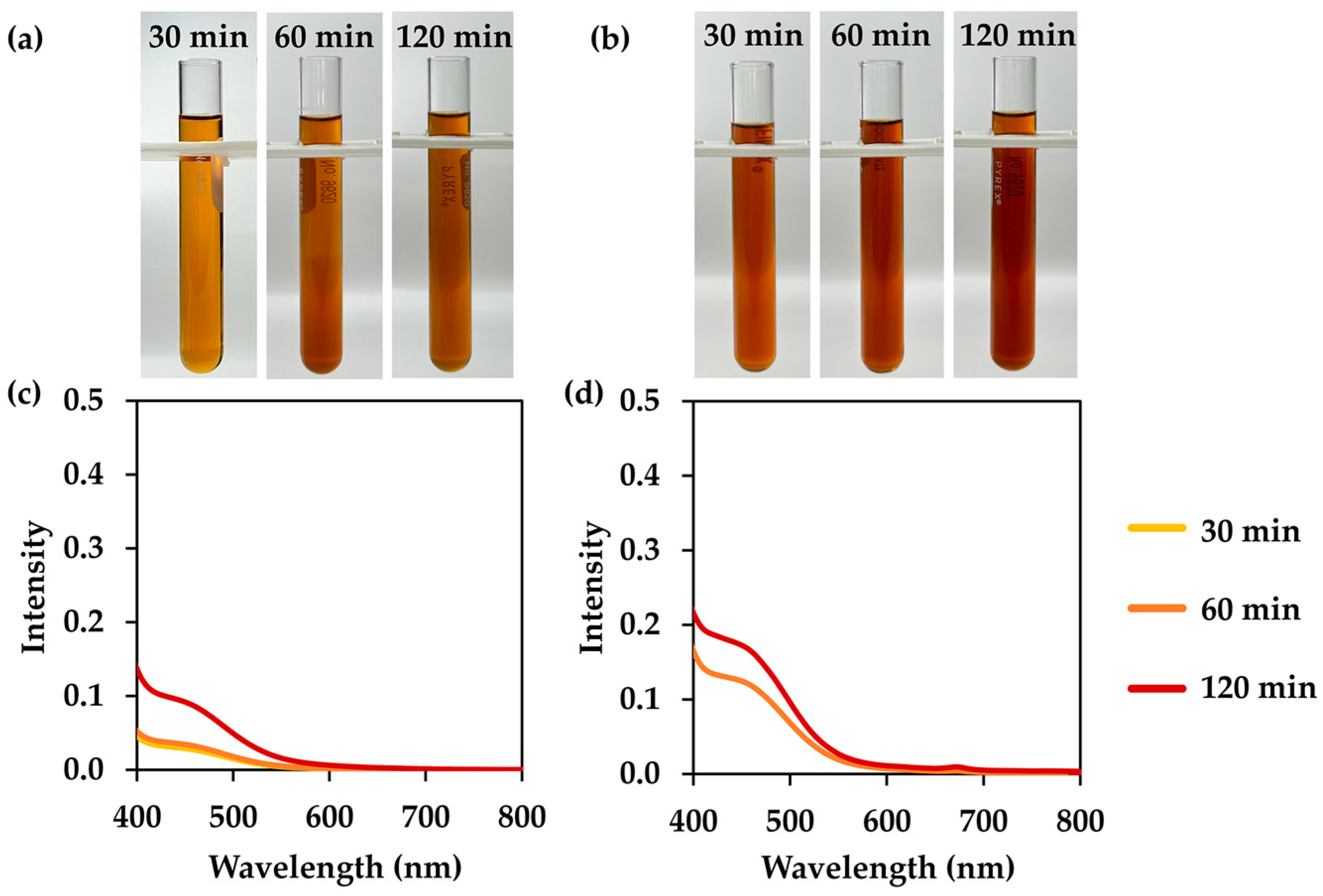
3.2. L. inermis Extracts
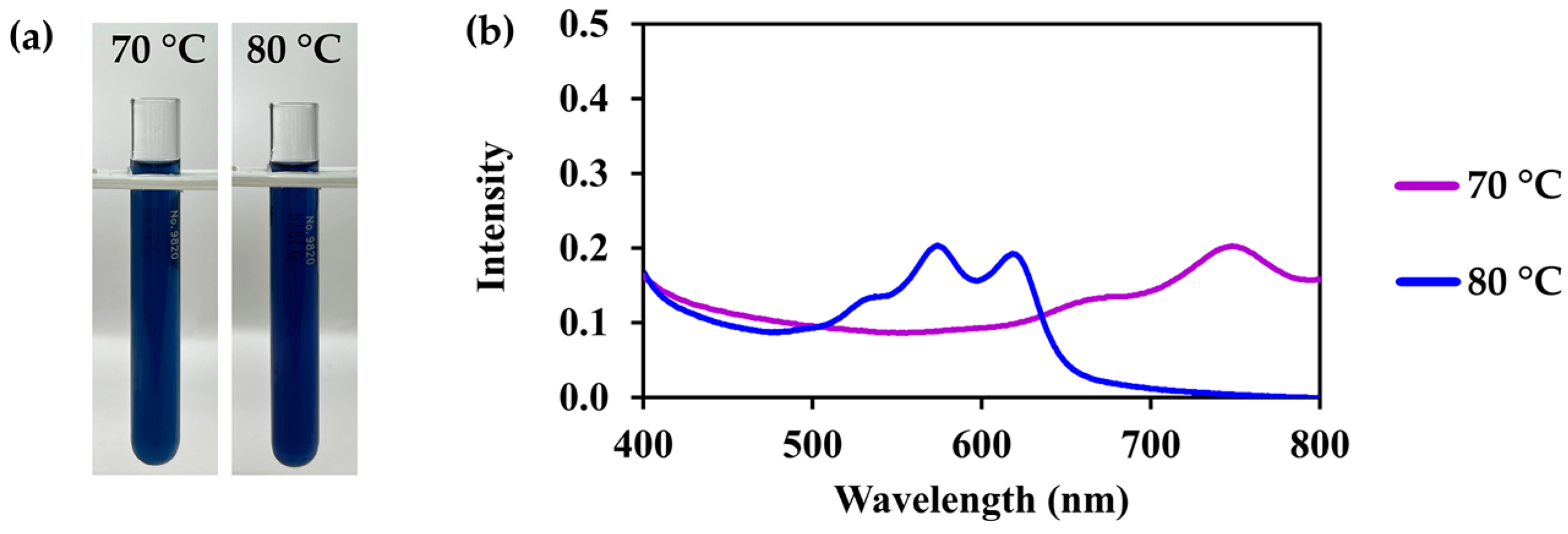
3.3. C. ternatea Extracts
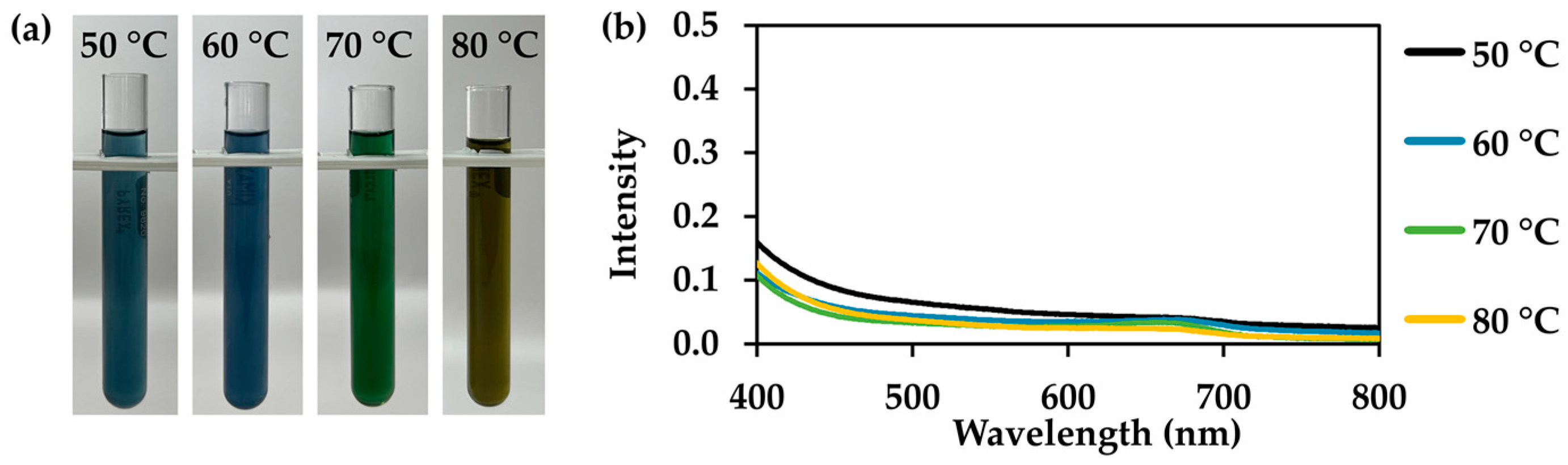
3.4. I. tinctoria Extracts
3.5. Comparison of Selected Natural Dye Extracts
3.6. Herbal Mixtures Containing L. inermis Leaves, C. ternatea Flowers, and I. tinctoria Leaves
0.000258 A × C − 0.000156 B × C,
3.7. Irritation Properties of Natual Extracts and Their Mixture (Formulation 15)
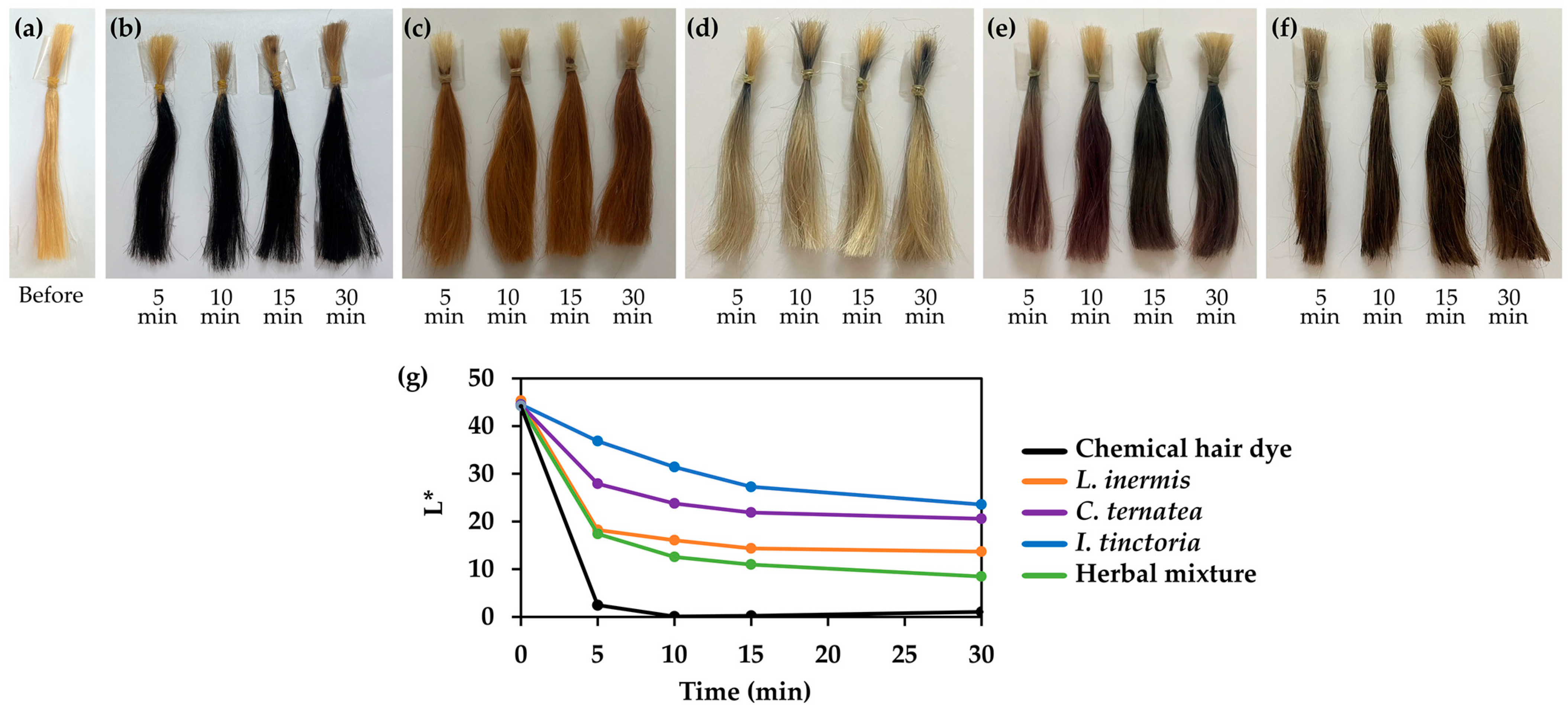
3.8. Color Staining Performance of Natual Extracts and Their Mixture
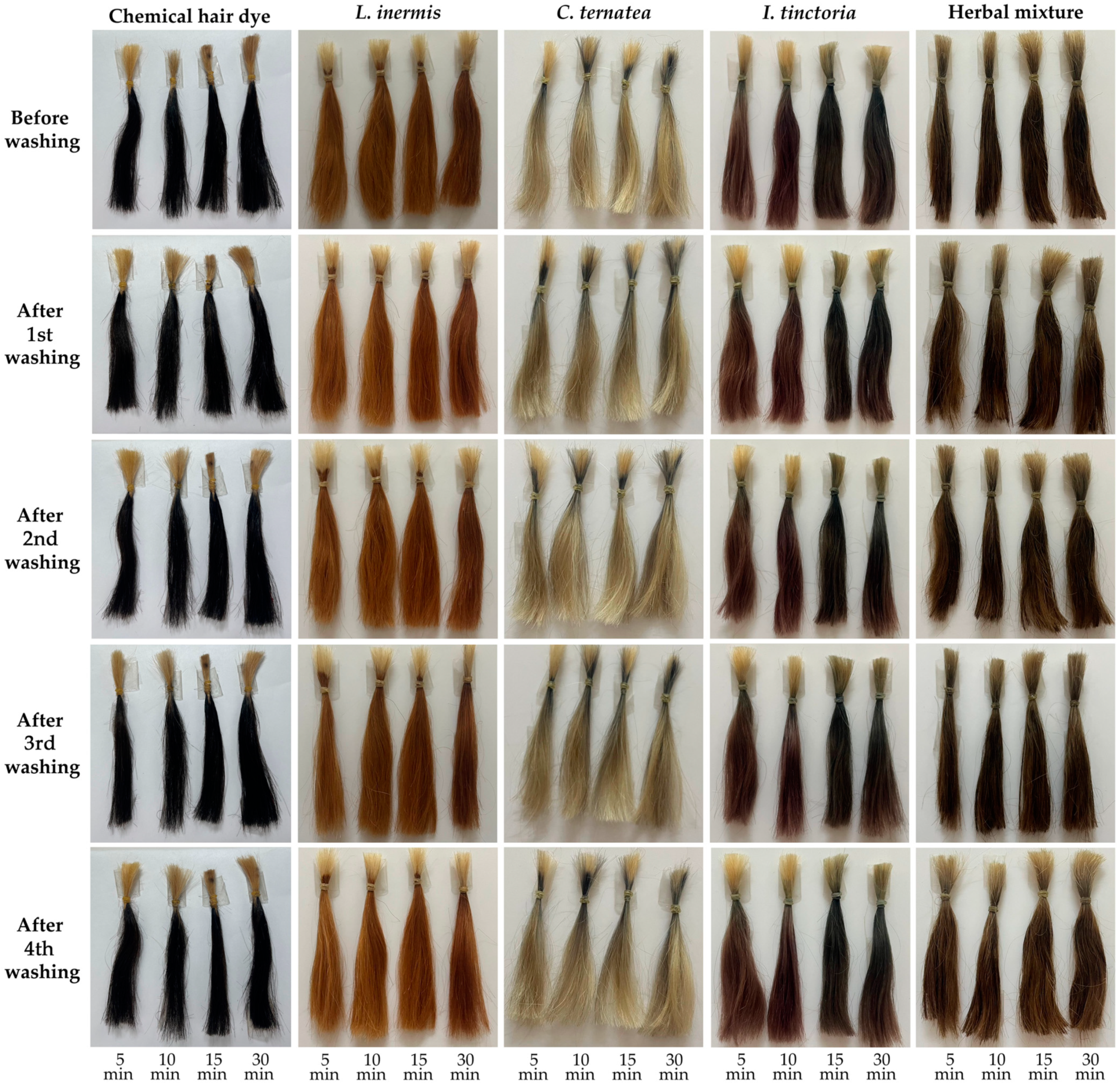
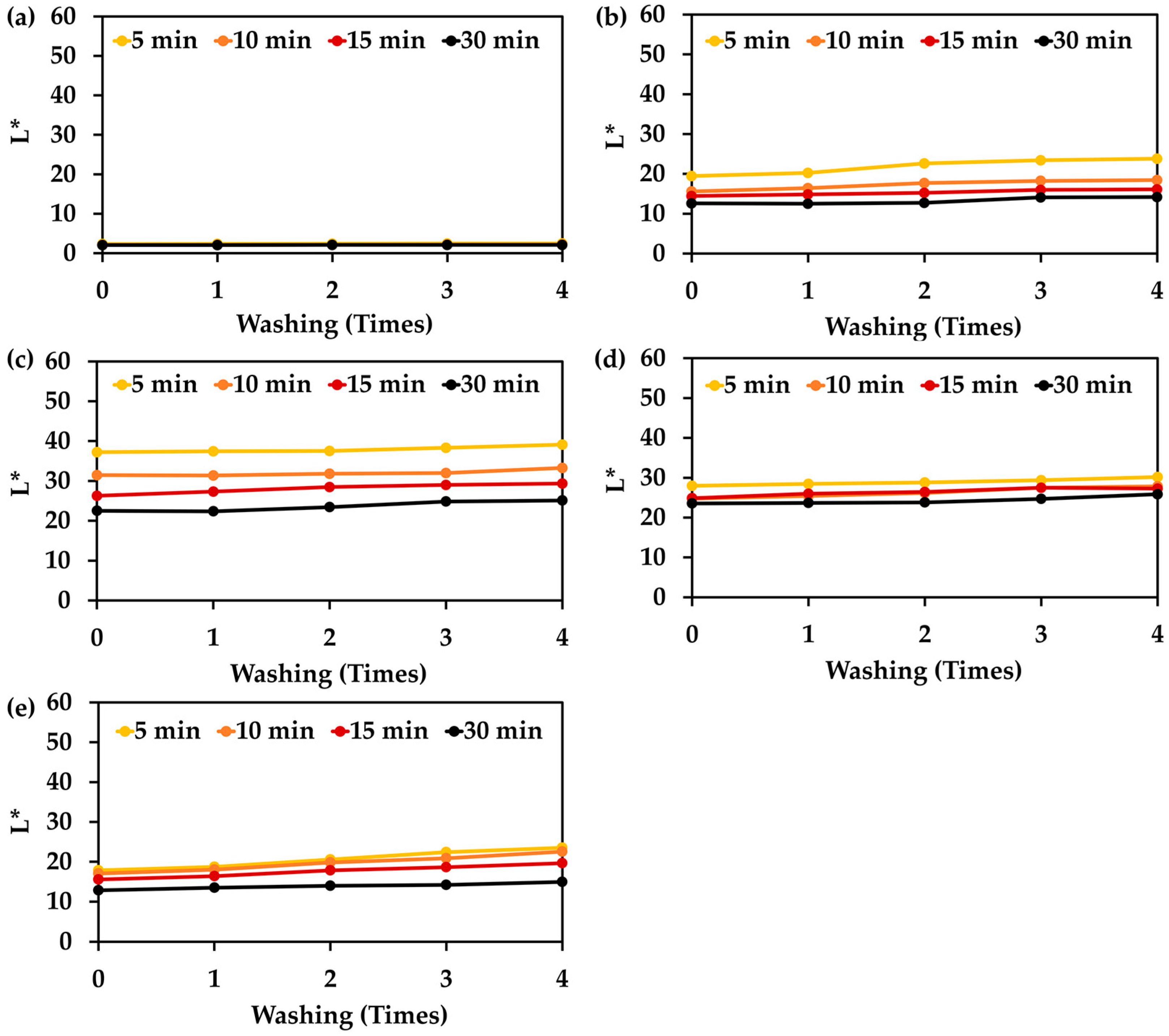
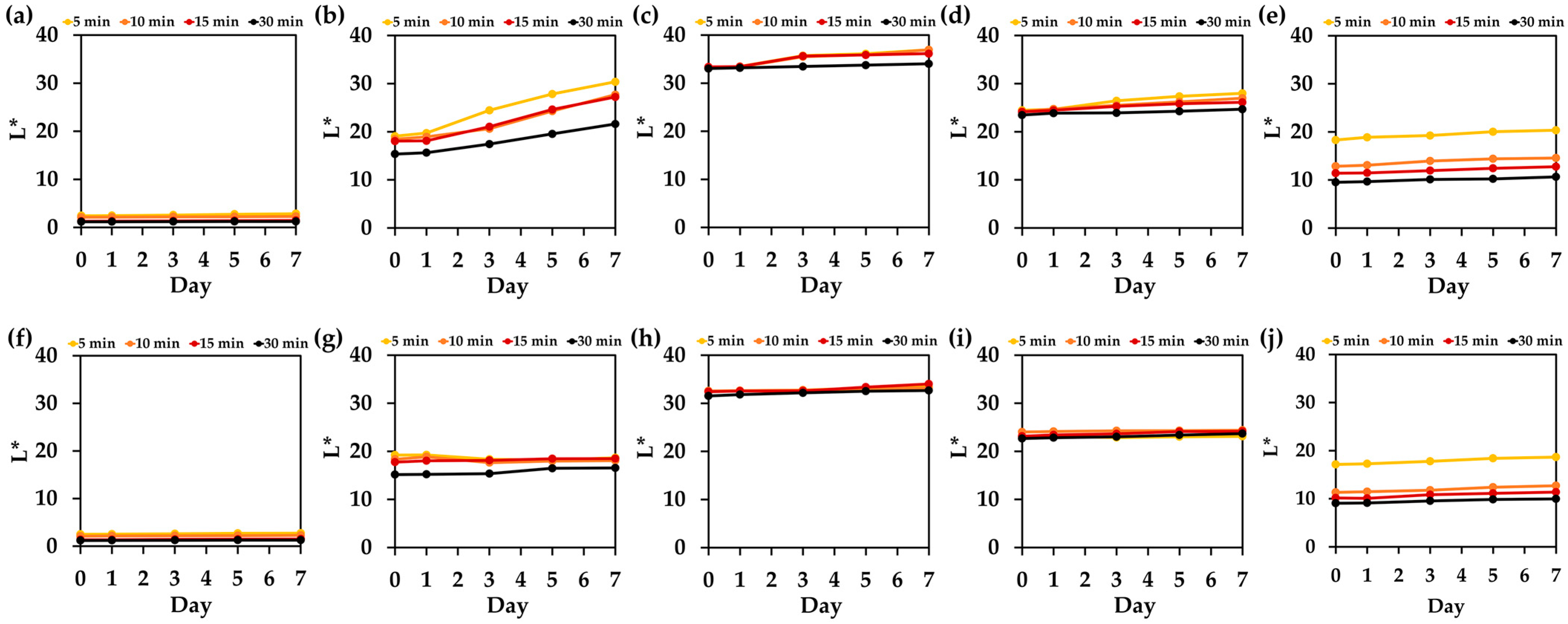
3.9. Color Stability After Washing of Hair Treated with Natual Extracts and Their Mixture
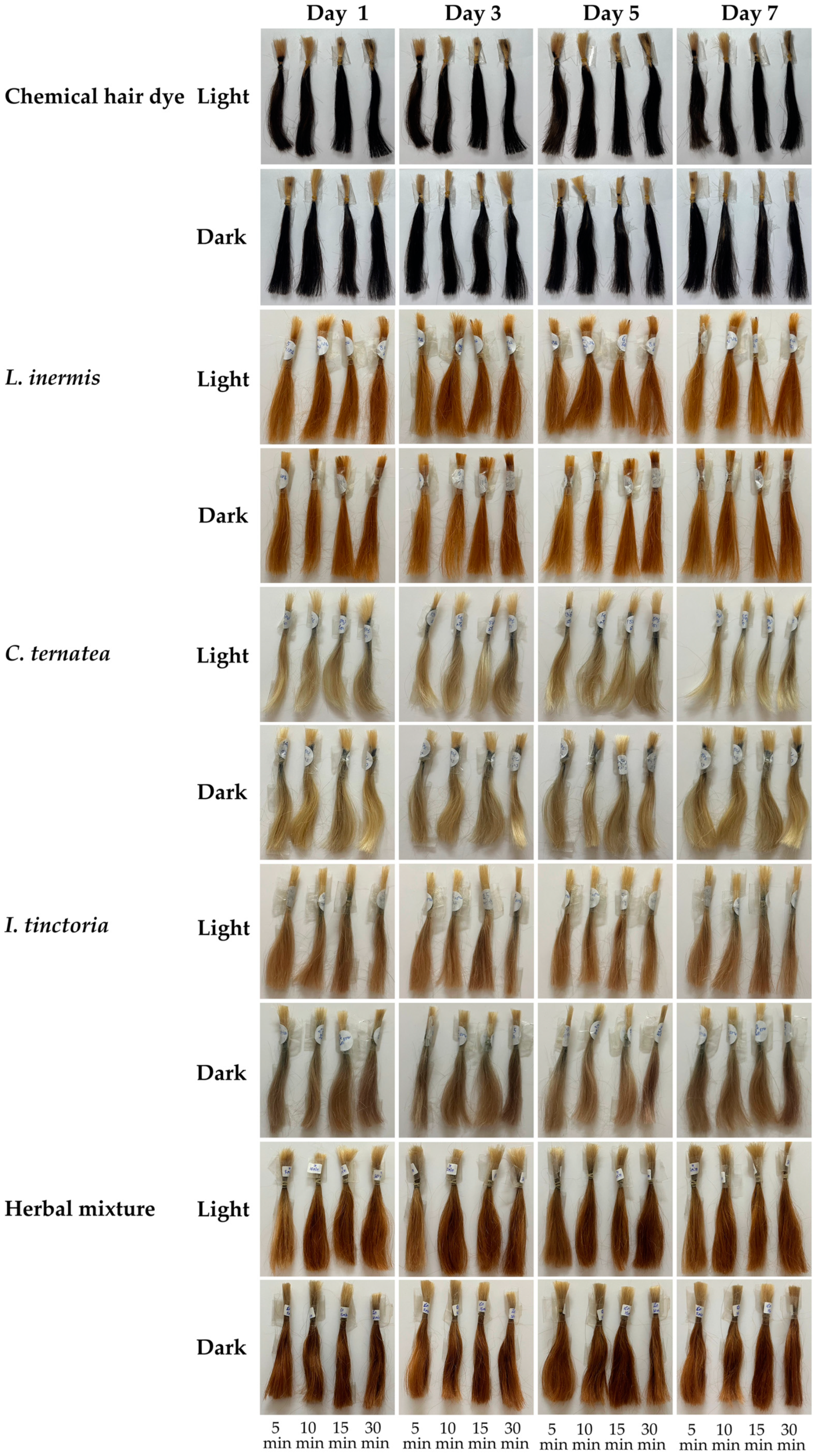
3.10. Color Stability After Light Exposure of Hair Treated with Natual Extracts and Their Mixture
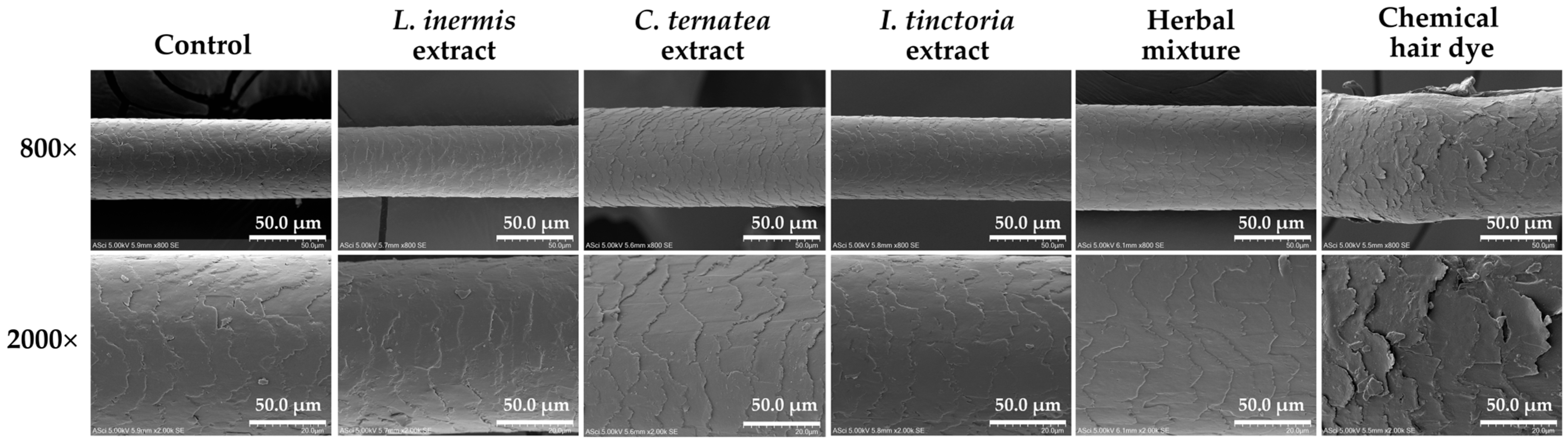
3.11. Hair Morphology
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cecil, V.; Pendry, L.F.; Salvatore, J.; Kurz, T. Women’s grey hair as an abomination of the body: Conceal and pass, or reveal and subvert. In Feminist Interrogations of Women’s Head Hair, 1st ed.; Routledge Journals, Taylor & Francis: Oxford, UK, 2018; pp. 124–140. [Google Scholar]
- Mirmirani, P. Age-related hair changes in men: Mechanisms and management of alopecia and graying. Maturitas. 2015, 80, 58–62. [Google Scholar] [CrossRef]
- Wang, S.; Kang, Y.; Qi, F.; Jin, H. Genetics of hair graying with age. Ageing Res. Rev. 2023, 89, 101977. [Google Scholar] [CrossRef]
- Tobin, D.J.; Paus, R. Graying: Gerontobiology of the hair follicle pigmentary unit. Exp. Gerontol. 2001, 36, 29–54. [Google Scholar] [CrossRef]
- Slominski, A.; Wortsman, J.; Plonka, P.M.; Schallreuter, K.U.; Paus, R.; Tobin, D.J. Hair follicle pigmentation. J. Investig. Dermatol. 2005, 124, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Affat, S.S. Classifications, advantages, disadvantages, toxicity effects of natural and synthetic dyes: A review. Qatar Univ. Sci. J. 2021, 8, 130–135. [Google Scholar]
- Ali, A.; Moinuddin; Allarakha, S.; Fatima, S.; Ali, S.A.; Habib, S. Risk of carcinogenicity associated with synthetic hair dyeing formulations: A biochemical view on action mechanisms, genetic variation and prevention. Indian J. Clin. Biochem. 2022, 37, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, P.P.; Chandrasena, N.R. Colonizing taxa (weeds) as sources of natural pigments and dyes. J. Asian Pac. Weed Sci. Soc. 2022, 4, 36–61. [Google Scholar]
- Pranta, A.D.; Rahaman, M.T. Extraction of eco-friendly natural dyes and biomordants for textile coloration: A critical review. Nano-Struct. Nano-Objects 2024, 39, 101243. [Google Scholar] [CrossRef]
- Bhuvaneswari, S.S.; Prabha, T.; Begum, S.S.; Sivakuamr, T.; Saranraj, P.; Manivannan, V.; Kumar, B.A. Formulation and evaluation, comparison of herbal hair dye with marketed formulation. Ann. Phytomed. 2021, 10, 175–181. [Google Scholar] [CrossRef]
- Assegaf, T.S.; Jusuf, N.K.; Pane, Y.S.; Rusda, M.; Darmani, E.H.; Amin, M.M.; Lubis, R.D.S.; Bachtiar, A. Anti-dandruff effects of butterfly pea flowers (Clitoria ternatea)-based shampoo: A pretest-posttest control study. Narra. J. 2024, 4, e876. [Google Scholar] [CrossRef]
- Chaksupa, N.; Sookvanichsilp, N.; Soonthornchareonnon, N.; Moongkarndi, P.; Gerdprasert, O. Effects of alcoholic extract from Clitoria ternatea flowers on the proliferation of human dermal papilla cells and hair growth in C57BL/6Mlac mice. Pharm. Sci. Asia. 2022, 49, 471–477. [Google Scholar] [CrossRef]
- Gerometta, E.; Grondin, I.; Smadja, J.; Frederich, M.; Gauvin-Bialecki, A. A review of traditional uses, phytochemistry and pharmacology of the genus Indigofera. J. Ethnopharmacol. 2020, 253, 112608. [Google Scholar] [CrossRef] [PubMed]
- Jisha, V.N.; Benjamin, S. Micropropagation and anti-microbial activity studies on Indigofera tinctoria. J. Trop. Med. Plants 2009, 10, 73–80. [Google Scholar]
- Caballero, A.C.; Jimenez, G.M.; Torres, I.C.; Diaz, L.A.C.; Muné, M.K.M. Calibration and conformity in the conductivity channels of pharmaceutical waters systems: A review. Glob. J. Pharm. Pharm. Sci. 2018, 6, 3–11. [Google Scholar] [CrossRef]
- Wyser, Y.; Vishtal, A.; Giardiello, M.I.; Pelletier, C.; Deantoni, F. Understanding barrier degradation during water vapour transmission rate testing of high barrier metallized paper. Packag. Technol. Sci. 2022, 35, 291–299. [Google Scholar] [CrossRef]
- Luepke, N.P.; Kemper, F.H. The HET-CAM test: An alternative to the Draize eye test. Food Chem. Toxicol. 1986, 24, 495–496. [Google Scholar] [CrossRef]
- Amnuaykan, P.; Juntrapirom, S.; Kanjanakawinkul, W.; Chaiyana, W. Enhanced antioxidant, anti-aging, anti-tyrosinase, and anti-inflammatory properties of Vanda coerulea Griff. Ex Lindl. Protocorm through elicitations with chitosan. Plants 2024, 13, 1770. [Google Scholar] [CrossRef]
- Sumbul, S.; Ahmad, M.A.; Asif, M.; Akhtar, M.; Saud, I. Physicochemical and phytochemical standardization of berries of Myrtus communis Linn. J. Pharm. Bioallied Sci. 2012, 4, 322–326. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Wang, L.; Liu, Q.; Yang, S.; Wang, C. Advancing herbal medicine: Enhancing product quality and safety through robust quality control practices. Front. Pharmacol. 2023, 14, 1265178. [Google Scholar] [CrossRef]
- Ali, S.; Hussain, T.; Nawaz, R. Optimization of alkaline extraction of natural dye from Henna leaves and its dyeing on cotton by exhaust method. J. Clean. Prod. 2009, 17, 61–66. [Google Scholar] [CrossRef]
- Nikolić, M.G.; Krstić, N.S.; Živanović, S.C.; Nikolić, G.M. The influence of Mg (II) and Ca (II) ions on the autoxidation of 4-methylcatechol in weakly alkaline aqueous solutions. React. Kinet. Mech. Catal. 2022, 135, 1373–1386. [Google Scholar] [CrossRef]
- Kanniappan, R. Assessment of dyeing properties and quality parameters of natural dye extracted from Lawsonia inermis. Eur. J. Exp. Biol. 2015, 5, 62–70. [Google Scholar]
- Abd El-Malek, Y.; El-Leithy, M.A.; Reda, F.A.; Khalil, M. Antimicrobial principles in leaves of Lawsonia inermis L. Zentralbl. Bakteriol. Parasitenkd. Infektionskr. Hyg. 1973, 128, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Elenen, M.N.A.; Elshobaky, A.R.; Elsayed, S.A. New Silver (I) Lawsone-based complexes: Synthesis, characterization, interaction with biological macromolecules, in vitro antineoplastic, antimetastatic, and antimicrobial activity. J. Mol. Struct. 2024, 1317, 139048. [Google Scholar] [CrossRef]
- Vijayalakshmi, R.A.; Ravindhran, R. Comparative fingerprint and extraction yield of Diospyrus ferrea (willd.) Bakh. root with phenol compounds (gallic acid), as determined by UV–vis and FT–IR spectroscopy. Asian Pac. J. Trop. Biomed. 2012, 2, S1367–S1371. [Google Scholar] [CrossRef]
- Kavepour, N.; Bayati, M.; Rahimi, M.; Aliahmadi, A.; Ebrahimi, S.N. Optimization of aqueous extraction of henna leaves (Lawsonia inermis L.) and evaluation of biological activity by HPLC-based profiling and molecular docking techniques. Chem. Eng. Res. Des. 2023, 195, 332–343. [Google Scholar] [CrossRef]
- Marpaung, A.M.; Paramaputri, A. UV-visible light spectra of Clitoria ternatea L. flower extract during aqueous extraction and storage. Int. Food Res. J. 2023, 30, 764–773. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Chen, S.M.; Li, C.H.; Zhu, X.R.; Deng, Y.M.; Sun, W.; Wang, L.S.; Chen, F.D.; Zhang, Z. The identification of flavonoids and the expression of genes of anthocyanin biosynthesis in the chrysanthemum flowers. Biol. Plant. 2012, 56, 458–464. [Google Scholar] [CrossRef]
- Jeyaraj, E.J.; Lim, Y.Y.; Choo, W.S. Effect of organic solvents and water extraction on the phytochemical profile and antioxidant activity of Clitoria ternatea flowers. ACS Food Sci. Technol. 2021, 1, 1567–1577. [Google Scholar] [CrossRef]
- Jaafar, N.F.; Ramli, M.E.; Salleh, R.M. Optimum extraction condition of Clitorea ternatea flower on antioxidant activities, total phenolic, total flavonoid and total anthocyanin contents. Trop. Life Sci. Res. 2020, 31, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Shiau, S.Y.; Wang, Y.; Yu, Y.; Cai, S.; Liu, Q. Phytochemical, antioxidant activity, and thermal stability of Clitoria ternatea flower extracts. Czech J. Food Sci. 2024, 42, 284–294. [Google Scholar] [CrossRef]
- Tello-Burgos, N.; López-Montes, A.M.; Ballesta-Claver, J.; Collado-Montero, F.J.; Blanc Garcia, M.D.R. Identification of Indigo dye (Indigofera tinctoria) and its degradation products by separation and spectroscopic techniques on historic documents and textile fibers. Stud. Conserv. 2021, 66, 7–22. [Google Scholar] [CrossRef]
- Kabish, A.K.; Abate, M.T.; Alemar, Z.A.; Girmay, S. The importance of natural indigo dye and its revitalization and ethiopian potential for indigo growing. Adv. Mater. Sci. Eng. 2023, 2023, 2135014. [Google Scholar] [CrossRef]
- Kiteto, M.K.; Mecha, C.A. Insight into the bouguer-beer-lambert law: A review. Sustain. Chem. Eng. 2024, 5, 567–587. [Google Scholar] [CrossRef]
- Fischer, K. Animal testing and marketing bans of the EU cosmetics legislation. Eur. J. Risk Regul. 2015, 6, 613–621. [Google Scholar] [CrossRef]
- Sreedhar, D.; Manjula, N.; Pise, A.; Pise, S. Ban of cosmetic testing on animals: A brief overview. Int. J. Curr. Res. Rev. 2020, 12, 113–116. [Google Scholar] [CrossRef]
- Öztürk, A.A.; Yenilmez, E.; Şenel, B.; Kıyan, H.T.; Güven, U.M. Effect of different molecular weight PLGA on flurbiprofen nanoparticles: Formulation, characterization, cytotoxicity, and in vivo anti-inflammatory effect by using HET-CAM assay. Drug Dev. Ind. Pharm. 2020, 46, 682–695. [Google Scholar] [CrossRef]
- Somwongin, S.; Chaiyana, W. Clinical efficacy in skin hydration and reducing wrinkles of nanoemulsions containing Macadamia integrifolia seed oil. Nanomaterials 2024, 14, 724. [Google Scholar] [CrossRef]
- Suresh, D.S.; Shbil, A.B.; Vijaykumar, S.P.; Sharanappa, S.; Ganesha, H. Optical Properties of Lawsone Dye Doped with Zinc Oxide for The Fabrication of Dye Sensitized Solar Cell. Proc. IOP Conf. Ser. Mater. Sci. Eng. 2024, 1300, 012002. [Google Scholar] [CrossRef]
- Bhushan, B. Nanoscale characterization of human hair and hair conditioners. Prog. Mater. Sci. 2008, 53, 585–710. [Google Scholar] [CrossRef]
- Cruz, C.F.; Costa, C.; Gomes, A.C.; Matamá, T.; Cavaco-Paulo, A. Human hair and the impact of cosmetic procedures: A review on cleansing and shape-modulating cosmetics. Cosmetics 2016, 3, 26. [Google Scholar] [CrossRef]
- Lee, M.S.; Chang, B.S. Morphological damage procedures of hair surface treated with repetitive oxidation coloring agent. Med. Legal Update 2019, 19, 533–539. [Google Scholar] [CrossRef]
- Harrison, S.; Sinclair, R. Hair colouring, permanent styling and hair structure. J. Cosmet. Dermatol. 2003, 2, 180–185. [Google Scholar] [CrossRef]
- Bianchi, S.; Bernardi, S.; Continenza, M.A.; Vincenti, E.; Antonouli, S.; Torge, D.; Macchiarelli, G. Scanning electron microscopy approach for evaluation of hair dyed with Lawsonia inermis powder: In vitro study. Int. J. Morphol. 2020, 38, 96–100. [Google Scholar] [CrossRef]

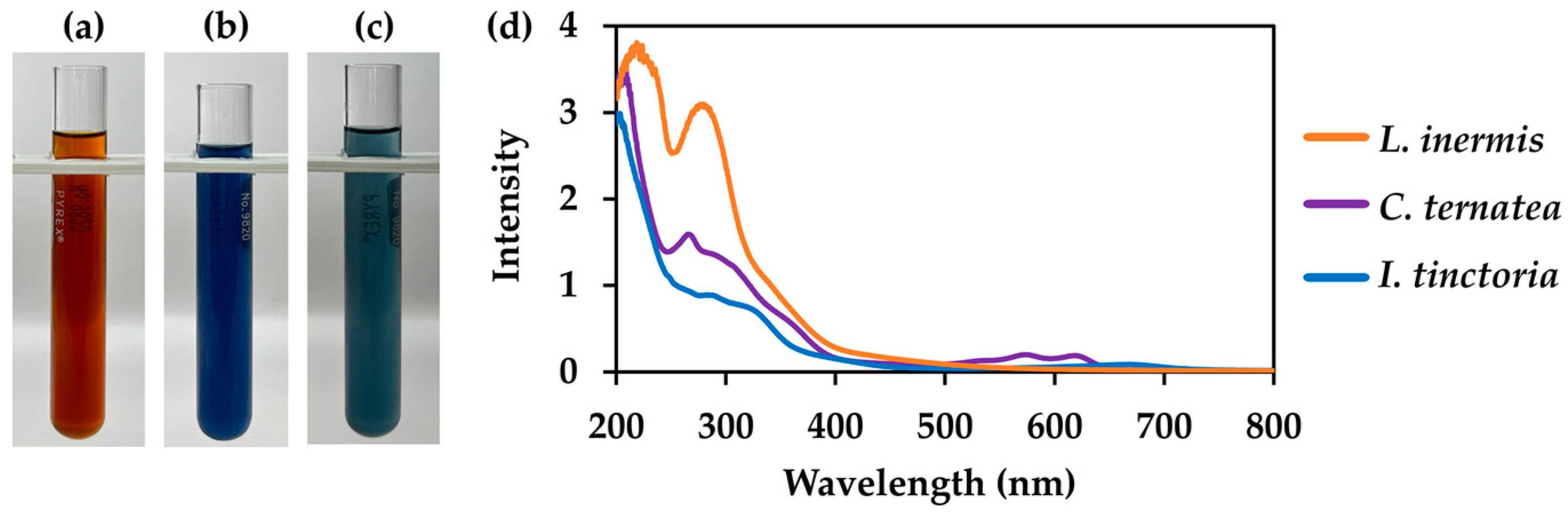
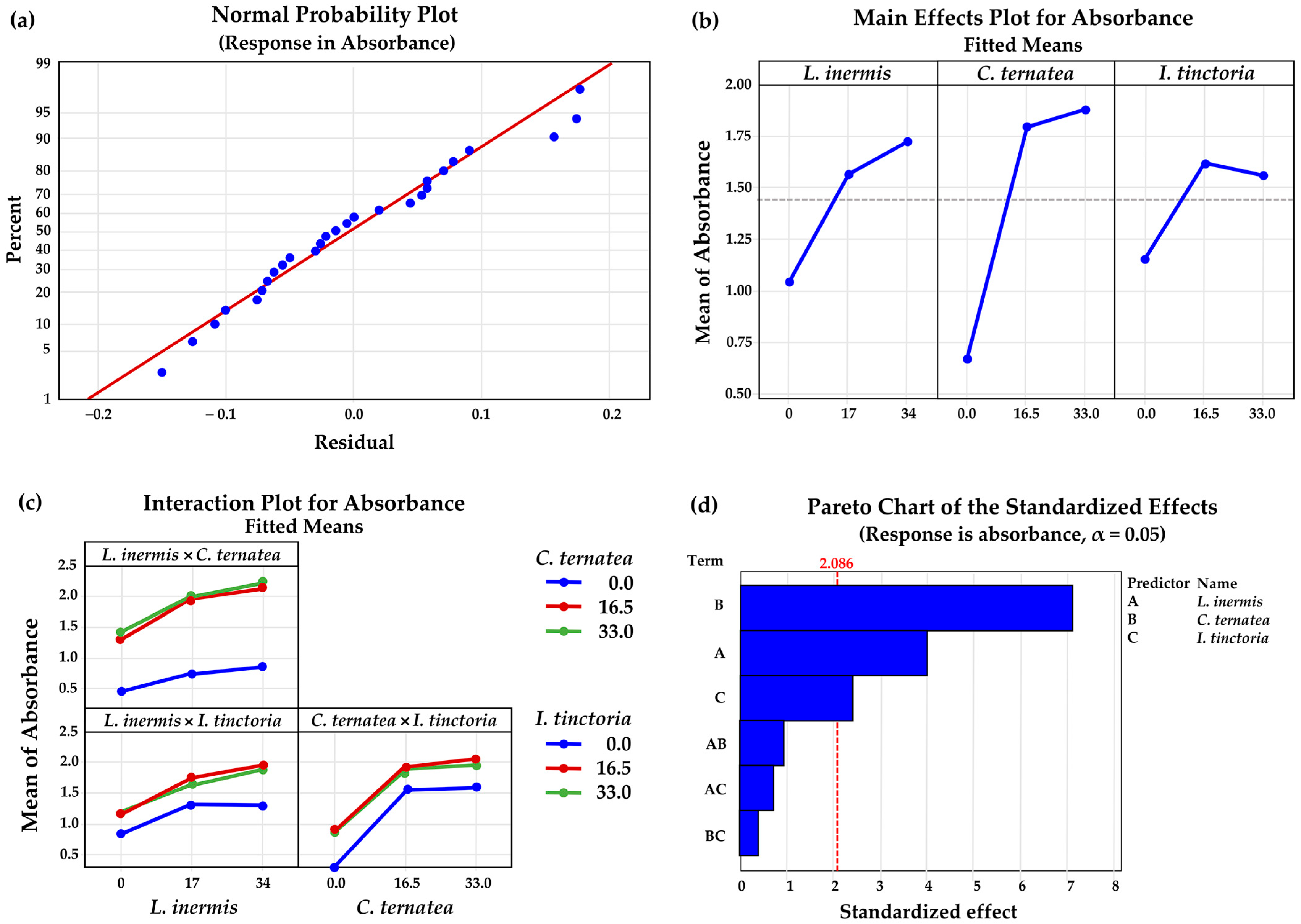

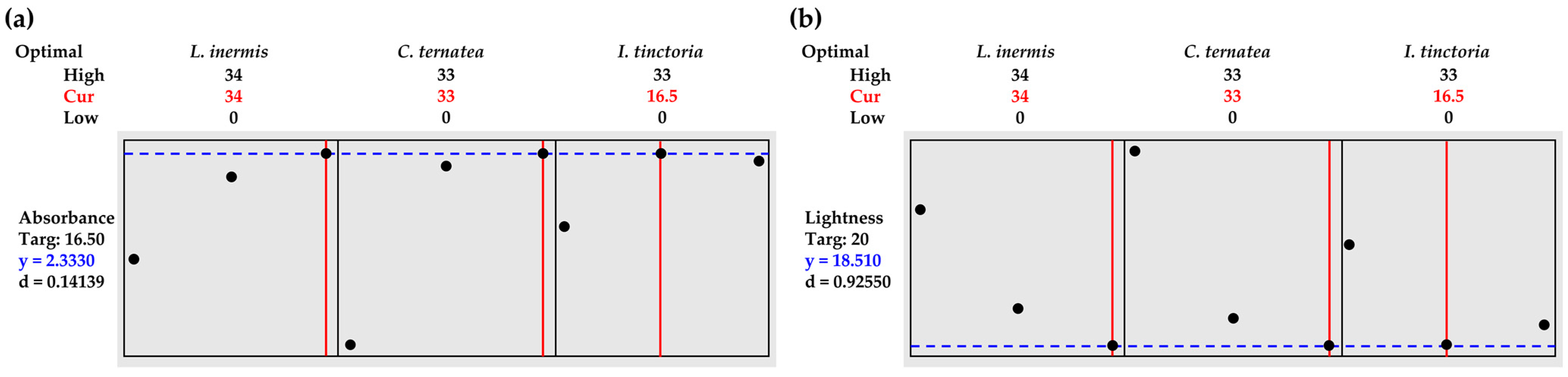
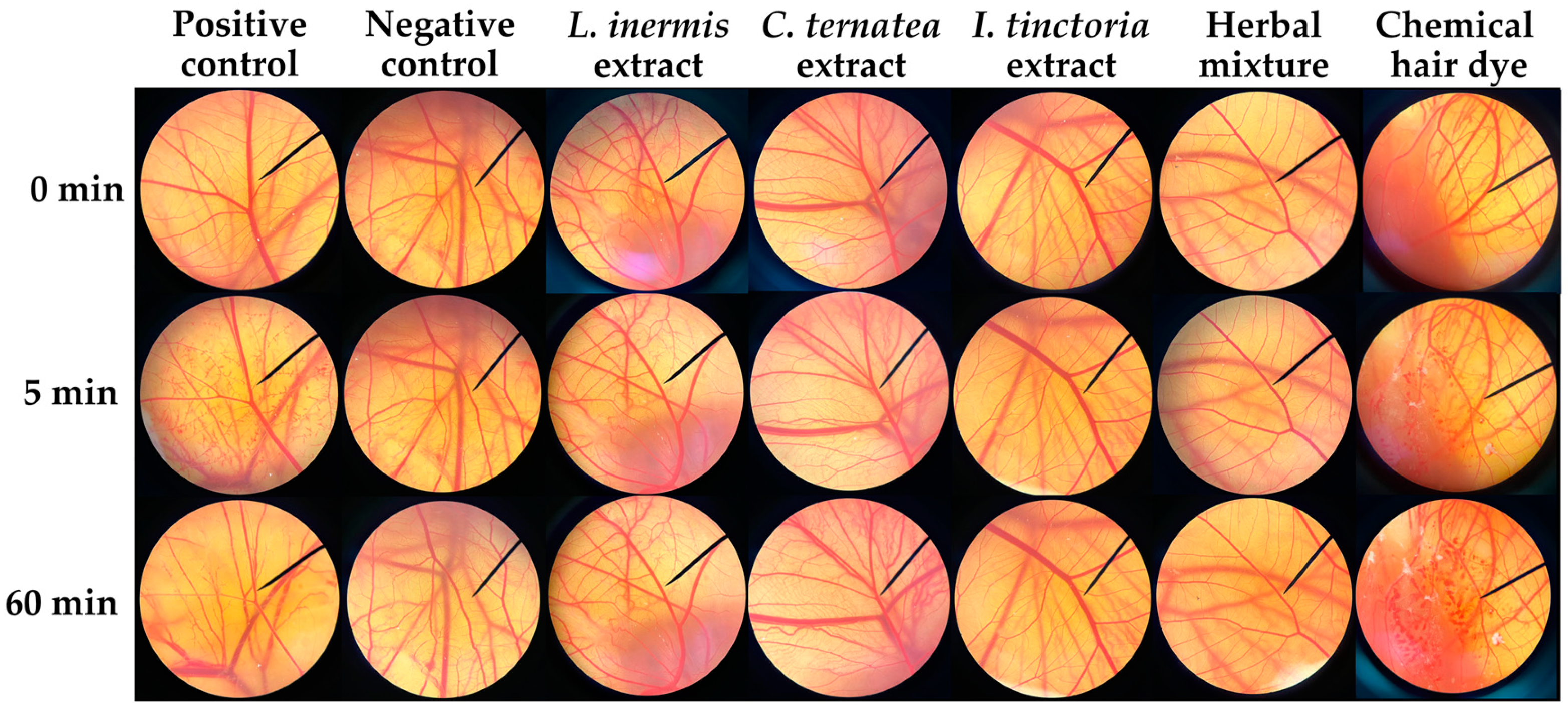
| Extraction Conditions | L. inermis Leaves | C. ternatea Flowers | I. tinctoria Leaves |
|---|---|---|---|
| Dried powder to solvent ratio (w/v) | 1:20 | 1:20 | 1:20 |
| Solvents | EtOH, water, and NaOH | Water | Water |
| Extraction methods | Orbital shaker and ultrasonication | Ultrasonication | Ultrasonication |
| Temperatures | RT and 50 °C | 70 and 80 °C | 50, 60, 70, and 80 °C |
| Extraction durations | 30, 60, and 120 min | 60 min | 30 min |
| Mixture | L. inerms (%) | C. ternatea (%) | I. tinctoria (%) | Water (%) | Absorbance | Lightness | Appearance | λmax (nm) |
|---|---|---|---|---|---|---|---|---|
| 1 (0, −1, +1) | 17 | 0 | 33 | 50 | 0.80 ± 0.05 i | 44.80 ± 0.02 v |  | 291 |
| 2 (−1, 0, 0) | 0 | 16.5 | 16.5 | 67 | 1.40 ± 0.01 f | 37.20 ± 0.01 p |  | 289 |
| 3 (−1, +1, 0) | 0 | 33 | 16.5 | 50.5 | 1.51 ± 0.01 f | 34.03 ± 0.02 m |  | 290 |
| 4 (0, −1, −1) | 17 | 0 | 0 | 83 | 0.38 ± 0.01 k | 52.55 ± 0.02 z |  | 289 |
| 5 (−1, 0, −1) | 0 | 16.5 | 0 | 83.5 | 1.15 ± 0.01 g | 41.85 ± 0.02 r |  | 289 |
| 6 (+1, +1, +1) | 34 | 33 | 33 | 0 | 2.41 ± 0.05 a | 23.43 ± 0.02 b |  | 290 |
| 7 (−1, +1, +1) | 0 | 33 | 33 | 34 | 1.35 ± 0.04 f | 35.15 ± 0.03 n |  | 289 |
| 8 (+1, 0, −1) | 34 | 16.5 | 0 | 49.5 | 1.70 ± 0.02 e | 31.14 ± 0.02 j |  | 290 |
| 9 (0, 0, −1) | 17 | 16.5 | 0 | 66.5 | 1.84 ± 0.01 d | 32.79 ± 0.02 l |  | 291 |
| 10 (0, +1, 0) | 17 | 33 | 16.5 | 33.5 | 2.20 ± 0.07 b | 26.27 ± 0.02 e |  | 289 |
| 11 (−1, −1, +1) | 0 | 0 | 33 | 67 | 0.76 ± 0.10 i | 46.44 ± 0.03 w |  | 288 |
| 12 (−1, 0, +1) | 0 | 16.5 | 33 | 50.5 | 1.32 ± 0.02 f | 36.42 ± 0.02 o |  | 289 |
| 13 (−1, −1, 0) | 0 | 0 | 16.5 | 83.5 | 0.53 ± 0.01 j | 47.20 ± 0.02 x |  | 291 |
| 14 (+1, +1, −1) | 34 | 33 | 0 | 33 | 1.70 ± 0.01 e | 29.50 ± 0.01 i |  | 291 |
| 15 (+1, +1, 0) | 34 | 33 | 16.5 | 16.5 | 2.53 ± 0.03 a | 20.04 ± 0.01 a |  | 291 |
| 16 (+1, −1, −1) | 34 | 0 | 0 | 66 | 0.51 ± 0.01 j | 50.02 ± 0.02 y |  | 290 |
| 17 (0, 0, +1) | 17 | 16.5 | 33 | 33.5 | 2.01 ± 0.05 c | 29.26 ± 0.02 h |  | 291 |
| 18 (0, 0, 0) | 17 | 16.5 | 16.5 | 50 | 2.01 ± 0.07 c | 28.60 ± 0.01 g |  | 291 |
| 19 (+1, −1, +1) | 34 | 0 | 33 | 33 | 0.95 ± 0.03 h | 43.03 ± 0.01 t |  | 289 |
| 20 (+1, −1, 0) | 34 | 0 | 16.5 | 49.5 | 1.04 ± 0.01 g,h | 42.31 ± 0.02 s |  | 291 |
| 21 (0, −1, 0) | 17 | 0 | 16.5 | 66.5 | 1.03 ± 0.01 g,h | 44.66 ± 0.02 u |  | 289 |
| 22 (+1, 0, 0) | 34 | 16.5 | 16.5 | 33 | 2.34 ± 0.04 a | 24.30 ± 0.02 c |  | 289 |
| 23 (0, +1, +1) | 17 | 33 | 33 | 17 | 2.12 ± 0.02 b,c | 28.23 ± 0.02 f |  | 291 |
| 24 (−1, +1, −1) | 0 | 33 | 0 | 67 | 1.37 ± 0.02 f | 40.41 ± 0.02 q |  | 291 |
| 25 (0, +1, −1) | 17 | 33 | 0 | 50 | 1.70 ± 0.01 e | 31.31 ± 0.02 k |  | 289 |
| 26 (+1, 0, +1) | 34 | 16.5 | 33 | 16.5 | 2.33 ± 0.05 a | 25.08 ± 0.04 d |  | 291 |
| Source | DF | Sum of Squares | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Regression | 6 | 9.6346 | 1.60576 | 12.37 | 0.000 * |
| L. inermis | 1 | 2.0808 | 2.08080 | 16.02 | 0.001 * |
| C. ternatea | 1 | 6.5884 | 6.58845 | 50.74 | 0.000 * |
| I. tinctoria | 1 | 0.7606 | 0.76056 | 5.86 | 0.025 * |
| L. inermis × C. ternatea | 1 | 0.1200 | 0.12000 | 0.92 | 0.348 |
| L. inermis × I. tinctoria | 1 | 0.0631 | 0.06308 | 0.49 | 0.494 |
| C. ternatea × I. tinctoria | 1 | 0.0217 | 0.02167 | 0.17 | 0.687 |
| Error | 20 | 2.5971 | 0.12985 | ||
| Total | 26 | 12.2317 |
| Source | DF | Sum of Squares | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Regression | 6 | 1575.92 | 262.653 | 3.14 | 0.025 * |
| L. inermis | 1 | 50.17 | 50.167 | 0.60 | 0.447 |
| C. ternatea | 1 | 583.00 | 582.997 | 6.98 | 0.016 * |
| I. tinctoria | 1 | 0.34 | 0.339 | 0.00 | 0.950 |
| L. inermis × C. ternatea | 1 | 514.04 | 514.044 | 6.15 | 0.022 * |
| L. inermis × I. tinctoria | 1 | 252.73 | 252.725 | 3.03 | 0.097 |
| C. ternatea × I. tinctoria | 1 | 175.64 | 175.644 | 2.10 | 0.163 |
| Error | 20 | 1670.89 | 83.544 | ||
| Total | 26 | 3246.80 |
| Response | Fit | SE Fit | Actual Values | 95% CI | 95% PI |
|---|---|---|---|---|---|
| Absorbance | 2.3330 | 0.0863 | 2.53 ± 0.03 | (2.1530, 2.5130) | (1.9363, 2.7297) |
| Lightness | 18.51 | 8.78 | 20.04 ± 0.01 | (−1.73, 38.75) | (−12.98, 50.00) |
| Sample | IS | Classification |
|---|---|---|
| Positive control | 13.42 ± 1.8 a | Severe irritation |
| Negative control | 0.00 ± 0.0 b | No irritation |
| L. inermis extract | 0.00 ± 0.0 b | No irritation |
| C. ternatea extract | 0.00 ± 0.0 b | No irritation |
| I. tinctoria extract | 0.00 ± 0.0 b | No irritation |
| Herbal mixture formulation 15 | 0.00 ± 0.0 b | No irritation |
| Chemical hair dye | 15.63 ± 1.5 a | Severe irritation |
| Treatment Duration (min) | Color Fading Rate (L* Per Washing Time) | ||||
|---|---|---|---|---|---|
| Chemical Hair Dye | L. inermis Extract | C. ternatea Extract | I. tinctoria Extract | Herbal Mixture Formulation 15 | |
| 5 | 0.03 ± 0.00 α (R2 = 0.964) | 1.20 ± 0.03 a,γ (R2 = 0.914) | 0.47 ± 0.02 a,ε (R2 = 0.880) | 0.53 ± 0.01 c,δ (R2 = 0.986) | 1.52 ± 0.01 a,β (R2 = 0.986) |
| 10 | 0.02 ± 0.00 α (R2 = 0.956) | 0.75 ± 0.04 b,δ (R2 = 0.911) | 0.43 ± 0.09 a,ε (R2 = 0.968) | 0.83 ± 0.00 a,γ (R2 = 0.979) | 1.39 ± 0.01 b,β (R2 = 0.991) |
| 15 | 0.01 ± 0.00 α (R2 = 0.983) | 0.45 ± 0.04 c,ε (R2 = 0.985) | 0.78 ± 0.01 b,γ (R2 = 0.950) | 0.58 ± 0.01 b,δ (R2 = 0.949) | 1.05 ± 0.02 c,β (R2 = 0.993) |
| 30 | 0.01 ± 0.00 α (R2 = 0.818) | 0.47 ± 0.03 c,δ (R2 = 0.817) | 0.76 ± 0.01 b,β (R2 = 0.868) | 0.57 ± 0.01 b,γ (R2 = 0.818) | 0.50 ± 0.01 d,δ (R2 = 0.976) |
| Samples | Color Fading Rate (L* Per Day) | |||||||
|---|---|---|---|---|---|---|---|---|
| 5 min | 10 min | 15 min | 30 min | |||||
| Light | Dark | Light | Dark | Light | Dark | Light | Dark | |
| Chemical hair dye | 0.06 ± 0.01 a (R2 = 0.987) | 0.04 ± 0.01 d,* (R2 = 0.991) | 0.03 ± 0.01 b (R2 = 0.999) | 0.02 ± 0.01 c,* (R2 = 0.966) | 0.02 ± 0.01 c (R2 = 0.985) | 0.02 ± 0.00 b (R2 = 0.912) | 0.01 ± 0.00 d (R2 = 0.793) | 0.01 ± 0.00 a (R2 = 0.992) |
| L. inermis extract | 3.08 ± 0.01 a (R2 = 0.986) | −0.21 ± 0.01 d,* (R2 = 0.474) | 2.39 ± 0.01 c (R2 = 0.965) | −0.14 ± 0.00 c,* (R2 = 0.281) | 2.49 ± 0.01 b (R2 = 0.981) | 0.19 ± 0.00 b,* (R2 = 0.909) | 1.16 ± 0.86 d (R2 = 0.988) | 0.40 ± 0.01 a,* (R2 = 0.875) |
| C. ternatea extract | 1.00 ± 0.00 a (R2 = 0.929) | 0.24 ± 0.00 a,* (R2 = 0.970) | 1.01 ± 0.01 a (R2 = 0.949) | 0.18 ± 0.01 c,* (R2 = 0.974) | 0.81 ± 0.01 b (R2 = 0.847) | 0.20 ± 0.01 b,* (R2 = 0.904) | 0.30 ± 0.01 c (R2 = 1.000) | 0.25 ± 0.01 a,* (R2 = 0.969) |
| I. tinctoria extract | 0.98 ± 0.01 a (R2 = 0.964) | 0.00 ± 0.00 c,* (R2 = 0.002) | 0.68 ± 0.01 b (R2 = 0.999) | 0.08 ± 0.01 b,* (R2 = 0.921) | 0.44 ± 0.16 c (R2 = 0.971) | 0.27 ± 0.01 a,* (R2 = 0.958) | 0.27 ± 0.01 d (R2 = 0.939) | 0.27 ± 0.01 a (R2 = 0.993) |
| Herbal mixture | 0.53 ± 0.00 a (R2 = 0.964) | 0.42 ± 0.01 a,* (R2 = 0.002) | 0.47 ± 0.01 b (R2 = 0.999) | 0.37 ± 0.00 b,* (R2 = 0.921) | 0.36 ± 0.00 c (R2 = 0.971) | 0.35 ± 0.01 c (R2 = 0.958) | 0.28 ± 0.02 d (R2 = 0.939) | 0.26 ± 0.02 d (R2 = 0.993) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lueadnakrob, K.; Juntrapirom, S.; Rongthong, T.; Kanjanakawinkul, W.; Chaiyana, W. Functional Performance and Safety Evaluation of Optimized Plant-Based Dye Mixtures for Intense Hair Coloration. Cosmetics 2025, 12, 78. https://doi.org/10.3390/cosmetics12020078
Lueadnakrob K, Juntrapirom S, Rongthong T, Kanjanakawinkul W, Chaiyana W. Functional Performance and Safety Evaluation of Optimized Plant-Based Dye Mixtures for Intense Hair Coloration. Cosmetics. 2025; 12(2):78. https://doi.org/10.3390/cosmetics12020078
Chicago/Turabian StyleLueadnakrob, Kodpaka, Saranya Juntrapirom, Thitiphorn Rongthong, Watchara Kanjanakawinkul, and Wantida Chaiyana. 2025. "Functional Performance and Safety Evaluation of Optimized Plant-Based Dye Mixtures for Intense Hair Coloration" Cosmetics 12, no. 2: 78. https://doi.org/10.3390/cosmetics12020078
APA StyleLueadnakrob, K., Juntrapirom, S., Rongthong, T., Kanjanakawinkul, W., & Chaiyana, W. (2025). Functional Performance and Safety Evaluation of Optimized Plant-Based Dye Mixtures for Intense Hair Coloration. Cosmetics, 12(2), 78. https://doi.org/10.3390/cosmetics12020078








