Abstract
Microneedling, also known as percutaneous collagen induction, using microneedling devices and fabricated microneedle patches, has been widely employed in cosmetic applications for acne scar treatment, skin care, hair loss, melasma, skin rejuvenation, and skin cancer. The micro-channels formed by microneedling through the stratum corneum facilitate the delivery of cosmetic agents and stimulate collagen and elastin production by inducing the wound-healing cascade, keeping the skin shiny and wrinkle-free. Several cosmetic agents, such as ascorbic acid, hyaluronic acid, retinoids, niacinamide, and peptides, have been delivered by microneedling. This review aims to highlight the use of microneedling devices and fabricated microneedle patches in facilitating the delivery of cosmetic agents through the skin layers. Moreover, the differences between the microneedling devices, commonly used alone or in combinational treatments with topical formulations, are explored. Furthermore, the safety of microneedling in terms of skin irritation, pain sensation, skin or systemic infection, and chemical and biological materials used in the fabrication of microneedles is discussed.
1. Introduction
Healthy skin has positive psychological and social impacts on individuals [1]. It has been shown that there is a direct link between skin conditions and psychology, where individuals with skin conditions are more likely to suffer from depression, social isolation, loneliness, and a lower quality of life [2]. Several strategies have been employed to improve skin conditions, such as topical medical preparations, chemical peeling, and ablative and non-ablative laser photo-rejuvenations. These above strategies have been widely used in treating skin wrinkles, aging, acne scars, and hyperpigmentation [3]. Moreover, the recent advances in cosmetic applications have opened doors to treat more complex skin conditions such as vitiligo, hair loss, melasma, and skin cancer [4].
Various topical/transdermal delivery platforms, such as nanoparticles, liposomes, dendrimers, gels, creams, lotions, patches, nanoemulsions, and microemulsions, have been widely used to deliver cosmetic agents to the skin [5,6]. However, these delivery platforms often cannot cross the major barrier of the skin, the stratum corneum (SC), hindering the penetration of the chemical molecules through the skin layers [7,8,9]. Additionally, this barrier limits the penetration of the hydrophilic and high-molecular-weight molecules (>500 Da) through the intact skin [10,11,12]. This will ultimately increase the frequency of application of the delivery platforms onto the skin so that a sufficient amount of cosmetic agents can penetrate the skin layers (epidermis and dermis), leading to poor patient compliance [1].
Recently, the microneedling technique, also known as percutaneous collagen induction (PCI), using microneedling devices such as Dermaroller and Dermapen, and fabricated microneedle patches (MNs), such as solid, hollow, dissolved, coated, and hydrogel MNs, have been widely employed in the cosmetics field [13]. Microneedling is characterized by its minimal invasiveness, painlessness, and self-administration, which can result in good patient compliance, in addition to reduced hazardous waste sharps [14]. These advantages suggest that there will be a broad market for microneedling applications in the cosmetics field.
Microneedling or PCI was introduced in 1990 to treat scars [15], and it then became more widely explored for the delivery of drugs after 1990 [14]. Recently, microneedling has been employed to enhance the delivery of active ingredients used in cosmetics, such as ascorbic acid (AA), retinoids, melanin, proteins, and peptides [1]. When applying microneedling devices and fabricated MNs, micro-sized needles can pass through the SC, creating micro-channels of aqueous transport pathways that enhance the transport of molecules through the skin at therapeutically relevant doses [16,17]. This will eventually increase the absorption of cosmetic agents compared with topical administration and effectively reduce the quantity delivered [1]. It is worth noting that the disruption of the molecular architecture of the SC by microneedling is reversible, resulting in less pain or bleeding compared with hypodermic needles, particularly for short microneedles of <600 μm in length [18,19]. It has been shown that the microneedle’s length dramatically correlates with pain and bleeding [20]. For instance, microneedles of 500–1500 μm in length would result in a sevenfold increase in pain score for human subjects [21]. This is because pain receptors innervate the epidermis and dermis; thus, the penetration of longer microneedles would excite more receptors [21]. Additionally, microneedles of 1000–1500 μm in length have been reported to puncture tiny blood capillaries, leaving blood spots on the skin surface [22,23]. It should also be noted that the thickness of the skin vastly varies within the human body, a factor that needs to be considered when applying microneedles to specific areas of the body [24].
The use of microneedling in cosmetic applications has previously been reviewed by McCrudden et al. [13] and Iriarte et al. [25]. McCrudden et al. [13] explored the chronology of using Dermaroller® and Dermapen®, developed based on Fernandes’s PCI innovation, and Dermastamp® in inducing collagen and treating acne, burn scars, and photo-aging. The design of the microneedling devices and some commercially available devices were reported in this review. In addition, the safety, regulatory considerations, and public perception relating to the use of these devices were addressed. This was followed by a review article by Iriarte et al. [25] that discussed the use of microneedling in combination with topical medications for treating various dermatological conditions, including scars, alopecia, pigmentary disorders, verruca, and actinic keratosis. The frequency and interval between treatments for optimal effects were also evaluated. A very recent review by Huang et al. [1] focused on describing the preparation methods, polymers, loading drugs, mechanical and penetrative properties, and types of fabricated MNs used in a wide range of cosmetic applications, such as whitening, moisturizing, wrinkle removal, fat reduction, scar removal, alopecia treatment, and acne treatment. Different drug loading methods, like simple physical mixing of active ingredients and matrix materials, drug carriers such as liposomes, polymeric micelles, nanoparticles, nanogels, and nanocapsules, and drug coupling through covalent bonds between drug molecules and polymer materials, were also discussed.
Therefore, this paper reviews the most recent update on the extensive use of microneedling in various cosmetic applications, including skin anti-aging, scar removal, skin whitening, skin moisturizing, skin rejuvenation, hair loss, melasma, vitiligo, and skin cancer. In addition, this review highlights the expansion of using microneedling as adjuvant therapy for various indications in dermatology. Moreover, this review focuses on the difference between microneedling devices used alone or in combinational treatments with topical formulations. Furthermore, it summarizes the types of fabricated MNs, polymers used in fabrication, approaches used to deliver cosmetic agents across the skin, clinical outcomes of the cosmetic-agent-loaded MNs, and their safety when applied to the skin.
2. Microneedling Devices
Microneedling using microneedling devices such as Dermaroller and Dermapen (Figure 1) in cosmetic applications is gaining prominence, particularly for improving the skin’s appearance and treating blemishes and scars [25,26,27,28,29]. The use of these devices has continuously evolved since their origin in the early twentieth century. Recently, dermatologists introduced microneedling in anti-aging and rejuvenation therapy to achieve smooth and young-looking skin [28]. These devices puncture the skin with micron-sized needles in a non-pathogenic manner, causing the underlying cells to produce more collagen and elastin, the crucial dermal components [28,30,31]. In addition, these devices have been used to enhance the delivery of cosmetic agents [31,32,33].
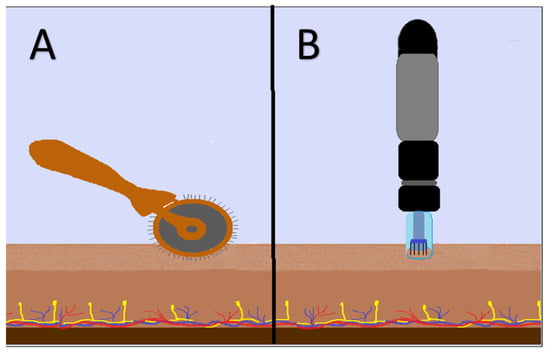
Figure 1.
Representative images of (A) Dermaroller and (B) Dermapen when applied to the skin.
Dermaroller® is a simple handheld microneedling device with fine needles of 200–3000 µm in length and an effective diameter of 0.1 mm [28]. The needles of the Dermaroller® are fabricated from silicon or stainless steel by reactive ion etching [28]. The silicon and stainless steel needles are known to be tough and durable [28,34]. Dermarollers® are commonly used for acne scar treatment [13,29], skin care [28], burn scars [35], pigmentary disorders [36], and PCI [37]. In addition, they can be used in facial rejuvenation, stretch marks, and hair loss [37,38]. However, because of the length of the needles, this treatment is not optimal, as it may cause skin bleeding [27].
Two mechanisms have been proposed for microneedling [39]: The first mechanism suggests that microneedling leads to the release of growth factors that stimulate the formation of collagen and elastin in the papillary dermis. Briefly, the needles penetrate the SC and create small holes (micropunctures) without damaging the epidermis. These micro-injuries lead to minimal superficial bleeding, inducing the wound-healing cascade and activating platelets and neutrophils to release growth factors (transforming growth factor (TGF)-alpha, TGF-beta, and platelet-derived growth factor) and stimulate the production of collagen and elastin in the papillary layer of the dermis. This ultimately results in the deposition of collagen by fibroblasts [25,39,40]. Moreover, micropores created by microneedling enhance the permeation of skin care formulations, thus boosting their efficacy [28]. The second mechanism suggests that microneedling generates a demarcation current rather than physical wounds when microneedles penetrate the skin. This current triggers a cascade of growth factors that facilitate the healing process. This mechanism is based on the concept of bioelectricity, where epidermal injury alters the electric potential within cells to a negative electric potential of −70 mV, compared to the epidermis, which has a positive potential. It is anticipated that the change in the potential stimulates the migration and proliferation of fibroblasts to the site of injury, leading to collagen deposition at the injury site [39,41].
Another microneedling device used in cosmetic applications is Dermapen® [38]. Dermapen® is a handheld pen-like device with disposable needles used to treat acne, burn scars, and photo-aging [13]. The needle’s length can be adjusted to drive the needle up and down the treatment site [25,28]. It has a battery that can be recharged. Nine to twelve needles are stacked in rows on the needle tip.
Moreover, Dermapen® has two operating modes: high speed (700 cycles per minute) and low speed (412 cycles per minute) [25]. Dermapen® has benefits over Dermaroller®, as it provides disposable needles, uniform application of pressure on the skin, and minimum risk of tips breaking in the skin [28,42]. In addition, it can treat delicate regions around the eyes, lips, and nose without damaging the skin [28]. Table 1 presents a general comparison between Dermaroller® and Dermapen®, without referring to any commercial brands.

Table 1.
Comparison between Dermaroller and Dermapen.
Recently, a miniature version of Dermaroller® (Dermastamp®, Figure 2) has been fabricated to reach the small, confined areas that are difficult to reach with Dermaroller® [13,40,50,51]. The Dermastamp® (Teoxy Beauty, Wuhan, China) is a non-oscillating device composed of microneedle arrays with 42 needles per stamp of 1000 µm in length, 0.12 mm in diameter, a 21–25 μm tip radius, and curved conical geometry, arranged at the base of the stamp [52]. In addition, the Dermastamp® is commercially available, with adjustable needle length ranges from 200–3000 µm and a diameter of 0.12 mm [53]. It is inserted into the skin in a vertical position. A recent study by Sabri et al. [37] evaluated the mechanical insertion of an oscillating Dermapen® (ZJChao, China) and a non-oscillating Dermastamp® (Teoxy Beauty, Wuhan, China), used to overcome the physiological barrier of the SC and, hence, enhance the delivery of molecules into and across the skin. The needles of Dermastamp® were 1000 μm in length, and those of Dermapen® were adjustable to the same length of 1000 μm. This study showed that greater force was required to puncture the skin with Dermastamp® compared to Dermapen®. In addition, Dermapen® was more effective in generating micro-channels across the skin, as it can penetrate deeper skin layers due to its oscillating microneedle system. Nevertheless, the ex vivo permeation study revealed that both microneedle systems exhibited similar permeation profiles for the model drug imiquimod across the skin after 24 h, since both systems breached the SC, epidermis, and most likely the superficial dermis [37]. Finally, a patented gold-plated micro-injection system known as AquaGold® Fine Touch (Aquavit Pharmaceuticals, NY, USA) was used to induce collagen and elastin and deliver cosmetic products deep into the skin layers, such as retinol, vitamin C, antioxidants, anti-aging serums, vitamins, growth factors, fillers (Botox and hyaluronic acid (HA)), plasma-rich protein, and toxins. The device contains thin, hollow, 24-karat gold needles of 600 µm in length and 130 µm in width that create tiny punctures in the skin and deposit products beneath the skin’s surface. This improves the skin’s quality and texture, reduces the appearance of fine lines, shrinks the pores, and boosts hydration [48,54].
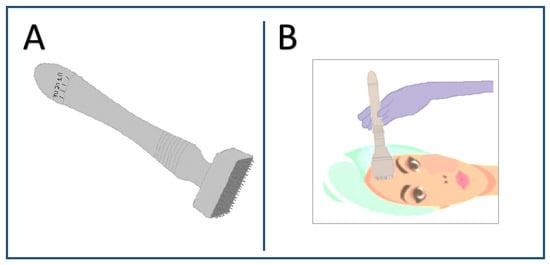
Figure 2.
Representative images of (A) Dermastamp and (B) Dermastamp when applied to the skin.
2.1. Microneedling in Cosmetic Applications
2.1.1. Treatment of Acne Scars
Microneedling is a technique used to improve cutaneous scarring by increasing the production of collagen and elastin in the dermis, collagen remodeling, and increasing the thickness of the epidermis and dermis [27]. The most frequently used alpha-hydroxy acid peel is the glycolic acid (GA) peel. Sharad [55] compared the effectiveness of a combination of microneedling using Dermaroller® MF8 and 35% GA peel for treating acne scars in skin types III–V versus microneedling alone. Microneedling was performed six times a week for six weeks, followed by a 35% GA peel three weeks later. The superficial or moderately deep scars were improved significantly. In addition, the skin’s texture was improved, and the post-acne pigmentation was reduced. Saadawi et al. [56] compared the efficacy of using a combination of microneedling using Dermapen® (Bomtech Electronics, Seoul, Republic of Korea) and GA peel versus each alone. Thirty participants with acne scars were included in this study. The participants were divided into three groups at random, each with ten patients. Six sessions were given to each patient at two-week intervals. The results showed that combining microneedling with GA peel was more effective in treating acne scars compared with monotherapy. In another study [57], microneedling using Dermaroller® with a topical application of platelet-rich plasma (PRP) was found to be effective in accelerating the wound healing of atrophic acne scars by enhancing nucleogenesis and inducing remodeling of acne scars. In this study [57], the authors found that a combination of microneedling and chemical peeling using 15% trichloroacetic acid (TCA) improved severe atrophic acne scars.
Furthermore, Costa and Costa [58] found that microneedling improved varicella scars in dark-skinned teenagers. Recently, Ali et al. [59] used microneedling using Dermapen® (Derma Stamp Electric Pen, Beijing, China) and Jessner’s peeling solution—a mixture of salicylic acid, resorcinol, and lactic acid in 95% ethanol—for the treatment of acne scars. The results showed that the combined technique of microneedling and Jessner’s solution was considered to be the best clinical treatment for atrophic acne scars, with the smallest number of sessions compared to each technique alone.
Additionally, microneedling using a Dermastamp® with stainless steel needles of 2.1 mm in length was used to treat hypertrophic burn scars. Five patients underwent 8–12 microneedling sessions every two weeks. During the treatment, the scar was pressed by the Dermastamp 3–4 times, producing 200–300 holes in the scar area. The results demonstrated a clinical improvement in the burn scars when evaluating the Vancouver Scar Scale score and the scar depth. The scar improvement was attributed to the microneedling of the scars using Dermastamp®, which induces the rearrangement of collagen fibers on scar tissue and thins the height of the scar [60].
Moreover, microneedling is a widely used procedure for improving the final appearance of surgical scars [61]. Microneedling creates controlled micro-injuries to the skin, which stimulate the natural wound-healing response of the body. The healing process leads to the production of new collagen and elastin, resulting in the remodeling of scar tissues, which ultimately improves the texture, color, and overall appearance of the scars [53]. Several studies have demonstrated the effectiveness of microneedling in treating various types of scars, including surgical scars [62,63,64].
2.1.2. Treatment of Vitiligo
Vitiligo is a chronic skin disorder characterized by depigmented white patches caused by the destruction of melanocytes, and it affects both sexes equally. These patches are known to resist conventional therapies such as topical creams of corticosteroids and calcineurin inhibitors, systemic corticosteroids, immunosuppressants, biologic medications, and ultraviolet light and laser therapy, in addition to combinations of these treatments [65,66]. Microneedling has shown its efficacy in treating vitiligo either as a monotherapy or as a combination therapy with topical treatment [45,65,67,68,69]. Microneedling can potentially stimulate melanocytes and skin re-pigmentation by creating micro-injuries, which lead to the release of the growth factors in the bulge area and the epidermis [70]. Bailey et al. [71] reported that microneedling facilitated re-pigmentation in vitiligo by increasing the penetration of topical therapies into the dermis, inducing neocollagenesis, and activating melanocytes. Thus, microneedling was an effective and well-tolerated adjuvant to topical therapy for vitiligo, as evidenced by increasing the rate of treatment to >25%. Studies have shown the efficacy of microneedling in treating vitiligo, particularly when combined with topical treatments [46,72,73,74]. For instance, a topical tacrolimus ointment (0.1%) combined with microneedling, performed with an electric Dermapen® (My M Micro Needle Therapy, Shanghai, China) with different needle ranging in size from 1500 to 2000 µm, was more efficient in treating patients with vitiligo than microneedling alone [46]. Additionally, twenty-seven patients with localized stable vitiligo were subjected to six sessions of microneedling with Dermapen® (Dr. Pen Derma Pen Ultima M5®) at 2-week intervals. Afterwards, a solution of 5-fluorouracil (5-FU, 5%) was applied once daily for two weeks over the affected areas. The results showed that the combination therapy (microneedling + 5-FU) yielded a better response (by 3.8 times) compared to microneedling alone [72]. Another study compared the efficacy of microneedling using Dermapen® (My M Micro Needle Therapy, Shanghai, China) with 5-FU solution (50 mg/mL) versus microneedling with topical tacrolimus ointment (0.03%) in the treatment of vitiligo. Excellent re-pigmentation and a higher clinical response were found in patients subjected to 5-FU with microneedling (48% vs. 16%, 5-FU vs. tacrolimus) [73]. Moreover, the efficacy of a combination of microneedling with Dermapen® (My M Micro Needle Therapy, Shanghai, China) and a topical combination of calcipotriol (0.05 mg/g) and betamethasone (0.5 mg) was compared with the efficacy of microneedling and topical tacrolimus ointment (0.03%). The results showed that microneedling with topical calcipotriol and betamethasone was superior to microneedling with topical tacrolimus (60% vs. 32%) in treating vitiligo [74].
2.1.3. Treatment of Hair Loss
Microneedling can be used for androgenic alopecia and alopecia areata [75]. In treating hair loss, scalp rollers with titanium needles are thought to be suitable for androgenic alopecia, where hair growth starts after 8–10 sessions [28]. Microneedling using Dermaroller® with a needle length of 1500 µm activates the stem cells in the hair bulge area under wound-healing conditions, which results in a new hair cycle and new hair growth. In addition, it facilitates the penetration of first-line medications [76]. The success of microneedling in stimulating hair growth has been reported in severe male and female alopecia [76], particularly in patients who cannot use systemic treatment [77]. Generally, there is no standard procedure for microneedling in hair loss. Typically, a needle length of 500–2500 µm is used, and the procedure involves multiple, repeated, and sequential movements of the Dermaroller® until pinpoint bleeding is visible [77]. Faghihi et al. [78] showed that a combination of microneedling, performed with an electrical pen-shaped device (Auto MTS, Republic of Korea) with adjustable penetration depth from 100 to 2000 µm, and minoxidil (5% lotion) has a better therapeutic effect in treating androgenic alopecia in terms of hair count, thickness, and growth than using the topical minoxidil lotion alone. This was because microneedling induced the release of platelet-derived growth factor via the activation of platelets and stimulated the overexpression of hair-growth-related genes [78]. Additionally, it is believed that microneedling stimulates the deposition of collagen, enhances the release of growth factors, and facilitates the penetration of medications such as minoxidil and topical steroids [79].
Recently, preclinical studies demonstrated the clear benefits of integrating MNs with therapeutic exosomes for hair regeneration. Yang et al. [80] prepared detachable MNs using a water-soluble HA base, integrated with therapeutic exosomes and a small-molecular drug (UK5099) for hair regeneration. The matrix of MNs was fabricated using the natural hair protein keratin. The hair follicle stem cells were loaded into exosomes, UK5099 was loaded into poly(lactic-co-glycolic acid) nanoparticles, and then both systems delivery were encapsulated into keratin-hydrogel-based MNs. The results showed that this system promoted pigmentation and hair regrowth in treated mice within 6 days by activating the telogen-to-antigen transition and acting as a depot inside the skin for sustaining the release of therapeutics. The MNs–exosomal system was clinically more effective when compared to the subcutaneous injection of exosomes and topical administration of UK5099. Moreover, MNs integrated with therapeutic exosomes were used to activate the dermal papilla cells, which play a key role in hair regeneration [81]. MNs were fabricated with HA and swellable PVA needles and loaded with chitosan lactate and exosome-encapsulated dermal papilla cells (DPCs). Chitosan lactate released L-lactate, promoting cell growth by activating lactate dehydrogenase, whereas exosomes sustained the release of DPC, stimulating cell proliferation by activating the Wnt/β-catenin signaling pathway, which plays a role in regenerating the hair follicles [82]. Therefore, this transdermally combined system of MNs and exosomes was able to promote hair regeneration by regulating hair follicle cycling, providing great potential for clinical application. Finally, a new hair growth technology inspired by seed germination (plowing, breeding, and lighting) was employed to promote hair regeneration [83]. MN patches were loaded with exosomes of stem cells (human amniotic mesenchymal stem cells (hAMSCs)) and hair-derived nanoparticles. The results showed that the MNs were able to penetrate the cuticle (plowing), facilitating the delivery of hAMSC exosomes into the dermis and stimulating the hair follicles’ stem cells (breeding). Moreover, the yellow-light irradiation alleviated the inflammation of hair follicles (lighting), further prompting hair regeneration. This strategy demonstrated effective hair growth within 7 days with minimum inflammation, offering a broad clinical application.
2.1.4. Treatment of Melasma
Melasma is a type of acquired hyperpigmentation disorder that affects the photo-exposed parts of the face and significantly influences the quality of life of those who suffer from it [84,85]. Microneedling plays a role in the treatment of melasma by facilitating the delivery of topical therapies to the epidermis and dermis [85,86,87]. For instance, Lima et al. [87] evaluated the effectiveness of microneedling performed with a Dermaroller® (Dr. Roller® Mooham Enterprise, Gyeonggi-do, Republic of Korea) with a needle length of 1500 µm, with a combination of depigmentation therapy and sunscreen in treating 22 patients with recalcitrant melasma that was unresponsive to topical lightening and sunscreen. Although it was suggested that the lightening of the skin was attainable due to modifications in the skin after a moderate injury caused by the Dermaroller, the exact mechanism of skin lightening via microneedling remained unclear. Over a 4-month treatment, Farshi et al. [88] compared the efficacy of microneedling performed with needles with meso-depigmentation solution (mesoneedling) to the standard microneedling performed with needles only. In this pilot study, 20 patients received microneedling on one side of their face and mesoneedling on the other side. The needles had a 1500 µm length and 0.25 mm diameter. The therapy involved rolling in all four directions four times (right–left and horizontal–vertical). The method was used to treat melasma areas. For four months, the treatment was repeated every month. The results showed that 2–4 sessions of mesoneedling were considerably more effective in treating melasma than microneedling alone [88]. Meymandi et al. [89] compared the effectiveness of microneedling combined with 4% tranexamic acid to 4% hydroquinone in treating melasma. In this study, 60 melasma patients were randomly assigned to one of the two groups: group A (microneedling + topical 4% tranexamic acid, monthly) or group B (topical 4% hydroquinone, nightly). The utilized microneedling device (Amiea Med, MT Derm GmbH) contained six fine needles of 1500 µm in length and 0.25 mm in width that could pierce the skin from 0.1 to 1.3 mm. The results showed that the efficacy of microneedling + topical 4% tranexamic acid in the treatment of melasma was comparable to that of 4% hydroquinone [89].
Microneedling shows promise in treating melasma (hyperpigmentation) and vitiligo (hypopigmentation) to varying degrees with different mechanisms [25,87]. The skin’s reaction to microneedling differs between melasma and vitiligo due to their distinct pathophysiology [90]. In melasma, microneedling may help by stimulating collagen production and facilitating the penetration of topical medications, potentially leading to improvement in hyperpigmented spots [86,87]. Additionally, microneedling may induce controlled injury, triggering a healing response that could lead to melanocyte activation and pigment dispersion [84]. Meanwhile, in vitiligo, microneedling may not directly address the underlying cause of depigmentation, but it could potentially aid in promoting melanocytes’ and keratinocytes’ proliferation and their migration to the hypopigmented areas, potentially aiding re-pigmentation [91,92]. As for the intelligence of wound-healing cells in modulating melanocytes, research suggests that fibroblasts, keratinocytes, and immune cells play critical roles in regulating melanocytes’ function and distribution. These cells release factors such as cytokines, growth factors, and extracellular matrix components, which influence melanocytes’ behavior in response to stimuli, including wound-healing processes [93].
2.1.5. Skin Rejuvenation
Subsurface resurfacing and laser toning are two concepts used to describe minimally invasive skin rejuvenation techniques [94]. These modalities are designed to improve wrinkles, skin laxity, and skin texture. Recently, microneedling has gained popularity in skin rejuvenation due to its efficacy, safety, and long-lasting and natural results [94,95]. For facial rejuvenation, El-Domyati et al. [94] investigated the use and effectiveness of microneedling combined with PRP or 15% trichloroacetic acid (TCA) peeling. The Dermaroller® (ADROLL, TD, Spain) was made of 600 stainless steel needles of 1000 µm in length. Twenty-four photo-aging volunteers were randomized into three equal groups based on the technique conducted on each side of the face (microneedling alone or in combination with PRP or 15% TCA peeling). The volunteers received six sessions of treatment, with one session every two weeks. When microneedling and PRP or microneedling and TCA were combined, the results were significantly better than microneedling alone, since there was a significant increase in the thickness of the epidermis, particularly following TCA treatment. In the three groups studied, there were organized collagen bundles with newly generated collagen formation. However, the use of microneedling in conjunction with PRP appeared to be more beneficial for facial rejuvenation than microneedling in conjunction with TCA. In another study [29], ten patients with Fitzpatrick skin types III and IV and Glogau wrinkle classes II to III received six skin microneedling sessions separated by two weeks. Microneedling was carried out with a Dermaroller® (Directive MDD, Germany) that had 192 needles arranged in eight rows, with a needle length of 1500 µm and diameter of 0.25 mm. The Dermaroller® passed six times in eight directions (vertical, up and down, horizontal, right and left, and in both diagonal directions). The results showed that microneedling improved the photo-aged skin significantly. However, to preserve the progress made, multiple sessions were frequently required.
Microneedling was combined with radiofrequency (RF) energy to stimulate the production of collagen [96]. Radiofrequency microneedling (RFM) is used to treat various skin conditions, such as acne scars, acne vulgaris, and skin rejuvenation [97]. The RFM devices are composed of an energy system with a 50 W output and a disposable tip with 49 insulated gold-plated needles. The depth of the needles can be adjusted from 0.5 to 3.5 mm [98]. The RF energy heats the skin to 65–70 °C, without thermally damaging the epidermis [97,99]. The insulated needles penetrate the epidermis with minimal heating while effectively delivering the desired energy to predetermined depths [99]. The needles’ adjustable depth allows for distinct electrothermal coagulation in different dermis layers [98]. Several studies have shown that fractional radiofrequency is safe and effective for treating moderate and severe acne scars in different skin types [98,100,101]. Kim et al. [102] reported the findings of a pilot study on the effect of an antioxidant topical formulation containing L-ascorbic acid, vitamin E, and ferulic acid on facial photo-aging after microneedling treatment with radiofrequency (FMRF). In this study, all patients were treated using a pulse-type fractional microneedle FMRF device (SylfirmTM, Seongnam, Republic of Korea) with 25 non-insulated microneedles in 5 × 5 arrays. The patients were instructed to apply four to five drops of the topical formulation to one side of the face immediately after FMRF treatment. The results showed that the laser-assisted delivery of the antioxidant formulation following FMRF was a safe and effective adjuvant approach for the treatment of photo-damaged skin.
2.1.6. Treatment of Skin Cancer
Microneedling has been combined with anticancer drugs to treat squamous- and basal-cell skin carcinoma and melanoma [103,104,105,106]. Ahmed et al. [105] found that microneedling by pretreating the skin with a Dermaroller® (SQY®, Guangdong, China) containing 40 needles of 500 μm in length and 50 μm in diameter enhanced the penetration of doxorubicin and celecoxib to tumor tissues by ~2-fold compared to passive delivery. Both drugs were encapsulated into liposomes and loaded into gels to be delivered topically, with and without microneedling. Almayahy and co-workers [106] reported that microneedling using Dermapen® (ZJchao, China) enhanced the penetration of imiquimod, a drug used to treat basal-cell carcinoma and characterized by its limited permeation into the skin. Porcine skin was initially pierced with the stainless steel Dermapen®, and the commercial product Aldara® cream containing imiquimod was applied onto the skin. In another study [103], microneedling using a Dermapen® (ZJchao, China) was also used to promote the intradermal delivery of imiquimod. A 5% w/w imiquimod cream was applied to the skin after pretreatment with either an oscillating Dermapen or a non-oscillating Dermastamp to overcome the limitations of drug permeation. The results of this study highlighted the significant increase in the intradermal permeation of imiquimod when the oscillating Dermapen was used compared to the limited dermal permeation of imiquimod with the non-oscillating Dermastamp. This approach would ultimately improve the treatment of basal-cell carcinoma, particularly in patients who do not prefer surgery. Naguib et al. [104] used microneedling (Dermaroller®, Cynergy, LLC, NV, USA) to effectively deliver a topical 5-FU cream into the target tissues to treat basal-cell carcinoma. The results showed that the pretreatment of the skin with microneedling increased the flux of 5-FU by up to 4.5-fold, improving its clinical efficacy. Therefore, the above studies confirmed that microneedling could effectively deliver the therapeutic agents to the target tissues, thus enhancing the tumor inhibition effect [107,108]. Table 2 summarizes the microneedling devices approved by the Food and Drug Administration (FDA) for cosmetic applications.

Table 2.
FDA-approved microneedling devices for cosmetic applications.
While microneedling has proven its efficacy in treating skin cancer, there are concerns regarding the potential spread of cancer cells if microneedling is performed over a skin lesion affected by cancer. Nevertheless, to the best of our knowledge, there are currently no published studies that definitively confirm the spread of existing skin cancer through microneedling.
3. Fabricated Microneedle Patches
Different materials have been used to fabricate microneedle patches (MNs) [113,114]. Previously, MNs were made of stainless steel, silicon, ceramic, and glass. However, these MNs lack biocompatibility and biodegradability. MNs have now become more popular and exhibit better breakage resistance due to their sufficient mechanical strength [115]. In addition, the use of biodegradable and biocompatible materials becomes desirable for the fabrication of microneedles [116].
Silicon, metals, ceramics, and polymeric materials are employed in the fabrication of MNs [117]. The first introduced MNs were made of silicon. Multiple shapes and types of MNs could be made from silicon owing to its flexible nature, which makes it a preferable material. However, a variety of drawbacks limit the usage of silicon in MN fabrication, including high cost, time-consuming and complex procedures, and high skin fracture potential, which may lead to skin infections. For handling such concerns, a compatible, biodegradable, nano-structured, porous silicon has been developed for the MNs’ tips. Thus, even if the tip is fractured and persists within the skin, it will be degraded in a few weeks. The manufacturing of silicon MNs with nano- and porous features significantly affects the skin’s permeability and results in improved drug delivery [109]. Metals, mainly stainless steel and titanium, exhibit decent mechanical characteristics; hence, they are prevalent in the production of MNs [23]. Before titanium, stainless steel was the first metal used in the manufacturing of MNs. It has been utilized over a few decades due to its biocompatibility under expanded clinical use and patient compliance [109]. Metallic materials are more rigid and difficult to fracture relative to silicon [114]. Although metal MNs are capable of penetrating skin, their application may lead to an allergic response [114]. Porous titanium MNs, which are relatively novel developments, have been investigated for various biomedical transdermal delivery systems, including the loading and delivery of macromolecule compounds like insulin [118]. Ceramic materials, such as alumina, calcium sulfate dihydrate, and calcium phosphate dihydrate, have been employed in the manufacturing of MNs owing to their valuable chemical characteristics, reliable resistance to compression, and biocompatibility [114]. On the other hand, these materials tend to have lower tensile strength, particularly alumina, which is fragile and readily broken within the patient’s body [119]. Currently, alumina is frequently utilized to fabricate micro- or nano-scale porous MNs for delivering fluids [119]. Polymers have gained a lot of interest in MNs’ fabrication due to their biocompatibility, biodegradability, cost-effectiveness, and distinct mechanical properties, such as their capacity to resist higher bending stresses without breaking down [120]. However, they are weaker than metals and silicon [121]. A variety of polymers, including poly (methyl methacrylate) (PMMA), polylactic acid (PLA), poly (carbonate), polystyrene, and SU-8 photoresist, have been utilized to fabricate MNs [114,122].
There are five types of MNs: solid, hollow, dissolvable, coated, and hydrogel [121] (Figure 3). Each type of MN has its own attributes, benefits, drawbacks, uses, and materials.
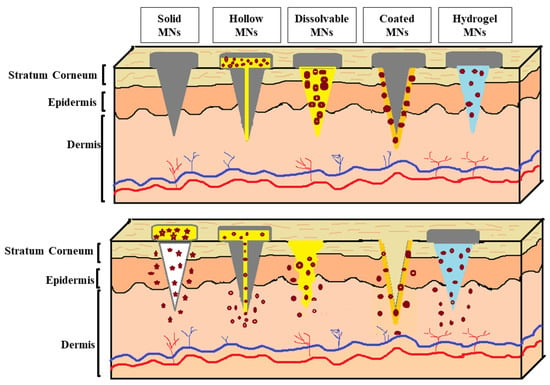
Figure 3.
Types of microneedles (solid, hollow, dissolvable, coated, and hydrogel MNs).
Solid MNs, also known as “poke and patch”, have attracted attention in transdermal delivery because of their capability to improve skin penetration by making holes in the skin over the SC [121]. Solid MNs can be made from various materials, such as silicon [123], metals [124], and polymers [125]. Additionally, solid MNs have several advantages, including low cost, a broad range of mass production technologies, and appropriate mechanical strength [126]. In this type of MN, the cosmetic agent is not encapsulated within the MNs. However, it is applied via semi-solid formulations onto skin pretreated with solid MNs to transport the active cosmetic molecules into the dermis through the pores [127]. Despite their numerous advantages, the use of solid MNs is not patient-friendly, as it requires a two-step application [128]. In addition, removing broken metal or silicon MNs from the skin is considered to be another major drawback for this type of MN [119].
Vibrating solid MNs are designed by connecting solid MNs with a motor-driven vibrator at different vibrational frequencies [129]. The results showed that solid MNs were more effective when vibrating at higher frequencies. This is because a higher vibration frequency creates more pores, which act as transdermal pathways, enhancing the permeability of drugs across the skin [129]. This type of MN has been used to enhance the transdermal delivery of ascorbic acid (AA) through the skin. Optimal conditions for the in vitro and in vivo transdermal delivery of AA were chosen based on vibrating the MNs at three levels of intensity, application time, and application power. A pharmacokinetics study of AA gel in rats found that the AUC0–∞ and Cmax increased by 1.35- and 1.44-fold, respectively, compared with those of AA gel obtained without using the vibrating solid MNs [130].
Hollow MNs, also known as “poke and flow”, contain a conical cavity internally fabricated with a biocompatible polymer using injection molding [121,131]. When the hollow MNs are applied to the skin, they provide a pressure-driven flow of the liquid formulations. The delivery rate can be modulated to rapid bolus injection, slow infusion, or other delivery rates [127]. On the other hand, hollow MNs usually require high-precision and high-cost manufacturing technologies [113]. In addition, after insertion, the tips of MNs press against the surrounding dense dermal tissue, obstructing the flow of the cosmetic agent solution and resulting in an inaccurate dose [132,133]. Although various researchers have studied hollow MNs for transdermal vaccination routes [134] and insulin delivery [135], as they can be used like tiny hypodermic needles for injection [136], studies have shown that they are not primarily used in cosmetic applications.
Contrarily, studies have shown that dissolvable MNs offer great potential in the cosmetics field [137], making them the most commonly used MNs for delivering active cosmetic agents. In the dissolved MNs, also known as “poke and release”, the cosmetic agent can be encapsulated within a dissolved or biodegradable matrix that is completely dissolved when introduced into the skin, without producing any biohazardous waste [121,138]. These MNs can deliver and release molecules immediately for short-term applications [139], or over prolonged/sustained periods [140], based on the degradation rates of the polymers [121]. The dissolvable MNs have several advantages, such as high loading efficiency and the absence of the requirement for the disposal of sharps, as well as the risk of MNs’ reuse [125,140]. Cosmetic agents delivered to the skin using dissolvable MNs are discussed in more detail in the following section.
Coated MNs, also known as “coat and patch”, are used to deliver ionic, polar, and water-soluble molecules like proteins and peptides [121,122]. This strategy is a one-step procedure that provides rapid delivery with a lower loading efficiency, making it ideal for delivering highly potent compounds [122,141]. In this type of MN, a formulation composed of polymer(s) and the loading compound is deposited on the tips of the MNs by dip-coating, casting deposition, spray-drying, and inkjet printing [121]. Metal or silicone MNs are commonly used as the base structure to ensure sufficient mechanical strength to pierce the skin. Additionally, studies have shown that drugs coated on MNs in a solid phase can be stable for a long time [142]. The delivery of high-molecular-weight compounds from coated MNs has proven attractive [122].
Finally, hydrogel MNs are made from super-swelling polymers [143]. The hydrophilic structure of the polymers allows them to absorb a considerable amount of water into their three-dimensional polymeric network, which regulates the release of the loading compounds. Due to the presence of interstitial fluid, these polymers swell when applied to the skin [144]. The rate of release of loading compounds can be controlled based on the degree of crosslinking of the hydrogel network, allowing for slow release over several days [121,144]. This may result in patient discomfort due to the long patch-wearing time [141]. In general, polymers used in the fabrication of MNs should be biocompatible, non-immunogenic, and mechanically strong enough to be inserted into the skin [109,121]. Aung et al. [145] fabricated dissolved MNs and hydrogel MNs to deliver the hydrophilic cosmetic agent alpha-arbutin (α-arbutin), commonly used for skin lightening. MNs were made from polyacrylic acid-co-maleic acid (PAMA) and polyvinyl alcohol (PVA) (1:4). Both types of MN were sharp, stable for 3 months at 25 °C, and showed high mechanical strength, allowing for their successful insertion into porcine skin. Needles of dissolved MNs were completely dissolved after 45 min, and those of hydrogel MNs swelled after 4 h. The in vitro permeation and in vivo studies demonstrated significant enhancement of α-arbutin-loaded dissolved and hydrogel MNs when compared to α-arbutin gel and α-arbutin commercial cream. The benefits, risks, and limitations of the fabricated MNs are summarized in Table 3. In addition, fabricated MNs approved by the FDA for cosmetic applications are summarized in Table 4.

Table 3.
Benefits, risks, and limitations of fabricated MNs.

Table 4.
FDA-approved fabricated MNs for cosmetic applications.
4. Cosmetic-Agent-Loaded Dissolvable Microneedles
Our literature search revealed that dissolvable MNs are most commonly used when delivering cosmetic agents through the skin. Cosmetic agents such as ascorbic acid (AA) [153], HA [154], retinoids [155], glutathione [156], acetyl hexapeptide-3 (AHP-3) [157,158], and other agents that are frequently used in various skin conditions and loaded into dissolvable MNs are discussed in detail below.
4.1. Ascorbic Acid (AA)
AA is an antioxidant agent with an anti-wrinkle effect [159], characterized by its limited skin permeation due to high aqueous solubility (333 mg/mL) and low log P (−1.85) [160]. AA-loaded dissolvable MNs showed a significant improvement in skin with wrinkles, offering a potential anti-wrinkle cosmetic application. Moreover, clinical results showed that these MNs were more convenient, safer, and did not induce skin irritation or sensitization [159]. Kim et al. [161] developed HA-dissolvable MNs to deliver magnesium ascorbyl phosphate (MAP) transdermally through porcine ear skin. The length of the needles was ~400 μm. The MNs were able to form 95–100 micro-channels after 2 min of treatment with ~125 μm depths, as determined by confocal microscopy. It was found that the application of HA-dissolvable MNs enhanced the delivery of MAP into the skin by twofold compared to the passive topical delivery of MAP (solution with no MNs) (96.8 ± 3.9 μg/cm2 vs. 44.9 ± 16.3 μg/cm2). Another study by Park et al. [162] showed that the biocompatible dissolvable carboxymethyl cellulose (CMC) MNs significantly enhanced the antioxidant activity of AA after crossing the skin: about a sixfold increase compared to the same amount of AA applied topically onto the skin without MNs. Additionally, amylopectin was used in combination with CMC to finely tune the dissolution and mechanical properties of MNs.
4.2. Hyaluronic Acid (HA)
HA is commonly used as a polymer matrix to fabricate MNs for cosmetic applications [32,159,161,163,164,165,166]. HA exhibited high compatibility, viscoelastic properties, and skin-moisturizing effects [32]. In addition, HA has a high volumizing effect due to its strong water-binding potential and stimulates the fibroblasts, leading to collagen synthesis [167]. Avcil et al. [165] demonstrated the usefulness of HA-dissolvable MNs loaded with bioactives of arginine/lysine polypeptide, acetyl octapeptide-3, palmitoyl tripeptide-5, adenosine, and seaweed extract in improving skin hydration, reducing wrinkles, and increasing the density of the skin and the thickness of the dermis. Choi et al. [167] investigated the efficacy of HA as an anti-wrinkle agent using HA-MNs and found that these MNs were more effective against wrinkles after an 8-week treatment, increasing the elasticity of the skin compared to topical applications containing HA. Additionally, the authors of this study reported that the application of HA-MNs was not associated with skin irritation. Moreover, a combination of HA and the herbal medicine Lonicerae flos ethanol extract (LEE) was loaded into the MN patch to improve skin moisturization [168]. Owing to the moisturizing effect of HA, the twenty female participants, who placed the patches on their forearms, demonstrated an increase in skin moisture content of between 29.6 and 88.5% compared to the untreated group, suggesting the addition of HA and LEE to the moisturizing formulations available on the market. In another study [169], dissolvable HA-MNs with a backing layer made of nanocellulose and loaded with the natural antioxidant rurin were developed for skin applications. The HA-MNs arrays of 200 μm base widths, 450 μm height, and 500 μm tip-to-tip distance showed sufficient mechanical properties to withstand skin insertion. Due to the hydrating and regenerative properties and volumizing effect of HA, the results showed that this novel system can potentially be used for skin cosmetic applications [169].
4.3. Retinoids
Retinoids are a group of compounds that include vitamin A and retinoic acid derivatives such as retinyl retinoate, all-trans retinoic acid (ATRA), retinal, and retinol. Retinoids are commonly used in photo-aged skin characterized by increasing wrinkles, thickening, inelasticity, dryness, roughness, shallowness, and pigmentary mottling of patchy and irregular colors of the skin [170,171,172]. Limcharoen et al. [173] developed a combination of two transdermal delivery systems using chitosan, MNs, and proretinal nanoparticles (PRNs) to directly target retinal into the epidermis and dermis. The amount of retinal in the dermis was investigated after applying three different forms (PRN-loaded MNs, PRN suspension, or conventional retinal solution). Imaging techniques confirmed the formation of micro-channels in the skin after applying PRN-loaded MNs, resulting in higher recovery of retinal in the dermis, which could benefit skin conditions such as atrophic scars and photo-aged skin. Recently, this research group loaded PRNs into detachable MNs made of HA and maltose at a 1:1 ratio. The arrays of MNs were embedded into the skin tissues, resulting in diffusion of PRNs in the epidermis and dermis layers, which, in turn, increased the epidermal thickness compared with unloaded microneedles [172]. In another study [174], the retinoid derivative ATRA was loaded into MNs (ATRA-MNs) to treat pigmented lesions commonly seen on the skin of elderly people. The ATRA-MNs were made of sodium hyaluronate as the base material. The ATRA-MNs were applied to the lesion site once a week for four weeks, and the local (skin irritation) and systemic adverse reactions were assessed during the study. These results showed that the ATRA-MNs were promising for whitening pigmented lesions; in addition, they were safe in humans. Additionally, a follow-up study [175] evaluated the use of the ATRA-MNs patches in treating seborrheic keratosis, one of the most common benign skin tumors in people > 50 years of age. The dissolvable MNs, made of sodium hyaluronate, were efficient in delivering ATRA into the epidermis and dermis without causing serious adverse events. The activity of ATRA was confirmed by inducing the epidermal hyperplasia and the expression of heparin-binding epidermal growth factor-like growth factor, and by accelerating epidermal cell turnover and stratum corneum turnover. This ultimately resulted in the seborrheic keratosis lesions falling off the surface of the skin.
4.4. Glutathione
Glutathione is a natural anti-aging agent that protects protein thiols from oxidation by reactive oxygen species. In addition, glutathione can be used as a skin-whitening agent in cosmetics because it prevents melanin synthesis via tyrosinase inhibition. Nevertheless, the poor permeability and unpleasant odor of glutathione limit its use in cosmetic applications [176]. To circumvent the drawbacks of the current glutathione delivery systems, Lee et al. [176] proposed fast-dissolving MNs made from deodorizing biopolymers that would improve glutathione efficacy and patient compliance. After screening different biopolymers for odorless glutathione formulations, HA was used to fabricate glutathione-loaded MNs. The glutathione MN arrays (10*10 MNs/cm2) were made by the solvent casting of aqueous solutions containing 10% dissolved solutions with variable glutathione concentrations. Based on the experimental findings presented in this study, the feasibility of HA-MNs as a potential platform for transdermal delivery of glutathione or drugs with unpleasant or unsatisfactory organoleptic qualities has been proven.
4.5. Acetyl-Hexapeptide-3 (AHP-3)
Acetyl hexapeptide-3 (AHP-3) or acetyl hexapeptide-8 (AHP-8), also marketed as Argireline®, are peptide mimetics or neurotransmitter-inhibiting peptides that are effective anti-aging ingredients for skin [177,178]. Several MN eye patches with varying geometries and curvatures were fabricated with resin using 3D printing for the delivery of the anti-wrinkle peptide AHP-3 [179]. The best MN geometry was then used to fabricate personalized MNs for anti-wrinkle therapy of 800 µm in height, with 100 µm tip diameters, 800 µm interspacing, and 400 µm base diameter across all curvatures. Using the personalized MNs, the in vitro skin permeation study revealed an improvement in the transdermal delivery of AHP-3 for wrinkle treatment [179]. In another study [180], critical parameters such as the mechanical strength, rate of polymerization, rate of swelling, 3D printing resolution, and safety profile of the final polymer were investigated using the two liquid monomers polyethylene glycol diacrylate (PEGDA) and vinyl pyrrolidone (VP), in various proportions. Based on the above factors, the best resin had a weight ratio of 7:3 (VP: PEGDA) for the delivery of the anti-wrinkle AHP-3 [180]. A personalized MN eye patch was produced using computer-assisted design (CAD) V 1.1.7 software and then fabricated using a Digital Light Processing (DLP) 3D printer with the best resin, utilizing a 3D-scanned face model. The ability of the fabricated MNs to penetrate human cadaver skin was demonstrated in vitro. It was found that the MNs remained intact after compression, and that the human dermal fibroblasts were also unaffected by the final polymer. As a result, the photopolymer-fabricated personalized MNs were considered to be a novel way to boost the transdermal delivery of AHP-3 for effective wrinkle reduction. Furthermore, An et al. [181] fabricated MN patches of mixed HA and crosslinked HA (CLHA). CLHA is commonly used in dermal fillers. The HA/CLHA MN patches were loaded with AHP-8 or epidermal growth factor (EGF) for wrinkle improvement. Korean females (n = 52) were enrolled in a double-blind, randomized clinical study conducted for 29 days. Based on treatment, the subjects were divided into three groups. The first group was treated with MNs alone, the second group with MNs/AHP-8, and the third group with MNs/EGF. The results revealed that the second and third groups treated with MNs/AHP-8 and MNs/EGF showed statistically significant improvements in wrinkles compared to those treated with MNs alone (p < 0.05). Additionally, no serious adverse events were noted for the fifty subjects who completed this study.
4.6. Niacinamide
Niacinamide, also known as vitamin B3, is a hydrophilic compound that is well recognized for its beneficial effects on skin aging, photo-aging, hyperpigmentation, anti-inflammatory effects in acne, and discolored skin patches [163,182]. Park et al. [183] developed niacinamide-loaded MNs using various compositions of sodium hyaluronate and CMC. In addition, amylopectin was added to the polymer matrix to increase the mechanical strength of the MNs. In this study [183], the effect of the MN composition on the skin permeability of niacinamide and the mechanical strength and solubility of the MNs were investigated. The results demonstrated that increasing the CMC concentration improved the mechanical properties of the MNs, leading to a dramatic increase in the permeability of niacinamide through the skin due to the high ability of the MNs to puncture the SC barrier.
Additionally, the niacinamide’s skin permeability was controlled by changing the polymeric composition of the MNs. In a recent study, Shin et al. [184] developed microneedle-like particles (MLPs), a modified approach to conventional MNs. MNs are currently only available as patches that can cover a limited skin area. Thus, this new platform allows for the application of MLPs over a large area of the skin surface, where needles disrupt the skin during the rubbing process due to their sufficient mechanical strength. The results of this study found that, after applying MLPs to the skin, the permeability of niacinamide increased by up to 200%. This was attributed to the fact that the MLPs were able to disrupt the skin, as confirmed by fluorescence images of porcine skin slices, where the fluorescent dye was able to penetrate deeper into the skin tissues.
4.7. Collagen
Collagen makes up a large proportion of the human skin, giving the skin smoothness, hydration, density, and elasticity [185]. As the skin ages, its collagen content decreases, resulting in wrinkled skin [186]. Dissolvable MNs were used as a platform to deliver collagen to skin layers to replace the collagen levels lost due to natural skin aging. For instance, Sun et al. [187] delivered various concentrations of type I collagen (1, 2, 4, and 8% w/w) into porcine and human skin utilizing PVP-MNs. The needles’ length was ~365 μm, with 135 μm in diameter at the base. The distribution of collagen I-labeled rhodamine B isocyanate through the skin was evaluated using fluorescence images. The penetration efficiency of the PVP-MNs varied based on the concentration of collagen, where the penetration decreased concomitant with an increase in collagen concentration. This is because increasing the collagen content resulted in poor mechanical strength of the MNs. The results showed that PVP-MNs were effective in delivering collagen I into the epidermis and dermis of porcine and human skin, offering a fast, safe, effective, and simple delivery system for cosmetic applications. Aditya et al. [188] developed dissolvable collagen MNs of various needle lengths (300–600 μm) to reach different targeted layers of the skin. The collagen MNs were fabricated from collagen and PVP at a ratio of 7:3. Various process parameters, such as pressure, temperature, and duration of solidification, were optimized using the Taguchi method. The results showed that the needles’ height and time of solidification played an important role in the MN fabrication. The histological images revealed the ability of collagen to penetrate the skin, indicating that collagen MNs may serve as a promising platform for delivering collagen for younger-looking skin.
Moreover, a mold-free approach was used to develop collagen MNs using a simple photolithographic method [189]. This approach used a photomask consisting of embedded micro-lenses to govern the MNs’ geometry with a shock-absorbing backing layer. The use of simple glass scaffolds controlled the length of needles. The collagen MNs were tested for their mechanical properties, insertion into human skin, and collagen delivery. The results illustrated that the needles were sharp, with two different lengths of 1336 and 957 μm. In addition, the MNs could resist fracture forces of up to 25 N, penetrate human skin when inserted with the force of a thumb, and enhance the permeation of collagen through rat skin. Thus, the mold-free approach proved that collagen could be delivered within the dermis for cosmetic applications [189]. Regardless of the reported benefits of the exogenous collagen delivered to the dermis, there is no strong evidence to support the notion that externally applied collagen can promote collagen remodeling and dermal improvement or reverse natural aging [190,191].
4.8. Combinations of Cosmetic Agents
Combinations of cosmetic agents were loaded into dissolvable microneedles. Park et al. [163] used polydimethylsiloxane (PDMS) molds fabricated using a laser-writing process to develop dissolvable MNs and enhance the skin permeability of the cosmetic agents AA and niacinamide. The dissolvable MNs were prepared from sodium hyaluronate and amylopectin. The results showed that adding amylopectin increased the mechanical strength of the needles but decreased their dissolution rate. In addition, MNs improved the skin permeability of AA and niacinamide compared to their application without MNs [32]. For wrinkle improvement, AA was combined with retinyl retinoate. The two cosmetic agents exhibited different hydrophilicity and were loaded into dissolvable HA-MN patches. The patches were safe and demonstrated efficacy in improving wrinkles, suggesting their potential application in cosmetics [32]. A recent study by Sawutdeechaikul et al. [164] developed special dissolvable MNs known as detachable dissolvable MNs (DDMNs), which allow the needles to detach from the base within 2 min post-administration. DDMNs were fabricated from HA and PVA and could effectively embed AA into the epidermis and dermis to lessen the spots of post-acne hyperpigmentation. Glutathione was co-loaded with AA into the detachable MNs (DDMNs) to stabilize AA. The experimental studies found that glutathione effectively stabilized AA in the DDMNs, compared to vitamin E and coenzyme Q10, due to its redox potential. No degradation was detected for AA in DDMNs for at least 6 months when stored at 25, 40, or 50 °C, corresponding to a shelf-life of >2 years at room temperature as estimated using the Arrhenius equation. In addition, the co-delivery of AA and glutathione to skin tissues resulted in a reduction in melanin, which, in turn, decreased skin hyperpigmentation. Recently, Jang et al. [192] studied the skin improvement effects of HA. The anti-wrinkle agent adenosine was loaded into high- and low-molecular-weight HA-dissolvable MN patches (Ad-HMN and Ad-LMN, respectively). Both Ad-HMN and Ad-LMN patches were evaluated for skin wrinkling, dermal density, elasticity, and safety. Clinical tests were performed for 12 weeks on 23 females, where patches were applied once every 3 days for 8 weeks to the designated crow’s feet area. The results showed significant skin improvement without adverse skin events for both Ad-HMN and Ad-LMN patches. Additionally, the Ad-HMN patch had a better skin effect than the Ad-LMN patches with a similar adenosine dose. Furthermore, the lipophilic compound horse oil was combined with the hydrophilic compound adenosine for wrinkle improvement and skin restoration [193]. A topical formulation of horse oil was co-loaded into adenosine-dissolvable MNs (Ad-MNs), forming a two-phase delivery system in a single patch (HO-Ad-MNs patch). Dissolvable Ad-MNs were fabricated using HA. The efficacy of HO-Ad-MN patches on skin elasticity, hydration, dermal density, and wrinkles was clinically evaluated for 20 women and compared with that of Ad-MNs. The microscopic images demonstrated the successful delivery of adenosine and horse oil into the skin through the micro-channels created by the HO-Ad-MN patches. Additionally, the HO-Ad-MN patches significantly improved skin restoration and wrinkles compared with Ad-MNs, without observing adverse events [193].
4.9. Other Cosmetic Agents
Cosmetic agents such as horse oil [194] and adenosine [195,196] have also been loaded into dissolvable MNs using HA as a matrix base. It is worth noting that other cosmetic agents, such as coenzyme Q10 [197], peptides [198], green tea extract [199], grape seed extract [200], vitamin E [201], and quercetin [202,203,204], can also be potential candidates for fabricated MNs (particularly dissolvable MNs) for cosmetic applications.
5. Safety of Microneedling
Microneedling using Dermaroller, Dermapen, Dermastamp, and fabricated MNs has safety issues related to transdermal delivery, such as skin irritation and pain sensation [204,205]. The FDA has reported that the risk associated with microneedling, including skin irritation, mild bleeding, bruising, redness, itching, rashes, and peeling, may last for a short time (few days) or a long time (few weeks) [28,206]. In addition, the use of the devices can sometimes be accompanied by skin infections [207], irritant and allergic contact dermatitis, hyperpigmentation, abnormal scarring, and irritant and allergic granulomas [208]. The FDA has addressed the safe use of these devices, such as cleaning and disinfecting the reusable parts between patients, where reusing needle cartridges can cause or spread infection. Furthermore, special care might be needed after microneedling, as skin might become more sensitive to the sun and skin care products containing retinol, glycolic acid, or alcohol [206]. Because microneedling is relatively similar to conventional hypodermic injection, they are supplied as sterile products. Additionally, the fabrication of devices from metals routinely used in a dermatological setup makes their sterilization and re-sterilization an easy process [13,28]. To minimize the side effects of microneedling, patients with recent sun exposure are recommended to delay the microneedling procedure until all traces of suntan have faded to avoid post-microneedling dyspigmentation. In addition, patients with oral herpes labialis might be at high risk of viral reactivation post-microneedling. Moreover, microneedling over inflammatory or active acne lesions may lead to bacterial micro-abscesses or granulomas. Furthermore, skin preparation and hygiene before the microneedling procedure are important, where proper skin cleansing removes makeup and debris from the skin’s surface and reduces the risk of introducing bacteria into the deeper skin layers, decreasing superficial skin infections [31].
For polymeric MNs, owing to the small size of MN arrays, sufficient mechanical strength, including the strength, the geometry of tips, the aspect ratio of height-to-base diameter, and the sharpness of needles, is required to insert the needle successfully into the skin without breakage [209]. It is worth noting that after MNs are inserted into the skin, the loading agent is delivered, and there is no way to remove the needles from the body [210]. The variation in the MNs’ safety depends on the types of MNs and materials used in the fabrication [109]. The microbiological characterization of hydrogel-forming MNs and the potential for microbes to pass into the skin following the penetration of MNs were described by Donnelly et al. [211]. There was no evidence of microbial penetration through swollen MNs. Investigation of human volunteers indicated that when MNs were employed for transdermal drug delivery, skin or systemic infections were relatively uncommon [211]. Altogether, the chemical and biological safety of materials used in the fabrication of MNs, generally regarded as safe (GRAS), should be considered to achieve successful and safe administration [18,204]. MNs should possess a fracture force greater than the insertion force required for successful microporation, where the higher the fracture force, the safer the MN insertion [212]. Microporation using MNs demonstrated minimal invasiveness, reduced pain, and less tissue trauma in human subjects, with a very low average pain score on a 0–100 mm visual analog pain scale compared to a hypodermic needle [20]. The pain sensation is inversely proportional to the MNs’ length and number [21]. Additionally, the pain intensity depends on the tip angle and shape of the needles [213]. MNs may induce bleeding, which depends on the length of the MN arrays and their penetration depth into the skin [58].
6. Regulations Related to Microneedling Products
The licensing of microneedling products is handled individually for each application (as product-specific approval), and not for each microneedle system (as specific MN systems) [116,141]. This approach usually delays the licensing process and commercialization of microneedling products, as several variables should be considered during licensing, including shape, formulation, sterilization, and packaging [141]. The FDA has raised several concerns regarding fabricated MNs, particularly those used to deliver therapeutic agents, including the stability of the formulation and the loaded therapeutic agent, content uniformity of MNs, risk analysis, sterility, and manufacturing [116]. Therefore, a thorough investigation is required to assess MNs, including cell, animal, and clinical studies. The FDA considered microneedles loaded with a therapeutic agent as a “combination product” composed of drug and device. The FDA regulations for combined products emphasized the safety and effectiveness of each product component and the product as a whole [116].
The guidance for industry and FDA staff was issued on 10 November 2020 for regulatory considerations for microneedling products. The guidance included definitions and classifications of microneedling products. Definitions of the stratum corneum, exfoliation, living layers of skin, and dermabrasion were included within the context of this guidance [214]. In addition, the guidance classified the microneedling products into (1) devices, (2) not devices, and (3) devices for aesthetic use (class II devices). It is noteworthy that microneedling combination products, acupuncture needles, hypodermic needles, tattoo machine needles, and dermabrasion devices are outside the scope of this guidance [214]. For the first category, the microneedling product is considered to be a device when it is intended for use in the diagnosis, treatment, or prevention of disease, or to affect the structure or any function of the body. Therefore, to determine whether the microneedling product is a device, the FDA considers any claim or statement that indicates penetration beyond the stratum corneum into living layers of skin (epidermis and dermis) to represent a device. Other claims, such as treating scars, wrinkles, facial lines, cellulite, dermatoses, acne, and alopecia and stimulating collagen and wound healing, also meet the definition of a device. Additionally, the FDA may evaluate the needles’ length, arrangement, sharpness, and degree of penetration into skin layers, all related to the product’s design and the technological features of the microneedling products that can be determined to be devices. Microneedling products are not considered to be devices when they do not penetrate living skin and claim only exfoliation of the skin, improving the appearance of the skin by giving it a smoother feel and a luminous look. However, these products may still be subject to other Federal Food, Drug, and Cosmetic (FD&C) Act requirements or other federal agencies. Finally, specific microneedling devices designed for aesthetic uses are classified by the FDA as class II devices (a de novo classification process). These devices use one or more needles to mechanically puncture the skin tissue for aesthetic use. This classification excludes devices intended for transdermal/topical delivery of cosmetic agents.
Additionally, according to FDA guidance, for a new microneedling device entering the market, the manufacturers should demonstrate a significant equivalent of their device to those legally available in the market. Based on the special controls described in 21 CFR 878.4430, the health risks associated with microneedling devices should be mitigated. These health risks include adverse tissue reaction or tissue damage, cross-contamination and skin infection, electrical shock, nerve and blood vessel damage, scarring, hyper/hypopigmentation, mechanical failure, and software failure. For new microneedling devices, the FDA may request clinical data and non-clinical testing, if needed. The clinical data should describe the following: (1) the clinical study protocol and representative subjects enrolled for the intended use of the device, (2) safety data collection to support the safe use of the device, (3) the proposed effectiveness endpoint of the new microneedling device, and (4) a follow-up period that ensures a reasonable assessment of the short-term and long-term performance of the device, including the safety and effectiveness of the device [214]. Nevertheless, the special controls in 21 CFR 878.4430 might differ based on the specific features of the new microneedling device, where a wireless microneedling device would require additional controls to mitigate electrical shock hazards, which are not essential in a roller with fixed needles [214].
7. Conclusions
Microneedling has now attracted great attention in the cosmetics field. This simple, inexpensive, painless, and self-administered application has shown promising results in various skin conditions, making this application widespread in the cosmeceuticals market and opening doors for many active cosmetic agents to be delivered via the microneedling technique. For microneedling, cosmetic agents can be delivered into the skin via topical preparations pretreated with microneedling devices. Meanwhile, in fabricated MNs, cosmetic agents can be loaded into the matrix of MNs. Nevertheless, due to the promising results of microneedling in cosmetic applications, numerous cosmetic agents that have not yet been delivered by microneedling applications can be regarded as potential candidates. Additionally, due to the recent advances in machine technology and materials science, microneedling, as a cosmetic application, is expected to commercially boom worldwide. One of the limitations of the clinical application of fabricated MN patches is their ability to cover small areas of the skin. However, attempts have been made to increase the size of the MN patches to cover a larger area of the skin surface by developing larger MN patches. In addition, current limitations include the complexity of the application when using a combinational treatment of microneedling and topical preparations. Furthermore, fabricated MNs possess a limited loading ability. To overcome the limitations of the clinical application of fabricated MN patches, alternative materials with desirable MN attributes, such as better mechanical properties (strength and flexibility) and biocompatibility, should be explored. In addition, new fabrication methods for MNs that can improve the delivery of several types of MNs for different therapeutic applications should be investigated. In future research, novel materials, new fabrication methods, and commercialization will be discussed in detail to ensure medical efficiency, cost-effectiveness, and mass production of fabricated MNs. In addition, advanced applications of MNs, including disease detection, management, monitoring, diagnosis, and personalized medicine, will be explored. Moreover, commercially available microneedling devices, particularly those used at home, need strict regulatory control over manufacturing. For example, it is necessary to strengthen the supervision of devices in terms of the materials used in manufacturing, mechanical properties, insertion force, dosing and release accuracy, and needle depth.
Author Contributions
R.H., B.J.A.N., A.Z.A., Y.A.-A. and R.O. contributed to the conception, design, and interpretation of the relevant literature in this review article; R.H., B.J.A.N., A.Z.A. and R.O. wrote the review article; R.H. and A.Z.A. revised the review article. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by the Deanship of Scientific Research and Innovation at Al-Zaytoonah University of Jordan.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Huang, Y.; Yu, H.; Wang, L.; Shen, D.; Ni, Z.; Ren, S.; Lu, Y.; Chen, X.; Yang, J.; Hong, Y. Research progress on cosmetic microneedle systems: Preparation, property and application. Eur. Polym. J. 2021, 163, 110942. [Google Scholar] [CrossRef]
- Krasuska, M.; Lavda, A.; Thompson, A.; Millings, A. The role of adult attachment orientation and coping in psychological adjustment to living with skin conditions. Br. J. Dermatol. 2018, 178, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Derm. Endocrinol. 2012, 4, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Lawson, C.N.; Hollinger, J.; Sethi, S.; Rodney, I.; Sarkar, R.; Dlova, N.; Callender, V.D. Updates in the understanding and treatments of skin & hair disorders in women of color. Int. J. Womens Dermatol. 2017, 3, S21–S37. [Google Scholar] [PubMed]
- Ganesan, P.; Choi, D.-K. Current application of phytocompound-based nanocosmeceuticals for beauty and skin therapy. Int. J. Nanomed. 2016, 11, 1987. [Google Scholar] [CrossRef] [PubMed]
- Abu-Huwaij, R.; Al-Assaf, S.F.; Hamed, R. Recent exploration of nanoemulsions for drugs and cosmeceuticals delivery. J. Cosmet. Dermatol. 2022, 21, 3729–3740. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; Ponec, M. The skin barrier in healthy and diseased state. Biochim. Biophys. Acta 2006, 1758, 2080–2095. [Google Scholar] [CrossRef]
- Hamed, R.; Al Baraghthi, T.; Alkilani, A.Z.; Abu-Huwaij, R. Correlation between rheological properties and in vitro drug release from penetration enhancer-loaded Carbopol® gels. J. Pharm. Innov. 2016, 11, 339–351. [Google Scholar] [CrossRef]
- Alkilani, A.Z.; Nasereddin, J.; Hamed, R.; Nimrawi, S.; Hussein, G.; Abo-Zour, H.; Donnelly, R.F. Beneath the Skin: A Review of Current Trends and Future Prospects of Transdermal Drug Delivery Systems. Pharmaceutics 2022, 14, 1152. [Google Scholar] [CrossRef]
- Bos, J.D.; Meinardi, M.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef]
- Andrews, S.N.; Jeong, E.; Prausnitz, M.R. Transdermal delivery of molecules is limited by full epidermis, not just stratum corneum. Pharm. Res. 2013, 30, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Hamed, R.; Mahmoud, N.N.; Alnadi, S.H.; Alkilani, A.Z.; Hussein, G. Diclofenac diethylamine nanosystems-loaded bigels for topical delivery: Development, rheological characterization, and release studies. Drug Dev. Ind. Pharm. 2020, 46, 1705–1715. [Google Scholar] [CrossRef] [PubMed]
- McCrudden, M.T.; McAlister, E.; Courtenay, A.J.; González-Vázquez, P.; Raj Singh, T.R.; Donnelly, R.F. Microneedle applications in improving skin appearance. Exp. Dermatol. 2015, 24, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Carrier, A.; Chen, Y.; Lin, S.; Wang, J.; Cui, S.; Zhang, X. Polymeric microneedles for controlled transdermal drug delivery. J. Control. Release 2019, 315, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Bonati, L.M.; Epstein, G.K.; Strugar, T.L. Microneedling in all skin types: A review. J. Drugs Dermatol. 2017, 16, 308–313. [Google Scholar] [PubMed]
- Henry, S.; McAllister, D.V.; Allen, M.G.; Prausnitz, M.R. Microfabricated microneedles: A novel approach to transdermal drug delivery. J. Pharm. Sci. 1998, 87, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Y.; Zhang, J.N.; Chen, B.Z.; Wang, Q.L.; Guo, X.D. A solid polymer microneedle patch pretreatment enhances the permeation of drug molecules into the skin. RSC Adv. 2017, 7, 15408–15415. [Google Scholar] [CrossRef]
- Park, J.-H.; Allen, M.G.; Prausnitz, M.R. Polymer microneedles for controlled-release drug delivery. Pharm. Res. 2006, 23, 1008–1019. [Google Scholar] [CrossRef]
- Ripolin, A.; Quinn, J.; Larrañeta, E.; Vicente-Perez, E.M.; Barry, J.; Donnelly, R.F. Successful application of large microneedle patches by human volunteers. Int. J. Pharm. 2017, 521, 92–101. [Google Scholar] [CrossRef]
- Nguyen, H.X. Safety of microneedles for transdermal drug delivery. J. Pharmacovigil. 2018, 6, e172. [Google Scholar] [CrossRef]
- Gill, H.S.; Denson, D.D.; Burris, B.A.; Prausnitz, M.R. Effect of microneedle design on pain in human volunteers. Clin. J. Pain 2008, 24, 585–594. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Mikszta, J.A.; Cormier, M.; Andrianov, A.K. Microneedle-based vaccines. Curr. Top. Microbiol. Immunol. 2009, 333, 369–393. [Google Scholar]
- Dharadhar, S.; Majumdar, A.; Dhoble, S.; Patravale, V. Microneedles for transdermal drug delivery: A systematic review. Drug Dev. Ind. Pharm. 2019, 45, 188–201. [Google Scholar] [CrossRef]
- Cheung, K.; Das, D.B. Microneedles for drug delivery: Trends and progress. Drug Deliv. 2016, 23, 2338–2354. [Google Scholar] [CrossRef]
- Iriarte, C.; Awosika, O.; Rengifo-Pardo, M.; Ehrlich, A. Review of applications of microneedling in dermatology. Clin. Cosmet. Investig. Dermatol. 2017, 10, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Larraneta, E.; Lutton, R.E.; Woolfson, A.D.; Donnelly, R.F. Microneedle arrays as transdermal and intradermal drug delivery systems: Materials science, manufacture and commercial development. Mater. Sci. Eng. R Rep. 2016, 104, 1–32. [Google Scholar] [CrossRef]
- Harris, A.G.; Naidoo, C.; Murrell, D.F. Skin needling as a treatment for acne scarring: An up-to-date review of the literature. Int. J. Womens Dermatol. 2015, 1, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Dsouza, L.; Ghate, V.M.; Lewis, S.A. Derma rollers in therapy: The transition from cosmetics to transdermal drug delivery. Biomed. Microdevices 2020, 22, 77. [Google Scholar] [CrossRef] [PubMed]
- El-Domyati, M.; Barakat, M.; Awad, S.; Medhat, W.; El-Fakahany, H.; Farag, H. Microneedling therapy for atrophic acne scars: An objective evaluation. J. Clin. Aesthet. Dermatol. 2015, 8, 36. [Google Scholar]
- Hou, A.; Cohen, B.; Haimovic, A.; Elbuluk, N. Microneedling: A comprehensive review. Dermatol. Surg. 2017, 43, 321–339. [Google Scholar] [CrossRef]
- Alster, T.S.; Graham, P.M. Microneedling: A review and practical guide. Dermatol. Surg. 2018, 44, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yang, H.; Kim, H.; Jung, H.; Jung, H. Novel cosmetic patches for wrinkle improvement: Retinyl retinoate-and ascorbic acid-loaded dissolving microneedles. Int. J. Cosmet. Sci. 2014, 36, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, H.; Kolli, C.S.; Banga, A.K. Characterization of microchannels created by metal microneedles: Formation and closure. AAPS J. 2011, 13, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zhao, D.; Wang, X.; Li, C.; Yang, T.; Du, L.; Wei, Z.; Cheng, Q.; Cao, H.; Liang, Z. Efficient delivery of nucleic acid molecules into skin by combined use of microneedle roller and flexible interdigitated electroporation array. Theranostics 2018, 8, 2361–2376. [Google Scholar] [CrossRef] [PubMed]
- Matilda, I.; Drejøe, J.B. Microneedling in Mature Burn Scars. J. Med. Case Rep. Rev. 2019, 2, 277–281. [Google Scholar]
- Fabbrocini, G.; De Vita, V.; Fardella, N.; Pastore, F.; Annunziata, M.; Mauriello, M.; Monfrecola, A.; Cameli, N. Skin needling to enhance depigmenting serum penetration in the treatment of melasma. Plast. Surg. Int. 2011, 2011, 158241. [Google Scholar] [CrossRef] [PubMed]
- Sabri, A.H.; Cater, Z.; Ogilvie, J.; Scurr, D.J.; Marlow, M.; Segal, J. Characterisation of mechanical insertion of commercial microneedles. J. Drug Deliv. Sci. Technol. 2020, 58, 101766. [Google Scholar] [CrossRef]
- Scott, J.A.; Banga, A.K. Cosmetic devices based on active transdermal technologies. Ther. Deliv. 2015, 6, 1089–1099. [Google Scholar] [CrossRef]
- Majid, I.; Sheikh, G.; September, P. Microneedling and its applications in dermatology. Prime Int. J. Aesthetic Anti-Ageing Med. Healthc. 2014, 4, 44–49. [Google Scholar]
- Doddaballapur, S. Microneedling with dermaroller. J. Cutan. Aesthet. Surg. 2009, 2, 110–111. [Google Scholar] [CrossRef]
- Fabbrocini, G.; Fardella, N.; Monfrecola, A.; Proietti, I.; Innocenzi, D. Acne scarring treatment using skin needling. Clin. Exp. Dermatol. 2009, 34, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Kochhar, J.S.; Tan, J.J.; Kwang, Y.C.; Kang, L. Recent trends in microneedle development & applications in medicine and cosmetics (2013–2018). In Microneedles for Transdermal Drug Delivery; Springer: Cham, Switzerland, 2019; pp. 95–144. [Google Scholar]
- Amer, M.; Farag, F.; Amer, A.; ElKot, R.; Mahmoud, R. Dermapen in the treatment of wrinkles in cigarette smokers and skin aging effectively. J. Cosmet. Dermatol. 2018, 17, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- McAlister, E.; McCrudden, M.T.; Donnelly, R.F. Microneedles in improving skin appearance and enhanced delivery of cosmeceuticals. In Microneedles for Drug and Vaccine Delivery and Patient Monitoring; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018. [Google Scholar]
- Zduńska, K.; Kołodziejczak, A.; Rotsztejn, H. Is skin microneedling a good alternative method of various skin defects removal. Dermatol. Ther. 2018, 31, e12714. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, H.M.; Albalate, W. Efficacy of microneedling combined with tacrolimus versus either one alone for vitiligo treatment. J. Cosmet. Dermatol. 2020, 19, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Soltani-Arabshahi, R.; Wong, J.W.; Duffy, K.L.; Powell, D.L. Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation. JAMA Dermatol. 2014, 150, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Lewis, W. Is Microneedling Really the Next Big Thing? Practice 2014, 7, 24–28. [Google Scholar]
- Fucci-da-Costa, A.P.C.; Camasmie, H.R. Drug delivery after microneedling: Report of an adverse reaction. Dermatol. Surg. 2018, 44, 593–594. [Google Scholar] [CrossRef] [PubMed]
- Bahuguna, A. Micro needling-facts and fictions. Asian J. Med. Sci. 2013, 4, 1–4. [Google Scholar] [CrossRef]
- Agarwal, M. Dermaroller: The Transepidermal Delivery System. In Aesthetic Medicine; Springer: Berlin/Heidelberg, Germany, 2012; pp. 273–275. [Google Scholar]
- Bin Sabri, A.H. Application of Microneedles for the Treatment of Nodular Basal Cell Carcinoma. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2021. [Google Scholar]
- Singh, A.; Yadav, S. Microneedling: Advances and widening horizons. Indian Dermatol. Online J. 2016, 7, 244–254. [Google Scholar]
- Koo, H. Effectiveness of Dyes as Skin Biopsy Markers; North Carolina State University: Raleigh, NC, USA, 2018; p. 165. [Google Scholar]
- Sharad, J. Combination of microneedling and glycolic acid peels for the treatment of acne scars in dark skin. J. Cosmet. Dermatol. 2011, 10, 317–323. [Google Scholar] [CrossRef]
- Saadawi, A.N.; Esawy, A.M.; Kandeel, A.H.; El-Sayed, W. Microneedling by dermapen and glycolic acid peel for the treatment of acne scars: Comparative study. J. Cosmet. Dermatol. 2019, 18, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Baveja, S. Combination therapy in the management of atrophic acne scars. J. Cutan. Aesthet. Surg. 2014, 7, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Costa, I.M.; Costa, M.C. Microneedling for varicella scars in a dark-skinned teenager. Dermatol. Surg. 2014, 40, 333–334. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; ElMahdy, N.; Elfar, N.N. Microneedling (Dermapen) and Jessner’s solution peeling in treatment of atrophic acne scars: A comparative randomized clinical study. J. Cosmet. Laser Ther. 2019, 21, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Jang, Y.-H.; Son, Y.-H.; Lee, C.-S.; Bae, J.-Y.; Park, J.-M. Management of Hypertrophic Scar after Burn Wound Using Microneedling Procedure (Dermastamp (R)). J. Korean. Burn. Soc. 2009, 121–124. [Google Scholar] [CrossRef]
- Claytor, R.B.; Sheck, C.G.; Chopra, V. Microneedling outcomes in early postsurgical scars. Plast. Reconst. Surg. 2022, 150, 557e–561e. [Google Scholar] [CrossRef] [PubMed]
- Ramaut, L.; Hoeksema, H.; Pirayesh, A.; Stillaert, F.; Monstrey, S. Microneedling: Where do we stand now? A systematic review of the literature. J. Plast. Reconstr. Aesthet. Surg. 2018, 71, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, M.L.; Cohen, J.L. Microneedling for the treatment of scars: An update for clinicians. Clin. Cosmet. Investig. Dermatol. 2020, 13, 997–1003. [Google Scholar] [CrossRef]
- Lima, E.; Lima, M.; Lima, E.; Lima, M. Correcting Post-surgical Scar Using PCI. In Percutaneous Collagen Induction with Microneedling: A Step-by-Step Clinical Guide; Springer: Cham, Switzerland, 2021; pp. 133–139. [Google Scholar]
- Salloum, A.; Bazzi, N.; Maalouf, D.; Habre, M. Microneedling in vitiligo: A systematic review. Dermatol. Ther. 2020, 33, e14297. [Google Scholar] [CrossRef]
- Dillon, A.B.; Sideris, A.; Hadi, A.; Elbuluk, N. Advances in vitiligo: An update on medical and surgical treatments. J. Clin. Aesthet. Dermatol. 2017, 10, 15–28. [Google Scholar]
- Lima, E.V.A.; Lima, M.M.D.A.; Miot, H.A. Induction of pigmentation through microneedling in stable localized vitiligo patients. Dermatol. Surg. 2020, 46, 434–435. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Sonthalia, S. 5-Fluorouracil as an adjuvant therapy along with microneedling in vitiligo. J. Am. Acad. Dermatol. 2019, 80, e75–e76. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bharti, R.; Agarwal, S. Microneedling with Dermaroller 192 needles along with 5-fluorouracil solution in the treatment of stable vitiligo. J. Am. Acad. Dermatol. 2019, 81, e67–e69. [Google Scholar] [CrossRef] [PubMed]
- Lei, T.-C.; Hearing, V.J. Deciphering skin re-pigmentation patterns in vitiligo: An update on the cellular and molecular events involved. Chin. Med. J. 2020, 133, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.J.-M.; Li, H.O.-Y.; Zheng, D.; Glassman, S.J.; Tan, M.G. Microneedling as an Adjuvant to Local Therapies for Vitiligo: A Systematic Review and Meta-Analysis. Dermatol. Surg. 2021, 47, 1314–1316. [Google Scholar] [CrossRef] [PubMed]
- Attwa, E.M.; Khashaba, S.A.; Ezzat, N.A. Evaluation of the additional effect of topical 5-fluorouracil to needling in the treatment of localized vitiligo. J. Cosmet. Dermatol. 2020, 19, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Mina, M.; Elgarhy, L.; Al-saeid, H.; Ibrahim, Z. Comparison between the efficacy of microneedling combined with 5-fluorouracil vs microneedling with tacrolimus in the treatment of vitiligo. J. Cosmet. Dermatol. 2018, 17, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Z.A.; Hassan, G.F.; Elgendy, H.Y.; Al-shenawy, H.A. Evaluation of the efficacy of transdermal drug delivery of calcipotriol plus betamethasone versus tacrolimus in the treatment of vitiligo. J. Cosmet. Dermatol. 2019, 18, 581–588. [Google Scholar] [CrossRef]
- Martinez-Lopez, A.; Montero-Vilchez, T.; Sierra-Sánchez, Á.; Molina-Leyva, A.; Arias-Santiago, S. Advanced medical therapies in the management of non-scarring alopecia: Areata and androgenic alopecia. Int. J. Mol. Sci. 2020, 21, 8390. [Google Scholar] [CrossRef]
- Starace, M.; Alessandrini, A.; Brandi, N.; Piraccini, B.M. Preliminary results of the use of scalp microneedling in different types of alopecia. J. Cosmet. Dermatol. 2020, 19, 646–650. [Google Scholar] [CrossRef]
- Ocampo-Garza, S.S.; Fabbrocini, G.; Ocampo-Candiani, J.; Cinelli, E.; Villani, A. Micro needling: A novel therapeutic approach for androgenetic alopecia, A Review of Literature. Dermatol. Ther. 2020, 33, e14267. [Google Scholar] [CrossRef] [PubMed]
- Faghihi, G.; Nabavinejad, S.; Mokhtari, F.; Fatemi Naeini, F.; Iraji, F. Microneedling in androgenetic alopecia; comparing two different depths of microneedles. J. Cosmet. Dermatol. 2021, 20, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Fertig, R.M.; Gamret, A.C.; Cervantes, J.; Tosti, A. Microneedling for the treatment of hair loss? J. Eur. Acad. Dermatol. Venereol. 2018, 32, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Chen, Q.; Wen, D.; Chen, Z.; Wang, J.; Chen, G.; Wang, Z.; Zhang, X.; Zhang, Y.; Hu, Q. A therapeutic microneedle patch made from hair-derived keratin for promoting hair regrowth. ACS Nano 2019, 13, 4354–4360. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhao, J.; Li, H.; Yu, M.; Zhang, W.; Qin, D.; Qiu, K.; Chen, X.; Kong, M. A Drug-Free, Hair Follicle Cycling Regulatable, Separable, Antibacterial Microneedle Patch for Hair Regeneration Therapy. Adv. Healthc. Mater. 2022, 11, 2200908. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y. Targeting Wnt/β-catenin pathway for developing therapies for hair loss. Int. J. Mol. Sci. 2020, 21, 4915. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Zhang, G.; Zhang, W.; Liu, J.; Zhang, J.; Chen, Y.; Peng, H.; Cheng, Y.; Ding, X.; Xin, H. Hair grows hair: Dual-effective hair regrowth through a hair enhanced dissolvable microneedle patch cooperated with the pure yellow light irradiation. Appl. Mater. Today 2021, 25, 101188. [Google Scholar] [CrossRef]
- Ogbechie-Godec, O.A.; Elbuluk, N. Melasma: An up-to-date comprehensive review. Dermatol. Ther. 2017, 7, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Z.; Muddasani, S.; Alam, M. A Systematic Review of the Efficacy and Safety of Microneedling in the Treatment of Melasma. Dermatol. Surg. 2020, 46, 1636–1641. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Soto, C. Microneedles: A therapeutic alternative in melasma. J. Dermat. Cosmetol. 2018, 2, 207–210. [Google Scholar] [CrossRef]
- Lima, E.d.A. Microneedling in facial recalcitrant melasma: Report of a series of 22 cases. An. Bras. Dermatol. 2015, 90, 919–921. [Google Scholar] [CrossRef] [PubMed]
- Farshi, S.; Mansouri, P. Study of efficacy of microneedling and mesoneedling in the treatment of epidermal melasma: A pilot trial. J. Cosmet. Dermatol. 2020, 19, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Shamsi Meymandi, S.; Mozayyeni, A.; Shamsi Meymandi, M.; Aflatoonian, M. Efficacy of microneedling plus topical 4% tranexamic acid solution vs 4% hydroquinone in the treatment of melasma: A single-blind randomized clinical trial. J. Cosmet. Dermatol. 2020, 19, 2906–2911. [Google Scholar] [CrossRef] [PubMed]
- Speeckaert, R.; Bulat, V.; Speeckaert, M.M.; van Geel, N. The Impact of Antioxidants on Vitiligo and Melasma: A Scoping Review and Meta-Analysis. Antioxidants 2023, 12, 2082. [Google Scholar] [CrossRef] [PubMed]
- Aust, M.C.; Fernandes, D.; Kolokythas, P.; Kaplan, H.M.; Vogt, P.M. Percutaneous collagen induction therapy: An alternative treatment for scars, wrinkles, and skin laxity. Plast. Reconstr. Surg. 2008, 121, 1421–1429. [Google Scholar] [CrossRef] [PubMed]
- Roohaninasab, M.; Gandomkar, K.; Goodarzi, A. Microneedling in vitiligo: A systematic review. Surg. Cosmet. Dermatol. 2022, 14, e20220123. [Google Scholar] [CrossRef]
- Gao, F.L.; Jin, R.; Zhang, L.; Zhang, Y.G. The contribution of melanocytes to pathological scar formation during wound healing. Int. J. Clin. Exp. Med. 2013, 6, 609–613. [Google Scholar] [PubMed]
- El-Domyati, M.; Abdel-Wahab, H.; Hossam, A. Combining microneedling with other minimally invasive procedures for facial rejuvenation: A split-face comparative study. Int. J. Dermatol. 2018, 57, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
- Alessa, D.; Bloom, J.D. Microneedling Options for Skin Rejuvenation, Including Non-temperature-controlled Fractional Microneedle Radiofrequency Treatments. Facial Plast. Surg. Clin. N. Am. 2020, 28, 1–7. [Google Scholar] [CrossRef]
- Dayan, E.; Chia, C.; Burns, A.J.; Theodorou, S. Adjustable depth fractional radiofrequency combined with bipolar radiofrequency: A minimally invasive combination treatment for skin laxity. Aesthet. Surg. J. 2019, 39 (Suppl. 3), S112. [Google Scholar] [CrossRef]
- Hidajat, D.; Murlistyarini, S. Successful treatment of rare adverse event after radiofrequency microneedle on Fitzpatrick skin type IV: A case report. J. Cosmet. Laser. Ther. 2023, 25, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, B.S.; Sriram, R.; Mysore, R.; Bhaskar, S.; Shetty, A. Evaluation of microneedling fractional radiofrequency device for treatment of acne scars. J. Cutan. Aesthet. Surg. 2014, 7, 93–97. [Google Scholar] [CrossRef]
- Baek, G.; Kim, M.H.; Jue, M.S. Efficacy of microneedle radiofrequency therapy in the treatment of senile purpura: A prospective study. Skin Res. Technol. 2022, 28, 856–864. [Google Scholar] [CrossRef]
- Eubanks, S.W.; Solomon, J.A. Safety and efficacy of fractional radiofrequency for the treatment and reduction of acne scarring: A prospective study. Lasers. Surg. Med. 2022, 54, 74–81. [Google Scholar] [CrossRef]
- Li, J.; Duan, F.; Kuang, J. Meta-analysis of fractional radiofrequency treatment for acne and/or acne scars. J. Cosmet. Dermatol. 2022, 21, 6754–6766. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.M.; Jung, B.K.; Oh, S.H.; Kim, Y.-K.; Lee, J.H. Laser-assisted Delivery of a Combined Antioxidant Formulation Enhances the Clinical Efficacy of Fractional Microneedle Radiofrequency Treatment: A Pilot Study. Med. Lasers Eng. Basic Res. Clin. Appl. 2021, 10, 161–169. [Google Scholar] [CrossRef]
- Sabri, A.; Ogilvie, J.; McKenna, J.; Segal, J.; Scurr, D.; Marlow, M. Intradermal Delivery of an Immunomodulator for Basal Cell Carcinoma; Expanding the Mechanistic Insight into Solid Microneedle-Enhanced Delivery of Hydrophobic Molecules. Mol. Pharm. 2020, 17, 2925–2937. [Google Scholar] [CrossRef]
- Naguib, Y.W.; Kumar, A.; Cui, Z. The effect of microneedles on the skin permeability and antitumor activity of topical 5-fluorouracil. Acta. Pharm. Sin. B 2014, 4, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.S.; Shan, X.; Mao, J.; Qiu, L.; Chen, J. Derma roller® microneedles-mediated transdermal delivery of doxorubicin and celecoxib co-loaded liposomes for enhancing the anticancer effect. Mater. Sci. Eng. C 2019, 99, 1448–1458. [Google Scholar] [CrossRef]
- Al-Mayahy, M.H.; Sabri, A.H.; Rutland, C.S.; Holmes, A.; McKenna, J.; Marlow, M.; Scurr, D.J. Insight into imiquimod skin permeation and increased delivery using microneedle pre-treatment. Eur. J. Pharm. Biopharm. 2019, 139, 33–43. [Google Scholar] [CrossRef]
- Zhi, D.; Yang, T.; Zhang, T.; Yang, M.; Zhang, S.; Donnelly, R.F. Microneedles for gene and drug delivery in skin cancer therapy. J. Control. Release 2021, 335, 158–177. [Google Scholar] [CrossRef] [PubMed]
- Seetharam, A.A.; Choudhry, H.; Bakhrebah, M.A.; Abdulaal, W.H.; Gupta, M.S.; Rizvi, S.M.D.; Alam, Q.; Siddaramaiah; Gowda, D.V.; Moin, A. Microneedles Drug Delivery Systems for Treatment of Cancer: A Recent Update. Pharmaceutics 2020, 12, 1101. [Google Scholar] [CrossRef] [PubMed]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Alba, M.; Tieu, T.; Tong, Z.; Minhas, R.S.; Rudd, D.; Voelcker, N.H.; Cifuentes-Rius, A.; Elnathan, R. Engineering Micro–Nanomaterials for Biomedical Translation. Adv. Biomed. Res. 2021, 1, 2100002. [Google Scholar] [CrossRef]
- Dugam, S.; Tade, R.; Dhole, R.; Nangare, S. Emerging era of microneedle array for pharmaceutical and biomedical applications: Recent advances and toxicological perspectives. Future J. Pharm. Sci. 2021, 7, 19. [Google Scholar] [CrossRef]
- Dalvi, M.; Kharat, P.; Thakor, P.; Bhavana, V.; Singh, S.B.; Mehra, N.K. Panorama of dissolving microneedles for transdermal drug delivery. Life Sci. 2021, 284, 119877. [Google Scholar] [CrossRef] [PubMed]
- Alkilani, A.Z.; McCrudden, M.T.C.; Donnelly, R.F. Transdermal drug delivery: Innovative pharmaceutical developments based on disruption of the barrier properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef] [PubMed]
- Aldawood, F.K.; Andar, A.; Desai, S. A Comprehensive Review of Microneedles: Types, Materials, Processes, Characterizations and Applications. Polymers 2021, 13, 2815. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Yang, S.; Yuan, W.; Jin, T. Challenges and strategies in developing microneedle patches for transdermal delivery of protein and peptide therapeutics. Curr. Pharm. Biotechnol. 2012, 13, 1292–1298. [Google Scholar] [CrossRef]
- Avcil, M.; Çelik, A. Microneedles in Drug Delivery: Progress and Challenges. Micromachines 2021, 12, 1321. [Google Scholar] [CrossRef]
- Luo, X.; Yang, L.; Cui, Y. Microneedles: Materials, fabrication, and biomedical applications. Biomed. Microdevices 2023, 25, 20. [Google Scholar] [CrossRef] [PubMed]
- Sargioti, N.; Levingstone, T.J.; O’Cearbhaill, E.D.; McCarthy, H.O.; Dunne, N.J. Metallic microneedles for transdermal drug delivery: Applications, fabrication techniques and the effect of geometrical characteristics. Bioengineering 2022, 10, 24. [Google Scholar] [CrossRef]
- Bao, L.; Park, J.; Bonfante, G.; Kim, B. Recent advances in porous microneedles: Materials, fabrication, and transdermal applications. Drug Deliv. Transl. Res. 2022, 12, 395–414. [Google Scholar] [CrossRef] [PubMed]
- Ebrahiminejad, V.; Rad, Z.F.; Prewett, P.D.; Davies, G.J. Fabrication and testing of polymer microneedles for transdermal drug delivery. Beilstein J. Nanotechnol. 2022, 13, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Al-Japairai, K.A.S.; Mahmood, S.; Almurisi, S.H.; Venugopal, J.R.; Hilles, A.R.; Azmana, M.; Raman, S. Current trends in polymer microneedle for transdermal drug delivery. Int. J. Pharm. 2020, 587, 119673. [Google Scholar] [CrossRef] [PubMed]
- Tucak, A.; Sirbubalo, M.; Hindija, L.; Rahić, O.; Hadžiabdić, J.; Muhamedagić, K.; Čekić, A.; Vranić, E. Microneedles: Characteristics, materials, production methods and commercial development. Micromachines 2020, 11, 961. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, W.; Li, Z. An improved manufacturing approach for discrete silicon microneedle arrays with tunable height-pitch ratio. Sensors 2016, 16, 1628. [Google Scholar] [CrossRef]
- Rajabi, M.; Roxhed, N.; Shafagh, R.Z.; Haraldson, T.; Fischer, A.C.; Wijngaart, W.v.d.; Stemme, G.; Niklaus, F. Flexible and stretchable microneedle patches with integrated rigid stainless steel microneedles for transdermal biointerfacing. PLoS ONE 2016, 11, e0166330. [Google Scholar] [CrossRef] [PubMed]
- Salwa; Chevala, N.T.; Jitta, S.R.; Marques, S.M.; Vaz, V.M.; Kumar, L. Polymeric microneedles for transdermal delivery of nanoparticles: Frontiers of formulation, sterility and stability aspects. J. Drug. Deliv. Sci. Technol. 2021, 65, 102711. [Google Scholar] [CrossRef]
- Parhi, R. Review of microneedle based transdermal drug delivery systems. Int. J. Pharm. Sci. Nanotechnol. (IJPSN) 2019, 12, 4511–4523. [Google Scholar] [CrossRef]
- Kim, Y.C.; Park, J.H.; Prausnitz, M.R. Microneedles for drug and vaccine delivery. Adv. Drug Deliv. Rev. 2012, 64, 1547–1568. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Singh, T.R.R.; Morrow, D.I.; Woolfson, A.D. Microneedle-Mediated Transdermal and Intradermal Drug Delivery; Wiley-Blackwell: Hoboken, NJ, USA, 2012. [Google Scholar]
- Liu, T.T.; Chen, K.; Wang, Q. Skin drug permeability and safety through a vibrating solid micro-needle system. Drug Deliv. Transl. Res. 2018, 8, 1025–1033. [Google Scholar] [CrossRef]
- Lee, C.A.; Baek, J.S.; Kwag, D.G.; Lee, H.J.; Park, J.; Cho, C.W. Enhancement of skin permeation of vitamin C using vibrating microneedles. Transl. Clin. Pharmacol. 2017, 25, 15–20. [Google Scholar] [CrossRef]
- Lhernould, M.S.; Deleers, M.; Delchambre, A. Hollow polymer microneedles array resistance and insertion tests. Int. J. Pharm. 2015, 480, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Kumar Singla, S.; Muthuraman, A.; Sahai, D.; Mangal, N.; Dhamodharan, J. Therapeutic applications of transdermal microneedles. Front. Biosci. (Elite Ed.) 2021, 13, 158–184. [Google Scholar] [PubMed]
- Yang, D.; Chen, M.; Sun, Y.; Jin, Y.; Lu, C.; Pan, X.; Quan, G.; Wu, C. Microneedle-mediated transdermal drug delivery for treating diverse skin diseases. Acta Biomater. 2021, 121, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Menon, I.; Bagwe, P.; Gomes, K.B.; Bajaj, L.; Gala, R.; Uddin, M.N.; D’souza, M.J.; Zughaier, S.M. Microneedles: A new generation vaccine delivery system. Micromachines 2021, 12, 435. [Google Scholar] [CrossRef] [PubMed]
- Vinayakumar, K.; Kulkarni, P.G.; Nayak, M.; Dinesh, N.; Hegde, G.M.; Ramachandra, S.; Rajanna, K. A hollow stainless steel microneedle array to deliver insulin to a diabetic rat. J. Micromech. Microeng. 2016, 26, 065013. [Google Scholar] [CrossRef]
- Lee, J.W.; Prausnitz, M.R. Drug Delivery Using Microneedle Patches: Not Just for Skin; Taylor & Francis: Abingdon, UK, 2018; pp. 541–543. [Google Scholar]
- Kim, J.; Jeong, D. Dissolvable Microneedles: Applications and Opportunities. ONdrugDelivery Mag. 2018, 84, 24–29. [Google Scholar]
- Obaidat, R.; BaniAmer, F.; Assaf, S.M.; Yassin, A. Fabrication and Evaluation of Transdermal Delivery of Carbamazepine Dissolving Microneedles. AAPS PharmSciTech 2021, 22, 253. [Google Scholar] [CrossRef]
- Lahiji, S.F.; Dangol, M.; Jung, H. A patchless dissolving microneedle delivery system enabling rapid and efficient transdermal drug delivery. Sci. Rep. 2015, 5, 7914. [Google Scholar] [CrossRef] [PubMed]
- McCrudden, M.T.; Alkilani, A.Z.; McCrudden, C.M.; McAlister, E.; McCarthy, H.O.; Woolfson, A.D.; Donnelly, R.F. Design and physicochemical characterisation of novel dissolving polymeric microneedle arrays for transdermal delivery of high dose, low molecular weight drugs. J. Control. Release 2014, 180, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Jin, S.G. Microneedle for transdermal drug delivery: Current trends and fabrication. J. Pharm. Investig. 2021, 51, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y. Development and Characterization of Polymeric Formulations and Microneedles for Dermal Drug Delivery. Ph.D. Thesis, Mercer University, Macon, GA, USA, 2020. [Google Scholar]
- Donnelly, R.F.; McCrudden, M.T.C.; Zaid Alkilani, A.; Larrañeta, E.; McAlister, E.; Courtenay, A.J.; Kearney, M.-C.; Singh, T.R.R.; McCarthy, H.O.; Kett, V.L. Hydrogel-forming microneedles prepared from “super swelling” polymers combined with lyophilised wafers for transdermal drug delivery. PLoS ONE 2014, 9, e111547. [Google Scholar] [CrossRef]
- Raj Singh, T.R.; McCarron, P.A.; Woolfson, A.D.; Donnelly, R.F. Investigation of swelling and network parameters of poly(ethylene glycol)-crosslinked poly(methyl vinyl ether-co-maleic acid) hydrogels. Eur. Polym. J. 2009, 45, 1239–1249. [Google Scholar] [CrossRef]
- Aung, N.N.; Ngawhirunpat, T.; Rojanarata, T.; Patrojanasophon, P.; Pamornpathomkul, B.; Opanasopit, P. Fabrication, characterization and comparison of α-arbutin loaded dissolving and hydrogel forming microneedles. Int. J. Pharm. 2020, 586, 119508. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.M.; Lim, S.-M.; Choi, S.; Kim, D.-H.; Jin, H.-E.; Jee, G.; Hong, K.-J.; Kim, J.Y. Microneedles: Quick and easy delivery methods of vaccines. Clin. Exp. Vaccine Res. 2017, 6, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Guillot, A.J.; Cordeiro, A.S.; Donnelly, R.F.; Montesinos, M.C.; Garrigues, T.M.; Melero, A. Microneedle-based delivery: An overview of current applications and trends. Pharmaceutics 2020, 12, 569. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.M.; Cornwell, M.; Hill, J.; Prausnitz, M.R. Precise microinjection into skin using hollow microneedles. J. Investig. Dermatol. 2006, 126, 1080–1087. [Google Scholar] [CrossRef]
- Tarbox, T.N.; Watts, A.B.; Cui, Z.; Williams, R.O. An update on coating/manufacturing techniques of microneedles. Drug Deliv. Transl. Res. 2018, 8, 1828–1843. [Google Scholar] [CrossRef]
- Mizuno, Y.; Takasawa, K.; Hanada, T.; Nakamura, K.; Yamada, K.; Tsubaki, H.; Hara, M.; Tashiro, Y.; Matsuo, M.; Ito, T. Fabrication of novel-shaped microneedles to overcome the disadvantages of solid microneedles for the transdermal delivery of insulin. Biomed. Microdevices 2021, 23, 38. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.G.; White, L.R.; Estrela, P.; Leese, H.S. Hydrogel-Forming Microneedles: Current Advancements and Future Trends. Macromol. Biosci. 2021, 21, 2000307. [Google Scholar] [CrossRef]
- Alimardani, V.; Abolmaali, S.S.; Yousefi, G.; Rahiminezhad, Z.; Abedi, M.; Tamaddon, A.; Ahadian, S. Microneedle arrays combined with nanomedicine approaches for transdermal delivery of therapeutics. J. Clin. Med. 2021, 10, 181. [Google Scholar] [CrossRef] [PubMed]
- Ravetti, S.; Clemente, C.; Brignone, S.; Hergert, L.; Allemandi, D.; Palma, S. Ascorbic acid in skin health. Cosmetics 2019, 6, 58. [Google Scholar] [CrossRef]
- Juncan, A.M.; Moisă, D.G.; Santini, A.; Morgovan, C.; Rus, L.-L.; Vonica-Țincu, A.L.; Loghin, F. Advantages of hyaluronic acid and its combination with other bioactive ingredients in cosmeceuticals. Molecules 2021, 26, 4429. [Google Scholar] [CrossRef]
- Zasada, M.; Budzisz, E. Retinoids: Active molecules influencing skin structure formation in cosmetic and dermatological treatments. Postep. Dermatol. Alergol. 2019, 36, 392–397. [Google Scholar] [CrossRef]
- Burger, P.; Landreau, A.; Azoulay, S.; Michel, T.; Fernandez, X. Skin whitening cosmetics: Feedback and challenges in the development of natural skin lighteners. Cosmetics 2016, 3, 36. [Google Scholar] [CrossRef]
- Raikou, V.; Varvaresou, A.; Panderi, I.; Papageorgiou, E. The efficacy study of the combination of tripeptide-10-citrulline and acetyl hexapeptide-3. A prospective, randomized controlled study. J. Cosmet. Dermatol. 2017, 16, 271–278. [Google Scholar] [CrossRef]
- Tadini, K.A.; Mercurio, D.G.; Campos, P.M.B.G.M. Acetyl hexapeptide-3 in a cosmetic formulation acts on skin mechanical properties-clinical study. Braz. J. Pharm. Sci. 2015, 51, 901–909. [Google Scholar] [CrossRef]
- Lee, C.; Yang, H.; Kim, S.; Kim, M.; Kang, H.; Kim, N.; An, S.; Koh, J.; Jung, H. Evaluation of the anti-wrinkle effect of an ascorbic acid-loaded dissolving microneedle patch via a double-blind, placebo-controlled clinical study. Int. J. Cosmet. Sci. 2016, 38, 375–381. [Google Scholar] [CrossRef]
- Zaid Alkilani, A.; Hamed, R.; Hussein, G.; Alnadi, S. Nanoemulsion-based patch for the dermal delivery of ascorbic acid. J. Dispers. Sci. Technol. 2021, 43, 1801–1811. [Google Scholar] [CrossRef]
- Kim, Y.; Bhattaccharjee, S.A.; Beck-Broichsitter, M.; Banga, A.K. Fabrication and characterization of hyaluronic acid microneedles to enhance delivery of magnesium ascorbyl phosphate into skin. Biomed. Microdevices 2019, 21, 104. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-H.; Ha, S.K.; Choi, I.; Kim, K.S.; Park, J.; Choi, N.; Kim, B.; Sung, J.H. Fabrication of degradable carboxymethyl cellulose (CMC) microneedle with laser writing and replica molding process for enhancement of transdermal drug delivery. Biotechnol. Bioprocess. Eng. 2016, 21, 110–118. [Google Scholar] [CrossRef]
- Park, Y.; Kim, K.S.; Chung, M.; Sung, J.H.; Kim, B. Fabrication and characterization of dissolving microneedle arrays for improving skin permeability of cosmetic ingredients. J. Ind. Eng. Chem. 2016, 39, 121–126. [Google Scholar] [CrossRef]
- Sawutdeechaikul, P.; Kanokrungsee, S.; Sahaspot, T.; Thadvibun, K.; Banlunara, W.; Limcharoen, B.; Sansureerungsikul, T.; Rutwaree, T.; Oungeun, M.; Wanichwecharungruang, S. Detachable dissolvable microneedles: Intra-epidermal and intradermal diffusion, effect on skin surface, and application in hyperpigmentation treatment. Sci. Rep. 2021, 11, 24114. [Google Scholar] [CrossRef]
- Avcil, M.; Akman, G.; Klokkers, J.; Jeong, D.; Çelik, A. Efficacy of bioactive peptides loaded on hyaluronic acid microneedle patches: A monocentric clinical study. J. Cosmet. Dermatol. 2020, 19, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Huh, I.; Kim, S.; Yang, H.; Jang, M.; Kang, G.; Jung, H. Effects of two droplet-based dissolving microneedle manufacturing methods on the activity of encapsulated epidermal growth factor and ascorbic acid. Eur. J. Pharm. Sci. 2018, 114, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Kwon, H.J.; Ahn, G.R.; Ko, E.J.; Yoo, K.H.; Kim, B.J.; Lee, C.; Kim, D. Hyaluronic acid microneedle patch for the improvement of crow’s feet wrinkles. Dermatol. Ther. 2017, 30, e12546. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-K.; Lee, S.-J.; Ha, H.-Y. Skin Moisturizing Effects of a Microneedle Patch Containing Hyaluronic Acid and Lonicerae flos. Processes 2021, 9, 321. [Google Scholar] [CrossRef]
- Fonseca, D.F.; Vilela, C.; Pinto, R.J.; Bastos, V.; Oliveira, H.; Catarino, J.; Faísca, P.; Rosado, C.; Silvestre, A.J.; Freire, C.S. Bacterial nanocellulose-hyaluronic acid microneedle patches for skin applications: In vitro and in vivo evaluation. Mater. Sci. Eng. C 2021, 118, 111350. [Google Scholar] [CrossRef]
- Kim, H.; Kim, B.; Kim, H.; Um, S.; Lee, J.; Ryoo, H.; Jung, H. Synthesis and in vitro biological activity of retinyl retinoate, a novel hybrid retinoid derivative. Bioorg. Med. Chem. 2008, 16, 6387–6393. [Google Scholar] [CrossRef]
- Kim, H.; Kim, N.; Jung, S.; Mun, J.; Kim, J.; Kim, B.; Lee, J.; Ryoo, H.; Jung, H. Improvement in skin wrinkles from the use of photostable retinyl retinoate: A randomized controlled trial. Br. J. Dermatol. 2010, 162, 497–502. [Google Scholar] [CrossRef]
- Toprangkobsin, P.; Banlunara, W.; Limcharoen, B.; Leelahavanichkul, A.; Asawanonda, P.; Kumtornrut, C.; Sansureerungsikul, T.; Rutwaree, T.; Wanichwecharungruang, S. Delivery and diffusion of retinal in dermis and epidermis through the combination of prodrug nanoparticles and detachable dissolvable microneedles. Drug Deliv. Transl. Res. 2022, 12, 2751–2761. [Google Scholar] [CrossRef]
- Limcharoen, B.; Toprangkobsin, P.; Kröger, M.; Darvin, M.E.; Sansureerungsikul, T.; Rutwaree, T.; Wanichwecharungruang, S.; Banlunara, W.; Lademann, J.; Patzelt, A. Microneedle-facilitated intradermal proretinal nanoparticle delivery. Nanomaterials 2020, 10, 368. [Google Scholar] [CrossRef]
- Hirobe, S.; Otsuka, R.; Iioka, H.; Quan, Y.-S.; Kamiyama, F.; Asada, H.; Okada, N.; Nakagawa, S. Clinical study of a retinoic acid-loaded microneedle patch for seborrheic keratosis or senile lentigo. Life Sci. 2017, 168, 24–27. [Google Scholar] [CrossRef]
- Hiraishi, Y.; Hirobe, S.; Iioka, H.; Quan, Y.-S.; Kamiyama, F.; Asada, H.; Okada, N.; Nakagawa, S. Development of a novel therapeutic approach using a retinoic acid-loaded microneedle patch for seborrheic keratosis treatment and safety study in humans. J. Control. Release 2013, 171, 93–103. [Google Scholar] [CrossRef]
- Lee, Y.; Kumar, S.; Kim, S.H.; Seong, K.-Y.; Lee, H.; Kim, C.; Jung, Y.-S.; Yang, S.Y. Odorless glutathione microneedle patches for skin whitening. Pharmaceutics 2020, 12, 100. [Google Scholar] [CrossRef]
- Gorouhi, F.; Maibach, H.I. Role of topical peptides in preventing or treating aged skin. Int. J. Cosmet. Sci. 2009, 31, 327–345. [Google Scholar] [CrossRef]
- Schagen, S.K. Topical peptide treatments with effective anti-aging results. Cosmetics 2017, 4, 16. [Google Scholar] [CrossRef]
- Lim, S.H.; Tiew, W.J.; Zhang, J.; Ho, P.C.-L.; Kachouie, N.N.; Kang, L. Geometrical optimisation of a personalised microneedle eye patch for transdermal delivery of anti-wrinkle small peptide. Biofabrication 2020, 12, 035003. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Kathuria, H.; Amir, M.H.B.; Zhang, X.; Duong, H.T.T.; Ho, P.C.; Kang, L. High resolution photopolymer for 3D printing of personalised microneedle for transdermal delivery of anti-wrinkle small peptide. J. Control. Release 2021, 329, 907–918. [Google Scholar] [CrossRef] [PubMed]
- An, J.H.; Lee, H.J.; Yoon, M.S.; Kim, D.H. Anti-wrinkle efficacy of cross-linked hyaluronic acid-based microneedle patch with acetyl hexapeptide-8 and epidermal growth factor on Korean skin. Ann. Dermatol. 2019, 31, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Bissett, D.L.; Oblong, J.E.; Berge, C.A. Niacinamide: AB vitamin that improves aging facial skin appearance. Dermatol. Surg. 2005, 31, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kim, B. Skin permeability of compounds loaded within dissolving microneedles dependent on composition of sodium hyaluronate and carboxymethyl cellulose. Korean J. Chem. Eng. 2017, 34, 133–138. [Google Scholar] [CrossRef]
- Shin, C.I.; Kim, M.; Kim, Y.-C. Delivery of niacinamide to the skin using microneedle-like particles. Pharmaceutics 2019, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Reilly, D.M.; Lozano, J. Skin collagen through the lifestages: Importance for skin health and beauty. Aesthet. Surg. J. 2021, 8, 10–20517. [Google Scholar] [CrossRef]
- Zhang, S.; Duan, E. Fighting against skin aging: The way from bench to bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Inayathullah, M.; Manoukian, M.A.; Malkovskiy, A.V.; Manickam, S.; Marinkovich, M.P.; Lane, A.T.; Tayebi, L.; Seifalian, A.M.; Rajadas, J. Transdermal delivery of functional collagen via polyvinylpyrrolidone microneedles. Ann. Biomed. Eng. 2015, 43, 2978–2990. [Google Scholar] [CrossRef] [PubMed]
- Aditya, A.; Kim, B.; Koyani, R.D.; Oropeza, B.; Furth, M.; Kim, J.; Kim, N.P. Kinetics of collagen microneedle drug delivery system. J. Drug Deliv. Sci. Technol. 2019, 52, 618–623. [Google Scholar] [CrossRef]
- Kochhar, J.S.; Anbalagan, P.; Shelar, S.B.; Neo, J.K.; Iliescu, C.; Kang, L. Direct microneedle array fabrication off a photomask to deliver collagen through skin. Pharm. Res. 2014, 31, 1724–1734. [Google Scholar] [CrossRef]
- Al-Atif, H. Collagen supplements for aging and wrinkles: A paradigm shift in the fields of dermatology and cosmetics. Dermatol. Pract. Concept. 2022, 12, e2022018. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.; Fauzi, M.B. Current update of collagen nanomaterials—Fabrication, characterisation and its applications: A review. Pharmaceutics 2021, 13, 316. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Baek, S.; Kang, G.; Yang, H.; Kim, S.; Jung, H. Dissolving microneedle with high molecular weight hyaluronic acid to improve skin wrinkles, dermal density and elasticity. Int. J. Cosmet. Sci. 2020, 42, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kim, S.; Jang, M.; Kim, H.; Lee, S.; Kim, Y.; Eom, Y.A.; Kang, G.; Chiang, L.; Baek, J.H.; et al. Two-phase delivery using a horse oil and adenosine-loaded dissolving microneedle patch for skin barrier restoration, moisturization, and wrinkle improvement. J. Cosmet. Dermatol. 2019, 18, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Eom, Y.A.; Yang, H.; Jang, M.; Jung, S.U.; Park, Y.O.; Lee, S.E.; Jung, H. Skin Barrier Restoration and Moisturization Using Horse Oil-Loaded Dissolving Microneedle Patches. Skin Pharmacol. Physiol. 2018, 31, 163–171. [Google Scholar] [CrossRef]
- Kang, G.; Tu, T.; Kim, S.; Yang, H.; Jang, M.; Jo, D.; Ryu, J.; Baek, J.; Jung, H. Adenosine-loaded dissolving microneedle patches to improve skin wrinkles, dermal density, elasticity and hydration. Int. J. Cosmet. Sci. 2018, 40, 199–206. [Google Scholar] [CrossRef]
- Hong, J.Y.; Ko, E.J.; Choi, S.Y.; Li, K.; Kim, A.R.; Park, J.O.; Kim, B.J. Efficacy and safety of a novel, soluble microneedle patch for the improvement of facial wrinkle. J. Cosmet. Dermatol. 2018, 17, 235–241. [Google Scholar] [CrossRef] [PubMed]
- El-Leithy, E.S.; Makky, A.M.; Khattab, A.M.; Hussein, D.G. Optimization of nutraceutical coenzyme Q10 nanoemulsion with improved skin permeability and anti-wrinkle efficiency. Drug Dev. Ind. Pharm. 2018, 44, 316–328. [Google Scholar] [CrossRef]
- Bradley, E.J.; Griffiths, C.E.; Sherratt, M.J.; Bell, M.; Watson, R.E. Over-the-counter anti-ageing topical agents and their ability to protect and repair photoaged skin. Maturitas 2015, 80, 265–272. [Google Scholar] [CrossRef]
- Hunt, K.J.; Hung, S.K.; Ernst, E. Botanical extracts as anti-aging preparations for the skin. Drugs Aging 2010, 27, 973–985. [Google Scholar] [CrossRef]
- Rafique, M.; Hussain Shah, S.N. Anti-Ageing Potential of a Cream (W/O Emulsion) Containing Grape Seed Extract (GSE): Formulation and in vivo Evaluation of Effectiveness Using Non-Invasive Biophysical Technique. J. Clin. Exp. Dermatol. Res. 2019, 10, 1000500. [Google Scholar] [CrossRef]
- Rattanawiwatpong, P.; Wanitphakdeedecha, R.; Bumrungpert, A.; Maiprasert, M. Anti-aging and brightening effects of a topical treatment containing vitamin C, vitamin E, and raspberry leaf cell culture extract: A split-face, randomized controlled trial. J. Cosmet. Dermatol. 2020, 19, 671–676. [Google Scholar] [CrossRef]
- Chondrogianni, N.; Kapeta, S.; Chinou, I.; Vassilatou, K.; Papassideri, I.; Gonos, E.S. Anti-ageing and rejuvenating effects of quercetin. Exp. Gerontol. 2010, 45, 763–771. [Google Scholar] [CrossRef]
- Sunoqrot, S.; Al-Shalabi, E.; Hasan Ibrahim, L.; Zalloum, H. Nature-Inspired Polymerization of Quercetin to Produce Antioxidant Nanoparticles with Controlled Size and Skin Tone-Matching Colors. Molecules 2019, 24, 3815. [Google Scholar] [CrossRef]
- Bal, S.M.; Caussin, J.; Pavel, S.; Bouwstra, J.A. In vivo assessment of safety of microneedle arrays in human skin. Eur. J. Pharm. Sci. 2008, 35, 193–202. [Google Scholar] [CrossRef]
- Jeong, H.-R.; Lee, H.-S.; Choi, I.-J.; Park, J.-H. Considerations in the use of microneedles: Pain, convenience, anxiety and safety. J. Drug Target. 2017, 25, 29–40. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Microneedling Devices: Getting to the Point on Benefits, Risks and Safety; U.S. Food & Drug Administration: Silver Spring, DA, USA, 2021.
- Donnelly, R.F.; Singh, T.R.R.; Larrañeta, E.; McCrudden, M.T. Microneedles for Drug and Vaccine Delivery and Patient Monitoring; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Cary, J.H.; Li, B.S.; Maibach, H.I. Dermatotoxicology of microneedles (MNs) in man. Biomed. Microdevices 2019, 21, 66. [Google Scholar] [CrossRef]
- Park, J.H.; Prausnitz, M.R. Analysis of Mechanical Failure of Polymer Microneedles by Axial Force. J. Korean Phys. Soc. 2010, 56, 1223–1227. [Google Scholar] [CrossRef]
- Hoesly, F.J.; Borovicka, J.; Gordon, J.; Nardone, B.; Holbrook, J.S.; Pace, N.; Ibrahim, O.; Bolotin, D.; Warycha, M.; Kwasny, M.; et al. Safety of a novel microneedle device applied to facial skin: A subject- and rater-blinded, sham-controlled, randomized trial. Arch. Dermatol. 2012, 148, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; Singh, T.R.; Alkilani, A.Z.; McCrudden, M.T.; O’Neill, S.; O’Mahony, C.; Armstrong, K.; McLoone, N.; Kole, P.; Woolfson, A.D. Hydrogel-forming microneedle arrays exhibit antimicrobial properties: Potential for enhanced patient safety. Int. J. Pharm. 2013, 451, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.P.; Landis, B.J.; Adams, Z.H.; Allen, M.G.; Prausnitz, M.R. Insertion of microneedles into skin: Measurement and prediction of insertion force and needle fracture force. J. Biomech. 2004, 37, 1155–1163. [Google Scholar] [CrossRef]
- Huang, H.; Fu, C. Different fabrication methods of out-of-plane polymer hollow needle arrays and their variations. J. Micromech. Microeng. 2007, 17, 393–402. [Google Scholar] [CrossRef]
- FDA. Regulatory Considerations for Microneedling Products. Guidance for Industry and Food and Drug Administration Staff. 2020. Available online: https://www.fda.gov/media/107708/download (accessed on 10 November 2020).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).