Jatrorrhizine Isolated from Phellodendron amurense Improves Collagen Homeostasis in CCD-986sk Human Dermal Fibroblast Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Jatrorrhizine Isolation and Identification
2.3. Cell Culture and Maintenance
2.4. Determination of Cytotoxicity
2.5. UV-B Irradiation and Treatment
2.6. Gene Expression
2.7. Statistical Analysis
3. Results and Discussion
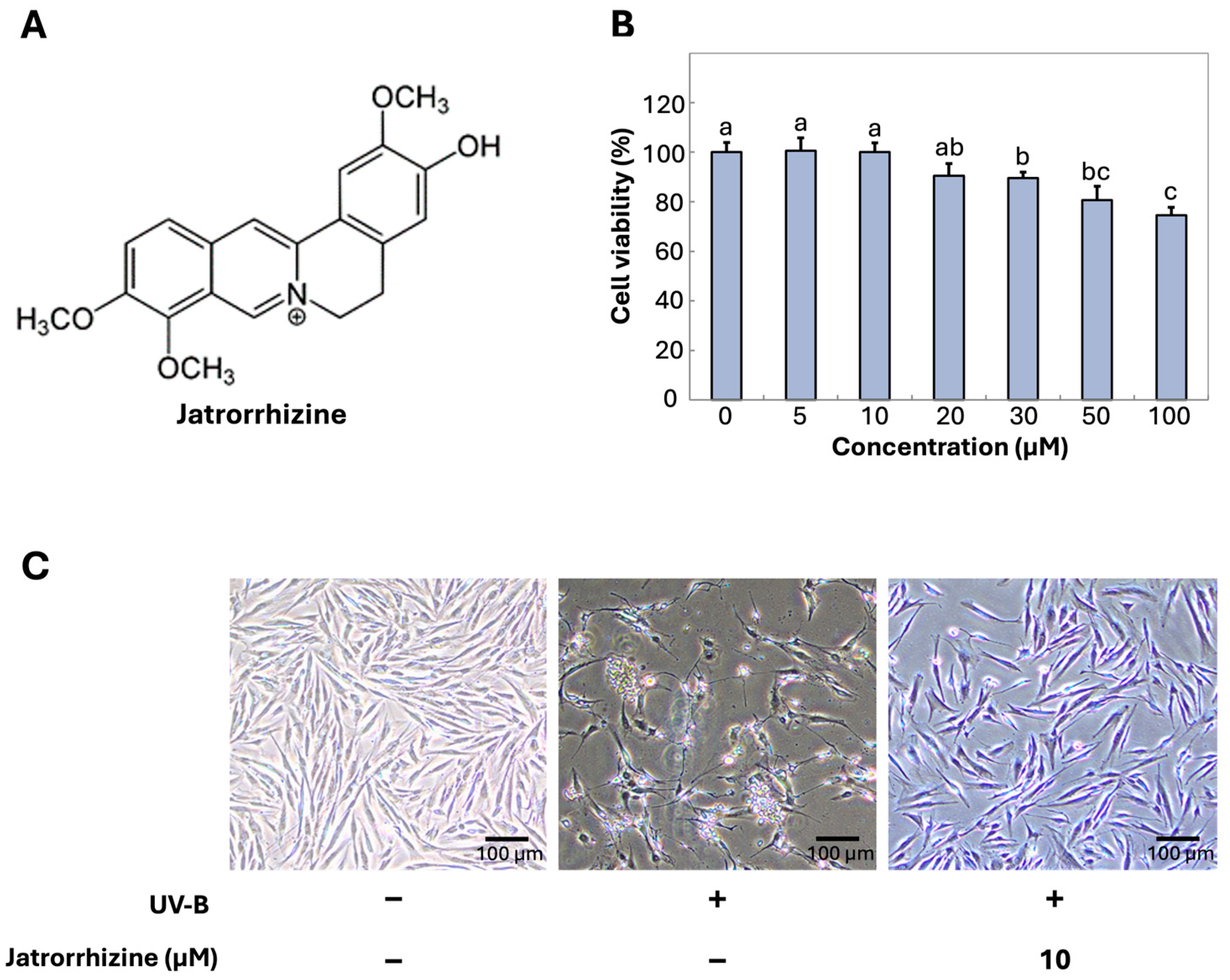
3.1. Cytotoxicity and Jatrorrhizine and UV-B Irradiation Recovery in CCD-986sk Fibroblast Cells
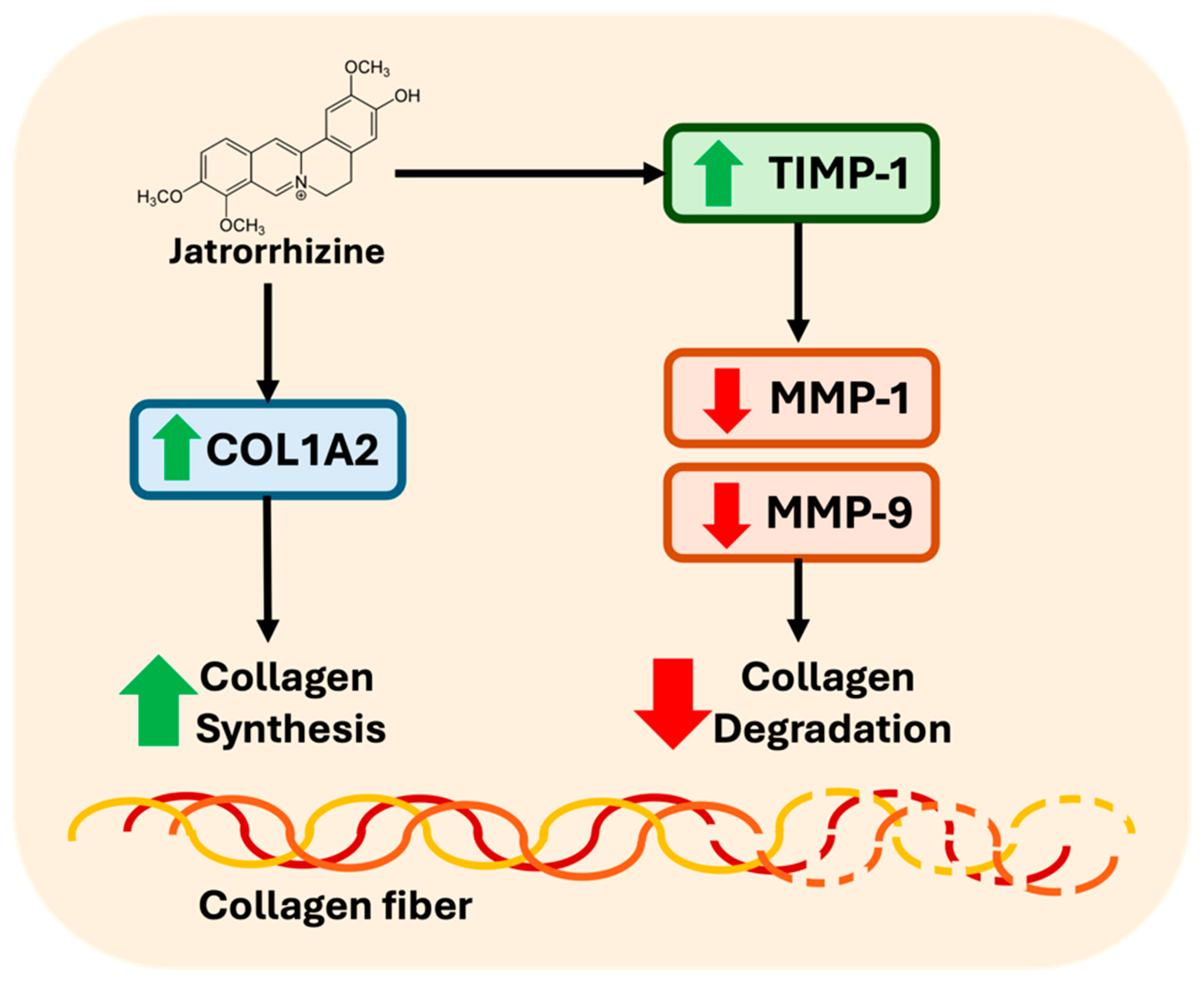
3.2. Jatrorrhizine Increase Procollagen Production and Inhibits Collagen Degradation in CCD-986sk Cells
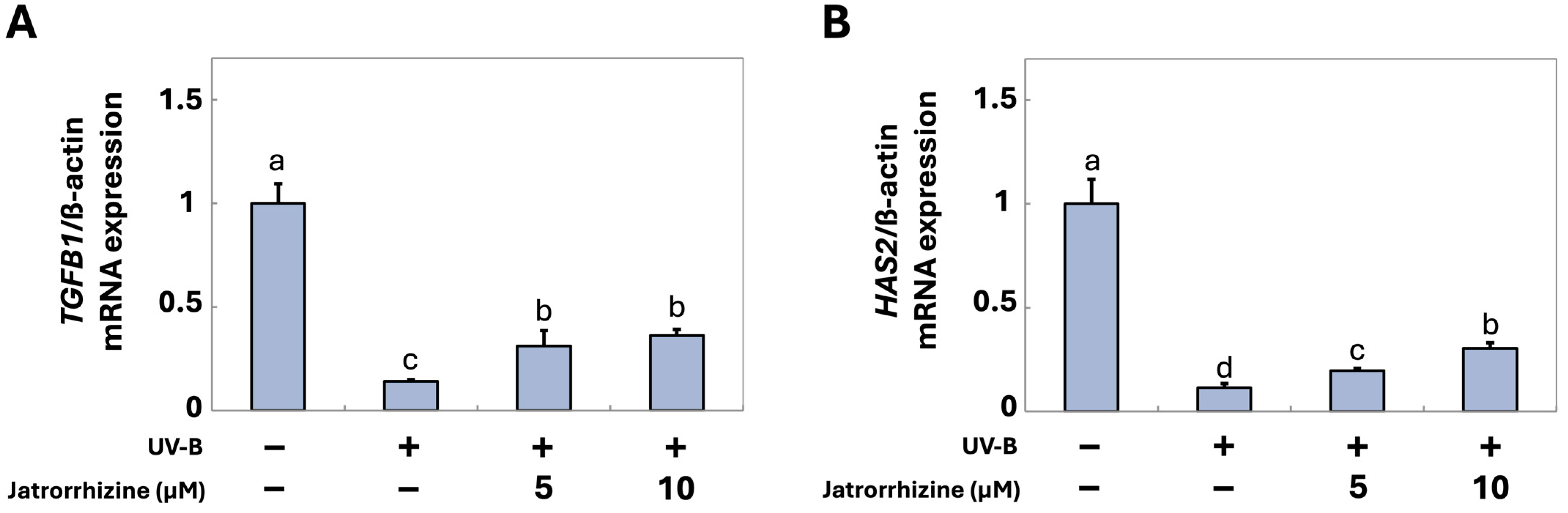
3.3. Jatrorrhizine Increases the Expression of TGFB1 and HAS2 in CCD-986sk Cells
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Read, R.A.; Zasada, J.C. Phellodendron amurense Rupr.: Amur corktree; U.S. Department of Agriculture: Washington DC, USA, 2008. [Google Scholar]
- Aryal, B.; Raut, B.K.; Bhattarai, S.; Bhandari, S.; Tandan, P.; Gyawali, K.; Sharma, K.; Ranabhat, D.; Thapa, R.; Aryal, D.; et al. Potential therapeutic applications of plant-derived alkaloids against inflammatory and neurodegenerative diseases. Evid.-Based Complement. Altern. Med. 2022, 2022, 7299778. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Kundu, A.; Kumar, A.; Gupta, M.; Lee, B.M.; Bhakta, T.; Dash, S.; Kim, H.S. Chapter 15—Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). In Recent Advances in Natural Products Analysis; Sanches Silva, A., Nabavi, S.F., Saeedi, M., Nabavi, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 505–567. [Google Scholar] [CrossRef]
- Cho, Y.-J. Anti-inflammatory effect of jatrorrhizine from Phellodendron amurense in lipopolysaccharide-stimulated Raw264.7 Cells. J. Appl. Biol. Chem. 2011, 54, 114–119. [Google Scholar] [CrossRef]
- Cho, Y.-J. Characteristics of cosmetic with whitening compounds from Phellodendron amurense. J. Appl. Biol. Chem. 2011, 54, 108–113. [Google Scholar] [CrossRef][Green Version]
- Brenneisen, P.; Sies, H.; Scharffetter-Kochanek, K. Ultraviolet-B irradiation and matrix metalloproteinases: From induction via signaling to initial events. Ann. N. Y. Acad. Sci. 2002, 973, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, O.; Thampan, R.V. Factors influencing collagen biosynthesis. J. Cell. Biochem. 2008, 104, 1150–1160. [Google Scholar] [CrossRef]
- Talwar, H.S.; Griffiths, C.E.; Fisher, G.J.; Hamilton, T.A.; Voorhees, J.J. Reduced type I and type III procollagens in photodamaged adult human skin. J. Investig. Dermatol. 1995, 105, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, M.P.; Brinckerhoff, C.E. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: Integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. Ther. 2002, 4, 157. [Google Scholar] [CrossRef]
- Galvez-Martin, P.; Soto-Fernandez, C.; Romero-Rueda, J.; Cabañas, J.; Torrent, A.; Castells, G.; Martinez-Puig, D. A novel hyaluronic acid matrix ingredient with regenerative, anti-aging and antioxidant capacity. Int. J. Mol. Sci. 2023, 24, 4774. [Google Scholar] [CrossRef]
- Ellenrieder, V.; Alber, B.; Lacher, U.; Hendler, S.F.; Menke, A.; Boeck, W.; Wagner, M.; Wilda, M.; Friess, H.; Büchler, M.; et al. Role of MT-MMPs and MMP-2 in pancreatic cancer progression. Int. J. Cancer 2000, 85, 14–20. [Google Scholar] [CrossRef]
- Jenkins, G. Molecular mechanisms of skin ageing. Mech. Ageing Dev. 2002, 123, 801–810. [Google Scholar] [CrossRef]
- Kim, Y.A.; Kim, D.H.; Yu, J.M.; Bin Park, C.; Park, T.S.; Park, B.J. Anti-wrinkle effects of extracts and solvent fractions from Nymphoides peltate on CCD-986sk. J. Appl. Biol. Chem. 2017, 60, 357–362. [Google Scholar] [CrossRef]
- Puizina-Ivić, N. Skin aging. Acta Dermatovenerol Alp Pannonica Adriat 2008, 17, 47–54. [Google Scholar] [PubMed]
- Matsuo, T.; Ito, S. The chemical structure of Kaki-tannin from immature fruit of the Persimmon (Diospyros kaki L.). Agric. Biol. Chem. 1978, 42, 1637–1643. [Google Scholar] [CrossRef]
- Hostettmann, K.; Hostettmann, M. Isolation techniques for flavonoids. In The Flavonoids; Harborn, J.B., Mabry, T.J., Eds.; Springer: Boston, MA, USA, 1982. [Google Scholar] [CrossRef]
- Li, Y.; He, W.; Liu, J.; Sheng, F.; Hu, Z.; Chen, X. Binding of the bioactive component Jatrorrhizine to human serum albumin. Biochim. Biophys. Acta 2005, 1722, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Parmar, V.S.; Bracke, M.E.; Philippe, J.; Wengel, J.; Jain, S.C.; Olsen, C.E.; Bisht, K.S.; Sharma, N.K.; Courtens, A.; Sharma, S.K.; et al. Anti-invasive activity of alkaloids and polyphenolics in vitro. Bioorganic Med. Chem. 1997, 5, 1609–1619. [Google Scholar] [CrossRef]
- Lee, K.-E.; Mun, S.; Pyun, H.-B.; Kim, M.-S.; Hwang, J.-K. Effects of macelignan isolated from Myristica fragrans (Nutmeg) on expression of matrix metalloproteinase-1 and type I procollagen in UVB-irradiated human skin fibroblasts. Biol. Pharm. Bull. 2012, 35, 1669–1675. [Google Scholar] [CrossRef]
- Ricard-Blum, S.; Ruggiero, F. The collagen superfamily: From the extracellular matrix to the cell membrane. Pathol. Biol. 2005, 53, 430–442. [Google Scholar] [CrossRef]
- Jang, Y.-A.; Yeom, B.-S.; Kim, S.-G.; Lee, J.-T. Evaluation of whitening activity and wrinkle inhibitory effect of ethanol extracts of Nelumbinis Rhizomatis Nodus. J. Life Sci. 2019, 29, 1192–1199. [Google Scholar]
- Lee, Y.-R.; Noh, E.-M.; Han, J.-H.; Kim, J.-M.; Hwang, J.-K.; Hwang, B.-M.; Chung, E.-Y.; Kim, B.-S.; Lee, S.-H.; Lee, S.J.; et al. Brazilin inhibits UVB-induced MMP-1/3 expressions and secretions by suppressing the NF-κB pathway in human dermal fibroblasts. Eur. J. Pharmacol. 2012, 674, 80–86. [Google Scholar] [CrossRef]
- Dragsbæk, K.; Neergaard, J.; Hansen, H.; Byrjalsen, I.; Alexandersen, P.; Kehlet, S.; Bay-Jensen, A.-C.; Christiansen, C.; Karsdal, M. Matrix metalloproteinase mediated type I collagen degradation—An independent risk factor for mortality in women. EBioMedicine 2015, 2, 723–729. [Google Scholar] [CrossRef]
- Nikolov, A.; Popovski, N. Role of gelatinases MMP-2 and MMP-9 in healthy and complicated pregnancy and their future potential as preeclampsia biomarkers. Diagnostics 2021, 11, 480. [Google Scholar] [CrossRef] [PubMed]
- Svensson, R.B.; Hassenkam, T.; Grant, C.A.; Magnusson, S.P. Tensile properties of human collagen fibrils and fascicles are insensitive to environmental salts. Biophys. J. 2010, 99, 4020–4027. [Google Scholar] [CrossRef]
- Vincenti, M.P.; White, L.A.; Schroen, D.J.; Benbow, U.; Brinckerhoff, C.E. Regulating expression of the gene for matrix metalloproteinase-1 (collagenase): Mechanisms that control enzyme activity, transcription, and mrna stability. Crit. Rev. Eukaryot. Gene Expr. 1996, 6, 391–411. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.S.; Crocker, S.J. An alternate perspective on the roles of TIMPs and MMPs in pathology. Am. J. Pathol. 2012, 180, 12–16. [Google Scholar] [CrossRef]
- Brew, K.; Nagase, H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim. Biophys. Acta 2010, 1803, 55–71. [Google Scholar] [CrossRef]
- Boon, L.; Ugarte-Berzal, E.; Martens, E.; Fiten, P.; Vandooren, J.; Janssens, R.; Blanter, M.; Yu, K.; Boon, M.; Struyf, S.; et al. Citrullination as a novel posttranslational modification of matrix metalloproteinases. Matrix Biol. 2021, 95, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Bosch, R.; Philips, N.; Suárez-Pérez, J.A.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; González, S. Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef]
- Brenneisen, P.; Oh, J.; Wlaschek, M.; Wenk, J.; Briviba, K.; Hommel, C.; Herrmann, G.; Sies, H.; Scharffetter-Kochanek, K. Ultraviolet B wavelength dependence for the regulation of two major matrix-metalloproteinases and their inhibitor TIMP-1 in human dermal fibroblast. Phtochem. Photobiol. 1996, 64, 877–885. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Lee, E.-H.; Cho, E.-B.; Kim, D.-H.; Kim, B.-O.; Kang, I.-K.; Jung, H.-Y.; Cho, Y.-J. Protective effect of galangin against UVB irradiation-induced photo-aging in CCD-986sk human skin fibroblasts. Appl. Biol. Chem. 2019, 62, 40. [Google Scholar] [CrossRef]
- Park, H.J.; Cho, J.H.; Hong, S.H.; Kim, D.H.; Jung, H.Y.; Kang, I.K.; Cho, Y.J. Whitening and anti-wrinkle activities of ferulic acid isolated from Tetragonia tetragonioides in B16F10 melnoma and CCD-986sk fibroblast cells. J. Nat. Med. 2018, 72, 127–135. [Google Scholar] [CrossRef]
- Zhang, Y.; Alexander, P.B.; Wang, X.-F. TGF-β Family signaling in the control of cell proliferation and survival. Cold Spring Harb. Perspect. Biol. 2017, 9, a022145. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Chen, Z.; Huang, R.; Yao, Y.; Ma, G. Transforming growth factor β1 induces the expression of collagen type I by DNA methylation in cardiac fibroblasts. PLoS ONE 2013, 8, e60335. [Google Scholar] [CrossRef]
- Yuan, W.; Varga, J. Transforming growth factor-β repression of matrix metalloproteinase-1 in dermal fibroblasts involves Smad3. J. Biol. Chem. 2001, 276, 38502–38510. [Google Scholar] [CrossRef]
- Cui, H.-M.; Zhang, Q.-Y.; Wang, J.-L.; Chen, J.-L.; Zhang, Y.-L.; Tong, X.-L. Poor permeability and absorption affect the activity of four alkaloids from Coptis. Mol. Med. Rep. 2015, 12, 7160–7168. [Google Scholar] [CrossRef]
- Zhong, F.; Chen, Y.; Chen, J.; Liao, H.; Li, Y.; Ma, Y. Jatrorrhizine: A review of sources, pharmacology, pharmacokinetics and toxicity. Front. Pharmacol. 2021, 12, 783127. [Google Scholar] [CrossRef]
- Aghababaei, F.; Hadidi, M. Recent advances in potential health benefits of quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Chen, R.; Lin, J.; Hong, J.; Han, D.; Zhang, A.D.; Lan, R.; Fu, L.; Wu, Z.; Lin, J.; Zhang, W.; et al. Potential toxicity of quercetin: The repression of mitochondrial copy number via decreased POLG expression and excessive TFAM expression in irradiated murine bone marrow. Toxicol. Rep. 2014, 1, 450–458. [Google Scholar] [CrossRef]
- Sergi, C.M. Epigallocatechin-3-Gallate Toxicity in Children: A Potential and Current Toxicological Event in the Differential Diagnosis with Virus-Triggered Fulminant Hepatic Failure. Front. Pharmacol. 2019, 10, 1563. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Lee, E.-H.; Cho, Y.-J.; Park, Y. Jatrorrhizine, an alkaloid isolated from Phellodendron amurense, reduces melanogenesis in mouse B16F10 melanoma cells. NFS J. 2025, 38, 100221. [Google Scholar] [CrossRef]

| Gene | Accession No. | Primer | Sequence (5′-3′) | Amplicon Size (bp) |
|---|---|---|---|---|
| COL1A2 | NM_000089.3 | Forward | AGAAACACGTCTGGCTAGGAG | 105 |
| Reverse | GCATGAAGGCAAGTTGGGTAG | |||
| MMP-1 | NM_002421.4 | Forward | TGGGAGGCAAGTTGAAAAGC | 135 |
| Reverse | CATCTGGGCTGCTTCATCAC | |||
| MMP-9 | NM_004994.3 | Forward | CCTGGGCAGATTCCAAACCT | 172 |
| Reverse | GTACACGCGAGTGAAGGTGA | |||
| TIMP-1 | NM_003254.3 | Forward | CTTCTGCAATTCCGACCTCGT | 79 |
| Reverse | ACGCTGGTATAAGGTGGTCTG | |||
| TGFB1 | NM_000660.6 | Forward | CAATTCCTGGCGATACCTCAG | 86 |
| Reverse | GCACAACTCCGGTGACATCAA | |||
| HAS2 | NM_005328.3 | Forward | GAGGACGACTTTATGACCAAGAGC | 121 |
| Reverse | TAAGCAGCTGTGATTCCAAGGAGG | |||
| β-actin | NM_007393.4 | Forward | CGTGCGTGACATCAAAGAGAA | 137 |
| Reverse | GCTCGTTGCCAATAGTGATGA | |||
| Gene | Real-Time PCR Condition | |||
| COL1A2 | 95 °C for 5 min, followed by undergoing 40 cycles of 95 °C for 10 s, 60 °C for 20 s, followed by each 95 °C, 60 °C, 95 °C for 15 s | |||
| MMP-1 | ||||
| MMP-9 | ||||
| TIMP-1 | ||||
| TGFB1 | ||||
| HAS2 | ||||
| ß-actin | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, J. Jatrorrhizine Isolated from Phellodendron amurense Improves Collagen Homeostasis in CCD-986sk Human Dermal Fibroblast Cells. Cosmetics 2025, 12, 70. https://doi.org/10.3390/cosmetics12020070
Cho J. Jatrorrhizine Isolated from Phellodendron amurense Improves Collagen Homeostasis in CCD-986sk Human Dermal Fibroblast Cells. Cosmetics. 2025; 12(2):70. https://doi.org/10.3390/cosmetics12020070
Chicago/Turabian StyleCho, Junhyo. 2025. "Jatrorrhizine Isolated from Phellodendron amurense Improves Collagen Homeostasis in CCD-986sk Human Dermal Fibroblast Cells" Cosmetics 12, no. 2: 70. https://doi.org/10.3390/cosmetics12020070
APA StyleCho, J. (2025). Jatrorrhizine Isolated from Phellodendron amurense Improves Collagen Homeostasis in CCD-986sk Human Dermal Fibroblast Cells. Cosmetics, 12(2), 70. https://doi.org/10.3390/cosmetics12020070






