Genomic Markers and Personalized Medicine in Androgenetic Alopecia: A Comprehensive Review
Abstract
1. Introduction
2. Androgenetic Alopecia
2.1. Genetics and Androgenetic Alopecia
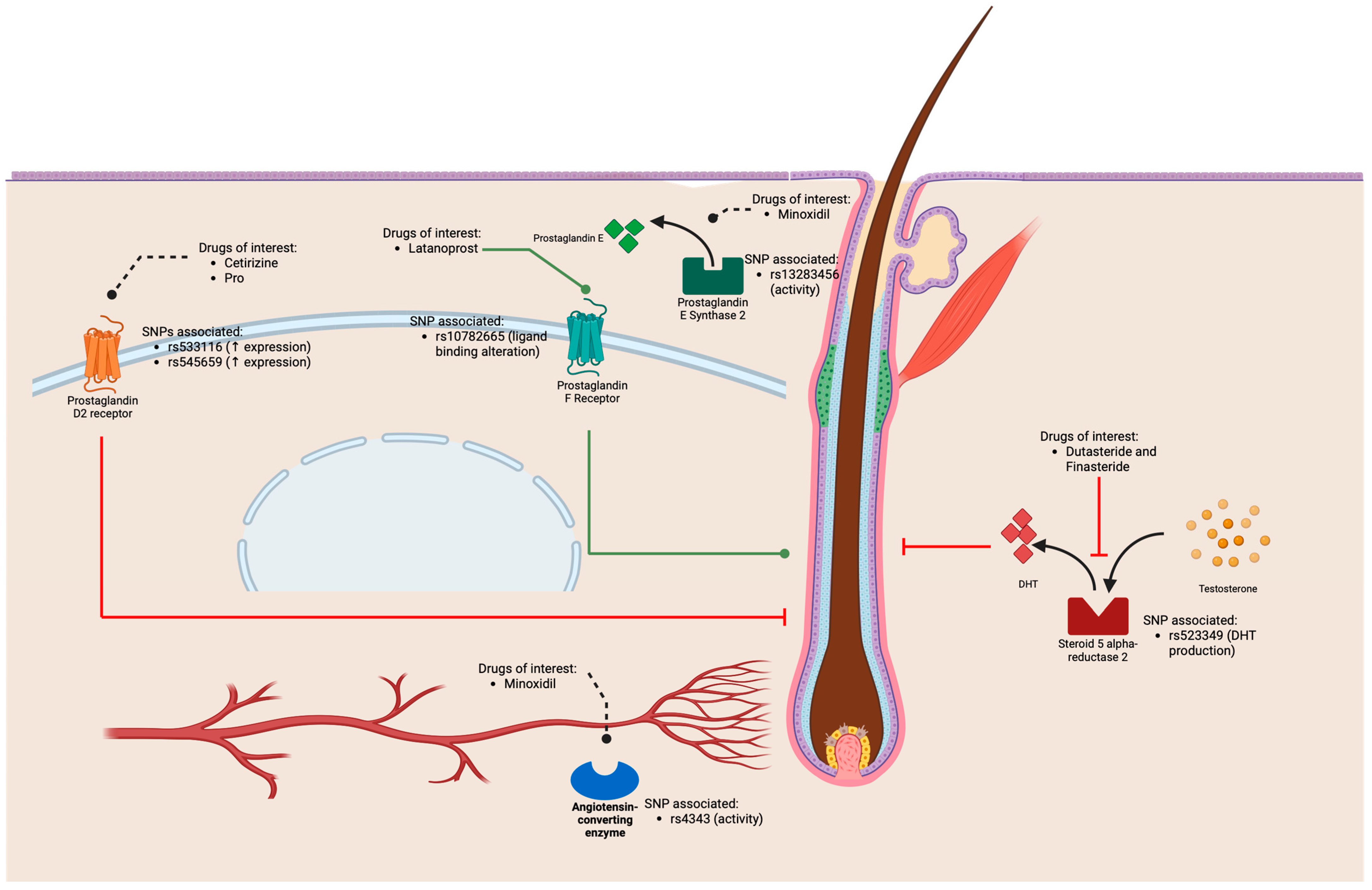
2.2. Pharmacological Therapy of Androgenetic Alopecia
2.3. Potential Use of Pharmacogenetics to Treat Androgenetic Alopecia
3. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Francès, M.P.; Vila-Vecilla, L.; Russo, V.; Caetano Polonini, H.; de Souza, G.T. Utilising SNP Association Analysis as a Prospective Approach for Personalising Androgenetic Alopecia Treatment. Dermatol. Ther. 2024, 14, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. Molecular Mechanisms of Androgenetic Alopecia. Exp. Gerontol. 2002, 37, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M.; Dias, M.F.R.G. Alopecia Areata: A Comprehensive Review of Pathogenesis and Management. Clin. Rev. Allergy Immunol. 2018, 54, 68–87. [Google Scholar] [CrossRef]
- Lolli, F.; Pallotti, F.; Rossi, A.; Fortuna, M.C.; Caro, G.; Lenzi, A.; Sansone, A.; Lombardo, F. Androgenetic Alopecia: A Review. Endocrine 2017, 57, 9–17. [Google Scholar] [CrossRef]
- Ho, B.S.-Y.; Vaz, C.; Ramasamy, S.; Chew, E.G.Y.; Mohamed, J.S.; Jaffar, H.; Hillmer, A.; Tanavde, V.; Bigliardi-Qi, M.; Bigliardi, P.L. Progressive Expression of PPARGC1α Is Associated with Hair Miniaturization in Androgenetic Alopecia. Sci. Rep. 2019, 9, 8771. [Google Scholar] [CrossRef]
- Adil, A.; Godwin, M. The Effectiveness of Treatments for Androgenetic Alopecia: A Systematic Review and Meta-Analysis. J. Am. Acad. Dermatol. 2017, 77, 136–141.e5. [Google Scholar] [CrossRef]
- Heilmann, S.; Kiefer, A.K.; Fricker, N.; Drichel, D.; Hillmer, A.M.; Herold, C.; Tung, J.Y.; Eriksson, N.; Redler, S.; Betz, R.C.; et al. Androgenetic Alopecia: Identification of Four Genetic Risk Loci and Evidence for the Contribution of WNT Signaling to Its Etiology. J. Investig. Dermatol. 2013, 133, 1489–1496. [Google Scholar] [CrossRef]
- Kaufman, K.D.; Olsen, E.A.; Whiting, D.; Savin, R.; DeVillez, R.; Bergfeld, W.; Price, V.H.; Van Neste, D.; Roberts, J.L.; Hordinsky, M.; et al. Finasteride in the Treatment of Men with Androgenetic Alopecia. J. Am. Acad. Dermatol. 1998, 39, 578–589. [Google Scholar] [CrossRef]
- Lee, M.J.; Cha, H.J.; Lim, K.M.; Lee, O.-K.; Bae, S.; Kim, C.-H.; Lee, K.-H.; Lee, Y.N.; Ahn, K.J.; An, S. Analysis of the MicroRNA Expression Profile of Normal Human Dermal Papilla Cells Treated with 5α-Dihydrotestosterone. Mol. Med. Rep. 2015, 12, 1205–1212. [Google Scholar] [CrossRef]
- Messenger, A.G.; Rundegren, J. Minoxidil: Mechanisms of Action on Hair Growth. Br. J. Dermatol. 2004, 150, 186–194. [Google Scholar] [CrossRef]
- Feldman, P.R.; Gentile, P.; Piwko, C.; Motswaledi, H.M.; Gorun, S.; Pesachov, J.; Markel, M.; Silver, M.I.; Brenkel, M.; Feldman, O.J.; et al. Hair Regrowth Treatment Efficacy and Resistance in Androgenetic Alopecia: A Systematic Review and Continuous Bayesian Network Meta-Analysis. Front. Med. 2023, 9, 998623. [Google Scholar] [CrossRef] [PubMed]
- Garza, L.A.; Liu, Y.; Yang, Z.; Alagesan, B.; Lawson, J.A.; Norberg, S.M.; Loy, D.E.; Zhao, T.; Blatt, H.B.; Stanton, D.C.; et al. Prostaglandin D 2 Inhibits Hair Growth and Is Elevated in Bald Scalp of Men with Androgenetic Alopecia. Sci. Transl. Med. 2012, 4, 126ra34. [Google Scholar] [CrossRef] [PubMed]
- Rundegren, J. A One-Year Observational Study with Minoxidil 5% Solution in Germany: Results of Independent Efficacy Evaluation by Physicians and Patients. J. Am. Acad. Dermatol. 2004, 50, P91. [Google Scholar] [CrossRef]
- Suchonwanit, P.; Thammarucha, S.; Leerunyakul, K. Minoxidil and Its Use in Hair Disorders: A Review. Drug Des. Devel. Ther. 2019, 13, 2777–2786. [Google Scholar] [CrossRef]
- Nieves, A.; Garza, L.A. Does Prostaglandin D2 Hold the Cure to Male Pattern Baldness? Exp. Dermatol. 2014, 23, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Devjani, S.; Ezemma, O.; Kelley, K.J.; Stratton, E.; Senna, M. Androgenetic Alopecia: Therapy Update. Drugs 2023, 83, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Betz, R.C.; Petukhova, L.; Ripke, S.; Huang, H.; Menelaou, A.; Redler, S.; Becker, T.; Heilmann, S.; Yamany, T.; Duvic, M.; et al. Genome-Wide Meta-Analysis in Alopecia Areata Resolves HLA Associations and Reveals Two New Susceptibility Loci. Nat. Commun. 2015, 6, 5966. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Brockschmidt, F.F.; Kiefer, A.K.; Stefansson, H.; Nyholt, D.R.; Song, K.; Vermeulen, S.H.; Kanoni, S.; Glass, D.; Medland, S.E.; et al. Six Novel Susceptibility Loci for Early-Onset Androgenetic Alopecia and Their Unexpected Association with Common Diseases. PLoS Genet. 2012, 8, e1002746. [Google Scholar] [CrossRef]
- Kaiser, M.; Abdin, R.; Gaumond, S.I.; Issa, N.T.; Jimenez, J.J. Treatment of Androgenetic Alopecia: Current Guidance and Unmet Needs. Clin. Cosmet. Investig. Dermatol. 2023, 16, 1387–1406. [Google Scholar] [CrossRef] [PubMed]
- Aukerman, E.L.; Jafferany, M. The Psychological Consequences of Androgenetic Alopecia: A Systematic Review. J. Cosmet. Dermatol. 2023, 22, 89–95. [Google Scholar] [CrossRef]
- Kidangazhiathmana, A.; Santhosh, P. Pathogenesis of Androgenetic Alopecia. Clin. Dermatol. Rev. 2022, 6, 69. [Google Scholar] [CrossRef]
- Hamilton, J.B. Patterned Loss of Hair in Man: Types and Incidence. Ann. N. Y. Acad. Sci. 1951, 53, 708–728. [Google Scholar] [CrossRef]
- Salman, K.E.; Altunay, I.K.; Kucukunal, N.A.; Cerman, A.A. Frequency, Severity and Related Factors of Androgenetic Alopecia in Dermatology Outpatient Clinic: Hospital-Based Cross-Sectional Study in Turkey. An. Bras. Dermatol. 2017, 92, 35–40. [Google Scholar] [CrossRef]
- Martinez-Jacobo, L.; Villarreal-Villarreal, C.; Ortiz-López, R.; Ocampo-Candiani, J.; Rojas-Martínez, A. Genetic and Molecular Aspects of Androgenetic Alopecia. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 263. [Google Scholar] [CrossRef]
- Heilmann-Heimbach, S.; Herold, C.; Hochfeld, L.M.; Hillmer, A.M.; Nyholt, D.R.; Hecker, J.; Javed, A.; Chew, E.G.Y.; Pechlivanis, S.; Drichel, D.; et al. Meta-Analysis Identifies Novel Risk Loci and Yields Systematic Insights into the Biology of Male-Pattern Baldness. Nat. Commun. 2017, 8, 14694. [Google Scholar] [CrossRef]
- Hillmer, A.M.; Hanneken, S.; Ritzmann, S.; Becker, T.; Freudenberg, J.; Brockschmidt, F.F.; Flaquer, A.; Freudenberg-Hua, Y.; Jamra, R.A.; Metzen, C.; et al. Genetic Variation in the Human Androgen Receptor Gene Is the Major Determinant of Common Early-Onset Androgenetic Alopecia. Am. J. Hum. Genet. 2005, 77, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hysi, P.; Maj, C.; Heilmann-Heimbach, S.; Spector, T.D.; Liu, F.; Kayser, M. Genetic Prediction of Male Pattern Baldness Based on Large Independent Datasets. Eur. J. Hum. Genet. 2023, 31, 321–328. [Google Scholar] [CrossRef]
- Hagenaars, S.P.; Hill, W.D.; Harris, S.E.; Ritchie, S.J.; Davies, G.; Liewald, D.C.; Gale, C.R.; Porteous, D.J.; Deary, I.J.; Marioni, R.E. Genetic Prediction of Male Pattern Baldness. PLoS Genet. 2017, 13, e1006594. [Google Scholar] [CrossRef]
- Pirastu, N.; Joshi, P.K.; de Vries, P.S.; Cornelis, M.C.; McKeigue, P.M.; Keum, N.; Franceschini, N.; Colombo, M.; Giovannucci, E.L.; Spiliopoulou, A.; et al. GWAS for Male-Pattern Baldness Identifies 71 Susceptibility Loci Explaining 38% of the Risk. Nat. Commun. 2017, 8, 1584. [Google Scholar] [CrossRef]
- Yap, C.X.; Sidorenko, J.; Wu, Y.; Kemper, K.E.; Yang, J.; Wray, N.R.; Robinson, M.R.; Visscher, P.M. Dissection of Genetic Variation and Evidence for Pleiotropy in Male Pattern Baldness. Nat. Commun. 2018, 9, 5407. [Google Scholar] [CrossRef]
- Ellis, J.A.; Stebbing, M.; Harrap, S.B. Genetic Analysis of Male Pattern Baldness and the 5α-Reductase Genes. J. Investig. Dermatol. 1998, 110, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Hillmer, A.M.; Flaquer, A.; Hanneken, S.; Eigelshoven, S.; Kortüm, A.-K.; Brockschmidt, F.F.; Golla, A.; Metzen, C.; Thiele, H.; Kolberg, S.; et al. Genome-Wide Scan and Fine-Mapping Linkage Study of Androgenetic Alopecia Reveals a Locus on Chromosome 3q26. Am. J. Hum. Genet. 2008, 82, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, F.L.; Xu, W.; Wang, L.; Wu, Y.; Xu, Z.L.; Zhao, J.Y. Androgen Receptor Gene Polymorphisms and Risk for Androgenetic Alopecia: A Meta-Analysis. Clin. Exp. Dermatol. 2012, 37, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Levy-Nissenbaum, E.; Bar-Natan, M.; Frydman, M. Elon Pras Confirmation of the Association between Male Pattern Baldness and the Androgen Receptor Gene. Eur. J. Dermatol. 2005, 15, 339–340. [Google Scholar] [PubMed]
- Prodi, D.A.; Pirastu, N.; Maninchedda, G.; Sassu, A.; Picciau, A.; Palmas, M.A.; Mossa, A.; Persico, I.; Adamo, M.; Angius, A.; et al. EDA2R Is Associated with Androgenetic Alopecia. J. Investig. Dermatol. 2008, 128, 2268–2270. [Google Scholar] [CrossRef]
- Shin, D.W. The Physiological and Pharmacological Roles of Prostaglandins in Hair Growth. Korean J. Physiol. Pharmacol. 2022, 26, 405–413. [Google Scholar] [CrossRef]
- Nitz, I.; Fisher, E.; Grallert, H.; Li, Y.; Gieger, C.; Rubin, D.; Boeing, H.; Spranger, J.; Lindner, I.; Schreiber, S.; et al. Association of Prostaglandin E Synthase 2 (PTGES2) Arg298His Polymorphism with Type 2 Diabetes in Two German Study Populations. J. Clin. Endocrinol. Metab. 2007, 92, 3183–3188. [Google Scholar] [CrossRef]
- Li, Y.; Yang, S.; Liao, M.; Zheng, Z.; Li, M.; Wei, X.; Liu, M.; Yang, L. Association between Genetically Predicted Leukocyte Telomere Length and Non-Scarring Alopecia: A Two-Sample Mendelian Randomization Study. Front. Immunol. 2023, 13, 1072573. [Google Scholar] [CrossRef]
- Foulquier, S.; Daskalopoulos, E.P.; Lluri, G.; Hermans, K.C.M.; Deb, A.; Blankesteijn, W.M. WNT Signaling in Cardiac and Vascular Disease. Pharmacol. Rev. 2018, 70, 68–141. [Google Scholar] [CrossRef]
- Choi, B.Y. Targeting Wnt/β-Catenin Pathway for Developing Therapies for Hair Loss. Int. J. Mol. Sci. 2020, 21, 4915. [Google Scholar] [CrossRef]
- Shin, D.W. The Molecular Mechanism of Natural Products Activating Wnt/β-Catenin Signaling Pathway for Improving Hair Loss. Life 2022, 12, 1856. [Google Scholar] [CrossRef] [PubMed]
- Lekven, A.C.; Lilie, C.J.; Gibbs, H.C.; Green, D.G.; Singh, A.; Yeh, A.T. Analysis of the Wnt1 Regulatory Chromosomal Landscape. Dev. Genes Evol. 2019, 229, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, Y.; Agalliu, D.; Vonica, A.; Luria, V.; Wajid, M.; Baumer, A.; Belli, S.; Petukhova, L.; Schinzel, A.; Brivanlou, A.H.; et al. APCDD1 Is a Novel Wnt Inhibitor Mutated in Hereditary Hypotrichosis Simplex. Nature 2010, 464, 1043–1047. [Google Scholar] [CrossRef]
- Kumar, A.; Girisa, S.; Alqahtani, M.S.; Abbas, M.; Hegde, M.; Sethi, G.; Kunnumakkara, A.B. Targeting Autophagy Using Long Non-Coding RNAs (LncRNAs): New Landscapes in the Arena of Cancer Therapeutics. Cells 2023, 12, 810. [Google Scholar] [CrossRef]
- Noto, M.; Noguchi, N.; Ishimura, A.; Kiyonari, H.; Abe, T.; Suzuki, T.; Hasunuma, N.; Taira, M.; Manabe, M.; Osada, S.-I. Sox13 Is a Novel Early Marker for Hair Follicle Development. Biochem. Biophys. Res. Commun. 2019, 509, 862–868. [Google Scholar] [CrossRef]
- Brockschmidt, F.F.; Heilmann, S.; Ellis, J.A.; Eigelshoven, S.; Hanneken, S.; Herold, C.; Moebus, S.; Alblas, M.A.; Lippke, B.; Kluck, N.; et al. Susceptibility Variants on Chromosome 7p21.1 Suggest HDAC9 as a New Candidate Gene for Male-Pattern Baldness. Br. J. Dermatol. 2011, 165, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Cauhe, J.; Vaño-Galvan, S.; Mehta, N.; Hermosa-Gelbard, A.; Ortega-Quijano, D.; Buendia-Castaño, D.; Fernández-Nieto, D.; Porriño-Bustamante, M.; Saceda-Corralo, D.; Pindado-Ortega, C.; et al. Hair Follicle Sulfotransferase Activity and Effectiveness of Oral Minoxidil in Androgenetic Alopecia. J. Cosmet. Dermatol. 2024. [Google Scholar] [CrossRef]
- Siegel, P.M.; Shu, W.; Cardiff, R.D.; Muller, W.J.; Massagué, J. Transforming Growth Factor β Signaling Impairs Neu-Induced Mammary Tumorigenesis While Promoting Pulmonary Metastasis. Proc. Natl. Acad. Sci. USA 2003, 100, 8430–8435. [Google Scholar] [CrossRef]
- Dijkers, P.F.; Birkenkamp, K.U.; Lam, E.W.-F.; Thomas, N.S.B.; Lammers, J.-W.J.; Koenderman, L.; Coffer, P.J. FKHR-L1 Can Act as a Critical Effector of Cell Death Induced by Cytokine Withdrawal. J. Cell Biol. 2002, 156, 531–542. [Google Scholar] [CrossRef]
- Shin, W.; Rosin, N.L.; Sparks, H.; Sinha, S.; Rahmani, W.; Sharma, N.; Workentine, M.; Abbasi, S.; Labit, E.; Stratton, J.A.; et al. Dysfunction of Hair Follicle Mesenchymal Progenitors Contributes to Age-Associated Hair Loss. Dev. Cell 2020, 53, 185–198.e7. [Google Scholar] [CrossRef]
- Raveh, E.; Cohen, S.; Levanon, D.; Groner, Y.; Gat, U. Runx3 Is Involved in Hair Shape Determination. Dev. Dyn. 2005, 233, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Premanand, A.; Reena Rajkumari, B. Bioinformatic Analysis of Gene Expression Data Reveals Src Family Protein Tyrosine Kinases as Key Players in Androgenetic Alopecia. Front. Med. 2023, 10, 1108358. [Google Scholar] [CrossRef]
- Boudjadi, S.; Chatterjee, B.; Sun, W.; Vemu, P.; Barr, F.G. The Expression and Function of PAX3 in Development and Disease. Gene 2018, 666, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Henne, S.K.; Aldisi, R.; Sivalingam, S.; Hochfeld, L.M.; Borisov, O.; Krawitz, P.M.; Maj, C.; Nöthen, M.M.; Heilmann-Heimbach, S. Analysis of 72,469 UK Biobank Exomes Links Rare Variants to Male-Pattern Hair Loss. Nat. Commun. 2023, 14, 5492. [Google Scholar] [CrossRef]
- Păun, M.; Torres, G.; Țiplica, G.S.; Cauni, V.M. Epidemiologic Study of Gene Distribution in Romanian and Brazilian Patients with Non-Cicatricial Alopecia. Medicina 2023, 59, 1654. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Santas, M.; Diaz-Guimaraens, B.; Saceda-Corralo, D.; Hermosa-Gelbard, A.; Muñoz-Moreno Arrones, O.; Pindado-Ortega, C.; Fernandez-Nieto, D.; Jimenez-Cauhe, J.; Ortega-Quijano, D.; Suarez-Valle, A.; et al. The State-of-the-Art in the Management of Androgenetic Alopecia: A Review of New Therapies and Treatment Algorithms. JEADV Clin. Pract. 2022, 1, 176–185. [Google Scholar] [CrossRef]
- Kanti, V.; Messenger, A.; Dobos, G.; Reygagne, P.; Finner, A.; Blumeyer, A.; Trakatelli, M.; Tosti, A.; del Marmol, V.; Piraccini, B.M.; et al. Evidence-Based (S3) Guideline for the Treatment of Androgenetic Alopecia in Women and in Men—Short Version. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 11–22. [Google Scholar] [CrossRef]
- Zhang, X.; Ji, Y.; Zhou, M.; Zhou, X.; Xie, Y.; Zeng, X.; Shao, F.; Zhang, C. Platelet-Rich Plasma for Androgenetic Alopecia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Cutan. Med. Surg. 2023, 27, 504–508. [Google Scholar] [CrossRef]
- Dhurat, R.; Sharma, A.; Rudnicka, L.; Kroumpouzos, G.; Kassir, M.; Galadari, H.; Wollina, U.; Lotti, T.; Golubovic, M.; Binic, I.; et al. 5-Alpha Reductase Inhibitors in Androgenetic Alopecia: Shifting Paradigms, Current Concepts, Comparative Efficacy, and Safety. Dermatol. Ther. 2020, 33, e13379. [Google Scholar] [CrossRef]
- Giordano, S.; Romeo, M.; Lankinen, P. Platelet-rich Plasma for Androgenetic Alopecia: Does It Work? Evidence from Meta Analysis. J. Cosmet. Dermatol. 2017, 16, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Nestor, M.S.; Ablon, G.; Gade, A.; Han, H.; Fischer, D.L. Treatment Options for Androgenetic Alopecia: Efficacy, Side Effects, Compliance, Financial Considerations, and Ethics. J. Cosmet. Dermatol. 2021, 20, 3759–3781. [Google Scholar] [CrossRef] [PubMed]
- Roden, D.M.; McLeod, H.L.; Relling, M.V.; Williams, M.S.; Mensah, G.A.; Peterson, J.F.; Van Driest, S.L. Pharmacogenomics. Lancet 2019, 394, 521–532. [Google Scholar] [CrossRef]
- Xiao, Q.; Wang, L.; Supekar, S.; Shen, T.; Liu, H.; Ye, F.; Huang, J.; Fan, H.; Wei, Z.; Zhang, C. Structure of Human Steroid 5α-Reductase 2 with the Anti-Androgen Drug Finasteride. Nat. Commun. 2020, 11, 5430. [Google Scholar] [CrossRef]
- Li, X.; Huang, Y.; Fu, X.; Chen, C.; Zhang, D.; Yan, L.; Xie, Y.; Mao, Y.; Li, Y. Meta-Analysis of Three Polymorphisms in the Steroid-5-Alpha-Reductase, Alpha Polypeptide 2 Gene (SRD5A2) and Risk of Prostate Cancer. Mutagenesis 2011, 26, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Hayes, V.M.; Severi, G.; Padilla, E.J.D.; Morris, H.A.; Tilley, W.D.; Southey, M.C.; English, D.R.; Sutherland, R.L.; Hopper, J.L.; Boyle, P.; et al. 5α-Reductase Type 2 Gene Variant Associations with Prostate Cancer Risk, Circulating Hormone Levels and Androgenetic Alopecia. Int. J. Cancer 2007, 120, 776–780. [Google Scholar] [CrossRef]
- Zeng, X.-T.; Su, X.-J.; Li, S.; Weng, H.; Liu, T.-Z.; Wang, X.-H. Association between SRD5A2 Rs523349 and Rs9282858 Polymorphisms and Risk of Benign Prostatic Hyperplasia: A Meta-Analysis. Front. Physiol. 2017, 8, 688. [Google Scholar] [CrossRef]
- Chen, X.; Xiang, H.; Yang, M. Topical Cetirizine for Treating Androgenetic Alopecia: A Systematic Review. J. Cosmet. Dermatol. 2022, 21, 5519–5526. [Google Scholar] [CrossRef]
- Moon, I.J.; Yoon, H.K.; Kim, D.; Choi, M.E.; Han, S.H.; Park, J.H.; Hong, S.W.; Cho, H.; Lee, D.K.; Won, C.H. Efficacy of Asymmetric SiRNA Targeting Androgen Receptors for the Treatment of Androgenetic Alopecia. Mol. Pharm. 2023, 20, 128–135. [Google Scholar] [CrossRef]
- Yun, S.I.; Lee, S.K.; Goh, E.A.; Kwon, O.S.; Choi, W.; Kim, J.; Lee, M.S.; Choi, S.J.; Lim, S.S.; Moon, T.K.; et al. Weekly Treatment with SAMiRNA Targeting the Androgen Receptor Ameliorates Androgenetic Alopecia. Sci. Rep. 2022, 12, 1607. [Google Scholar] [CrossRef] [PubMed]
| Gene | SNP | Chromosome Location | Functional Role | Study References |
|---|---|---|---|---|
| AR | rs12558842 | X | Major determinant of AGA; affects androgen metabolism | [31,32,33,34,35] |
| AR | rs2497938 | X | Influences severity of hair loss through androgen receptor activity | [26,34,36,37] |
| SRD5A2 | rs523349 | 2p23.1 | Encodes 5α-reductase type 2, converts testosterone to DHT | [9,38,39] |
| EDA2R | rs1385699 | 1p36.12 | Associated with increased risk of AGA, involved in hair follicle development | [33,36,40,41] |
| PTGDS | rs245324 | 9q34.2 | Involved in prostaglandin pathways affecting hair growth | [1,12,15,42,43,44] |
| WNT10A | rs7349332 | 2q35 | Implicated in hair follicle development through WNT signaling pathway | [1,7,33,38,44,45,46,47,48,49] |
| APCDD1 | rs7349333 | 18p11.22 | Inhibits WNT signaling, affecting hair follicle growth and cycling | [39,42,49] |
| LINC01475 | rs7349341 | 6q23.2 | Non-coding RNA influencing hair follicle function | [27,30,50] |
| HDAC9 | rs12021639 | 7p21.1 | Histone deacetylase affecting gene expression in hair follicles | [38,51,52] |
| SULT2A1 | rs11085258 | 19q13.3 | Sulfotransferase involved in DHT metabolism | [30,38,53] |
| TERT | rs2736098 | 5p15.33 | Involved in telomere maintenance, associated with cellular aging and hair loss | [31,38] |
| TGFBR2 | rs6784615 | 3p24.1 | Transforming growth factor-beta receptor, involved in cell growth and differentiation | [34,54] |
| FOXO1 | rs4946936 | 13q14.11 | Forkhead box protein O1, involved in cell cycle regulation and apoptosis | [55] |
| RUNX3 | rs2456449 | 1p36.11 | Involved in hair follicle morphogenesis | [56,57,58,59] |
| PAX3 | rs619847 | 2q36.1 | Paired box 3, involved in early development of hair follicles | [58] |
| SOX13 | rs7717630 | 1q32.1 | SRY-box 13, transcription factor involved in hair growth | [38,51] |
| MUC1 | rs4072037 | 1q22 | Mucin 1, cell surface-associated, involved in cell adhesion and signaling | [1,31] |
| ACE | rs4343 | 17q23.3 | Angiotensin I-converting enzyme, involved in blood pressure regulation | [60] |
| COL1A1 | rs1800012 | 17q21.33 | Collagen type I alpha 1 chain, involved in collagen structure | [1,7,25] |
| PTGFR | rs10782665 | 1p31.1 | Prostaglandin F receptor, involved in prostaglandin signaling | [55] |
| EBF1 | rs17643057 | 5q33.3 | Involved in B-cell development | [25,31] |
| GPR44 | rs533116 | 9q34.3 | Prostaglandin D2 receptor, involved in hair growth inhibition | [1,11] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vila-Vecilla, L.; Russo, V.; de Souza, G.T. Genomic Markers and Personalized Medicine in Androgenetic Alopecia: A Comprehensive Review. Cosmetics 2024, 11, 148. https://doi.org/10.3390/cosmetics11050148
Vila-Vecilla L, Russo V, de Souza GT. Genomic Markers and Personalized Medicine in Androgenetic Alopecia: A Comprehensive Review. Cosmetics. 2024; 11(5):148. https://doi.org/10.3390/cosmetics11050148
Chicago/Turabian StyleVila-Vecilla, Laura, Valentina Russo, and Gustavo Torres de Souza. 2024. "Genomic Markers and Personalized Medicine in Androgenetic Alopecia: A Comprehensive Review" Cosmetics 11, no. 5: 148. https://doi.org/10.3390/cosmetics11050148
APA StyleVila-Vecilla, L., Russo, V., & de Souza, G. T. (2024). Genomic Markers and Personalized Medicine in Androgenetic Alopecia: A Comprehensive Review. Cosmetics, 11(5), 148. https://doi.org/10.3390/cosmetics11050148





