Interaction of Perfumes with Cytochrome P-450 19
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Methods
2.2.1. Perfume Exposure to UV Light
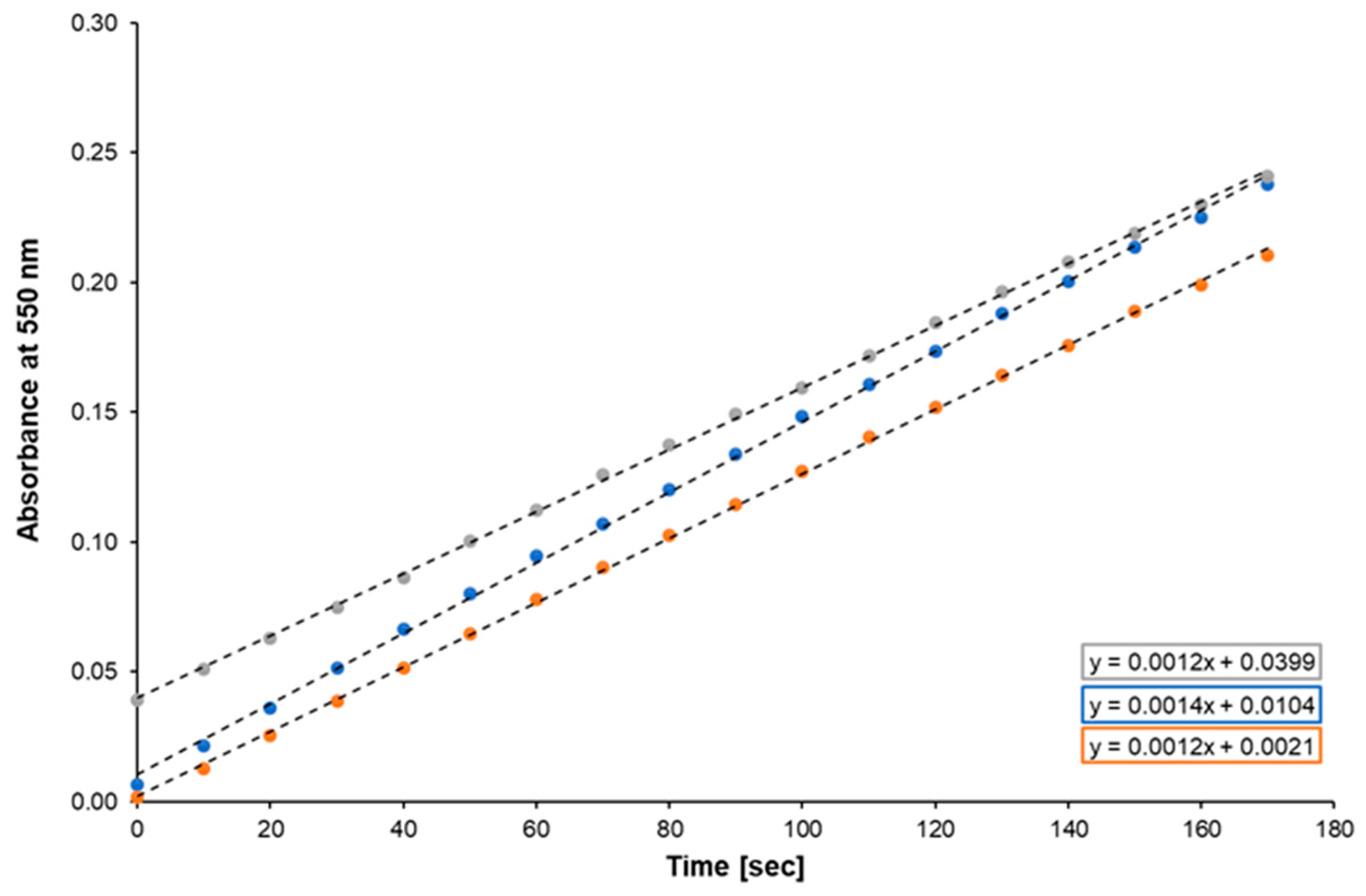
2.2.2. Cytochrome c Reduction by CYPOR
2.2.3. CYP19 Activity Assay
2.2.4. TLC Analysis
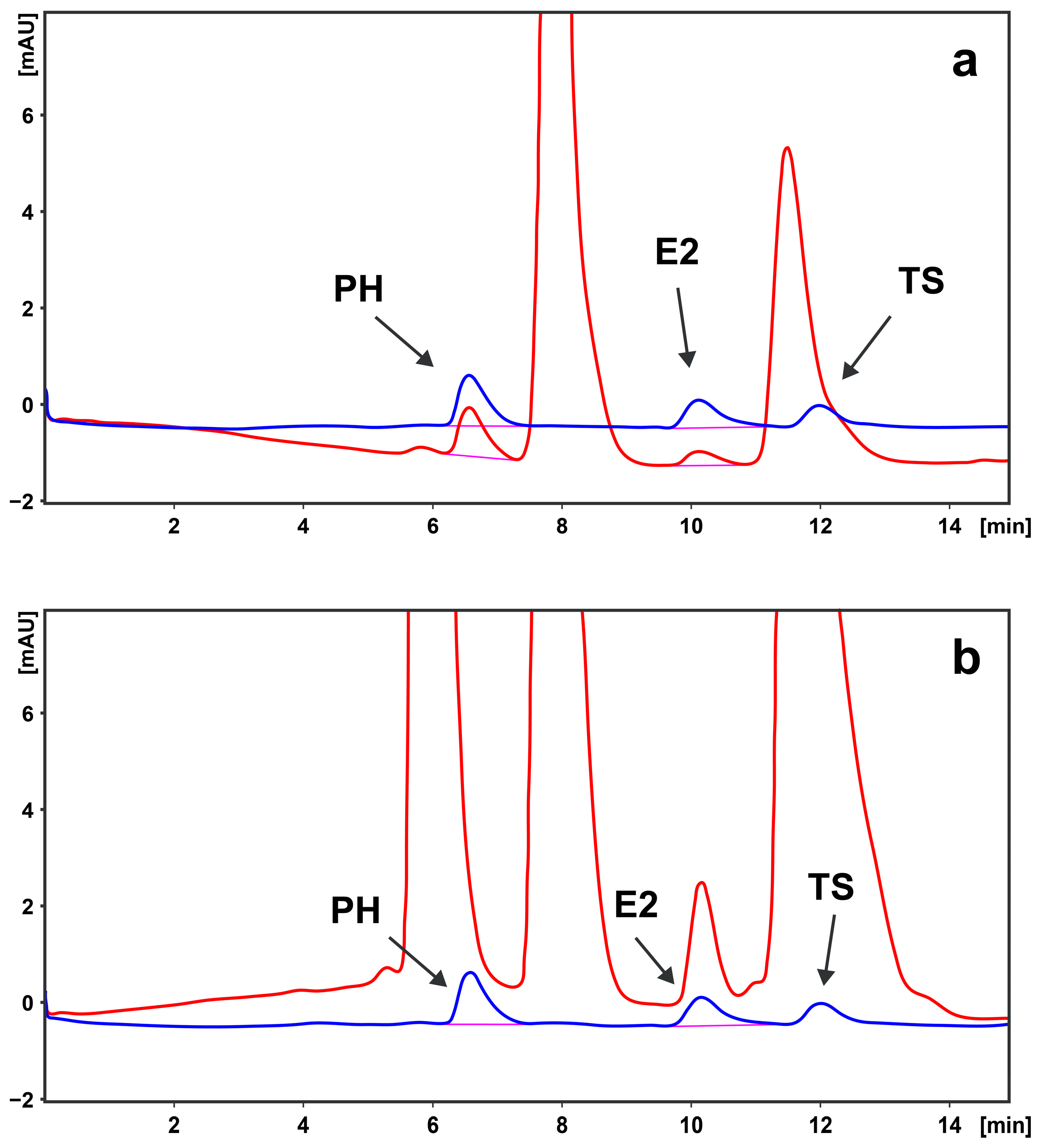
2.2.5. LC Analysis
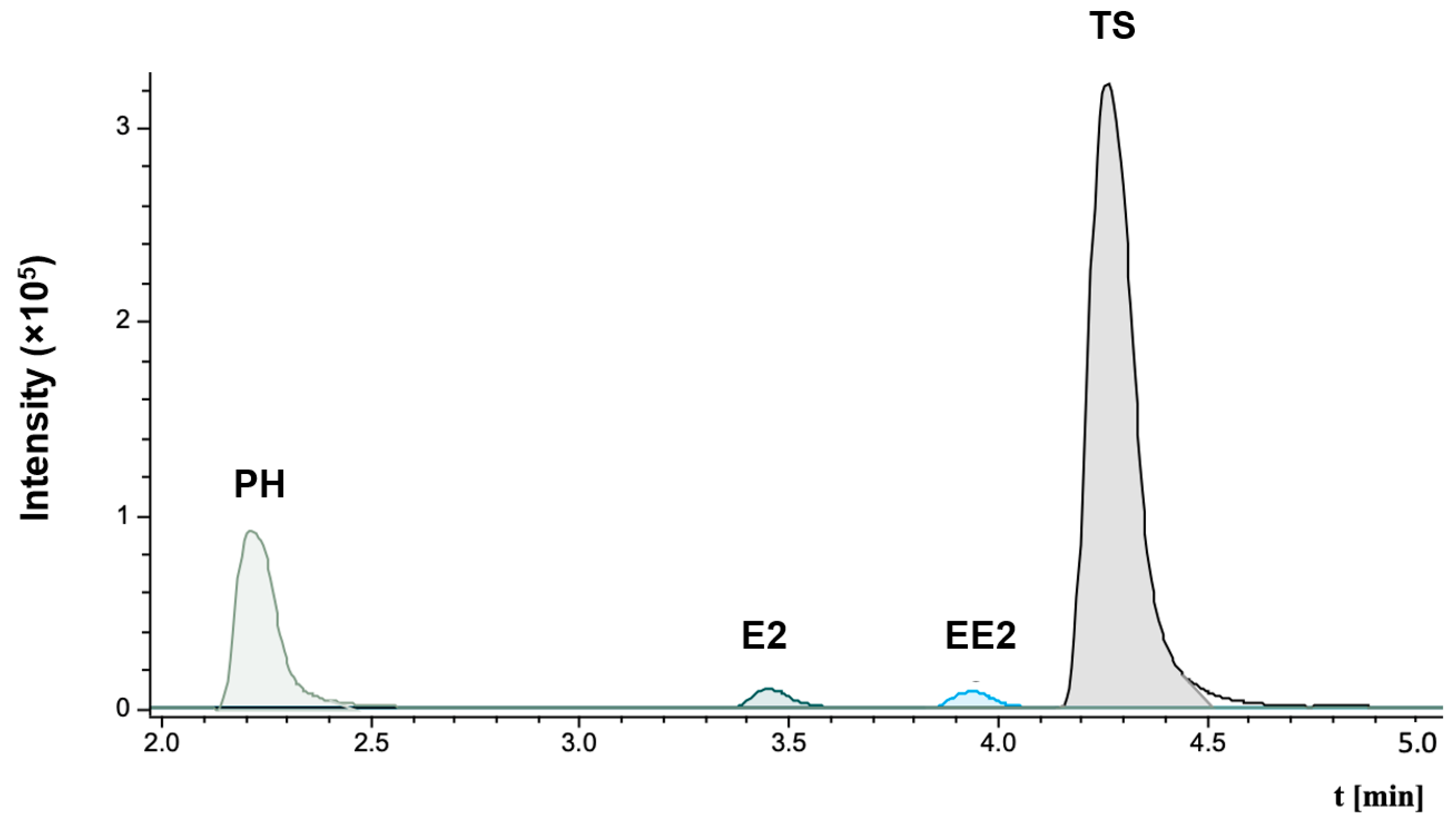
2.2.6. LC–MS/MS Analysis
2.2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Perfume Brand Name | Sample | Ingredients |
|---|---|---|
| Bvlgari® Man Wood Essence | #1 | Alcohol denat. (SD alcohol 39-c), parfum (fragrance), aqua (water), benzyl salicylate, coumarin, ethylhexyl methoxycinnamate, benzyl alcohol, limonene, linalool, alpha-isomethyl ionone, butyl methoxydibenzoylmethane, ethylhexyl salicylate, citronellol, geraniol, eugenol, citral, benzyl benzoate, CI 19140 (Yellow 5), BHT, CI 42090 (Blue 1), CI 60730 (Ext. Violet 2) |
| Dolce Gabbana® Q | #2 | Alcohol denat., parfum (fragrance), aqua (water), benzyl salicylate, limonene, hydroxycitronellal, butyl methoxydibenzoylmethane, ethylhexyl salicylate, linalool, citral, alcohol, citronellol, hexyl cinnamal, tris(tetramethylhydroxypiperidinol) citrate, coumarin, benzyl alcohol, pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate, eugenol, geraniol, CI 14700 (Red 4), CI 60730 (Ext. Violet 2), CI 19140 (Yellow 5) |
| Montale® Intense Roses Musk | #3 | Parfum/fragrance, alcohol denat., aqua/water, citronellol, limonene, geraniol, citral, linalool |
| Narciso Rodriguez® Pure Musc For Her | #4 | Alcohol, parfum (fragrance), aqua (water), benzyl salicylate, linalool, butyl methoxydibenzoylmethane, ethylhexyl methoxycinnamate, hexyl cinnamal, hydroxycitronellal, cinnamyl alcohol, benzyl alcohol, limonene, isoeugenol, BHT, benzyl benzoate, farnesol |
| Sisley® Soir d’Orient | #5 | Alcohol, fragrance (parfum), water/eau (aqua), tetrasodium EDTA, citronellol, hexyl cinnamal, limonene, alpha-isomethyl ionone, benzyl salicylate, geraniol, linalool, eugenol, citral, benzyl benzoate, benzyl alcohol |
| Karl Lagerfeld® Her | #6 | Alcohol denat. (SD alcohol 39-c), parfum (fragrance), aqua (water), ethylhexyl methoxycinnamate, benzyl salicylate, limonene, hexyl cinnamal, hydroxycitronellal, linalool, ethylhexyl salicylate, butyl methoxydibenzoylmethane, geraniol, alpha-isomethyl ionone, BHT, citral, citronellol, benzyl alcohol, isoeugenol, CI 14700 (Red 4), CI 15985 (Yellow 6), CI 19140 (Yellow 5), CI 60730 (Ext. Violet 2) benzoate, CI 19140 (YELLOW 5), CI 42090 (Blue 1) |
| Marc Jacobs® Daisy Eau So Intense | #7 | Alcohol denat., parfum/fragrance, aqua/water/eau, ethylhexyl methoxycinnamate, butyl methoxydibenzoylmethane, alpha-isomethyl ionone, citronellol, linalool, octocrylene, hydroxycitronellal, limonene, hexyl cinnamal, geraniol, benzyl benzoate, methyl 2-octynoate, BHT, FD&C Yellow no. 5 (CI 19140), FD&C Red no. 4 (CI 14700), FD&C Blue no. 1 (CI 42090) |
| Dior® Sauvage Elixir | #8 | Alcohol, parfum (fragrance), aqua (water), linalool, limonene, coumarin, citronellol, eugenol, butyl methoxydibenzoylmethane, triethyl citrate, pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate, geraniol, cinnamal, citral, evernia furfuracea (treemoss) extract, farnesol, isoeugenol, CI 60730 (Ext. Violet 2), CI 19140 (Yellow 5), CI 42090 (Blue 1) |
| Kayali® Invite Only Amber 23 | #9 | Alcohol denat./SD alcohol 40-b, fragrance/parfum, water/aqua/eau, avobenzone/butyl methoxydibenzoylmethane, alcohol, tris (tetramethylhydroxypiperidinol) citrate, alpha-isomethyl ionone, benzyl benzoate, cinnamal, cinnamyl alcohol, coumarin, linalool, citral, citronellol, eugenol, farnesol, geraniol, limonene, benzyl alcohol, Red 4/CI 14700, Blue 1/CI 42090, Ext. Violet 2/CI 60730 |
| Mugler® Aura | #10 | Alcohol, parfum/fragrance, aqua/water/eau, alpha-isomethyl ionone, limonene, ethylhexyl methoxycinnamate, linalool, benzyl salicylate, benzyl alcohol, coumarin, ethylhexyl salicylate, butyl methoxydibenzoylmethane, citronellol, geraniol, hexyl cinnamal, benzyl benzoate, pentaerythrityl |
References
- Calkin, R.R.; Jellinek, J.S. Perfumery: Practice and Principles; John Wiley & Sons: New York, NY, USA, 1994. [Google Scholar]
- Reiner, J.L.; Wong, C.M.; Arcaro, K.F.; Kannan, K. Synthetic musk fragrances in human milk from the United States. Environ. Sci. Technol. 2007, 41, 3815–3820. [Google Scholar] [CrossRef]
- Rimkus, G.G.; Wolf, M. Polycyclic musk fragrances in human adipose tissue and human milk. Chemosphere 1996, 33, 2033–2043. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Reiner, J.L.; Yun, S.H.; Perrotta, E.E.; Tao, L.; Johnson-Restrepo, B.; Rodan, B.D. Polycyclic musk compounds in higher trophic level aquatic organisms and humans from the United States. Chemosphere 2005, 61, 693–700. [Google Scholar] [CrossRef]
- Aqil, M.; Ahad, A.; Sultana, Y.; Ali, A. Status of terpenes as skin penetration enhancers. Drug Discov. Today 2007, 12, 1061–1067. [Google Scholar] [CrossRef]
- Patel, S. Fragrance compounds: The wolves in sheep’s clothings. Med. Hypotheses 2017, 102, 106–111. [Google Scholar] [CrossRef]
- Gomez, E.; Pillon, A.; Fenet, H.; Rosain, D.; Duchesne, M.J.; Nicolas, J.C.; Balaguer, P.; Casellas, C. Estrogenic activity of cosmetic components in reporter cell lines: Parabens; UV screens; and musks. J. Toxicol. Environ. Health A 2005, 68, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Lymperi, S.; Giwercman, A. Endocrine disruptors and testicular function. Metabolism 2018, 86, 79–90. [Google Scholar] [CrossRef]
- Darbre, P.D.; Charles, A.K. Environmental oestrogens and breast cancer: Evidence for combined involvement of dietary; household and cosmetic xenoestrogens. Anticancer. Res. 2010, 30, 815–827. [Google Scholar]
- Harvey, P.W. Parabens, oestrogenicity, underarm cosmetics and breast cancer: A perspective on a hypothesis. J. Appl. Toxicol. 2003, 23, 285–288. [Google Scholar] [CrossRef]
- Taylor, K.M.; Weisskopf, M.; Shine, J. Human exposure to nitro musks and the evaluation of their potential toxicity: An overview. Environ. Health 2014, 13, 14. [Google Scholar] [CrossRef]
- Nohynek, G.J.; Antignac, E.; Re, T.; Toutain, H. Safety assessment of personal care products/cosmetics and their ingredients. Toxicol. Appl. Pharmacol. 2010, 243, 239–259. [Google Scholar] [CrossRef]
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009, on Cosmetic Products—ANNEX VI. Available online: https://eur-lex.europa.eu/eli/reg/2009/1223/oj (accessed on 5 January 2024).
- ECHA–European Chemicals Agency. Available online: https://echa.europa.eu/ (accessed on 12 January 2024).
- Vrtačnik, P.; Ostanek, B.; Mencej-Bedrač, S.; Marc, J. The many faces of estrogen signaling. Biochem. Med. 2014, 24, 329–342. [Google Scholar] [CrossRef]
- Boon, W.C.; Chow, J.D.; Simpson, E.R. The multiple roles of estrogens and the enzyme aromatase. Prog. Brain Res. 2010, 181, 209–232. [Google Scholar]
- Brodie, A.M. Recent advances in studies on estrogen biosynthesis. J. Endocrinol. Investig. 1979, 2, 445–460. [Google Scholar] [CrossRef]
- Smedsland, S.K.; Vandraas, K.F.; Bøhn, S.K.; Dahl, A.A.; Kiserud, C.E.; Brekke, M.; Falk, R.S.; Reinertsen, K.V. Sexual activity and functioning in long-term breast cancer survivors; exploring associated factors in a nationwide survey. Breast Cancer Res. Treat. 2022, 193, 139–149. [Google Scholar] [CrossRef]
- Del Giudice, F.; Busetto, G.M.; De Berardinis, E.; Sperduti, I.; Ferro, M.; Maggi, M.; Gross, M.S.; Sciarra, A.; Eisenberg, M.L. A systematic review and meta-analysis of clinical trials implementing aromatase inhibitors to treat male infertility. Asian J. Androl. 2020, 22, 360–367. [Google Scholar] [PubMed]
- Rochira, V.; Carani, C. Estrogens, Male Reproduction and Beyond. In Endotext (Internet); Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2023. [Google Scholar]
- OECD. Test No. 456: H295R Steroidogenesis Assay, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2023. [Google Scholar]
- Strobel, H.W.; Dignam, J.D. Purification and properties of NADPH-cytochrome P-450 reductase. Methods Enzymol. 1978, 52, 89–96. [Google Scholar] [PubMed]
- Šulc, M.; Indra, R.; Moserová, M.; Schmeiser, H.H.; Frei, E.; Arlt, V.M.; Stiborová, M. The impact of individual cytochrome P450 enzymes on oxidative metabolism of benzo[a]pyrene in human livers. Environ. Mol. Mutagen. 2016, 57, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Chabi, K.; Sleno, L. Estradiol, Estrone and Ethinyl Estradiol Metabolism Studied by High Resolution LC-MS/MS Using Stable Isotope Labeling and Trapping of Reactive Metabolites. Metabolites 2022, 12, 931. [Google Scholar] [CrossRef] [PubMed]
- Wooding, K.M.; Hankin, J.A.; Johnson, C.A.; Chosich, J.D.; Baek, S.W.; Bradford, A.P.; Murphy, R.C.; Santoro, N. Measurement of estradiol, estrone, and testosterone in postmenopausal human serum by isotope dilution liquid chromatography tandem mass spectrometry without derivatization. Steroids 2015, 96, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Zarerad, E.; Niksalehi, K.; Armandeh, M.; Sani, M.A.; Ataei, M.; Mousavi, T.; Maghsoudi, A.S.; Hassani, S. Polychlorinated Biphenyls: A Review of Recent Updates on Food Safety and Environmental Monitoring, Health and Toxicological Implications, and Analysis. Mini Rev. Med. Chem. 2023, 23, 1390–1411. [Google Scholar]
- FDA–U.S. Food and Drug Administration. Development & Approval Process|Drugs. 2022. Available online: https://www.fda.gov/drugs/development-approval-process-drugs (accessed on 5 January 2024).
- EMA–European Medicines Agency. Authorisation of Medicines. 2020. Available online: https://www.ema.europa.eu/en/about-us/what-we-do/authorisation-medicines (accessed on 5 January 2024).
- Batke, M.; Damm, G.; Foth, H.; Freyberger, A.; Gebel, T.; Gundert-Remy, U.; Hengstler, J.; Mangerich, A.; Partosch, F.; Röhl, C.; et al. The EU chemicals strategy for sustainability: Critical reflections on proposed regulatory changes for endocrine disruptors and mixture toxicity. Arch. Toxicol. 2022, 96, 1133–1135. [Google Scholar] [CrossRef] [PubMed]
- FDA–U.S. Federal Food; Drug; and Cosmetic Act. 2013. Available online: https://www.fda.gov/Cosmetics/GuidanceRegulation/LawsRegulations (accessed on 5 January 2024).
- Lilienblum, W.; Dekant, W.; Foth, H.; Gebel, T.; Hengstler, J.G.; Kahl, R.; Kramer, P.J.; Schweinfurth, H.; Wollin, K.M. Alternative methods to safety studies in experimental animals: Role in the risk assessment of chemicals under the new European Chemicals Legislation (REACH). Arch. Toxicol. 2008, 82, 211–236. [Google Scholar] [CrossRef] [PubMed]
- Bråred Christensson, J.; Hagvall, L.; Karlberg, A.-T. Fragrance allergens; overview with a focus on recent developments and understanding of abiotic and biotic activation. Cosmetics 2016, 3, 19. [Google Scholar] [CrossRef]
- van Meeuwen, J.A.; van Son, O.; Piersma, A.H.; de Jong, P.C.; van den Berg, M. Aromatase inhibiting and combined estrogenic effects of parabens and estrogenic effects of other additives in cosmetics. Toxicol. Appl. Pharmacol. 2008, 230, 372–382. [Google Scholar] [CrossRef]
- Li, Z.; Yin, N.; Liu, Q.; Wang, C.; Wang, T.; Wang, Y.; Qu, G.; Liu, J.; Cai, Y.; Zhou, Q.; et al. Effects of polycyclic musks HHCB and AHTN on steroidogenesis in H295R cells. Chemosphere 2013, 90, 1227–1235. [Google Scholar] [CrossRef]
- Kar, S.; Leszczynski, J. Exploration of computational approaches to predict the toxicity of chemical mixtures. Toxics 2019, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Ji, K. Identification of combinations of endocrine disrupting chemicals in household chemical products that require mixture toxicity testing. Ecotoxicol. Environ. Saf. 2022, 240, 113677. [Google Scholar] [CrossRef]
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef]
- FDA–U.S. Food and Drug Administration. Fragrances in Cosmetics. 2022. Available online: https://www.fda.gov/cosmetics/cosmetic-ingredients/fragrances-cosmetics (accessed on 5 January 2024).
- Hutter, H.P.; Wallner, P.; Moshammer, H.; Hartl, W.; Sattelberger, R.; Lorbeer, G.; Kundi, M. Blood concentrations of polycyclic musks in healthy young adults. Chemosphere 2005, 59, 487–492. [Google Scholar] [CrossRef]
- Cal, K. Skin penetration of terpenes from essential oils and topical vehicles. Planta Med. 2006, 72, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Darbre, P.D.; Aljarrah, A.; Miller, W.R.; Coldham, N.G.; Sauer, M.J.; Pope, G.S. Concentrations of parabens in human breast tumours. J. Appl. Toxicol. 2004, 24, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.R.; Bulun, S.E. Estrogen production and action. J. Am. Acad. Dermatol. 2001, 45, S116–S124. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2022/1176 of 7 July 2022 Amending Regulation (EC) No 1223/2009 of the European Parliament and of the Council as Regards the Use of Certain UV Filters in Cosmetic Products. Available online: http://data.europa.eu/eli/reg/2022/1176/oj (accessed on 10 January 2024).
- Li, Y.; He, Y.; Lam, C.H.; Nah, T. Environmental photochemistry of organic UV filter butyl methoxydibenzoylmethane: Implications for photochemical fate in surface waters. Sci. Total Environ. 2022, 839, 156145. [Google Scholar] [CrossRef]
- Notino, S.R.O. Available online: https://www.notino.cz (accessed on 5 January 2024).




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drejslarová, I.; Ječmen, T.; Hodek, P. Interaction of Perfumes with Cytochrome P-450 19. Cosmetics 2024, 11, 33. https://doi.org/10.3390/cosmetics11020033
Drejslarová I, Ječmen T, Hodek P. Interaction of Perfumes with Cytochrome P-450 19. Cosmetics. 2024; 11(2):33. https://doi.org/10.3390/cosmetics11020033
Chicago/Turabian StyleDrejslarová, Iva, Tomáš Ječmen, and Petr Hodek. 2024. "Interaction of Perfumes with Cytochrome P-450 19" Cosmetics 11, no. 2: 33. https://doi.org/10.3390/cosmetics11020033
APA StyleDrejslarová, I., Ječmen, T., & Hodek, P. (2024). Interaction of Perfumes with Cytochrome P-450 19. Cosmetics, 11(2), 33. https://doi.org/10.3390/cosmetics11020033






