Histological, Clinical Assessment, and Treatment of a Permanent Filler Complication in the Upper Lip: A Case Report with 16-Year Follow-Up
Abstract
1. Introduction
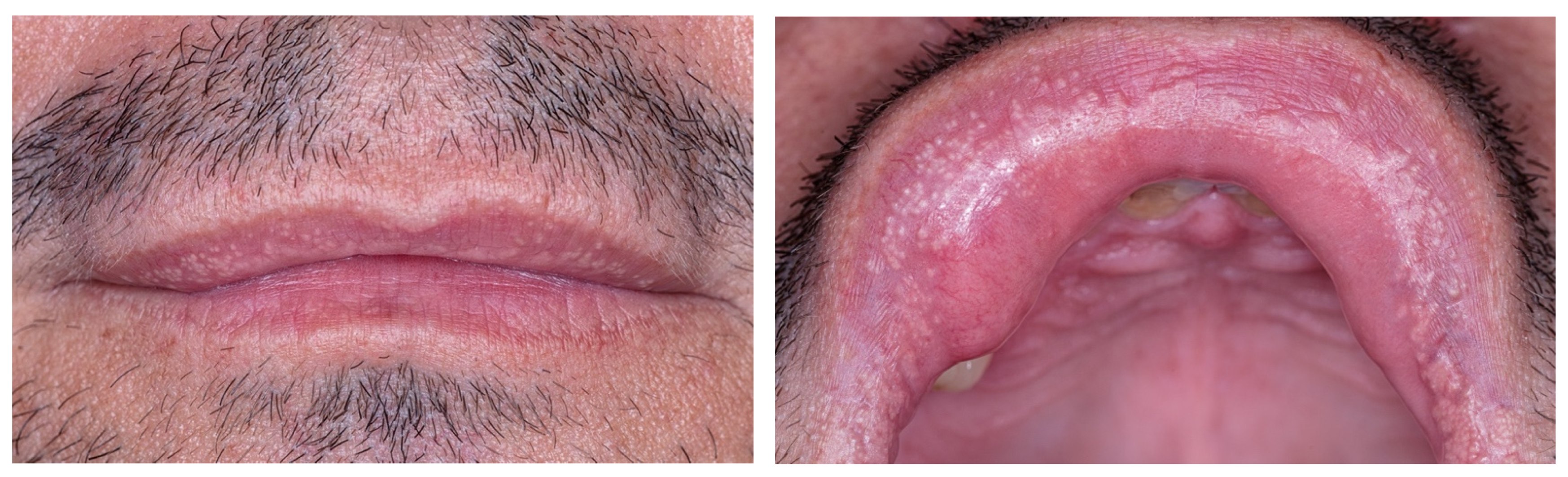
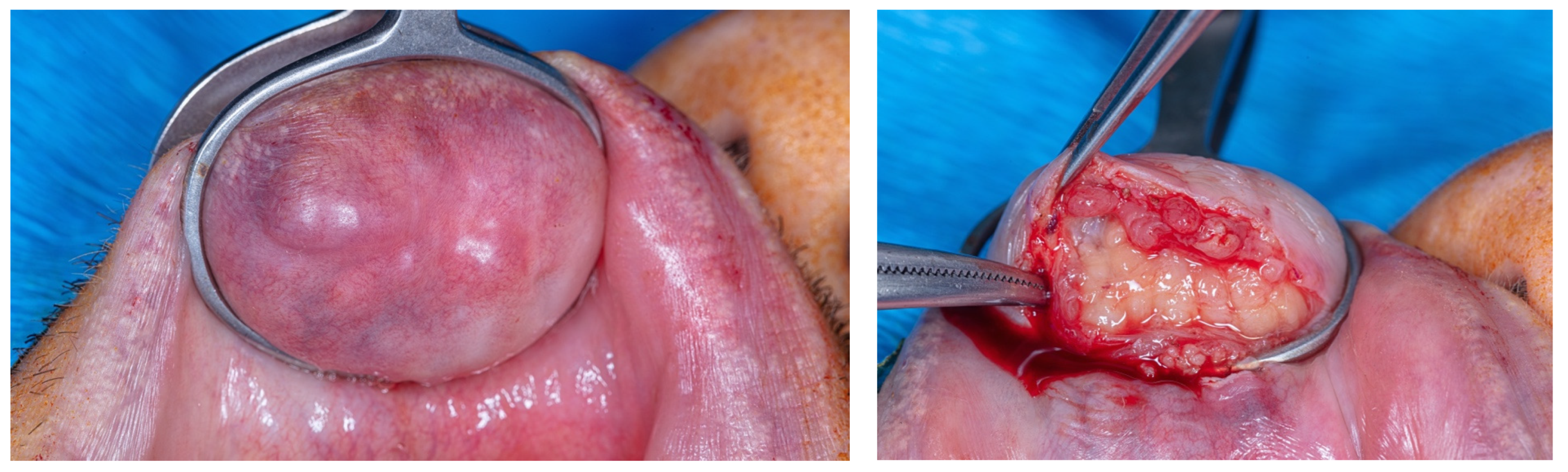
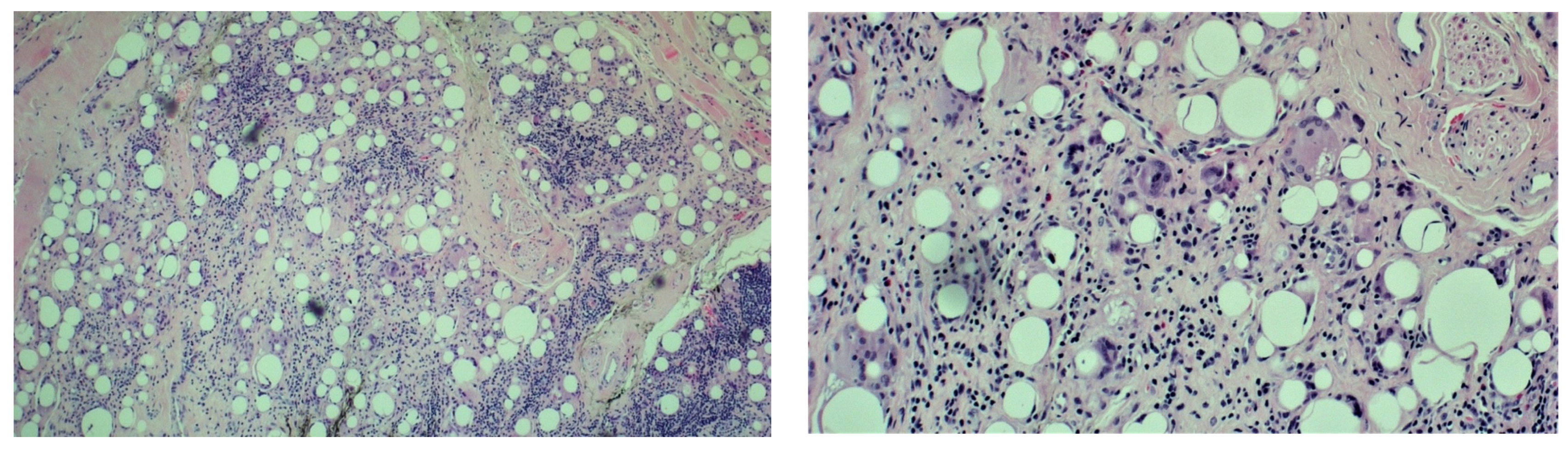
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmidt-Westhausen, A.; Frege, J.; Reichart, P. Abscess formation after lip augmentation with silicone: Case report. Int. J. Oral Maxillofac. Surg. 2004, 33, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Miller, P.J. Management of lip complications. Facial Plast. Surg. Clin. N. Am. 2019, 27, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Rivkin, A.; Weinkle, S.H.; Hardas, B.; Weiss, R.A.; Glaser, D.A.; Biesman, B.S.; Schumacher, A.; Murphy, D.K. Safety and effectiveness of repeat treatment with VYC-15L for lip and perioral enhancement: Results from a prospective multicenter study. Aesthetic Surg. J. 2019, 39, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Ajuz, D.; Oliveira, M.D.; Fernandes, J.C.H.; Fernandes, G.V.d.O. Facial Hemiplegia Treated with Botulinum Toxin: A Case Report. Diseases 2022, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Lemperle, G.; Morhenn, V.; Charrier, U. Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesthetic Plast. Surg. 2003, 27, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Johl, S.S.; Burgett, R.A. Dermal filler agents: A practical review. Curr. Opin. Ophthalmol. 2006, 17, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Clymer, M.A. Evolution in techniques: Lip augmentation. Facial Plast. Surg. 2007, 23, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Rauso, R. 5-year study of a polyacrylamide hydrogel-based filler for rehabilitation of HIV-related facial lipoatrophy. Aesthetic Surg. J. 2015, 35, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Moscona, R.A.; Fodor, L. A retrospective study on liquid injectable silicone for lip augmentation: Long-term results and patient satisfaction. J. Plast. Reconstr. Aesthetic Surg. 2010, 63, 1694–1698. [Google Scholar] [CrossRef]
- Alster, T.S.; West, T.B. Human-derived and new synthetic injectable materials for soft-tissue augmentation: Current status and role in cosmetic surgery. Plast. Reconstr. Surg. 2000, 105, 2515–2525. [Google Scholar] [CrossRef]
- Smith, K.C. Reversible versus nonreversible fillers in facial aesthetics: Concerns and considerations. Dermatol. Online J. 2008, 14, 3. [Google Scholar] [PubMed]
- Hirsch, R.J.; Stier, M. Complications of soft tissue augmentation. J. Drugs Dermatol. 2008, 7, 841–845. [Google Scholar] [PubMed]
- Abtahinaeini, B.; Faghihi, G.; Shahmoradi, Z.; Saffaei, A. Filler migration and extensive lesions after lip augmentation: Adverse effects of polydimethylsiloxane filler. J. Cosmet. Dermatol. 2018, 17, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Fulton, J.E., Jr.; Porumb, S.; Caruso, J.C.; Shitabata, P.K. Lip augmentation with liquid silicone. J. Dermatol. Surg. 2005, 31, 1577–1586. [Google Scholar] [CrossRef]
- Pallua, N.; Wolter, T.P. A 5-year assessment of safety and aesthetic results after facial soft-tissue augmentation with polyacrylamide hydrogel (Aquamid): A prospective multicenter study of 251 patients. Plast. Reconstr. Surg. 2010, 125, 1797–1804. [Google Scholar] [CrossRef]
- Hevia, O. Six-year experience using 1,000-centistoke silicone oil in 916 patients for soft-tissue augmentation in a private practice setting. Dermatol. Surg. 2009, 35 (Suppl. S2), 1646–1652. [Google Scholar] [CrossRef] [PubMed]
- Kavoussi, H.; Ebrahimi, A. Delayed gel indurations as an adverse effect of polyacrylamide filler and its easy treatment. Dermatol. Res. Pract. 2012, 2012, 539153. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.R.; Holmes, R.E. Artecoll: A long-lasting injectable wrinkle filler material: Report of a controlled, randomized, multicenter clinical trial of 251 subjects. Plast. Reconstr. Surg. 2004, 114, 964–976. [Google Scholar] [CrossRef] [PubMed]
- Rauso, R.; Califano, L.; Rugge, L.; Chirico, F.; Tartaro, G. Late onset complications secondary to polyacrylamide hydrogel-based filler for rehabilitation of HIV-related facial lipoatropy. Aesthetic Surg. J. 2018, 38, N170–N174. [Google Scholar] [CrossRef] [PubMed]
- Salles, A.G.; Lotierzo, P.H.; Gemperli, R.; Besteiro, J.M.; Ishida, L.C.; Gimenez, R.P.; Menezes, J.; Ferreira, M.C. Complications after polymethylmethacrylate injections: Report of 32 cases. Plast. Reconstr. Surg. 2008, 121, 1811–1820. [Google Scholar] [CrossRef]
- Park, T.H.; Seo, S.W.; Kim, J.-K.; Chang, C.H. Clinical experience with polymethylmethacrylate microsphere filler complications. Aesthetic Plast. Surg. 2012, 36, 421–426. [Google Scholar] [CrossRef]
- Rauso, R.; Curinga, G.; Rusciani, A.; Colella, G.; Amore, R.; Tartaro, G. Safety and efficacy of one-step rehabilitation of human immunodeficiency virus-related facial lipoatrophy using an injectable calcium hydroxyapatite dermal filler. Dermatol. Surg. 2013, 39, 1887–1894. [Google Scholar] [CrossRef]
- Rohrich, R.J. Advances in facial rejuvenation: Botulinum toxin A, hyaluronic acid dermal fillers, and combination therapies-consensus recommendations. Plast. Reconstr. Surg. 2008, 121, 5S–30S. [Google Scholar]
- Bergeret-Galley, C.; Latouche, X.; Illouz, Y.G. The value of a new filler material in corrective and cosmetic surgery: DermaLive and DermaDeep. Aesthetic Plast. Surg. 2001, 25, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Requena, C.; Izquierdo, M.J.; Navarro, M.; Martínez, A.; Vilata, J.J.; Botella, R.; Amorrortu, J.; Sabater, V.; Aliaga, A.; Requena, L. Adverse reactions to injectable aesthetic microimplants. Am. J. Dermatopathol. 2001, 23, 197–202. [Google Scholar] [CrossRef]
- Lemperle, G.; Rullan, P.P.; Gauthier-Hazan, N. Avoiding and treating dermal filler complications. Plast. Reconstr. Surg. 2006, 118 (Suppl. S3), 92S–107S. [Google Scholar] [CrossRef] [PubMed]
- Gladstone, H.B.; Cohen, J.L. Adverse effects when injecting facial fillers. Semin. Cutan. Med. Surg. 2007, 26, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Broder, K.W.; Cohen, S.R. An overview of permanent and semipermanent fillers. Plast. Reconstr. Surg. 2006, 118 (Suppl. S3), 7S–14S. [Google Scholar] [CrossRef]
- Sanchis-Bielsa, J.M.; Bagán, J.V.; Poveda, R.; Salvador, I. Foreign body granulomatous reactions to cosmetic fillers: A clinical study of 15 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 237–241. [Google Scholar] [CrossRef]
- Lombardi, T.; Samson, J.; Plantier, F.; Husson, C.; Küffer, R. Orofacial granulomas after injection of cosmetic fillers. Histopathologic and clinical study of 11 cases. J. Oral Pathol. Med. 2004, 33, 115–120. [Google Scholar] [CrossRef]
- Jham, B.C.; Nikitakis, N.G.; Scheper, M.A.; Papadimitriou, J.C.; Levy, B.A.; Rivera, H. Granulomatous foreign-body reaction involving oral and perioral tissues after injection of biomaterials: A series of 7 cases and review of the literature. J. Oral Maxillofac. Surg. 2009, 67, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Stolman, L.P. To the Editor: Human collagen reactions. Dermatol. Surg. 2005, 31 Pt 2, 1634. [Google Scholar] [CrossRef] [PubMed]
- Funt, D.; Pavicic, T. Dermal fillers in aesthetics: An overview of adverse events and treatment approaches. Clin. Cosmet. Investig. Dermatol. 2013, 6, 295–316. [Google Scholar] [CrossRef] [PubMed]
- Monheit, G.D.; Rohrich, R.J. The nature of long-term fillers and the risk of complications. Dermatol. Surg. 2009, 35 (Suppl. S2), 1598–1604. [Google Scholar] [CrossRef]
- Trinh, L.N.; McGuigan, K.C.; Gupta, A. Delayed Granulomas as a Complication Secondary to Lip Augmentation with Dermal Fillers: A Systematic Review. Surg. J. 2022, 8, e69–e79. [Google Scholar] [CrossRef] [PubMed]
- Botti, G.; Botti, C.; Cella, A. A simple surgical remedy for iatrogenic excessively thick lips. Plast. Reconstr. Surg. 2002, 110, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Sage, R.J.; Chaffins, M.L.; Jouba, D.J. Granulomatous foreign body reaction to hyaluronic acid: Report of a case after nasolabial fold augmentation and review of management. Dermatol. Surg. 2009, 35, 1696–1700. [Google Scholar] [CrossRef] [PubMed]
- Arin, M.J.; Bäte, J.; Krieg, T.; Hunzelmann, N. Silicone granuloma of the face treated with minocycline. J. Am. Acad. Dermatol. 2005, 52, S53–S56. [Google Scholar] [CrossRef]
- Baumann, L.S.; Halem, M.L. Lip silicone granulomatous foreign body reaction treated with aldara (imiquimod 5%). Dermatol. Surg. 2003, 29, 429–432. [Google Scholar] [CrossRef]
- Akrish, S.; Dayan, D.; Taicher, S.; Adam, I.; Nagler, R.M. Foreign body granulomas after injection of bio-alcamid for lip augmentation. Am. J. Otolaryngol. 2009, 30, 356–359. [Google Scholar] [CrossRef]
- Hubmer, M.G.; Hoffmann, C.; Popper, H.; Scharnagl, E. Expanded polytetrafluoroethylene threads for lip augmentation induce foreign body granulomatous reaction. Plast. Reconstr. Surg. 1999, 103, 1277–1279. [Google Scholar] [CrossRef] [PubMed]
- Mercer, S.E.; Kleinerman, R.; Goldenberg, G.; Emanuel, P.O. Histopathologic identification of dermal filler agents. J. Drugs Dermatol. 2010, 9, 1072–1078. [Google Scholar] [PubMed]
- Requena, L.; Requena, C.; Christensen, L.; Zimmermann, U.S.; Kutzner, H.; Cerroni, L. Adverse reactions to injectable soft tissue fillers. J. Am. Acad. Dermatol. 2011, 64, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Conejo-Mir, J.S.; Sanz Guirado, S.; Angel Muñoz, M. Adverse granulomatous reaction to Artecoll treated by intralesional 5-fluorouracil and triamcinolone injections. Dermatol. Surg. 2006, 32, 1079–1082. [Google Scholar] [PubMed]
- Christensen, L.; Breiting, V.; Janssen, M.; Vuust, J.; Hogdall, E. Adverse reactions to injectable soft tissue permanent fillers. Aesthetic Plast. Surg. 2005, 29, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Pasternack, F.R.; Fox, L.P.; Engler, D.E. Silicone granulomas treated with etanercept. Arch. Dermatol. 2005, 141, 13–15. [Google Scholar] [CrossRef]
- Kästner, S.; Gonser, P.; Paprottka, F.; Kaye, K.O. Removal of polyacrylamide gel (Aquamid) from the lip as a solution for late-onset complications: Our 8-year experience. Aesthetic Plast. Surg. 2018, 42, 791–797. [Google Scholar] [CrossRef]
- Fanous, N.; Brousseau, V.J.; Yoskovitch, A. The ‘bikini lip reduction’: A detailed approach to hypertrophic lips. Can. J. Plast. Surg. Winter 2007, 15, 205–210. [Google Scholar] [CrossRef]
- Rauso, R.; Califano, L.; Rugge, L.; Chirico, F.; Tartaro, G. Surgical lip remodeling after injection of permanent filler. Aesthetic Surg. J. 2019, 39, 565–571. [Google Scholar] [CrossRef]
- Pascali, M.; Chirico, F.; Rugge, L.; Rauso, R. Aesthetic Surgical Pathway in Permanent Facial Filler Removal. Facial Plast. Surg. 2024, 40, 19–30. [Google Scholar] [CrossRef]
- El-Nahas, M.A.; Ghareeb, F.M. Complicated facial filler management by facelift. Menoufia Med. J. 2021, 33, 1347–1351. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiuza, S.; Marques, T.; Padin, I.; Carvalho, M.T.; Veiga, N.; Fernandes, J.C.H.; Fernandes, G.V.O.; Couto, P. Histological, Clinical Assessment, and Treatment of a Permanent Filler Complication in the Upper Lip: A Case Report with 16-Year Follow-Up. Cosmetics 2024, 11, 50. https://doi.org/10.3390/cosmetics11020050
Fiuza S, Marques T, Padin I, Carvalho MT, Veiga N, Fernandes JCH, Fernandes GVO, Couto P. Histological, Clinical Assessment, and Treatment of a Permanent Filler Complication in the Upper Lip: A Case Report with 16-Year Follow-Up. Cosmetics. 2024; 11(2):50. https://doi.org/10.3390/cosmetics11020050
Chicago/Turabian StyleFiuza, Samuel, Tiago Marques, Irving Padin, Maria Teresa Carvalho, Nelio Veiga, Juliana Campos Hasse Fernandes, Gustavo Vicentis Oliveira Fernandes, and Patrícia Couto. 2024. "Histological, Clinical Assessment, and Treatment of a Permanent Filler Complication in the Upper Lip: A Case Report with 16-Year Follow-Up" Cosmetics 11, no. 2: 50. https://doi.org/10.3390/cosmetics11020050
APA StyleFiuza, S., Marques, T., Padin, I., Carvalho, M. T., Veiga, N., Fernandes, J. C. H., Fernandes, G. V. O., & Couto, P. (2024). Histological, Clinical Assessment, and Treatment of a Permanent Filler Complication in the Upper Lip: A Case Report with 16-Year Follow-Up. Cosmetics, 11(2), 50. https://doi.org/10.3390/cosmetics11020050







