Abstract
Pachyrhizus erosus (L.) Urb. is a tropical perennial vine plant native to southern Mexico, Southeast Asia, Central America, and Africa. In this study, we analyzed and identified various polyphenolic compounds and isoflavones present in ethanolic P. erosus root extracts and investigated their potential anti-obesity activity as a natural health food resource. The extraction process involved drying the yam bean, followed by extraction with 70% ethanol, evaporation, and freeze-drying. Fractionation was achieved through layer separation using n-hexane, ethyl acetate (EtOAc), butanol (BuOH), and water. The EtOAc fraction exhibited the highest antioxidant activity among the experimental groups, with an IC50 value of 531.77 µg/mL for ABTS radical scavenging. In α-glucosidase and lipase inhibition assays, IC50 values were determined to be 873.07 µg/mL and 915.02 µg/mL, respectively. Using HPLC and LC-MS/MS, we detected isoflavone components in P. erosus root extracts, identifying daidzein, genistein, and rotenone among them. Daidzein was the most abundant isoflavone in P. erosus root extracts. To validate the anti-obesity activity in the EtOAc fraction and daidzein, we used 3T3-L1 preadipocytes treated with MDI (3-isobutyl-1-methylxanthine, dexamethasone, insulin) for 8 days. Oil Red O staining experiments demonstrated a concentration-dependent reduction in lipid content in the EtOAc fraction and daidzein treatment groups. Additionally, we examined the expression pattern of proteins related to the leptin-PPAR-FAS Pathway, revealing a concentration-dependent decrease in obesity-related proteins.
1. Introduction
The worldwide incidence of obesity has become a significant societal issue in many countries, defined as the excessive or abnormal accumulation of fat [1]. Obesity is where excess neutral fat accumulates, primarily in visceral adipose tissue. Still, it gradually extends to other organs, such as the liver, muscles, and pancreas, disrupting their normal functions and contributing to various diseases [2]. Fatty tissue serves as a reservoir for surplus energy storage. It is recognized as an endocrine organ due to its synthesis and secretion of multiple adipokines, broadly influencing systemic metabolic processes [3].
Especially when excessive fat accumulates in visceral adipose tissue, it leads to abnormal secretion of adipokines such as leptin, adiponectin, resistin, TNF-α, and plasminogen activator inhibitor-1, increased expression of NADPH oxidase, enhanced immune cell infiltration, and increased production of free radicals, specifically reactive oxygen species (ROS) [4]. These factors contribute to chronic inflammation and oxidative stress, promoting the development of insulin resistance, diabetes, metabolic syndrome, cardiovascular diseases, and neurodegenerative disorders like Alzheimer’s disease and cancer, among others [5,6,7].
Obesity is not merely a cosmetic concern but a medical condition across various age groups, elevating the risk of numerous health complications. Intracellular fat accumulation results from the proliferation and differentiation of preadipocytes into mature adipocytes during adipogenesis [8]. Excessive addition of preadipocytes or an increased number of differentiated adipocytes leads to an elevated adipocyte count. As the intra-adipocyte lipid storage increases, adipocyte size also increases, ultimately culminating in obesity [9]. The process of preadipocyte differentiation into adipocytes is complex, involving concurrent changes in cell morphology, hormone sensitivity, and the interaction of various genes [10]. Obesity can be mitigated by inhibiting adipocyte proliferation and differentiation, suppressing intra-adipocyte lipid accumulation, and promoting the breakdown of accumulated fat [11]. The regulation of adipocyte differentiation involves the expression of transcription factors, including CCAAT/enhancer-binding protein alpha (C/EBPα), sterol regulatory element-binding protein 1c (SREBP-1c), and activation of peroxisome proliferator-activated receptor gamma (PPARγ). Among various transcription factors, SREBP-1c is a pivotal regulator that activates critical transcription factors in lipid metabolism, such as acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS). Unlike PPAR, C/EBP is not a transcription factor for fatty acid synthase (FAS) synthesis. The influence on FAS expression is primarily attributed to PPARγ, a direct factor in synthesizing new fatty acids [12]. The intricate pathophysiology of obesity, characterized by an energy imbalance due to factors such as genetic predisposition, metabolic anomalies, and lifestyle choices, necessitates multifaceted interventions [13]. Genetic factors may predispose individuals to obesity through effects on appetite, satiety, and energy metabolism, while metabolic contributors include insulin resistance and alterations in lipid metabolism that promote adipose tissue accumulation [13,14]. Among dietary approaches, including low-calorie, high-fiber foods are critical for weight management and metabolic health [15]. In this context, P. erosus with its low glycemic index and high dietary fiber content, represents a promising addition to the diet that can support satiety, reduce overall calorie intake, and improve glycemic control, potentially alleviating some symptoms [16].
The scientific name of the Yam bean (or Jicama) is Pachyrhizus erosus, which is also referred to as the Mexican yam bean. It is a tropical vine plant. It is primarily cultivated in Mexico but grown in Brazil, Indonesia, China, the United States, Nigeria, the Philippines, and other regions [2].
P. erosus is considered one of the top ten health foods globally, with a low glycemic index compared to potatoes and sweet potatoes [17]. It is rich in dietary fiber, low in calories, and abundant in various nutrients, vitamins, and minerals [17]. Obesity is closely related to the leptin hormone, and it is a hormone that can initiate adipogenesis [18].
In previous studies, the investigation into the anti-obesity properties of P. erosus primarily centered around the polysaccharide inulin. Moreover, most existing studies have primarily focused on P. erosus’s nutritional value and potential as a processed food ingredient. However, this study shifted its focus to the isoflavone components of P. erosus a research aspect that had not been explored in prior studies. Moreover, this investigation delved into its potential as an anti-obesity agent from EtOAc fraction and daidzein contained in P. ersus through enzyme inhibition and 3T3-L1 cells.
2. Materials and Methods
2.1. Chemicals
Daidzein, genistein, rotenone, quercetin, 2,2-diphenyl-1-picrylhydrazyl (DPPH), potassium persulfate, gallic acid, sodium carbonate, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), aluminum chloride, 0.2 M Folin–Ciocalteu’s phenol reagent, potassium acetate, acarbose, orlistat, α-glucosidase (Type Ⅰ, EC Number 232-604-7), 4-nitrophenyl β-glucuronide, lipase (Type II, EC Number 232-619-9), 4-nitrophenyl butyrate, dimethyl sulfoxide (DMSO), insulin, thiazolyl blue tetrazolium bromide (MTT), 3-isobutyl-1-methylxanthine (IBMX), and dexamethasone were sourced from Sigma Aldrich (St. Louis, MO, USA). n-Hexane, ethanol, n-butanol (BuOH), acetonitrile, methanol, and ethyl acetate (EtOAc) were acquired from OCI (Soul, KR). All other chemicals and reagents were commercially available and of high-quality grade. Fetal bovine serum (FBS), penicillin, and Dulbecco’s modified Eagle’s medium (DMEM) were procured from Hy-Clone (Boston, MA, USA). Antibodies against sterol regulatory element-binding protein (SREBP) and peroxisome proliferator-activated receptor-γ (PPARγ) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS) antibodies were purchased from Cell Signaling (Boston, MA, USA). Horseradish peroxidase-conjugated goat anti-rabbit and anti-mouse antibodies were sourced from Invitrogen (Carlsbad, CA, USA).
2.2. Preparation of Extracts and Solvent Fractions
The Pachyrhizus erosus (L.) Urb. species are mainly distributed in Jeonnam province, Republic of Korea. The plant name has been checked with http://www.theplantlist.org (accessed on 2 May 2023). P. erosus roots used in this study were collected in the summer season (from June to August) in 2022 by Yongjin eco-friendly farming in Hampyeong-gun, Jeollanam-do, Republic of Korea (35.127831436322275°, 126.42414132351274). A voucher specimen has been officially archived in the Department of Chemical Engineering at Chosun University for future reference. The P. erosus root employed in this experiment underwent the following processes: it was initially washed, sliced into thin pieces, dried at 60 °C for 24 h, subsequently ground, and finally subjected to extraction with 70% EtOH (Figure 1). For extraction, 1 L of 70% EtOH was added to 100 g of dried P. erosus matter and left to soak at room temperature for 2 days. Each resulting extract was filtered through Whatman filter paper (0.45 µm), concentrated, lyophilized, and utilized as a sample. The concentrated 70% EtOH extract was dissolved in 170 mL of 50% methanol (MeOH) for solvent-specific fractionation. Based on polarity, a fractional funnel was employed to fractionate the solution into n-hexane, EtOAc, BuOH, and water fractions. This fractionation process was repeated three times using 340 mL of each solvent. Subsequently, each fraction was concentrated and lyophilized to serve as a sample, and the percentage yield was determined using the following equation.
Yield (%) = (weight of the sample after freezing drying (g)/weight of the sample before extraction (g)) × 100
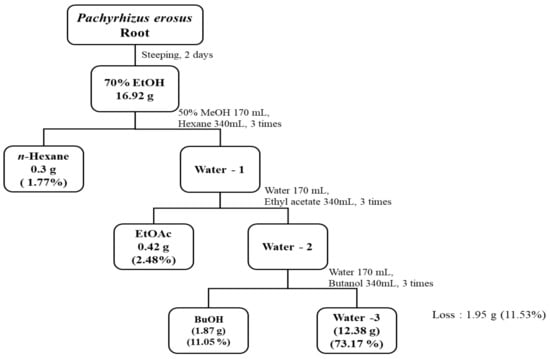
Figure 1.
Isolation scheme of 70% EtOH extracts and solvent fractionation from P. erosus roots. Extraction yields are indicated in parentheses.
2.3. Antioxidants Activity Assay
2.3.1. DPPH Free Radical Activity
In this study, we utilized the stable free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) to evaluate the antioxidant activity of P. erosus roots. DPPH exhibits a peak at 520 nm when measured using UV-vis spectrophotometry, appearing purple. Upon scavenging DPPH free radicals, it changes color to yellow, indicating high antioxidant capability [19]. The DPPH free radical scavenging capacity in this experiment was evaluated by modifying Blois’ method [20]. First, sample solutions of the 70% EtOH extract and its respective fractions were prepared at 100 to 5000 μg/mL concentrations. Each concentration was mixed with 200 μL of the sample and 800 μL of a 0.2 mM DPPH reagent. The reaction proceeded for 15 min in a light-protected environment. Daidzein was the positive control at 1 to 50 µg/mL concentrations and underwent the same reaction with the DPPH reagent. Subsequently, absorbance at 517 nm was measured using a Biotek Synergy HT multi-detection microplate reader. The scavenging activity for each sample was computed using the following equation.
Radical scavenging activity (%) = (Abscontrol − Abssample)/Abscontrol × 100
2.3.2. ABTS Radical Activity
ABTS does not spontaneously generate radicals; instead, it undergoes oxidation to produce ABTS radicals. The interaction between ABTS radicals and antioxidants leads to removing radicals, transitioning from a deep green-blue color to a transparent state [21]. In this experiment, we assessed the ABTS radical scavenging activity by modifying the method proposed by Sato [22]. To generate ABTS radicals, a 1:1 mixture of 7 mM ABTS solution and 2 mM potassium persulfate solution was incubated in the dark for 18 h to prepare the reagent. Subsequently, the ABTS solution was diluted eight times with PBS (pH 7.4) to produce the ABTS reagent. The 70% EtOH extract samples and specific solvent fractions used in the experiment were prepared at 50 to 1000 μg/mL concentrations. Daidzein was a positive control at 1 to 50 µg/mL concentrations. 200 μL of the sample was mixed with 1000 μL of ABTS reagent for each concentration. After a 15 min reaction in a light-protected chamber, the absorbance was measured at 730 nm using the Biotek Synergy HT multi-detection microplate reader. The scavenging activity of each sample was determined using the following formula.
Radical scavenging activity (%) = (Abscontrol − Abssample)/Abscontrol × 100
2.4. Total Polyphenol and Flavonoid Contents
2.4.1. Total Polyphenol Contents (TPC)
Polyphenols are secondary metabolites in plants that eliminate reactive oxygen species in the human body. A representative polyphenolic compound is catechins found in green tea. Examining the structure of polyphenols reveals multiple hydroxyl groups (-OH) attached to the phenol group, allowing easy interaction with other compounds and exhibiting antioxidant, anti-inflammatory, and various physiological effects [23]. This experiment used a modified Folin–Ciocalteu method to measure the total polyphenol content in P. erosus roots [24]. For each sample, samples of P. erosus root, 70% EtOH extract, and solvent-specific fractions were prepared at 1000 μg/mL each. According to the experimental protocol, a 500 μL aliquot was mixed with 500 μL of 0.2 M Folin-Ciocalteu phenol reagent and 500 μL of 2% sodium carbonate (w/v). The mixture was reacted at room temperature for 30 min, and absorbance values at 750 nm for each sample were measured using the Biotek Synergy HT multi-detection microplate reader (Synergy™ HT, Biotek Instruments, Winooksi, VT, USA). The measured absorbance values were used to calculate the total polyphenol content in each sample regarding gallic acid equivalents (GAE) per gram, referring to the standard calibration curve.
y = 15.366x − 0.0444, R2 = 0.997, where x is the gallic acid equivalent (µg/g) and y is the absorbance.
2.4.2. Total Flavonoid Contents (TFC)
A subgroup of polyphenols, referred to as flavonoids, is characterized by the presence of an aglycone and a sugar moiety combined into a glycoside, and they exhibit various physiological effects, including antioxidative, antimicrobial, and anti-inflammatory activities [25]. The total flavonoid content was quantified according to the outlined procedure [26]. Sample solutions of the 70% EtOH extract and solvent-specific fractions were prepared at a 1000 µg/mL concentration. Subsequently, 500 μL of each prepared sample was successively mixed with 1.5 mL of methanol, 100 μL of 1 M potassium acetate, and 1.4 mL of distilled water. The mixture was allowed to react at room temperature for 40 min. Following this, the absorbance at 415 nm was measured using a Biotek Synergy HT multi-detection microplate reader. Based on a standard calibration curve, the recorded value was then used to determine the total flavonoid content, expressed as the equivalent amount of quercetin (QUE) per 1 g of the sample.
y = 3.3503x + 0.0436, R2 = 0.999, where x is the quercetin equivalent (µg/g) and y is the absorbance.
2.5. High-Performance Liquid Chromatography with Diode-Array Detection (HPLC–DAD) Analysis
We performed an HPLC analysis to identify the most abundant polyphenols, such as daidzein, genistein, rotenone, and others in the P. erosus extract. We prepared the 70% EtOH extract and the EtOAc fraction at a concentration of 1000 µg/mL and conducted the analysis using an HPLC system (SPD-20A, Shimadzu Co., Kyoto, Japan). A 10 µL sample was filtered through a 0.45 µm syringe filter and analyzed on a C18 column (Shimpack GIS-ODS, 4.6 × 250 mm, 5.0 µm, Shimadzu Co., Kyoto, Japan) at a flow rate of 1.0 mL/min, with detection at 280 nm. Daidzein, genistein, and rotenone were used as reference standards, and the mobile phase consisted of a mixture of water (A) and acetonitrile (B). The HPLC elution gradient was as follows: 0–10 min: B (10–30%), 10–20 min: B (30–70%), 20–30 min: B (70–80%), 30–40 min: B (80–100%), 40–50 min: B (100–100%).
2.6. Quantitative Analysis of Active Ingredient Using LC-MS/MS
The LC-MS/MS analysis method was conducted following the reference of Kim et al.’s method [27]. An AB SCIEX 4000 Q Trap LC/MS/MS System (Shimadzu LC 20A System, Kyoto, Japan) was utilized for the analysis, with the mobile phases consisting of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). The analysis conditions were implemented as a gradient as follows: 0–0.1 min, 5% (B); 0.1–2 min, 40% (B); 2–3 min, 80% (B); 3–5 min, 80% (B); 5–5.1 min, 5% (B). The analysis duration was 8 min, and both negative and positive ionization modes were analyzed using Turbo Ion Spray.
2.7. Anti-Obesity Enzyme Inhibition Assay
2.7.1. α-Glucosidase Inhibition Assay
During digestion, alpha-amylase breaks down polysaccharides, and subsequent hydrolysis into monosaccharides by α-glucosidase can lead to elevated blood glucose levels. Inhibiting this enzymatic process has been shown to impact blood glucose levels and fat accumulation [28]. We assessed α-glucosidase inhibitory activity according to the protocol outlined [29]. In this study, we utilized the 70% EtOH extract, the EtOAc fraction exhibiting the highest antioxidant activity, and daidzein as a positive control due to its identification as the most abundant component through efficacy analysis. The extract concentrations ranged from 50 to 1000 µg/mL, while the positive control was prepared at 5 to 500 µg/mL concentrations. For each concentration, 100 µL of the sample was mixed with 25 μL of 1 unit/mL α-glucosidase (derived from Saccharomyces cerevisiae) and allowed to react at 37 °C for 10 min in a water bath. Subsequently, 50 μL of 5 mM ρ-nitrophenyl α-D-glucopyranoside was added, and the mixture was reacted at 37 °C for an additional 10 min. Absorbance was then measured at 405 nm using a Biotek Synergy HT multi-detection microplate reader.
2.7.2. Lipase Inhibition Assay
Lipase is an enzyme responsible for the hydrolysis of triglycerides (TG) in adipocytes, resulting in the release of glycerol and fatty acids. Inhibition of lipase activity can reduce the digestion and absorption of dietary fat, making it an effective strategy for addressing obesity and diabetes [30]. We assessed lipase inhibition using a modified version of Kim’s method [31]. An enzyme buffer was prepared by mixing 5 mg of pancreatic lipase (derived from porcine pancreas) with 1 mL of 10 mM MOPS/1 mM EDTA (pH 6.8). Sample solutions were prepared at 500 to 5000 µg/mL concentrations. Subsequently, 200 μL of the enzyme buffer, 200 μL of each sample concentration, and 200 μL of 100 mM Tris-HCl/5 mM CaCl2 (pH 6.8) were sequentially mixed and allowed to react at 37 °C for 5 min. Following this, 100 μL of 10 mM p-nitrophenyl butyrate (p-NPB) was added, and the mixture was further incubated for 30 min at 37 °C, with absorbance measured at 410 nm using a Biotek Synergy HT multi-detection microplate reader.
2.8. Inhibition of Adipogenesis and Fatty Acid Synthesis in 3T3-L1 Cells
2.8.1. Cell Culture and Adipocyte Differentiation
3T3-L1 cells are a type of stem cell. They are so-called committed stem cells that are differentiated into adipocytes. These cells are differentiated into adipocytes when treated with a differentiation inducer. In this experiment, 3T3-L1 preadipocytes were procured from the American Type Culture Collection (Rockville, MD, USA). The cells were maintained in DMEM supplemented with 10% FBS, 50 units/mL of penicillin, and 50 μg/mL of streptomycin in a humidified environment with 5% CO2 at 37 °C for two days [32]. Following this initial culture period, 3T3-L1 preadipocytes were induced to undergo differentiation by modifying the culture medium. During the differentiation process, the preadipocytes were incubated in a growth medium containing MDI (0.5 μM of IBMX, 1 μM of dexamethasone, and 1 μg/mL of insulin) in conjunction with the test samples (EtOAc and daidzein) for 3 days. Subsequently, the cells were cultured in a growth medium supplemented with 1 μg/mL of insulin for an additional 3 days, with medium renewal occurring every 2 days. Following a total culture period of 10 days, the cells were utilized for experimental purposes.
2.8.2. Cell Viability Assay
In this study, to evaluate the cytotoxicity of P. erosus roots, cells were seeded in 12-well plates at 1 × 104 cells per well, and an MTT assay was performed according to other cells previously [33]. The battery was kept at 37 °C for 48 h while maintaining the test samples. MTT (0.2 mg/mL) was continued for 40 min. After removing the supernatant of the repaired cells, we will use DMSO to obtain formazan crystals. Finally, the absorption light intensity of each well was measured at 570 nm using a microplate reader.
2.8.3. Oil Red O Staining
The Oil Red O staining method searches for triglycerides (Fat), which are widely used to identify lipomas, fatty degeneration, and related substances. Oil red O can easily detect and distinguish things challenging to see or determine in Hematoxylin and eosin Staining. In this study, cells differentiated into adipocytes were stained with Oil Red O solution at day 10 to assess cellular lipid content according to a standardized protocol [34]. Cells were rinsed with PBS and then fixed in 4% formalin for 1 h at room temperature. After fixation, cells were stained with Oil Red O working solution and incubated for an additional hour at room temperature. Cells were washed twice with PBS and imaged using a microscope (Eclipse TS 100, Nikon Corporation, Kyoto, Japan).
2.8.4. Western Blot Analysis
Western blotting is a technique employed to selectively identify a specific protein (antigen) within a mixture of proteins, utilizing an antibody that recognizes the antigen epitope of the target protein. In this experiment, the 3T3-L1 cell culture medium was fully lysed using RIPA buffer, and subsequent protein extraction was performed. Protein concentration was determined through the Bradford assay. A 12% and 7.5% sodium dodecyl sulfate-polyacrylamide gel was prepared for the quantified proteins, and electrophoresis (SDS-PAGE) was conducted to separate the proteins [35]. Subsequently, the nitrocellulose membrane was incubated with the primary antibody and allowed to react at 4 °C for 24 h. The membrane was washed with PBST 5 to 7 times for over 1 h and then incubated with the secondary antibody. Antibodies targeting proteins directly associated with anti-obesity activity (SREBP, PPARγ, FAS, ACC) were utilized, with β-actin as the housekeeping gene. Finally, the protein expression pattern was visualized using an ECL chemiluminescence detector (Amersham Biosciences, Buckinghamshire, UK).
2.9. Statistical Analysis
All experiments performed in this experiment were repeated more than three times, and the results are presented as the mean ± standard deviation (SD). Data analysis was carried out using analysis of variance (ANOVA) followed by Dunnett’s multiple-range test (p < 0.05) with SPSS 27 (IBM, Armonk, NY, USA).
3. Results
3.1. Extraction Yield of P. erosus Root Extracts
In this study, P. erosus roots were extracted with 70% ethanol and subsequently fractionated using various solvents. The yield of the 70% ethanol extract, obtained from 100 g of P. erosus roots, was approximately 16.92 g, corresponding to an approximate yield of 17%. The 70% EtOH extract was further fractionated into n-hexane, EtOAc, BuOH, and water fractions, resulting in yields of approximately 0.30 g, 0.42 g, 1.87 g, and 12.38 g, respectively. The yields of each fraction were determined to be 1.77%, 2.48%, 11.05%, and 73.17%, with the water fraction exhibiting the highest yield.
3.2. Antioxidant Activity of P. erosus Root Extracts
In this study, we conducted DPPH and ABTS radical scavenging assays to assess the antioxidant activity of P. erosus 70% EtOH extract and its solvent-specific fractions. We measured the radical scavenging capacity in each experiment and calculated the IC50 values, representing the concentration at which 50% of the radicals were reduced (Figure 2, Table 1). According to the DPPH and ABTS assay results, the EtOAc fraction exhibited the highest antioxidant activity. The DPPH and ABTS IC50 values for the EtOAc fraction were determined to be 1054.66 µg/mL and 531.77 µg/mL, respectively, while daidzein showed values of 14.80 µg/mL and 11.31 µg/mL, respectively. In the DPPH assay, the activity order, apart from the EtOAc fraction, was observed as water, BuOH, n-hexane, and 70% EtOH extract. In the ABTS assay, the activity order was BuOH, water, n-hexane, and 70% EtOH extract, following the EtOAc fraction.
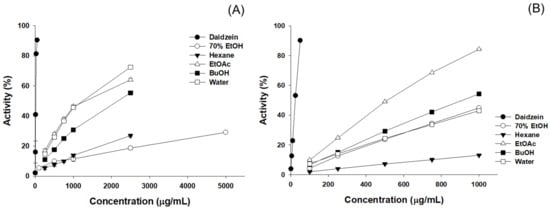
Figure 2.
Antioxidant activity results of 70% EtOH extracts and solvent fraction from P. erosus roots. (A) DPPH free radical scavenging activity; (B) ABTS radical scavenging activity.

Table 1.
Antioxidant activity results of 70% EtOH extracts and solvent fraction from P. erosus roots.
3.3. Total Polyphenol and Total Flavonoid Contents
To determine the polyphenol and flavonoid contents of P. erosus 70% EtOH extract and solvent-specific fractions, we conducted measurements of total polyphenol and total flavonoid contents (Table 2). The experimental results revealed that the EtOAc fraction exhibited the highest polyphenol and flavonoid contents among all the fractions. In the EtOAc fraction, the total polyphenol and flavonoid contents were determined to be 2105.08 GAE µg/g and 1062.99 QUE µg/g, respectively. In contrast, the n-hexane fraction had the lowest contents, with 1254.72 GAE µg/g and 24.28 QUE µg/g. This aligns with the lower antioxidant activity observed in the n-hexane fraction.

Table 2.
Identifying polyphenol compounds in P. erosus 70% EtOH extracts and EtOAc fraction by LC-MS/MS (Unit: μg/g).
3.4. Analysis of Polyphenol Content Using HPLC and LC-MS/MS
Previous studies have reported the presence of compounds such as daidzein, genistein, and rotenone in P. erosus extracts, which are known to contribute to antioxidant and skin-whitening activities [36,37]. In this study, we used HPLC to analyze polyphenolic components present in P. erosus 70% extracts and the EtOAc fraction, which exhibited the highest antioxidant activity (Figure 3). The HPLC analysis confirmed the presence of daidzein in both the 70% EtOH extract and the EtOAc fraction, with the highest content of 337.86 µg/g observed in the EtOAc fraction. Additionally, rotenone was not detected in the 70% EtOH extract or the EtOAc fraction.
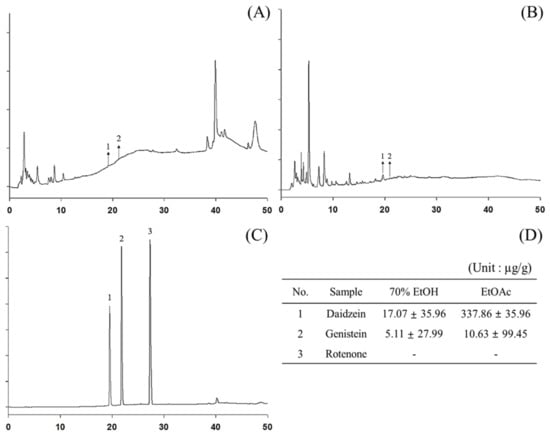
Figure 3.
High-performance liquid chromatography (HPLC) profiles of (A) P. erosus root 70% EtOH extract, (B) EtOAc fraction, and (C) standard mixture using diode array detection at 280 nm. (D) Identified polyphenols in P. erosus root 70% EtOH extract and EtOAc fraction, quantified through HPLC. (1) Daidzein; (2) Genistein; (3) Rotenone.
This study analyzed 22 bioactive compounds in the 70% EtOH extract and the EtOAc fraction using LC-MS/MS. Notably, among the compounds identified through HPLC, daidzein exhibited the highest content. The quantitative analysis by LC-MS/MS revealed that the polyphenolic content in the 70% EtOH extract and the EtOAc fraction was 207.24 μg/g and 1286.96 μg/g, respectively. In the 70% EtOH extract, nine compounds were identified, with fumaric acid, 4-hydroxybenzoic acid, allopurinol, and daidzein being the most abundant in descending order (Figure S1, Table 2). In the EtOAc fraction, a total of 22 compounds were detected, with 4-hydroxybenzoic acid, daidzein, fumaric acid, vanillic acid, and genistein being the predominant constituents in descending order (Figure S2, Table 2).
3.5. Results of the Anti-Obesity Enzyme Inhibition Activity
To evaluate the anti-obesity enzyme inhibition activity of the 70% EtOH extract and the EtOAc fraction of P. erosus, α-glucosidase inhibition, and lipase inhibition assays were conducted (Figure 4, Table 3). The experimental results revealed that the EtOAc extract exhibited superior enzyme inhibition activity. The IC50 values for α-glucosidase and lipase inhibition assays were 873.07 µg/mL and 915.02 µg/mL, respectively, for the EtOAc fraction. Daidzein, when assessed for its anti-obesity enzyme inhibition activity, showed IC50 values of 87.59 µg/mL and 84.85 µg/mL, respectively, in the α-glucosidase and lipase inhibition assays, indicating a high inhibitory effect.
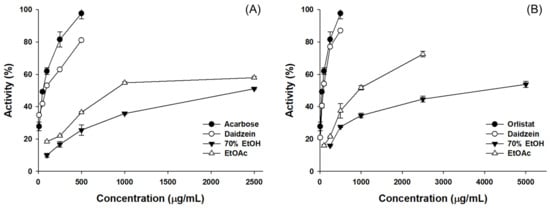
Figure 4.
Results of anti-obesity enzyme inhibition activity for 70% EtOH extracts and solvent fraction from P. erosus roots. (A) α-Glucosidase inhibition assay; (B) Lipase inhibition assay.

Table 3.
Results of anti-obesity enzyme inhibition activity for 70% EtOH extracts and EtOAc fraction from P. erosus roots.
3.6. Inhibition of Adipogenesis and Fatty Acid Synthesis in 3T3-L1 Cells
To determine the cytotoxicity of EtOAc fractions in 3T3-L1 cells, 10–100 μg/mL of EtOAc fractions were treated for 48 h and evaluated by MTT assay. Treatment of 3T3-L1 preadipocytes with EtOAc extract at concentrations ranging from 10 to 100 μg/mL for 48 h showed no significant difference in cytotoxicity or cell growth (Figure 5A). This study used concentrations of 100 μg/mL or lower for experimentation. To investigate the impact of EtOAc extract on the number and size of lipid droplets within 3T3-L1 cells, Oil Red O staining was performed on fully differentiated 3T3-L1 adipocytes. Undifferentiated 3T3-L1 preadipocytes did not form lipid droplets, while the positively induced control group, treated with MDI to induce differentiation, displayed a significant number of lipid droplets (Figure 5C). To evaluate the adipogenesis inhibitory activity of the EtOAc extract in 3T3-L1 cells, Oil Red O staining was used to confirm the adipogenesis inhibitory activity (Figure 5C). The positively induced control group, differentiated into adipocytes by MDI treatment, exhibited approximately a twofold higher absorbance than undifferentiated cells. Furthermore, the EtOAc extract-treated groups showed lipid synthesis inhibition activity of 7.95%, 13.75%, and 19.32% at concentrations of 25, 50, and 100 μg/mL, respectively, compared to the positive control group (Figure 5B).
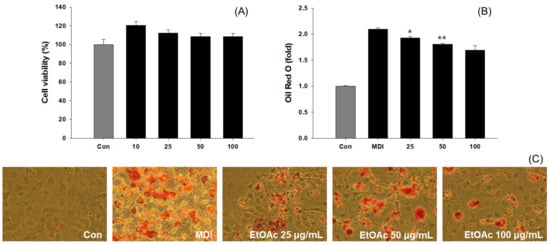
Figure 5.
Evaluation results of EtOAc fractions’ cytotoxic and lipogenesis inhibitory activities from P. erosus against 3T3-L1 cells. (A) Cytotoxicity measurement results; (B) Measurement results of Oil Red O staining; (C) 3T3-L1 Oil Red O staining. * p < 0.05, ** p < 0.01, compared with MDI treatment.
To determine the cytotoxicity of Daidzein, 3T3-L1 cells were treated with a concentration of 10–100 μg/mL for 48 h and subjected to MTT assay. The experimental results indicated no cytotoxicity at concentrations below 25 μg/mL (Figure 6A). Similar to the EtOAc fraction, Oil Red O staining was conducted to investigate the effects of daidzein on the number and size of lipid droplets within 3T3-L1 cells. The results of the experiment confirmed the adipogenesis inhibitory activity of daidzein in 3T3-L1 cells (Figure 5C). Furthermore, daidzein-treated groups exhibited lipid synthesis inhibition activity of 27.92%, 32.57%, and 37.28% at concentrations of 5, 10, and 25 μg/mL, respectively, when compared to the positive control group (Figure 6B).
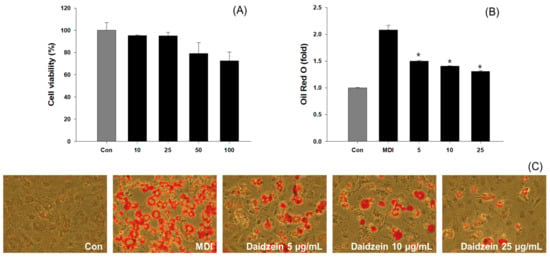
Figure 6.
Evaluation results of cytotoxic and lipogenesis inhibitory activities of daidzein against 3T3-L1 cells. (A) Cytotoxicity measurement results; (B) Measurement results of Oil Red O staining; (C) 3T3-L1 Oil Red O staining. * p < 0.05, compared with MDI treatment.
Obesity arises due to the differentiation of preadipocytes and the process of adipogenesis [38]. Adipocyte differentiation is regulated by the expression of transcription factors such as C/EBPα, PPARγ, and SREBP-1c. Particularly, SREBP-1c activates transcription factors involved in fat metabolism (fat transport, synthesis, and accumulation), such as FAS and ACC [39].
In this study, we analyzed the impact of P. erosus EtOAc fraction and daidzein on the protein expression of transcription factors related to fat synthesis and lipid generation, including PPARγ, SREBP, FAS, and ACC, in 3T3-L1 cells (Figure 7 and Figure 8). The results revealed a concentration-dependent inhibitory effect on fat synthesis and lipid generation protein expression in 3T3-L1 cells for the EtOAc fraction (Figure 7). Similarly, daidzein also exhibited a concentration-dependent inhibitory effect on the expression of fat synthesis and lipid generation proteins (Figure 8). Notably, daidzein showed a stronger inhibitory effect than the EtOAc fraction, with the highest inhibition activity observed at 25 μg/mL.
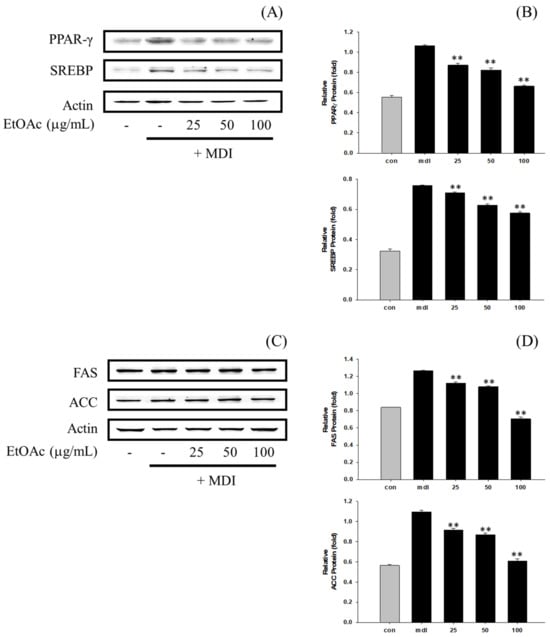
Figure 7.
(A) Impact of EtOAc fractions’ on PPARγ and SREBP protein expression in 3T3-L1 cells. The loading control was assessed using a β-actin antibody. (B) Quantitative analysis of PPARγ and SREBP by Western blotting. (C) Effect of daidzein on FAS and ACC protein expression in 3T3-L1 cells. The loading control was assessed using a β-actin antibody. (D) Quantitative analysis of FAS and ACC by Western blotting. ** p < 0.01, compared with MDI treatment.
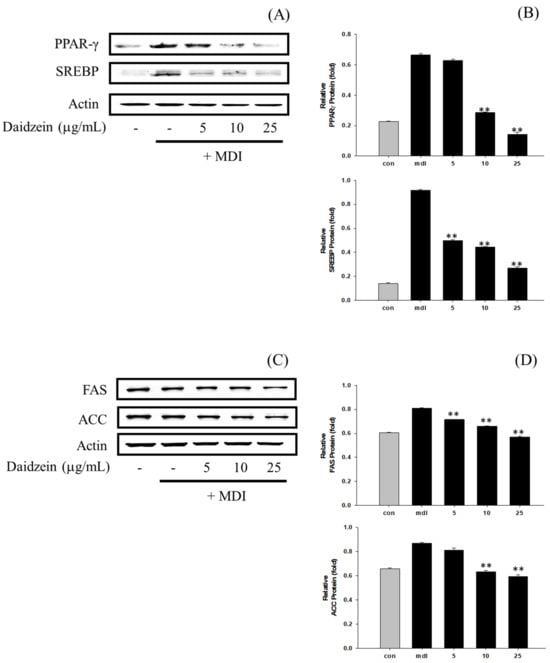
Figure 8.
(A) Impact of daidzein on PPARγ and SREBP protein expression in 3T3-L1 cells. The loading control was assessed using a β-actin antibody. (B) Quantitative analysis of PPARγ and SREBP by Western blotting. (C) Effect of daidzein on FAS and ACC protein expression in 3T3-L1 cells. The loading control was assessed using a β-actin antibody. (D) Quantitative analysis of FAS and ACC by Western blotting. ** p < 0.01, compared with MDI treatment.
4. Discussion
P. erosus, a perennial plant from the Fabaceae family, thrives in warm, humid tropical climates, with growth observed up to 1400 m above sea level [40]. This legume vine produces pods similar to beans; however, the seeds within these pods are toxic and not suitable for consumption [41]. Instead, the plant forms potato-like tubers on its roots, rich in water and various nutrients [42]. Notably, the nutritional composition of P. erosus can vary depending on factors such as harvest timing and environmental conditions, with the highest proportions found in moisture, reducing sugars, starch, crude fiber, and carbohydrates among its constituents [40].
P. erosus boasts a plethora of phytochemicals that contribute to its diverse biological activities, including anti-cancer, antifungal, and antiviral properties, among others [40]. P. erosus is not a source of insulin which is produced by beta cells in the pancreas, instead, P. erosus has been proven to reduce insulin resistance due to its antioxidant activity [40,43]. It contains a significant amount of dietary fiber, which has proven effective in alleviating constipation, preventing intestinal diseases, and reducing cholesterol levels. Studies have demonstrated positive outcomes in managing diet-induced metabolic disorders and inflammation using 10% P. erosus fiber [43].
This study focused on polyphenols and isoflavones, which had not been previously conducted, among various P. erosus components. HPLC and LC-MS/MS were employed to identify indicator components and quantify polyphenols and isoflavones. 4-Hydroxybenzoic acid was found to have the highest content among the polyphenols present, while daidzein exhibited the highest isoflavone content. Previous studies have indicated that genistein, daidzein, and other related phytoestrogens exhibit weak binding to receptors and induce transcription at significantly lower levels than aglycones [44].
The main characteristic of obesity is an increase in the number and size of adipocytes, and the differentiation process from preadipocytes to mature adipocytes is associated with fat production [45]. 3T3-L1 cells are preadipocytes widely used in research on adipogenesis and accumulate neutral fat by transcription factors and hormones related to adipocytes [46]. When 3T3-L1 cells are cultured in an MDI medium containing the adipocyte-inducing complex insulin, dexamethasone, and IBMX, differentiation into adipocytes is induced, accompanied by the expression of related genes [47]. During the onset of adipocyte differentiation, the expression of proteins belonging to the SREBP-1c and C/EBP families increases (Figure 9). Through the ER-mediated pathway, soy isoflavones demonstrate anti-adipogenic effects by decreasing SREBP-1 expression [48]. C/EBPβ and δ, members of the C/EBP family, are activated by dexamethasone and IBMX, and then C/EBPα and PPARγ, which are significant regulators of adipogenesis, increase adipogenesis, induce differentiation and maturation [49] and induce the conversion into adipocytes.
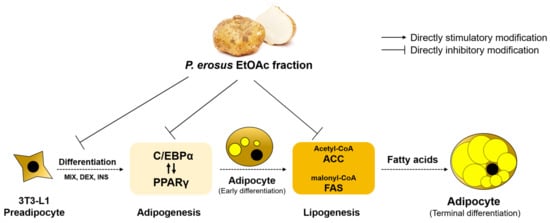
Figure 9.
Anti-obesity mechanism of P. erosus in MDI-treated 3T3-L1 cells through Leptin-PPAR-FAS signaling pathway.
PPARγ is one of the nuclear hormone receptors that increase insulin sensitivity and fat production and induce fatty acid uptake and triglyceride accumulation in mature adipocytes [50]. C/EBPα is expressed early in differentiation along with PPARγ, and target genes of PPARγ include adipocyte fatty acid binding protein 2 (aP2) and lipoprotein lipase and also regulate the expression of FAS and leptin [51,52]. Leptin and aP2 regulate the expression of heavy tissue-specific genes in the final stage of differentiation, and after differentiation, enzymes such as FAS, ACC, and hormone-sensitive lipase are activated. It synthesizes and regulates lipid metabolism [53,54]. Therefore, inhibition of preadipocyte differentiation will suppress the incidence of obesity by reducing the mass of adipose tissue and will help prevent and treat diseases related to obesity [55].
Prior research explored the anti-diabetic properties of P. erosus and observed anti-obesity effects in mice [56]. Previous studies have demonstrated the effectiveness of P. erosus fiber in preventing excessive blood sugar and weight gain when fed to rats fed a high-sugar diet [56]. Additionally, recent investigations have highlighted the efficacy of P. erosus tuber fiber in ameliorating energy metabolism irregularities and obesity in adult male BALB/c mice subjected to a high-fat diet [56]. These findings provide compelling evidence supporting the potential of P. erosus tuber fiber as a dietary supplement to mitigate disruptions in energy homeostasis and address the issue of obesity [57]. In previous studies, daidzein was observed to inhibit adipogenesis through the stimulation of lipolysis [58]. Inhibition by acarbose against the α-glucosidase from S. cerevisiae is weaker than the enzyme from mammalian intestinal [59]. We did not perform the effect of daidzein on mammalian a-glucosidase. To validate the effectiveness of daidzein compared to acarbose, kinetic and docking studies will be done in the near future. This study confirmed the anti-obesity activity of P. erosus EtOAc fraction and daidzein, which had not been previously verified, through the Leptin-PPAR-FAS Pathway. These results suggest the potential of polyphenols in P. erosus as anti-obesity and diet-healthy functional food ingredients.
5. Conclusions
This study investigated the anti-obesity activity of P. erosus extract and the isoflavone it contains. Antioxidant experiments revealed that the EtOAc fraction exhibited the highest antioxidant activity in both DPPH and ABTS radical scavenging assays. HPLC and LC-MS/MS experiments identified daidzein as a representative isoflavone component in P. erosus. The analysis of protein expression related to adipogenesis and lipogenesis in 3T3-L1 cells, including transcription factors PPARγ, SREBP, FAS, and ACC, revealed concentration-dependent reductions for both the P. erosus-derived EtOAc fraction and daidzein. Notably, daidzein exhibited higher inhibitory effects than the EtOAc fraction, with the highest inhibition observed at 25 μg/mL. These results indicate that P. erosus extract has excellent carbohydrate-digestive enzyme inhibitory effects, suggesting its potential use as a functional food ingredient for diabetes and obesity prevention.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cosmetics10060164/s1, Figure S1. LC-MS/MS profile of the P. erosus 70% EtOH extract. Figure S2. LC-MS/MS profile of the EtOAc fraction from P. erosus 70% EtOH extract.
Author Contributions
Conceptualization, M.-H.C. and H.-J.S.; methodology, S.-H.Y. and Y.-J.L.; software, S.-H.Y. and Y.-J.L.; validation, M.-H.C. and H.-J.S.; formal analysis, M.-H.C. and H.-J.S.; investigation, Y.-J.L. and J.H.S.; resources, S.-H.Y.; data curation, M.-H.C. and J.H.S.; writing—original draft preparation, M.-H.C., S.-H.Y. and Y.-J.L.; writing—review and editing, H.-J.S.; visualization, S.-H.Y.; supervision, K.S.L. and H.-J.S.; project administration, K.S.L. and H.-J.S.; funding acquisition, K.S.L. and H.-J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This paper has been written with the support of Jeollannam-do (‘2022 R&D supporting program’ operated by Jeonnam Technopark).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Fruh, S.M. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J. Am. Assoc. Nurse. Pract. 2017, 29, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Ramos-de-la-Peña, A.M.; Renard, C.M.; Wicker, L.; Montañez, J.; de la Luz Reyes-Vega, M.; Voget, C.; Contreras-Esquivel, J.C. Enzymatic liquefaction of Jicama (Pachyrhizus erosus) tuberous roots and characterization of the cell walls after processing. LWT-Food Sci. Technol. 2012, 49, 257–262. [Google Scholar] [CrossRef]
- Baranowska-Bik, A.; Bik, W. The association of obesity with autoimmune thyroiditis and thyroid function-possible mechanisms of Bilateral Interaction. Int. J. Endocrinol. 2020, 2020, 8894792. [Google Scholar] [CrossRef] [PubMed]
- Azorín-Ortuño, M.; Urbán, C.; Cerón, J.J.; Tecles, F.; Allende, A.; Tomás-Barberán, F.A.; Espín, J.C. Effect of low inulin doses with different polymerisation degree on lipid metabolism, mineral absorption, and intestinal microbiota in rats with fat-supplemented diet. Food Chem. 2009, 113, 1058–1065. [Google Scholar] [CrossRef]
- Nam, Y.R.; Won, S.B.; Chung, Y.S.; Kwak, C.S.; Kwon, Y.H. Inhibitory effects of Doenjang, Korean traditional fermented soybean paste, on oxidative stress and inflammation in adipose tissue of mice fed a high-fat diet. Nutr. Res. Pract. 2015, 9, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, G.F.; Santos, I.B.; de Bem, G.F.; Cordeiro, V.S.C.; da Costa, C.A.; de Carvalho, L.C.R.M.; Ognibene, D.T.; Resende, A.C.; de Moura, R.S. The beneficial effect of anthocyanidin-rich Vitis vinifera L. grape skin extract on metabolic changes induced by high-fat diet in mice involves antiinflammatory and antioxidant actions. Phytother. Res. 2017, 31, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Charradi, K.; Elkahoui, S.; Limam, F.; Aouani, E. High-fat diet induced an oxidative stress in white adipose tissue and disturbed plasma transition metals in rat: Prevention by grape seed and skin extract. J. Physiol. Sci. 2013, 63, 445–455. [Google Scholar] [CrossRef]
- Xu, S.P.; Mao, X.Y.; Ren, F.Z.; Che, H.L. Attenuating effect of casein glycomacropeptide on proliferation, differentiation, and lipid accumulation of in vitro Sprague-Dawley rat preadipocytes. J. Dairy Sci. 2011, 94, 676–683. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Cires, M.J.; Gotteland, M. Quercetin and epigallocatechin gallate in the prevention and treatment of obesity: From molecular to clinical studies. J. Med. Food 2019, 22, 753–770. [Google Scholar] [CrossRef]
- Lin, X.; Li, H. Obesity: Epidemiology, pathophysiology, and therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef]
- Wen, X.; Zhang, B.; Wu, B.; Xiao, H.; Li, Z.; Li, R.; Xu, X.; Li, T. Signaling pathways in obesity: Mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 298. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.H.; Seo, M.J.; Choi, H.S.; Lee, B.Y. Pycnogenol® inhibits lipid accumulation in 3T3-L1 adipocytes with the modulation of reactive oxygen species (ROS) production associated with antioxidant enzyme responses. Phytother. Res. 2012, 26, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Olejnik, A.; Szwajgier, D.; Olkowicz, M. Inhibitory activity of chokeberry, bilberry, raspberry and cranberry polyphenol-rich extract towards adipogenesis and oxidative stress in differentiated 3T3-L1 adipose cells. PLoS ONE 2017, 12, e0188583. [Google Scholar] [CrossRef] [PubMed]
- Hinds, T.D.; Burns, K.A.; Hosick, P.A.; McBeth, L.; Nestor-Kalinoski, A.; Drummond, H.A.; AlAmodi, A.A.; Hankins, M.W.; Vanden Heuvel, J.P.; Stec, D.E. Biliverdin reductase A attenuates hepatic steatosis by inhibition of glycogen synthase kinase (GSK) 3β phosphorylation of serine 73 of peroxisome proliferator-activated receptor (PPAR) α. J. Biol. Chem. 2016, 291, 25179–25191. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.; Weickert, M.O. The health benefits of dietary fiber. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- Sabarathinam, S. A glycemic diet improves the understanding of glycemic control in diabetes patients during their follow-up. Future Sci. OA 2023, 9, FSO843. [Google Scholar] [CrossRef]
- Suryana, A.L.; Kristanto, A.A.; Putri, F. Effect consumption of Papaya and Jicama juice to total cholesterol levels in hypercholesterolemia patients. Int. J. Pharma. Bio-Med. Sci. 2023, 3, 152–155. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N.; et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001, 7, 941–946. [Google Scholar] [CrossRef]
- Mungmai, L.; Kanokwattananon, C.; Thakang, S.; Nakkrathok, A.; Srisuksomwong, P.; Tanamatayarat, P. Physicochemical properties, antioxidant and anti-tyrosinase activities of Durio zibethinus Murray and value added for cosmetic product formulation. Cosmetics 2023, 10, 87. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Ahn, G.Y.; Bae, S.H. Strategies for the safe use of nonsteroidal anti-inflammatory drugs. J. Korean Med. Assoc. 2018, 61, 367–375. [Google Scholar] [CrossRef]
- Sato, K.; Hiraga, Y.; Yamaguchi, Y.; Sakaki, S.; Takenaka, H. Anti-melanogenic and anti-oxidative effects of Nostoc verrucosum (ashitsuki) extracts. Cosmetics 2023, 10, 30. [Google Scholar] [CrossRef]
- Kurzawa, M.; Wilczyńska, E.; Brudzyńska, P.; Sionkowska, A. Total phenolic content, antioxidant capacity and UV Radiation Protection Properties of Marigold (Calendula officinalis), carrot (Daucus carota), tomato (Solanum lycopersicum) and hop (Humulus lupulus) Extracts. Cosmetics 2022, 9, 134. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Rungsang, T.; Srivilai, J.; Rakasawapokin, P.; Rakasawapokin, P.; Mungmai, L.; Saesue, K.; Aoonboontum, P.; Plukham, N.; Siriwipanan, P.; Chaichanathawikit, P.; et al. Assessment of antioxidant, anti-lipid peroxidation, antiglycation, anti-inflammatory and anti-tyrosinase properties of Dendrobium sulcatum Lindl. Cosmetics 2023, 10, 43. [Google Scholar] [CrossRef]
- Phosri, S.; Kiattisin, K.; Intharuksa, A.; Janon, R.; Na Nongkhai, T.; Theansungnoen, T. Anti-aging, anti-acne, and cytotoxic activities of Houttuynia cordata extracts and phytochemicals analysis by LC-MS/MS. Cosmetics 2022, 9, 136. [Google Scholar] [CrossRef]
- Kim, J.M.; Jung, I.A.; Kim, J.M.; Choi, M.H.; Yang, J.H. Anti-Inflammatory Effect of Cinnamomum japonicum Siebold’s Leaf through the Inhibition of p38/JNK/AP-1 Signaling. Pharmaceuticals 2023, 16, 1402. [Google Scholar] [CrossRef]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Eom, S.H.; Lee, S.H.; Yoon, N.Y.; Jung, W.K.; Jeon, Y.J.; Kim, S.K.; Lee, M.S.; Kim, Y.M. α-Glucosidase- and α-amylase-inhibitory activities of phlorotannins from Eisenia bicyclis. J. Sci. Food Agric. 2012, 92, 2084–2090. [Google Scholar] [CrossRef]
- Gupta, R.; Rathi, P.; Gupta, N.; Bradoo, S. Lipase assays for conventional and molecular screening: An overview. Biotechnol. Appl. Biochem. 2003, 37, 63–71. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.J.; Kim, C.; Jung, H.; Kim, Y.O.; Ju, J.Y.; Shin, C.S. Development of lipase inhibitors from various derivatives of monascus pigment produced by Monascus fermentation. Food Chem. 2007, 101, 357–364. [Google Scholar] [CrossRef]
- Tafuri, S.R. Troglitazone enhances differentiation, basal glucose uptake, and Glut1 protein levels in 3T3-L1 adipocytes. Endocrinology 1996, 137, 4706–4712. [Google Scholar] [CrossRef] [PubMed]
- Gerlier, D.; Thomasset, N. Use of MTT colorimetric assay to measure cell activation. J. Immunol. Methods 1986, 94, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Zacarias, J.L.; Castro-Munozledo, F.; Kuri-Harcuch, W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry 1992, 97, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Yang, P.C. Western blot: Technique, theory, and trouble shooting. N. Am. J. Med. Sci. 2012, 4, 429. [Google Scholar]
- Lukitaningsih, E.; Bahi, M.; Holzgrabe, U. Tyrosinase inhibition type of isolated compounds obtained from Pachyrhizus erosus. Aceh Int. J. Sci. Technol. 2013, 2, 98–102. [Google Scholar] [CrossRef][Green Version]
- Estrella-Parra, E.A.; Gomez-Verjan, J.C.; González-Sánchez, I.; Vázquez-Martínez, E.R.; Vergara-Castañeda, E.; Cerbón, M.A.; Alavez-Solano, D.; Reyes-Chilpa, R. Rotenone isolated from Pachyrhizus erosus displays cytotoxicity and genotoxicity in K562 cells. Nat. Prod. Res. 2014, 28, 1780–1785. [Google Scholar] [CrossRef]
- Sethi, J.K.; Vidal-Puig, A.J. Thematic review series: Adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J. Lipid Res. 2007, 48, 1253–1262. [Google Scholar] [CrossRef]
- Ràfols, M.E. Adipose tissue: Cell heterogeneity and functional diversity. Endocrinol. Nutr. 2014, 61, 100–112. [Google Scholar] [CrossRef]
- Jaiswal, V.; Chauhan, S.; Lee, H.J. The bioactivity and phytochemicals of Pachyrhizus erosus (L.) Urb.: A multifunctional underutilized crop plant. Antioxidants 2021, 11, 58. [Google Scholar] [CrossRef]
- Fu, P.K.; Wang, P.Y. Toxic leukoencephalopathy due to yam bean seeds poisoning. Neurologist 2012, 18, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Levaj, B.; Pelaić, Z.; Galić, K.; Kurek, M.; Ščetar, M.; Poljak, M.; Hunjek, D.D.; Pedisić, S.; Balbino, S.; Čošić, Z.; et al. Maintaining the quality and safety of fresh-cut potatoes (Solanum tuberosum): Overview of recent findings and approaches. Agronomy 2023, 13, 2002. [Google Scholar] [CrossRef]
- Santoso, P.; Maliza, R.; Insani, S.J.; Fadhila, Q.; Rahayu, R. Preventive effect of Jicama (Pachyrhizus erosus) fiber against diabetes development in mice fed with high-fat diet. J. Appl. Pharm. Sci. 2021, 11, 137–143. [Google Scholar]
- Wang, S.; Wang, Y.; Pan, M.H.; Ho, C.T. Anti-obesity molecular mechanism of soy isoflavones: Weaving the way to new therapeutic routes. Food Funct. 2017, 8, 3831–3846. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Shou, Q.; Lu, Y.; Wang, G.; Qiu, J.; Wang, J.; He, L.; Chen, J.; Jiao, J.; Zhang, Y. Arachidonic acid sex-dependently affects obesity through linking gut microbiota-driven inflammation to hypothalamus-adipose-liver axis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2715–2726. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rong, Y.; Bao, L.; Nie, B.; Ren, G.; Zheng, C.; Amin, R.; Arnold, R.D.; Jeganathan, R.B.; Huggins, K.W. Suppression of adipocyte differentiation and lipid accumulation by stearidonic acid (SDA) in 3T3-L1 cells. Lipids Health Dis. 2017, 16, 181. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Cha, J.Y.; Seok, J.W.; Choi, Y.; Yoon, B.K.; Choi, H.; Yu, J.H.; Song, S.J.; Kim, A.; Lee, H.; et al. Dexras1 links glucocorticoids to insulin-like growth factor-1 signaling in adipogenesis. Sci. Rep. 2016, 6, 28648. [Google Scholar] [CrossRef]
- Han, S.I.; Komatsu, Y.; Murayama, A.; Steffensen, K.R.; Nakagawa, Y.; Nakajima, Y.; Suzuki, M.; Oie, S.; Parini, P.; Vedin, L.L.; et al. Estrogen receptor ligands ameliorate fatty liver through a nonclassical estrogen receptor/Liver X receptor pathway in mice. Hepatology 2014, 59, 1791–1802. [Google Scholar] [CrossRef]
- Wu, C.; Fang, S.; Zhang, H.; Li, X.; Du, Y.; Zhang, Y.; Lin, X.; Wang, L.; Ma, X.; Xue, Y.; et al. Long noncoding RNA XIST regulates brown preadipocytes differentiation and combats high-fat diet induced obesity by targeting C/EBPα. Mol. Med. 2022, 28, 6. [Google Scholar] [CrossRef]
- He, Y.F.; Liu, F.Y.; Zhang, W.X. Tangeritin inhibits adipogenesis by down-regulating C/EBPα, C/EBPβ, and PPARγ expression in 3T3-L1 fat cells. Genet. Mol. Res. 2015, 14, 13642–13648. [Google Scholar] [CrossRef]
- Han, L.; Shen, W.J.; Bittner, S.; Kraemer, F.B.; Azhar, S. PPARs: Regulators of metabolism and as therapeutic targets in cardiovascular disease. Part II: PPAR-β/δ and PPAR-γ. Future Cardiol. 2017, 13, 279–296. [Google Scholar] [CrossRef] [PubMed]
- Rayner, M.L.D.; Grillo, A.; Williams, G.R.; Tawfik, E.; Zhang, T.; Volitaki, C.; Craig, D.Q.M.; Healy, J.; Phillips, J.B. Controlled local release of PPARγ agonists from biomaterials to treat peripheral nerve injury. J. Neural Eng. 2020, 17, 046030. [Google Scholar] [CrossRef] [PubMed]
- Baldini, F.; Fabbri, R.; Eberhagen, C.; Voci, A.; Portincasa, P.; Zischka, H.; Vergani, L. Adipocyte hypertrophy parallels alterations of mitochondrial status in a cell model for adipose tissue dysfunction in obesity. Life Sci. 2021, 265, 118812. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Bok, S.H.; Kim, M.H.; Lee, K.S.; Park, D.H. Pachyrhizus erosus inhibits adipogenesis via the Leptin-PPARγ-FAS Pathway in a high-fat diet-induced mouse model. Processes 2023, 11, 735. [Google Scholar] [CrossRef]
- Meriga, B.; Parim, B.; Chunduri, V.R.; Naik, R.R.; Nemani, H.; Suresh, P.; Ganapathy, S.; Uddandrao, V.V. Antiobesity potential of Piperonal: Promising modulation of body composition, lipid profiles and obesogenic marker expression in HFD-induced obese rats. Nutr. Metab. 2017, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Santoso, P.; Amelia, A.; Rahayu, R. Jicama (Pachyrhizus erosus) fiber prevents excessive blood glucose and body weight increase without affecting food intake in mice fed with high-sugar diet. J. Adv. Vet. Anim. Res. 2019, 6, 222. [Google Scholar] [CrossRef]
- Santoso, P.; Maliza, R.; Insani, S.J.; Fadhilah, Q. Effect of Jicama (Pachyrhizus erosus) fiber on energy intake and adipose tissue profiles in mice fed with high-fat diet. J. Phys. Conf. Ser. 2021, 1940, 012055. [Google Scholar] [CrossRef]
- Kim, M.H.; Park, J.S.; Jung, J.W.; Byun, K.W.; Kang, K.S.; Lee, Y.S. Daidzein supplementation prevents non-alcoholic fatty liver disease through alternation of hepatic gene expression profiles and adipocyte metabolism. Int. J. Obes. 2011, 35, 1019–1030. [Google Scholar] [CrossRef]
- Martin, A.E.; Montgomery, P.A. Acarbose: An a-glucosidase inhibitor. Am. J. Health Syst. Pharm. 1996, 53, 2277–2290. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).