Enhancing Skin Anti-Aging through Healthy Lifestyle Factors
Abstract
1. Introduction
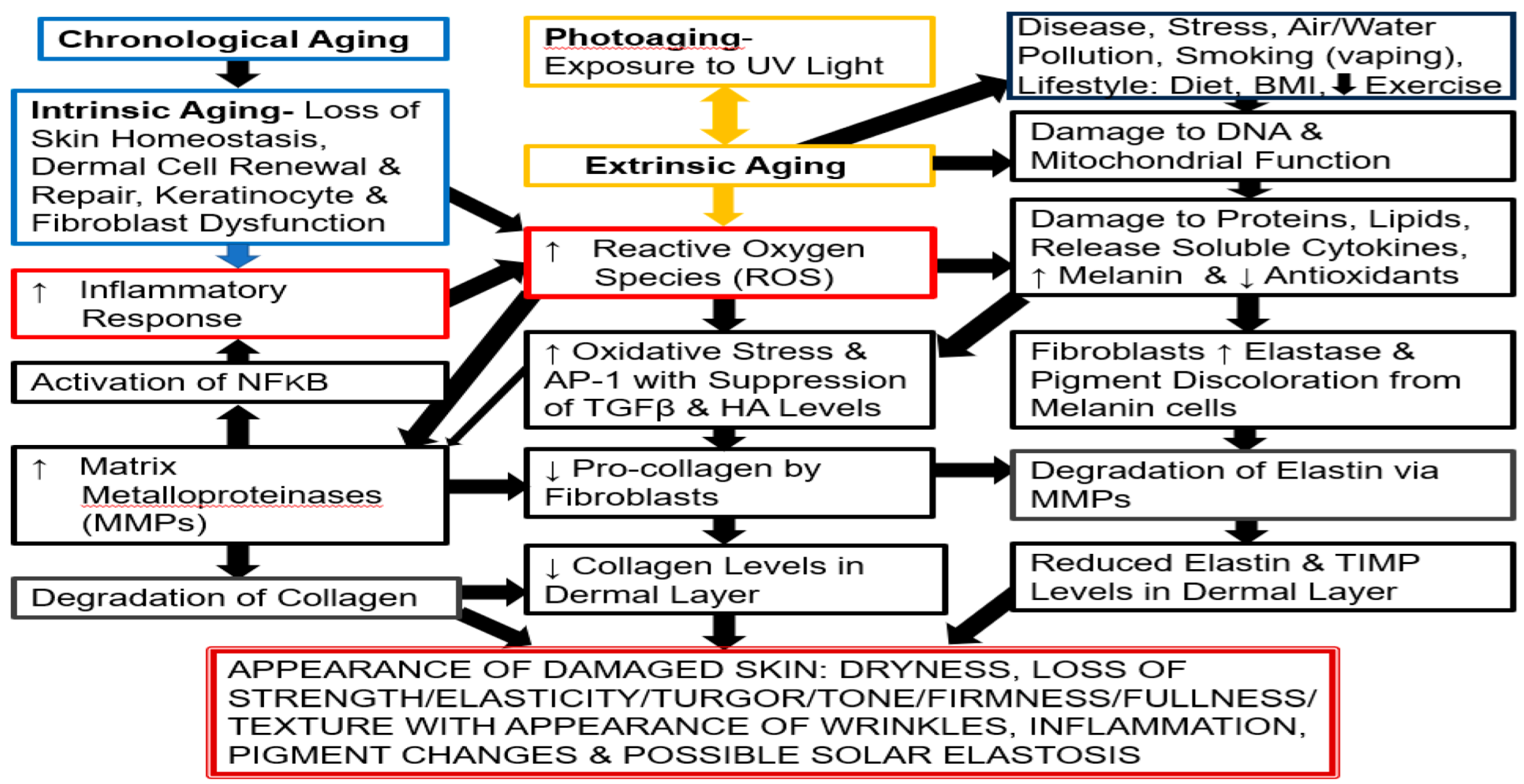
2. Skin Aging
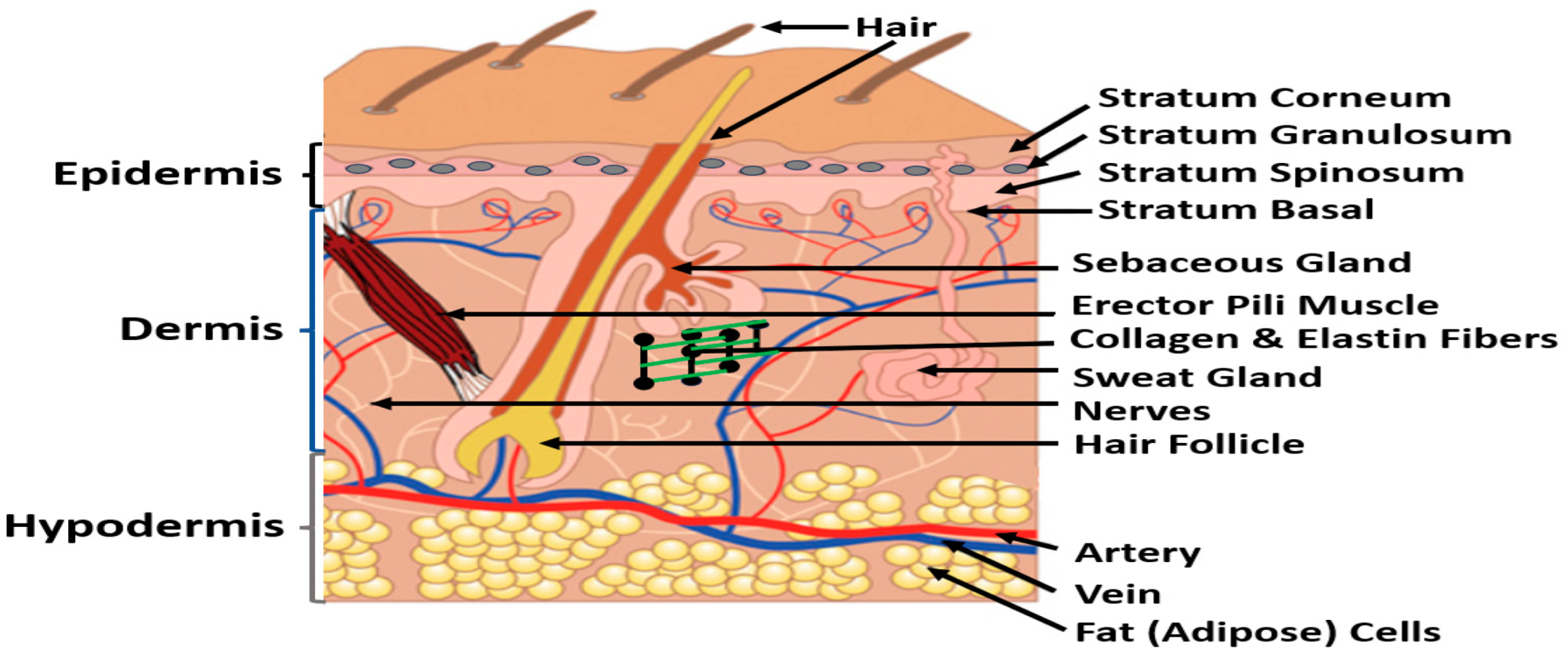
2.1. Skin
2.2. Skin: Chronological Aging and Photoaging Aging
2.2.1. Extrinsic Skin Aging
2.2.2. Intrinsic Skin Aging
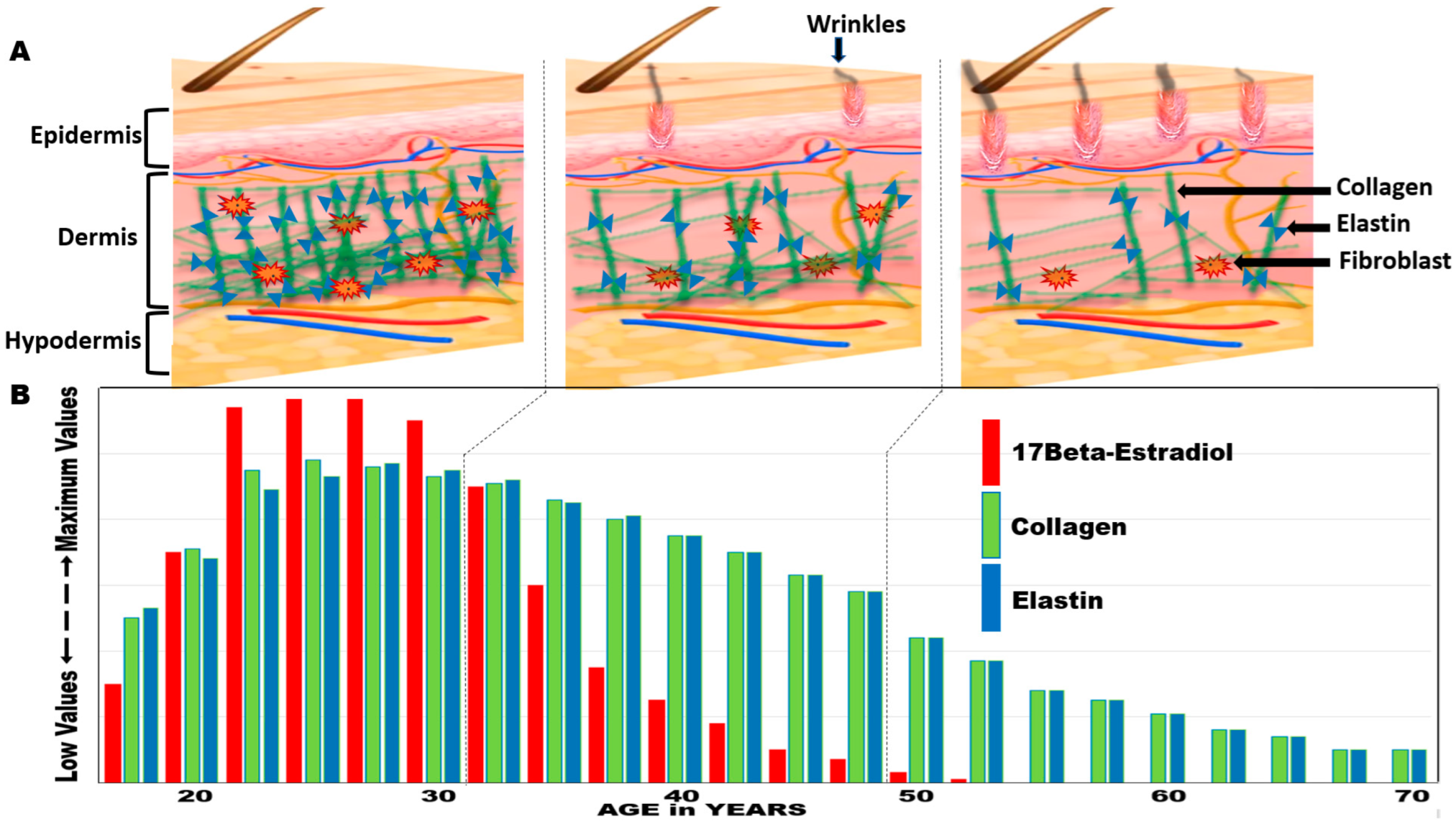
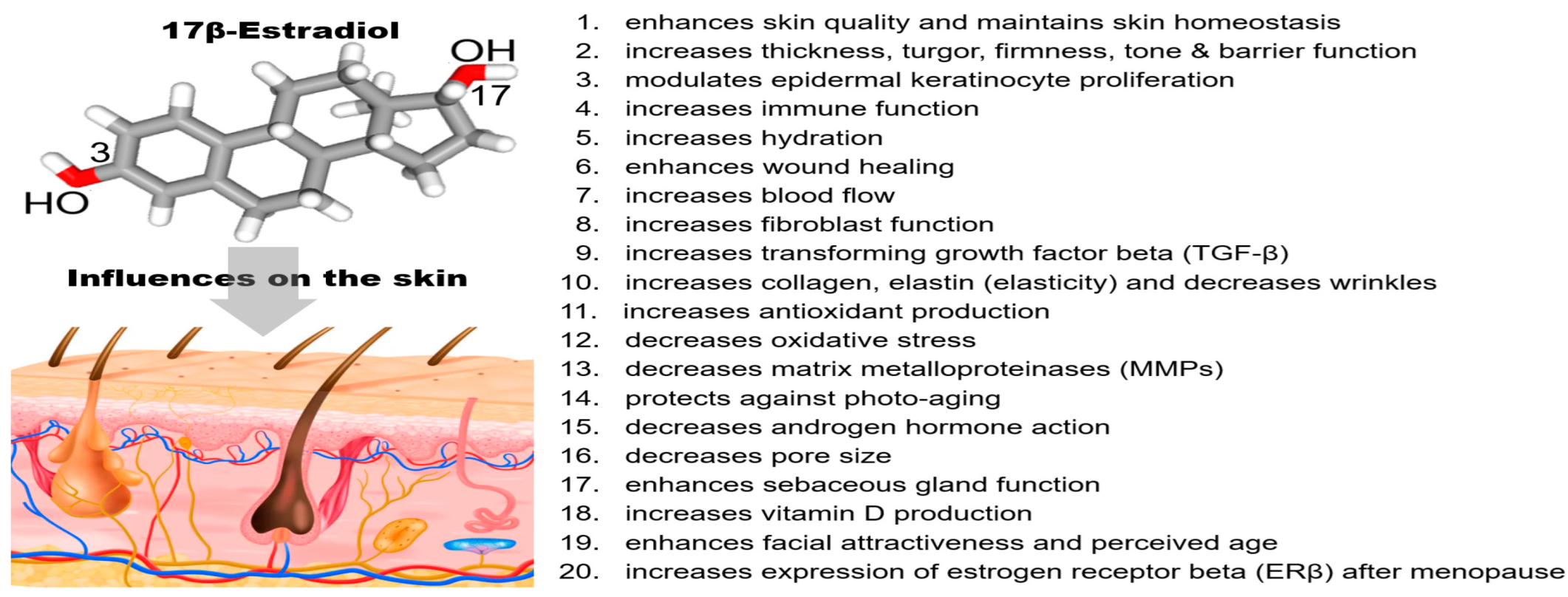
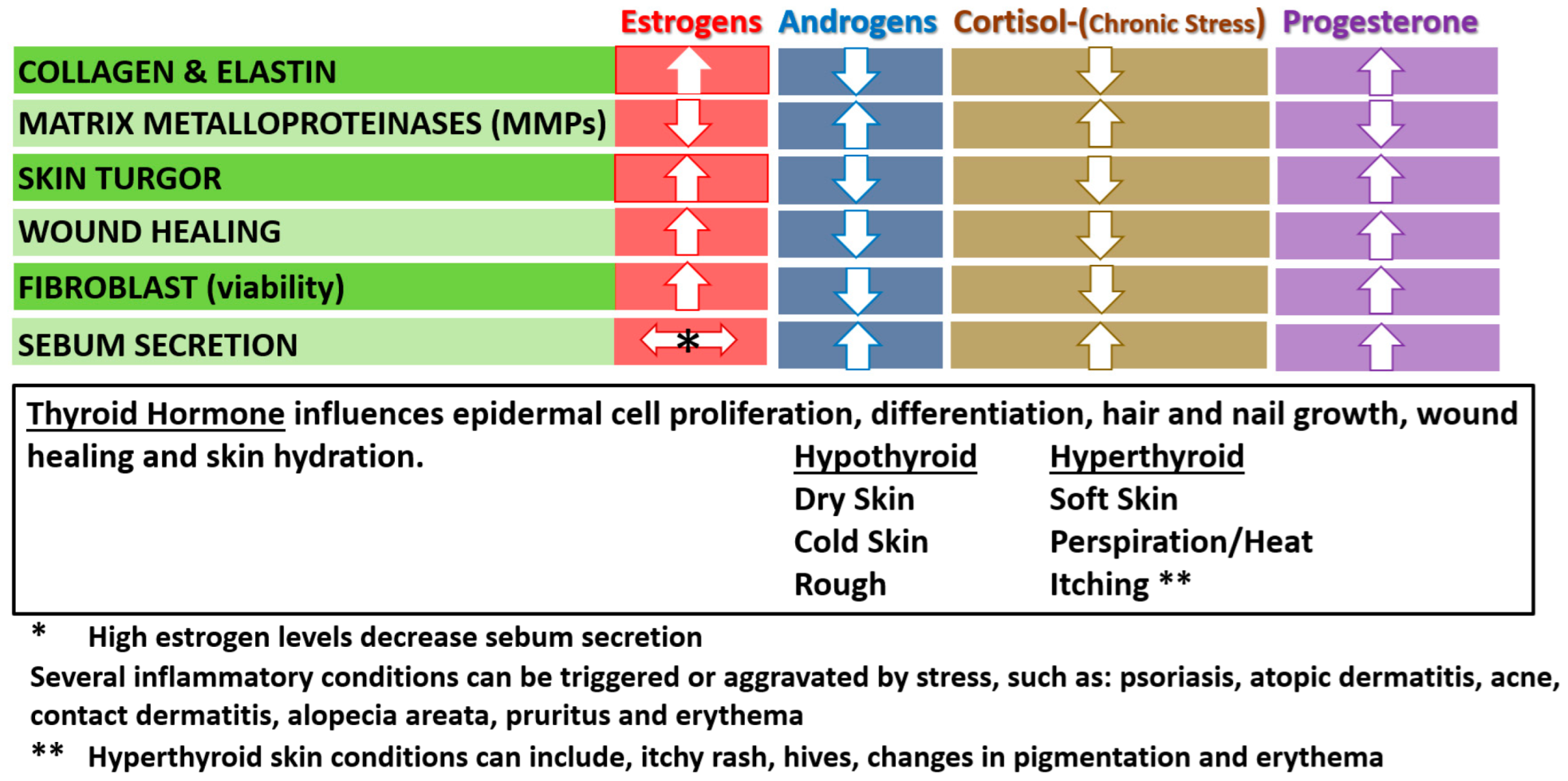
2.3. Hormonal Benefits/Changes with Aging (Estrogen in Women)
2.4. Other Hormonal Influences on Skin
3. Health Lifestyle Factors
3.1. Aging
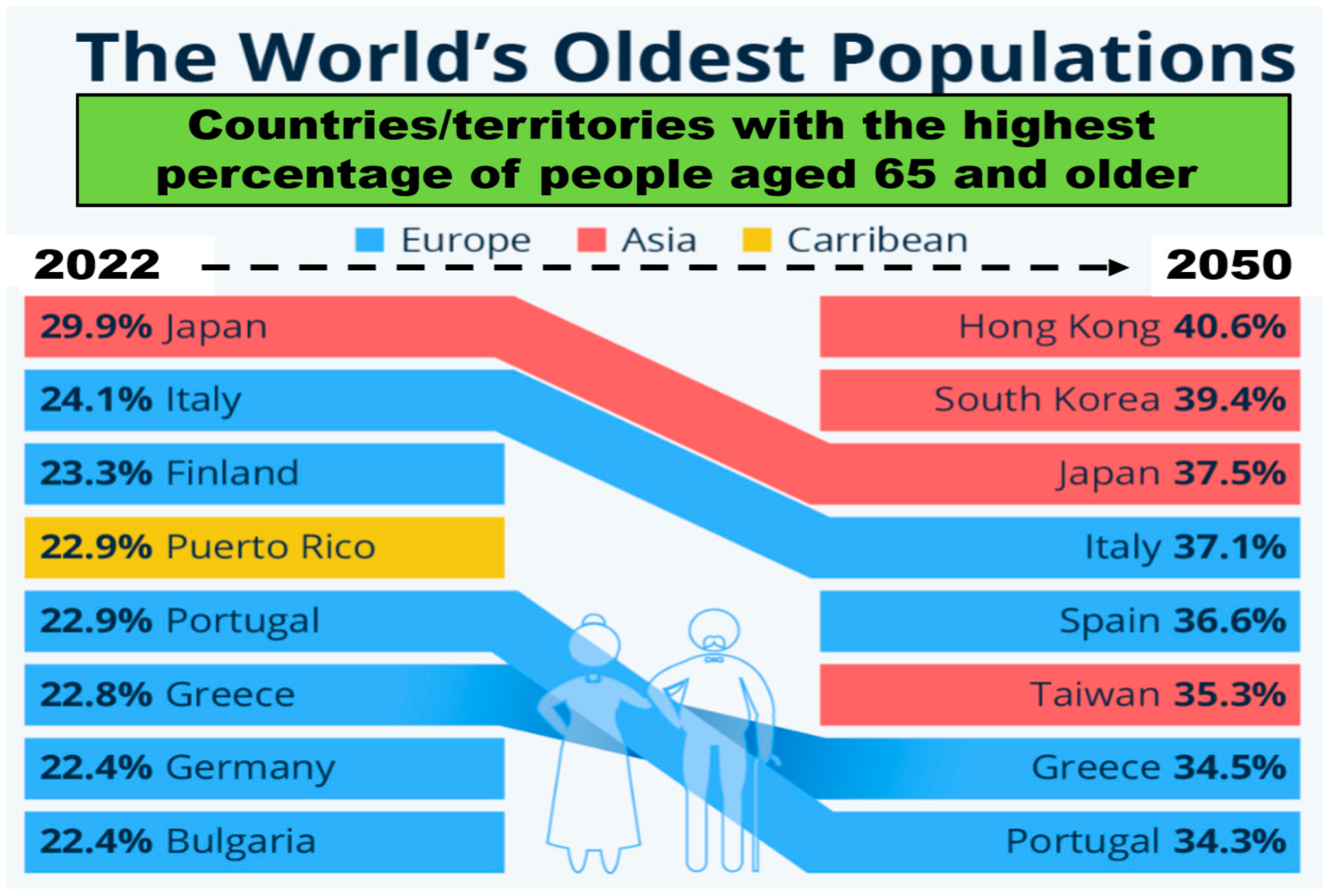
3.2. Population Aging around the World
3.3. Aging Related-Disorders
3.4. Aging and the Global Burden of Cancer Attributable to Lifestyle Risk Factors
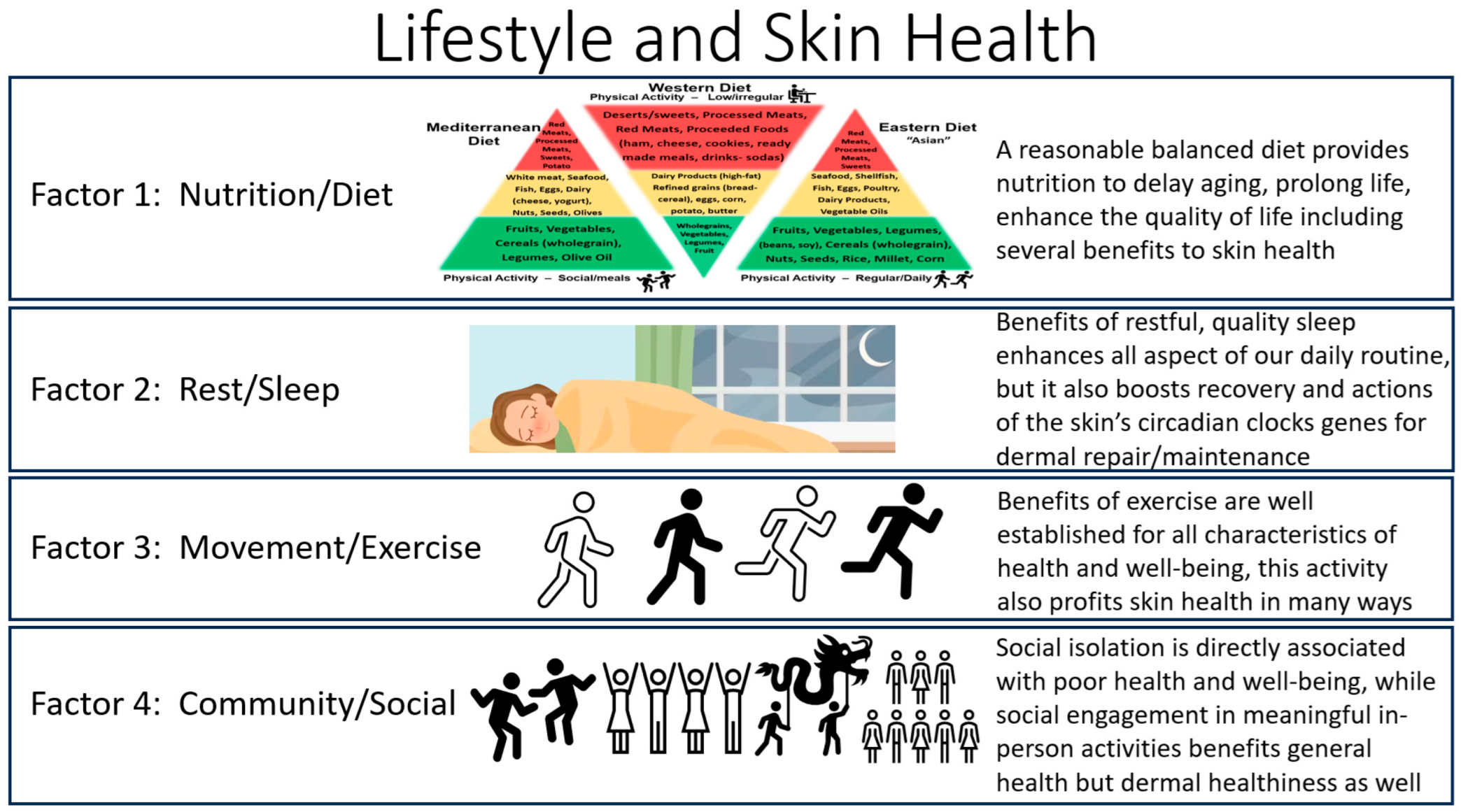
4. Factors of Lifestyle Health
Different Types of Lifestyle Health
5. Four Factors of Lifestyle Health
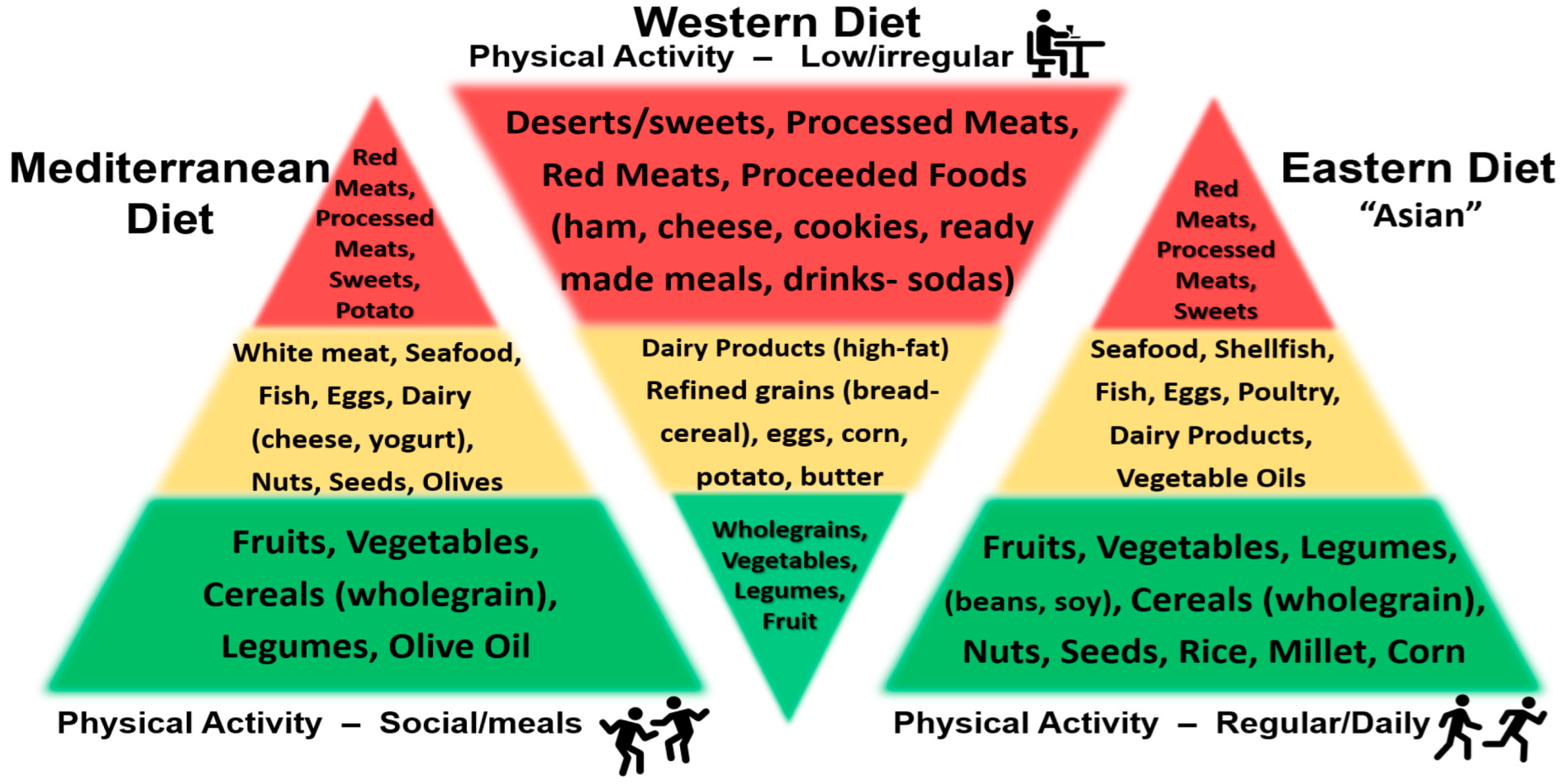
5.1. Factor 1: Lifestyle Health—Nutrition, Diet and Skin Health
5.2. Nutrition-Diet Lifestyle Benefits
5.3. Nutrition, Diet and Skin Health
5.4. Lifestyle/Daily Habits- Negative Impact on Skin Health [AGEs, Alcohol, Smoking, High Fat, Body Mass Index (BMI)]
6. Factor 2: Lifestyle Health—Rest, Relax, Recover (RRR) and Manage Stressors
6.1. Sleep and Skin Health
6.2. Skin Health and Circadian Factors
6.3. Skin Health and Exposome Factors (Stressors)
7. Factor 3: Lifestyle Health—Physical Exercise and Skin Health
8. Factor 4: Lifestyle Skin Health—Social/Community and Skin Health
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krutmann, J.; Bouloc, A.; Sore, G.; Bernard, B.A.; Passerson, T. The skin aging exposome. J. Dermatol. Sci. 2017, 85, 152–161. [Google Scholar] [CrossRef]
- Lephart, E.D.; Naftolin, F. Factors influencing skin aging and the important role of estrogens and selective estrogen receptor modulators (SERMs). Clin. Cosmet. Investig. Dermatol. 2022, 15, 1695–1709. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Su, W.; Wang, F. Skin aging: A progressive, multi-factorial condition demanding and integrated, multilayer-targeted remedy. Clin. Cosmet. Investig. Dermatol. 2023, 16, 1215–1229. [Google Scholar] [CrossRef] [PubMed]
- Rippe, J.M. Lifestyle Medicine: The health promoting power of daily habits and practices. Am. J. Lifestyle Med. 2018, 12, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Rippe, J.M. Lifestyle Medicine, 3rd ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2019; pp. 1–1404. [Google Scholar]
- US Department of Health and Human Services; US Department of Agriculture. 2020–2025 Dietary Guidelines for Americans, 9th ed.; US Department of Health and Human Services: Washington, DC, USA; US Department of Agriculture: Washington, DC, USA, 2020. Available online: https://www.dietaryguidelines.gov/sites/default/files/2021-03/Dietary_Guidelines_for_Americans-2020-2025.pdf (accessed on 31 July 2023).
- Abe, M.; Abe, H. Lifestyle medicine- An evidence based approach to nutrition, sleep, physical activity, and stress management on health and chronic illness. Pers. Med. Universe 2019, 8, 3–9. [Google Scholar] [CrossRef]
- Cena, H.; Calder, P.C. Defining a healthy diet: Evidence for the role of contemporary dietary patterns in health and disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [PubMed]
- Santos, L. The impact of nutrition and lifestyle modification on health. Eur. J. Intern. Med. 2022, 97, 18–25. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Research (NIHR). Healthy Lifestyles Increase Life Expectancy in People with Multiple Conditions (Multimorbidity) by as Much as in Other Groups. Public Health 2021. Available online: https://evidence.nihr.ac.uk/alert/healthy-lifestyles-increase-life-expectancy-in-people-with-multiple-conditions-multimorbidity-by-as-much-as-in-other-groups/#:~:text=The%20healthier%20the%20lifestyle%2C%20the,compared%20with%20the%20unhealthiest%20lifestyles (accessed on 31 July 2023).
- Wong, V.W.-H.; Ho, F.Y.-Y.; Wong, Y.S.-H.; Chung, F.-K.; Yeung, W.-F.; Ng, C.H.; Sarris, J. Efficacy of lifestyle medicine on sleep quality: A meta-analysis of randomized controlled trails. J. Affect. Disord. 2023, 330, 125–138. [Google Scholar] [CrossRef]
- Wong, V.W.-H.; Ho, F.Y.-Y.; Shi, N.-G.; Sarris, J.; Ng, C.H.; Tam, O.K.-Y. Lifestyle medicine for anxiety symptoms: A meta-analysis of randomized controlled trials. J. Affect. Disord. 2022, 10, 354–368. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, N.; Wang, S.; Hong, J.; Li, F.; Guo, H.; Lv, Z.; Wang, Y.; Wang, W.; Wu, W. Circadian rhythms and sleep quality among undergraduate students in China: The mediating role of health-promoting lifestyle behaviours. J. Affect. Disord. 2023, 333, 225–232. [Google Scholar] [CrossRef]
- Murad, H. Cultural stress: The undiagnosed epidemic of our time. J. Integr. Med. 2023. ahead-of-print. [Google Scholar] [CrossRef] [PubMed]
- Yanping, L.; Pan, A.; Wang, A.A.; Liu, Z.; Dhana, K.; Franco, O.H.; Kaptoge, S.; Di Angelantonio, E.; Stampfer, M.; Willett, W.C.; et al. Impact of health lifestyle factors on life expectancies in the US population. Circulation 2018, 138, 345–355. [Google Scholar]
- Hu, P.; Zheng, M.; Huang, J.; Fan, H.-Y.; Fan, C.-J.; Ruan, H.-H.; Yuan, Y.-S.; Zhao, W.; Wang, H.H.X.; Deng, H.; et al. Effect of healthy lifestyle index and lifestyle patterns on the risk of mortality: A community-based cohort study. Front. Med. 2022, 9, 920760. [Google Scholar] [CrossRef]
- Rozanski, A.; Blumenthal, J.A.; Hinderliter, A.L.; Cole, S.; Lavie, G.J. Cardiology and lifestyle medicine. Prog. Cardiovasc. Dis. 2023, 77, 4–13. [Google Scholar] [CrossRef]
- Cudjoe, T.K.M.; Roth, D.L.; Szanton, S.L.; Wolff, J.L.; Boyd, C.M.; Thorpe, R.J. The epidemiology of social isolation: National health and aging trends study. J. Gerontol. B Psycol. Sci. Soc. Sci. 2020, 75, 107–113. [Google Scholar] [CrossRef]
- Ding, Z.; Leung, P.-Y.; Lee, T.-I.; Chan, A.S. Effectiveness of lifestyle medicine on cognitive functions in mild cognitive impairments and dementia: A systematic review of randomized controlled trails. Ageing Res. Rev. 2023, 86, 101886. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.X.; Sun, L.; Wang, L.; Khoo, A.L.Y.; Lim, K.X.; Lu, G.; Yu, L.; Li, C.; Maier, A.B.; Feng, L. The role of lifestyle factors in cognitive health and dementia in oldest-old: A systematic review. Neurosci. Biobehav. Rev. 2023, 152, 105286. [Google Scholar] [CrossRef]
- JoJack, B. Eight Healthy Habits that May Add 24 Years to Your Lifespan. Medical News Today. 25 July 2023. Available online: https://www.medicalnewstoday.com/articles/8-healthy-longevity-habits-add-24-years-to-lifespan (accessed on 31 July 2023).
- Standing, S. Skin and its appendage. In Gray’s Anatomy, 42nd ed.; Standing, S., Ed.; Elsevier: Philadelphia, PA, USA, 2021; Chapter 7. [Google Scholar]
- Lephart, E.D.; Naftolin, F. Estrogen action and gut microbiome metabolism in dermal health. Dermatol. Ther. 2022, 12, 1535–1550. [Google Scholar] [CrossRef]
- Bernatchez, S.; Bichel, J. The science of skin: Measuring damage and assessing risk. Adv. Wound Care 2023, 12, 187–204. [Google Scholar] [CrossRef]
- Jansen van Rensburg, S.; Franken, A.; Du Plessis, J.L. Measurement of transepidermal water loss, stratum corneum hydration and skin surface pH in occupational settings: A review. Skin Res. Technol. 2019, 25, 595–605. [Google Scholar] [CrossRef]
- Haniffa, M.; Gunawan, M.; Jardine, L. Human skin dendritic cells in health and disease. J. Dermatol. Sci. 2015, 77, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.; Yosipovitch, G. Skin pH: From basic science to basic skin care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Lupa, D.M.; Fakalah, F.; Safferling, K.; Boukamp, P.; Poschmann, G.; Volpo, E.; Gotz-Rosch, C.; Bernerd, F.; Haag, L.; Huebenthal, U.; et al. Characterization of skin aging- Associated secreted proteins (SSAAP) produced by dermal fiboblasts isolated from intrinsically aged skin. J. Investig. Dermatol. 2015, 135, 1954–1968. [Google Scholar] [CrossRef]
- Bonté, F.; Girard, D.; Archambault, J.-C. Chapter 10, Skin changes during aging, 9n. In Biochemistry & Cell Biology of Aging: Part II Clinical Sciences, Subcellular Biochemistry 91; Harris, J.R., Korolchuk, V.I., Eds.; Springer Nature: Singapore, 2019; pp. 249–280. [Google Scholar]
- Karim, P.L.; Npriyati, I.A.A. Anatomy and histology of intrinsic aging skin. Biosci. Med. 2021, 5, 1065–1077. [Google Scholar]
- Huang, A.H.; Chien, A.L. Photoaging: A review of the current literature. Curr. Dermatol. Rep. 2020, 9, 22–29. [Google Scholar] [CrossRef]
- Sparavigna, A. Role of the extracellular matrix in skin aging and dedicated treatment—State of the art. Plast. Aesthet. Res. 2020, 7, 14. [Google Scholar] [CrossRef]
- Lee, A.-Y. Skin pigmentation abnormalities and their possible relationship with skin aging. Int. J. Mol. Sci. 2021, 22, 3727. [Google Scholar] [CrossRef]
- Sant Anna Addor, F.A. Beyond photoaging: Additional factors involved in the process of skin aging. Clin. Cosmet. Investig. Dermatol. 2018, 11, 437–443. [Google Scholar] [CrossRef]
- Woodby, B.; Penta, K.; Pecorelli, A.; Lila, M.A.; Valacchi, G. Skin health from the inside out. Annu. Rev. Food Sci. Technol. 2020, 11, 235–254. [Google Scholar] [CrossRef]
- Lephart, E.D. Skin aging and oxidative stress: Equol’s anti-aging effects via biochemical and molecular mechanisms. Ageing Res. Rev. 2016, 31, 36–54. [Google Scholar] [CrossRef]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.G.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronically aged skin. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D.; Naftolin, F. Menopause and the skin: Old favorites and new innovations in cosmeceuticals for estrogen-deficient skin. Dermatol. Ther. 2021, 11, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D. A review of the role of estrogen in dermal aging and facial attractiveness in women. J. Cosmet. Dermatol. 2018, 17, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and age-related disease: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef]
- Phuoung, C.; Maibach, H.I. Biological effects of estrogen on skin. In Textbook of Aging Skin; Farage, M.A., Miller, K.W., Maibach, H.I., Eds.; Springer: Berlin/Heidelburg, Germany, 2015; pp. 1–12. [Google Scholar]
- Reilly, D.M.; Lozano, J. Skin collagen through the lifestages: Importance for skin health and beauty. Plast. Aesthet. Res. 2021, 8, 2. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Blume-Peytavi, U.; Kosmadaki, M.; Roo, E.; Kerob, D.V.-R.D.; Goldstein, S.R. Skin, hair and beyond: The impact of menopause. Climacteric 2022, 25, 434–442. [Google Scholar] [CrossRef]
- Santen, R.J.; Simpson, E.R. History of estrogen: Its purification, structure, synthesis, biological actions, and clinical implications. Endocrinology 2019, 160, 605–625. [Google Scholar] [CrossRef]
- Rzepecki, A.K.; Murase, J.E.; Juran, R.; Fabi, S.G.; McLellan, N. Estrogen-deficient skin: The role of topical therapy. Int. J. Women’s Dermatol. 2019, 5, 85–90. [Google Scholar] [CrossRef]
- Thornton, M.J. Estrogens and skin aging. Dermatoendcrinology 2013, 5, 264–270. [Google Scholar] [CrossRef]
- Ceccarelli, I.; Bioletti, B.; Peparini, S. Estrogens and phytoestrogens in body functions. Neurosci. Biobehav. Rev. 2022, 132, 648–663. [Google Scholar] [CrossRef]
- Hong, H.-C.; Chang, W.-H.; Yeh, C.-C. Estrogen effects on wound healing. Int. J. Mol. Sci. 2017, 18, 2325. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. A role of estrogen in skin aging and dermal biomechanics. Mech. Ageing Dev. 2021, 197, 111513. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D. Phytoestrogens (resveratrol and equol) for estrogen-deficient skin-controversies/misinformation versus anti-aging in vitro and clinical evidence via nutraceutical-cosmetics. Int. J. Mol. Sci. 2021, 11, 11218. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zbytek, B.; Nikolakis, G.; Manna, P.R.; Skobowiat, C.; Zmijewski, M.; Li, W.; Janjetovic, Z.; Postlethwaite, A.; Zouboulis, C.C. Steroidogenesis in the skin: Implications for local immune function. J. Steroid Biochem. Mol. Biol. 2013, 137, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Antonini, D.; Siblo, A.; Dentice, M.; Missero, C. An intimate relationship between thyroid hormone and skin: Regulation of gene expression. Front. Endocrinol. 2013, 4, 104. [Google Scholar] [CrossRef]
- Safer, J.D. Thyroid hormone action on skin. Derm. Endocrinol. 2011, 3, 211–215. [Google Scholar] [CrossRef]
- Chen, Y.; Luga, J. Brain-skin connection: Stress, inflammation and skin health. Inflam. Allergy Drug Targets 2014, 13, 177–190. [Google Scholar] [CrossRef]
- Choe, S.J.; Kim, D.; Kim, E.J.; Ahn, J.-S.; Choi, E.-J.; Son, E.D.; Lee, T.R.; Choi, E.H. Psychological stress deteriorates skin barrier function by activating 11β-hydroxy steroid dehydrogenase 1 and the HPA axis. Sci. Rep. 2018, 8, 6344. [Google Scholar] [CrossRef]
- Huber, J.; Gruber, C. Immunological and dermatological impact of progesterone. Gynecol. Endocrinol. 2001, S6, 18–21. [Google Scholar] [CrossRef]
- Phillips, N.; Devaney, J. Beneficial regulation of type 1 collagen and matrixmetalloproteinase-1 expression by estrogen, progesterone, and its combination in skin fibroblasts. J. Am. Aging Assoc. 2003, 26, 59–62. [Google Scholar] [CrossRef][Green Version]
- Gasser, S.; Heidemeyer, K.; von Wolff, M.; Stute, P. Impact of progesterone on skin and hair in menopause-a comprehensive review. Climacteric 2021, 24, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://nrd.gov/resource/detail/14117290/National+Institute+on+Aging (accessed on 5 August 2023).
- Available online: https://www.un.org/development/desa/dspd/2023/01/world-social-report-2023/ (accessed on 28 July 2023).
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Cesare, M.; Anton, S.; Marzetti, E.; Giovannini, S.; Seo, A.Y.; Carter, C.; Yu, B.P.; Leeuwenburgh, C. Molecular inflammation: Underpinnings of aging and age-related disease. Ageing Res. Rev. 2009, 8, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Dumic, I.; Nordin, T.; Jecmenica, M.; Lalosevic, M.S.; Milosavljevic, T.; Milovanovic, T. Gastrointestinal tract disorders in older age. Can. J. Gastroenterol. Hepatol. 2019, 19, 6757524. [Google Scholar] [CrossRef]
- City of Hope. At What Age Is Cancer Most Common? 6 June 2023. Available online: https://www.cancercenter.com/community/blog/2023/06/cancer-risk-by-age#:~:text=By%20and%20large%2C%20the%20biggest,in%20between%20ages%2035%2D44 (accessed on 7 August 2023).
- Murray, C.J.L. (GBD 2019 Cancer Risk Factors Collaborators). The global burden of cancer attributed to risk factors, 2010–2019: A systematic analysis for the global burden of disease study 2019. Lancet 2022, 400, 563–591. [Google Scholar]
- Alegria-Torres, J.A.; Baccarelli, A.; Bollati, V. Epigenetics and lifestyle. Epigenetics 2011, 3, 267–277. [Google Scholar] [CrossRef]
- Kassis, A.; Fichot, M.-C.; Horcajada, M.-N.; Horstman, A.M.H.; Duncan, P.; Bergonzelli, G.; Preitner, N.; Zimmermann, D.; Bosco, N.; Vidal, K.; et al. Nutritional and lifestyle management of the aging journey: A narrative review. Front. Nutr. 2023, 9, 1087505. [Google Scholar] [CrossRef]
- Loef, B.; Herber, G.-C.M.; Wong, A.; Janssen, N.A.H.; Hoekstra, J.; Picavet, H.S.J.; Verschuren, W.M.M. Predictors of healthy physiological aging across generations in a 30-year population-based cohort study: The Doetinchem Cohort Study. BMC Geiratrics 2023, 23, 107. [Google Scholar] [CrossRef]
- Bosnes, I.; Norhahl, H.M.; Stordal, E.; Bosnes, O.; Myklebust, T.A.; Almkvist, O. Lifestyle predictors of successful aging: A 20-year prospective HUNT study. PLoS ONE 2019, 14, e0219200. [Google Scholar] [CrossRef]
- Katz, S. Active and successful aging. Lifestyle as a gerontological idea. Rech. Sociol. Et Anthropol. 2013, 44, 33–49. Available online: https://journals.openedition.org.ras.910 (accessed on 17 August 2023). [CrossRef]
- Sakaniwa, R.; Noguchi, M.; Imano, H.; Shirai, K.; Tamakoshi, A.; Iso, H.; The JACC Study Group. Impact of modifiable health lifestyle adoption on lifetime gain form middle to older age. Age Ageing 2022, 51, afac080. [Google Scholar] [CrossRef] [PubMed]
- Wahl, D.; Solon-Biet, S.M.; Cogger, V.C.; Fontana, L.; Simpson, S.J.; Le Couteur, D.G.; Ribeiro, R.V. Aging, lifestyle and dementia. Neurobiol. Dis. 2019, 130, 104481. [Google Scholar] [CrossRef] [PubMed]
- Buettner, D.; Skemp, S. Blue zones: Lessons from the world’s longest lived. Am. J. Lifestyle Med. 2016, 10, 318–322. [Google Scholar] [CrossRef] [PubMed]
- National Center for Chronic Disease Prevention and Health Promotion (NCCDPHP). Poor Nutrition. Available online: https://www.cdc.gov/chronicdisease/resources/publications/factsheets/nutrition.htm#:~:text=A%20healthy%20diet%20helps%20children,2%20diabetes%2C%20and%20certain%20cancers (accessed on 9 August 2023).
- Stewart, K.L.; Lephart, E.D. Overview of BPH: Symptom relief with dietary polyphenols, vitamins, and phytochemicals by nutraceutical supplements with implications to the prostate microbiome. Int. J. Mol. Sci. 2023, 24, 5486. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean diet and health status: Active ingredients and pharmacological mechanisms. Br. J. Pharmacol. 2020, 177, 1241–1257. [Google Scholar] [CrossRef]
- Raits, E.; Kirse-Ozolina, A. Modern dietary patterns based on territorial origin—A review. Foodblat 2019. [Google Scholar] [CrossRef]
- Maleaza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Madry, E. High-fat, Western-style diet, systematic inflammation, and gut microbiota: A narrative review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef]
- Mazza, E.; Ferro, Y.; Pujia, R.; Mare, R.; Maurotti, T.; Puijia, A. Mediterranean diet in healthy aging. J. Nutr. Health Aging 2021, 25, 1076–1083. [Google Scholar] [CrossRef]
- Galbete, C.; Schwingshackl, L.; Schwedheln, C.; Boeing, H.; Schulze, M.B. Evaluation of the Mediterranean diet and risk of chronic disease in cohort studies: An umbrella review of meta-analyses. Eur. J. Epidermiol. 2018, 33, 909–931. [Google Scholar] [CrossRef]
- Stefler, D.; Brett, D.; Sarkadi-Nagy, E.; Kopczynska, E.; Detchev, S.; Bati, A.; Scrob, M.; Koenker, D.; Aleksov, B.; Douarin, E.; et al. Traditional Eastern European diet and mortality: Prospective evidence for the HAPIEE study. Eur. J. Nutr. 2021, 60, 1091–1100. [Google Scholar] [CrossRef]
- World Heart Federation. 2023. Available online: https://world-heart-federation.org/where-we-work/europe-central-asia/ (accessed on 11 August 2023).
- Cao, C.; Xiao, Z.; Wu, Y.; Ge, C. Diet and skin aging-from the perspective of food nutrition. Nutrients 2020, 12, 870. [Google Scholar] [CrossRef]
- Palma, L.; Marques, L.T.; Bujan, J.; Rodrigues, L.M. Dietary water affects human skin hydration and biomechanics. Clin. Cosmet. Investig. Dermatol. 2015, 8, 413–421. [Google Scholar]
- Popkin, B.M.; Rosenberg, I.H. Water, hydration and health. Nutr. Rev. 2010, 68, 439–458. [Google Scholar] [CrossRef] [PubMed]
- Akdeniz, M.; Tomova-Simithieva, T.; Dobos, G.; Blume-Peytavi, U.; Kottner, J. Does dietary fluid intake affect skin hydration in healthy humans? A systematic literature review. Skin Res. Technol. 2018, 24, 459–465. [Google Scholar] [CrossRef]
- Wu, G. Dietary protein intake and human health. Food Funct. 2016, 7, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, D.L.; West, V.A.; Lephart, E.D. Enhancing skin health: By oral administration of natural compounds and minerals with implications to the dermal microbiome. Int. J. Mol. Sci. 2018, 19, 3059. [Google Scholar] [CrossRef]
- Nieves, D.S.; Goldsmith, L.A. Cutaneous changes in nutritional disease. Chapter 145. In Fitzpatrick’s Dermatology in General Medicine, 6th ed.; Freedburg, I.M., Eisen, A., Wolff, K., Austen, K.F., Goldsmith, L.A., Katz, S.I., Eds.; McGraw-Hill: London, UK, 2003; pp. 1399–1411. [Google Scholar]
- Choi, F.D.; Sung, C.T.; Juhasz, J.L.W.; Mesinkovsk, N.A. Oral collagen supplementation: A systematic review of dermatological applications. J. Drugs Dermatol. 2019, 18, 9–16. [Google Scholar] [PubMed]
- de Miranda, R.B.; Weimer, R.; Rossi, R. Effects of hydrolyzed collagen supplementation on skin aging: A systematic review and meta-analysis. Int. J. Dermatol. 2021, 60, 1449–1461. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.G.; Lee, J.; Choi, S.; Suk, J.; Lee, J.H.; Yang, J.H.; Yang, J.S.; Kim, J. Oral supplementation of low-molecular weight collagen peptides reduces skin wrinkles and improves biophysical properties of skin: A randomized, double-blind, placebo-controlled study. J. Med. Food 2022, 24, 1146–1154. [Google Scholar] [CrossRef]
- Coerdt, K.M.; Goggins, C.A.; Khachemoune, A. Vitamins A, B, C, and D: A short review for the dermatologist. Altern. Ther. Health Med. 2021, 27, 41–49. [Google Scholar]
- Draelos, Z.D. An oral supplement and the nutrition-Skin connection. J. Clin. Aesthet. Dermatol. 2019, 12, 13–16. [Google Scholar] [PubMed]
- Faria-Silva, C.; Ascenso, A.; Costa, A.M.; Marto, J.; Carbalheiro, M.; Ribeiro, H.M.; Simoes, S. Feeding the skin: A new trend in food and cosmetics convergence. Trends Food Sci. Technol. 2020, 95, 21–32. [Google Scholar] [CrossRef]
- Liu, T.; Li, N.; Yan, Y.; Liu, Y.; Xiong, K.; Liu, Y.; Xia, Q.-M.; Zhang, H.; Liu, Z.-D. Recent advances in the anti-aging effects of phytoestrogens on collagen, water content, and oxidative stress. Phytotherapy Res. 2020, 34, 435–447. [Google Scholar] [CrossRef]
- Juturu, V.; Bowman, J.P.; Deshpande, J. Overall skin tone and skin-lightening-improving effects with oral supplementation of lutein and zeaxanthin isomers: A double-blind, placebo-controlled clinical trial. Clin. Cosmet. Investig. Derm. 2016, 9, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Fam, V.W.; Charoenwoodhipong, P.; Sivamani, R.J.; Holt, R.R.; Keen, C.L.; Hackman, R.M. Plant-based foods for skin health: A narrative review. J. Acad. Nutr. Diet. 2020, 122, 614–629. [Google Scholar] [CrossRef]
- Michalak, M. Plant-derived antioxidants: Significance in skin health and the ageing process. Int. J. Mol. Sci. 2022, 23, 585. [Google Scholar] [CrossRef]
- Darbre, P.D. Chapter 5-Plant-based ingredients in personal care products. In Personal Care Products and Human Health; Darbre, P.D., Ed.; Academic Press-Elsevier: London, UK, 2023; pp. 97–1112. [Google Scholar]
- Lee, J.H.; Park, J.; Skin, D.W. The molecular mechanism of polyphenols with anti-aging activity in aged human dermal fibroblasts. Molecules 2022, 27, 4351. [Google Scholar] [CrossRef]
- Solway, J.; McBride, M.; Haq, F.; Abdul, W.; Miller, R. Diet and dermatology: The role of a whole-food, plant-based diet in preventing and reversing skin aging, a review. J. Clin. Aesthet. Dermatol. 2020, 13, 38–43. [Google Scholar]
- Lephart, E.D. Determination of S-equol and/or R-equol in plant-based food products and efficacy of topical or oral 4′,7-isoflavandiol (R/S equol) to improve skin health in adult men, a placebo-controlled study. J. Funct. Foods 2021, 83, 104563. [Google Scholar] [CrossRef]
- Teng, Y.; Huang, Y.; Xu, D.; Tao, X.; Fan, Y. The role of probiotics in skin photoaging and related mechanisms: A review. Clin. Cosmet. Investig. Derm. 2022, 15, 2455–2464. [Google Scholar] [CrossRef]
- De Almeida, C.V.; Antiga, E.; Lulli, M. Oral and topical probiotics and postbiotics in skincare and dermatological therapy: A concise review. Microorganisms 2023, 11, 1420. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Wang, X.; Li, Y.; Ren, F. The role of probiotics in skin health and related gut-skin axis: A review. Nutrients 2023, 15, 3123. [Google Scholar] [CrossRef]
- Szanto, M.; Dozsa, A.; Antal, D.; Szabo, K.; Kemeny, L.; Bai, P. Targeting the gut-skin axis-Probiotics as a new tool for skin disorder management. Exp. Dermatol. 2019, 28, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Khmaladze, I.; Leonardi, M.; Fabre, S.; Messaraa, C.; Mavon, A. The skin interactome: A holistic “genone-microbiome-exposome” approach to understand and modulate skin health and aging. Clin. Cosmet. Investig. Dermatol. 2020, 13, 1021–1040. [Google Scholar] [CrossRef] [PubMed]
- Souak, D.; Barreau, M.; Courtois, A.; Andre, V.; Poc, C.D.; Feuilloley, M.G.J.; Gault, M. Challenging cosmetic innovation: The skin microbiota and probiotics protect the skin from UV-induced damage. Microorganisms 2021, 9, 936. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qu, L.; Mijakovic, I.; Wei, Y. Advances in the human skin microbiota and its roles in cutaneous diseases. Microb. Cell Factories 2022, 21, 176. [Google Scholar] [CrossRef]
- Galland, L. Diet and inflammation. Nutr. Clin. Pract. 2010, 25, 634–640. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Melnickova, U.I.; Porozov, Y.B.; Terrentiev, A.A. Oxidative stress and advanced lipoxidation and glycation end products (ALEs and AGEs) in aging and age-related diseases. Oxidative Med. Cell. Longev. 2019, 2019, 3085756. [Google Scholar] [CrossRef]
- Lai, S.W.T.; de Jesus Lopez Gonzales, E.; Zoukari, T.; Ki, P.; Shuck, S.C. Methylglyoxal and its adducts: Induction, repair, and association with disease. Chem. Res. Toxicol. 2022, 35, 1720–1746. [Google Scholar] [CrossRef]
- Kellow, N.J.; Coughlan, M.T.; Reid, C.M. Association between habitual dietary and lifestyle behaviours and skin autofluorescence (SAF), a marker of tissue accumulation of advanced glycation endproducts (AGEs), in healthy adults. Eur. J. Nutr. 2018, 57, 2209–2216. [Google Scholar] [CrossRef]
- Clatici, V.G.; Racoceanu, D.; Dalle, C.; Voicu, C.; Tomas-Aragones, L.; Marron, S.E.; Wollina, U.; Fica, S. Perceived age and life style. The specific contributions of seven factors involved in health and beauty. MAEDICA—J. Clin. Med. 2017, 12, 191–201. [Google Scholar]
- Hu, S.; Anand, P.; Laughter, M.; Maymone, M.B.C.; Dellavalle, R.P. Holistic dermatology: An evidence-based review of modifiable lifestyle factor associations with dermatologic disorders. J. Am. Acad. Dermatol. 2022, 86, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, V.; Singh, A.; Tripathi, S.; Kumar, A. Various aging factors significantly impact skin and its conclusion. Res. Rev. J. Health Prof. 2023, 13, 1–10. [Google Scholar]
- Soliman, Y.S.; Hashim, P.A.; Farberg, A.S.; Goldenberg, G. The role of diet in preventing photoaging and treating common skin conditions. Cosmet. Derm. 2019, 103, 153–156. [Google Scholar]
- Madden, S.K.; Flanagan, K.L.; Jones, G. How lifestyle factors and their associated pathogenetic mechanisms impact psoriasis. Clin. Nutr. 2020, 39, 1026–1040. [Google Scholar] [CrossRef]
- Jamochian, M.; Alamgir, M.; Rao, B. Diet and dermatology: Review of diet’s influence on the conditions of rosacea, hidradenitis, suppurativa, herpes labials, and vitiligo. Am. J. Lifestyle Med. 2021, 17, 152–160. [Google Scholar] [CrossRef]
- Musumeci, M.L.; Nasca, M.R.; Boscaglia, S.; Micali, G. The role of lifestyle and nutrition in psoriasis: Current status of knowledge and interventions. Dermatol. Ther. 2022, 35, e15685. [Google Scholar] [CrossRef]
- Hoonhorst, S.J.M.; Lo Tam Loi, A.T.; Pouwels, S.D.; Faiz, A.; Telenga, E.D.; van den Berge, M.; Koenderman, L.; Lammers, J.-W.J.; Boezen, H.M.; van Oosterhout, A.J.M.; et al. Advanced glycation endproducts and their receptor in different body compartments in COPD. Respir. Res. 2016, 17, 46. [Google Scholar] [CrossRef]
- Palimeri, S.; Palioura, E.; Diamanti-Kandarakis, E. Current perspectives on the health risks associated with the consumption of advanced glycation end products: Recommendations for dietary management. Diabetes Metab. Syndr. Obes. Targets Ther. 2015, 8, 415–426. [Google Scholar] [CrossRef]
- Rugratanwanich, W.; Qu, Y.; Wang, X.; Essa, M.M.; Song, B.-J. Advanced glycation end products (AGEs) and other adducts in aging-related disease and alcohol-mediated tissue injury. Exp. Mol. Med. 2021, 53, 168–188. [Google Scholar] [CrossRef]
- Goodman, G.D.; Kaufman, J.; Day, D.; Wiess, R.; Kawata, A.K.; Garcia, J.K.; Santangelo, S.; Gallagher, C.J. Impact of smoking and alcohol use on facial aging in women: Results of a large multinational, multiracial, cross-sectional survey. J. Clin. Aesthet. Dermatol. 2019, 12, 28–39. [Google Scholar] [PubMed]
- Mitri, A.; Lin, G.; Waldman, R.A.; Grant-Kels, J.M. Effects of tobacco and vaping on the skin. Clin. Dermatol. 2021, 39, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Tackett, A.P.; Urman, R.; Barrington-Trimis, J.; Liu, F.; Hong, H.; Pentz, M.A.; Islam, T.S.; Eckel, S.P.; Rebuli, M.; Leventhal, A.; et al. Prospective study of e-cigarette use and respiratory symptoms in adolescents and young adults. Thorax 2023. ahead-of-print. [Google Scholar] [CrossRef] [PubMed]
- Gunn, D.A.; Larson, L.A.; Lall, J.S. Mortality is written on the face. J. Gerontol. Ser. A Biol. Med. Sci. 2016, 71, 72–77. [Google Scholar] [CrossRef]
- Emanuele, M.A.; Wezeman, F.; Emanuele, N.V. Alcohol’s effects on female reproductive function. Alcohol Res. Health 2002, 26, 274–281. [Google Scholar]
- McDivit, A.M.; Greendale, G.A.; Stanczyk, F.Z.; Huang, M.-H. Effects of alcohol and cigarette smoking on change in serum estrone levels in postmenopausal woman randomly assigned to fixed doses of conjugated equine estrogens with or without progestin. Menopause 2008, 15, 382–385. [Google Scholar] [CrossRef]
- Division Nutrition, Physical Activity, and Obesity, National Center for Chronic Disease Prevention and Health Promotion (CDC). Defining Adult Overweight and Obesity. 3 June 2022. Available online: https://www.cdc.gov/obesity/basics/adult-defining.html#:~:text=Adult%20Body%20Mass%20Index&text=If%20your%20BMI%20is%20less,falls%20within%20the%20obesity% (accessed on 17 August 2023).
- Boutari, C.; Mantzoros, C.S. A 2022 update on the epidemiology of obesity and a call to action: As its twin COVID-19 pandemic appears to be receding, the obesity and dysmetabolism pandemic continues to rage on. Metabolism 2022, 133, 155217. [Google Scholar] [CrossRef]
- The GBD 2015 Obesity Collaborators. Health effects of overweight and obesity in 195 countries over 25 years. New Engl. J. Med. 2017, 377, 13–27. [Google Scholar]
- Darlenski, R.; Mihaylova, V.; Handjieva-Darlenska, T. The link between obesity and the skin. Front. Nutr. 2022, 9, 855573. [Google Scholar] [CrossRef]
- Frasca, D.; Strbo, N. Effects of obesity on infections with emphasis on skin infections and wound healing. J. Dermatol. Skin Sci. 2022, 4, 5–10. [Google Scholar] [CrossRef]
- Nakamizo, S.; Honda, T.; Kabashima, K. Obesity and inflammatory skin diseases. Trends Immunother. 2019, 3, 50–57. [Google Scholar] [CrossRef]
- Palanivel, J.A.; Millington, G.W.M. Obesity-induced immunological effects on the skin. Ski. Health Dis. 2023, 3, e160. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Vukmanovic-Stejic, M. Skin barrier immunity and ageing. Immunology 2019, 160, 116–125. [Google Scholar] [CrossRef]
- Trompette, A.; Ubags, N.D. Skin barrier immunology from early life to adulthood. Mucosal Immunol. 2023, 16, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, G.; Curatolo, S.; Busa, B.; Belfore, A.; Gullo, D. Two birds one stone: Semaglutide is highly effective against severe psoriasis in a type 2 diabetic patient. Endocrinol. Diabetes Metab. Case Rep. 2021. ahead-of-print. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lin, L.; Chen, P.; Yu, Y.; Chen, S.; Chen, X.; Shao, Z. Treatment with liraglutide, a glucagon-like peptide-1 analogue, improves effectively skin lesions of psoriasis patients with type 2 diabetes: A prospective cohort study. Diabetes Res. Clin. Pract. 2019, 150, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J.-P.; Dutil, C.; Featherstone, R.; Ross, R.; Giangregorio, L.; Saunders, T.J.; Janssen, I.; Poitras, V.J.; Kho, M.E.; Ross-White, A.; et al. Sleep timing, sleep consistency, and health in adults: A systematic review. Appl. Physiol. Nutr. Metab. 2020, 45, S232–S247. [Google Scholar] [CrossRef]
- McCarter, S.J.; Hagen, P.T.; St Louis, E.K.; Rieck, T.M.; Haider, C.R.; Holmes, D.R.; Morgenthaler, T.I. Physiological markers of sleep quality: A scoping review. Sleep Med. Rev. 2022, 64, 101657. [Google Scholar] [CrossRef] [PubMed]
- Troynikov, O.; Watson, C.G.; Nawaz, N. Sleep environments and sleep physiology: A review. J. Therm. Biol. 2018, 78, 192–203. [Google Scholar] [CrossRef]
- Lv, Y.; Jiang, G.; Tan, X.; Bao, W.; Chen, L.; Liu, L. Association of sleep patterns and lifestyles with incident hypertension: Evidence from a large population-based cohort study. Front. Cardiovasc. Med. 2022, 9, 847452. [Google Scholar] [CrossRef]
- Sejbuk, M.; Mironczuk-Chodakowska, I.; Witkowska, A.M. Sleep Quality: A narrative review on nutrition, stimulants, and physical activity as important factors. Nutrients 2022, 14, 1912. [Google Scholar] [CrossRef] [PubMed]
- Gangwisch, J.; Hale, L.; St-Onge, M.-P.; Choi, L.; LeBlanc, E.S.; Malaspina, D.; Opler, M.G.; Shadyab, A.H.; Shikany, J.M.; Snetselaar, L. High gylcemic index and glycemic load diets as risk factors for insomnia: Analyses from the women’s health initiative. Am. J. Clin. Nutr. 2020, 111, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Binks, H.; Vincent, G.E.; Gupta, C.; Irwin, C.; Khalesi, S. Effects of diet on sleep: A narrative review. Nutrients 2020, 12, 936. [Google Scholar] [CrossRef] [PubMed]
- Stutanto, C.N.; Loh, W.W.; Toh, D.W.K.; Lee, D.P.S.; Kim, J.E. Association between dietary protein intake and sleep quality in middle-aged and older adults in Singapore. Front. Nutr. 2022, 9, 832341. [Google Scholar] [CrossRef]
- O’Callaghan, F.; Muurlink, O.; Reid, N. Effects of caffeine on sleep quality and daytime functioning. Risk Manag. Health Policy 2018, 11, 263–271. [Google Scholar] [CrossRef]
- Majid, M.S.; Ahmad, H.S.; Bizhan, H.; Hosein, H.Z.M.; Mohammad, A. The effects of vitamin D supplement on the score and quality of sleep in 20-50 year-old people with sleep disorders compared to control group. Nutr. Neurosci. 2018, 21, 511–519. [Google Scholar] [CrossRef]
- Zengin, L.; Aylaz, R. The effects of sleep hygiene education and reflexology on sleep quality and fatigue in patients receiving chemotherapy. Eur. J. Cancer Care 2019, 28, e13020. [Google Scholar] [CrossRef]
- Stein, M.D.; Friedman, P.D. Disturbed sleep and its relationship to alcohol use. Subst. Abus. 2006, 26, 1–13. [Google Scholar] [CrossRef]
- Purani, H.; Friedrichsen, S.; Allen, A.M. Sleep quality in cigarette smokers: Associations with smoking-related outcomes and exercise. Addict. Behav. 2018, 90, 71–76. [Google Scholar] [CrossRef]
- Al Ryalat, S.A.; Kussad, S.; Khatib, O.; Hamad, I.; Al-Tanjy, A.; Alshnneikat, M.; AbuMahfouz, B. Assessing the effect of nicotine dose in cigarette smoking on sleep quality. Sleep Breath. 2012, 25, 1319–1324. [Google Scholar] [CrossRef]
- Wilchens, K.A.; Erickson, K.I.; Wheeler, M.E. Physical activity and cognition: A mediating role of efficient sleep. Behav. Sleep Med. 2018, 16, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Christie, A.D.; Seery, E.; Kent, J.A. Physical activity, sleep quality, and self-reported fatigue across the adult lifespan. Exp. Gerontol. 2016, 77, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Dedhia, P.; Maurer, R. Sleep and health- A lifestyle medicine approach. J. Fam. Pract. 2022, 71, S30–S34. [Google Scholar] [CrossRef]
- Oyetakin-White, P.; Suggs, A.; Koo, B. Does poor sleep quality affect skin aging? Clin. Exp. Dermatol. 2015, 40, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Sundelin, T.; Lekander, M.; Kecklund, G.; Van Sommeren, E.J.; Olsson, A.; Axelsson, J. Cues of fatigue: Effect of sleep deprivation on facial appearance. Sleep 2013, 36, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Oliveria, C.; Torres, T. More than skin deep: The systemic nature of atopic dermatitis. Eur. J. Dermatol. 2019, 29, 250–258. [Google Scholar] [CrossRef]
- Xerfan, E.M.S.; Andersen, M.L.; Facina, A.S.; Tufik, S.; Tomimori, J. Rosacea, poor sleep quality, and obstructive sleep apnea: A commentary on potential interconnected features. J. Cosmet. Dermatol. 2022, 21, 4234–4236. [Google Scholar] [CrossRef]
- Mann, C.; Gorai, S.; Staubach-Renz, P.; Goldust, M. Sleep disorders in dermatology-a comprehensive review. JDDG J. Dtsch. Dermatol. Ges. 2023, 21, 577–584. [Google Scholar] [CrossRef]
- Duan, G.Y. Sleep impairment in patients with chronic inflammatory skin diseases: A review of mechanisms and management. J. Am. Acad. Dermatol. 2022, 88, 421–427. [Google Scholar] [CrossRef]
- Afzal, U.M.; Ali, F.R. Sleep deprivation and the skin. Clin. Exp. Dermatol. 2023, 48, 1113–1116. [Google Scholar] [CrossRef]
- Duan, J.; Greenberg, E.N.; Karri, S.S.; Anderson, B. The circadian clock and diseases of the skin. FEBS Lett. 2021, 595, 2413–2436. [Google Scholar] [CrossRef]
- Camilion, J.V.; Khanna, S.; Anasseri, S.; Laney, C.; Mayrovitz, H.N. Physiological, pathological, and circadian factors impacting skin hydration. Cureus 2022, 14, e27666. [Google Scholar] [CrossRef]
- Lyons, A.B.; Moy, L.; Moy, R.; Tung, R. Circadian rhythm and the skin: A review of the literature. J. Clin. Aesthet. Dermatol. 2019, 12, 42–45. [Google Scholar]
- Matsui, M.S.; Pelle, E.; Dong, K.; Pernodet, N. Biological rhythms in the skin. Int. J. Mol. Sci. 2016, 17, 801. [Google Scholar] [CrossRef] [PubMed]
- Pernodet, N.; Pelle, E. Chronobiology of the Skin, Skin Circadian Rhythm and Clock Genes: A New Approach to Slowing Down the Aging Process, 9th ed.; Chemical Publishing: Los Angeles, CA, USA, 2015; Volume 2. [Google Scholar]
- Sarkar, S.; Gaddameedhi, S. UV-B-Induced erythema in human skin: The circadian clock is ticking. J. Investig. Dermatol. 2018, 138, 248–251. [Google Scholar] [CrossRef]
- Nikkola, V.; Miettinen, M.E.; Karisola, P.; Gronroos, M.; Yianttia, L.; Alenius, H.; Snellman, E.; Pateonen, T. Ultraviolet B radiation modifies circadian time in epidermal skin and in subcutaneous adipose tissue. Photodermatol. Photoimmunol. Photomed. 2019, 35, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A. Perceptions of sensitive skin: Changes in perceived severity and associations with environmental causes. Contact Dermat. 2008, 59, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Farage, M.A.; Miller, K.W.; Wippel, A.M.; Berardesca, E.; Misery, L.; Mailbach, H. Sensitive skin in the United States: Survey of regional differences. Fam. Med. Med. Sci. Res. 2013, 2, 3. [Google Scholar]
- Lanuti, E.L.; Kirsner, R.S. Effects of pollution on skin aging. J. Investig. Dermatol. 2010, 130, 2696. [Google Scholar] [CrossRef]
- Vierkötter, A.; Schikowski, T.; Ranft, U.; Sugiri, D.; Matsui, M.; Krämer, U.; Krutmann, J. Airborne particle exposure and extrinsic skin aging. J. Investig. Dermatol. 2010, 130, 2719–2726. [Google Scholar] [CrossRef]
- CDC. Exposome. Available online: https://www.cdc.gov/niosh/topics/exposome/default.html#:~:text=The%20exposome%20can%20be%20defined,%2C%20diet%2C%20lifestyle%2C%20etc (accessed on 22 August 2023).
- Lephart, E.D. Equol’s anti-aging effects protect against environmental assaults by increasing skin antioxidant defense and ECM proteins while decreasing oxidative stress and inflammation. Cosmetics 2018, 5, 16. [Google Scholar] [CrossRef]
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental stressors on skin aging. Mechanistic insights. Front. Pharm. 2019, 10, 759. [Google Scholar] [CrossRef] [PubMed]
- Passeron, T.; Zouboulis, C.C.; Tan, J.; Andersen, M.L.; Katta, R.; Jyu, X.; Aguilar, L.; Kerob, D.; Morita, A.; Krutmann, J. Adult skin acute stress responses to short-term environmental and internal aggression from exposome factors. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1963–1975. [Google Scholar] [CrossRef] [PubMed]
- Reis-Mansur, M.C.P.; Goncalves da Luz, B.; Pereira dos Santos, E. Consumer behavior, skin phototype, sunscreens, and tools for photoprotection: A review. Cosmetics 2023, 10, 39. [Google Scholar] [CrossRef]
- Suozzi, K.; Turban, J.; Girardi, M. Cutaneous photoprotection: A review of the current status and evolving strategies. Yale J. Biol. Med. 2020, 93, 55–67. [Google Scholar]
- Pethank, N.; Pollard, K.J. Lifestyle medicine prescriptions for personal and planetary health. J. Clim. Change Health 2021, 4, 100077. [Google Scholar] [CrossRef]
- Ferro de Oliveira, C.S.; Tavaria, F.K. The impace of bioactive textiles on human skin microbiota. Eur. J. Pharm. Biopharm. 2023, 188, 66–77. [Google Scholar] [CrossRef]
- Farkas, A.; Farkes, G. Effects of spaceflight on human skin. Skin Pharm. Physiol. 2021, 34, 239–245. [Google Scholar] [CrossRef]
- Qui, Y.; Ferandez-Garcia, B.; Lehmann, H.I.; Li, G.; Kroemer, G.; Lopez-Otin, C.; Ziao, J. Exercise sustains the hallmarks of health. J. Sports Health Sci. 2023, 12, 8–35. [Google Scholar]
- Ruegsegger, G.N.; Booth, F.W. Health benefits of exercise. Cold-Spring Harb. Perspect. Med. 2018, 8, a029694. [Google Scholar] [CrossRef]
- Giacomello, M.; Pyakurel, A.; Glytsou, C.; Scorrano, L. The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Biol. 2020, 21, 204–224. [Google Scholar] [CrossRef] [PubMed]
- Yeb, C.; Flately, E.; Elkattawy, O.; Berger, L.; Rao, B. Exercise in dermatology: Exercise’s influence on skin aging, skin cancer, psoriasis, venous ulcers, androgenetic alopecia. J. Am Acad. Dermatol. Res. Lett. 2022, 87, 183–184. [Google Scholar]
- Ryosuke, O.; Yoshie, S.; Hiromi, A. The association between activity levels and skin moisturizing function in adults. Dermatol. Rep. 2021, 13, 8811. [Google Scholar]
- Franca, K.; Lotti, T. (Eds.) Advances in Integrative Dermatology; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- United States Environmental Protection Agency (EPA). Ultraviolet (UV) Radiation and Sun Exposure. Available online: https://www.epa.gov/radtown/ultraviolet-uv-radiation-and-sun-exposure (accessed on 21 August 2023).
- Leigh-Hunt, N.; BAgguley, D.; Bash, K.; Tirner, V.; Turnbull, S.; Valtorta, N.; Cann, W. An overview of systematic reviews on the public health consequences of social isolation and loneliness. Public Health 2017, 152, 157–171. [Google Scholar] [CrossRef]
- Cene, C.W.; Beckie, T.M.; Sims, M.; Suglia, S.F.; Aggarwal, B.; Moise, N.; Jimenez, M.C.; Gaye, B.; McCullough, L.D. Effects of objective and perceived social isolation on cardiovascular and brain health: A scientific statement from the American Heart Association. J. Am. Heart Assoc. 2022, 11, e026493. [Google Scholar] [CrossRef]
- Cudjoe, T.K.M.; Selvakumar, S.; Chung, S.-E.; Latkin, C.A.; Roth, D.L.; Thorpe, R.J.; Boyd, C.M. Getting under the skin: Social isolation and biological markers in the National Health and Aging trend study. J. Am. Geriatr. Soc. 2022, 70, 408–414. [Google Scholar] [CrossRef]
- Kottner, J.; Fastner, A.; Lintzeri, D.-A.; Griffths, C.E.M.; Blume-Peytavi, U. Improving skin health of community-dwelling older people: A scoping review protocol. BMJ Open 2023, 13, E071313. [Google Scholar] [CrossRef]
- Usgu, S.; Akinci, B.; Bali, K. Complementary therapies for women with body image issues. Anti-Aging East. Eur. 2023, 2, 97–108. [Google Scholar] [CrossRef]
- Rapaport, M.H.; Schettler, P.; Bresee, C. A preliminary study of the effects of repeated massage on hypothalamic-pituitary-adrenal and immune function in healthy individuals: A study of mechanisms of action and dosage. J. Altern. Comp. Med. 2012, 8, 789–797. [Google Scholar] [CrossRef]
- Almutairi, N.; Schwartz, R.A. COVID-19 with dermatologic manifestations and implications: An unfolding conundrum. Dermatol. Ther. 2020, 33, e13544. [Google Scholar] [CrossRef]
- Afshar, Z.M.; Babaxadeh, A.; Hasanpour, A.; Barray, M.; Sayad, S.; Janbakhsh, A.; Aryanian, Z.; Ebrahimpour, S. Dermatological manifestations associated with COVID-19: A comprehensive review of the current knowledge. J. Med. Virol. 2021, 93, 5756–5767. [Google Scholar] [CrossRef] [PubMed]
- Mangini, C.S.M.; Farias de Vaconcelos, R.C.; Rodriguez, E.V.R.; Lorenzon de Oliveira, I.R. Social isolation: Main dermatosis and impact of stress during the COVID-19 pandemic. Einstein 2022, 20, eAO6320. [Google Scholar] [CrossRef] [PubMed]
- Flandroy, L.; Poutadhidis, T.; Berg, G.; Clakrke, G.; Dao, M.-C.; Decaestecker, E.; Furman, E.; Haahtela, T.; Massart, S.; Plovier, H.; et al. The impact of human activities and lifestyle on the interlinked microbiota and health of humans and of ecosystems. Sci. Total Environ. 2018, 627, 1018–1038. [Google Scholar] [CrossRef]
- Umberson, D.; Montez, J.K. Social relationships and health: A flashpoint for health policy. J. Health Soc. Behav. 2010, 51, S54–S66. [Google Scholar] [CrossRef]
- Petkovic, J.; Duench, S.; Trawin, J.; Dewidar, O.; Pardo, J.; Simeon, R.; DesMeules, M.; Gagnon, D.; Robert, H.; Hossain, A.; et al. Behavioural interventions delivered through interactive social media for health behaviour change, health outcomes, and health equity in the adult population. Cochrane Database Syst. Rev. 2021, 31, CD012932. [Google Scholar]
- White, N.D.; Bautista, V.; Lenz, T.; Cosimano, A. Using the SMART-EST goals in lifestyle medicine prescription. Am. J. Lifestyle Med. 2020, 14, 271–273. [Google Scholar] [CrossRef] [PubMed]

| Nutrient/Diet/Habit | Influence on Skin Health | Reference |
|---|---|---|
| Water | skin hydration-biomechanics | [2,22,84,85] |
| Proteins | support/repair, extracellular matrix (collagen/elastin) | [88,90,91,92] |
| Trace Elements | ||
| Copper | extracellular matrix/angiogenesis | [89,94,95] |
| Iron | wound healing/antioxidant capacity | [89,94,95] |
| Selenium | keratinocyte function/antioxidant function | [88] |
| Zinc | /development-keratinocytes | [88] |
| Vitamins and Other Compounds | ||
| A | aging, improves wrinkles, stimulates dermis | [89,93,94,95] |
| B-Complex (below) | [88,89,94,95] | |
| B1 (thiamine) | hydration/anti-inflammatory | |
| B2 (riboflavin) | skin tone/radiant balance | |
| B3 (niacinamide) | keratin/barrier function | |
| B5 (pantothenic acid) | hydration/wound healing/anti-inflammatory | |
| B6 (pyridoxine) | anti-inflammatory/skin balance | |
| B7 (biotin) | if deficiency-improves skin, hair/nails | |
| B9 (folate) | skin support/tone/turgor | |
| B12 (cobalamin) | collagen/hydration, anti-inflammatory, deficiency-hyperpigmentation | |
| C | antioxidant/boost collagen/hydration | [89,93,94,95] |
| D | anti-inflammatory, skin protectant | [89,93,94,95] |
| E | antioxidant, anti-aging, improves wrinkles | [89,93,94,95] |
| Dietary Intake/Supplementation | ||
| Coenzyme Q 10 (CoQ10) | antioxidant (↓ oxidative stress), enhance vitamin E | [88] |
| Collagen peptides | boost collagen and elasticity (skin, hair, nails) | [88,90,91,92] |
| Carotenoids | antioxidant, increases collagen, elastin, | |
| Astaxanthin, Lutein | reduces appearance of wrinkles by inhibiting | |
| Zeaxanthin, Lycopene | oxidative stress and MMPs | [88,97,98,99,100,101] |
| Chlorophyll | antioxidant/anticancer | [102] |
| Polyphenols | antioxidant, anti-inflammatory, boost collagen and | |
| Resveratrol, Flavonoids, | elastin, TIMP, SOD, Nrf2, inhibits oxidative stress, | |
| Isoflavonoids, Green Tea, etc. | NFkappKB, Matrix Metalloproteinases (MMPs) | [8,36,50,75,96,98,99,100,101] |
| Probiotics | reduces oxidative stress and photoaging | [104,105,106] |
| Daily Habits/Negative Impact | ||
| Advanced Glycation | toxins causing skin damage | |
| End Products (AGEs) | skin inflammation/stiffening | [114,115] |
| Alcohol | dehydration/inflammation decrease skin barrier | [115,116,125] |
| Smoking (vaping) | damage skin/skin aging many negative influences | [125,126] |
| High Fat | skin inflammation/damage/infections | [83,134,135,136,137] |
| Increased BMI (Obesity) | skin inflammation/damage/infections | [83,134,135,136,137] |
| Factors Improving Sleep Quality | Factors Deteriorating Sleep Quality | References | |
|---|---|---|---|
| Diet | |||
| Balanced diet | Intake of inflammatory foods (sugar, alcohol, etc.) | [146,147] | |
| Carbohydrates | Low-carb diet and High-carb diet (>70% energy) | [146,147] | |
| Fats | Polyunsaturated Fatty Acids (PUFA) | Saturated Fatty Acids | [146,148] |
| (omega-3 > than omega-6 fatty acids) | Trans Fats | [146,148] | |
| Proteins | Appropriate intake of proteins | Too Low Protein or Too High Protein Intake; | |
| Red Meats and Processed Meats | [146,149] | ||
| Other Factors: | |||
| Caffeine (chocolate, coffee, tea, energy drinks) | [146,150] | ||
| Adequate Vitamin D Levels | Low Vitamin D Levels | [151] | |
| Alcohol (beer, wine, etc.) | [152,153] | ||
| Nicotine (cigarettes, chewing gum, e-cigarettes) | [154,155] | ||
| Regular Physical Exercise | Lack of exercise or obesity | [156,157] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knaggs, H.; Lephart, E.D. Enhancing Skin Anti-Aging through Healthy Lifestyle Factors. Cosmetics 2023, 10, 142. https://doi.org/10.3390/cosmetics10050142
Knaggs H, Lephart ED. Enhancing Skin Anti-Aging through Healthy Lifestyle Factors. Cosmetics. 2023; 10(5):142. https://doi.org/10.3390/cosmetics10050142
Chicago/Turabian StyleKnaggs, Helen, and Edwin D. Lephart. 2023. "Enhancing Skin Anti-Aging through Healthy Lifestyle Factors" Cosmetics 10, no. 5: 142. https://doi.org/10.3390/cosmetics10050142
APA StyleKnaggs, H., & Lephart, E. D. (2023). Enhancing Skin Anti-Aging through Healthy Lifestyle Factors. Cosmetics, 10(5), 142. https://doi.org/10.3390/cosmetics10050142






