Abstract
Exposure to ultraviolet (UV) radiation is a primary risk factor for various skin disorders, including erythema, sunburn, and skin cancer. Sunscreens containing UV filters, categorized as organic or inorganic, are widely utilized to mitigate these effects. Among inorganic UV filters, titanium dioxide (TiO2) and zinc oxide (ZnO) are prominently used due to their favorable safety and achievable broad-spectrum protection profiles. This review focuses on the properties, safety, and efficacy of TiO2 and ZnO in sunscreens, emphasizing their mechanisms of action, photostability, and impacts on human health and the environment. Key factors influencing their performance include particle size, surface coatings, and formulation pH. Despite recognized advantages, concerns about toxicity—particularly related to nanoparticle penetration and reactive oxygen species generation—highlight the need for robust safety assessments. Additionally, the environmental impacts of inorganic UV filters, including bioaccumulation and effects on aquatic ecosystems, warrant consideration. Advances in nanoparticle synthesis, bioactive compound integration, and environmentally friendly formulations offer pathways to enhance sunscreen efficacy and safety, providing opportunities for innovation in photoprotection.
1. Introduction
It is well recognized that unprotected exposition to ultraviolet (UV) radiation is the main risk factor associated with erythema, sunburn, and the development of many skin disorders, such as hyperpigmentation, and skin cancer; however, it can be prevented using sunscreens [1]. Unprotected skin is prone to many complex chemical and morphological reactions when exposed to UV radiation, such as the formation of reactive oxygen species (ROS), histochemical conditions, thickening of the stratum corneum, and alterations of the dermo-epidermal junction, which may compromise the barrier function of the skin [2,3], facilitating the development of skin disorders, including atopic dermatitis, chronic xerosis, and infections [3]. It is also known that UV radiation can damage genetic materials such as DNA, cause the oxidation of lipids, and formation of free radicals, leading to inflammation and, eventually, the weakening of the skin’s immune system, triggering undesirable skin disorders [4] and the formation of cis-urocanic acid as well [5].
Sunscreens are dermocosmetics used to protect the skin from solar UV radiation and its harmful effects [6]. Essentially, they are UV filters associated with an adequate vehicle for topical use, like emulsions, gels, aerosols, and sticks, among others [7]. UV filters are active compounds that can be classified as organic (chemical) and inorganic (physical), capable of transforming, dispersing, scattering, and/or absorbing UV radiation turning it harmless [8]. An ideal sunscreen formulation not only reduces the radiation reaching the skin surface but also is stable under all application conditions, including heating and electromagnetic irradiation, and has a pleasant sensorial profile [4,9,10]. Along with these properties, it should be water resistant, flavorless, odorless, and colorless, among some other desirable characteristics. Additionally, UV filters must be innocuous, non-phototoxic, compatible with dermocosmetic vehicles, stay only on the skin surface without suffering absorption, and must not generate ROS and/or other harmful radicals [4,11]. Apart from sunscreen formulations, these compounds are also found in many other cosmetics and personal care products, such as make-up, moisturizers, antiaging products, BB/CC/DD creams, lip balms, and perfumes. They are also found in products other than cosmetics, as in many daily-use ones, including paints and plastics, improving their longevity and protecting them from the effects of UV radiation exposure [12].
Organic UV filters are molecules that absorb the incident radiation, due to the presence of suitable chromophore groups, and transform it into innocuous, less harmful radiation, e.g., fluorescence or infrared radiation (heat). Essentially, these molecules are aromatic compounds with a carboxyl group and usually an electron-donating amine or methoxy group in the aromatic ring’s ortho or para position [2]. Some examples of these compounds are octocrylene, avobenzone, and ethylhexyl methoxycinnamate. The absorption of a photon promotes an electron situated at π HOMO (High Occupied Molecular Orbitals) to an unoccupied π* LUMO (Low Unoccupied Molecular Orbitals). The molecule in such an excited state can return to its ground state, releasing the excess energy as a photon (fluorescence) or heat (infrared radiation). The electronic transitions occur only when the energy of a photon matches the energy difference between the ground and an excited electronic state [7,13,14,15]. These molecules are classified as UVA, UVB, or broad-spectrum filters, depending on the range of protection against UV radiation. Considering that the wavelength is inversely proportional to the energy, an energy reduction will lead to a longer wavelength. So, organic UV filter molecules presenting a narrower HOMO-LUMO gap will absorb longer wavelength photons, thus absorbing UVA radiation and vice-versa [7]. Electronically excited UV filters that do not decay by destructive routes (e.g., fragmentation, biomolecular, and isomerization reactions) and have a fast conversion rate are classified as photostable molecules [15,16]. Broad-spectrum sunscreens can be developed by combining organic filter molecules with distinct, complementary absorption spectra spanning the desired wavelength range [2,17]. However, the association of different UV filters can also enhance the allergenic potential of the resulting formulation or even induce photoinstability [7], such as the combination of avobenzone and ethylhexyl methoxycinnamate (EHMC), which is known for its photounstable profile after UV irradiation [18].
In contrast, inorganic UV filters are insoluble metal oxide particles, typically zinc oxide (ZnO) and titanium dioxide (TiO2), which form an opaque film on the skin that disperse, scatter, and absorb part of the incident UV radiation [2]. These metal oxide particles should stay suspended in the cosmetic formulation, and their particle size determines the product’s appearance and sensorial on the skin surface, as well as the UV filter efficacy [7]. The most preeminent issue regarding inorganic UV filters is the formation of a characteristic white chalky texture film over the skin surface due to their properties to reflect and scatter visible light in addition to UV radiation [8,19,20]. Since the maximum scattering occurs at wavelengths close to the particle size, a way to decrease that effect is by diminishing the size below the shortest visible light wavelength, i.e., below about 400 nm [7]. The use of both ZnO and TiO2 nanoparticles (NPs) in the formulation brings advantages and consumer satisfaction, enabling the development of safer, transparent, non-greasy formulations that have reasonable costs and provide broad-spectrum protection against UV radiation [1].
The most known efficacy parameter of the protective effect of sunscreens is the Sun Protection Factor (SPF), defined as the numerical ratio of the minimum UV radiation dose required to produce erythema (minimal erythemal dose, MED) in sunscreen-protected and non-protected skin. The minimal erythemal dose (MED) is defined as the minimal UV radiation energy dose, or the time interval of exposure to a calibrated UV radiation source, necessary to induce perceptible erythema on the skin [21]. For SPF testing, the Mercosur, European, Canadian, Australian, and Japanese Regulations Act is in accordance with ISO 24.444:2019 by in vivo evaluation [9]. As a standard, 2.0 mg/cm2 of the product must be evenly applied on the skin to determine the SPF. When determining the SPF of sunscreens, it is essential to apply the formulation correctly. Otherwise, the result will not be reproducible or accurate [9,22].
The importance of the use of sunscreens is undeniable, but many controversies regarding its safety can be found in the literature. For example, organic UV filters, and their potentially cytotoxic byproducts and intermediate compounds, can lead to side effects including skin rashes, photoallergy, endocrine disruptions, and possible systemic absorption in long-term use [9,23]. The possibility of such adverse effects is prompting researchers to develop more stable/photostable organic UV filters with a wider range of absorption in the UV region, ideally, with much lower or no adverse effects [16]. Some strategies can be applied to increase the photostability of organic UV filters, such as the use of synergic blends of UV filters, which have the properties of stabilizing each other, antioxidants, and/or use micro or nanoencapsulation to increase the molecule’s stability [9]. Also, inorganic UV filters can be an alternative to develop safer sunscreens, as they are broad-spectrum and cause less cutaneous irritation than organic UV filters. Thus, they are the first option to lower the allergenic potential of formulations and develop products that are more suitable for children and individuals with sensitive skin [2,20,24,25]. Jesus et al. [26] analyzed 444 sunscreens (379 sunscreens for adults and 65 for children) in the Portuguese market in 2021. It was demonstrated that 54% of the sunscreen formulations for children contained at least one type of inorganic UV filter, in contrast to 38% of adult sunscreens [26].
Traditionally, the group of inorganic UV filters includes many compounds, such as iron oxide, red veterinary petrolatum, talc, calamine, kaolin, ZnO, and TiO2, with the last two, currently, being the only ones that the regulatory agencies of the USA, Europe, Brazil, and Japan approve to be used in sunscreens [2,8,20,27,28,29]. There are also some innovative suggestions in the literature, such as hydroxyapatite (Hap), cerium dioxide (CeO2), mesoporous silica, TiO2, ZnO or silicon oxide (SiO) rich clays, and hydrotalcite [20,24,30,31,32,33,34]. In this review, we focused on zinc oxide and titanium dioxide, as they are the only inorganic UV filters approved for sunscreen use, classified as category I—“GRASE” (Generally Recognized as Safe and Effective) by the US FDA [27,35].
2. UV Radiation—Characteristics and Biological Effects
The solar radiation that reaches the surface of our planet comprises 3–5% UV radiation, 52–55% infrared radiation, and 42–43% visible light [36]. The UV radiation is classified into three types based on their wavelength interval: UVA (320–400 nm), UVB (290–320 nm), and UVC (100–290 nm). Fortunately, the most harmful UVC radiation is completely absorbed by the ozone layer and atmosphere, but UVA and UVB can cross those barriers, reaching the surface of the Earth [37]. UVA may also be classified as UVA1 (320–340 nm) and UVA2 (340–400 nm). The UV fraction of the sunlight present in the ambiance is predominantly composed of UVA (90–95%) and 5–10% of UVB radiations [14]. Some examples of benefits and potential harmful effects on skin of exposure to UV radiation are described in Table 1.

Table 1.
Examples of benefits and negative effects of exposure to UV radiation on humans.
Furthermore, it was reported that 80% of skin aging is caused by UV radiation exposure, which can be prevented with the use of strategies of photoprotection. These strategies/methods include limiting the time of solar exposure, especially in midday sun, seeking shade when outdoors, wearing a brimmed hat, use of adequate photoprotective clothing and sunglasses, and applying broad-spectrum sunscreens with FPS equal or greater to 30 with a critical wavelength of, at least, 370 nm on the exposed sites of the body. Additionally, it is advisable not to use artificial tanning devices, as they can increase the risk of developing skin cancer and suffering from other deleterious consequences of UV radiation exposure [37,41,43].
UVA radiation has the longest wavelengths and hence is less energetic, but it can penetrate deeper into the skin, reaching more internal layers, such as the dermis [16]. This radiation is more abundant and cannot be blocked by glass [14]. Exposition to this type of radiation leads to immediate skin tanning, due to the darkening of already formed melanin via photooxidation of leucomelanin present in the epidermis outer layer cells. Also, UVA causes dermic and epidermic alterations, leading to a reduction in skin elasticity and damage to the peripheral vascular system. It can cause the formation of free radicals and ROS, responsible for photoaging and the development of skin cancer, due to the indirect oxidative stress on DNA nucleotide bases [14,16]. This oxidative stress can lead to DNA oxidative base modifications and strand breaks, which may result in cell mutations and carcinogenesis [38]. It is also related to the rise in matrix metalloproteinases (MMPs) levels, which accelerates collagen degradation and may induce apoptosis of dermal fibroblasts [41].
UVB radiation has intermediary photon energy and the capacity to penetrate the skin until the epidermis. It is responsible for the development of acute skin disorders, such as sunburn and erythema, as well as skin cancer [4]. This radiation causes erythema about 4 h after exposition and can stimulate the production of more melanin [16]. Furthermore, UVB radiation comprises approximately 6% of all the UV radiation that reaches the surface of our planet. It is considered more cytotoxic than UVA radiation, causing DNA damage when absorbed, inducing mutations and carcinogenesis, thus being highly related to the development of squamous cell carcinomas. It also induces the formation of matrix metalloproteinases (MMPs), reactive oxygen species (ROS), and elastases, which are involved in the skin aging process [41].
3. General Characteristics of TiO2 and ZnO
Titanium dioxide (TiO2) and zinc oxide (ZnO) are the only inorganic UV filters currently approved for use in sunscreen formulations [44]. The inorganic UV filters have a wider UV absorption spectrum than the organic ones, as they combine different mechanisms of action, including absorption, reflection, and scattering of UV radiation. As TiO2 mostly absorbs in the UVB range and ZnO interacts with UVB and UVA radiations, the combination of both enables the development of broad-spectrum sunscreen systems [14,19,45]. The typical absorption spectra of both ZnO and TiO2 are presented in Figure 1, but their SPF values vary according to each particle’s characteristics among other contexts. A study by Couteau et al. [46] evaluated the in vitro SPF of 9 ZnO and 8 TiO2 samples from different manufacturers. The samples were dispersed in an O/W emulsion at 25 wt%, and the SPF of these formulations was evaluated by diffuse reflectance spectroscopy with an integrating sphere (UV Transmittance Analyzer UV1000S, Labsphere, North Sutton, US). The reported in vitro SPF values ranged from 5.00 to 10.13 for the ZnO samples and from 5.01 to 38.60 for the TiO2 ones [46]. Notwithstanding, it is recommended that formulations must have a combination of organic and inorganic UV filters to obtain a synergic effect, enhancing the efficacy of the photoprotective system [14].
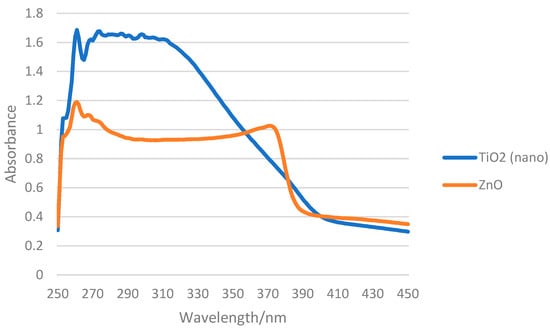
Figure 1.
Absorption spectra of O/W formulations containing 10% of TiO2 and ZnO obtained by spectroscopy of diffuse reflectance with integrating sphere (Labsphere UV-2000S). Source: authors.
TiO2 is largely applied in many industries due to its high stability, biocompatibility, photocatalytic properties, and affordability [47,48]. The population is in constant contact with TiO2 particles, since this metal oxide is used, for example, as a white pigment in paints, papers, plastics, processed foods, pharmaceuticals, and cosmetics (makeup, and toothpastes, for instance). These particles are usually found in daily products such as sweets (i.e., chewing gums, chocolate with hard white coating, white-colored dairy products, powdered sugar), personal care (i.e., white-colored shampoos, shaving foam, lip balms, sunscreens, toothpaste) and paints [49]. They are applied in the packaging of light-sensitive food products, since they have a great refraction index and UV filtering properties, which ensure longer food durability [50]. TiO2-containing materials can also be used as antimicrobials for water purification and medical applications (e.g., drug delivery systems, antibacterial devices, bone and drug-releasing implant applications, chemotherapeutic agents, and bone implants) [48,49,50,51]. Furthermore, they can be applied in environmental remediation as a photocatalyst for organic contaminants, in the development of super hydrophilic coatings, and in solar cells due to the high surface area and photoreactive properties [52]. TiO2 nanoparticles (NPs) can assist in the process of pollutant removal since their large surface areas and electrical charge facilitate the process of attracting and flocculating the pollutants [53].
As a UV filter, TiO2 and ZnO can be added to sunscreens at a maximum of 25% per weight, according to the Scientific Committee on Consumer Safety (SCCS). In 2014, the SCCS concluded that nanosized TiO2 at a concentration of up to 25% was “considered to not pose any risk of adverse effects to humans after application on healthy, intact or sunburnt skin”. The ZnO nanoparticles in the same concentration were also considered “not to pose a risk of adverse effects in humans after dermal application”. However, the application of such nanoparticles in products that may be inhaled (e.g., powders and sprayable products) is not recommended, as there are concerns regarding inhalation exposure and the development of respiratory disorders [54]. In 2019, the US FDA issued a proposed order to revise the regulation of sunscreens, maintaining TiO2 and ZnO as GRASE I (generally recognized as safe and effective) at concentrations of up to 25% [27,35]. The Mercosur and European Commission legislations also allow a maximum concentration of 25% of inorganic UV filters (TiO2 and ZnO) in cosmetic formulations [28,55,56].
TiO2 particles are found in different crystalline structures: anatase, rutile, and brookite. Each polymorph has a characteristic shape, structure density, and refractive index. Rutile is the most thermodynamically stable form among all three [50], and anatase is the most photochemically active form, as it can generate six times more ROS than rutile [47]. These compounds also have a wide bandgap: 3.2 eV for anatase and 3.0 eV for rutile [57]. The forms of TiO2 generally used in sunscreens are pure rutile or a rutile and anatase combination, with anatase rarely being used alone [58]. All three forms have a high refractive index, with rutile having the highest one, thus being an optimal pigment. Therefore, rutile is the form that better scatters the incident light, while anatase is better at absorbing the radiation [50]. On the other hand, ZnO presents two different forms, wurtzite and zinc-blende, with wurtzite being the most common and stable polymorph [19] with a bandgap of 3.37 eV [59].
The size of the particles affects the absorption spectrum of the UV filters since the absorption edge wavelength decreases as the particle gets smaller and shifts into the UVB region [20]. Smaller TiO2 particles tend to be more effective as UV filters, as the reduction in size leads to a change in the absorption properties, increasing this characteristic in the UV spectrum, and decreasing the scattering effect of visible light, which reduces the whitening aspect on the skin, thus leading to a more cosmetically acceptable product [60]. The size reduction also benefits the film formation on the skin, as the particles may be more evenly spread enhancing the photoprotective effect. Also, micronized particles are more prone to form aggregates and agglomerates due to electrostatic effects, which tend to decrease their efficacy [61]. A study by Lin [61], analyzed the efficacy of sub-micron and nanosized TiO2 particles, indicating a great discrepancy in the SPF values. The sub-micron particle showed an SPF value of 3.09 ± 0.27 in contrast to 16.08 ± 1.28 of the nanosized particle, both at 20 wt% [61]. However, nanosized particles have increased photocatalytic activity [62]. Rutile absorption starts around 380–400 nm, whereas anatase starts absorbing at 360–380 nm [63]; therefore, rutile is more effective against UVA radiation than anatase [14]. The visible light scattering is also directly affected by particle size, the most efficient being those around 150–250 nm large [63]. Accordingly, smaller ZnO and TiO2 particles have better UVB absorption and decreased UVA absorption; thus it is important to have a combination of various-sized particles, small nanosized and larger micronized particles, to achieve broad-spectrum protection [19].
As aforementioned, the issue regarding the formation of a white chalky texture film on the skin surface is a characteristic of inorganic UV filters that displeases the consumer [8]. The refraction indexes of both ZnO and TiO2 are high (1.9 for ZnO and 2.6 for TiO2), contributing to enhancing the whitish aspect of the protected skin [8,19,20]. Accordingly, ZnO tends to exhibit a lesser whitening effect than TiO2 when applied on the skin as a UV filter [19]. To improve this characteristic, it is possible to use inorganic UV filters with particle sizes smaller than about 200 nm since the efficiency of visible light scattering decreases exponentially as the wavelength decreases [19]. In fact, nanosized inorganic UV filters have been increasingly included in sunscreen formulations to improve aesthetic and sensorial characteristics, as they appear transparent, can be less greasy and easier to apply, and the amount of UV filters to be included in the formulation can be reduced given the contribution of UV radiation scattering, which is positive to increase the photoprotection efficiency [14,50,64]. Nanoparticles may have positive aspects; however, the smaller UV filter particles tend to absorb in the range of UVA2 and UVB, diminishing the absorption in the visible and UVA radiations [14,50,64].
TiO2 and ZnO NPs may cause the generation of ROS, such as hydrogen peroxide (H2O2), hydroxyl radicals (•OH−), or singlet oxygen (1O2), which can penetrate the stratum corneum, inducing photoallergenic contact dermatitis and photoaging upon prolonged exposure [14,38,65]. To decrease the photoactivity, the particles are usually coated with unreactive coating materials, such as aluminum oxide (Al2O3), zirconium oxide (ZrO2), silicon dioxide (SiO2), silicon tetrahydride (SiH4), or polymers, among others. SiO2 and Al2O3 coatings have the properties to increase the energy band gap of the TiO2, decreasing the formation of ROS [50,66,67,68]. The surface coating has the capability of diminishing the photocatalytic properties of these metal oxides, as it covers the particle surface inhibiting the occurrence of redox reactions when UV-irradiated, increasing safety without compromising efficacy. Even though the surface coating can increase the safety of the particles, it is not possible to completely eliminate the photoreactivity [50,67]. Furthermore, TiO2 NPs usually are coated with lipophilic materials such as polydimethylsiloxane (PDMS), dimethicone, or stearic acid, which reduces their photoactivity and enhances dispersibility, improving the compatibility among the ingredients of the sunscreen formulation [50,58,67,69].
According to Lewicka et al. [70], only TiO2 coated with alumina and simethicone was not photoactive and did not result in the production of ROS. Remarkably, all ZnO-containing sunscreen samples had a high level of photoactivity, which indicated that ZnO intrinsically generated ROS similarly to TiO2, but, differently from TiO2, ZnO particles seemed not to be coated in the analyzed samples [70]. Janczarek and Szaferski [71] also confirmed that titania coated with SiO2 and/or Al2O3 has a reduced photocatalytic activity, suppressing the generation of ROS. The researchers demonstrated that the thickness and the homogeneity of the surface coating were crucial factors in determining the effectiveness of the inhibition of ROS production [71].
In addition to UV radiation, infrared (700 nm–1 mm) and visible light (400–700 nm), predominantly blue light (380–455 nm), are also related to photoaging and photodamage. Visible light can cause ROS production, proinflammatory cytokines, MMP-1 expression and potentiate the effects of UV radiation. This suggests the relevance of developing sunscreen formulations that can protect against these types of radiation since they also contribute to the photodamage processes [41]. Pigmentary-grade ZnO and TiO2 can reflect visible light since they must be opaque; however, they have the disadvantage of leaving a whitish layer on the skin after application. It is a strategy for the development of formulations containing these types of particles to incorporate iron oxide to match the color of the consumer’s skin tone, solving the aesthetical issues [41] and providing protection not only in the UV but also in the visible region [41,72].
4. Green Synthesis Methods of TiO2 and ZnO Nanoparticles
Nanoparticles can be synthesized by distinct methods, which can be classified as top-down or bottom-up synthesis. The top-down synthesis takes bulk materials and turns them into nanosized particles by a kind of grinding/comminution process, whereas the latter builds up the particles from individual atoms and molecules to produce the nanoparticles [73]. In top-down methods, the bulk material is used to produce nanosized particles using both chemical and physical techniques. However, it results in particles with imperfections and defects on the surface, as well as aggregation processes, which can affect physical characteristics and the product’s surface properties. On the other hand, the bottom-up methods have the advantage of enabling better control over the particles’ characteristics, such as size, homogeneity, and morphology, which are essential to determine the properties of the NPs [74]. Both bottom-up and top-down methods are depicted in Figure 2.
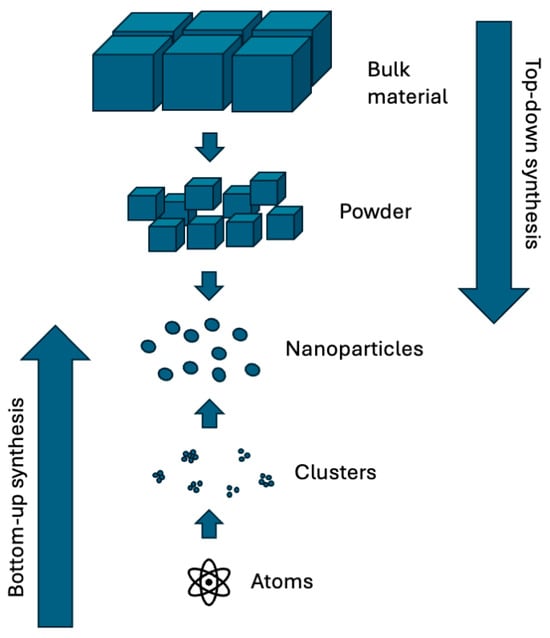
Figure 2.
The top-down and bottom-up methods.
TiO2 and ZnO NPs can be synthesized by sol–gel, hydrothermal, solvothermal methods, precipitation/coprecipitation, electrochemical deposition, and chemical vapor deposition process [59,74]. The size and morphology of the NPs can be conveniently modified by alteration of the experimental parameters, such as the type of solvents, precursors, and reaction conditions, e.g., pH value, calcination temperature, and the duration of the reaction, considering the different methods [59,75]. Usually, the precursors for the synthesis of TiO2 and ZnO are titanium (IV) alkoxide and a zinc (II) salt solution (zinc acetate or zinc acetate dehydrate, for instance) [59,76]. Some of these methods require specific conditions and may be high-costly, which can be an obstacle to the preparation of these compounds. The most attractive technique among all these is the sol–gel method, which is a cost-effective, simple, highly reproducible, and reliable method with the advantage of the possibility to control the physical characteristics and morphology of the particles [59,77]. It also has a low reaction temperature and results in a product with high purity and homogeneity [77].
Traditional chemical synthesis methods have a large yield, enabling production scalability and control over the particle’s morphology and size. Nevertheless, they require the use of large amounts of solvents, high temperatures, and/or pressure, resulting in a dangerous working environment in addition to requiring a great amount of energy. Furthermore, the chemical reactions may produce potentially hazardous and toxic byproducts, which can pose a risk to human health and the environment applied to produce TiO2 and ZnO NPs, in which the molecules in the plant extracts act as capping agents. Such molecules can be extracted from different parts of the plants, such as the roots, leaves, fruits, seeds, or beans [74,75], and their concentration can influence the physical properties and morphology of the particles [74,75,77]. Some interesting and innovative examples are described in the literature, such as the study by Porrawatkul et al. [78], which synthesized Na- and Al-doped ZnO NPs using the extract of star-fruit (Averrhoa carambola) as a reducing agent [78], and a study by Roopan et al. [79] with the synthesis of rutile type TiO2 using agricultural waste derived from custard apple (Annona squamosa L.) peel [79]. In addition, a noteworthy possibility of using a modified sol–gel method with only water as a solvent for the synthesis of these NPs was also reported [59].
As nanoparticles are increasingly being applied in daily-use products, the identification of these materials is critical to ensure their safety to consumer health. The particles in the nanoscale size have different properties in comparison to their bulk materials and can be characterized by particle size and size distribution, surface area, composition and functional groups, shape and crystalline phase, aggregation and agglomeration state, solubility and dispersibility, and porosity [80]. For cosmetics, the SCCS states the importance of particle characterization, suggesting that information regarding these parameters should be collected by methodologies such as transmission electron microscopy (TEM), laser confocal microscopy, X-ray diffraction (XRD), dynamic light scattering (DLS), nuclear magnetic resonance (NMR) and atomic force microscopy (AFM) [81,82]. In particular, the aggregation and agglomeration processes may modify the characteristics and properties of the nanoparticles, which can alter their toxicological profile, thus the US FDA recommends that the analysis ought to be conducted with the free and the agglomerate/aggregate NPs [82]. However, the characterization of nanoparticles in cosmetic formulations poses a challenge, as the characteristics of the products, such as the pH and viscosity, but especially the presence of organic components, may interfere with the analysis [83,84].
5. Health Concerns
The population is in constant contact with TiO2 and ZnO particles present in many daily-use products. However, there are concerns regarding the potential toxicity of these nanosized particles. According to the IARC (International Agency for Research on Cancer), TiO2 is classified as possibly carcinogenic to humans and is included in the IARC group 2B of carcinogenic materials, with hazard statement H351 (suspected of causing cancer—inhalation), when at least 1% of the particles have a diameter of 1 µm or less. This statement is based on evidence showing that high concentrations of this pigment and ultrafine TiO2 dust can lead to the development of respiratory tract cancer in rats [50,85]. The inhalation of TiO2 NPs is also recognized to induce direct damage to the brain since they can reach it directly via the olfactory bulb and extend to the hippocampus region. When in the brain, these particles can cause cell toxicity, DNA damage, apoptosis, inflammation, and oxidative stress, leading to neurodegeneration and neurodegenerative diseases. Also, it is reported in the literature that the incidence of lung cancer among workers exposed to TiO2 dust in TiO2 factories is significantly higher in comparison to other individuals [47,86]. The IARC considered this information relevant, because of the possible health hazards due to occupational exposure during the production of these materials [87]. Thus, it is not advisable to develop aerosolized sunscreens containing such NPs. Regarding the potential oral toxicity of TiO2 particles, some studies using animal models indicated that ingestion exceeding the recommended daily limit may cause damage due to its accumulation in tissues, including stomach and intestine inflammation, liver cell necrosis, lesions on cardiovascular tissue, and even enhancement of anxiety [50]. However, an in vivo acute oral toxicity study in rats with dosages up to 5000 mg/kg of nanoscale coated rutile/anatase TiO2, evaluated over 14 days after exposition, following the OECD (Organization for Economic Co-operation and Development) Test Guideline, concluded that the oral LD50 for this substance is >5000 mg/kg bw. In addition, a study with nine participants receiving a single oral dose of 5 mg/kg of different particle sizes (15 nm, 100 nm, and <5000 nm) reported insignificant absorption of these particles in the gastrointestinal tract, and thus, little to no toxicity of TiO2. TiO2 particles designed for food applications are pigmentary grade, i.e., over 100 nm, not reaching the nanoscale. When ingested, most of these particles are not absorbed, as they are excreted from the gastrointestinal tract [88]. Also, an in vitro study demonstrated that human intestinal cells are protected from the potential TiO2 NPs reactivity regardless of concentration, even when the cells are in their most sensitive phase [50].
ZnO is “generally recognized as safe” by the US FDA when used as an inorganic UV filter according to the cosmetic directives. However, both ZnO and TiO2 can induce the formation of free radicals and ROS when exposed to UV radiation [89], leading to deleterious effects, such as lipid peroxidation, the elevation of the glutathione peroxidase levels, catalase, superoxide dismutase, depletion of cellular antioxidants, and oxidative stress-induced apoptosis in TiO2 NPs-exposed cells due to the elevation of ROS levels [47,64,90]. These mechanisms are associated with the toxicity of the TiO2 NPs which can lead to cell damage, inflammation, cell death, and organelle disruption. These processes are also involved in genotoxic consequences due to DNA damage and micronuclei formation, which indicates the induction of chromosomal aberrations is related to carcinogenesis [47]. In opposition to similar reports in the literature about the harmful photocatalytic effects of these metal oxides, a study by Osmond-McLeod et al. [45] with chronic exposure during 36 weeks of hairless mice to commercially available sunscreens containing TiO2 and ZnO NPs had a reassuring result. The study analyzed three sunscreens labeled as SPF 30+ purchased from a local retail store in Sydney, Australia, one containing only ZnO (200 mg/g), the second a mixture of TiO2 (40 mg/mL), octylmethoxycinnamate (70 mg/mL) and butyl-methoxydibenzoylmethane (40 mg/mL), and the last with only organic filters octylmethoxycinnamate (99 mg/mL, butyl-methoxydibenzoylmethane (19.8 mg/mL), 4-metylbenzylidene camphor (39.6 mg/mL) and octocrylene (9.9 mg/mL). The mice were exposed to the sunscreens once a week, applying 2 mg/cm2 to their body with a gloved fingertip, and after 20 min, they were exposed to UVR. After the study period, the researchers observed that, even though the particles had photocatalytic properties, they did not cause adverse effects in long-term application under the research experimental conditions. Additionally, there was no increase in Zn levels in the serum and internal organs/tissues, except for a small increase in Ti levels in the liver, probably associated with low-level ingestion of TiO2 [45].
The application of innovative nanomaterials and/or nanostructures is increasingly being considered in cosmetic formulations as they can, for instance, improve aesthetic and sensorial characteristics and bring greater skin penetration, enhancing the active ingredient’s efficiency. However, these particles may cause potential risks to human health, which are mainly related to their size, ability to evade immunological defense systems, form complexes with proteins, and generate free radicals [89]. Considering the size range of the metal oxide NPs, researchers suppose that they suffer dermal, respiratory, or gastrointestinal penetration, reaching circulation and thus posing a greater risk to the consumer’s health [58]. The characteristics that may influence the penetration of NPs into the skin, despite the size, are the type of coating, surface charge, and dispersion, as well as the formulation matrix and their interaction with the constituents of the outer skin layers [91]. Also, the cytotoxic effect of TiO2 NPs is size-dependent, so the smaller the particle, the greater tends to be its toxicity [47]. The penetration of nanoparticles into the human skin could be minimized by the application of nanoparticles designed to adhere to the skin surface [92]. Furthermore, the different physicochemical characteristics of the particles, e.g., particle size, presence of coating, and crystalline structure, can influence the toxicity of these materials [85].
Specialized literature demonstrates controversial results on the eventual dermal absorption of these particles as some studies indicated that TiO2 NPs can pass through cell membranes and cause damage to human dermal fibroblasts, whereas others reported that these NPs do not trespass the skin surface or the stratum corneum and cannot reach deeper layers of the skin [38]. Studies support the last statement, as the stratum corneum layer is a natural barrier under normal conditions that protects against, for example, UV radiation effects and exogenous particles. Nevertheless, when this barrier is compromised, the particles may cross it and reach deeper cutaneous layers [47]. Thereby, it was proposed that damaged skin could have a greater permeability, however, most of the literature described that slightly compromised skin has no greater susceptibility to the penetration of those agents. Skin affected by psoriasis tends to have a hyperkeratotic epidermis, so it exhibits less penetration susceptibility. In the case of skin affected by eczema, the stratum corneum is disrupted, thus enhancing the penetration of topically applied agents more than in psoriatic skin [14,89]. Also, a study demonstrated that TiO2 NPs could not reach viable cells, regardless of being applied to healthy or psoriatic skin [58]. In addition, an increase in the absorption of topically applied TiO2 and ZnO-containing sunscreen formulations was not observed even in skin damaged by UVB radiation [88]. The TiO2 absorption into the skin is unlikely to occur due to its lack of solubility in aqueous media and biological fluids, regardless of the concentration or formulation of the sunscreen [14].
The majority of the in vitro studies regarding the skin penetration of TiO2 NPs indicate that these particles mainly remain within the stratum corneum and hair follicles. A study with repeated application of formulations containing 5% of coated and uncoated TiO2 particles with a size range of 20–500 nm detected the presence of these particles in the dermis. However, it was not clear in the study if these particles penetrated the skin or were found in the hair follicles [19,93]. It is important to highlight that the potential skin penetration of nanoparticles is prone to be affected by the size and coating of the particles used in the study, as well as the dose and number of applications, duration of the study, and the exposure or not to UV radiation [47]. In addition, the permeability of the skin is dependent on the animal species, as rabbit skin tends to be more permeable than rodent skin, which is larger than pig skin, which is larger than human skin [89]. For in vitro skin penetration studies and evaluation of topical products, the porcine ear skin model is usually used as a model for human skin [21].
An in vitro skin absorption test performed using porcine skin indicated that none of the rutile type TiO2 samples (diameter of 120 nm) reached deeper layers of the skin, implying no risks of systemic exposure to these particles since more than 70% of the TiO2 was recovered from the surface of the skin, 20–30% was present in the stratum corneum and epidermis, and less than 3% reached the dermis [63]. Moreover, it was demonstrated that TiO2 NPs coated with cetyl phosphate, manganese dioxide, or triethoxycaprylylsilane reduced the penetration in pig skin, not penetrating beyond the stratum corneum [58].
Another in vitro study performed by Kubác et al. [63] indicated that, despite the TiO2 photoactivity, there was no cytotoxicity or phototoxicity even in concentrations of 1000 µg/mL, recommended by the OECD (Organization for Economic Co-operation and Development) Test Guideline. Also, there were no alterations in cell viability, demonstrating that the photoactivity of the samples was low enough to be safe, and the free radicals produced during irradiation did not negatively affect the cell culture [63]. However, TiO2 NPs were detected beyond stratum corneum at the viable cells in the epidermis of intact and UVB sunburned skin in an in vivo study by Næss et al. [91] with two subjects after seven days of application of 2.0 mg/cm2 on a skin area of 600 cm2, six times a day of a commercial sunscreen containing nano-TiO2 [91].
In a review paper, Smijs and Pavel [19] analyzed seven articles about in vitro, and eleven in vivo human skin penetration studies using TiO2 and/or ZnO NPs-containing formulations, including commercially available sunscreens, and O/W emulsions and dispersions with different concentrations of these metal oxides. The researchers concluded that the nanoparticles were found in deeper stratum corneum layers and hair follicles but generally remained in more superficial layers. Additionally, oily dispersions facilitate the penetration of TiO2 into the skin compared with an aqueous dispersion [19].
The dermal absorption of ZnO NPs is unlikely in normal conditions of usage of sunscreens, and, similarly to TiO2 NPs, the penetration would be limited to the stratum corneum. Some amount of Zn2+, present due to the dissociation of ZnO, is absorbed by human skin through the skin folds, furrows, and hair follicles [94]. This increase in Zn2+ ions seems to be directly correlated with the irradiation intensity, being mainly related to UVB radiation, which causes the dissociation of ZnO and, consequently, the accumulation of free Zn2+ ions. An in vitro study also demonstrated that the Zn2+ ions induced the elevation of ROS levels, causing cytotoxic effects and oxidative stress to human epidermal keratinocytes. Moreover, the oxidative stress caused by ZnO particles is associated with decreased collagen content in the skin [14,94], as the presence of ROS can alter the biosynthesis of collagen, cause collagen oxidation and degradation, and damage keratinocytes and fibroblasts [95,96,97].
A study of rats by Ryu et al. [94] with long-term topical application of ZnO NPs, with the samples being applied to an area of skin corresponding to 10% of the total body surface area, as described in the OECD guideline 411, reported no internal organ toxicity, only temporary, dose-dependent inflammation of the skin at the application site. In conclusion, there were no adverse effects observed in rats with doses as high as 1000 mg/kg body weight [94]. Furthermore, many in vitro studies with excised human skin and in vivo studies evaluating the penetration of ZnO in healthy and psoriatic patients’ skin after a single topical application indicated that its penetration was limited to the superficial layers, the stratum corneum, without reaching viable epidermal cells [92].
A study by Mohammed et al. [92] evaluated if ZnO NPs in sunscreens could penetrate the skin in repeated application in vivo in five subjects. The results demonstrate that, even with hourly or daily topical application of the nano-ZnO-containing formulations, there was no significative elevation of ZnO levels in the skin, evidencing no penetration to the viable epidermis. This demonstrated that the nano-ZnO particles did not penetrate human skin or cause visible morphological changes or changes due to redox reactions. In an ex vivo study by the same research group with human skin samples, with repeated hourly application of coated and uncoated nano-ZnO, an increase in soluble Zn species was detected compared with the control. The most probable form of Zn penetrating the skin was Zn2+, originating from the solubilization of nano-ZnO particles accumulated on the stratum corneum and in skin furrows due to the naturally acidic pH of the human skin. In this case, the Zn2+ ions could reach the circulation and cause a nonsignificant increase in systemic Zn levels. Also, no evidence of morphological changes in skin cells, necrosis, or apoptosis was found after the topical application of nano-ZnO-containing formulation in vivo for five days, indicating that there were no toxic effects. This concludes that the toxic effects detected in in vitro studies may not correlate to in vivo assays, i.e., when applied in vivo to human skin under realistic in-use conditions [92].
A recent study by Alves et al. [44] evaluating the penetration of coated and uncoated ZnO NPs in topical formulations containing 20% of the metal oxide using vertical diffusion cells concluded that the samples did not reach viable epidermis. Both NPs accumulated around the hair follicles and did not reach deeper structures, although the coated ZnO accumulated on the skin furrows [44].
A study by Osmond-McLeod et al. [45] on the topical use of sunscreens containing nanosized ZnO and TiO2 in hairless mice demonstrated no statistically relevant histological outcomes with the long-term use of the samples. Also, an increase in Zn samples in internal organ tissues was not observed. Only a minimal increase in Ti in the liver was detected, probably due to residual TiO2 ingestion [45].
The main mechanism of TiO2 and ZnO NPs toxicity is still not fully elucidated, but it is proposed to be related to photochemical reactions leading to the production of free radicals and ROS [47]. However, their potential toxicity would only be a concern if they effectively penetrated viable cutaneous cells [89]; however, studies indicated that nanosized ZnO and TiO2 particles were not able to permeate intact normal skin when topically applied [98]. The major risk is related to their inhalation since they can trigger inflammatory and cardiovascular diseases and promote the development of some types of lung cancer [14]. It was also reported that ZnO NPs administered via respiratory routes caused damage to the liver and lungs [94]. Therefore, it is imperative to be careful when using sunscreens containing inorganic filters, such as sprays and aerosols [14]. When inhaled, these nanoparticles stay mainly in the upper airways but can also reach the lungs and alveoli due to their small size. The clearance of these particles from the upper airways is usually by the mucociliary mechanism and cough, which is the t1/2 for two to four hours in healthy individuals. However, 10% of these insoluble particles may remain in the lungs and have a slow clearance rate [58].
As mentioned in a previous section, the surface coating of TiO2 and ZnO NPs exerts great influence on their reactivity, significantly reducing their photocatalytic activity. Hence, either uncoated or coated TiO2, presenting imperfections in its surface coating, exhibited significant photocatalytic activity and significant cellular damage in cultured human skin cells, which reinforces the importance of the surface coating to reduce the reactivity of these particles [14]. However, it was also observed that some coatings may increase their cytotoxicity, as TiO2 with alumina and silica seems to induce a higher pulmonary inflammatory response in comparison with uncoated particles [47].
Nowadays, the importance of bacterial microbiota to human health is vastly studied, thus the evaluation of the impact of sunscreens in the growth and development of these organisms is of great interest, as the disruption of the microbiota is related to the development of skin disorders [99]. Studies evaluating the effect of rutile type TiO2 particles on the skin residential bacterial flora indicated that they had insignificant effects on such lifeforms, and thus, they do not cause any alteration in that microbiota [63]. However, a study by Rowenczyk et al. [99] evaluating the effect of coated TiO2 in bacterial microbiota demonstrated that different coatings have distinct effects on bacterial growth due to their structure and properties, not intrinsic toxicity. It was observed that the presence of NPs altered the growth kinetics of S. aureus and P. fluorescens, increasing their generation time [99].
At present, there is no evidence implying that TiO2 and ZnO NPs can cause pathogenic modifications in the skin, as the majority of the studies indicated that they do not reach viable cells or the circulation through the deeper layers of the skin, remaining at the stratum corneum [8,58]. In addition, current manufacturing processes generally limit the TiO2 particle sizes above 100 nm, thus reducing the concerns regarding skin penetration [62].
6. Environmental Aspects
The increasing manufacturing and consumption of sunscreens may result in environmental impacts due to the growing release of their ingredients in aquatic milieus, such as rivers, lakes, oceans, and other water bodies. With the progress of tourism worldwide, especially in coastal and marine outdoors, associated with people’s knowledge of the necessity to protect the cutaneous tissue against UV radiation, the release of sunscreens’ ingredients in water bodies also augmented in recreational aquatic activities and discharge in wastewater [100,101]. In tropical countries, it is estimated that 25% of the sunscreen applied on the skin eventually reaches the water bodies [53,101]. Due to their toxicity, non-biodegradability, and chemical structures, these compounds are known as emerging pollutants, presenting a risk to aquatic organisms [102], as organic UV filters pose a challenge to be removed from wastewater, since they are highly lipophilic, have a high organic carbon–water coefficient and very low solubility in water, and most water treatment plants are designed to remove particulates from water [103,104]. A study by da Silva [105] analyzed the presence of organic UV filters in water treatment plants localized in the state of São Paulo, Brazil, over a period of six months to one year. The results indicate the presence of benzophenone-3 (BP-3) and ethylhexyl methoxycinnamate (EHMC) in low but detectable concentrations in raw water and water from public water supply, especially during summer [105]. Accordingly, other studies also have indicated that the increase in aquatic recreational activities in coastal areas and the concentration of UV filters in the water bodies are directly correlated [101]. A study conducted in Korea indicated that the concentration of UVR filters in water bodies increased by 25% during summer, confirming this correlation. In addition, when released in chlorinated water, these organic UV filters may generate toxic byproducts resulting from reactions with chlorine, which can be even more hazardous [104].
The main concern about the presence of these components in the environment is related to the impact on the ecosystem. Organic UV filters in sunscreens can provoke unfavorable effects on aquatic organisms, including bleaching coral reefs and the disruption of hormonal balance in marine animals [53,102]. Considering these outcomes, among other justified points of view, commercial sunscreens containing oxybenzone and octinoxate had their sale and distribution banned by the Hawaiian state, starting in 2021 [53,106]. The possibility of imposing a similar ban is also being discussed in other locations, such as Brazil and the European Union [103].
As a result of the possibilities of the environmental impact of organic UV filters, the interest in sunscreens containing inorganic UV filters has increased since they are considered safer and more eco-friendly [53]. There is also a general belief that inorganic filters are considered “reef-safe” due to their natural origin and are attracting attention from both industry and consumers as most people are looking for products labeled as harmless to the environment [101].
The toxicity of inorganic UV filters is mainly related to their photocatalytic properties, which result in photooxidation and photodegradation, as they generate ROS when irradiated with visible and UV radiations [53,100]. This mechanism is the main cause of toxicity to aquatic organisms such as algae, arthropods, mollusks, echinoderms, and marine vertebrates, as they generate oxidative stress [101]. However, ZnO toxicity is also related to its solubility and the release of Zn2+, which is critical to aquatic organisms, as most of the uptake of Zn by these organisms is in its ionic form, as demonstrated by a study by Wu [107], who evaluated the toxicity of ZnO NPs and microparticles and ZnCl2 in four different species (i.e., C. reinhardtii, E. coli, D. magna and zebrafish). The toxicity of Zn is mostly related to the disruption of homeostasis due to Zn2+ uptake, cytotoxicity, and formation of ROS, and, consequently, oxidative stress [53,107,108]. Additionally, nano-ZnO is more prone to dissolution in seawater than non-nano-ZnO or Fe-doped nano-ZnO. On the other hand, nano-TiO2 is relatively stable and insoluble in water. Thus, it tends to form larger particles via aggregation, remaining suspended or set onto the bottom of the water bodies [53]. Despite its higher toxicity to aquatic organisms in comparison to TiO2, ZnO is the most used inorganic UV filter in commercially available cosmetic formulations labeled as “reef-safe” [109].
Metal accumulation is also an important factor related to the toxicity of these particles since they can enter bioaccumulation and biomagnification processes, even reaching other ecosystems [101]. The bioaccumulation process is facilitated due to the high stability of the inorganic UV filters, which can accumulate in the different organisms and transfer along food chains, reaching both low-trophic and higher-trophic levels, and even affecting humans [53]. Studies in Switzerland, Spain, and Norway indicated the presence of low levels of UVR filters, mostly 4-methylbenzylidene camphor (4-MBC), oxybenzone, and octocrylene in some species of fish. Despite being at low concentrations, it was observed that the concentration of these UVR filters was higher in these organisms than in water, which corroborates with the bioaccumulation process [103,104].
The surface coating, as well as the crystalline structure of the materials, affects the biological and environmental reactivity of the particles [70]. To diminish the negative effects related to the photocatalytic property of inorganic UV filters, an adequate surface coating is required, as they increase stability and reduce the ROS generation when UV-irradiated [53,110]. Additionally, the surface coating significantly decreases the dissolution rate of inorganic filters, acting as a physical barrier that prevents electrons and photons from reaching the particle’s surface [53]. Studies demonstrated that uncoated TiO2 NPs are toxic to dinoflagellates, which are symbiotic organisms vital for coral survival. On the other hand, coated TiO2 NPs were not related to coral bleaching and caused minimal effects on dinoflagellates [101]. In addition, these inorganic NPs can attach to the surface of aquatic organisms, such as phytoplankton, leading to the shading effect on photosynthesis, generating deleterious effects on these organisms [53]. Furthermore, the photodegradation byproducts of ZnO may cause increased morphological deformities in zebrafish, which suggests that these particles can release toxic byproducts [111].
A study carried out at the Old Danube Recreational Lake in Vienna, Austria, evaluated the presence of TiO2 NPs possibly released to water from commercially available sunscreens during aquatic recreational activities. According to estimations of the maximum amount of TiO2 possibly released by sunscreens in the lake, it was predicted that the particles would remain suspended in the water column during the bathing season, generating a final concentration of 27.1 µg/L of the metal oxide. However, it was observed that the NPs did not remain suspended for a long time, posteriorly settling on the bottom of the water body. Nevertheless, the total TiO2 concentration in lake water during the different seasons of the year was lower than 1.7 µg/L [110]. When quantifying the concentration of TiO2 particles, other possible sources of these metal oxides should also be considered, as they are present in many materials, including natural sources, such as titania, a common element in nature. Therefore, a correlation between the increase in Ti ratios in the water bodies to the use of TiO2-containing sunscreens cannot be confirmed [110]. Since ZnO and TiO2 particles are naturally abundant in the environment, it is a challenge to differentiate the natural particles from the manufactured ones, posing a contest to quantify correctly the particles specifically released from sunscreens [1].
Currently, the environmental risk associated with these materials is considered extremely low. Studies have shown that these metal oxide NPs do not remain freely suspended in water; instead, they tend to aggregate and precipitate to the bottom of aquatic environments [1]. This sedimentation limits the penetration/passage of sunlight, thereby reducing the potential for photocatalytic reactions and, consequently, their toxicity. Furthermore, the detected concentration of TiO2 in natural environments is estimated to be in the range of 10–100 µg/L, which is very low [104]. Notably, studies reporting negative impacts of inorganic UV filters on aquatic life, such as zooxanthellae and corals, utilized significantly higher testing concentrations (6.3 mg/L for nano-ZnO and 10 mg/L for nano-TiO2) compared with the concentrations typically observed in natural water bodies [101].
7. Establishment of the Efficacy of Inorganic UV Filters—How Scientific/Specialized Literature Deals with This Task
An ideal sunscreen must provide, at least, despite safety properties, broad-spectrum protection and be photostable [111]. The presence of inorganic UV filters can modify the stability of cosmetic formulations, leading to undesirable outcomes [14]. For instance, Ghamarpoor et al. [62] reported stability challenges for formulations containing more than 10% of TiO2 [62].
Accordingly, the pH value of the cosmetic sample must not be near the isoelectric point of the particles, pH(I), since in this pH the surface charge of the compounds goes to zero, inducing coalescence. The surface coating of these particles exerts influence on their pH(I) value and must be carefully controlled [7]. Ghamarpoor et al. [62] evaluated the effectiveness of TiO2 NPs in sunscreens and described the effect of pH on SPF and UVAPF. At the pH range of 5.0–8.5, there was a decrease in the SPF due to the increased agglomeration of the particles. Particularly, at the range of 6.0–7.5, the formulations presented the lowest SPF and UVAPF values. According to their results, the authors suggested the production of sunscreens preferably in the pH range of 5–6, since this interval is also biocompatible with the skin [62].
Another relevant characteristic to be considered when developing formulations containing inorganic UV filters is the particle size, which greatly influences their performance. The application of particles at the nanoscale increased in the sunscreen market, as they do not leave a white chalky texture on the skin and have a better performance in UVR absorption. When at a range of 200–500 nm, these metal oxide particles mostly reflect visible light, and thus confer a whitish appearance on skin, resulting in an unpleasant sensorial and aesthetic aspect [61]. Smaller particles reduce the scattering of visible light and shift the absorption range to shorter UV wavelengths, improving the UV protective effect [61,62] The literature describes that TiO2 particles with 62 nm are the most effective for protecting skin against UVB radiation [112]. A recent study by Nakamura et al. [113] presented interesting results combining ZnO particles with different sizes, non-nano-ZnO (300 nm), and nano-ZnO (25–35 nm), which resulted in an approximately 18% increase in its SPF value, showing a possible synergistic effect between these particles and the importance of applying particles with different sizes for a better photoprotective performance [113].
The presence of clumps in cosmetic products causes a non-uniform spreading on the skin, interfering with the sensorial, film formation, efficacy, and aesthetic properties of the applied sample [8]. Also, the formation of aggregates and agglomerates of particles decreases the efficacy of these types of UV filters, as the particles overlap, leading to a reduction of their absorbing efficiency, since they do not cover the skin surface completely [61]. Keeping the inorganic UV filter adequately dispersed in vehicles is of relevance; however, avoiding particle agglomeration generally is a challenge in the development of sunscreens [67]. To solve this concern, surface coatings can be useful, as they can reduce the agglomeration of the particles, conferring a better dispersion stability, and safety, as it also reduces the generation of ROS [114,115]. The surface coating also decreases the dissolution rate of the inorganic UV filters, as it acts as a physical barrier that prevents electrons and photons from reaching the particles. In short, uncoated TiO2 or ZnO NPs may lead to problems related to their photoactivity and stability, thus reducing the efficacy of the sunscreen system [53]. Furthermore, the coating also determines the affinity of the particle with the dispersing medium, which influences the manufacturing process [67]. It is also recommended that formulations have a combination of organic and inorganic filters to obtain a synergic effect, enhancing the efficacy of these active ingredients [14]. However, metal oxide NPs may decrease the efficacy of sunscreens containing organic filters, since they may promote the formation of potentially toxic byproducts, which can be avoided by the use of coated particles [111,116].
Specialized literature reports new proposals for inorganic UV filters, such as the composite developed by Reinosa et al. [117] combining 15% of TiO2 NPs with 85% of ZnO microparticles by dry dispersion. The SPF of the formulations containing the composite was determined in vitro by UV-vis diffuse reflectance spectroscopy and indicated that this blend resulted in a 60% increase in SPF value assigned to the anchoring of the TiO2 NPs onto the ZnO microparticles. Such ZnO-TiO2 composite is a nano-free UV filter and was considered safer for consumers’ health and the environment [117]. Furthermore, compounds other than ZnO and TiO2, such as the zinc oxide–ceria nanocomposite with CeO2 NPs decorating the ZnO particle’s surface, increased and led to a more selective absorption of UV radiation. The CeO2 also reduced the photocatalytic characteristic of ZnO NPs, as it reduced the degradation of crystal violet dye by nearly 97% within a 30 min exposure to UV radiation and about 99% within a 30 min exposure to simulated sunlight [14]. In addition, a study by Marcelino et al. [24] proposed a synergistic effect of TiO2 incorporated into mesoporous silica. The SPF was determined in vitro by diffuse reflectance spectrophotometer with an integration sphere, indicating that the TiO2-containing mesoporous silica had an elevation of 50% in its SPF and greater absorption in the UVB and UVA regions in comparison with TiO2 [24].
The crystalline morphology also influences the efficiency of these metal oxides as demonstrated by a recent study by Machado et al. [25] in which they synthesized and evaluated the photoprotective efficiency of star-shaped ZnO nanoaggregates in comparison with commercially available ZnO samples. The nanoaggregates and ZnO samples were incorporated in a formulation with a blend of organic UV filters (i.e., BMDBM and EHMC) and had their SPF evaluated by diffuse reflectance spectrophotometer with an integration sphere. The results indicate that the star-shaped ZnO nanoaggregates increased the BMDBM/EHMC sample photoprotective efficacy by 1096% (from 26 ± 6 to 285 ± 57) [25].
An actual trend in the sunscreen market is the association of UV filters with bioactive compounds, making the final product with multiple benefits. The association of bioactive compounds, such as caffeine, rutin, and ferulic acid, among others, and UV filters can enhance the protective effect against UV radiation, adding to the sunscreens more functions, like an antioxidant action [118]. The presence of antioxidants in sunscreens has an important role in preventing and dampening free radicals and diminishing oxidative stress [41]. Thus, these associations improve skin health. For example, an in vivo study by Rosado et al. [118] indicated that the association of TiO2 (5.0%), avobenzone (3.0%), ethylhexyl methoxycinnamate (7.5%), and caffeine (2.5%) increased the SPF value in approximately 25% of the abovementioned sunscreen system [118].
Finally, it is well known that the amount of sunscreen applied to the skin influences its effectiveness. Studies indicated that most sunscreen users apply an amount of product that guarantees 20 to 50% of the labeled SPF, since 0.5 to 1.5 mg/cm3 of product is applied in real-life conditions, usually close to 1.0 mg/cm2, whereas 2.0 mg/cm2 is used in the SPF testing [23,42,119]. This scenario highlights the importance of applying enough amount of sunscreen as homogeneously as possible [119]. It is of utmost relevance to develop sunscreens that blend easily and provide evenly dispersed coatings on the skin, maximizing the efficacy of real application conditions [120].
8. Conclusions
The importance of sunscreen use is undeniable, as excessive unprotected exposure to UV radiation poses significant cumulative health risks. The development of effective and environmentally friendly sunscreens presents a significant challenge. Inorganic UV filters have emerged as promising alternatives due to their demonstrated safety for children and sensitive skin, along with a lower environmental impact compared with organic UV filters.
Currently, TiO2 and ZnO are the primary inorganic filters approved for use in sunscreens, offering broad-spectrum protection, particularly when used in synergistic combinations. Particle size plays a crucial role in determining their efficacy. While there is a growing trend towards using NPs to enhance sunscreen efficacy, sensorial properties, and aesthetics, concerns regarding their toxicity and safety remain.
Significant toxicity has been observed with the inhalation of these compounds. However, most studies investigating the skin penetration of TiO2 and ZnO NPs have shown limited penetration beyond the viable epidermis following topical application. Phototoxicity is another concern, but the photoinduced production of ROS can be mitigated through appropriate particle coatings. The environmental risk associated with inorganic UV filters is generally considered low.
In conclusion, the development of innovative broad-spectrum inorganic UV filters holds significant promise, contingent upon careful control of factors influencing phototoxicity, stability, and efficacy within sunscreen formulations. This area presents exciting avenues for research and the development of novel and improved suncare products.
Author Contributions
Conceptualization, S.M.A. and A.R.B.; methodology, S.M.A. and A.R.B.; formal analysis, S.M.A. and A.R.B.; investigation, S.M.A. and A.R.B.; data curation, S.M.A. and A.R.B.; writing—original draft preparation, S.M.A.; writing—review and editing, S.M.A. and A.R.B.; visualization, S.M.A. and A.R.B.; supervision, A.R.B.; project administration, S.M.A. and A.R.B.; funding acquisition, A.R.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil (CNPq, Process 303862/2022-0); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil (CAPES, Finance Code 001); and Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil (FAPESP, grant number 2024/01920-0).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
S.M.A. is thankful to CAPES for the master’s scholarship. A.R.B. is thankful to CNPq-Brazil for the research productivity scholarship and to the FAPESP, Brazil.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Schneider, S.; Lim, H.W. A Review of Inorganic UV Filters Zinc Oxide and Titanium Dioxide 2018. Photodermatol. Photoimmunol. Photomed. 2019, 35, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Balogh, T.S.; Valéria, M.; Velasco, R.; Pedriali, C.A.; Kaneko, T.M.; Baby, A.R. Proteção à Radiação Ultravioleta: Recursos Disponíveis Na Atualidade Em Fotoproteção. An. Bras. Dermatol. 2011, 86, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Biniek, K.; Levi, K.; Dauskardt, R.H. Solar UV Radiation Reduces the Barrier Function of Human Skin. Proc. Natl. Acad. Sci. USA 2012, 109, 17111–17116. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, T.S.; De Souza, S.O. Protetores Solares e Os Efeitos Da Radiação Ultravioleta. Sci. Plena 2008, 4, 1–7. [Google Scholar]
- Lima, F.V.; Martins, T.E.A.; Morocho-Jácome, A.L.; Almeida, I.F.; Rosado, C.F.; Velasco, M.V.R.; Baby, A.R. Analytical Tools for Urocanic Acid Determination in Human Samples: A Review. J. Sep. Sci. 2021, 44, 438–447. [Google Scholar] [CrossRef]
- Parrado, C.; Gilaberte, Y.; Philips, N.; Juarranz, A.; Gonzalez, S. Fern Extract, Oxidative Stress, and Skin Cancer. In Cancer; Elsevier: Amsterdam, The Netherlands, 2021; pp. 387–398. [Google Scholar]
- Flor, J.; Davolos, M.R.; Correa, M.A. Protetores Solares. Química Nova 2007, 30, 153–158. [Google Scholar] [CrossRef]
- Manaia, E.B.; Kaminski, R.C.K.; Corrêa, M.A.; Chiavacci, L.A. Inorganic UV Filters. Braz. J. Pharm. Sci. 2013, 49, 201–209. [Google Scholar] [CrossRef]
- Addor, F.A.S.; Barcaui, C.B.; Gomes, E.E.; Lupi, O.; Marçon, C.R.; Miot, H.A. Sunscreen Lotions in the Dermatological Prescription: Review of Concepts and Controversies. An. Bras. Dermatol. 2022, 97, 204–222. [Google Scholar] [CrossRef]
- Azim, S.A.; Bainvoll, L.; Vecerek, N.; DeLeo, V.A.; Adler, B.L. Sunscreens Part 2: Regulation and Safety. J. Am. Acad. Dermatol. 2024, 92, 689–698. [Google Scholar] [CrossRef]
- Serpone, N.; Dondi, D.; Albini, A. Inorganic and Organic UV Filters: Their Role and Efficacy in Sunscreens and Suncare Products. Inorganica Chim. Acta 2007, 360, 794–802. [Google Scholar] [CrossRef]
- Couteau, C.; Philippe, A.; Galharret, J.-M.; Metay, E.; Coiffard, L. UV Filters in Everyday Cosmetic Products, a Comparative Study. Environ. Sci. Pollut. Res. 2023, 31, 2976–2986. [Google Scholar] [CrossRef] [PubMed]
- Herzog, B.; Hüglin, D.; Borsos, E.; Stehlin, A.; Luther, H. New UV Absorbers for Cosmetic Sunscreens—A Breakthrough for the Photoprotection of Human Skin. Chimia 2004, 58, 554. [Google Scholar] [CrossRef]
- Serpone, N. Sunscreens and Their Usefulness: Have We Made Any Progress in the Last Two Decades? Photochem. Photobiol. Sci. 2021, 20, 189–244. [Google Scholar] [CrossRef] [PubMed]
- Herzog, B.; Giesinger, J.; Settels, V. Insights into the Stabilization of Photolabile UV-Absorbers in Sunscreens. Photochem. Photobiol. Sci. 2020, 19, 1636–1649. [Google Scholar] [CrossRef]
- Lopes, F.M.; Da Cruz, R.D.O.; de Aleluia Batista, K. Radiação Ultravioleta E Ativos Utilizados Nas Formulações De Protetores Solares Resumo. Ens. Ciência Ciências Biológicas Agrárias Da Saúde 2012, 16, 183–199. [Google Scholar]
- Freire, T.B.; de Castro Lima, C.R.R.; de Oliveira Pinto, C.A.S.; Borge, L.F.; Baby, A.R.; Velasco, M.V.R. Evaluation of Interaction between Natural Antioxidants and Chemical Sunscreens Aiming the Photoprotective Efficacy. J. Therm. Anal. Calorim. 2022, 147, 7829–7836. [Google Scholar] [CrossRef]
- de Oliveira Pinto, C.A.S. Influência Da Rutina Na Fotoestabilização Da Avobenzona (Filtro UVA) e Do ρ-Metoxicinamato de Octila (Filtro UVB); Universidade de São Paulo: São Paulo, Brazil, 2014. [Google Scholar]
- Smijs, T.G.; Pavel, S. Titanium Dioxide and Zinc Oxide Nanoparticles in Sunscreens: Focus on Their Safety and Effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95–112. [Google Scholar] [CrossRef]
- Nery, É.M.; Martinez, R.M.; Velasco, M.V.R.; Baby, A.R. A Short Review of Alternative Ingredients and Technologies of Inorganic UV Filters. J. Cosmet. Dermatol. 2021, 20, 1061–1065. [Google Scholar] [CrossRef]
- Infante, V.H.P.; Maia Campos, P.M.B.G.; Calixto, L.S.; Darvin, M.E.; Kröger, M.; Schanzer, S.; Lohan, S.B.; Lademann, J.; Meinke, M.C. Influence of Physical–Mechanical Properties on SPF in Sunscreen Formulations on Ex Vivo and in Vivo Skin. Int. J. Pharm. 2021, 598, 120262. [Google Scholar] [CrossRef]
- Bispo, M.d.O.; Morocho-Jácome, A.L.; Escudeiro, C.C.; Martinez, R.M.; de Oliveira Pinto, C.A.S.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. Photoprotective Efficacy of the Association of Rosmarinic Acid 0.1% with Ethylhexyl Methoxycinnamate and Avobenzone. Cosmetics 2023, 10, 11. [Google Scholar] [CrossRef]
- Diffey, B. When Should Sunscreen Be Applied: The Balance between Health Benefit and Adverse Consequences to Humans and the Environment. Int. J. Cosmet. Sci. 2023, 45, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Marcelino, P.d.S.; Martinez, R.M.; Daneluti, A.L.M.; Morocho-Jácome, A.L.; Pessoa, F.V.L.S.; Rijo, P.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. In Vitro Photoprotection and Functional Photostability of Sunscreen Lipsticks Containing Inorganic Active Compounds. Cosmetics 2023, 10, 46. [Google Scholar] [CrossRef]
- Machado, G.T.; Chiabai, C.R.; Pinheiro, M.S.; Pinto, C.A.S.d.O.; Baby, A.R.; Andrade, G.R.S.; Pessoa, F.V.L.S. In Vitro Photoprotective Efficacy and Photostability of Synthesized Star-Shaped ZnO Nanoaggregates Associated with Ethylhexyl Methoxycinnamate and Butyl Methoxydibenzoylmethane. J. Photochem. Photobiol. B 2024, 261, 113068. [Google Scholar] [CrossRef] [PubMed]
- Jesus, A.; Augusto, I.; Duarte, J.; Sousa, E.; Cidade, H.; Cruz, M.T.; Lobo, J.M.S.; Almeida, I.F. Recent Trends on UV Filters. Appl. Sci. 2022, 12, 12003. [Google Scholar] [CrossRef]
- US FDA. U.S. Food and Drug Administration Over-the-Counter Monograph M020: Sunscreen Drug Products for Over-the-Counter Human Use. Available online: https://dps-admin.fda.gov/omuf/omuf/sites/omuf/files/primary-documents/2022-09/Final%20Administrative%20Order%20OTC000006_M020-Sunscreen%20Drug%20Products%20for%20OTC%20Human%20Use.pdf (accessed on 2 April 2025).
- ANVISA. Brazilian Health Regulatory Agency. Resolução—RDC No 600, de 9 de fevereiro de 2022. Available online: https://anvisalegis.datalegis.net/action/ActionDatalegis.php?acao=abrirTextoAto&tipo=RDC&numeroAto=00000600&seqAto=000&valorAno=2022&orgao=RDC/DC/ANVISA/MS&codTipo=&desItem=&desItemFim=&cod_menu=1696&cod_modulo=134&pesquisa=true (accessed on 3 April 2025).
- EU. European Union. Regulation (Ec) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:02009R1223-20190813 (accessed on 3 April 2025).
- Olejnik, A.; Goscianska, J. Hybrid Systems Based on Avobenzone–Wrinkled Mesoporous Silica as Ingredients of Sunscreen Formulations. Microporous Mesoporous Mater. 2024, 367, 112949. [Google Scholar] [CrossRef]
- Ambrogi, V.; Latterini, L.; Marmottini, F.; Pagano, C.; Ricci, M. Mesoporous Silicate MCM-41 as a Particulate Carrier for Octyl Methoxycinnamate: Sunscreen Release and Photostability. J. Pharm. Sci. 2013, 102, 1468–1475. [Google Scholar] [CrossRef]
- Daneluti, A.L.M.; Guerra, L.O.; Velasco, M.V.R.; do Rosário Matos, J.; Baby, A.R.; Kalia, Y.N. Preclinical and Clinical Studies to Evaluate Cutaneous Biodistribution, Safety and Efficacy of UV Filters Encapsulated in Mesoporous Silica SBA-15. Eur. J. Pharm. Biopharm. 2021, 169, 113–124. [Google Scholar] [CrossRef]
- Daneluti, A.L.M.; Neto, F.M.; Ruscinc, N.; Lopes, I.; Robles Velasco, M.V.; Do Rosário Matos, J.; Baby, A.R.; Kalia, Y.N. Using Ordered Mesoporous Silica SBA-15 to Limit Cutaneous Penetration and Transdermal Permeation of Organic UV Filters. Int. J. Pharm. 2019, 570, 118633. [Google Scholar] [CrossRef]
- Sarruf, F.D.; Contreras, V.J.P.; Martinez, R.M.; Velasco, M.V.R.; Baby, A.R. The Scenario of Clays and Clay Minerals Use in Cosmetics/Dermocosmetics. Cosmetics 2024, 11, 7. [Google Scholar] [CrossRef]
- US FDA. U.S. Food and Drug Administration. Questions and Answers: FDA Posts Deemed Final Order and Proposed Order for over-the-Counter Sunscreen. Available online: https://www.fda.gov/drugs/understanding-over-counter-medicines/questions-and-answers-fda-posts-deemed-final-order-and-proposed-order-over-counter-sunscreen (accessed on 21 April 2024).
- Wang, L.; Yu, J. Principles of Photocatalysis. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–52. [Google Scholar]
- CDC. Centers for Disease Control and Protection. Ultraviolet Radiation. Available online: https://www.cdc.gov/radiation-health/features/uv-radiation.html?CDC_AAref_Val=https://www.cdc.gov/nceh/features/uv-radiation-safety/index.html (accessed on 10 April 2024).
- Ngoc, L.T.N.; Tran, V.V.; Moon, J.-Y.; Chae, M.; Park, D.; Lee, Y.-C. Recent Trends of Sunscreen Cosmetic: An Update Review. Cosmetics 2019, 6, 64. [Google Scholar] [CrossRef]
- Jansen, R.; Wang, S.Q.; Burnett, M.; Osterwalder, U.; Lim, H.W. Photoprotection: Part I. Photoprotection by naturally occurring, physical, and systemic agents. J. Am. Acad. Dermatol. 2013, 69, e1–e853. [Google Scholar] [CrossRef] [PubMed]
- Jesus, A.; Sousa, E.; Cruz, M.; Cidade, H.; Lobo, J.; Almeida, I. UV Filters: Challenges and Prospects. Pharmaceuticals 2022, 15, 263. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.L.; Lim, H.W.; Mohammad, T.F. Sunscreens and Photoaging: A Review of Current Literature. Am. J. Clin. Dermatol. 2021, 22, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Young, A.R.; Claveau, J.; Rossi, A.B. Ultraviolet Radiation and the Skin: Photobiology and Sunscreen Photoprotection. J. Am. Acad. Dermatol. 2017, 76, S100–S109. [Google Scholar] [CrossRef]
- WHO. World Health Organization. Ultraviolet Radiation. Available online: https://www.who.int/news-room/fact-sheets/detail/ultraviolet-radiation#:~:text=Wear%20protective%20clothing.,cannot%20be%20covered%20by%20clothes (accessed on 2 March 2024).
- de Alves, G.A.D.; Cuelho, C.H.F.; Fonseca, M.J.V.; Maia Campos, P.M.B.G. Development of Topical Formulations Containing 20% of Coated and Uncoated Zinc Oxide Nanoparticles: Stability Assessment and Penetration Evaluation by Reflectance Confocal Laser Microscopy. Cosmetics 2023, 11, 6. [Google Scholar] [CrossRef]
- Osmond-McLeod, M.J.; Oytam, Y.; Rowe, A.; Sobhanmanesh, F.; Greenoak, G.; Kirby, J.; McInnes, E.F.; McCall, M.J. Long-Term Exposure to Commercially Available Sunscreens Containing Nanoparticles of TiO2 and ZnO Revealed No Biological Impact in a Hairless Mouse Model. Part Fibre Toxicol. 2015, 13, 44. [Google Scholar] [CrossRef]
- Couteau, C.; Alami, S.; Guitton, M.; Paparis, E.; Coiffard, L.J.M. Mineral Filters in Sunscreen Products—Comparison of the Efficacy of Zinc Oxide and Titanium Dioxide by in Vitro Method. Pharmazie 2008, 63, 58–60. [Google Scholar]
- Grande, F.; Tucci, P. Titanium Dioxide Nanoparticles: A Risk for Human Health? Mini-Rev. Med. Chem. 2016, 16, 762–769. [Google Scholar] [CrossRef]
- Jafari, S.; Mahyad, B.; Hashemzadeh, H.; Janfaza, S.; Gholikhani, T.; Tayebi, L. Biomedical Applications of TiO2 Nanostructures: Recent Advances. Int. J. Nanomed. 2020, 15, 3447–3470. [Google Scholar] [CrossRef]
- Weir, A.; Westerhoff, P.; Fabricius, L.; Hristovski, K.; von Goetz, N. Titanium Dioxide Nanoparticles in Food and Personal Care Products. Environ. Sci. Technol. 2012, 46, 2242–2250. [Google Scholar] [CrossRef]
- Racovita, A.D. Titanium Dioxide: Structure, Impact, and Toxicity. Int. J. Environ. Res. Public Health 2022, 19, 5681. [Google Scholar] [CrossRef] [PubMed]
- Neta, I.A.B.; Mota, M.F.; Lira, H.L.; Neves, G.A.; Menezes, R.R. Nanostructured Titanium Dioxide for Use in Bone Implants: A Short Review. Cerâmica 2020, 66, 440–450. [Google Scholar] [CrossRef]
- de Paula, L.R.; Parussulo, A.L.A.; Araki, K.; Toma, H.E. Reliable and Fast Sensor for in Vitro Evaluation of Solar Protection Factor Based on the Photobleaching Kinetics of a Nanocrystalline TiO2/Dye UV-Dosimeter. Sens. Actuators B Chem. 2011, 156, 325–331. [Google Scholar] [CrossRef]
- Yuan, S.; Huang, J.; Jiang, X.; Huang, Y.; Zhu, X.; Cai, Z. Environmental Fate and Toxicity of Sunscreen-Derived Inorganic Ultraviolet Filters in Aquatic Environments: A Review. Nanomaterials 2022, 12, 699. [Google Scholar] [CrossRef]
- SCCS. Scientific Committee on Consumer Safety. Opinion for Clarification of the Meaning of the Term “Sprayable Applications/Products” for the Nano Forms of Carbon Black CI 77266, Titanium Oxide and Zinc Oxide. Available online: https://health.ec.europa.eu/publications/revision-opinion-clarification-term-sprayable-applicationsproducts-nano-forms-carbon-black-ci-77266_en (accessed on 2 April 2025).
- EU. European Union. Commission Regulation (EU) 2016/621 of 21 April 2016 Amending Annex VI to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on Cosmetic Products. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32016R0621 (accessed on 4 April 2025).
- EU. European Union. Commission Regulation (EU) 2016/1143 of 13 July 2016 Amending Annex VI to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on Cosmetic Products. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32016R1143 (accessed on 4 April 2025).
- Aravind, M.; Amalanathan, M.; Mary, M.S.M. Synthesis of TiO2 Nanoparticles by Chemical and Green Synthesis Methods and Their Multifaceted Properties. SN Appl. Sci. 2021, 3, 409. [Google Scholar] [CrossRef]
- Dréno, B.; Alexis, A.; Chuberre, B.; Marinovich, M. Safety of Titanium Dioxide Nanoparticles in Cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 34–46. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A Review of ZnO Nanoparticles as Solar Photocatalysts: Synthesis, Mechanisms and Applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Cole, C.; Shyr, T.; Ou-Yang, H. Metal Oxide Sunscreens Protect Skin by Absorption, Not by Reflection or Scattering. Photodermatol. Photoimmunol. Photomed. 2016, 32, 5–10. [Google Scholar] [CrossRef]
- Lin, C.-C.; Lin, W.-J. Sun Protection Factor Analysis of Sunscreens Containing Titanium Dioxide Nanoparticles. J. Food Drug Anal. 2011, 19, 5. [Google Scholar] [CrossRef]
- Ghamarpoor, R.; Fallah, A.; Jamshidi, M. Investigating the Use of Titanium Dioxide (TiO2) Nanoparticles on the Amount of Protection against UV Irradiation. Sci. Rep. 2023, 13, 9793. [Google Scholar] [CrossRef]
- Kubáč, L.; Akrman, J.; Kejlová, K.; Bendová, H.; Klánová, K.; Hladíková, Z.; Pikal, P.; Kovaříková, L.; Kašparová, L.; Jírová, D. Characteristics of Titanium Dioxide Microdispersions with Different Photo-Activity Suitable for Sunscreen Formulations. Int. J. Pharm. 2015, 481, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Acharya, D.; Hala, V.; Daware, S.; Vora, L.K. Sunscreens: A Comprehensive Review with the Application of Nanotechnology. J. Drug. Deliv. Sci. Technol. 2023, 86, 104720. [Google Scholar] [CrossRef]
- Mascarenhas-Melo, F.; Mathur, A.; Murugappan, S.; Sharma, A.; Tanwar, K.; Dua, K.; Singh, S.K.; Mazzola, P.G.; Yadav, D.N.; Rengan, A.K.; et al. Inorganic Nanoparticles in Dermopharmaceutical and Cosmetic Products: Properties, Formulation Development, Toxicity, and Regulatory Issues. Eur. J. Pharm. Biopharm. 2023, 192, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Morsella, M.; d’Alessandro, N.; Lanterna, A.E.; Scaiano, J.C. Improving the Sunscreen Properties of TiO 2 through an Understanding of Its Catalytic Properties. ACS Omega 2016, 1, 464–469. [Google Scholar] [CrossRef]
- Catalano, R.; Masion, A.; Ziarelli, F.; Slomberg, D.; Laisney, J.; Unrine, J.M.; Campos, A.; Labille, J. Optimizing the Dispersion of Nanoparticulate TiO2-Based UV Filters in a Non-Polar Medium Used in Sunscreen Formulations—The Roles of Surfactants and Particle Coatings. Colloids Surf. A Physicochem. Eng. Asp. 2020, 599, 124792. [Google Scholar] [CrossRef]
- Jansen, R.; Osterwalder, U.; Wang, S.Q.; Burnett, M.; Lim, H.W. Photoprotection: Part II. Sunscreen: Development, efficacy and controversies. J. Am. Acad. Dermatol. 2013, 69, e1–e867. [Google Scholar] [CrossRef]
- Rossano, M.; Hucher, N.; Picard, C.; Colletta, D.; Le Foll, F.; Grisel, M. Effects of Aging on Structure and Stability of TiO2 Nanoparticle-Containing Oil-in-Water Emulsions. Int. J. Pharm. 2014, 461, 89–96. [Google Scholar] [CrossRef]
- Lewicka, Z.A.; Yu, W.W.; Oliva, B.L.; Contreras, E.Q.; Colvin, V.L. Photochemical Behavior of Nanoscale TiO2 and ZnO Sunscreen Ingredients. J. Photochem. Photobiol. A Chem. 2013, 263, 24–33. [Google Scholar] [CrossRef]
- Janczarek, M.; Szaferski, W. Titanium Dioxide as a Safe Additive to Sunscreen Emulsions. Chem. Process Eng. 2023, 43, 483–490. [Google Scholar] [CrossRef]
- Lyons, A.B.; Trullas, C.; Kohli, I.; Hamzavi, I.H.; Lim, H.W. Photoprotection beyond Ultraviolet Radiation: A Review of Tinted Sunscreens. J. Am. Acad. Dermatol. 2021, 84, 1393–1397. [Google Scholar] [CrossRef]
- Huston, M.; DeBella, M.; DiBella, M.; Gupta, A. Green Synthesis of Nanomaterials. Nanomaterials 2021, 11, 2130. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Al-Dossari, M.; Singh, J.; Rawat, M.; Kordy, M.G.M.; Shaban, M. A Review on Green Synthesis of TiO2 NPs: Photocatalysis and Antimicrobial Applications. Polymers 2022, 14, 1444. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Huang, Y.; Zhu, S.; Abbes, N.; Jing, X.; Zhang, L. A Review of the Green Synthesis of ZnO Nanoparticles Using Plant Extracts and Their Prospects for Application in Antibacterial Textiles. J. Eng. Fiber Fabr. 2021, 16, 155892502110462. [Google Scholar] [CrossRef]
- Meulenkamp, E.A. Synthesis and Growth of ZnO Nanoparticles. J. Phys. Chem. B 1998, 102, 5566–5572. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Mahmoud, Z.H.; Abdullaev, S.; Ali, F.K.; Ali Naeem, Y.; Mzahim Mizher, R.; Morad Karim, M.; Abdulwahid, A.S.; Ahmadi, Z.; Habibzadeh, S.; et al. Nano Titanium Oxide (Nano-TiO2): A Review of Synthesis Methods, Properties, and Applications. Case Stud. Chem. Environ. Eng. 2024, 9, 100626. [Google Scholar] [CrossRef]
- Porrawatkul, P.; Nuengmatcha, P.; Kuyyogsuy, A.; Pimsen, R.; Rattanaburi, P. Effect of Na and Al Doping on ZnO Nanoparticles for Potential Application in Sunscreens. J. Photochem. Photobiol. B 2023, 240, 112668. [Google Scholar] [CrossRef]
- Roopan, S.M.; Bharathi, A.; Prabhakarn, A.; Abdul Rahuman, A.; Velayutham, K.; Rajakumar, G.; Padmaja, R.D.; Lekshmi, M.; Madhumitha, G. Efficient Phyto-Synthesis and Structural Characterization of Rutile TiO2 Nanoparticles Using Annona Squamosa Peel Extract. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 98, 86–90. [Google Scholar] [CrossRef]
- Rasmussen, K.; Rauscher, H.; Mech, A.; Riego Sintes, J.; Gilliland, D.; González, M.; Kearns, P.; Moss, K.; Visser, M.; Groenewold, M.; et al. Physico-Chemical Properties of Manufactured Nanomaterials—Characterisation and Relevant Methods. An Outlook Based on the OECD Testing Programme. Regul. Toxicol. Pharmacol. 2018, 92, 8–28. [Google Scholar] [CrossRef]
- SCCS. Scientific Committee on Consumer Safety. Guidance on the Safety Assessment of Nanomaterials in Cosmetics. Available online: https://health.ec.europa.eu/publications/sccs-guidance-safety-assessment-nanomaterials-cosmetics-2nd-revision_en (accessed on 4 April 2025).
- US FDA. U.S. Food and Drug Administration. Guidance for Industry: Safety of Nanomaterials in Cosmetic Products. Available online: https://www.fda.gov/media/83957/download (accessed on 4 April 2025).
- Lu, P.J.; Fang, S.W.; Cheng, W.L.; Huang, S.C.; Huang, M.C.; Cheng, H.F. Characterization of Titanium Dioxide and Zinc Oxide Nanoparticles in Sunscreen Powder by Comparing Different Measurement Methods. J. Food Drug Anal. 2018, 26, 1192–1200. [Google Scholar] [CrossRef]
- Lu, P.-J.; Huang, S.-C.; Chen, Y.-P.; Chiueh, L.-C.; Shih, D.Y.-C. Analysis of Titanium Dioxide and Zinc Oxide Nanoparticles in Cosmetics. J. Food Drug Anal. 2015, 23, 587–594. [Google Scholar] [CrossRef]
- Bonetta, S.; Macrì, M.; Acito, M.; Villarini, M.; Moretti, M.; Bonetta, S.; Bosio, D.; Mariella, G.; Bellisario, V.; Bergamaschi, E.; et al. DNA Damage in Workers Exposed to Pigment Grade Titanium Dioxide (TiO2) and Association with Biomarkers of Oxidative Stress and Inflammation. Environ. Toxicol. Pharmacol. 2024, 105, 104328. [Google Scholar] [CrossRef] [PubMed]
- Hansa, J.; Merzenich, H.; Cascant Ortolano, L.; Klug, S.J.; Blettner, M.; Gianicolo, E. Health Risks of Titanium Dioxide (TiO2) Dust Exposure in Occupational Settings—A Scoping Review. Int. J. Hyg. Environ. Health 2023, 252, 114212. [Google Scholar] [CrossRef] [PubMed]
- IARC. International Agency for Research on Cancer. Carbon Black, Titanium Dioxide, and Talc IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Carbon-Black-Titanium-Dioxide-And-Talc-2010 (accessed on 8 February 2025).
- Warheit, D.B.; Donner, E.M. Risk Assessment Strategies for Nanoscale and Fine-Sized Titanium Dioxide Particles: Recognizing Hazard and Exposure Issues. Food Chem. Toxicol. 2015, 85, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.D.; Stotland, M.; Ellis, J.I. The Safety of Nanosized Particles in Titanium Dioxide– and Zinc Oxide–Based Sunscreens. J. Am. Acad. Dermatol. 2009, 61, 685–692. [Google Scholar] [CrossRef]
- El-Said, K.S.; Ali, E.M.; Kanehira, K.; Taniguchi, A. Molecular Mechanism of DNA Damage Induced by Titanium Dioxide Nanoparticles in Toll-like Receptor 3 or 4 Expressing Human Hepatocarcinoma Cell Lines. J. Nanobiotechnol. 2014, 12, 48. [Google Scholar] [CrossRef]
- Næss, E.M.; Hofgaard, A.; Skaug, V.; Gulbrandsen, M.; Danielsen, T.E.; Grahnstedt, S.; Skogstad, A.; Holm, J. Titanium Dioxide Nanoparticles in Sunscreen Penetrate the Skin into Viable Layers of the Epidermis: A Clinical Approach. Photodermatol. Photoimmunol. Photomed. 2016, 32, 48–51. [Google Scholar] [CrossRef]
- Mohammed, Y.H.; Holmes, A.; Haridass, I.N.; Sanchez, W.Y.; Studier, H.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Support for the Safe Use of Zinc Oxide Nanoparticle Sunscreens: Lack of Skin Penetration or Cellular Toxicity after Repeated Application in Volunteers. J. Investig. Dermatol. 2019, 139, 308–315. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, R.K.; Gaur, K.; Cátala Torres, J.F.; Loza-Rosas, S.A.; Torres, A.; Saxena, M.; Julin, M.; Tinoco, A.D. Fueling a Hot Debate on the Application of TiO2 Nanoparticles in Sunscreen. Materials 2019, 12, 2317. [Google Scholar] [CrossRef]
- Ryu, H.J.; Seo, M.Y.; Jung, S.K.; Meang, E.-H.; Lee, S.-Y.; Jang, D.-H.; Lee, T.-J.; Jo, K.-Y.; Kim, Y.-R.; Cho, K.-B.; et al. Zinc Oxide Nanoparticles: A 90-Day Repeated-Dose Dermal Toxicity Study in Rats. Int. J. Nanomed. 2014, 9, 137–144. [Google Scholar] [CrossRef]
- Surekha, P.; Kishore, A.S.; Srinivas, A.; Selvam, G.; Goparaju, A.; Reddy, P.N.; Murthy, P.B. Repeated Dose Dermal Toxicity Study of Nano Zinc Oxide with Sprague-Dawley Rats. Cutan. Ocul. Toxicol. 2012, 31, 26–32. [Google Scholar] [CrossRef]
- Panchatcharam, M.; Miriyala, S.; Gayathri, V.S.; Suguna, L. Curcumin Improves Wound Healing by Modulating Collagen and Decreasing Reactive Oxygen Species. Mol. Cell. Biochem. 2006, 290, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Okada, T.; Konishi, H.; Tsuji, T. The Effect of Reactive Oxygen Species on the Biosynthesis of Collagen and Glycosaminoglycans in Cultured Human Dermal Fibroblasts. Arch. Dermatol. Res. 1993, 285, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Gamer, A.O.; Leibold, E.; van Ravenzwaay, B. The in Vitro Absorption of Microfine Zinc Oxide and Titanium Dioxide through Porcine Skin. Toxicol. Vitr. 2006, 20, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Rowenczyk, L.; Duclairoir-Poc, C.; Barreau, M.; Picard, C.; Hucher, N.; Orange, N.; Grisel, M.; Feuilloley, M. Impact of Coated TiO2-Nanoparticles Used in Sunscreens on Two Representative Strains of the Human Microbiota: Effect of the Particle Surface Nature and Aging. Colloids Surf. B Biointerfaces 2017, 158, 339–348. [Google Scholar] [CrossRef]
- Sendra, M.; Sánchez-Quiles, D.; Blasco, J.; Moreno-Garrido, I.; Lubián, L.M.; Pérez-García, S.; Tovar-Sánchez, A. Effects of TiO2 Nanoparticles and Sunscreens on Coastal Marine Microalgae: Ultraviolet Radiation Is Key Variable for Toxicity Assessment. Environ. Int. 2017, 98, 62–68. [Google Scholar] [CrossRef]
- Chatzigianni, M.; Pavlou, P.; Siamidi, A.; Vlachou, M.; Varvaresou, A.; Papageorgiou, S. Environmental Impacts Due to the Use of Sunscreen Products: A Mini-Review. Ecotoxicology 2022, 31, 1331–1345. [Google Scholar] [CrossRef]
- Tran, H.-T.; Dang, B.-T.; Thuy, L.T.T.; Hoang, H.-G.; Bui, X.-T.; Le, V.-G.; Lin, C.; Nguyen, M.-K.; Nguyen, K.-Q.; Nguyen, P.-T.; et al. Advanced Treatment Technologies for the Removal of Organic Chemical Sunscreens from Wastewater: A Review. Curr. Pollut. Rep. 2022, 8, 288–302. [Google Scholar] [CrossRef]
- Narla, S.; Lim, H.W. Sunscreen: FDA Regulation, and Environmental and Health Impact. Photochem. Photobiol. Sci. 2020, 19, 66–70. [Google Scholar] [CrossRef]
- Schneider, S.L.; Lim, H.W. Review of Environmental Effects of Oxybenzone and Other Sunscreen Active Ingredients. J. Am. Acad. Dermatol. 2019, 80, 266–271. [Google Scholar] [CrossRef]
- da Silva, C.P.; Emídio, E.S.; de Marchi, M.R.R. The Occurrence of UV Filters in Natural and Drinking Water in São Paulo State (Brazil). Environ. Sci. Pollut. Res. 2015, 22, 19706–19715. [Google Scholar] [CrossRef]
- Suh, S.; Pham, C.; Smith, J.; Mesinkovska, N.A. The Banned Sunscreen Ingredients and Their Impact on Human Health: A Systematic Review. Int. J. Dermatol. 2020, 59, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Harper, B.J.; Harper, S.L. Comparative Dissolution, Uptake, and Toxicity of Zinc Oxide Particles in Individual Aquatic Species and Mixed Populations. Environ. Toxicol. Chem. 2019, 38, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Sibiya, A.; Jeyavani, J.; Santhanam, P.; Preetham, E.; Freitas, R.; Vaseeharan, B. Comparative Evaluation on the Toxic Effect of Silver (Ag) and Zinc Oxide (ZnO) Nanoparticles on Different Trophic Levels in Aquatic Ecosystems: A Review. J. Appl. Toxicol. 2022, 42, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.B.; Pawlowski, S.; Kellermann, M.Y.; Petersen-Thiery, M.; Moeller, M.; Nietzer, S.; Schupp, P.J. Toxic Effects of UV Filters from Sunscreens on Coral Reefs Revisited: Regulatory Aspects for “Reef Safe” Products. Environ. Sci. Eur. 2021, 33, 74. [Google Scholar] [CrossRef]
- Gondikas, A.P.; von der Kammer, F.; Reed, R.B.; Wagner, S.; Ranville, J.F.; Hofmann, T. Release of TiO2 Nanoparticles from Sunscreens into Surface Waters: A One-Year Survey at the Old Danube Recreational Lake. Environ. Sci. Technol. 2014, 48, 5415–5422. [Google Scholar] [CrossRef]
- Ginzburg, A.L.; Blackburn, R.S.; Santillan, C.; Truong, L.; Tanguay, R.L.; Hutchison, J.E. Zinc Oxide-Induced Changes to Sunscreen Ingredient Efficacy and Toxicity under UV Irradiation. Photochem. Photobiol. Sci. 2021, 20, 1273–1285. [Google Scholar] [CrossRef]
- Ko, H.-H.; Chen, H.-T.; Yen, F.-L.; Lu, W.-C.; Kuo, C.-W.; Wang, M.-C. Preparation of TiO2 Nanocrystallite Powders Coated with 9 Mol% ZnO for Cosmetic Applications in Sunscreens. Int. J. Mol. Sci. 2012, 13, 1658–1669. [Google Scholar] [CrossRef]
- Nakamura, I.; Mastelaro, G.; Borges, I.; Shao, Y.; Cruz, K.; Schlossman, D. Evaluating the Synergy Between Naturally Coated Zinc Oxides in SPF 50+/UVA Balanced Sunscreens. Available online: https://www.koboproductsinc.com/Downloads/KoboProducts-IFSCC-2024-ZnO-Size-Synergy.pdf (accessed on 8 February 2025).
- Couteau, C.; Coiffard, L. The Interest in Nanomaterials for Topical Photoprotection. Cosmetics 2015, 2, 394–408. [Google Scholar] [CrossRef]
- Kim, N.; Kim, Y.; Yun, J.-M.; Jeong, S.-K.; Lee, S.; Lee, B.Z.; Shim, J. Surface Coating of Titanium Dioxide Nanoparticles with a Polymerizable Chelating Agent and Its Physicochemical Property. ACS Omega 2023, 8, 18743–18750. [Google Scholar] [CrossRef]
- Kockler, J.; Oelgemöller, M.; Robertson, S.; Glass, B.D. Photostability of Sunscreens. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 91–110. [Google Scholar] [CrossRef]
- Reinosa, J.J.; Leret, P.; Álvarez-Docio, C.M.; del Campo, A.; Fernández, J.F. Enhancement of UV Absorption Behavior in ZnO–TiO2 Composites. Boletín Soc. Española Cerámica Vidr. 2016, 55, 55–62. [Google Scholar] [CrossRef]
- Rosado, C.; Tokunaga, V.K.; Sauce, R.; de Oliveira, C.A.; Sarruf, F.D.; Parise-Filho, R.; Maurício, E.; de Almeida, T.S.; Velasco, M.V.R.; Baby, A.R. Another Reason for Using Caffeine in Dermocosmetics: Sunscreen Adjuvant. Front. Physiol. 2019, 10, 519. [Google Scholar] [CrossRef] [PubMed]
- Couteau, C.; Diarra, H.; Coiffard, L. Effect of the Product Type, of the Amount of Applied Sunscreen Product and the Level of Protection in the UVB Range on the Level of Protection Achieved in the UVA Range. Int. J. Pharm. 2016, 500, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Portilho, L.; Aiello, L.M.; Vasques, L.I.; Bagatin, E.; Leonardi, G.R. Effectiveness of Sunscreens and Factors Influencing Sun Protection: A Review. Braz. J. Pharm. Sci. 2022, 58, e20693. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).