Mechanistic Insights into Pigmented Rice Bran in Mitigating UV-Induced Oxidative Stress, Inflammation, and Pigmentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Retrieval
2.2. Screening and Eligibility
2.3. Annotated Bibliography
3. Results
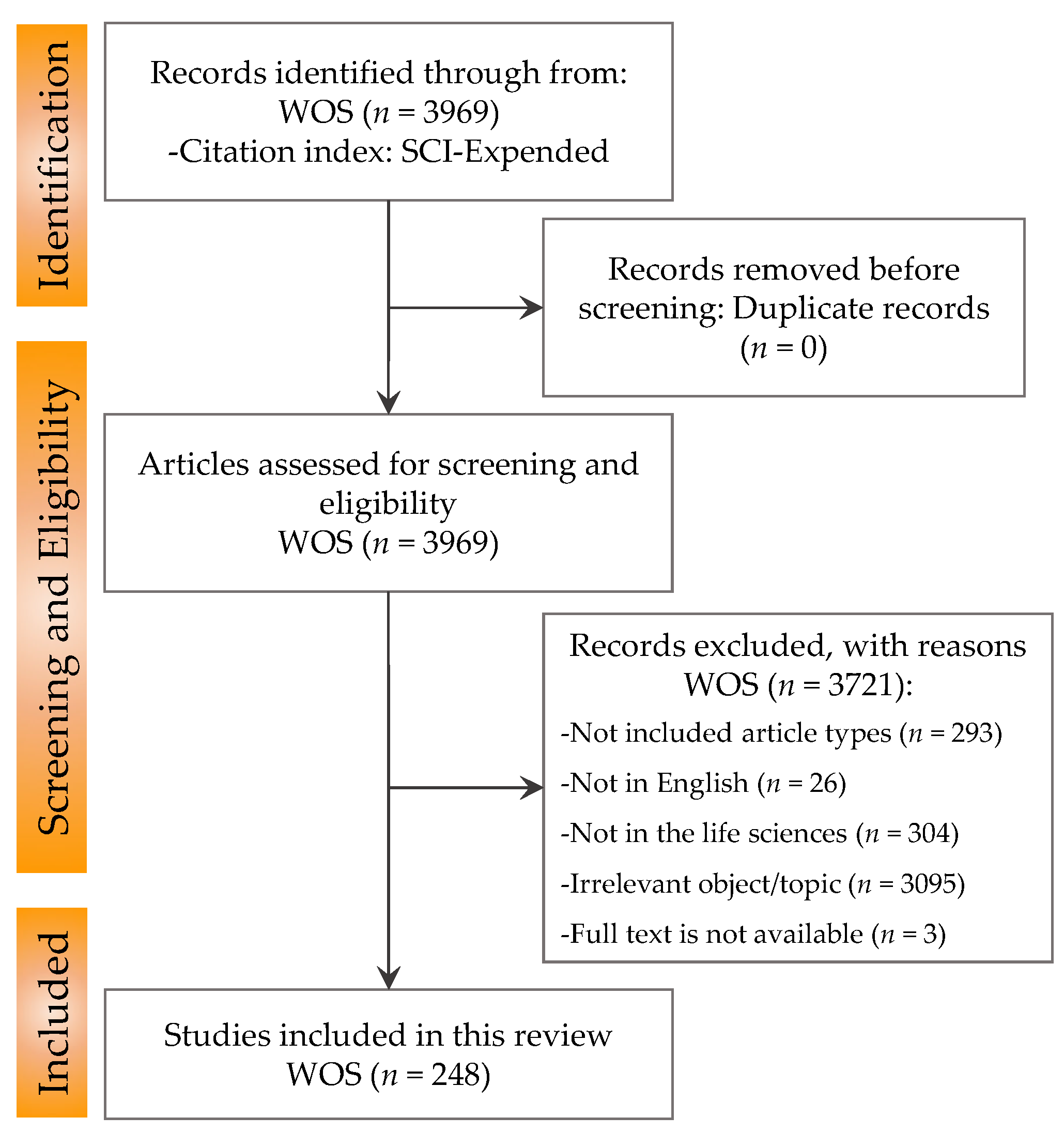
3.1. Search Results and Study Inclusion
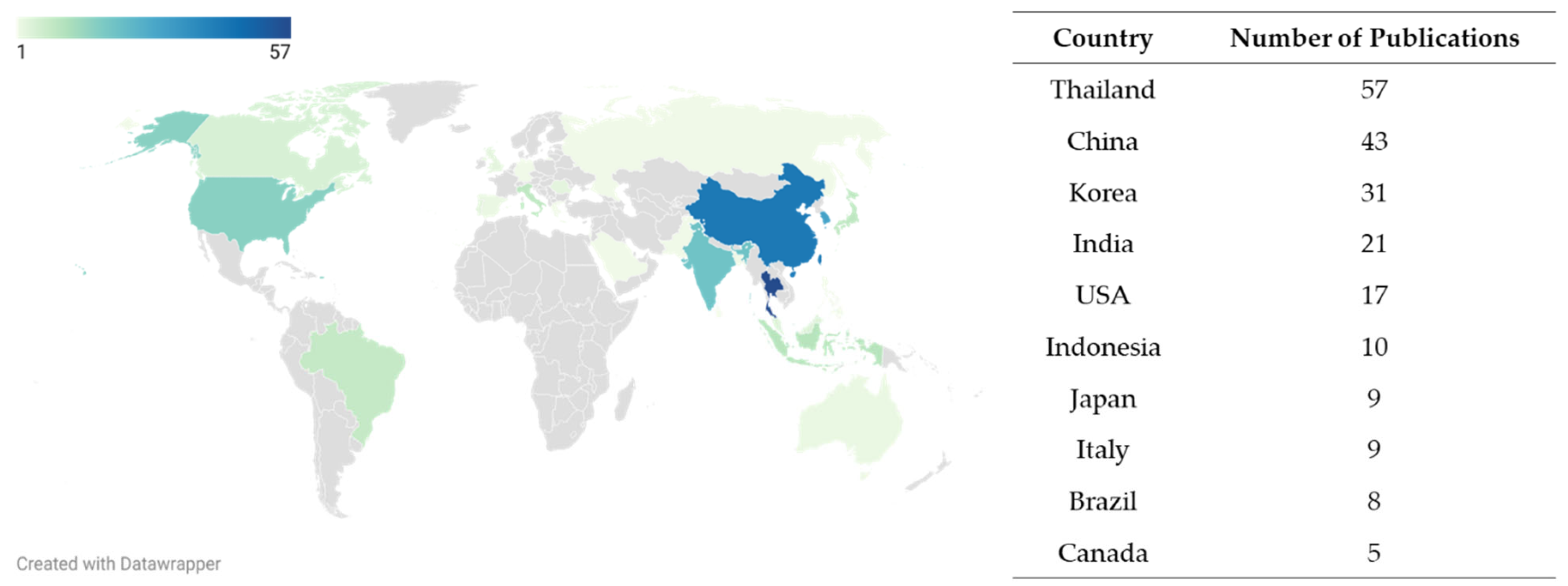
3.2. Study Characteristics
3.3. Chemical Composition of Pigmented Rice Bran
3.3.1. Pigment Compounds
3.3.2. Phenolic Compounds
3.3.3. Flavonoids
3.3.4. Functional Lipids
3.3.5. Vitamins and Minerals
3.3.6. Bioactive Peptides
3.4. Mechanisms of Sun Protection and Antiphotoaging of Pigmented Rice
3.4.1. UV Absorption Capacity
3.4.2. Antioxidant Effects
3.4.3. Anti-Inflammatory Effect
3.4.4. Inhibition of Matrix Metalloproteinases (MMPs)
3.4.5. Inhibition of Tyrosinase Activity
3.4.6. Enhancement of Skin Barrier Function
4. Discussion
4.1. Applications in Functional Skincare and Challenges
4.2. Technological Challenges
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AP-1 | Activator protein-1 |
| BRE | Black rice extract |
| BRP | Peptides from germinated black rice |
| COL1A1 | Collagen type 1 alpha 1 |
| COX-2 | Cyclooxygenase-2 |
| CPD | Cyclobutane pyrimidine dimer |
| ECM | Extracellular matrix |
| FLG | Filaggrin |
| GR | Glutathione reductase |
| HPLC | High-performance liquid chromatography |
| HAS2 | Hyaluronan synthase 2 |
| 4-HNE | 4-hydroxynonenal |
| MDA | Malondialdehyde |
| MMPs | Matrix metalloproteinases |
| NF-κB | Nuclear factor kappa B |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| PGE2 | Prostaglandin E2 |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| ROS | Reactive oxygen species |
| RRE | Red rice extract |
| SPF | Sun protection factor |
| TGF-β | Transforming growth factor-beta |
| TGM-1 | Transglutaminase-1 |
| TNF-α | Tumor necrosis factor-alpha |
| WOS | Web of Science |
References
- Gromkowska-Kępka, K.J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. The impact of ultraviolet radiation on skin photoaging—Review of in vitro studies. J. Cosmet. Dermatol. 2021, 20, 3427–3431. [Google Scholar] [CrossRef] [PubMed]
- Saewan, N.; Jimtaisong, A. Natural products as photoprotection. J. Cosmet. Dermatol. 2015, 14, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Sun, Y.; Zhu, L.; Li, L.Y.; Zhao, Y. Study on the Skincare Effects of Red Rice Fermented by Aspergillus oryzae In Vitro. Molecules 2024, 29, 2066. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xie, L.; Wang, G.; Jiao, J.; Zhao, J.; Yu, Q.; Chen, Y.; Shen, M.; Wen, H.; Ou, X.; et al. Anthocyanins-natural pigment of colored rice bran: Composition and biological activities. Food Res. Int. 2024, 175, 113722. [Google Scholar] [CrossRef]
- Kothapalli, L.; Kale, S.; Dharade, M.; Thomas, A.; Godse, A. Nutritional and Medicinal Value of Red Rice. Curr. Tradit. Med. 2023, 9, 64–73. [Google Scholar] [CrossRef]
- Samyor, D.; Das, A.B.; Deka, S.C. Pigmented rice a potential source of bioactive compounds: A review. Int. J. Food Sci. Technol. 2017, 52, 1073–1081. [Google Scholar] [CrossRef]
- Masisi, K.; Beta, T.; Moghadasian, M.H. Antioxidant properties of diverse cereal grains: A review on in vitro and in vivo studies. Food Chem. 2016, 196, 90–97. [Google Scholar] [CrossRef]
- Rungratanawanich, W.; Memo, M.; Uberti, D. Redox Homeostasis and Natural Dietary Compounds: Focusing on Antioxidants of Rice (Oryza sativa L.). Nutrients 2018, 10, 1605. [Google Scholar] [CrossRef]
- Choi, S.P.; Kim, S.P.; Kang, M.Y.; Nam, S.H.; Friedman, M. Protective Effects of Black Rice Bran against Chemically-Induced Inflammation of Mouse Skin. J. Agric. Food Chem. 2010, 58, 10007–10015. [Google Scholar] [CrossRef]
- Callcott, E.T.; Thompson, K.; Oli, P.; Blanchard, C.L.; Santhakumar, A.B. Coloured rice-derived polyphenols reduce lipid peroxidation and pro-inflammatory cytokines ex vivo. Food Funct. 2018, 9, 5169–5175. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Zhang, R.; Yang, X.; Sun, Y.; Shi, L.; Xue, P. Optimization of ultrasound-assisted extraction by response surface methodology, antioxidant capacity, and tyrosinase inhibitory activity of anthocyanins from red rice bran. Food Sci. Nutr. 2020, 8, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.C.; Yan, Y.X.; Al-Ansi, W.; Qian, H.F.; Li, Y.; Rao, Z.M.; Wang, L. Germination-induced changes in anthocyanins and proanthocyanidins: A pathway to boost bioactive compounds in red rice. Food Chem. 2024, 433, 9. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Min, C.W.; Jung, J.Y.; Ham, T.H.; Jeon, J.S.; Cho, L.H.; Kwon, S.W.; Kim, S.T. Proteome profiling highlights mechanisms underlying pigment and tocopherol accumulation in red and black rice seeds. Proteomics 2023, 23, 5. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.A.; Cañizares, L.D.C.; Meza, S.L.R.; Peres, B.B.; Jappe, S.N.; Timm, N.D.; de Oliveira, M.; Coradi, P.C. A Review of the Influence of Genotype, Environment, and Food Processing on the Bioactive Compound Profile of Red Rice (Oryza sativa L.). Agronomy 2024, 14, 16. [Google Scholar] [CrossRef]
- Shao, Y.F.; Hu, Z.Q.; Yu, Y.H.; Mou, R.X.; Zhu, Z.W.; Beta, T. Phenolic acids, anthocyanins, proanthocyanidins, antioxidant activity, minerals and their correlations in non-pigmented, red, and black rice. Food Chem. 2018, 239, 733–741. [Google Scholar] [CrossRef]
- Goufo, P.; Trindade, H. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci. Nutr. 2014, 2, 75–104. [Google Scholar] [CrossRef]
- Sapna, I.; Jayadeep, A. Influence of enzyme concentrations in enzymatic bioprocessing of red rice bran: A detailed study on nutraceutical compositions, antioxidant and human LDL oxidation inhibition properties. Food Chem. 2021, 351, 9. [Google Scholar] [CrossRef]
- Gong, E.S.; Liu, C.M.; Li, B.; Zhou, W.; Chen, H.Y.; Li, T.; Wu, J.Y.; Zeng, Z.C.; Wang, Y.H.; Si, X.; et al. Phytochemical profiles of rice and their cellular antioxidant activity against ABAP induced oxidative stress in human hepatocellular carcinoma HepG2 cells. Food Chem. 2020, 318, 12. [Google Scholar] [CrossRef]
- Irakli, M.N.; Samanidou, V.F.; Katsantonis, D.N.; Biliaderis, C.G.; Papadoyannis, I.N. Phytochemical Profiles and Antioxidant Capacity of Pigmented and Non-pigmented Genotypes of Rice (Oryza sativa L.). Cereal Res. Commun. 2016, 44, 98–110. [Google Scholar] [CrossRef]
- Li, X.; Liu, C.; Li, Y.; Yuan, K.; Zhang, W.; Cai, D.; Peng, Z.; Hu, Y.; Sun, J.; Bai, W. Bioactivity and application of anthocyanins in skin protection and cosmetics: An extension as a functional pigment. Phytochem. Rev. 2023, 22, 1441–1467. [Google Scholar] [CrossRef]
- Correia, P.; Araújo, P.; Ribeiro, C.; Oliveira, H.; Pereira, A.R.; Mateus, N.; de Freitas, V.; Brás, N.F.; Gameiro, P.; Coelho, P.; et al. Anthocyanin-Related Pigments: Natural Allies for Skin Health Maintenance and Protection. Antioxidants 2021, 10, 1038. [Google Scholar] [CrossRef] [PubMed]
- Asadollahi, L.; Abbaspour-Ravasjani, S.; Kim, K.A.; Maghsoodi, M.; Hamishehkar, H.; Kosari-Nasab, M.; Kim, K.H. Rice (Oryza sativa) Stem Cells as a Novel Promising Active Ingredient with Anti-Proliferative Effects for Potential Skin Cancer Prevention and Skin Whitening Activity. Foods 2024, 13, 2803. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Bae, J.S.; Ban, J.J.; Shin, H.S.; Lee, D.H.; Chung, J.H. Black rice (Oryza sativa L.) extract modulates ultraviolet-induced expression of matrix metalloproteinases and procollagen in a skin cell model. Int. J. Mol. Med. 2018, 41, 3073–3080. [Google Scholar] [CrossRef]
- Limtrakul, P.; Yodkeeree, S.; Punfa, W.; Srisomboon, J. Inhibition of the MAPK signaling pathway by red rice extract in UVB-irradiated human skin fibroblasts. Nat. Prod. Commun. 2016, 11, 1934578X1601101226. [Google Scholar] [CrossRef]
- Shin, H.Y.; Kim, S.M.; Lee, J.H.; Lim, S.T. Solid-state fermentation of black rice bran with Aspergillus awamori and Aspergillus oryzae: Effects on phenolic acid composition and antioxidant activity of bran extracts. Food Chem. 2019, 272, 235–241. [Google Scholar] [CrossRef]
- Yodkeeree, S.; Thippraphan, P.; Punfa, W.; Srisomboon, J.; Limtrakul, P. Skin anti-aging assays of proanthocyanidin rich red rice extract, oryzanol and other phenolic compounds. Nat. Prod. Commun. 2018, 13, 1934578X1801300812. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Brit. Med. J. 2021, 372, 9. [Google Scholar]
- Kim, T.J.; Kim, S.Y.; Park, Y.J.; Lim, S.-H.; Ha, S.-H.; Park, S.U.; Lee, B.; Kim, J.K. Metabolite Profiling Reveals Distinct Modulation of Complex Metabolic Networks in Non-Pigmented, Black, and Red Rice (Oryza sativa L.) Cultivars. Metabolites 2021, 11, 367. [Google Scholar] [CrossRef]
- Francavilla, A.; Joye, I.J. Anthocyanins in Whole Grain Cereals and Their Potential Effect on Health. Nutrients 2020, 12, 2922. [Google Scholar] [CrossRef]
- Sivasinprasasn, S.; Tocharus, J.; Mahatheeranont, S.; Nakrat, S.; Tocharus, C. Anthocyanin-Rich Fraction of Black Rice Bran Extract Protects against Amyloid β-Induced Oxidative Stress, Endoplasmic Reticulum Stress, and Neuronal Apoptosis in SK-N-SH Cells. Pharmaceuticals 2024, 17, 1039. [Google Scholar] [CrossRef]
- Min, B.; McClung, A.M.; Chen, M.-H. Phytochemicals and Antioxidant Capacities in Rice Brans of Different Color. J. Food Sci. 2011, 76, C117–C126. [Google Scholar] [CrossRef] [PubMed]
- Pattananandecha, T.; Apichai, S.; Sirilun, S.; Julsrigival, J.; Sawangrat, K.; Ogata, F.; Kawasaki, N.; Sirithunyalug, B.; Saenjum, C. Anthocyanin Profile, Antioxidant, Anti-Inflammatory, and Antimicrobial against Foodborne Pathogens Activities of Purple Rice Cultivars in Northern Thailand. Molecules 2021, 26, 5234. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, R.; Zhang, Y.; Lu, Y.; Cai, S.; Xiong, Q. Metabolomics Reveals Antioxidant Metabolites in Colored Rice Grains. Metabolites 2024, 14, 120. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Alagarsamy, K.; Thangaleela, S.; Bharathi, M.; Kesika, P.; Chaiyasut, C. Composition, Microbiota, Mechanisms, and Anti-Obesity Properties of Rice Bran. Foods 2023, 12, 1300. [Google Scholar] [CrossRef]
- Oladzadabbasabadi, N.; Mohammadi Nafchi, A.; Ghasemlou, M.; Ariffin, F.; Singh, Z.; Al-Hassan, A.A. Natural anthocyanins: Sources, extraction, characterization, and suitability for smart packaging. Food Packag. Shelf 2022, 33, 100872. [Google Scholar] [CrossRef]
- Verma, D.K.; Srivastav, P.P. Bioactive compounds of rice (Oryza sativa L.): Review on paradigm and its potential benefit in human health. Trends Food Sci. Technol. 2020, 97, 355–365. [Google Scholar] [CrossRef]
- Chen, M.-H.; McClung, A.M.; Bergman, C.J. Concentrations of oligomers and polymers of proanthocyanidins in red and purple rice bran and their relationships to total phenolics, flavonoids, antioxidant capacity and whole grain color. Food Chem. 2016, 208, 279–287. [Google Scholar] [CrossRef]
- Gunaratne, A.; Wu, K.; Li, D.; Bentota, A.; Corke, H.; Cai, Y.Z. Antioxidant activity and nutritional quality of traditional red-grained rice varieties containing proanthocyanidins. Food Chem 2013, 138, 1153–1161. [Google Scholar] [CrossRef]
- Seechamnanturakit, V.; Karrila, T.T.; Sontimuang, C.; Sukhoom, A. The natural pigments in pigmented rice bran and their relation to human health: A literature review. Kmutnb Int. J. Appl. Sci. Technol. 2018, 11, 3–13. [Google Scholar] [CrossRef]
- Belefant-Miller, H.; Grunden, E. Carotenoid metabolism is induced in rice bran during very high temperature stress. J. Sci. Food Agric. 2014, 94, 1808–1815. [Google Scholar] [CrossRef]
- Melini, V.; Acquistucci, R. Health-Promoting Compounds in Pigmented Thai and Wild Rice. Foods 2017, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Mamatha, B.S.; Sangeetha, R.K.; Baskaran, V. Provitamin-A and xanthophyll carotenoids in vegetables and food grains of nutritional and medicinal importance. Int. J. Food Sci. Technol. 2011, 46, 315–323. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. β-Carotene and other carotenoids in protection from sunlight. Am. J. Clin. Nutr. 2012, 96, 1179s–1184s. [Google Scholar] [CrossRef] [PubMed]
- Gruszecki, W.I.; Strzałka, K. Carotenoids as modulators of lipid membrane physical properties. Biochim. Biophys. Acta 2005, 1740, 108–115. [Google Scholar] [CrossRef]
- Anbualakan, K.; Tajul Urus, N.Q.; Makpol, S.; Jamil, A.; Mohd Ramli, E.S.; Md Pauzi, S.H.; Muhammad, N. A Scoping Review on the Effects of Carotenoids and Flavonoids on Skin Damage Due to Ultraviolet Radiation. Nutrients 2023, 15, 92. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, S.Y.; Chu, S.M.; Lim, S.H.; Suh, S.-C.; Lee, Y.-T.; Cho, H.S.; Ha, S.-H. Variation and Correlation Analysis of Flavonoids and Carotenoids in Korean Pigmented Rice (Oryza sativa L.) Cultivars. J. Agric. Food Chem. 2010, 58, 12804–12809. [Google Scholar] [CrossRef]
- Huang, Y.-P.; Lai, H.-M. Bioactive compounds and antioxidative activity of colored rice bran. J. Food Drug Anal. 2016, 24, 564–574. [Google Scholar] [CrossRef]
- Pang, Y.; Ahmed, S.; Xu, Y.; Beta, T.; Zhu, Z.; Shao, Y.; Bao, J. Bound phenolic compounds and antioxidant properties of whole grain and bran of white, red and black rice. Food Chem. 2018, 240, 212–221. [Google Scholar] [CrossRef]
- Gosangi, A. Phytochemicals, Antioxidant Activity and Grain Quality in Pigmented and Non-Pigmented Rice; Punjab Agricultural University: Punjab, India, 2023. [Google Scholar]
- Loypimai, P.; Moongngarm, A.; Chottanom, P. Phytochemicals and antioxidant capacity of natural food colorant prepared from black waxy rice bran. Food Biosci. 2016, 15, 34–41. [Google Scholar] [CrossRef]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef]
- Ying Zhu, Y.Z.; Qu, C.; Bai, J.; Zhao, Y.; Xiao, X. Fermented red rice improved the antioxidant activity, bioaccessibility of polyphenols, and lipid-lowering activity in C. elegans. Food Bioeng. 2024, 3, 160–171. [Google Scholar]
- Peanparkdee, M.; Patrawart, J.; Iwamoto, S. Physicochemical stability and in vitro bioaccessibility of phenolic compounds and anthocyanins from Thai rice bran extracts. Food Chem. 2020, 329, 127157. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Yoshida, N.; Kuriyama, I.; Kanamori, M.; Sakamoto, Y.; Mizushina, Y. Characteristics of Fatty Acid Distribution in Different Acyl Lipids of Colored Rice Bran Cultivars. Food Sci. Technol. Res. 2014, 20, 121–127. [Google Scholar] [CrossRef]
- Moongngarm, A.; Daomukda, N.; Khumpika, S. Chemical Compositions, Phytochemicals, and Antioxidant Capacity of Rice Bran, Rice Bran Layer, and Rice Germ. APCBEE Procedia 2012, 2, 73–79. [Google Scholar] [CrossRef]
- Chinvongamorn, C.; Sansenya, S. The γ-oryzanol Content of Thai Rice Cultivars and the Effects of Gamma Irradiation on the γ-oryzanol Content of Germinated Thai Market Rice. Orient. J. Chem. 2020, 36, 812–818. [Google Scholar] [CrossRef]
- Xu, Z.; Hua, N.; Godber, J.S. Antioxidant activity of tocopherols, tocotrienols, and gamma-oryzanol components from rice bran against cholesterol oxidation accelerated by 2,2′-azobis(2-methylpropionamidine) dihydrochloride. J. Agric. Food Chem. 2001, 49, 2077–2081. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, J.B.; Shanmugavelan, P.; Kim, S.N.; Cho, Y.S.; Kim, H.R.; Lee, J.T.; Jeon, W.T.; Lee, D.J. Evaluation of γ-oryzanol content and composition from the grains of pigmented rice-germplasms by LC-DAD-ESI/MS. BMC Res. Notes 2013, 6, 149. [Google Scholar] [CrossRef]
- Mas’ud, F.; Mahendradatta, M.; Laga, A.; Zainal, Z. Component, fatty acid and mineral composition of rice bran oil extracted by multistage with hexane and ethanol. Int. J. Sci. Technol. Res. 2017, 6, 63–69. [Google Scholar]
- Xu, D.; Hao, J.; Wang, Z.; Liang, D.; Wang, J.; Ma, Y.; Zhang, M. Physicochemical properties, fatty acid compositions, bioactive compounds, antioxidant activity and thermal behavior of rice bran oil obtained with aqueous enzymatic extraction. LWT 2021, 149, 111817. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Z.; Blanchard, C. Impact on the nutritional attributes of rice bran following various stabilization procedures. Crit. Rev. Food Sci. Nutr. 2019, 59, 2458–2466. [Google Scholar] [CrossRef]
- Zhao, M.; Lin, Y.; Chen, H. Improving nutritional quality of rice for human health. Theor. Appl. Genet. 2020, 133, 1397–1413. [Google Scholar] [CrossRef] [PubMed]
- Sankam, P.; Punvittayagul, C.; Sringam, K.; Chaiyasut, C.; Wongpoomchai, R. Antimutagenicity and anticlastogenicity of glutinous purple rice hull using in vitro and in vivo testing systems. Mol. Cell. Toxicol. 2013, 9, 169–176. [Google Scholar] [CrossRef]
- Chariyakornkul, A.; Punvittayagul, C.; Taya, S.; Wongpoomchai, R. Inhibitory effect of purple rice husk extract on AFB1-induced micronucleus formation in rat liver through modulation of xenobiotic metabolizing enzymes. BMC Complement. Altern. Med. 2019, 19, 11. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Strappe, P.; Shang, W.T.; Zhou, Z.K. Functional peptides derived from rice bran proteins. Crit. Rev. Food Sci. Nutr. 2019, 59, 349–356. [Google Scholar] [CrossRef]
- Mo, Q.; You, S.; Fu, H.; Wang, D.; Zhang, J.; Wang, C.; Li, M. Purification and Identification of Antioxidant Peptides from Rice Fermentation of Lactobacillus plantarum and Their Protective Effects on UVA-Induced Oxidative Stress in Skin. Antioxidants 2022, 11, 2333. [Google Scholar] [CrossRef]
- Sim, G.S.; Lee, D.H.; Kim, J.H.; An, S.K.; Choe, T.B.; Kwon, T.J.; Pyo, H.B.; Lee, B.C. Black rice (Oryza sativa L. var. japonica) hydrolyzed peptides induce expression of hyaluronan synthase 2 gene in HaCaT keratinocytes. J. Microbiol. Biotechnol. 2007, 17, 271–279. [Google Scholar]
- Saha, S.; Singh, J.; Paul, A.; Sarkar, R.; Khan, Z.; Banerjee, K. Anthocyanin Profiling Using UV-Vis Spectroscopy and Liquid Chromatography Mass Spectrometry. J. AOAC Int. 2020, 103, 23–39. [Google Scholar] [CrossRef]
- Guo, X.; He, L.; Sun, J.; Ye, H.; Yin, C.; Zhang, W.; Han, H.; Jin, W. Exploring the Potential of Anthocyanins for Repairing Photoaged Skin: A Comprehensive Review. Foods 2024, 13, 3506. [Google Scholar] [CrossRef]
- Tikapunya, T.; Pompimon, W.; Khamjainuk, P.; Sansomchai, P. Biological activity and its related compounds of Red Jasmine rice extracts linked to normal fibroblast viability for cosmetic product. Curr. Chem. Lett. 2022, 11, 69–74. [Google Scholar] [CrossRef]
- Li, S.; Huang, K.; Zhong, M.; Guo, J.; Wang, W.-z.; Zhu, R. Comparative studies on the interaction of caffeic acid, chlorogenic acid and ferulic acid with bovine serum albumin. Spectrochim. Acta A 2010, 77, 680–686. [Google Scholar] [CrossRef]
- Teeranachaideekul, V.; Wongrakpanich, A.; Leanpolchareanchai, J.; Thirapanmethee, K.; Sirichaovanichkarn, C. Characterization, biological activities and safety evaluation of different varieties of Thai pigmented rice extracts for cosmetic applications. Pharm. Sci. Asia 2018, 45, 140–153. [Google Scholar] [CrossRef]
- Milutinov, J.; Pavlović, N.; Ćirin, D.; Atanacković Krstonošić, M.; Krstonošić, V. The Potential of Natural Compounds in UV Protection Products. Molecules 2024, 29, 5409. [Google Scholar] [CrossRef] [PubMed]
- Farhamzah, F.; Kusumawati, A.; Alkandahri, M.; Hidayah, H.; Sujana, D.; Gunarti, N.; Yuniarsih, N.; Apriana, S.; Agustina, L. Sun protection factor activity of black glutinous rice emulgel extract (Oryza sativa var glutinosa). Indian J. Pharm. Educ. 2022, 56, 302–310. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Y.; Xu, Y.; Ungsurungsie, M. Red jasmine rice bran extract for the prevention of the blue light induced photodamges. Front. Med. Sci. Res. 2024, 6, 67–71. [Google Scholar]
- Zhang, T.; Ungsurungsie, M.; Yue, L.; Li, J.; Zhuang, B.; Ding, W.; Li, X. Skin Photobiological effect Induced by BL: Clinical manifestation, Mechanisms, and Protection against Photodamages. J. Dermatol. Sci. Cosm. Technol. 2024, 1, 100042. [Google Scholar] [CrossRef]
- Zeinali, M.; Abbaspour-Ravasjani, S.; Soltanfam, T.; Paiva-Santos, A.C.; Babaei, H.; Veiga, F.; Hamishehkar, H. Prevention of UV-induced skin cancer in mice by gamma oryzanol-loaded nanoethosomes. Life Sci. 2021, 283, 119759. [Google Scholar] [CrossRef]
- Bianchini Silva, L.S.; Perasoli, F.B.; Carvalho, K.V.; Vieira, K.M.; Paz Lopes, M.T.; Bianco de Souza, G.H.; Henrique dos Santos, O.D.; Freitas, K.M. Melaleuca leucadendron (L.) L. flower extract exhibits antioxidant and photoprotective activities in human keratinocytes exposed to ultraviolet B radiation. Free Radic. Biol. Med. 2020, 159, 54–65. [Google Scholar] [CrossRef]
- Cheng, W.; Shi, X.; Zhang, J.; Li, L.; Di, F.; Li, M.; Wang, C.; An, Q.; Zhao, D. Role of PI3K-AKT Pathway in Ultraviolet Ray and Hydrogen Peroxide-Induced Oxidative Damage and Its Repair by Grain Ferments. Foods 2023, 12, 806. [Google Scholar] [CrossRef]
- Jun, H.I.; Song, G.S.; Yang, E.I.; Youn, Y.; Kim, Y.S. Antioxidant activities and phenolic compounds of pigmented rice bran extracts. J. Food Sci. 2012, 77, C759–C764. [Google Scholar] [CrossRef]
- Tammasakchai, A.; Reungpatthanaphong, S.; Chaiyasut, C.; Rattanachitthawat, S.; Suwannalert, P. Red strain Oryza sativa-unpolished thai rice prevents oxidative stress and colorectal aberrant crypt foci formation in rats. Asian Pac. J. Cancer Prev. 2012, 13, 1929–1933. [Google Scholar] [CrossRef]
- Zhang, T.; Zhuang, B.X.; Liu, Y.; Zhang, Z.H.; Sun, Q. An application of red rice extraction and the method of extraction and preparation. CN 119055730A, 3 December 2024. [Google Scholar]
- Nas, J.S.B.; Manalo, R.V.M.; Medina, P.M.B. Peonidin-3-glucoside extends the lifespan of Caenorhabditis elegans and enhances its tolerance to heat, UV, and oxidative stresses. Sci. Asia 2021, 47, 457. [Google Scholar] [CrossRef]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Yarosh, D.; Both, D.; Kibitel, J.; Anderson, C.; Elmets, C.; Brash, D.; Brown, D. Regulation of TNFalpha production and release in human and mouse keratinocytes and mouse skin after UV-B irradiation. Photodermatol. Photoimmunol. Photomed. 2000, 16, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Limtrakul, P.; Yodkeeree, S.; Pitchakarn, P.; Punfa, W. Anti-inflammatory effects of proanthocyanidin-rich red rice extract via suppression of MAPK, AP-1 and NF-κB pathways in Raw 264.7 macrophages. Nutr. Res. Pract. 2016, 10, 251–258. [Google Scholar] [CrossRef]
- Gu, Y.; Han, J.; Jiang, C.; Zhang, Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020, 59, 101036. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Lee, E.-H.; Cho, E.-B.; Kim, D.-H.; Kim, B.-O.; Kang, I.-k.; Jung, H.-Y.; Cho, Y.-J. Protective effects of galangin against UVB irradiation-induced photo-aging in CCD-986sk human skin fibroblasts. Appl. Biol. Chem. 2019, 62, 1–8. [Google Scholar] [CrossRef]
- Yakaew, S.; Phimnuan, P.; Tiensomjitr, K.; Nakyai, W.; Nuengchamnong, N.; Ross, G.; Viyoch, J. Hom–Kularb–Dang rice bran extract for the prevention of UVB-damage against human skin fibroblast. CMU J. Nat. Sci 2020, 19, 34–50. [Google Scholar] [CrossRef]
- Peng, Z.; Chen, B.; Zheng, Q.; Zhu, G.; Cao, W.; Qin, X.; Zhang, C. Ameliorative Effects of Peptides from the Oyster (Crassostrea hongkongensis) Protein Hydrolysates against UVB-Induced Skin Photodamage in Mice. Mar. Drugs 2020, 18, 288. [Google Scholar] [CrossRef]
- Tembhre, M.K. Shipra Low-Dose Melittin Enhanced Pigment Production Through the Upregulation of Tyrosinase Activity and Dendricity in Melanocytes by Limiting Oxidative Stress: A Therapeutic Implication for Vitiligo. Antioxidants 2024, 13, 1424. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, A.; Wairkar, S. Management of hyperpigmentation: Current treatments and emerging therapies. Pigm. Cell Melanoma Res. 2021, 34, 1000–1014. [Google Scholar] [CrossRef] [PubMed]
- Rodboon, T.; Okada, S.; Suwannalert, P. Germinated riceberry rice enhanced protocatechuic acid and vanillic acid to suppress melanogenesis through cellular oxidant-related tyrosinase activity in B16 cells. Antioxidants 2020, 9, 247. [Google Scholar] [CrossRef]
- Wijayanti, E.D.; Safitri, A.; Siswanto, D.; Fatchiyah, F. Indonesian purple rice ferulic acid as a candidate for anti-aging through the inhibition of collagenase and tyrosinase activities. Indones. J. Chem. 2023, 23, 475–488. [Google Scholar] [CrossRef]
- Vardhani, A.; Jufri, M.; Purwaningsih, E. Potency of γ-Oryzanol rich black Rice bran (Oryza sativa L. indica) extract for Tyrosinase inhibition. Int. J. Pharm. Pharm. Sci. 2020, 12, 90–93. [Google Scholar] [CrossRef]
- Batubara, I.; Maharni, M.; Sadiah, S. The potency of white rice (Oryza sativa), black rice (Oryza sativa L. indica), and red rice (Oryza nivara) as antioxidant and tyrosinase inhibitor. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2017; p. 012017. [Google Scholar]
- Deng, G.F.; Xu, X.R.; Zhang, Y.; Li, D.; Gan, R.Y.; Li, H.B. Phenolic compounds and bioactivities of pigmented rice. Crit. Rev. Food Sci. Nutr. 2013, 53, 296–306. [Google Scholar] [CrossRef]
- Linsaenkart, P.; Ruksiriwanich, W.; Jantrawut, P.; Chittasupho, C.; Rachtanapun, P.; Jantanasakulwong, K.; Sommano, S.R.; Prom-U-Thai, C.; Jamjod, S.; Arjin, C. Natural melanogenesis inhibitor, antioxidant, and collagen biosynthesis stimulator of phytochemicals in rice bran and husk extracts from purple glutinous rice (Oryza sativa L. cv. Pieisu 1 CMU) for cosmetic application. Plants 2023, 12, 970. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, J.G.; Jang, Y.A.; Bayazid, A.B.; Ou Lim, B. Fermented black rice and blueberry with Lactobacillus plantarum MG4221 improve UVB-induced skin injury. Food Agric. Immunol. 2021, 32, 499–515. [Google Scholar] [CrossRef]
- Raab, S.; Yatskayer, M.; Lynch, S.; Manco, M.; Oresajo, C. Clinical Evaluation of a Multi-Modal Facial Serum That Addresses Hyaluronic Acid Levels in Skin. J. Drugs Dermatol. 2017, 16, 884–890. [Google Scholar]
- Sudjaroen, Y.; Kakatum, N.; Thongmuang, P. Evaluation of rice bran oil from riceberry rice (Oryza sativa L.) as active ingredient in skincare application. J. Pharm. Negat. Result. 2022, 13, 121–127. [Google Scholar]
- Jufri, M.; Vardhani, A.; Purwaningsih, E. Evaluating the efficacy of lotion containing black rice bran (Oryza sativa L. indica) extract as skin brightening agent: A clinical trial. Jundishapur J. Nat. Pharm. Prod. 2021, 16, e114152. [Google Scholar] [CrossRef]
- Palungwachira, P.; Tancharoen, S.; Phruksaniyom, C.; Klungsaeng, S.; Srichan, R.; Kikuchi, K.; Nararatwanchai, T. Antioxidant and anti-inflammatory properties of anthocyanins extracted from Oryza sativa L. in primary dermal fibroblasts. Oxid. Med. Cell. Longev. 2019, 2019, 2089817. [Google Scholar] [CrossRef]
- Sangkaew, O.; Yompakdee, C. Fermented unpolished black rice (Oryza sativa L.) inhibits melanogenesis via ERK, p38, and AKT phosphorylation in B16F10 melanoma cells. J. Microbiol. Biotechnol. 2020, 30, 1184. [Google Scholar] [CrossRef]


| No. | Eligibility Criteria |
|---|---|
| 1 | Article types not included, e.g., reviews, proceedings, features, editorial material |
| 2 | Not in the life sciences |
| 3 | Irrelevant object/topic |
| 4 | Full text not available |
| 5 | Not in English |
| Pigmented Rice Varieties | Preparation | Experiment Type | Methods | Animal/Cell | Target/Gene Regulation | Various Key Points | Ref. |
|---|---|---|---|---|---|---|---|
| Red jasmine rice bran | Extraction | In vitro | ROS detection, melanin inhibition test, collagen gene expression | HaCaT, HFF, and B16 | COL1A1 ↑ | The extract from red jasmine rice bran demonstrated the ability to mitigate ROS accumulation in keratinocytes induced by blue light exposure, as well as to inhibit melanin production triggered by blue light. | [75] |
| Red rice | Extraction | In vitro | Collagen synthesis, collagenase activity assay, MMP-2 activity assay, hyaluronic acid (HA) synthesis assay, melanin content detection, mushroom tyrosinase activity, cellular tyrosinase activity, DPPH free radical scavenging capacity | Primary human skin fibroblasts, B16-F10 | —— | Proanthocyanidin and catechin effectively inhibited collagenase and MMP-2, promoted collagen and hyaluronic acid synthesis in human fibroblasts, and reduced melanin content and tyrosinase activity in B16-F10 melanoma cells. They also showed strong DPPH radical scavenging activity. Oryzanol reduced melanin but did not affect tyrosinase activity and had a minimal impact on DPPH scavenging. Hydroxybenzoic acid, vanillic acid, and oryzanol did not influence collagenase or MMP-2, and compounds from red rice extract did not affect mushroom tyrosinase. | [26] |
| Red rice | Extraction | In vitro | DPPH, superoxide anion scavenging activity, tyrosinase inhibitory activity | RAW264.7, HGF, HaCaT | —— | The extract had good antioxidant and tyrosinase inhibition activities. | [11] |
| Red rice | Fermentation | In vitro | qPCR, ROS detection assay, 3D epidermal model moisture content test, melanin content test | HaCaT, 3D epidermal model, human primary melanocytes (MCs), human dermal fibroblasts (FB) | mRNA: Aquaporin 3 (AQP3) ↑, Filaggrin (FLG) ↑, Hyaluronan Synthase 1 (HAS1) ↑, Claudin 1 (CLDN1) ↑, Involucrin (IVL) ↑, Zonula Occludens-1 (ZO-1) ↑ | Anthocyanins inhibit collagen degradation and scavenge free radicals. | [3] |
| Thai red Hom–Kularb–Drice (HKD) rice bran | Extraction | In vitro | DPPH assay, ELISA | Primary Human skin fibroblasts | MMP-1 ↓, type I procollagen protein ↑ | The HKD extract at a concentration of 20 µg/mL exhibited protective effects in UVB-irradiated primary skin fibroblasts, evidenced by a reduction in MMP-1 expression and an enhancement in type I procollagen production. | [91] |
| Purple rice | Extraction | In clinic | qPCR, Bioinstrumentation measurements were taken, including corneometer, tewameter, ultrasound, and standardized digital imaging | —— | hyaluronan synthase 2 ↑, collagen type 1a1 ↑ | The skin showed a marked increase in HA content following 4 weeks of treatment. | [103] |
| Purple Glutinous Rice (Oryza sativa L. cv. Pieisu 1 CMU) (PES1CMU-DFRB) | Extraction | In vitro | Tyrosinase activity, melanin content test, DPPH, ABTS, collagen-stimulating effect, MDA | B16 melanoma, fibroblast cells | MMP-2 ↓ | Diminishes the activity of the tyrosinase enzyme responsible for a melanogenesis inhibitor as a skin-whitening agent. PES1CMU-DFRB illustrated impressive antioxidant capacities against DPPH, ABTS radicals, and malondialdehyde production. Reduces melanin production, protects the lipid membrane of fibroblasts, and decreases the destruction of collagen. | [101] |
| Purple rice (riceberry rice), rice bran oil (RBO) | Extraction | In vitro | DPPH, NO radical scavenging activity, anti-elastase enzyme, anti-tyrosinase activity, wound healing, antimicrobial activity | RAW264.7, human skin fibroblast cells | —— | RBO demonstrated antioxidant properties through the scavenging of DPPH and NO radicals, as well as anti-inflammatory effects by reducing NO radical production in LPS-induced macrophage cells. RBO marginally stimulated skin cell proliferation without exhibiting toxicity at concentrations ranging from 0.0001 to 0.1 mg/mL; however, a concentration of 1 mg/mL was found to be cytotoxic. RBO did not inhibit tyrosinase or elastase enzyme activities. Furthermore, no wound healing was observed following the incubation of RBO with scratched human skin fibroblast cells. | [104] |
| Purple rice and ferulic acid | Extraction | In vitro | Ferric reducing antioxidant potential (FRAP), inhibiting collagenase and tyrosinase activity, tyrosinase inhibitory activity | —— | —— | IPR demonstrated potent reducing power, anti-collagenase, and anti-tyrosinase activity. | [97] |
| Fermented black rice and blueberry with Lactobacillusplantarum | Fermentation | In vitro | DPPH and ABTS radical scavenging activity, ferric-reducing antioxidant power, prevention of oxidative DNA damage, measurement of intracellular ROS production, Western blot, qPCR, measurement of skin moisture, serum biochemical analysis, histological analysis | HaCaT, hairless mice | FLG ↑, TGM ↑, MMP-9 ↓, COL1A1 ↑, INV ↑, TGM ↑ | The fermented mixture significantly reduced DPPH and ABTS radicals, FBBBR inhibited both extracellular and intracellular free radicals, and the declining presence of these two enzymes (caspase-3 and PARP) indicated that FBBBR protected cells from apoptosis by regulating the caspase pathway; FBBBR enhances skin barrier function by modulating the expression of FLG, TGM, MMP-9, and COL1A1, thereby preventing UVB-induced collagen breakdown and moisture depletion in HaCaT cells. | [102] |
| Black rice bran (BRB) | fermentation | In vitro | DPPH radical scavenging activity, Tyrosinase inhibitory activity | —— | —— | Fungal fermentation was effective in enhancing the antioxidant activity of BRB | [25] |
| Black rice (Oryza sativa L.) | Extraction | In vitro | Western, qPCR, ROS detection | HaCaT, primary HDF | MMP-1 ↓, AP-1 (c-Jun/c-Fos) ↓, ERK ↓, JNK ↓, and p38 ↓ | BRE mitigates indicators of photoaging, such as the reduction in collagen and the elevation of MMPs in skin cells. The underlying mechanism contributing to these advantageous effects may involve the inhibition of ROS generation and AP-1 activation in vitro. | [23] |
| Black Rice Bran (Oryza sativa L. indica) | Extraction | In clinic | Measurement of Skin Brightness and Erythema Level | —— | —— | It effectively reduced skin melanin production | [105] |
| Pigmented rice (four red rice and one black rice) | Extraction | In vitro | DPPH, ABTS | —— | —— | The results confirmed that the content of total phenolics and the flavonoid content, as well as the antioxidant capacity (DPPH and ABTS assays) of pigmented rice, was several-fold greater than non-pigmented ones (4, 4, 3, and 5 times, respectively). | [19] |
| Anthocyanins (ANT) from Black rice (Oryza sativa L.) | Extraction | In vitro | Copper ion reduction activity, qPCR, cell migration assay, total collagen estimation, Western blot, immunofluorescence staining, ELISA | Rat primary dermal fibroblasts (RDFs) | mRNA expression of COL1A2 ↑ and type I collagen protein levels ↑ | ANT enhanced the migration of rat RDFs and showed antioxidant effects. It boosted the mRNA expression of collagen type I alpha 2 (COL1A2) and increased type I collagen protein levels in H2O2-stimulated RDFs without causing cytotoxicity. Compared to untreated cells, ANT treatment in the presence of H2O2 activated ERK1/2 and Akt signaling pathways, while significantly inhibiting IκBα phosphorylation and suppressing the activation of NF-κB subunits p50 and p65, which are linked to inflammation. | [106] |
| Black glutinous rice (Oryza sativa var. glutinosa) | Extraction | In vitro | Sun protection factor (SPF) assay | —— | —— | Anti-UV activity is demonstrated by the SPF value, with higher doses producing higher SPF values. | [74] |
| Fermented unpolished black rice (Oryza sativa L.) (FUBR) | Fermentation | In vitro | DPPH, melanin content test, qPCR, intracellular tyrosinase activity, Western blot | B16F10, Hs68 | mRNA and protein expression levels of tyrosinase, tyrosinase-related protein 1 (TYRP-1) ↓, TYRP-2 ↓, and microphthalmia-associated transcription factor ↓, phosphorylated ERK ↑, p38 ↑, and Akt ↑ | Decreased cellular tyrosinase activity by FUBRS, decreased the expression level of melanogenesis-related proteins by FUBRS, and induced the phosphorylation of the Erk1/2, p38, and Akt signaling pathways by FUBRS. | [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Zuo, H.-L.; Liu, Y.; Huang, H.-Y.; Li, S.-F.; Li, J.; Li, L.-P.; Chen, Y.-G.; Lin, T.-S.; Huang, S.-H.; et al. Mechanistic Insights into Pigmented Rice Bran in Mitigating UV-Induced Oxidative Stress, Inflammation, and Pigmentation. Cosmetics 2025, 12, 51. https://doi.org/10.3390/cosmetics12020051
Zhang T, Zuo H-L, Liu Y, Huang H-Y, Li S-F, Li J, Li L-P, Chen Y-G, Lin T-S, Huang S-H, et al. Mechanistic Insights into Pigmented Rice Bran in Mitigating UV-Induced Oxidative Stress, Inflammation, and Pigmentation. Cosmetics. 2025; 12(2):51. https://doi.org/10.3390/cosmetics12020051
Chicago/Turabian StyleZhang, Tao, Hua-Li Zuo, Yue Liu, Hsi-Yuan Huang, Shang-Fu Li, Jing Li, Li-Ping Li, Yi-Gang Chen, Ting-Syuan Lin, Sheng-Han Huang, and et al. 2025. "Mechanistic Insights into Pigmented Rice Bran in Mitigating UV-Induced Oxidative Stress, Inflammation, and Pigmentation" Cosmetics 12, no. 2: 51. https://doi.org/10.3390/cosmetics12020051
APA StyleZhang, T., Zuo, H.-L., Liu, Y., Huang, H.-Y., Li, S.-F., Li, J., Li, L.-P., Chen, Y.-G., Lin, T.-S., Huang, S.-H., Lin, Y.-C.-D., & Huang, H.-D. (2025). Mechanistic Insights into Pigmented Rice Bran in Mitigating UV-Induced Oxidative Stress, Inflammation, and Pigmentation. Cosmetics, 12(2), 51. https://doi.org/10.3390/cosmetics12020051










