Abstract
Turmeric and ginger, widely used rhizomes in culinary arts, have several beneficial biological activities, such as hypoglycemic, hepato-protective, antimicrobial, and antioxidant properties. This work investigated the effects of three phenolic extracts isolated from turmeric and ginger rhizomes on anti-inflammatory and healing properties using the solid–liquid extraction method. Wistar rats were used as a biological model. The anti-inflammatory activity was evaluated on induced edema in the rat’s hind paw using carrageenan (1%). Paw volume was measured at 0 min, 45 min, 3 h, and 5 h. Treatment with turmeric and ginger extracts, administered at a dose of 100 mg/kg, revealed a reduction in edema volume by 98.8%, 94.8%, and 98.3% using an aqueous extract of turmeric, ethanolic extract of turmeric, and methanolic extract of ginger, respectively. The healing activity parameters of induced burns on the rat’s dorsal region in nine groups (7 rats each) were monitored daily throughout the experiment’s duration. Results showed that the application of creams composed of petroleum jelly dispersing turmeric and ginger extracts to wounds at a dose of 100 mg/kg g induced complete healing after 19 days while the negative control was only 60% cured. On day 14, the aqueous, ethanolic, and methanolic turmeric extracts nearly resulted in complete tissue repair by 95.26%, 98.34%, and 87.39%, respectively. According to the chromatographic analysis (Sephadex G50 column), there is a variation in the molecular weight distribution of phenolic compounds (polymers, oligomers, and monomers) in the three studied extracts, which has a differential effect on the anti-inflammatory and wound healing activities of the extracts.
1. Introduction
The failure of conventional medications has become an absolute public health problem that is linked to the development of allergies with respect to many natural and synthetic chemical compounds. This has led to a trend towards finding new natural sources of compounds, thus intensifying the need for treatments of certain pathologies using plants or their extracts [1]. Indeed, the latter are rarely considered effective treatments, so many herbal remedies do not undergo scientific research. Recently, scientists have been led to find innovative solutions from medicinal plants and to develop new natural products to capitalize on the traditional knowledge of using these plants to treat certain current emerging pathologies (inflammations, chronic diseases, etc.) that are probably associated with the new lifestyle of Moroccan citizens.
Despite the improvements in modern medicine, chronic wounds are difficult to treat due to the specific physiologies between patients. Serious injuries negatively affect millions of people by causing severe consequences in their lifetime [2,3]. Subsequently, appropriate wound treatment that facilitates healthy healing is reasonable in order to maintain normal bodily functions and improve patient health [4].
Inflammation serves as a vital protective mechanism within the body, participating in various acute and chronic diseases. Yet, inadequately managed inflammation can lead to substantial damage and the development of chronic inflammatory conditions [5]. This intricate process involves enzyme activation, mediator release, fluid extravasation, cell migration, and tissue repair [6]. Therapeutic interventions targeting specific phases of the inflammatory response play a pivotal role in managing disorders like rheumatoid arthritis, infections, and tissue damage [7].
Traditional wound care and treatment practices have been used for millennia, offering a wide range of potential wound healing treatments [8]. However, these practices are limited by the availability of plant or animal resources. This further indicates the importance and timeliness of identifying potential medicinal resources for wound healing based on traditional knowledge and usage [9].
The epidermis presents an immediate defense function and has exhibited distinguished performances in skin regeneration in large animals [10]. The transmutation of this phenomenon in the process of wound healing regeneration is intriguingly complex and involves a number of highly regulated factors working in harmony to restore injured skin [11]. If the rupture of the epidermis progresses it can further extend to other tissues such as subcutaneous tissues, muscles, nerves, vessels, and bones. It can be said that skin, the largest human organ, is particularly exposed to damage because it can be easily burned or injured by trauma or surgical intervention [12].
The physiology of wound healing is complex and involves the related processes of tissue repair and regeneration [13,14]. Understanding wound healing physiology is important since it helps update the clinical management techniques for wounds.
In order to treat different cutaneous pathologies, phytotherapy has been used since antiquity to treat wounds by natural agents. This is because of scientific progress, improvements in extractive techniques, and the active discovery of new principles, which present a renewal of interest due to their exploitation in several domains, namely pharmaceutical, agri-food, and cosmetic industries. In this perspective, we have chosen two spices belonging to the Zingiberaceae family to evaluate their beneficial effects on the decrease in inflammation by reducing the volume of paw and wound healing caused by burns on rat skin.
Curcuma longa L. and Zingiber officinale rhizomes, commonly known as turmeric and ginger, respectively, are herbaceous perennial rhizomatous plants used in culinary arts and medicine [15,16]. As culinary products, ginger and turmeric are vital spices that are commonly consumed and play a key role in disease prevention, especially arthritis [17]. Turmeric (Curcuma longa) is a plant that is commonly used as a spice in food preparation due to its flavor and color [18]. It is also used as a medicinal plant due to its antioxidant, anti-inflammatory, antimutagenic, antimicrobial, and anticancer properties [19]. In cosmetology, ginger is widely valued for its highly appreciated essential oil [20].
Herbal products have been used in the treatment of human and animal diseases since ancient times because of their easy availability and good therapeutic effectiveness [21]. In order to cure various skin-related problems, including wound healing and inflammation, the present study examined the effect of creams based on turmeric and ginger extracts on improving anti-inflammatory and wound healing processes.
In Morocco, the two spices (Curcuma longa L. and Zingiber officinale) are used in traditional cuisine, but they are even better known for their benefits on human health, which is likely due to their richness in phenolic compounds that are responsible for numerous biological activities. Thus, this research work aimed at studying the phenolic compounds’ distribution and phytochemical composition of turmeric and ginger to prepare an ointment based on different extracts, viz., aqueous, ethanolic, and methanolic extracts, in order to evaluate their effectiveness on inflammation induced by carrageenan and on wound healing caused by thermal burns on Wistar rat’s dorsal areas.
2. Materials and Methods
2.1. Vegetal Material
Rhizomes of Curcuma longa L. and Zingiber officinale subjected to this study were purchased from herbalists in Fez city (Northern inland Morocco) in dry form and then ground into fine powder (particle size < 0.1 mm) using an M 20 Universal mill (IKA model).
2.2. Extracts Preparation
Sample powder (100 g) was added to 3000 mL (3 × 1000 mL) of solvents (distilled water, ethanol, and methanol) and stirred (100 RPM) for 2 h at room temperature and filtered (MF-Millipore™ Membrane Filter, 0.45 µm-ϕ pore size, Merck, Germany). Under reduced pressure, the filtrate was concentrated into dry extracts using a BUCHI Rotavapor™ R-300 (BÜCHI Labortechnik AG, Flawil/Switzerland). Afterwards, extracts were defatted thrice with hexane and then dried at 45 °C for several days to obtain a homogeneous powder extract.
2.3. Animal Model
Male rats (Wister strain) were used as animal models, and they possessed a body weight between 180 and 200 g. The animals were raised in the Fez Faculty of Medicine and Pharmacy (FFMP) animal house under standard lighting conditions (12 h of white light/12 h of darkness) at a temperature of 25 ± 1 °C and humidity of 55 ± 5%. Each animal was placed in individual plexiglass cages, received a portion of standard food, and drank tap water as much as they required.
The rats’ care and handling followed the guidelines provided by international standards for animal use. The Institutional Animal Care Committee (FFMP) approved the protocol according to French technical specifications for producing, caring for, and using laboratory animals at Sidi Mohamed Ben Abdellah University, Faculty of Science and Technology, Fez, Morocco.
2.4. Biochemical Study
To characterize the obtained extracts, a spectrophotometer (Zuzi 4255/50, Auxilab S.L., Navarra, Spain) was used. Total phenolic content was determined using the Folin–Ciocalteu reagent as reported by Atanassova et al. [22], flavonoids were determined using the aluminium trichloride (AlCl3) method as cited by Barros et al. [23], and carotenoid content was determined according to Lee and Castle’s method [24]. Vitamin C content was determined using a colorimetric assay, as described by Ahodegnon et al. [25].
2.5. Exclusion Chromatography
To study the molecular weight profiles of phenolic compounds in spice extracts, exclusion chromatography was used to separate the molecules of turmeric and ginger extracts in order to explain the difference in the obtained results from the tests carried out on wound healing and anti-inflammation. The molecules were eluted according to their decreasing molecular weight (MW) using a glass column (50 cm length × 2.5 cm diameter) containing Sephadex G50 as the stationary phase and a buffer solution formed from lithium chloride as the mobile phase (5 mM NaOH, 2.5 mM LiCl). Milliliter fractions were collected from the column at a flow rate of 1 mL/min. The isolated fractions were analyzed using a spectrophotometer (Zuzi 4255/50, Auxilab S.L., Navarra, Spain) at a wavelength of 380 nm [26]. Monocyclic phenolic compounds were localized by phenol as a molecular weight marker, while polycyclic peaks were positioned using the quercitrin molecule (Sigma Aldrich, Munich, Germany).
2.6. Effect of Extracts on Acute Inflammation Induced by 1% Carrageenan in Rats
The investigation of anti-inflammatory properties was evaluated by measuring plantar edema induced by 1% carrageenan according to the method described, in [27]. Rats were divided into 9 groups of 7 rats each and separated as follows: negative control rats treated with neutral cream (petroleum jelly: GRIFFON Mark) (NC); positive control rats treated with a pharmaceutical anti-inflammatory product (Diclofenac 1%) (PC); rats treated with a 10% aqueous turmeric-extract-based cream (TAE); rats treated with a 10% ethanolic turmeric-extract-based cream (TEE); rats treated with a 10% methanolic turmeric-extract-based cream (TME); rats treated with a 10% aqueous ginger-extract-based cream (GAE); rats treated with a 10% ethanolic ginger-extract-based cream (GEE); rats treated with a 10% methanolic ginger-extract-based cream (GME).
The creams were formulated by dispersing 10 g of ginger and turmeric extract in 100 g of valine (petroleum jelly). The negative control group was treated with Vaseline while the positive control was treated with an anti-inflammatory drug (Diclofenac 1%).
At about 30 min before chemical inflammation application, neutral cream (petroleum jelly: negative control), diclofenac (positive control), and creams prepared from extracts were applied to the rat’s paw. An injection of 10 µL of 1% carrageenan under the rat’s plantar fascia hind paw was induced. The anti-inflammatory effect was assessed by measuring paw volume edema before and after the injection of 1% carrageenan at 0 min, 45 min, 3 h, and 5 h using a digital caliper. Edema extent was assessed by determining the animal’s paw volume increase percentage (%PVI) (Equation (1)) [27]:
Anti-inflammatory activity was evaluated by calculating the edema inhibition percentage (%EIP) according to the following relationship (Equation (2)) [27]:
2.7. Effects of Turmeric and Ginger Rhizome Extracts on Induced Wound Healing in Wistar Rats
A wound is assessed by examining which tissue components are lost and which tissues are exposed. It is, therefore, necessary to precisely identify the factors involved and take the necessary measures promptly to prevent and heal wounds. Thus, it is essential to understand natural human or animal tissues and how they function [28].
Healing activity was performed on 40 Wister rats, which were used as an experimental model for thermal burn induction. The animals were divided into eight batches with five rats each as follows: negative control rats treated with neutral cream (petroleum jelly: GRIFFON Mark) (NC); positive control rats treated with H.E.C. (hemostatic and healing ointment) (PC); rats treated with a 10% aqueous turmeric-extract-based cream (TAE); rats treated with a 10% ethanolic turmeric-extract-based cream (TEE); rats treated with a 10% methanolic turmeric-extract-based cream (TME); rats treated with a 10% aqueous ginger extract-based cream (GAE); rats treated with a 10% ethanolic ginger-extract-based cream (GEE), and rats treated with a 10% methanolic ginger-extract-based cream (GME).
The creams were formulated by dispersing 10 g of ginger and turmeric extract in 100 g of valine (petroleum jelly). The negative control group was treated with Vaseline while the positive control was treated with a wound healing drug (hemostatic and healing ointment).
To create wounds, animals were anesthetized via diethyl ether inhalation [29]. The burns were introduced to the skin dorsal region (aiming to introduce burns easily and for wound evolution monitoring). Experimental burns were induced using a 1 cm diameter metal cylinder that was heated in water (100 °C) for 5 min and then applied by lightly pressing on the shaved skin surface of rats to induce second-degree burns [30]. Treatments were applied once every 24 h, and wound diameters were measured every day throughout 22 days; wound surface photos were taken to monitor the wound’s evolution using image processing software (ImageJ® version 1.53o). In order to allow the software to calculate burn areas, it must be calibrated first using a graduated ruler, which is included in the photographic frame to enable ImageJ calibration. Digital planimetry methods have the advantage of being fast, precise, and objective.
All animals were monitored regularly until the wounds were completely healed. The percentage of wound shrinkage (or contraction) was calculated using the following formula (Equation (3)) [26]:
2.8. Statistical Analysis
The effect of turmeric and ginger extracts on the healing phenomenon was carried out by performing kinetics that targeted and induced plantar edema volume reduction, wound surface reduction, and a percentage of wound contraction (%WC). Student’s test (t-test) was used to compare the performance of the healing phenomenon and the means obtained using the treatment with turmeric and ginger extracts to those obtained separately with both controls (C− and C+). To compare the means obtained between the positive control, negative control, and the treatments with tested turmeric and ginger extracts, unidirectional analysis of variance was applied (ANOVA 1). The same procedure was undertaken for biochemical analysis. Data were processed using the “SYS-TAT 12” software.
3. Results
3.1. Biochemical and Phytochemical Characteristics of the Studied Spice Extracts
The biochemical and phytochemical characteristics of the studied spice extracts are summarized in the following table (Table 1).

Table 1.
Biochemical and phytochemical characteristics of the studied rhizome (Curcuma longa L. and Zingiber officinale) extracts.
The outcomes of biochemical compositions (Table 1) showed that turmeric and ginger are rich in antioxidants such as phenolic compounds, flavonoids, vitamin C, carotenoids, and essential oils. The healing effects of flavonoids, polyphenols, vitamin C, and carotenoids increase antioxidant activity, collagen deposition, hydroxyproline formation, and antibacterial activity [31]. We can therefore conclude the anti-inflammatory and skin wound healing potential of turmeric and ginger. Such traits partly motivated the choice of these two spices in realizing this research study.
3.2. Gel Filtration Chromatography
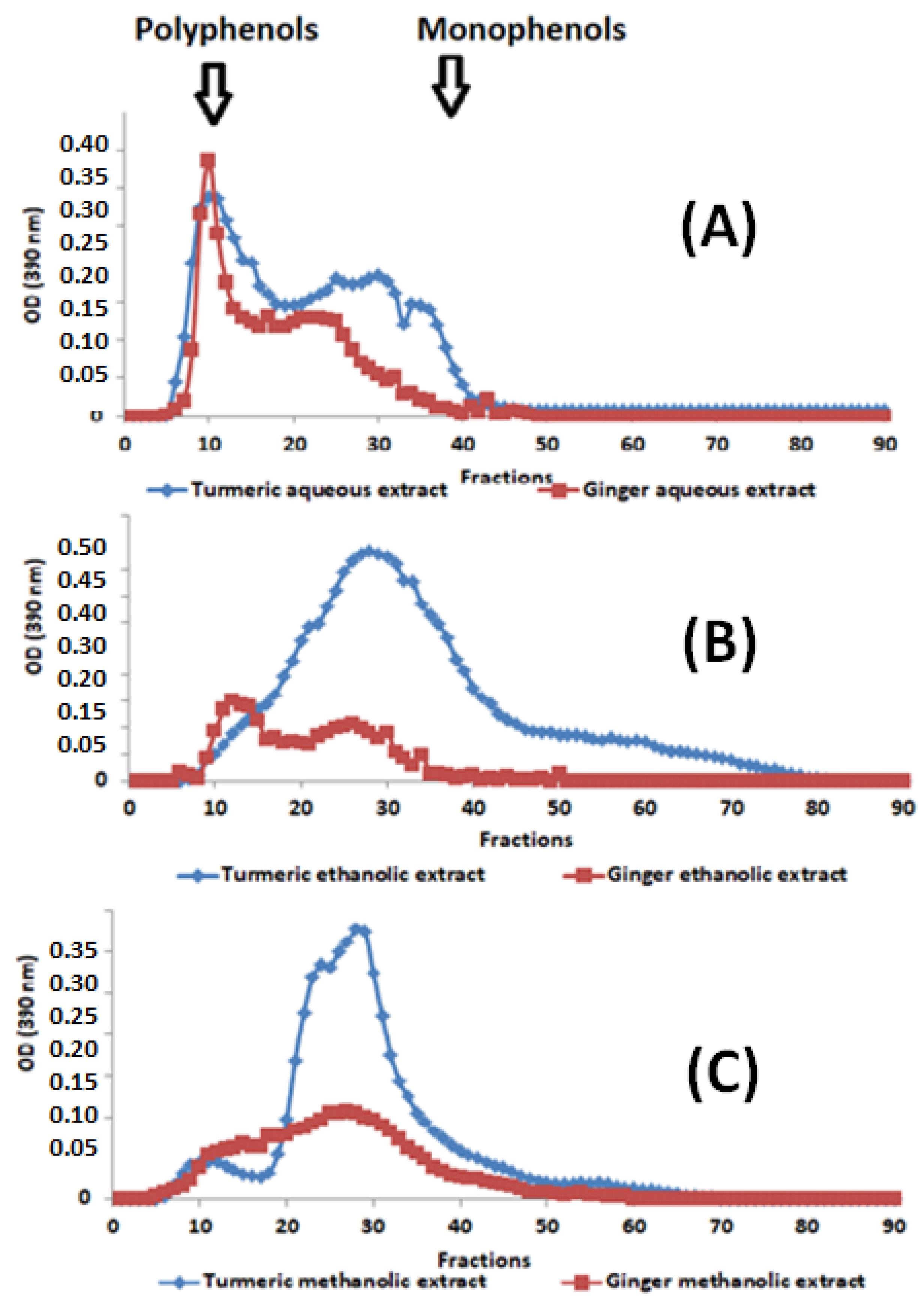
Phenolic compounds are significant constituents of the studied spice extracts, so their polymerization profile was analyzed to compare their molecular weights and assess their potential for anti-inflammatory and wound healing properties. Indeed, polyphenols are rapidly eluted due to their exclusion by Sephadex gel beads. Monophenols are eluted later due to their delay in the gel pores, resulting in their slow migration.
The following figure shows the exclusion’s chromatographic results obtained after the spectrophotometric analysis of turmeric and ginger extracts (Figure 1).
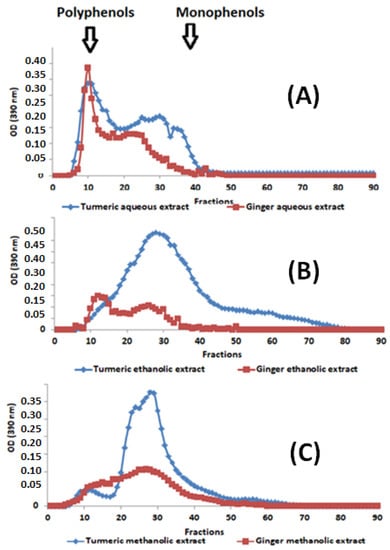
Figure 1.
Elution profiles from Sephadex G-50 of (A) aqueous, (B) ethanolic, and (C) methanolic extracts of turmeric and ginger. Arrows indicate the positions of molecular weight markers used to locate polymeric and monomeric phenolic compounds.
By comparing the phenolic compounds’ elution profiles (Figure 1A–C), it can be observed that the studied extracts, examined using gel filtration, comprise significant variations in molecular weight distribution. In this elution, we found that phenolic compounds extracted with water (Figure 1A) exhibit two characteristic peaks of the two large families of phenolic compounds (polymers (Fractions 5 to 20), oligomers (Fractions 20 to 30), and monomers (fractions 30 to 42)). On the other hand, the ethanolic (Figure 1B) and methanolic (Figure 1C) extracts have a significant shortage of polymeric phenolic compounds and an abundance of oligomeric and monomeric forms. It is also noted that the ethanolic extract is quantitatively and qualitatively richer in oligomeric and monomeric phenolic compounds compared to aqueous and methanolic extracts, demonstrating that the ethanolic extract is the poorest in phenolic polymer compounds. Moreover, extracted curcumin is richer than ginger in monomeric, oligomeric, and polymeric phenolic compounds. This difference in distribution is very likely to have consequences on the biological activities targeted by this study.
3.3. Anti-Inflammatory Activity
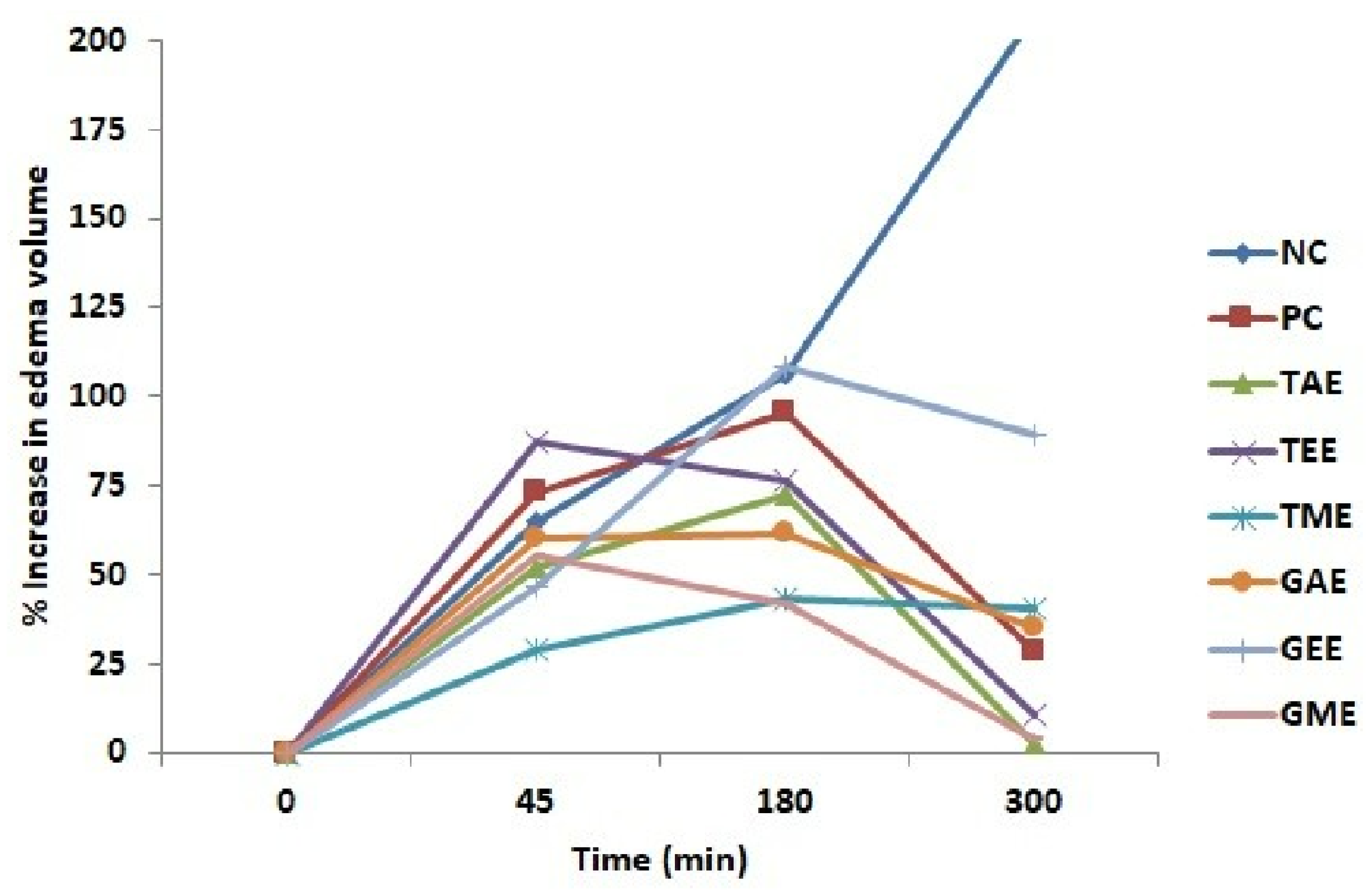
The results of the anti-inflammatory effects of turmeric and ginger are shown in Figure 2 and Figure 3. The injection of 10 µL of 1% carrageenan under the rat’s hind paw plantar fascia to cause edema increased gradually with time and reached the maximum at 3 h (180 min). Figure 2 shows the percentages of paw volume increase in rats treated with aqueous, ethanolic, methanolic extracts, diclofenac (positive control), and untreated rats (negative control).
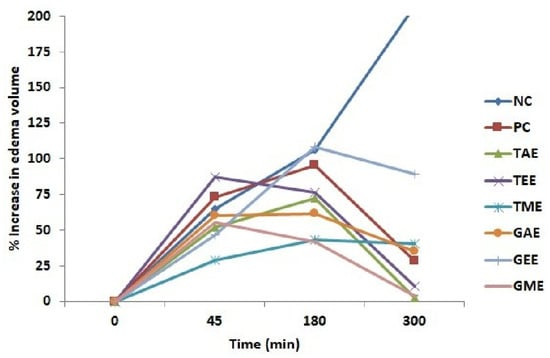
Figure 2.
Percentage increase (average ± standard deviation) in the plantar paw of rats after carrageenan injection. Note: NC: Negative control; PC: positive Control; TAE: turmeric aqueous extract; TEE: turmeric ethanolic extract; TME: turmeric methanolic extract; GAE: ginger aqueous extract; GEE: ginger ethanolic extract; GME: ginger methanolic extract.
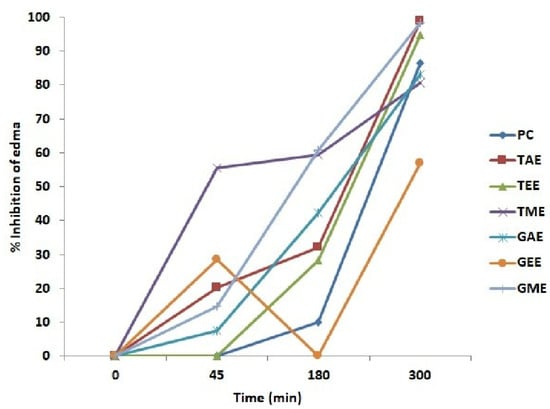
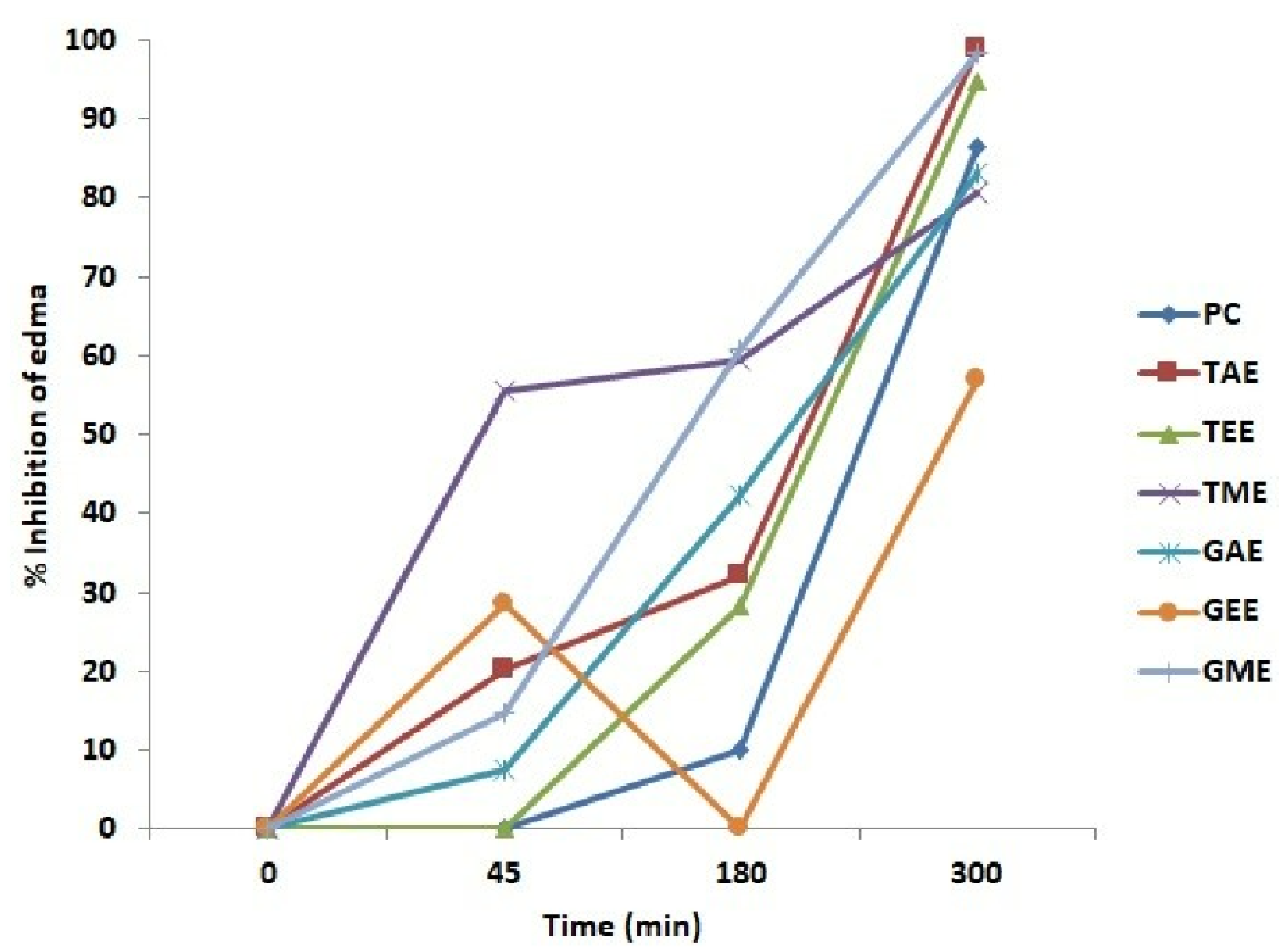
Figure 3.
Anti-inflammatory activity (average ± standard deviation) of tested extracts expressed as the percentage inhibition of edema induced by carrageenan injection. Note: PC: Positive control; TAE: turmeric aqueous extract; TEE: turmeric ethanolic extract; TME: turmeric methanolic extract; GAE: ginger aqueous extract; GEE: ginger ethanolic extract; GME: ginger methanolic extract.
From the obtained results, it can be disclosed that the carrageenan injection caused a progressive increase in the edema volume in all rats. Indeed, extracts (ethanolic turmeric, aqueous, and methanolic ginger) manifested their inhibitory effects on edema formation after 45 min. Animals from the negative control, positive control, and ethanolic ginger-extract-treated groups showed the highest paw volume at 3 h compared to the other groups. At 5 h (300 min), the groups that received tested extracts and diclofenac caused a reduction in paw volume. In contrast, the negative control batch showed a prolonged increase in volume.
From these results, one could calculate the edema inhibition percentage (%EIP) of carrageenan-induced paw edema in rats using turmeric, ginger extracts, and Diclofenac. The obtained results are presented in Figure 3, proving that rats treated with plant extracts have considerable anti-inflammatory activity. At 5 h, the rat’s treatment with aqueous and ethanolic extracts of turmeric and methanolic extracts of ginger produced a maximum decrease in edema with an inhibition percentage of 98.8, 94.8, and 98.3%, respectively, when compared to the positive control treated with diclofenac (86.4%), which was less effective indeed. However, the lowest anti-inflammatory activity was observed in the group treated with the ethanolic ginger extract, where the edema inhibition percentage was merely 56.8%.
3.4. Wound Healing Activity of Turmeric and Ginger Extracts
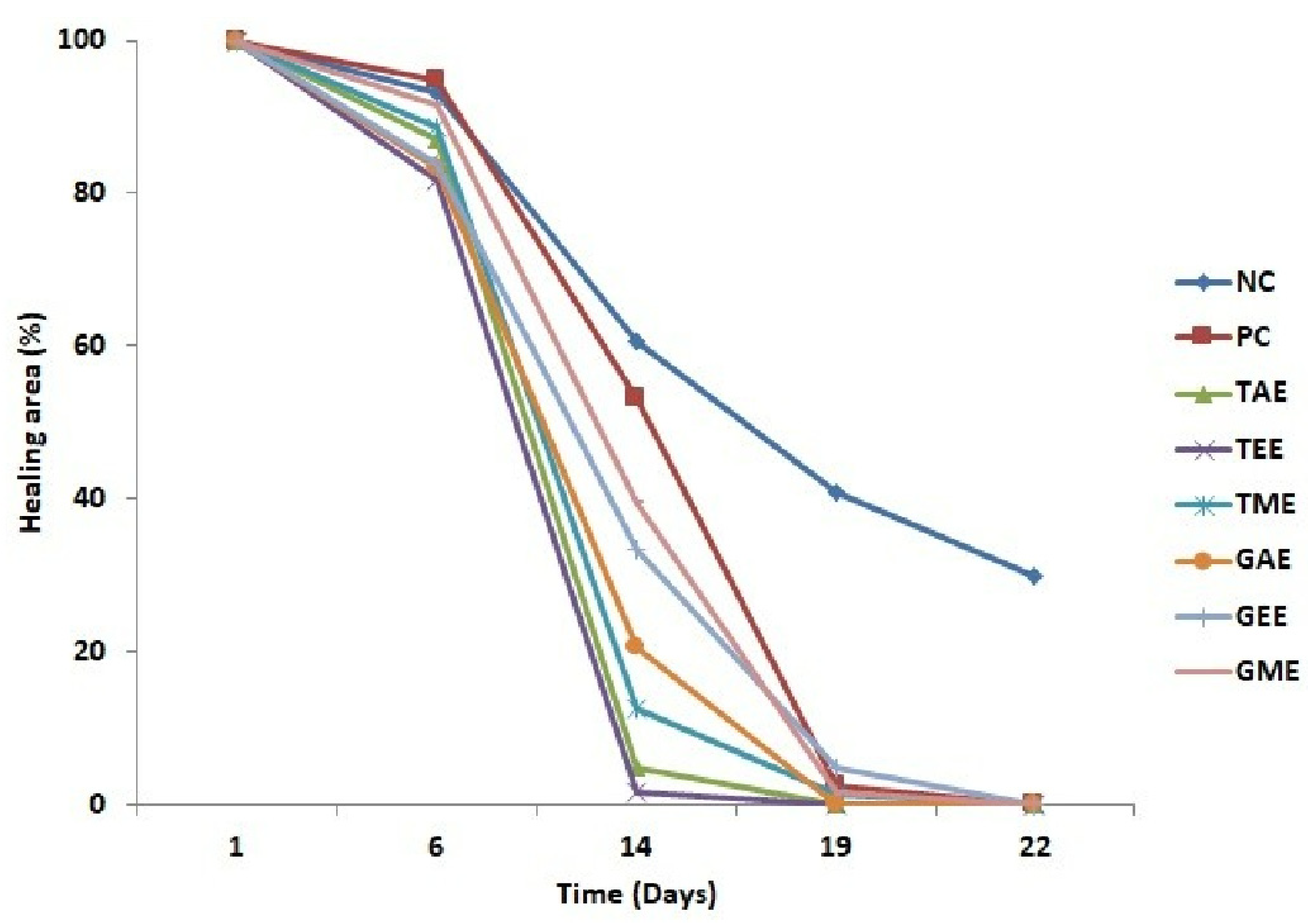
This part of the research was undertaken to evaluate the efficiency of aqueous, ethanolic, and methanolic turmeric and ginger extracts relative to wound healing in the rats’ dorsal region. Overall, there was a progressive reduction in the wound’s area over time in different treatments (Figure 4). On day 1, wound surfaces of the eight experimental group batches were homogeneous and showed the same signs of inflammation. However, the batch of animals treated with plant extracts showed significant reductions in their healing surfaces compared to the negative and positive control batches throughout the study. On day 14, a maximum reduction in wound area was observed in batches treated with aqueous and ethanolic turmeric extracts (4.7 and 1.6%, respectively). On the other hand, negative and positive control groups showed very low wound closure (60.6 and 53.3%, respectively). On day 19 of the trial, burned surface reduction percentages for all batches were attained and presented almost complete healing except for the negative control group (41%).
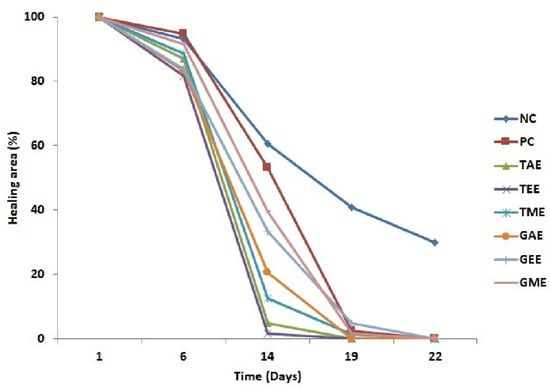
Figure 4.
Variation in mean burn areas (average ± standard deviation) of the eight batches throughout the experiment. Note: NC: Negative control; PC: positive control; TAE: turmeric aqueous extract; TEE: turmeric ethanolic extract; TME: turmeric methanolic extract; GAE: ginger aqueous extract; GEE: ginger ethanolic extract; GME: ginger methanolic extract.
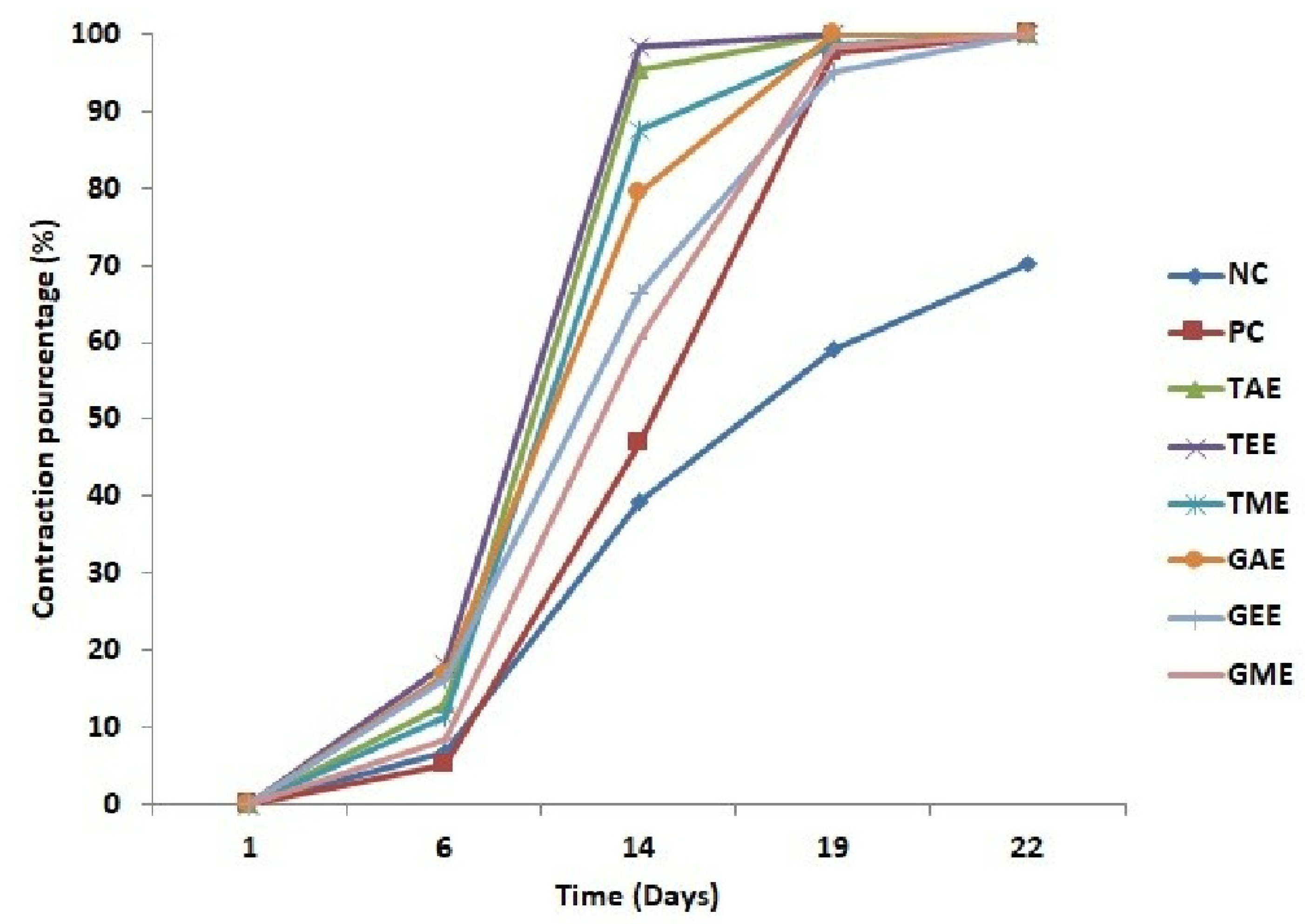
The evolution of wound contraction percentages induced in rats is presented in Figure 5. The obtained results show a progressive retraction of wounds that resulted in complete healing for all batches, but the negative control batch presented a lower percentage of contraction ranging from 6.87 to 39.37% from day 6 to 14 and from 59.08 to 70% from day 19 to 22, followed by the positive control batch that recorded a contraction percentage ranging from 5.17 to 46.74% from day 6 to 14 and from 97.41 to 100% from day 19 to 22. The healing process evolution of batches treated with plant extracts indicated that the highest percentage of wound contraction was obtained in wounds treated with turmeric extracts (aqueous, ethanolic, and methanolic) on day 14 at 95.26, 98.34, and 87.39%, respectively. On day 19, batches treated with aqueous and ethanolic turmeric extracts and aqueous ginger extracts exhibited complete healing (100%), followed by batches treated with methanolic turmeric extracts and ethanolic and methanolic ginger extracts ranging from 98.70, 95.16, and 98.27 to 100% on day 22, respectively. On this date (19th day), the negative control was only 60% cured.
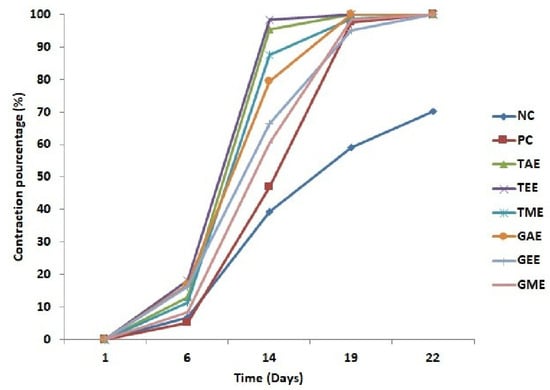
Figure 5.
Evolution of the average percentages of burn contraction (average ± standard deviation) in controls and treated batches. Note: NC: Negative control; PC: positive control; TAE: turmeric aqueous extract; TEE: turmeric ethanolic extract; TME: turmeric methanolic extract; GAE: ginger aqueous extract; GEE: ginger ethanolic extract; GME: ginger methanolic extract.
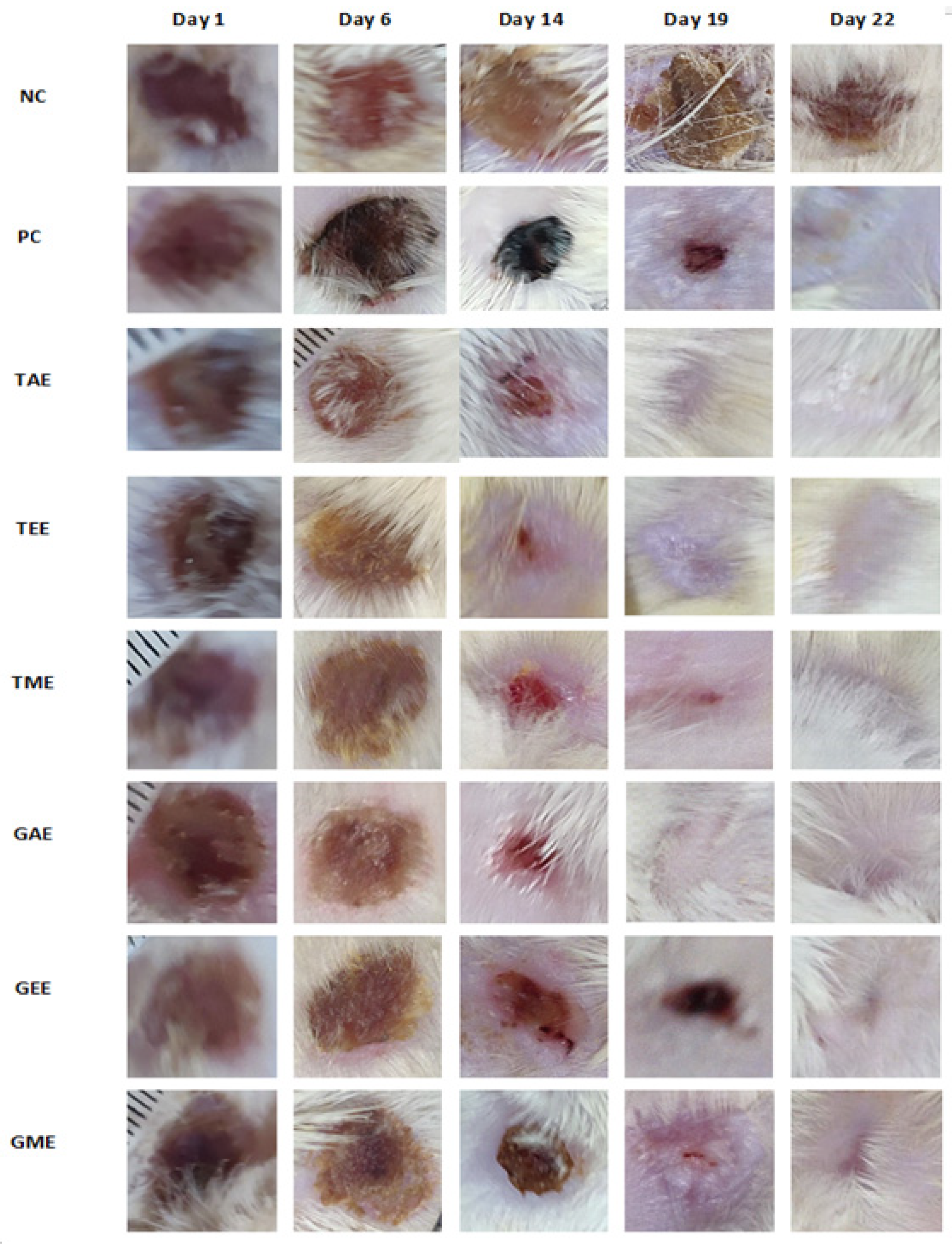
Wound healing image progressions during the application of turmeric and ginger extract creams, marketed ointment (H.E.C: “pomade Hémostatique et Cicatrisante”, in French), and negative control (no treatment) are shown in Figure 6. Regarding overall external appearance and based on the photos taken on days 1, 6, 14, 19, and 22 of the experiment, healing quality was better in turmeric and ginger extracts, which showed their preliminary effect from day 6 to day 14. Figure 6 clearly shows rapid and almost complete healing on day 19 of the application for groups treated with the plant extracts under study.
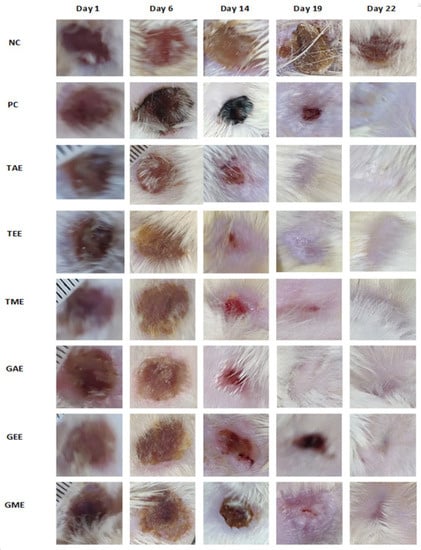
Figure 6.
Healing evolution activity of turmeric and ginger extracts in a deep second-degree burn. Note: NC: Negative control; PC: positive control; TAE: turmeric aqueous extract; TEE: turmeric ethanolic extract; TME: turmeric methanolic extract; GAE: ginger aqueous extract; GEE: ginger ethanolic extract; GME: ginger methanolic extract.
These results (Figure 3, Figure 4 and Figure 5) agree with those obtained using Sephadex G50 gel filtration, where it was found that the ethanolic extract exhibited the lowest phenolic polymer compounds. This lets us conclude that the anti-inflammatory activity is ensured by at least one polymeric or oligomeric molecule. These results raise several research questions focused on the effectiveness of the extract’s healing potential, which may be mainly due to these natural agents’ richness in phytochemical compounds, which, in turn, act as therapeutic agents for treating wounds and skin regeneration [32]. In a previous study carried out by our research group on the assessment of the wound healing potential of Moroccan Henna (Lawsonia inermis) aqueous extracts, as a source of phenolic compounds, similar results were found for a well-defined variety of Henna (Henne AQ-LI 3) [26]. On the other hand, Chelu et al. [33] reported similar results with respect to Aloe vera-based hydrogels where the total healing period was 21 days, a time which remains comparable to that found in the present study on ginger and turmeric and also in certain Henna varieties (Henna varieties AQ-LI 1 and AQ-LI 2), as already reported [26].
4. Discussion
Utilizing herbal products to treat human and animal ailments has been a practice since ancient times due to their wide availability and therapeutic efficacy [21]. Addressing various skin-related issues, including wound healing and inflammation, the current study focuses on evaluating the effects of creams containing extracts from turmeric and ginger on the enhancement of anti-inflammatory and wound healing processes.
To assess non-steroidal anti-inflammatory properties, the carrageenan-induced paw edema method was employed [34]. Carrageenan, a natural polysaccharide extracted from red algae, triggers local inflammatory responses that are characterized by five cardinal signs: swelling, redness, warmth, loss of function, and local hypersensitivity [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. The formation of edema comprises two phases: an immediate phase (1 h post-carrageenan injection) marked by mediator release such as serotonin and histamine and a subsequent phase (starting after 1 h and lasting 4 h) involving the release of prostaglandin-mediated pathways, including bradykinin [6,7].
Our trial unequivocally demonstrated that extracts from turmeric and ginger exhibit potent anti-inflammatory properties at a dosage of 100 mg/kg. Specifically, aqueous and ethanolic extracts of turmeric, along with methanolic extracts of ginger, remarkably attenuated carrageenan-induced edema. These natural agents’ ability to inhibit paw edema, as observed during experimentation, can be attributed to their richness in bioactive compounds, particularly phenolic compounds such as tannins and flavonoids. These phenolic compounds play a vital role in suppressing the synthesis of pro-inflammatory mediators [35].
Comparable results align with previous research, where hydroalcoholic extracts of Curcuma longa and Zingiber officinale, as well as their combination, exhibited potent and dose-dependent anti-inflammatory effects [36]. Earlier studies by Srinivasan et al. emphasized the anti-inflammatory prowess of curcuminoids derived from turmeric, demonstrating their efficacy in mitigating oxidative stress—a primary trigger of inflammation [37]. Furthermore, Prathamesh et al. highlighted the anti-inflammatory potential of aqueous and methanolic ginger extracts, which, at a dosage of 200 mg/kg, significantly reduced paw edema percentages compared to standard aspirin, a substance linked to potential gastric ulcer and hepatotoxicity issues [38].
In this study, anti-inflammatory activity was assessed before evaluating wound healing activity since the first phenomenon (anti-inflammatory) is one of the mechanisms involved in wound healing [9]. This anti-inflammatory activity is a very complex phenomenon that is supported by antioxidant activities strongly correlated with the contents of phenolic compounds, especially their flavonoids [39]. The involved second mechanism is the antimicrobial activity of phenolic compounds, which limits the proliferation of microorganisms that prevent successful healing [40]. The flavonoids in turn contribute differently because they improve capillary formation, which supplies wounds with the materials necessary for the structuring of tissues [41].
Phytochemical compounds, encompassing alkaloids, terpenes, steroids, flavonoids, and glycosides, function as therapeutic agents by combating free radicals and expediting the wound healing process [42]. These natural agents, present in plant extracts, effectively support proper wound healing processes without disrupting the sequence of events. However, it is important to note that subsequent bacterial infections and immunosuppression can potentially delay wound healing [43]. In the current study, we observed that control batches experienced delayed healing as their epidermal layers remained unhealed even after a 22-day duration. Conversely, turmeric and ginger extracts administered to surgically induced wounds demonstrated rapid and effective healing, as evidenced by the increase in wound contraction percentages. This augmentation in wound contraction can be attributed to the high concentration of collagen and the stabilization of collagen fibers present in the extracts [44]. Turmeric and ginger are enriched with antioxidants that effectively reduce free radicals and inflammation products, along with flavonoids that curtail lipid peroxidation and promote vascularization via capillarization [17,45,46].
Previous studies have consistently affirmed the wound healing potential of turmeric and ginger extracts. Research by [47] evaluated curcumin’s healing activity and highlighted its capacity to decrease inflammatory cell counts while promoting fibroblast proliferation. Another study conducted by [48] showcased the synergistic effect of curcumin conjugated with hyaluronic acid, exhibiting superior wound healing in comparison to individual curcumin or hyaluronic acid treatments. Furthermore, ginger-treated rat batches exhibited accelerated wound closure rates compared to negative control batches, which is attributed to increased collagen levels; improved fibroblast migration; and enhanced epithelial cell recruitment [49]. These results collectively underline the consistent positive impact of turmeric and ginger extracts on wound healing.
5. Conclusions
In conclusion, the present study establishes that rhizome extracts of Curcuma longa L. and Zingiber officinale possess both anti-inflammatory and wound healing properties. This confirms the interest in using plants in traditional medicine to treat various health problems. This should encourage pharmaceutical industries to think about making remedies based on these spices and contribute to the socio-economic and environmentally sustainable commercial exploitation of these natural products. The healing effects are due to bioactive molecules in the studied spices, such as flavonoids, phenolic compounds, vitamin C, and carotenoids, which increase antioxidant activity, collagen deposition, hydroxyproline formation, and antibacterial activity. Therefore, it can be concluded that the skin healing potential of Curcuma longa L. and Zingiber officinale could benefit therapeutic and cosmetic practices.
Furthermore, the use of phytochemicals as therapeutic agents presents certain difficulties, such as low solubility and bioavailability, and a lack of clinical research on the long-term effects of these medicinal agents. Thus, additional testing should be directed at potential healing agents to better understand their specific mechanisms of action and subsequent physiological effects. This can help develop effective formulations of these phytochemicals in emerging wound healing knowledge, such as micelles, liposomes, nanoparticles, and phospholipid complexes.
Author Contributions
Conceptualization, F.E. and R.C.; methodology, L.E.G. and S.M.R.; validation, R.C. and A.Z.; investigation, C.B. and S.M.R.; resources, C.B. and N.C.; writing—original draft preparation, C.B.; writing—review and editing, F.E., A.Z. and J.M.R.; supervision, F.E. and S.M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by USMBA University (Morocco) and the facilities of Functional Ecology and Environment Engineering (FEEE) and Human Pathology, Biomedicine and Environment (HPBE) Laboratories.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of the Fes Hospital-University Ethics Committee (CEHUF) protocol (code 005, approval date 2021) for studies involving animals.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available from the corresponding author upon request.
Acknowledgments
The authors would like to acknowledge the COST Action 18101 SOURDOMICS—Sourdough biotechnology network towards novel, healthier and sustainable food and bioprocesses (https://sourdomics.com/; https://www.cost.eu/actions/CA18101/, accessed on 31 July 2023), where the author A.Z. is a member of working groups 2, 3, 4, 5, 7, and 8, and the author J.M.R. is the Chair and Grant Holder Scientific Representative and is supported by COST (European Co-operation in Science and Technology) (https://www.cost.eu/, accessed on 31 July 2023). COST is a funding agency for research and innovation networks. Author J.M.R. also acknowledges the Universidade Católica Portuguesa, CBQF—Centro de Biotecnologia e Química Fina—Laboratório Associado, Escola Superior de Biotecnologia, Porto, Portugal, as well as the support provided by LA/P/0045/2020 (ALiCE) and UIDB/00511/2020-UIDP/00511/2020 (LEPABE), which are funded by national funds via FCT/MCTES (PIDDAC).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Najmi, A.; Javed, S.A.; Al Bratty, M.; Alhazmi, H.A. Modern Approaches in the Discovery and Development of Plant-Based Natural Products and Their Analogues as Potential Therapeutic Agents. Molecules 2022, 27, 349. [Google Scholar] [CrossRef] [PubMed]
- Thangapazham, R.L.; Sharad, S.; Maheshwari, R.K. Phytochemicals in Wound Healing. Adv. Wound Care 2014, 5, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Trinh, X.; Long, N.; Van Anh, L.T.; Nga, P.T.; Giang, N.N. A Comprehensive Review of Natural Compounds for Wound Healing: Targeting Bioactivity Perspective. Int. J. Mol. Sci. 2022, 23, 9573. [Google Scholar] [CrossRef] [PubMed]
- Loggenberg, S.R.; Twilley, D.; De Canha, M.N.; Meyer, D.; Mabena, E.C.; Lall, N. Evaluation of Wound Healing and Antibacterial Potential of Greyia Radlkoferi Szyszyl. Ethanolic Leaf Extract. Front. Pharmacol. 2022, 13, 806285. [Google Scholar] [CrossRef] [PubMed]
- Qaswal, A.B. A Theoretical Study to Explain the Referred Pain Phenomenon and Its Characteristics via Quantum Tunneling of Potassium Ions Through the Channels of Neuronal Membrane. NeuroQuantology 2019, 17, 43–52. [Google Scholar] [CrossRef]
- Bouyahya, A.; Guaouguaou, F.; El, N.; El, N.; Balahbib, A.; El-shazly, M.; Bakri, Y. Anti-Inflammatory and Analgesic Properties of Moroccan Medicinal Plants: Phytochemistry, in Vitro and in Vivo Investigations, Mechanism Insights, Clinical Evidences and Perspectives. J. Pharm. Anal. 2022, 12, 35–57. [Google Scholar] [CrossRef]
- Ratan, Z.A.; Jeong, D.; Sung, N.Y.; Shim, Y.Y.; Reaney, M.J.T.; Yi, Y.-S.; Cho, J.Y. LOMIX, a Mixture of Flaxseed Linusorbs, Exerts Anti-Inflammatory Effects through Src and Syk in the NF-κB Pathway. Biomolecules 2020, 10, 859. [Google Scholar] [CrossRef]
- Schilrreff, P.; Alexiev, U. Chronic Inflammation in Non-Healing Skin Wounds and Promising Natural Bioactive Compounds Treatment. Int. J. Mol. Sci. 2022, 23, 4928. [Google Scholar] [CrossRef]
- Criollo-Mendoza, M.S.; Contreras-Angulo, L.A.; Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Jiménez-Ortega, L.A.; Heredia, J.B. Wound Healing Properties of Natural Products: Mechanisms of Action. Molecules 2023, 28, 598. [Google Scholar] [CrossRef]
- Zhou, F.; Hong, Y.; Liang, R.; Zhang, X.; Liao, Y. Rapid Printing of Bio-Inspired 3D Tissue Constructs for Skin Regeneration. Biomaterials 2020, 258, 120287. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Okur, M.E.; Karantas, I.D.; Siafaka, P.I. Recent Trends on Wound Management: New Therapeutic Choices Based on Polymeric Carriers. Asian J. Pharm. Sci. 2020, 15, 661–684. [Google Scholar] [CrossRef] [PubMed]
- Karppinen, S.; Heljasvaara, R.; Gullberg, D.; Tasanen, K.; Pihlajaniemi, T. Toward Understanding Scarless Skin Wound Healing and Pathological Scarring [Version 1; Peer Review: 2 Approved]. F1000Research 2019, 8, 787. [Google Scholar] [CrossRef]
- Gonzalez, A.C.d.O.; Costa, T.F.; de Araújo Andrade, Z.; Medrado, A.R.A.P. Wound Healing—A Literature Review *. Bras Dermatol 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Muflihah, Y.M.; Gollavelli, G.; Ling, Y.C. Correlation Study of Antioxidant Activity with Phenolic and Flavonoid Compounds in 12 Indonesian Indigenous Herbs. Antioxidants 2021, 10, 1530. [Google Scholar] [CrossRef]
- Vitale, S.; Colanero, S.; Placidi, M.; Di Emidio, G.; Tatone, C.; Amicarelli, F.; D’Alessandro, A.M. Phytochemistry and Biological Activity of Medicinal Plants in Wound Healing: An Overview of Current Research. Molecules 2022, 27, 3566. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, Z.; Nadeem, M.T.; Arshad, M.U.; Saeed, F.; Ahmed, M.H.; Bader, H.; Ain, U.; Anjum, F.M.; Hussain, S.; Mushtaq, Z.; et al. Exploring the Biochemical and Antioxidant Potential of Ginger (Adric) and Turmeric (Haldi). Int. J. Food Prop. 2019, 22, 1642–1651. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92–98. [Google Scholar] [CrossRef]
- Reeta, V.; Kalia, S. Turmeric: A Review of Its’ Effects on Human Health. J. Med. Plants Stud. 2022, 10, 61–63. [Google Scholar]
- Schaller, T.; Schieberle, P. Comparison of the Key Aroma Compounds in Fresh, Raw Ginger ( Zingiber o Ffi Cinale Roscoe) from China and Roasted Ginger by Application of Aroma Extract Dilution Analysis. J. Agric. Food Chem. 2020, 68, 15292–15300. [Google Scholar] [CrossRef]
- Khan, B.A.; Ullah, S.; Khan, M.K.; Uzair, B.; Menaa, F.; Braga, V.A. Fabrication, Physical Characterizations, and In Vitro, In Vivo Evaluation of Ginger Extract-Loaded Gelatin/Poly(Vinyl Alcohol) Hydrogel Films Against Burn Wound Healing in Animal Model. AAPS PharmSciTech 2020, 21, 323. [Google Scholar] [CrossRef]
- Atanassova, M.; Georgieva, S.; Ivancheva, K. Total Phenolic and Total Flavonoid Contents, Antioxidant Capacity and Biological Contaminants in Medicinal Herbs. J. Univ. Chem. Technol. Metall. 2011, 46, 81–88. Available online: https://journal.uctm.edu/node/j2011-1/12_Maria_Atanasova.pdf (accessed on 9 September 2023).
- Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Exotic Fruits as a Source of Important Phytochemicals: Improving the Traditional Use of Rosa Canina Fruits in Portugal. Food Res. Int. 2011, 44, 2233–2236. [Google Scholar] [CrossRef]
- Lee, H.S.; Castle, W.S. Seasonal Changes of Carotenoid Pigments and Color in Hamlin, Earlygold, and Budd Blood Orange Juices. J. Agric. Food Chem. 2001, 49, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Ahodegnon, D.K.; Gnansounou, M.; Bogninou, R.G.S.; Kanfon, R.; Chabi, B.; Dossa, P.C.A.; Anago, E.A.; Ahoussi, E.; Wotto, V.; Sohounhloue, D.C.K.; et al. Biochemical Profile and Antioxidant Activity of Parkia Biglobosa and Tamarindus Indica Fruits Acclimated in Benin. Int. J. Adv. Res. 2018, 6, 702–711. [Google Scholar] [CrossRef]
- El Massoudi, S.; Zinedine, A.; Rocha, J.M.; Benidir, M.; Najjari, I.; El Ghadraoui, L.; Benjelloun, M.; Errachidi, F. Phenolic Composition and Wound Healing Potential Assessment of Moroccan Henna (Lawsonia Inermis) Aqueous Extracts. Cosmetics 2023, 10, 92. [Google Scholar] [CrossRef]
- Ouda, A.N.; Fatiha, M.; Sadia, M.; Zohra, S.F.; Noureddine, D. In Vivo Anti-Inflammatory Activity of Aqueous Extract of Carthamus Caeruleus L Rhizome Against Carrageenan-Induced Inflammation in Mice. Jordan J. Biol. Sci. 2021, 14, 529–535. [Google Scholar] [CrossRef]
- Mani, R.; Romanelli, M.; Shukla, V. (Eds.) Measurements in Wound Healing; Springer: London, UK, 2013; ISBN 978-1-4471-2986-8. [Google Scholar]
- Bhaskar, A.; Nithya, V. Evaluation of the Wound-Healing Activity of Hibiscus Rosa Sinensis L (Malvaceae) in Wistar Albino Rats. Indian J. Pharmacol. 2012, 44, 694–698. [Google Scholar] [CrossRef]
- Hoşnuter, M.; Gürel, A.; Babucçu, O.; Armutcu, F.; Kargi, E.; Işikdemir, A. The Effect of CAPE on Lipid Peroxidation and Nitric Oxide Levels in the Plasma of Rats Following Thermal Injury. Burns 2004, 30, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Dahmani, S.; Chabir, R.; Errachidi, F.; Berrada, W.; Lansari, H.; Benidir, M.; El Ghadraoui, L.; Bour, A. Evaluation of In Vivo Wound Healing Activity of Moroccan Citrus Reticulata Peel Extract. Clin. Phytoscience 2020, 6, 121–125. [Google Scholar] [CrossRef]
- Asif, A.H.; Mulla, S.M.; Shariff, A.; Sreeharsha, N.; Meravanige, G.; Shiroorkar, P.N.; Mohammed, S.; Asdaq, B.; Anwer, K.; Roopashree, T.S.; et al. Exploring the Topical Gel of Thespesia Populnea Leaf Extract for In Vivo Wound Healing Efficacy. Pharmacogn. Mag. 2022, 13, 519–523. [Google Scholar]
- Chelu, M.; Musuc, A.M.; Popa, M.; Moreno, J.C. Aloe Vera-Based Hydrogels for Wound Healing: Properties and Therapeutic Effects. Gels 2023, 9, 539. [Google Scholar] [CrossRef]
- Jisha, N.; Vysakh, A.; Vijeesh, V.; Latha, M.S. Anti-Inflammatory Efficacy of Methanolic Extract of Muntingia calabura L. Leaves in Carrageenan Induced Paw Edema Model. Pathophysiology 2019, 8, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Bagad, A.S.; Joseph, J.A.; Bhaskaran, N.; Agarwal, A. Comparative Evaluation of Anti-Inflammatory Activity of Curcuminoids, Turmerones, and Aqueous Extract of Curcuma longa. Adv. Pharmacol. Sci. 2013, 2013, 805756. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K. Anti-Inflammatory Influences of Culinary Spices and Their Bioactives Anti-Inflammatory Influences of Culinary Spices and Their. Food Rev. Int. 2020, 38, 1–17. [Google Scholar] [CrossRef]
- Pakale, P.V.; Khanwelkar, C.C.; Jadhav, S.A. Study of Anti-Inflammatory Activity of Aqueous and Methanolic Extracts of Dry Powder of Zingiber officinale (SUNTH) in Wistar Rats. Int. J. Health Sci. 2022, 6, 3535–3542. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Sandhiutami, N.M.D.; Fahleni, F.; Miftahurrohmah, N.; Widhiyasari, N.K.A.; Azalia, A.; Amalia, I. Enhanced Wound Healing Effect of Areca catechu L. Ointment via Antibacterial Activity and Anti-Inflammatory Process at Grade IIA Burns in Rats. J. HerbMed Pharmacol. 2023, 12, 388–398. [Google Scholar] [CrossRef]
- Zulkefli, N.; Che Zahari, C.N.M.; Sayuti, N.H.; Kamarudin, A.A.; Saad, N.; Hamezah, H.S.; Bunawan, H.; Baharum, S.N.; Mediani, A.; Ahmed, Q.U.; et al. Flavonoids as Potential Wound-Healing Molecules: Emphasis on Pathways Perspective. Int. J. Mol. Sci. 2023, 24, 4607. [Google Scholar] [CrossRef]
- Paulraj, M.S.; Rajkumar, S.R.J. Phytochemicals as a Potential Source for Anti- Microbial, Anti-Oxidant and Wound Healing—A Review. MOJ Bioorganic Org. Chem. Rev. 2018, 2, 61–70. [Google Scholar] [CrossRef]
- Epa, C.; Itou, R.E.; Ossibi, A.E.; Attibayeba, O.P.R.; Abena, A.A. Effet Anti-Inflammatoire et Cicatrisant Des Extraits Aqueux et Éthanolique Des Écorces Du Tronc de Buchholzia Coriacea Engl. (Capparidaceae). J. Appl. Biosci. 2015, 94, 8858. [Google Scholar] [CrossRef]
- Nagar, H.K.; Srivastava, A.K.; Srivastava, R.; Kurmi, M.L.; Chandel, H.S.; Ranawat, M.S. Pharmacological Investigation of the Wound Healing Activity of Cestrum nocturnum (L.) Ointment in Wistar Albino Rats. J. Pharm. 2016, 94, 8858. [Google Scholar] [CrossRef] [PubMed]
- Deldar, Y.; Pilehvar-soltanahmadi, Y.; Dadashpour, M.; Saheb, M.; Rahmati-yamchi, M.; Zarghami, N. An in Vitro Examination of the Antioxidant, Cytoprotective and Anti-Inflammatory Properties of Chrysin-Loaded Nanofibrous Mats for Potential Wound Healing Applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Mishra, S.R.; Soni, H. Efficacy of Hydrogel Containing Rutin in Wound Healing. EAS J. Pharm. Pharmacol. 2021, 3, 161–167. [Google Scholar] [CrossRef]
- Seyhan, N. Evaluation of the Healing Effects of Hypericum Perforatum and Curcumin on Burn Wounds in Rats. Evid.-Based Complement. Altern. Med. 2020, 2020, 6462956. [Google Scholar] [CrossRef]
- Sharma, M.; Sahu, K.; Singh, S.P.; Jain, B. Wound Healing Activity of Curcumin Conjugated to Hyaluronic Acid: In Vitro and In Vivo Evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1009–1017. [Google Scholar] [CrossRef]
- Mohamed, A.H.B.; Osman, A.A.F. Antibacterial and Wound Healing Potential of Ethanolic Extract of Zingiber officinale in Albino Rats Antibacterial and Wound Healing Potential of Ethanolic Extract of Zingiber officinale in Albino Rats. J. Dis. Med. Plants 2021, 3, 1–6. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).