Bleached Hair as Standard Template to Insight the Performance of Commercial Hair Repair Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Hair Type
2.2. Commercial Repair Products
2.3. Hair Treatment
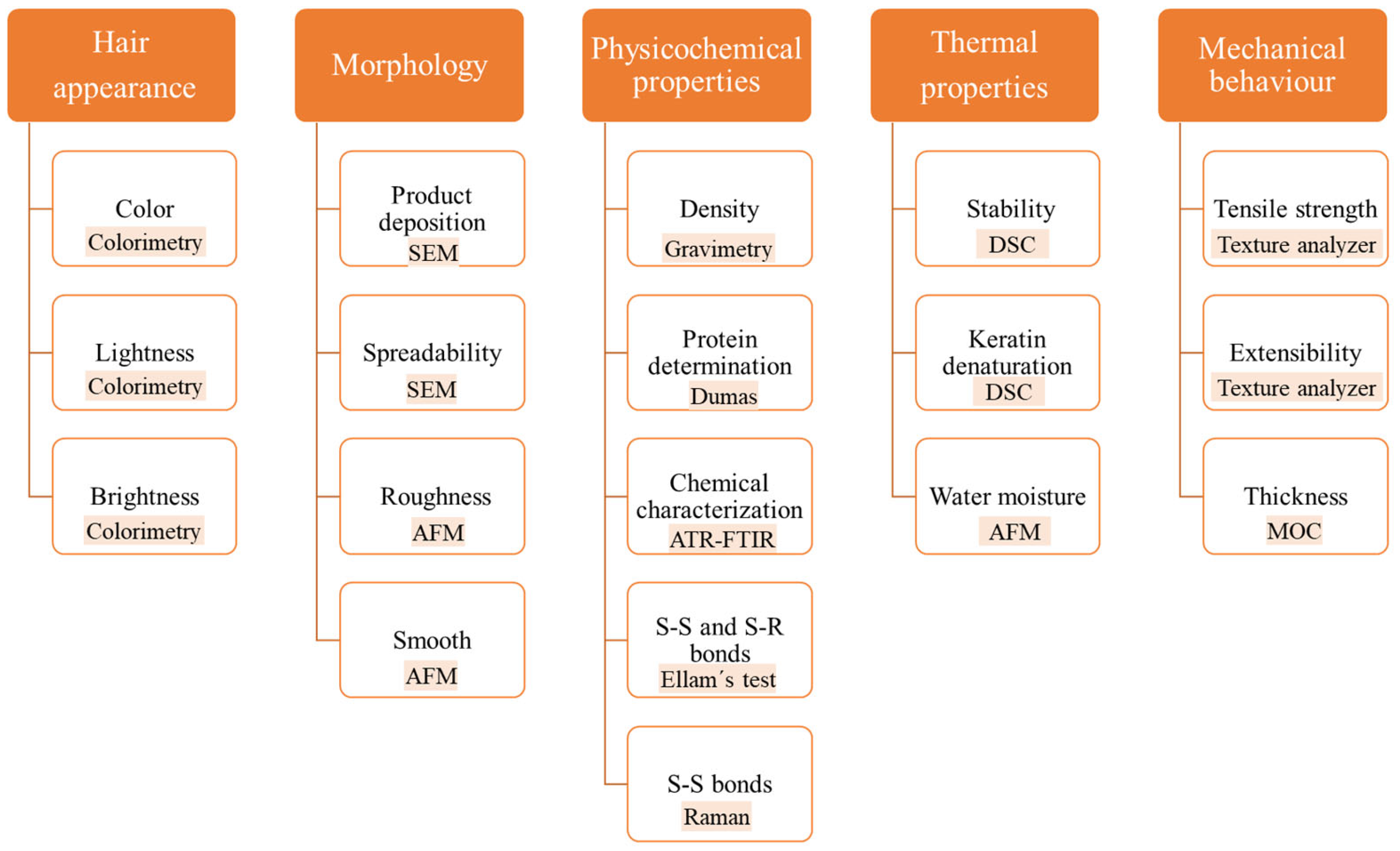
2.4. Hair Characterization
2.4.1. Hair Appearance
2.4.2. Morphological Analysis
2.4.3. Physical and Chemical Analysis
2.4.4. Thermal Analysis
2.4.5. Mechanical Behavior
3. Statistical Analysis
4. Results and Discussion
4.1. Hair Appearance
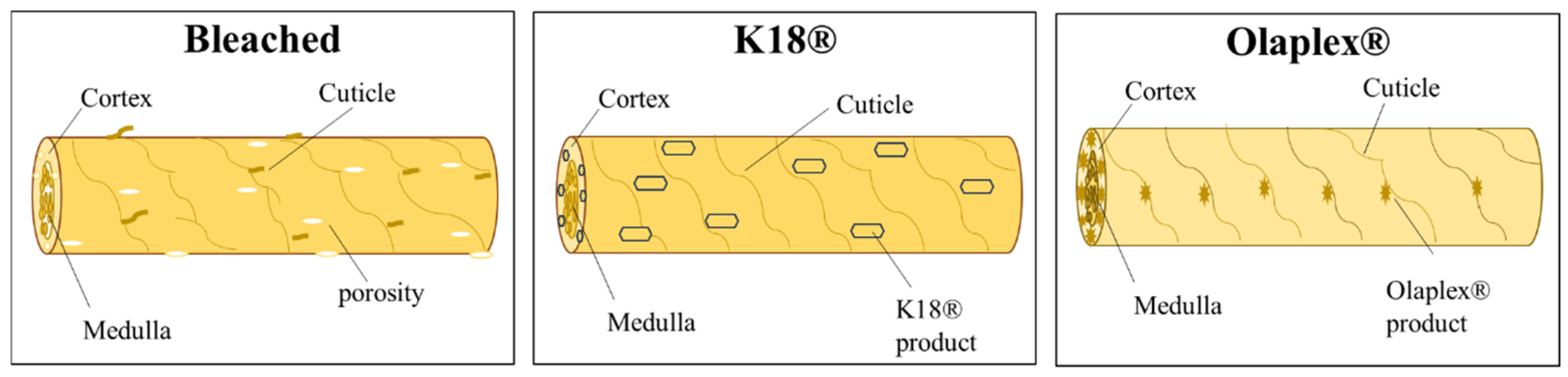
4.2. Morphological Analysis
4.2.1. SEM Micrographic Analysis
4.2.2. AFM Analysis
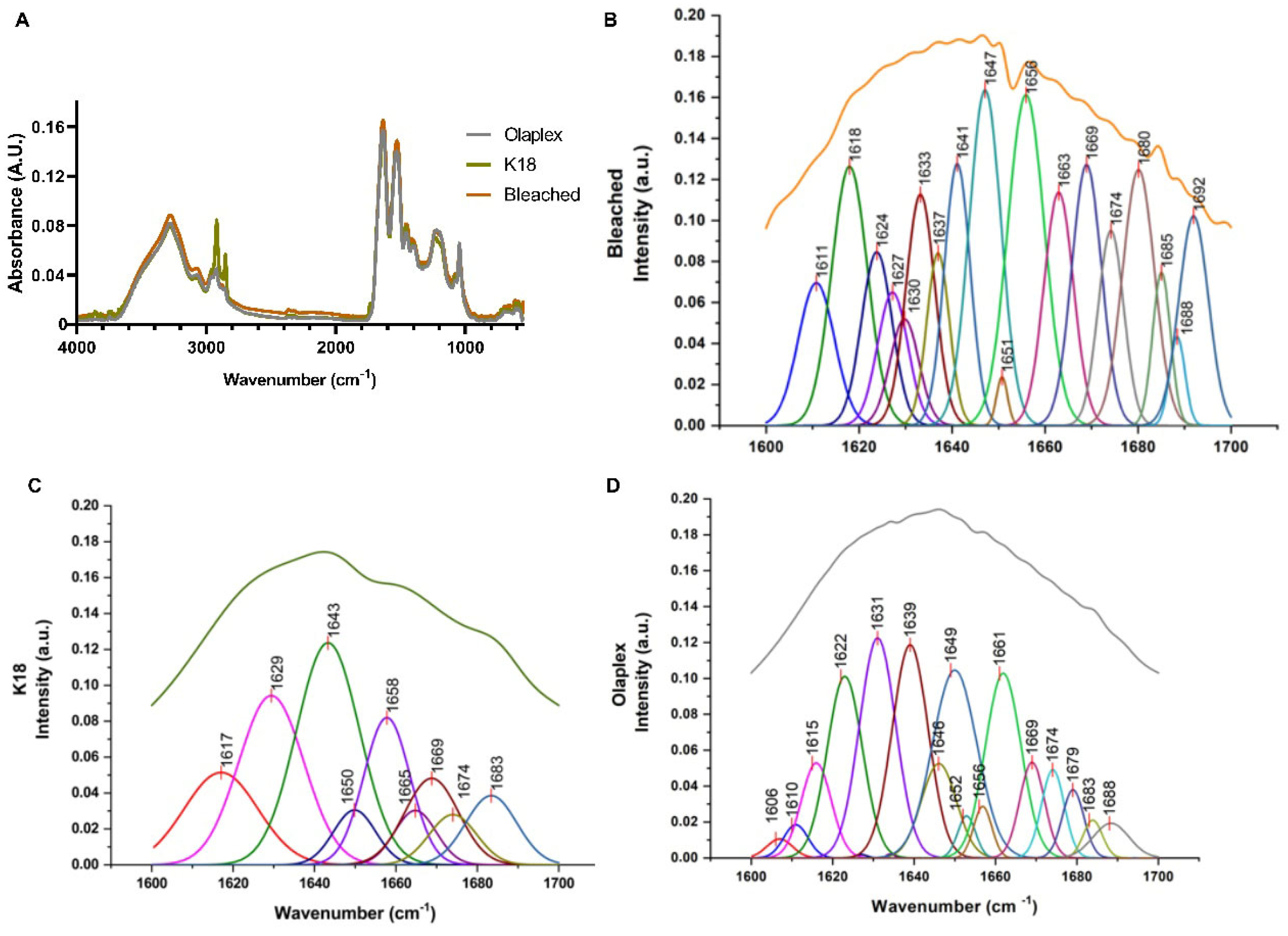
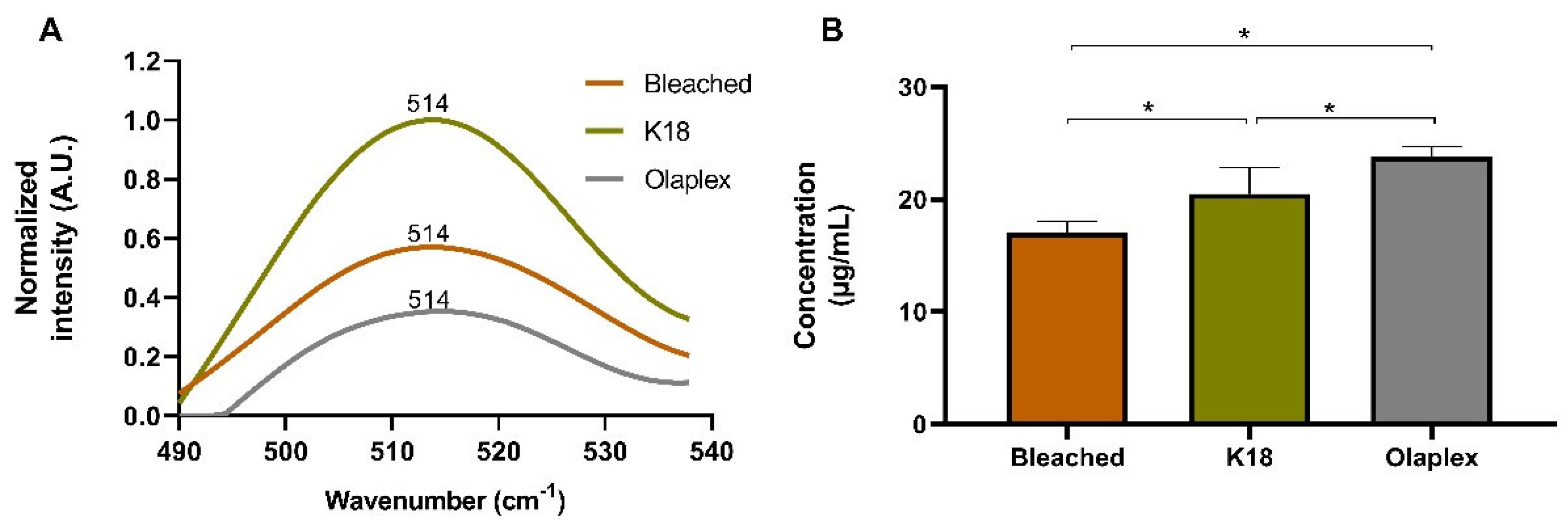
4.2.3. Physical and Chemical Analysis
Functional Groups and Bonds
4.2.4. Thermal Analysis
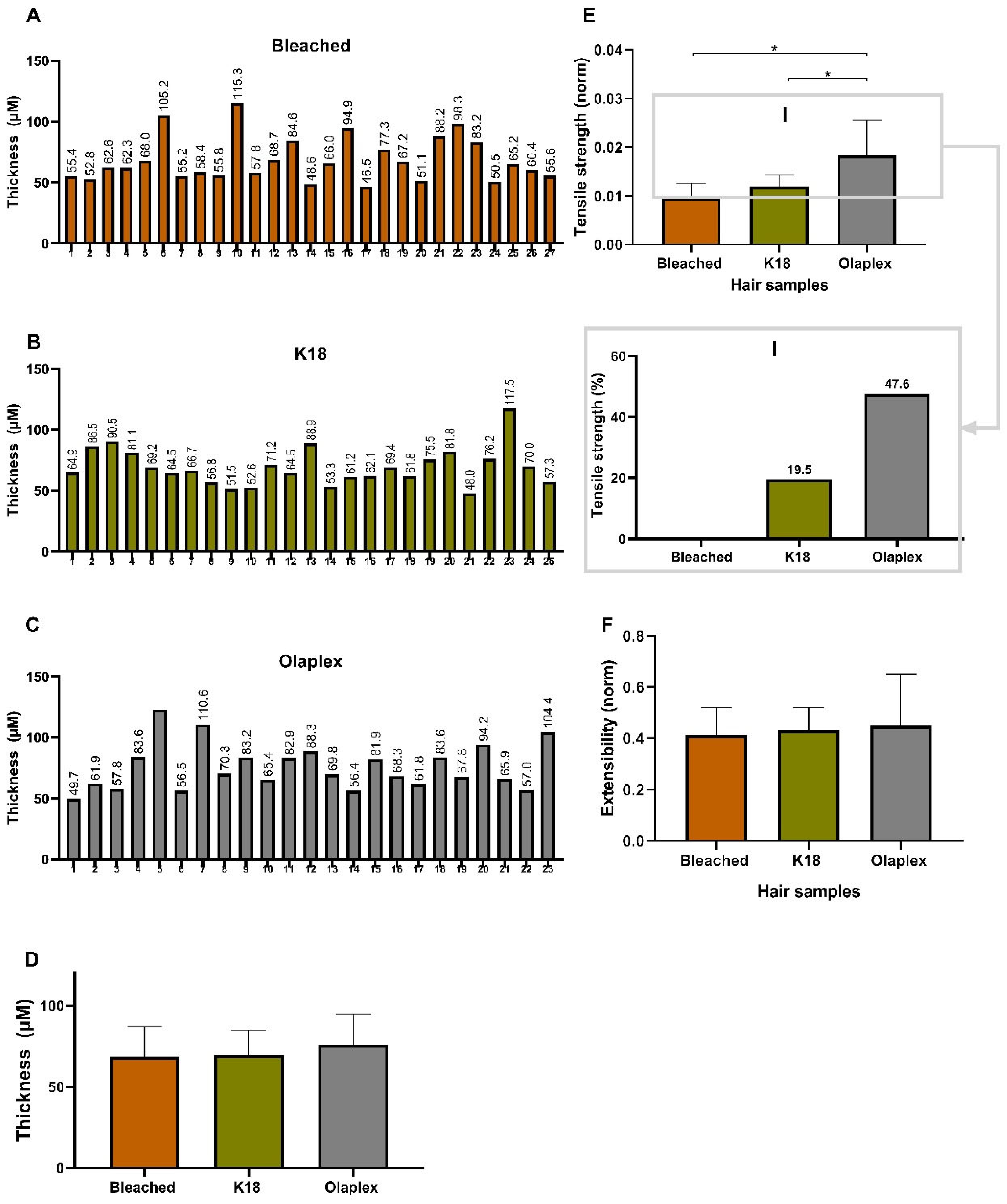
4.2.5. Mechanical Behavior
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Foggia, M.; Boga, C.; Micheletti, G.; Nocentini, B.; Taddei, P. Structural investigation on damaged hair keratin treated with α, β-unsaturated Michael acceptors used as repairing agents. Int. J. Biol. Macromol. 2021, 167, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Jerold, F. Biocosmetics: Technological advances and future outlook. Environ. Sci. Pollut. Res. 2023, 30, 25148–25169. [Google Scholar] [CrossRef] [PubMed]
- Benzarti, M.; Tkaya, M.B.; Mattei, C.P.; Zahouani, H. Hair mechanical properties depending on age and origin. World Acad. Sci. Eng. Technol. 2011, 74, 471–477. [Google Scholar]
- Fink, B.; Neuser, F.; Deloux, G.; Röder, S.; Matts, P.J. Visual attention to and perception of undamaged and damaged versions of natural and colored female hair. J. Cosmet. Dermatol. 2013, 12, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.R.; Lee, S.M.; Lim, N.R.; Chung, H.W.; Ahn, H.S. Association between hair mineral and age, BMI and nutrient intakes among Korean female adults. Nutr. Res. Pract. 2009, 3, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Almohanna, H.M.; Ahmed, A.A.; Tsatalis, J.P.; Tosti, A. The Role of Vitamins and Minerals in Hair Loss: A Review. Dermatol. Ther. 2019, 9, 51–70. [Google Scholar] [CrossRef]
- Flanagan, R.F.; Cai, J.X. Untangling the Link between Gastroparesis, Micronutrient Deficiency, and Hair Loss. Dig. Dis. Sci. 2023, 68, 1086–1088. [Google Scholar] [CrossRef]
- LaTorre, C.; Bhushan, B. Nanotribological effects of hair care products and environment on human hair using atomic force microscopy. J. Vac. Sci. Technol. A Vac. Surf. Film. 2005, 23, 1034–1045. [Google Scholar] [CrossRef]
- McMullen, R.; Schiess, T.; Kulcsar, L.; Foltis, L.; Gillece, T. Evaluation of the surface properties of hair with acoustic emission analysis. Int. J. Cosmet. Sci. 2021, 43, 88–101. [Google Scholar] [CrossRef]
- McMullen, R.L.; Kelty, S.P. Investigation of human hair fibers using lateral force microscopy. Scanning 2001, 23, 337–345. [Google Scholar] [CrossRef]
- Igarashi, K.; Maeda, K. Research on hair bleach that causes less hair damage and smells less pungent than ammonium hydroxide. Cosmetics 2018, 5, 39. [Google Scholar] [CrossRef]
- Robbins, C.R.; Robbins, C.R. Chemical and Physical Behavior of Human Hair; Springer: Berlin/Heidelberg, Germany, 2012; Volume 4. [Google Scholar]
- Kojima, T.; Yamada, H.; Isobe, M.; Yamamoto, T.; Takeuchi, M.; Aoki, D.; Matsushita, Y.; Fukushima, K. Compositional changes of human hair melanin resulting from bleach treatment investigated by nanoscale secondary ion mass spectrometry. Ski. Res. Technol. 2014, 20, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Pötsch, L. A discourse on human hair fibers and reflections on the conservation of drug molecules. Int. J. Leg. Med. 1996, 108, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Imai, T. The influence of hair bleach on the ultrastructure of human hair with special reference to hair damage. Okajimas Folia Anat. Jpn. 2011, 88, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. The use of personal hair dye and its implications for human health. Environ. Int. 2016, 89, 222–227. [Google Scholar] [CrossRef]
- Qu, W.; Guo, X.; Xu, G.; Zou, S.; Wu, Y.; Hu, C.; Chang, K.; Wang, J. Improving the mechanical properties of damaged hair using low-molecular weight hyaluronate. Molecules 2022, 27, 7701. [Google Scholar] [CrossRef]
- Robbins, C.R.; Robbins, C.R. Bleaching and oxidation of human hair. In Chemical and Physical Behavior of Human Hair; Springer: Berlin/Heidelberg, Germany, 2012; pp. 263–328. [Google Scholar]
- Tinoco, A.; Martins, M.; Cavaco-Paulo, A.; Ribeiro, A. Biotechnology of functional proteins and peptides for hair cosmetic formulations. Trends Biotechnol. 2022, 40, 591–605. [Google Scholar] [CrossRef]
- Guthrie, J.; Kazlauciunas, A.; Rongong, L.; Rush, S. The characterisation of treated and dyed hair. Dye. Pigment. 1995, 29, 23–44. [Google Scholar] [CrossRef]
- Camargo, F.B., Jr.; Minami, M.M.; Rossan, M.R.; Magalhaes, W.V.; Porto Ferreira, V.T.; Maia Campos, P.M.B.G. Prevention of chemically induced hair damage by means of treatment based on proteins and polysaccharides. J. Cosmet. Dermatol. 2022, 21, 827–835. [Google Scholar] [CrossRef]
- Pereira-Silva, M.; Martins, A.M.; Sousa-Oliveira, I.; Ribeiro, H.M.; Veiga, F.; Marto, J.; Paiva-Santos, A.C. Nanomaterials in hair care and treatment. Acta Biomater. 2022, 142, 14–35. [Google Scholar] [CrossRef]
- Tinoco, A.C.M. The Potential of Multifunctional Proteins and Peptides for Hair Protection and Coloring. Ph.D. Thesis, Universidade do Minho, Braga, Portugal, 2020. [Google Scholar]
- Neelima, T.; Riyamol, K.; Harikumar, N. Science and Technology of Hair Fibers. In Handbook of Biomass; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–19. [Google Scholar]
- Malinauskyte, E.; Shrestha, R.; Cornwell, P.; Gourion-Arsiquaud, S.; Hindley, M. Penetration of different molecular weight hydrolysed keratins into hair fibres and their effects on the physical properties of textured hair. Int. J. Cosmet. Sci. 2021, 43, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Kitagawa, M.; Yamamoto, S.; Sogabe, A.; Imura, T.; Fukuoka, T.; Kitamoto, D. Glycolipid biosurfactants, mannosylerythritol lipids, repair the damaged hair. J. Oleo Sci. 2010, 59, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Shi, J.; Wei, X.; Xie, Z.; Cheng, M.; Cao, X.; Zhou, Y.; Zhan, Y.; Yan, Y. Bioinspired peptides designed for hair perming and dyeing with potential for repair. Acta Biomater. 2023, 168, 440–457. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.F.R.G. Hair cosmetics: An overview. Int. J. Trichology 2015, 7, 2–15. [Google Scholar] [CrossRef]
- Grosvenor, A.; Deb-Choudhury, S.; Middlewood, P.; Thomas, A.; Lee, E.; Vernon, J.; Woods, J.; Taylor, C.; Bell, F.; Clerens, S. The physical and chemical disruption of human hair after bleaching—Studies by transmission electron microscopy and redox proteomics. Int. J. Cosmet. Sci. 2018, 40, 536–548. [Google Scholar] [CrossRef]
- Dias, M.F.R.G.; de Almeida, A.M.; Cecato, P.M.R.; Adriano, A.R.; Pichler, J. The shampoo pH can affect the hair: Myth or reality? Int. J. Trichology 2014, 6, 95–99. [Google Scholar] [CrossRef]
- Barve, K.; Dighe, A. The Chemistry and Applications of Sustainable Natural Hair Products; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Marsh, J.; Brown, M.; Felts, T.; Hutton, H.; Vatter, M.; Whitaker, S.; Wireko, F.; Styczynski, P.; Li, C.; Henry, I. Gel network shampoo formulation and hair health benefits. Int. J. Cosmet. Sci. 2017, 39, 543–549. [Google Scholar] [CrossRef]
- Hashim, P.; Mat Hashim, D. A review of cosmetic and personal care products: Halal perspective and detection of ingredient. Pertanika J. Sci. Technol. 2013, 21, 281–292. [Google Scholar]
- Gray, J. Hair care and hair care products. Clin. Dermatol. 2001, 19, 227–236. [Google Scholar] [CrossRef]
- Benzarti, M.; Pailler-Mattei, C.; Jamart, J.; Zahouani, H. The effect of hydration on the mechanical behaviour of hair. Exp. Mech. 2014, 54, 1411–1419. [Google Scholar] [CrossRef]
- Da Gama, R.M.; Balogh, T.S.; França, S.; Dias, T.C.S.; Bedin, V.; Baby, A.R.; do Rosário Matos, J.; Velasco, M.V.R. Thermal analysis of hair treated with oxidative hair dye under influence of conditioners agents. J. Therm. Anal. Calorim. 2011, 106, 399–405. [Google Scholar] [CrossRef]
- Coderch, L.; Alonso, C.; García, M.T.; Pérez, L.; Martí, M. Hair Lipid Structure: Effect of Surfactants. Cosmetics 2023, 10, 107. [Google Scholar] [CrossRef]
- Wang, N.; Barfoot, R.; Butler, M.; Durkan, C. Effect of surface treatments on the nanomechanical properties of human hair. ACS Biomater. Sci. Eng. 2018, 4, 3063–3071. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B. Nanoscale characterization of human hair and hair conditioners. Prog. Mater. Sci. 2008, 53, 585–710. [Google Scholar] [CrossRef]
- Sinclair, R.D. Healthy hair: What is it? J. Investig. Dermatol. Symp. Proc. 2007, 12, 2–5. [Google Scholar] [CrossRef]
- Rogers, G.E. Known and unknown features of hair cuticle structure: A brief review. Cosmetics 2019, 6, 32. [Google Scholar] [CrossRef]
- Fellows, A.P.; Casford, M.T.; Davies, P.B. Nanoscale molecular characterization of hair cuticle cells using integrated atomic force microscopy—Infrared laser spectroscopy. Appl. Spectrosc. 2020, 74, 1540–1550. [Google Scholar] [CrossRef]
- Wolfram, L.J. Human hair: A unique physicochemical composite. J. Am. Acad. Dermatol. 2003, 48, S106–S114. [Google Scholar] [CrossRef]
- Humphry, R.; Wang, N.; Durkan, C. Site-specific variations in surface structure and Young’s modulus of human hair surfaces at the nanometer scale as induced through bleach treatment. J. Mech. Behav. Biomed. Mater. 2022, 126, 105001. [Google Scholar] [CrossRef]
- Garcia, M.L.E.J.A.; Yare, R.S. Normal cuticle-wear patterns in human hair. J. Soc. Cosmet. Chem. 1978, 29, 155. [Google Scholar]
- Bhattarai, D.; Banday, A.Z.; Sadanand, R.; Arora, K.; Kaur, G.; Sharma, S.; Rawat, A. Hair microscopy: An easy adjunct to diagnosis of systemic diseases in children. Appl. Microsc. 2021, 51, 18. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Medronho, B.; Alves, L.; Rasteiro, M.G. On Hair Care Physicochemistry: From Structure and Degradation to Novel Biobased Conditioning Agents. Polymers 2023, 15, 608. [Google Scholar] [CrossRef]
- Malepfane, N.; Muchaonyerwa, P. Hair from different ethnic groups vary in elemental composition and nitrogen and phosphorus mineralisation in soil. Environ. Monit. Assess. 2017, 189, 76. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yang, W.; Wang, B.; Meyers, M.A. Structure and mechanical behavior of human hair. Mater. Sci. Eng. C 2017, 73, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Samanta, A.; Bhattacharya, M.; Dalui, S.; Acharya, M.; Das, P.S.; Chanda, D.K.; Acharya, S.D.; Sivaraman, S.K.; Nath, S.; Mandal, A.K.; et al. Nanomechanical responses of human hair. J. Mech. Behav. Biomed. Mater. 2016, 56, 229–248. [Google Scholar] [CrossRef]
- De Cássia Comis Wagner, R.; Kiyohara, P.K.; Silveira, M.; Joekes, I. Electron microscopic observations of human hair medulla. J. Microsc. 2007, 226, 54–63. [Google Scholar] [CrossRef]
- Deedrick, D.W.; Koch, S.L. Microscopy of hair part 1: A practical guide and manual for human hairs. Forensic Sci. Commun. 2004, 6, 1. [Google Scholar]
- Ly, B.C.K.; Dyer, E.B.; Feig, J.L.; Chien, A.L.; Del Bino, S. Research techniques made simple: Cutaneous colorimetry: A reliable technique for objective skin color measurement. J. Investig. Dermatol. 2020, 140, 3–12.e11. [Google Scholar] [CrossRef]
- Ordóñez-Santos, L.E.; Martínez-Girón, J.; Arias-Jaramillo, M.E. Effect of ultrasound treatment on visual color, vitamin C, total phenols, and carotenoids content in Cape gooseberry juice. Food Chem. 2017, 233, 96–100. [Google Scholar] [CrossRef]
- ASTM D792−20; Standard Test Methods for Density and Specific Gravity (Relative Density) of Plastics by Displacement. ASTM International Organization for Standardization: West Conshohocken, PA, USA, 2020.
- Cruz, C.F.; Azoia, N.G.; Matamá, T.; Cavaco-Paulo, A. Peptide–Protein interactions within human hair keratins. Int. J. Biol. Macromol. 2017, 101, 805–814. [Google Scholar] [CrossRef]
- ISO-5079-2020.(E); Textile Fibres—Determination of Breaking Force and Elongation at Break of Individual Fibres. 3rd ed. International Organization for Standardization: Geneva, Switzerland, 2020.
- Payne, C.E.; Rockson, A.; Ashrafi, A.; McDonald, J.A.; Bethea, T.N.; Barrett, E.S.; Llanos, A.A. Beauty Beware: Associations between Perceptions of Harm and Safer Hair-Product-Purchasing Behaviors in a Cross-Sectional Study of Adults Affiliated with a University in the Northeast. Int. J. Environ. Res. Public Health 2023, 20, 7129. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kwon, K.H. Considering the risk of a coloring shampoo with the function of gray hair cover cosmetology and skin barrier: A systematic review. Health Sci. Rep. 2023, 6, e1271. [Google Scholar] [CrossRef]
- Yun, K.; Ahn, C. Effect of surfactant type on the dyeability and color resistance of semi-permanent basic hair dye. Fash. Text. 2023, 10, 4. [Google Scholar] [CrossRef]
- Xu, L.; Dong, J. Click chemistry: Evolving on the fringe. Chin. J. Chem. 2020, 38, 414–419. [Google Scholar] [CrossRef]
- Pressly, E.D.; Hawker, C.J. Methods for Fixing Hair and Skin. 2015. Available online: https://patentimages.storage.googleapis.com/e6/92/5f/e237aaac6b0003/US9095518.pdf (accessed on 24 July 2024).
- Vaughn, M.; Van Oorschot, R.; Baindur-Hudson, S. Hair color measurement and variation. Am. J. Phys. Anthropol. Off. Publ. Am. Assoc. Phys. Anthropol. 2008, 137, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Murugan, D.B. A Study on Rural Customers’ Perception—A Case of Shampoo. SSRN Electron. J. 2010. [Google Scholar] [CrossRef]
- Labarre, L.; Squillace, O.; Liu, Y.; Fryer, P.J.; Kaur, P.; Whitaker, S.; Marsh, J.M.; Zhang, Z.J. Hair surface interactions against different chemical functional groups as a function of environment and hair condition. Int. J. Cosmet. Sci. 2023, 45, 224–235. [Google Scholar] [CrossRef]
- Morel, O.; Christie, R.M.; Greaves, A.; Morgan, K.M. Enhanced model for the diffusivity of a dye molecule into human hair fibre based on molecular modelling techniques. Color. Technol. 2008, 124, 301–309. [Google Scholar] [CrossRef]
- Yang, W.; Yu, Y.; Ritchie, R.O.; Meyers, M.A. On the strength of hair across species. Matter 2020, 2, 136–149. [Google Scholar] [CrossRef]
- Barba, C.; Oliver, M.A.; Martí, M.; Kreuzer, M.; Coderch, L. Lipid distribution on ethnic hairs by Fourier transform infrared synchrotron spectroscopy. Ski. Res. Technol. 2022, 28, 75–83. [Google Scholar] [CrossRef]
- Smith, R.; Garrett, B.; Naqvi, K.; Fülöp, A.; Godfrey, S.; Marsh, J.; Chechik, V. Mechanistic insights into the bleaching of melanin by alkaline hydrogen peroxide. Free Radic. Biol. Med. 2017, 108, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.; Mantsch, H.H. The use and misuse of FTIR spectroscopy in the determination of protein structure. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 95–120. [Google Scholar] [CrossRef]
- Essendoubi, M.; Andre, N.; Granger, B.; Clave, C.; Manfait, M.; Thuillier, I.; Piot, O.; Ginestar, J. New approach for hair keratin characterization: Use of the confocal Raman spectroscopy to assess the effect of thermal stress on human hair fibre. Int. J. Cosmet. Sci. 2022, 44, 588–601. [Google Scholar] [CrossRef] [PubMed]
- Oliver, M.A.; Coderch, L.; Carrer, V.; Barba, C.; Marti, M. Ethnic hair: Thermoanalytical and spectroscopic differences. Ski. Res. Technol. 2020, 26, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Istrate, D.; Popescu, C.; Möller, M. Non-Isothermal Kinetics of Hard α-Keratin Thermal Denaturation. Macromol. Biosci. 2009, 9, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Cao, J. Melting study of the α-form crystallites in human hair keratin by DSC. Thermochim. Acta 1999, 335, 5–9. [Google Scholar] [CrossRef]
- Lima, C.R.R.d.C.; Machado, L.D.B.; Velasco, M.V.R.; Matos, J.d.R. DSC measurements applied to hair studies. J. Therm. Anal. Calorim. 2018, 132, 1429–1437. [Google Scholar] [CrossRef]
- Popescu, C.; Gummer, C. DSC of human hair: A tool for claim support or incorrect data analysis? Int. J. Cosmet. Sci. 2016, 38, 433–439. [Google Scholar] [CrossRef]
- Monteiro, V.F.; Maciel, A.; Longo, E. Thermal analysis of caucasian human hair. J. Therm. Anal. Calorim. 2005, 79, 289–293. [Google Scholar] [CrossRef]
- Yang, F.-C.; Zhang, Y.; Rheinstädter, M.C. The structure of people’s hair. PeerJ 2014, 2, e619. [Google Scholar] [CrossRef]
- Franbourg, A.; Hallegot, P.; Baltenneck, F.; Toutaina, C.; Leroy, F. Current research on ethnic hair. J. Am. Acad. Dermatol. 2003, 48, S115–S119. [Google Scholar] [CrossRef]
- Vaughn, M.R.; Brooks, E.; van Oorschot, R.A.; Baindur-Hudson, S. A comparison of macroscopic and microscopic hair color measurements and a quantification of the relationship between hair color and thickness. Microsc. Microanal. 2009, 15, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Sayahi, E.; Harizi, T.; Msahli, S.; Sakli, F. Physical and mechanical properties of T unisian women hair. Int. J. Cosmet. Sci. 2016, 38, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.F.; Martins, M.; Egipto, J.; Osório, H.; Ribeiro, A.; Cavaco-Paulo, A. Changing the shape of hair with keratin peptides. RSC Adv. 2017, 7, 51581–51592. [Google Scholar] [CrossRef]
- McMichael, A.J. Hair breakage in normal and weathered hair: Focus on the Black patient. J. Investig. Dermatol. Symp. Proc. 2007, 12, 6–9. [Google Scholar] [CrossRef]
- Wei, G.; Bhushan, B. Nanotribological and nanomechanical characterization of human hair using a nanoscratch technique. Ultramicroscopy 2006, 106, 742–754. [Google Scholar] [CrossRef]
- Barba, C.; Méndez, S.; Martí, M.; Parra, J.; Coderch, L. Water content of hair and nails. Thermochim. Acta 2009, 494, 136–140. [Google Scholar] [CrossRef]
- Dawber, R. Hair follicle structure, keratinisation and the physical properties of hair. In Diseases of the Hair and Scalp, 3rd ed.; Dawber, R., Ed.; Blackwell Science: Oxford, UK, 1997; pp. 23–50. [Google Scholar]
- Tanaka, S.; Iimura, H.; Sugiyama, T. Study of the test method of reduction and recovery of disulfide bond in human hair. J. Soc. Cosmet. Chem. Jpn. 1992, 25, 232–239. [Google Scholar] [CrossRef]






| Samples | Event 1 (°C) | Event 2 (°C) | Max. Weight Loss (%) | Temp. 1 (°C) | DTG 1 (%) | Temp. 2 (°C) | DTG 2 (%) |
|---|---|---|---|---|---|---|---|
| Bleached | 130.0 ± 0.0 | 235.6 ± 0.7 | 60.0 ± 4.7 | 246.0 ± 2.9 | 0.277 ± 0.013 | 301.9 ± 0.2 | 0.416 ± 0.041 |
| K18 | 130.6 ± 0.0 | 235.2 ± 0.7 | 66.7 ± 2.5 | 244.4 ± 0.3 | 0.291 ± 0.011 | 303.5 ± 0.5 | 0.481 ± 0.009 |
| Olaplex | 130.5 ± 0.1 | 237.3 ± 1.3 | 67.0 ± 5.1 | 244.4 ± 1.1 | 0.267 ± 0.014 | 301.2 ± 0.4 | 0.453 ± 0.026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, E.; Castro, P.; Ribeiro, A.B.; Pereira, C.F.; Casanova, F.; Vilarinho, R.; Moreira, J.; Ramos, Ó.L. Bleached Hair as Standard Template to Insight the Performance of Commercial Hair Repair Products. Cosmetics 2024, 11, 150. https://doi.org/10.3390/cosmetics11050150
Martins E, Castro P, Ribeiro AB, Pereira CF, Casanova F, Vilarinho R, Moreira J, Ramos ÓL. Bleached Hair as Standard Template to Insight the Performance of Commercial Hair Repair Products. Cosmetics. 2024; 11(5):150. https://doi.org/10.3390/cosmetics11050150
Chicago/Turabian StyleMartins, Eva, Pedro Castro, Alessandra B. Ribeiro, Carla F. Pereira, Francisca Casanova, Rui Vilarinho, Joaquim Moreira, and Óscar L. Ramos. 2024. "Bleached Hair as Standard Template to Insight the Performance of Commercial Hair Repair Products" Cosmetics 11, no. 5: 150. https://doi.org/10.3390/cosmetics11050150
APA StyleMartins, E., Castro, P., Ribeiro, A. B., Pereira, C. F., Casanova, F., Vilarinho, R., Moreira, J., & Ramos, Ó. L. (2024). Bleached Hair as Standard Template to Insight the Performance of Commercial Hair Repair Products. Cosmetics, 11(5), 150. https://doi.org/10.3390/cosmetics11050150










