Abstract
Skin squamous cell carcinoma (SCC) represents a major public health concern due to its high incidence and potential for local invasion and metastasis. Compared to local recurrence, metastatic SCC represents an even greater therapeutic challenge. Once distant metastasis occurs, the disease becomes incurable, and treatment focuses on palliation and prolonging survival. The immune microenvironment of SCC is characterized by an infiltration of immune cells, including tumor-infiltrating lymphocytes. In addition to its direct cytotoxic effects, radiotherapy also induces immunomodulatory effects within the tumor microenvironment. Radiation can promote the release of tumor-associated antigens and induce immunogenic cell death, thereby enhancing the recognition of tumor cells by the immune system. Immunotherapy and radiotherapy have emerged as promising therapeutic modalities for metastatic SCC. This literature review aims to evaluate the potential synergy between these treatments and shed light on their combined efficacy. Within the manuscript, we present a compelling case report of a patient with advanced SCC who exhibited resistance to the combined regimen of immunotherapy and radiotherapy, leading to disease progression. Despite the increasing evidence supporting the synergy between these modalities, this case underscores the complex nature of treatment response and the importance of considering individual patient characteristics.
1. Introduction
Skin squamous cell carcinoma (SCC) is a common malignancy originating from the keratinocytes of the epidermis. It accounts for a significant proportion of non-melanoma skin cancers and is responsible for substantial morbidity and mortality worldwide. The incidence of SCC has been increasing over the past few decades, primarily attributed to factors such as increased sun exposure, aging populations, and immunosuppression. SCC commonly affects sun-exposed areas of the skin, such as the head, neck, and extremities, but it can also develop on non-sun-exposed regions [1].
1.1. Skin Squamous Cell Carcinoma: Epidemiology and Clinical Significance
Skin SCC represents a major public health concern due to its high incidence and potential for local invasion and metastasis. It is estimated that about one million instances of SCC are diagnosed each year in the United States [2]. Although surgical excision can effectively treat the majority of SCC cases, roughly 5–10% of patients will develop locally progressed or metastatic illness [3].
Risk factors for SCC development include prolonged and cumulative sun exposure, fair skin, advanced age, male gender, immunosuppression, chronic skin inflammation, genetic susceptibility, and exposure to certain carcinogens such as arsenic and ionizing radiation. Individuals with a history of actinic keratosis, a precursor lesion of SCC, are also at an increased risk [4,5].
The clinical significance of SCC lies in its potential for aggressive behavior. Locally progressed SCC has the potential to infect neighboring structures, resulting in deformity and functional impairment. Furthermore, metastasis to the regional lymph nodes or distant organs can occur, resulting in a poor prognosis. SCC has a higher propensity for lymphatic spread compared to basal cell carcinoma, another common type of skin cancer [6]. Moreover, patients with SCC have an increased risk of developing secondary primary tumors, emphasizing the need for vigilant long-term surveillance [7].
1.2. Challenges in the Management of Advanced or Metastatic SCC
The management of advanced or metastatic SCC presents challenges due to its aggressive nature and limited treatment options. Surgical excision alone may not be curative, necessitating adjuvant therapies to improve outcomes [8].
Locoregional recurrence is a significant concern, requiring extensive surgery with potential functional and cosmetic consequences. Lymph node involvement is linked to poorer survival outcomes and a higher risk of distant metastasis [8].
Metastatic SCC is particularly challenging, with common sites of distant metastasis including the regional lymph nodes, lungs, bones, liver, and brain. Once metastasis occurs, treatment focuses on palliation and survival prolongation [4].
Conventional treatments like radiation therapy, chemotherapy, and targeted therapy offer limited efficacy, with durable responses being uncommon [9]. Chemotherapy, including cisplatin-based regimens, has modest response rates but significant toxicities. Targeted therapies, like epidermal growth factor receptor (EGFR) inhibitors, have limited effectiveness in SCC, and resistance often develops over time [9].
Immunoradiotherapy, a promising treatment approach for SCC, combines immunotherapy and radiotherapy to maximize therapeutic benefits [10,11,12,13]. Based on the current literature, this review briefly discusses the mechanisms of cancer immunotherapy and RT, as well as the research progress of several immunotherapies combined with RT. It attempts to explore the challenges of synergistic treatment strategies for the two tumor treatment methods.
2. Role of the Immune System in the Antitumor Activity
2.1. Tumor Immune Microenvironment in SCC
Skin squamous cell carcinoma (SCC), a common non-melanoma skin cancer, originates from epidermal squamous cells and is greatly impacted by its immunological microenvironment, which includes complex interactions between tumor cells, immune cells, and stromal elements. A thorough understanding of these dynamics is essential to developing immunotherapeutic approaches that work [14]. Central to this microenvironment are tumor-infiltrating lymphocytes (TILs), including cytotoxic CD8+ T-cells, helper CD4+ T-cells, and regulatory T-cells [15]. High infiltration levels of TILs, particularly CD8+ T-cells, correlate with improved SCC outcomes, including reduced recurrence risk and enhanced overall survival, primarily due to their cytotoxic activity and pro-inflammatory cytokine release [16,17]. Conversely, the presence of Tregs within SCC tumors is linked to adverse outcomes, with their immunosuppressive role inhibiting effector T-cell function, fostering tumor aggressiveness and metastasis, and diminishing survival rates through cytokine secretion and direct inhibition of effector T-cells [18,19].
Moreover, within the SCC tumor microenvironment, several immunosuppressive factors impact cancer progression. Programmed cell death-ligand 1 (PD-L1), upregulated in response to inflammatory cues, engages programmed cell death protein 1 (PD-1) on activated T-cells, inducing T-cell exhaustion and immune response inhibition. Elevated PD-L1 expression in SCC is linked to poor prognosis, enabling tumor immune evasion [20]. Additional immunosuppressive factors, including indoleamine 2,3-dioxygenase (IDO) and prostaglandin E2 (PGE2), contribute to immune evasion and SCC progression. IDO catalyzes tryptophan conversion into kynurenine, depleting tryptophan and accumulating immunosuppressive metabolites, while PGE2 hampers immune cell function, promotes Treg expansion, and enhances immunosuppressive factor expression [21,22]. Moreover, myeloid-derived suppressor cells (MDSCs) are becoming more prevalent in SCC and are linked to worse prognoses because they block T-cell activation and promote tumor growth. When combined, these immunosuppressive elements in the tumor microenvironment provide a setting that inhibits the growth and progression of SCC [23].
2.2. Immunotherapeutic Approaches in SCC
Immunotherapy has transformed cancer treatment by targeting inhibitory pathways that suppress T-cell responses. Key targets in SCC include the PD-1/PD-L1 pathway and the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) pathway. PD-1, expressed on activated T-cells, interacts with PD-L1 on tumor or immune cells, leading to T-cell exhaustion and inhibition [24]. PD-1/PD-L1 inhibitors restore T-cell function and enhance antitumor immunity, showing promise in SCC clinical trials with improved response rates and survival outcomes [25]. CTLA-4, another immune checkpoint, competes with CD28 to bind to co-stimulatory molecules, suppressing T cell activation [26]. CTLA-4 inhibitors, like ipilimumab, have been effective in various cancers and are under investigation in SCC [27]. Additionally, adoptive cell therapy (ACT) is a promising approach, featuring CAR-T cells for recognizing tumor antigens and TIL therapy for isolating and expanding tumor-specific TILs [28,29]. Cytokines like interleukin-2 [IL-2] promote T-cell activation, with high-dose IL-2 therapy showing success in metastatic SCC [30]. Furthermore, immunomodulatory agents like interferons and immune checkpoint agonists stimulate antitumor immunity and hold potential in SCC immunotherapy [31,32].
2.3. Immunoradiotherapy Mechanisms
In the treatment of SCC, the combination of radiation and immunotherapy shows promise. Radiotherapy, a standard SCC treatment, can induce immunogenic cell death (ICD), triggering the release of danger-associated molecular patterns (DAMPs) and tumor antigen presentation. DAMPs like calreticulin and HMGB1 stimulate dendritic cells to capture and present tumor antigens to T-cells, enhancing immune cell activation and fostering an antitumor response [33,34]. Furthermore, radiotherapy can evoke the abscopal effect, a systemic immune response where localized tumor cell death leads to distant tumor regression. Immune cell activation and pro-inflammatory cytokine release mediate this effect, offering systemic antitumor immunity potential [14,35]. Radiotherapy also modulates the tumor microenvironment by increasing TIL infiltration, elevating tumor antigen expression, and reducing immunosuppressive factors, making it conducive for immunotherapy [36]. Additionally, radiotherapy upregulates immune checkpoint molecules like PD-L1, providing a basis for combining radiotherapy with checkpoint inhibitors, a synergy demonstrated in preclinical models and clinical studies, potentially improving SCC treatment outcomes [37,38].
3. Preclinical Evidence on Immunoradiotherapy
In preclinical research, several immunotherapy and radiation combination techniques were investigated in SCC models. For instance, Paulson et al. [39] demonstrated that combining immune checkpoint inhibitors, such as anti-PD-1 antibodies, with local radiotherapy resulted in enhanced antitumor immune responses and improved tumor control in a murine SCC model. Similarly, the authors of another trial [40] showed that the combination of radiotherapy and adoptive cell therapy using TILs led to synergistic effects, resulting in enhanced tumor regression and prolonged survival in SCC-bearing mice. These findings highlight the potential of combining immunotherapy and radiotherapy for the treatment of SCC.
The timing and sequencing of immunotherapy and radiotherapy have also been investigated in preclinical studies. The results of a recent trial [41] demonstrated that administering radiotherapy prior to the adoptive transfer of TILs resulted in improved tumor control and prolonged survival in SCC models, suggesting a potential benefit of priming the tumor microenvironment with radiation before immunotherapy. Conversely, Demaria et al. [35] showed that simultaneous treatment with anti-CTLA-4 antibodies and radiotherapy resulted in enhanced antitumor immune responses and improved tumor control in a SCC model. These studies highlight the importance of optimizing treatment schedules and sequencing to maximize therapeutic efficacy.
Preclinical research has yielded important mechanistic insights into the immunological responses caused by immunoradiotherapy in SCC models [33]. One key mechanism underlying the efficacy of this treatment approach is the induction of immunogenic cell death (ICD) by radiotherapy. ICD is characterized by the release of danger-associated molecular patterns (DAMPs) from dying tumor cells, which can stimulate the activation of innate and adaptive immune responses [42]. Several DAMPs, including calreticulin and high mobility group box 1 (HMGB1), have been shown to play a crucial role in the immunogenicity of irradiated SCC cells [33].
Furthermore, immunoradiotherapy has been found to modulate the tumor immune microenvironment in SCC models [33,43]. Radiotherapy has been shown to enhance the infiltration of tumor-infiltrating lymphocytes (TILs) into the tumor bed [44]. TILs play a critical role in mediating antitumor immune responses, and their presence has been associated with improved prognoses in SCC patients [45]. Additionally, radiotherapy can modulate the expression of immune checkpoint molecules, such as PD-1 and PD-L1, in the tumor microenvironment [43]. These findings suggest that immunoradiotherapy can promote a more favorable immune microenvironment by enhancing TIL infiltration and overcoming immune checkpoint-mediated immunosuppression.
Overall, preclinical studies investigating immunoradiotherapy in SCC models have provided valuable insights into its efficacy and underlying mechanisms. Combination strategies, treatment schedules, and mechanistic insights have been elucidated, highlighting the potential of immunoradiotherapy as an innovative approach for the treatment of skin squamous cell carcinoma [46,47].
4. Clinical Trials and Outcomes of Immunoradiotherapy in SCC
4.1. Locally Advanced Disease
Neoadjuvant immunoradiotherapy is the administration of immunotherapy and radiotherapy prior to surgical tumor excision. This approach aims to downsize the tumor, enhance local control, and stimulate systemic antitumor immune responses. Several clinical trials have investigated neoadjuvant immunoradiotherapy in locally advanced SCC [43]. For instance, a phase II clinical trial by Zhang et al. [48] explored the combination of neo-adjuvant radiotherapy with immune checkpoint inhibitors in patients with locally advanced SCC. The study reported a significant reduction in tumor size and an increase in immune infiltrates, indicating a favorable immunological response. Similar neoadjuvant approaches using adoptive cell therapy or cytokine-based immunotherapy have also shown promising results in early-phase clinical trials [43,49].
Adjuvant immunoradiotherapy, on the other hand, involves the administration of immunotherapy and radiotherapy after surgical resection to eliminate residual disease and prevent recurrence. Several clinical trials have investigated the efficacy of adjuvant immuno-radiotherapy in SCC patients [43]. Recent randomized trials, such as the one from Burns et al. [50] which resulted in improved disease-free survival and overall survival in the radiotherapy and immunotherapy groups compared to the sequential treatment groups, suggest the potential of immunoradiotherapy in improving treatment outcomes. Adjuvant approaches using TIL therapy or cytokine-based immunotherapy have also shown promising results in clinical trials [51,52].
Clinical trials evaluating immunoradiotherapy in locally advanced SCC have reported improved clinical outcomes and survival data in locally advanced tumors [53,54,55]. A previous trial [53] demonstrated that the combination of immune checkpoint inhibitors and radiotherapy led to a significant increase in overall survival and progression-free survival in patients with locally advanced SCC. Other clinical trials have also reported improved clinical responses and survival outcomes in patients receiving immunoradiotherapy [54,55,56]. Furthermore, long-term follow-up studies have shown sustained responses and durable remissions in a subset of patients treated with immunoradiotherapy, indicating the potential for long-term disease control and improved quality of life in SCC patients [57,58].
4.2. Metastatic Disease
Several case reports and early clinical trials [27,59,60] have described the use of immunoradiotherapy in metastatic SCC. A recently published manuscript [59] suggested that patients with metastatic SCC who received a combination of immune checkpoint inhibitors and radiotherapy resulted in significant tumor regression and prolonged survival. Similarly, early-phase clinical trials have reported objective responses and disease control in patients with metastatic SCC receiving immunoradiotherapy [27,60]. These reports highlight the potential of immunoradiotherapy to induce tumor regression and achieve durable responses in metastatic disease. Despite the promising results [27,59,60], challenges exist in the successful implementation of immunoradiotherapy in metastatic SCC.
Clinical Scenario
We present a case that highlights that in cases with resistance to immunotherapy and radiotherapy, further treatment efficacy is negatively influenced and disease progression is extremely likely to occur.
A 40-year-old woman with no significant past medical history presented in another healthcare facility in June 2022 with a vegetative superficial sacrococcygeal soft tissue tumor (Figure 1A). The tumor was spontaneously bleeding and had a diameter of 10 cm. From the patient’s anamnesis, the tumor was clinically visible starting 6 months prior to admission, revealing a significant increase in size in the last month.
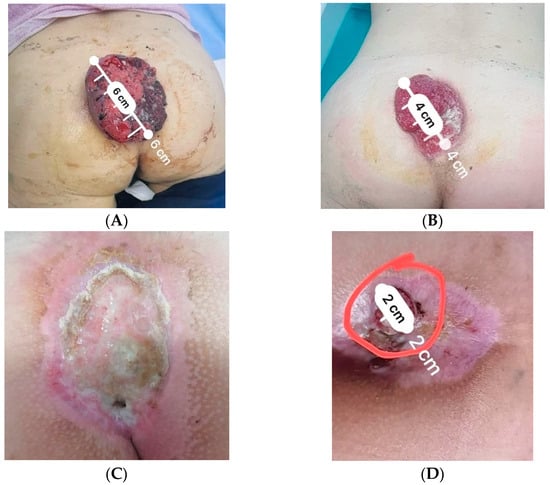
Figure 1.
Sacrococcygeal skin tumoral mass in different stages during medical care: (A) at initial presentation; (B) relapse following local excision; (C) clinical complete response following radiotherapy; (D) local relapse following concurrent radiotherapy and immunotherapy. The macroscopic relapse tumor is enclosed inside the red circle.
In July 2022, a whole-body computer tomography (CT) scan was performed in observation for lung metastasis, revealing a locally invasive tumor in the perirectal tissue and also multiple micronodules located bilaterally in the pulmonary bases (Figure 2A).
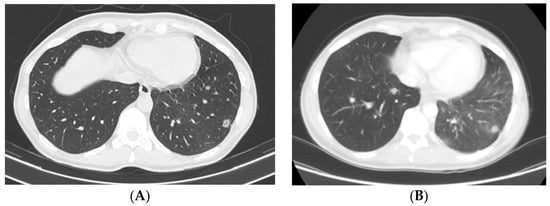
Figure 2.
Computer tomography imaging showing lung nodules progression following immunotherapy with Cemiplimab: (A) prior to immunotherapy initiation (July 2022); (B) following 6 cycles of immunotherapy (February 2023).
Upfront surgery was performed with a partial excision of the tumoral mass up to the sacrum, with macroscopic tumoral margin invasion, but with no possibility of suture due to the large size of the wound.
The results of the anatomopathological, immunohistochemistry, and genetic examinations established the diagnosis of moderately differentiated squamous cell carcinoma, ulcerated, invasive in the hypodermis, with lymphovascular invasion, but no perineural invasion, CKHMW positive, S100 positive, Ki67 approximately 30%, and RAS mutation in exon 2-KRAS (p.G12V) present. The tumor was staged pT3 pNx.
One month following surgery, tumor progression was diagnosed (Figure 1B), therefore the patient was subsequently referred to as “Prof. Dr. Alexandru Trestioreanu’’ Oncology Institute from Bucharest. Considering the decision of the multidisciplinary oncological committee, it was decided to initiate external beam radiotherapy (EBRT) and immunotherapy. The patient underwent EBRT, using a 6MV single-field 3D conformal technique, up to a total dose of 55 Gy. Radiotherapy was administered with a hypofractionated regimen of 2.75 Gy/fraction, from Monday to Friday. This was performed with a Clinac iX Varian linear Accelerator. Radiotherapy was well tolerated with minimal side effects. Concurrently, the patient underwent Libtayo-Cemiplimab, produced by Sanofi Romania, 350 mg every 21 days [q3w], from September 2022 to January 2023, with good clinical and hematological tolerance.
Six weeks following the end of radiotherapy, at the clinical exam, a complete local response was observed (Figure 1C). However, a whole-body CT scan from February 2023 revealed progressive disease in the lungs, therefore immunotherapy was discontinued and replaced with platinum salt (Carboplatin AUC 5, produced by TEVA, 450 mg) and taxanes (Paclitaxel-Sindaxel, produced by TEVA, 230 mg) chemotherapy. Six weeks following the end of radiotherapy, at the clinical exam, a complete local response was observed (Figure 1C). However, a whole-body CT scan revealed progressive disease in the lungs, therefore immunotherapy was discontinued and replaced with platinum salt and taxane chemotherapy.
Following four cycles of chemotherapy, clinical and imaging exams revealed progressive disease. At the whole-body CT scan, bone and ocular metastasis (Figure 3) were identified, supplementary to the progression of the disease both locally (Figure 1D) and at the lung level (Figure 2B). Considering this, the multidisciplinary board’s decision was to further refer the patient to the palliative care department.
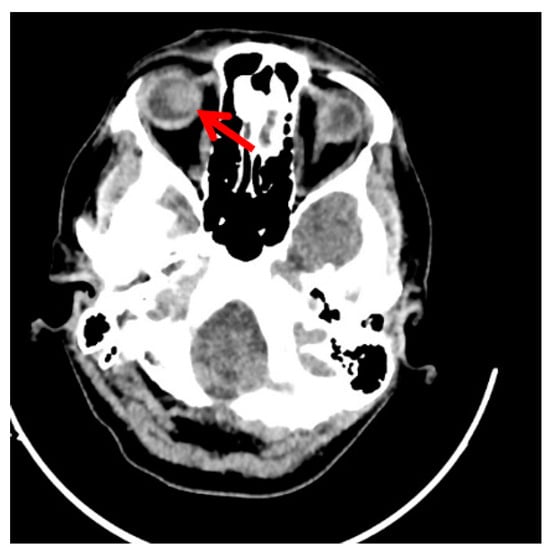
Figure 3.
Follow-up computer tomography imaging of the skull showing disease progression in the right eye following immunotherapy with Cemiplimab. (The red arrow marks the righ eye globe metastasis).
5. Discussion
The management of cutaneous SCC presents significant hurdles when the condition shows resistance to both immunotherapy and radiation therapy, as this case presentation illustrates [61,62]. We report the case of a 40-year-old female patient with a sacrococcygeal area cSCC that was aggressive and resistant to treatment. The patient had no notable medical history. The tumor, characterized by its vegetative nature and spontaneous bleeding, had a rapid progression and treatment resistance, deviating significantly from the typical clinical course of cSCC [61].
This instance emphasizes the reality that resistance to radiation and immunotherapy can result in significant disease progression when compared to data from the present literature [57,58]. Initial management involved surgical excision of the tumor, but its extensive size and margin invasion posed significant difficulties. Subsequent immunohistochemistry and genetic examinations confirmed a moderately differentiated squamous cell carcinoma with characteristics indicative of aggressiveness [61]. These findings align with the notion that specific genetic mutations, such as the RAS mutation identified here, can confer resistance to standard treatments [61,62].
Following surgical intervention, local recurrence emerged within a month. As per available international recommendations [62], the treatment was continued considering the multidisciplinary oncological team’s decision, with external beam radiotherapy (EBRT) and immunotherapy, specifically Cemiplimab. Cemiplimab is a very effective human IgG4 monoclonal antibody (mAb) that targets the PD-1 receptor and is hinge-stabilized. It is approved for use in patients with metastatic or locally advanced cutaneous squamous cell carcinoma (SCC) who are not candidates for radiation therapy or surgery [63]. While EBRT resulted in a complete local response in our patient, the subsequent development of lung metastases highlighted the unpredictable and relentless nature of advanced SCC.
The complexity of the case deepened as immunotherapy was replaced with platinum-based chemotherapy due to lung metastasis progression. Despite this shift, disease progression continued, affecting not only the lungs but also the bones and eyes, which are uncommon sites of tumor spread for cutaneous SCC [64].
In light of this case, it becomes evident that while immunoradiotherapy holds great potential for SCC treatment, its efficacy can vary significantly among patients [61,62]. Factors such as genetic mutations, tumor microenvironment, and individual patient responses can influence treatment outcomes [65]. Also, it should be noted that there is no direct evidence of PDL1 positivity at the level of the sacrococcygeal tumor formation, this being tested from the sample obtained following bronchoscopy and broncho-pulmonary lavage. Given these elements, it is possible that the treatment carried out was not individualized for the particularities and needs of the patient. This case exemplifies the pressing need for continued research into novel therapies and precision medicine approaches to address the complexities of advanced SCC effectively. Moreover, it highlights the importance of early detection and intervention. Altogether, treatments with different monoclonal antibodies (Mabs) have shown excellent efficacy along with acceptable safety in the SCC of different organs. Therefore, Mabs are considered excellent options for treating SCC, especially for the advanced stages of the disease. Overall, two highly potent types of Mabs in SCC therapy are anti-EGFR Mabs and checkpoint inhibitors, especially cetuximab, nimotuzumab, and PD-1 inhibitors [66]. Precisely because there are several options, it is extremely important to thoroughly test the tumors at the beginning of the treatment, to make sure that the line proposed to the patient is the most suitable for his particularities.
As the study was intended as a literature review, the following limitations should be considered when interpreting the case report: lack of data regarding HPV testing (e.g., p16 and p40 immunostains and in situ hybridization of HPV-HR) and supplementary data regarding PD-L1 immunotesting (e.g., primary antibodies and automated immunostainer). These limitations notwithstanding, this study contributes valuable insights into the challenges faced in the management of advanced SCC and highlights the need for further research to address these limitations and expand our understanding of this complex disease.
6. Future Directions
6.1. Biomarkers for Patient Selection and Treatment Response Prediction
One of the key challenges in the field of immunoradiotherapy for SCC is patient selection and prediction of treatment response. Biomarkers were found to play a crucial role in identifying patients who are likely to benefit from immunoradiotherapy and monitoring treatment response [67].
Efforts are underway to identify reliable biomarkers that can guide patient selection and treatment decisions [68,69,70]. These biomarkers may include immune cell profiles, tumor mutational burden, expression of immune checkpoint molecules, and immune-related gene signatures [68,69]. Ongoing research aims to validate these biomarkers and establish their clinical utility in predicting treatment response and prognosis [70].
6.2. Combination Approaches: Immunoradiotherapy with Targeted Therapies
Combination approaches hold great promise in maximizing treatment efficacy and overcoming resistance in SCC. Immunoradiotherapy can be combined with targeted therapies that specifically inhibit key signaling pathways implicated in SCC pathogenesis [71,72].
For example, the combination of immune checkpoint inhibitors with targeted therapies against oncogenic driver mutations, such as EGFR inhibitors or BRAF inhibitors, may enhance antitumor immune responses and improve treatment outcomes [71,73]. Preclinical and early-phase clinical trials investigating these combination strategies are underway, and their results will shed light on their potential clinical relevance in SCC treatment [73,74].
Additionally, the identification of predictive biomarkers can help guide the selection of targeted therapies in combination with immunoradiotherapy, ensuring personalized treatment approaches tailored to individual patients [67].
6.3. Emerging Technologies and Innovative Approaches
Advancements in technology and innovative treatment approaches are expanding the landscape of immunoradiotherapy in SCC. Several exciting developments hold promise for improving treatment outcomes and patient care [75,76].
Novel immunotherapeutic agents, such as bispecific antibodies, chimeric antigen receptor T-cell therapy, and immune agonists, are being investigated for their potential to enhance antitumor immune responses in SCC. These agents aim to target specific anti-gens expressed on SCC cells and stimulate immune cell activation and tumor cell killing [77,78].
Furthermore, the field of radiomics, which involves the extraction of quantitative imaging features from medical images, offers the potential for the non-invasive assessment of treatment response and the prediction of outcomes in SCC patients receiving immunoradiotherapy [79]. Radiomics-based approaches, coupled with machine learning algorithms, may help optimize treatment strategies and improve patient outcomes [80].
7. Conclusions
Immunoradiotherapy has emerged as a promising treatment approach for SCC, offering the potential for enhanced treatment responses and improved survival outcomes. The combination of immunotherapy and radiotherapy harnesses the power of the immune system to target tumor cells and elicit robust antitumor immune responses.
Current clinical evidence supports the efficacy of immunoradiotherapy in locally advanced SCC, with neoadjuvant and adjuvant approaches showing improved clinical outcomes and survival data. In metastatic SCC, early clinical trials and case reports have demonstrated encouraging results, although challenges and resistance mechanisms need to be addressed.
In light of the case report presented in this manuscript, it is essential to acknowledge the existence of treatment resistance and the challenges encountered in achieving successful outcomes with immunoradiotherapy in SCC. Despite the overall promising results observed in clinical trials and studies, this case serves as a reminder that not all patients may respond favorably to the combination treatment.
In order to optimize the therapeutic potential of immunoradiotherapy in SCC, future research endeavors should concentrate on unraveling the variables contributing to treatment resistance and investigating alternate techniques to overcome resistance mechanisms. By addressing these challenges, we can strive to improve patient outcomes and tailor treatment approaches to individualized needs in the pursuit of effective management of SCC.
Future directions in the field of immunoradiotherapy for SCC focus on the development of biomarkers for patient selection and treatment response prediction, as well as the exploration of combination approaches with targeted therapies. They should also focus on elucidating the factors contributing to treatment resistance and exploring alternative strategies to overcome resistance mechanisms, ultimately optimizing the therapeutic potential of immunoradiotherapy in SCC. Emerging technologies, such as radiomics and innovative immunotherapeutic agents, hold promise for optimizing treatment strategies and improving patient outcomes.
Author Contributions
Conceptualization, M.T.G., B.S.M.M. and R.I.M. (Raluca Ioana Mihaila); methodology, O.G.T. and R.I.M. (Radu Iulian Mitrica); validation, O.G.T., G.L.S. and M.T.G.; formal analysis, G.L.S. and C.B.; investigation, A.N. and A.G.; resources, M.T.G. and C.B.; data curation, O.G.T., R.I.M. (Raluca Ioana Mihaila) and G.L.S.; writing—original draft preparation, A.N., B.S.M.M. and M.T.G.; writing—review and editing, M.T.G. and D.E.G.; visualization, D.E.G., R.I.M. (Radu Iulian Mitrica) and A.G.; supervision, M.T.G., A.N. and D.E.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study is a narrative literature review and a retrospective case report presentation. Written consent for study participation was included in the written consent form for oncological treatments. For the presented study, no data/tissues/procedures were collected or performed outside the diagnostic-therapeutic routine. The study was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The data presented in the manuscript are available upon request from the corresponding author. The data are not publicly available due to privacy concerns.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lomas, A.; Leonardi-Bee, J.; Bath-Hextall, F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br. J. Dermatol. 2012, 166, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.W.; Weinstock, M.A.; Harris, A.R.; Coldiron, B.M. Incidence estimate of nonmelanoma skin cancer [keratinocyte carcinomas] in the U.S. population, 2012. JAMA Dermatol. 2015, 151, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Stratigos, A.; Garbe, C.; Lebbe, C.; Malvehy, J.; del Marmol, V.; Pehamberger, H.; Peris, K.; Becker, J.C.; Zalaudek, I.; Saiag, P.; et al. Diagnosis and treatment of invasive squamous cell carcinoma of the skin: European consensus-based interdisciplinary guideline. Eur. J. Cancer 2015, 51, 1989–2007. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Ratner, D. Cutaneous squamous-cell carcinoma. N. Engl. J. Med. 2001, 344, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Madan, V.; Lear, J.T.; Szeimies, R.M. Non-melanoma skin cancer. Lancet 2010, 375, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Green, A.C.; Olsen, C.M. Cutaneous squamous cell carcinoma: An epidemiological review. Br. J. Dermatol. 2017, 177, 373–381. [Google Scholar] [CrossRef]
- Bradford, P.T.; Freedman, D.M.; Goldstein, A.M.; Tucker, M.A. Increased risk of second primary cancers after a diagnosis of melanoma. Arch. Dermatol. 2010, 146, 265–272. [Google Scholar] [CrossRef]
- De Henau, O.; Rausch, M.; Winkler, D.; Campesato, L.F.; Liu, C.; Cymerman, D.H.; Budhu, S.; Ghosh, A.; Pink, M.; Tchaicha, J.; et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kγ in myeloid cells. Nature 2021, 539, 443–447. [Google Scholar] [CrossRef]
- Razi, S.; Khan, S.; Truong, T.M.; Zia, S.; Khan, F.F.; Uddin, K.M.; Rao, B.K. Cutaneous Squamous Cell Carcinoma: An Up-to-Date Comprehensive Review with a Focus on Contemporary Optical Imaging Diagnostic Modalities. Dermato 2023, 3, 161–181. [Google Scholar] [CrossRef]
- Whiteside, T.L. What are regulatory T cells [Treg] regulating in cancer and why? Semin Cancer Biol. 2012, 22, 327–334. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buqué, A.; Kepp, O.; Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017, 17, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Formenti, S.C.; Demaria, S. Combining radiotherapy and cancer immunotherapy: A paradigm shift. J. Natl. Cancer Inst. 2013, 105, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Ladányi, A. Prognostic and predictive significance of immune cells infiltrating cutaneous melanoma. Pigment Cell Melanoma Res. 2015, 28, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.W.; Moon, J.H.; Lee, J.H.; Kang, K.W.; Lee, H.S.; Eom, H.S.; Lee, E.; Lee, J.H.; Lee, J.O.; Park, S.K.; et al. Real-world data of long-term survival in patients with T-cell lymphoma who underwent stem cell transplantation. Blood Cancer J. 2023, 13, 95. [Google Scholar] [CrossRef]
- Wang, H.Y.; Lee, D.A.; Peng, G.; Guo, Z.; Li, Y.; Kiniwa, Y.; Shevach, E.M.; Wang, R.F. Tumor-specific human CD4+ regulatory T cells and their ligands: Implications for immunotherapy. Immunity 2004, 20, 107–118. [Google Scholar] [CrossRef]
- June, C.H.; O’Connor, R.S.; Kawalekar, O.U.; Ghassemi, S.; Milone, M.C. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef]
- Robbins, P.F.; Kassim, S.H.; Tran, T.L.; Crystal, J.S.; Morgan, R.A.; Feldman, S.A.; Yang, J.C.; Dudley, M.E.; Wunderlich, J.R.; Sherry, R.M.; et al. A pilot trial using lymphocytes genetically engineered with an NY-ESO-1-reactive T-cell receptor: Long-term follow-up and correlates with response. Clin. Cancer Res. 2015, 21, 1019–1027. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Restifo, N.P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015, 348, 62–68. [Google Scholar] [CrossRef]
- Li, B.; Severson, E.; Pignon, J.C.; Zhao, H.; Li, T.; Novak, J.; Jiang, P.; Shen, H.; Aster, J.C.; Rodig, S.; et al. Comprehensive analyses of tumor immunity: Implications for cancer immunotherapy. Genome Biol. 2016, 17, 174. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Andreu, P.; Coussens, L.M. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. 2010, 29, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Noy, R.; Pollard, J.W. Tumor-associated macrophages: From mechanisms to therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, A.H.; Pauken, K.E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018, 18, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Chambers, C.A.; Kuhns, M.S.; Egen, J.G.; Allison, J.P. CTLA-4-mediated inhibition in regulation of T cell responses: Mechanisms and manipulation in tumor immunotherapy. Annu. Rev. Immunol. 2001, 19, 565–594. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Parkhurst, M.R.; Yang, J.C.; Langan, R.C.; Dudley, M.E.; Nathan, D.A.; Feldman, S.A.; Davis, J.L.; Morgan, R.A.; Merino, M.J.; Sherry, R.M.; et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol. Ther. 2011, 19, 620–626. [Google Scholar] [CrossRef]
- Chinnasamy, D.; Yu, Z.; Theoret, M.R.; Zhao, Y.; Shrimali, R.K.; Morgan, R.A.; Feldman, S.A.; Restifo, N.P.; Rosenberg, S.A. Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice. J. Clin. Investig. 2010, 120, 3953–3968. [Google Scholar] [CrossRef]
- Atkins, M.B.; Lotze, M.T.; Dutcher, J.P.; Fisher, R.I.; Weiss, G.; Margolin, K.; Abrams, J.; Sznol, M.; Parkinson, D.; Hawkins, M.; et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J. Clin. Oncol. 1999, 17, 2105–2116. [Google Scholar] [CrossRef]
- Versteven, M.; Van den Bergh, J.M.J.; Marcq, E.; Smits Evelien, L.J.; Van Tendeloo Viggo, F.I.; Hobo, W.; Lion, E. Dendritic Cells and Programmed Death-1 Blockade: A Joint Venture to Combat Cancer. Front. Immunol. 2018, 9, 394. [Google Scholar] [CrossRef] [PubMed]
- Bulliard, Y.; Jolicoeur, R.; Windman, M.; Rue, S.M.; Ettenberg, S.; Knee, D.A.; Wilson, N.S.; Dranoff, G.; Brogdon, J.L. Activating Fc γ receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J. Exp. Med. 2013, 210, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Obeid, M.; Panaretakis, T.; Joza, N.; Tufi, R.; Tesniere, A.; van Endert, P.; Zitvogel, L.; Kroemer, G. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007, 14, 1848–1850. [Google Scholar] [CrossRef] [PubMed]
- Apetoh, L.; Ghiringhelli, F.; Tesniere, A.; Obeid, M.; Ortiz, C.; Criollo, A.; Mignot, G.; Maiuri, M.C.; Ullrich, E.; Saulnier, P.; et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 2007, 13, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Demaria, S.; Ng, B.; Devitt, M.L.; Babb, J.S.; Kawashima, N.; Liebes, L.; Formenti, S.C. Ionizing radiation inhibition of distant untreated tumors [abscopal effect] is immune mediated. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Golden, E.B.; Chhabra, A.; Chachoua, A.; Adams, S.; Donach, M.; Fenton-Kerimian, M.; Friedman, K.; Ponzo, F.; Babb, J.S.; Goldberg, J.; et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: A proof-of-principle trial. Lancet Oncol. 2015, 16, 795–803. [Google Scholar] [CrossRef]
- Lim, J.Y.; Gerber, S.A.; Murphy, S.P.; Lord, E.M. Type I interferons induced by radiation therapy mediate recruitment and effector function of CD8[+] T cells. Cancer Immunol. Immunother. 2014, 63, 259–271. [Google Scholar] [CrossRef]
- Zeng, J.; See, A.P.; Phallen, J.; Jackson, C.M.; Belcaid, Z.; Ruzevick, J.; Durham, N.; Meyer, C.; Harris, T.J.; Albesiano, E.; et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 343–349. [Google Scholar] [CrossRef]
- Paulson, K.G.; Lahman, M.C.; Chapuis, A.G.; Brownell, I. Immunotherapy for skin cancer. Int. Immunol. 2019, 31, 465–475. [Google Scholar] [CrossRef]
- Vaidya, P.; Mehta, A.; Ragab, O.; Lin, S.; In, G.K. Concurrent radiation therapy with programmed cell death protein 1 inhibition leads to a complete response in advanced cutaneous squamous cell carcinoma. JAAD Case Rep. 2019, 5, 763–766. [Google Scholar] [CrossRef]
- Spranger, S.; Sivan, A.; Corrales, L.; Gajewski, T.F. Tumor and Host Factors Controlling Antitumor Immunity and Efficacy of Cancer Immunotherapy. Adv. Immunol. 2016, 130, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2017, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Krishnakumar, H.N.; Saladi, S.V. Current and Future Biomarkers for Immune Checkpoint Inhibitors in Head and Neck Squamous Cell Carcinoma. Curr. Oncol. 2022, 29, 4185–4198. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Auh, S.L.; Wang, Y.; Burnette, B.; Wang, Y.; Meng, Y.; Beckett, M.; Sharma, R.; Chin, R.; Tu, T.; et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: Changing strategies for cancer treatment. Cancer Res. 2009, 69, 3927–3936. [Google Scholar] [CrossRef]
- Valpione, S.; Mundra, P.A.; Galvani, E.; Campana, L.G.; Lorigan, P.; De Rosa, F.; Gupta, A.; Weightman, J.; Mills, S.; Dhomen, N.; et al. The T cell receptor repertoire of tumor infiltrating T cells is predictive and prognostic for cancer survival. Nat. Commun. 2021, 12, 4098. [Google Scholar] [CrossRef]
- Elbanna, M.; Chowdhury, N.N.; Rhome, R.; Fishel, M.L. Clinical and Preclinical Outcomes of Combining Targeted Therapy With Radiotherapy. Front. Oncol. 2021, 11, 749496. [Google Scholar] [CrossRef] [PubMed]
- Saddawi-Konefka, R.; Msari, R.A.; Jones, R.N.; Sadat, S.; Franiak-Pietryga, I.; Sharabi, A.; Gutkind, J.S.; Califano, J.A. Califano Neoadjuvant Immunoradiotherapy in a Tobacco-Signature Preclinical Oral Squamous Cell Carcinoma Model. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, e7. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, B.; Peng, G.; Xiao, G.; Huang, J.; Ding, Q.; Yang, C.; Xiong, X.; Ma, H.; Shi, L.; et al. Neoadjuvant Chemoimmunotherapy for the Treatment of Locally Advanced Head and Neck Squamous Cell Carcinoma: A Single-Arm Phase 2 Clinical Trial. Clin. Cancer Res. 2022, 28, 3268–3276. [Google Scholar] [CrossRef]
- Newman, J.G.; Hall, M.A.; Kurley, S.J.; Cook, R.W.; Farberg, A.S.; Geiger, J.L.; Koyfman, S.A. Adjuvant therapy for high-risk cutaneous squamous cell carcinoma: 10-year review. Head Neck 2021, 43, 2822–2843. [Google Scholar] [CrossRef]
- Burns, C.; Kubicki, S.; Nguyen, Q.-B.; Aboul-Fettouh, N.; Wilmas, K.M.; Chen, O.M.; Doan, H.Q.; Silapunt, S.; Migden, M.R. Advances in Cutaneous Squamous Cell Carcinoma Management. Cancers 2022, 14, 3653. [Google Scholar] [CrossRef]
- Sharpe, M.; Mount, N. Genetically modified T cells in cancer therapy: Opportunities and challenges. Dis. Models Mech. 2015, 8, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.K.; Mukherjee, S. Current Headway in Cancer Immunotherapy Emphasizing the Practice of Genetically Engineered T Cells to Target Selected Tumor Antigens. Crit. Rev. Immunol. 2021, 41, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Chin, R.I.; Gastman, B.; Thorstad, W.; Yom, S.S.; Reddy, C.A.; Nussenbaum, B.; Wang, S.J.; Knackstedt, T.; Vidimos, A.T.; et al. Association of Disease Recurrence with Survival Outcomes in Patients with Cutaneous Squamous Cell Carcinoma of the Head and Neck Treated With Multimodality Therapy. JAMA Dermatol. 2019, 155, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Vermorken, J.B.; Remenar, E.; van Herpen, C.; Gorlia, T.; Mesia, R.; Degardin, M.; Stewart, J.S.; Jelic, S.; Betka, J.; Preiss, J.H.; et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N. Engl. J. Med. 2015, 16, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Kringel, R.; Lamszus, K.; Mohme, M. Chimeric Antigen Receptor T Cells in Glioblastoma—Current Concepts and Promising Future. Cells 2023, 12, 1770. [Google Scholar] [CrossRef]
- Ribas, A.; Hamid, O.; Daud, A.; Hodi, F.S.; Wolchok, J.D.; Kefford, R.; Joshua, A.M.; Patnaik, A.; Hwu, W.J.; Weber, J.S.; et al. Association of pembrolizumab with tumor response and survival among patients with advanced melanoma. N. Engl. J. Med. 2019, 381, 2229–2239. [Google Scholar] [CrossRef]
- Leidner, R.; Crittenden, M.; Young, K.; Xiao, H.; Wu, Y.; A Couey, M.; A Patel, A.; Cheng, A.C.; Watters, A.L.; Bifulco, C.; et al. Neoadjuvant immunoradiotherapy results in high rate of complete pathological response and clinical to pathological downstaging in locally advanced head and neck squamous cell carcinoma. J. Immunother. Cancer 2021, 9, e002485. [Google Scholar] [CrossRef]
- Shen, P.; Qiao, B.; Jin, N.; Wang, S. Neoadjuvant immunoradiotherapy in patients with locally advanced oral cavity squamous cell carcinoma: A retrospective study. Investig. New Drugs 2022, 40, 1282–1289. [Google Scholar] [CrossRef]
- Jiang, M.; Hu, Y.; Lin, G.; Chen, C.; Li, H. Radiotherapy combined with immune checkpoint inhibitors in locally advanced/metastatic esophageal squamous cell carcinoma: Clinical trials, efficacy and future directions. Front. Immunol. 2023, 14, 1177085. [Google Scholar] [CrossRef]
- Ralli, M.; Botticelli, A.; Visconti, I.C.; Angeletti, D.; Fiore, M.; Marchetti, P.; Lambiase, A.; de Vincentiis, M.; Greco, A. Immunotherapy in the Treatment of Metastatic Melanoma: Current Knowledge and Future Directions. J. Immunol. Res. 2020, 2020, 9235638. [Google Scholar] [CrossRef]
- Available online: https://www.mayoclinic.org/diseases-conditions/squamous-cell-carcinoma/diagnosis-treatment/drc-20352486 (accessed on 29 September 2023).
- Treating Squamous Cell Carcinoma|Squamous Cell Cancer Treatment|American Cancer Society. Available online: https://www.cancer.org/cancer/types/basal-and-squamous-cell-skin-cancer/treating/squamousl-cell-carcinoma.html (accessed on 29 September 2023).
- Gambale, E.; Fancelli, S.; Caliman, E.; Petrella, M.C.; Doni, L.; Pillozzi, S.; Antonuzzo, L. Immune checkpoint blockade with anti-programmed cell death 1 (PD-1) monoclonal antibody (mAb) cemiplimab: Ongoing and future perspectives in rare genital cancers treatment. J. Immunother. Cancer 2022, 10, e003540. [Google Scholar] [CrossRef] [PubMed]
- Swamy, N.N.; Tasneem, A.F. Ocular metastasis in a patient with squamous cell carcinoma tongue: A rare case. J. Clin. Res. Ophthalmol. 2020, 7, 001–003. [Google Scholar] [CrossRef]
- Fania, L.; Didona, D.; Di Pietro, F.R.; Verkhovskaia, S.; Morese, R.; Paolino, G.; Donati, M.; Ricci, F.; Coco, V.; Ricci, F.; et al. Cutaneous Squamous Cell Carcinoma: From Pathophysiology to Novel Therapeutic Approaches. Biomedicines 2021, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, A.; Tamimi, A.; Sorkheh, F.; Asl, S.M.; Ghafari, A.; Karimi, A.G.; Erabi, G.; Pourmontaseri, H.; Deravi, N. Monoclonal antibodies for the treatment of squamous cell carcinoma: A literature review. Cancer Rep. 2023, 6, e1802. [Google Scholar] [CrossRef] [PubMed]
- Affolter, A.; Kern, J.; Bieback, K.; Scherl, C.; Rotter, N.; Lammert, A. Biomarkers and 3D models predicting response to immune checkpoint blockade in head and neck cancer (Review). Int. J. Oncol. 2022, 61, 88. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.G.; Shen, B.; Sun, X.Q. Significant Biomarkers Identification Associated with Cutaneous Squamous Cell Carcinoma Progression. Int. J. Gen. Med. 2022, 15, 2347–2360. [Google Scholar] [CrossRef] [PubMed]
- Fumet, J.D.; Truntzer, C.; Yarchoan, M.; Ghiringhelli, F. Tumour mutational burden as a biomarker for immunotherapy: Current data and emerging concepts. Eur. J. Cancer 2020, 131, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X. An Immune-Related Gene Signature Can Predict Clinical Outcomes and Immunotherapeutic Response in Oral Squamous Cell Carcinoma. Front. Genet. 2020, 13, 870133. [Google Scholar] [CrossRef]
- Li, B.; Jin, J.; Guo, D.; Tao, Z.; Hu, X. Immune Checkpoint Inhibitors Combined with Targeted Therapy: The Recent Advances and Future Potentials. Cancers 2023, 15, 2858. [Google Scholar] [CrossRef]
- Plavc, G.; Jesenko, T.; Oražem, M.; Strojan, P. Challenges in Combining Immunotherapy with Radiotherapy in Recurrent/Metastatic Head and Neck Cancer. Cancers 2020, 12, 3197. [Google Scholar] [CrossRef]
- Wu, J.H.; Cohen, D.N.; Rady, P.L.; Tyring, S.K. BRAF inhibitor-associated cutaneous squamous cell carcinoma: New mechanistic insight, emerging evidence for viral involvement and perspectives on clinical management. Br. J. Dermatol. 2017, 177, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Ansary, T.M.; Hossain, M.R.; Komine, M.; Ohtsuki, M. Immunotherapy for the Treatment of Squamous Cell Carcinoma: Potential Benefits and Challenges. Int. J. Mol. Sci. 2022, 23, 8530. [Google Scholar] [CrossRef]
- Pan, C.; Liu, H.; Robins, E.; Song, W.; Liu, D.; Li, Z.; Zheng, L. Next-generation immuno-oncology agents: Current momentum shifts in cancer immunotherapy. J. Hematol. Oncol. 2020, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Shevtsov, M.; Sato, H.; Multhoff, G.; Shibata, A. Novel Approaches to Improve the Efficacy of Immuno-Radiotherapy. Front. Oncol. 2019, 9, 156. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Qiu, T.; Li, F.; Ren, S. Current status and future perspectives of bispecific antibodies in the treatment of lung cancer. Chin. Med. J. 2023, 136, 379–393. [Google Scholar] [CrossRef]
- Wang, H.-Q.; Fu, R.; Man, Q.-W.; Yang, G.; Liu, B.; Bu, L.-L. Advances in CAR-T Cell Therapy in Head and Neck Squamous Cell Carcinoma. J. Clin. Med. 2023, 12, 2173. [Google Scholar] [CrossRef]
- Dercle, L.; McGale, J.; Sun, S.; Marabelle, A.; Yeh, R.; Deutsch, E.; Mokrane, F.Z.; Farwell, M.; Ammari, S.; Schoder, H.; et al. Artificial intelligence and radiomics: Fundamentals, applications, and challenges in immunotherapy. J. Immunother. Cancer 2022, 10, e005292. [Google Scholar] [CrossRef]
- Tabari, A.; Cox, M.; D’Amore, B.; Mansur, A.; Dabbara, H.; Boland, G.; Gee, M.S.; Daye, D. Machine Learning Improves the Prediction of Responses to Immune Checkpoint Inhibitors in Metastatic Melanoma. Cancers 2023, 15, 2700. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).