Hair Lipid Structure: Effect of Surfactants
Abstract
1. Introduction
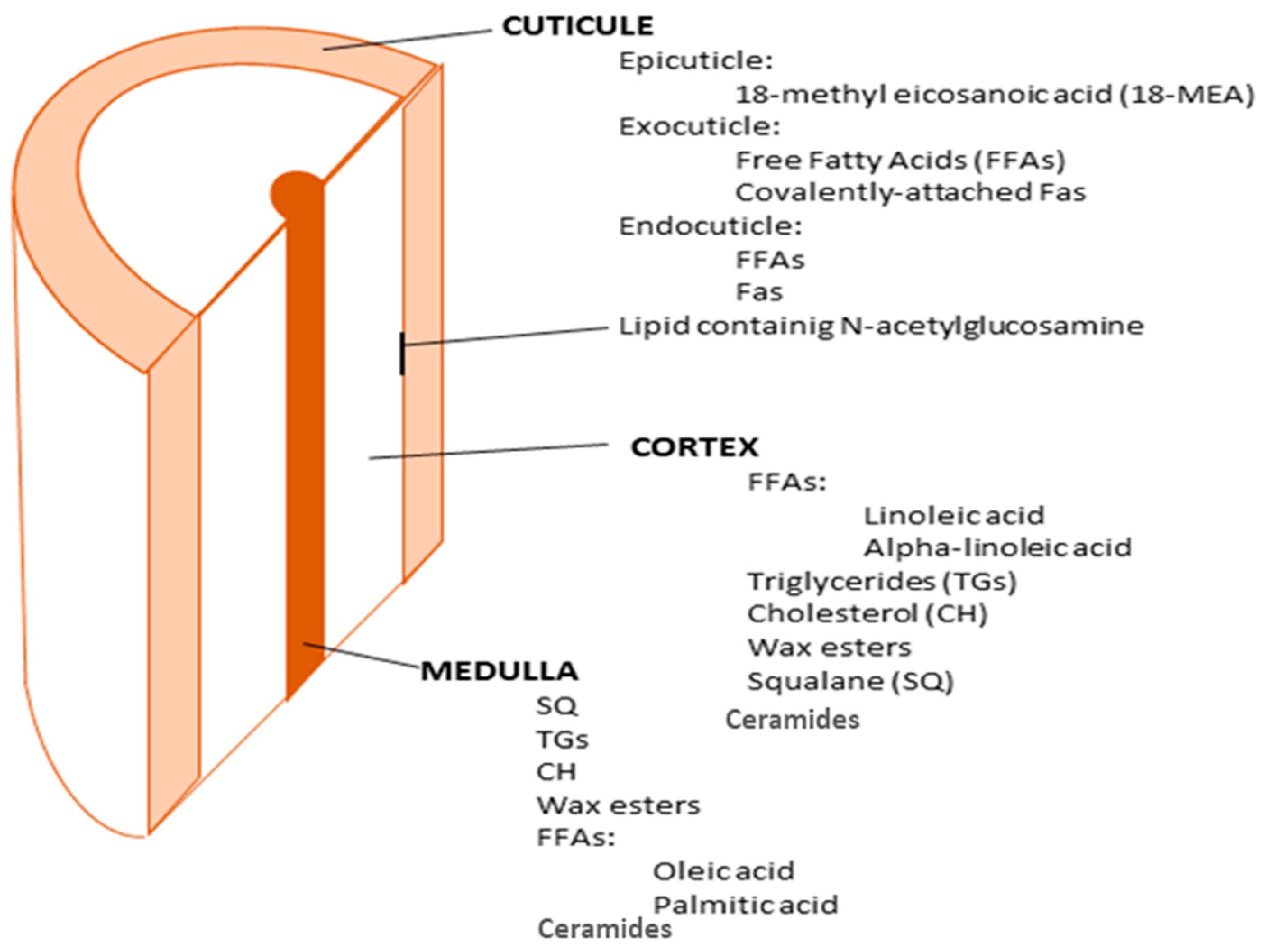
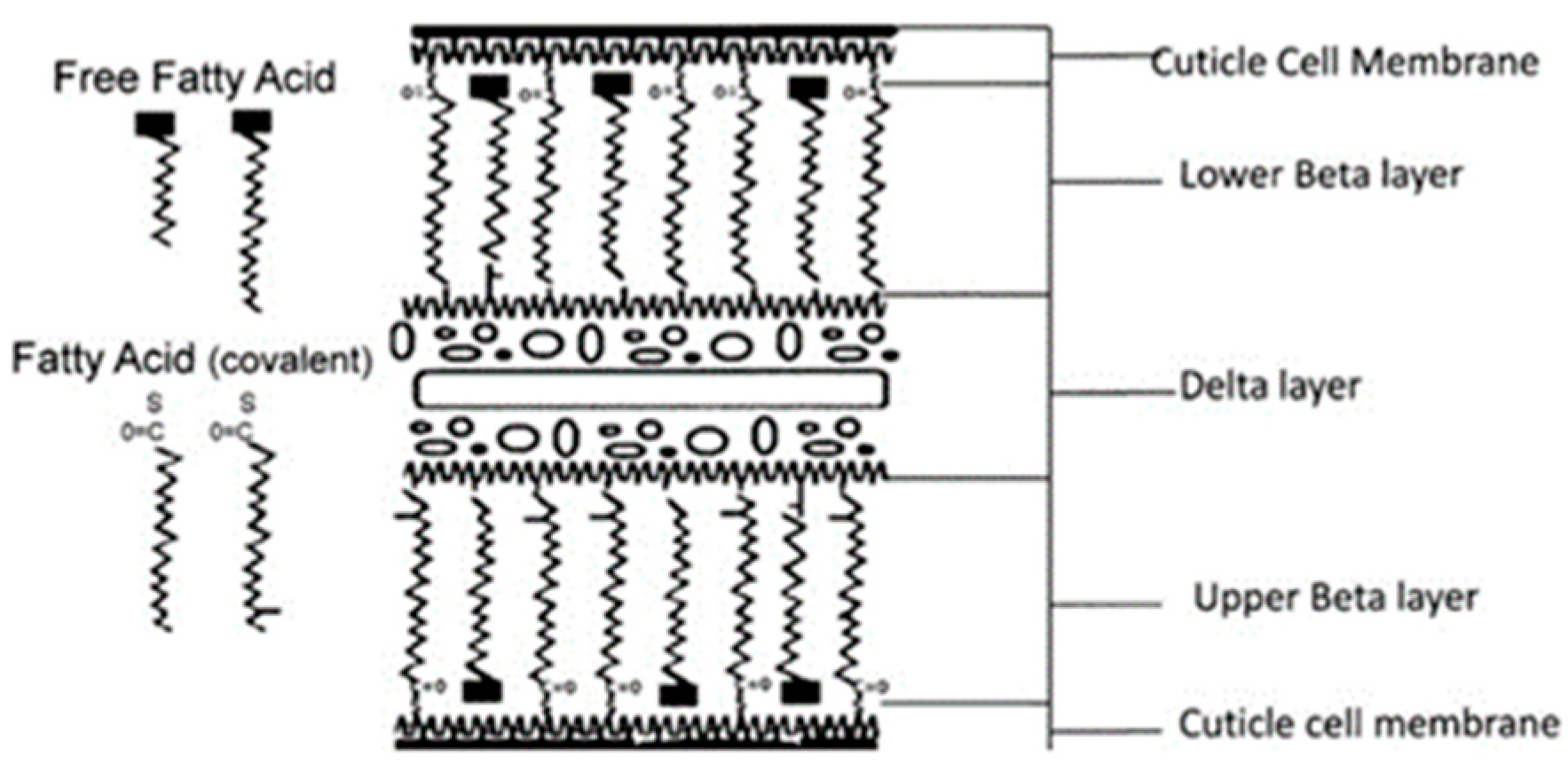
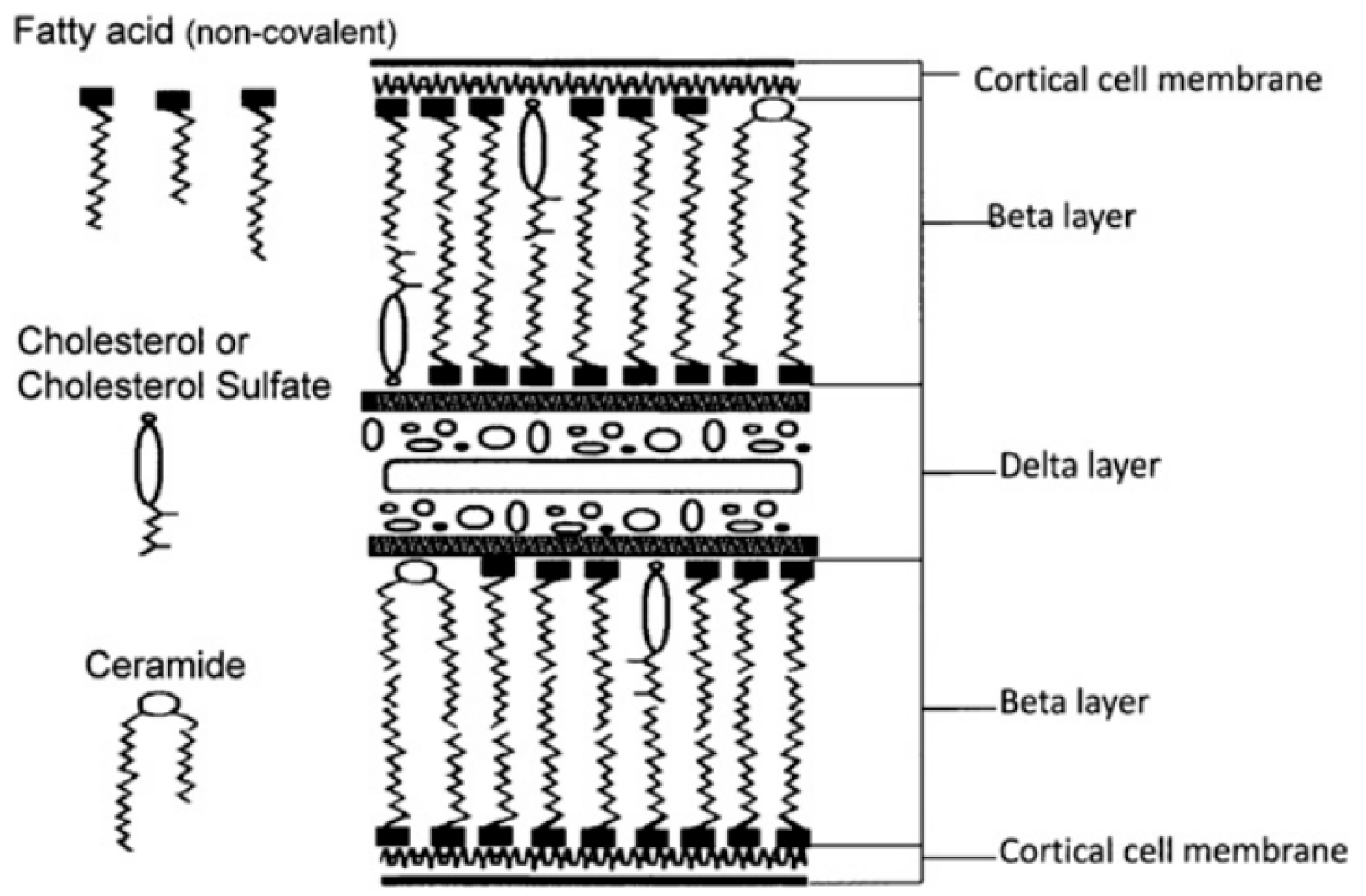
2. Lipids of Hair—Chemical Composition and Structure
3. Lipid Role in Hair Properties
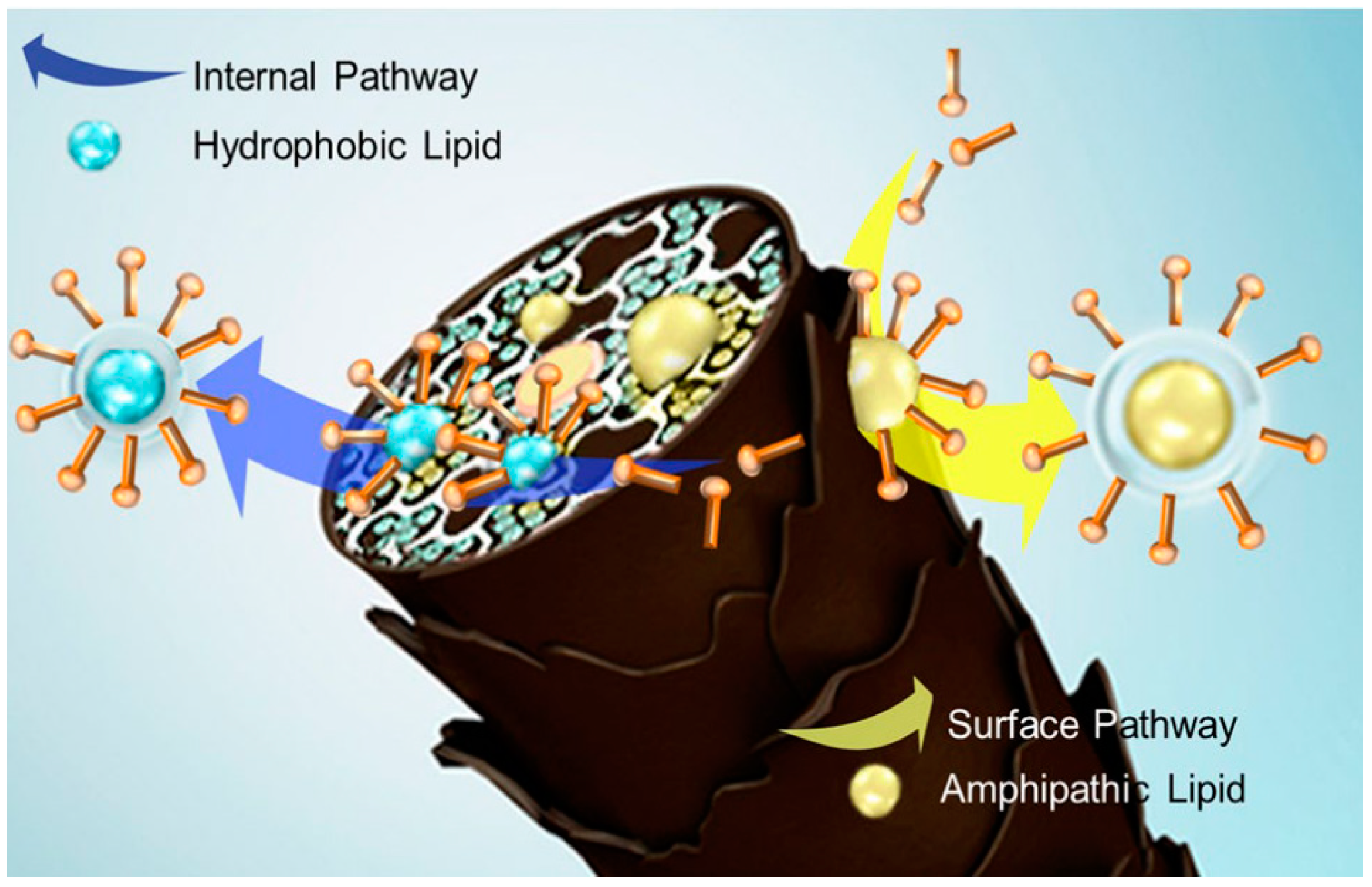
4. Effect of Surfactants on Hair Lipid Depletion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robbins, C.R. Chapter 2. Chemical Composition of Different Hair Types. In Chemical and Physical Behavior of Human Hair, 5th ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 105–166. [Google Scholar]
- McMullen, R.; Laura, D.; Chen, S.; Koelmel, D.; Gillece, T. Determination of physiochemical properties of delipidized hair. J. Cosmet. Sci. 2013, 64, 355–370. [Google Scholar] [PubMed]
- Marsh, J.M.; Whitaker, S.; Felts, T.; Shearouse, W.; Vatter, M.; Määttä, A.; Thompsonm, M.; Hawkins, T.J. Role of Internal Lipids in Hair Health. J. Cosmet. Sci. 2018, 69, 347–356. [Google Scholar]
- Förster, T.; Issberner, U.; Hensen, H. Lipid/surfactant compounds as a new tool to optimize skin-care properties of personal-cleansing products. J. Surfactants Deterg. 2000, 3, 345–352. [Google Scholar] [CrossRef]
- Song, S.; Lim, J.Y.; Son, S.K.; Choi, J.; Kang, N.; Lee, S. Prevention of lipid loss from hair surface and internal modification. Sci. Rep. 2019, 9, 9834. [Google Scholar]
- Joo, K.-M.; Kim, A.-R.; Kim, S.-N.; Kim, B.-M.; Lee, H.K.; Bae, S.; Lee, J.-H.; Lim, K.-M. Metabolomic analysis of amino acids and lipids in human hair altered by dyeing, perming and bleaching. Exp. Dermatol. 2016, 25, 729–731. [Google Scholar] [CrossRef] [PubMed]
- Bantignies, J.L.; Fuchs, G.; Carr, G.L.; Williams, G.P.; Lutz, D.; Marull, S. Organic reagent interaction with hair spatially characterized by infrared microspectroscopy using synchrotron radiation. Int. J. Cosmet. Sci. 1998, 20, 381–394. [Google Scholar] [CrossRef]
- Breakspear, S.; Smith, J.R.; Luengo, G. Effect of the covalently linked fatty acid 18-MEA on the nanotribology of hair’s outermost surface. J. Struct. Biol. 2005, 149, 235–242. [Google Scholar] [CrossRef]
- Hilterhaus-Bong, S.; Zahn, H. Contributions to the chemistry of human hair: II. Lipid chemical aspects of permanently waved hair. Int. J. Cosmet. Sci. 1989, 11, 167–174. [Google Scholar] [CrossRef]
- Csuka, D.A.; Csuka, E.A.; Juhász, M.L.; Sharma, A.N.; Mesinkovska, N.A. A systematic review on the lipid composition of human hair. Int. J. Dermatol. 2023, 62, 404–415. [Google Scholar] [CrossRef]
- Clay, C.; Cook, K.; Rough, J.I. Studies in the composition of human hair. J. Am. Chem. Soc. 1940, 62, 2709–2710. [Google Scholar] [CrossRef]
- Simmonds, D.H. The amino acid composition of keratins. Text. Res. J. 1958, 28, 314–317. [Google Scholar] [CrossRef]
- Robbins, C.R.; Kelly, C.H. Amino acid composition of human hair. Text. Res. J. 1970, 40, 891–896. [Google Scholar] [CrossRef]
- Robbins, C.R. Chemical aspects of bleaching human hair. J. Soc. Cosmet. Chem. 1971, 22, 339–348. [Google Scholar]
- Tanaka, S.; Imura, H.; Sugiyama, T. Study of the test method of reduction and recovery of disulfide bond in human hair. J. Soc. Cosmet. Chem. Jpn. 1992, 25, 232–239. [Google Scholar] [CrossRef]
- Lee, W.S.; Oh, T.H.; Chun, S.H. Integral lipid in human hair follicle. J. Investig. Dermatol. Symp. Proc. 2005, 10, 234–237. [Google Scholar] [CrossRef]
- Coderch, L.; Oliver, M.A.; Martínez, V.; Manich, A.M.; Rubio, L.; Martí, M. Exogenous and endogenous lipids of human hair. Skin. Res. Technol. 2017, 23, 479–485. [Google Scholar] [CrossRef]
- Naito, S.; Ooshika, M.; Yorinoto, N.; Kuroda, Y. The structure of bound lipids of human hair fibers and its physical properties. Proc. 9th Int. Wool Text. Res. Conf. Biella, Italy 1995, 2, 367–374. [Google Scholar]
- Marcott, C.; Lo, M.; Kjoller, K.; Fiat, F.; Baghdadli, N.; Balooch, G.; Luengo, G. Localization of human hair structural lipids using nanoscale infrared spectroscopy and imaging. Appl. Spectrosc. 2014, 68, 564–569. [Google Scholar] [CrossRef]
- Zhang, G.; Senak, L.; Moore, D.J. Measuring changes in chemistry, composition, and molecular structure within hair fibers by infrared and Raman spectroscopic imaging. J. Biomed. Opt. 2011, 16, 056009. [Google Scholar] [CrossRef]
- Kreplak, L.; Briki, F.; Duvault, Y.; Doucet, J.; Merigoux, C.; Leroy, F.; Lévêque, J.L.; Miller, L.; Carr, G.L.; Williams, G.P.; et al. Profiling lipids across Caucasian and Afro-American hair transverse cuts, using synchrotron infrared microspectrometry. Int. J. Cosmet. Sci. 2001, 23, 369–374. [Google Scholar] [CrossRef]
- Wertz, P.W.; Downing, D.T. Integral lipids of human hair. Lipids 1988, 23, 878–881. [Google Scholar] [CrossRef]
- Ryu, H.K.; Jung, B.H.; Kim, K.M.; Yoo, E.A.; Woo, J.-T.; Chung, B.C. Determination of cholesterol in human hair using gas chromatography-mass spectrometry. Biomed. Chromatogr. 2006, 20, 999–1003. [Google Scholar] [CrossRef]
- Robbins, C.R. Chemical and Physical Behavior of Human Hair; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Okamoto, M.; Ishikawa, K.; Tanji, N.; Aoyagi, S.; Kita, I.; Migita, C.T. Structural analysis of the outermost hair surface using TOF-SIMS with C60 depth profiling technique. E-J. Surf. Sci. Nanotechnol. 2012, 10, 234–238. [Google Scholar] [CrossRef]
- Lshikawa, K.; Okamotoy, M.; Aoyag, S. Structural analysis of the outermost hair surface using TOF-SIMS with gas cluster ion beam sputtering. Biointerphases 2016, 11, 02A315. [Google Scholar] [CrossRef]
- Rogers, G.E. Known and unknown features of hair cuticle structure: A brief review. Cosmetics 2019, 6, 32. [Google Scholar] [CrossRef]
- Fellows, A.P.; Casford, M.T.L.; Davies, P.B. Nanoscale molecular characterization of hair cuticle cells using integrated atomic force microscopy-infrared laser spectroscopy. Appl. Spectrosc. 2020, 74, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Destaillats, F.; Guitard, M.; Cruz-Hernandez, C. Identification of D6-monounsaturated fatty acids in human hair and nail samples by gas-chromatography-mass-spectrometry using ionic-liquid coated capillary column. J. Chromatogr. A 2011, 1218, 9384–9389. [Google Scholar] [CrossRef]
- Takahashi, T.; Yoshida, S. A highly resistant structure between cuticle and cortex of human hair. Int. J. Cosmet. Sci. 2017, 39, 327–336. [Google Scholar] [CrossRef]
- Takahashi, T.; Yoshida, S. Distribution of glycolipid and unsaturated fatty acids in human hair. Lipids 2014, 49, 905–917. [Google Scholar] [CrossRef]
- Dawber, R. Hair: Its structure and response to cosmetic preparations. Clin. Dermatol. 1996, 14, 105–112. [Google Scholar] [CrossRef]
- Fellows, A.P.; Casford, M.T.; Davies, P.B. Using hybrid atomic force microscopy and infrared spectroscopy (AFM-IR) to identify chemical components of the hair medulla on the nanoscale. J. Microsc. 2021, 284, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Barba, C.; Oliver, M.A.; Martí, M.; Kreuzer, M.; Coderch, L. Lipid distribution on ethnic hairs by Fourier transform infrared synchrotron spectroscopy. Skin. Res. Technol. 2022, 28, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Ymazaki, J.; Maeda, K. Analysis of lipids in the medulla of Japanese hair and their function. Cosmetics 2018, 5, 27. [Google Scholar] [CrossRef]
- Kaneta, D.; Goto, M.; Hagihara, M.; Leproux, P.; Couderc, V.; Egawa, M.; Kano, H. Visualizing intra-medulla lipids in human hair using ultra-multiplex CARS, SHG, and THG microscopy. Analyst 2021, 146, 1163–1168. [Google Scholar] [CrossRef]
- Sandt, C.; Borondics, F. A new typology of human hair medullas based on lipid composition analysis by synchrotron FTIR microspectroscopy. Analyst 2021, 146, 3942–3954. [Google Scholar] [CrossRef]
- Negri, A.P.; Cornell, H.J.; Rivett, D.E. A model for the surface of keratin fibers. Text Res. J. 1993, 63, 109–115. [Google Scholar]
- Swift, J.A. Human hair cuticle: Biologically conspired to the owner’s advantage. J. Cosmet. Sci. 1999, 50, 23–47. [Google Scholar]
- Luengo, G.; Schmitt, F.J.; Hill, R.; Israelachvili, J. Thin film rheology and tribology of confined polymer melts: Contrast with bulk properties. Macromolecules 1997, 30, 2482–2494. [Google Scholar] [CrossRef]
- Zahn, H.; Messenger, H.; Hocker, H. Covalently linked fatty acids at the surface of wool: Part of the cuticle cell envelope. Text. Res. J. 1994, 64, 554–555. [Google Scholar] [CrossRef]
- Robbins, C.R. The cell membrane complex: Three related but different cellular cohesion components of mammalian hair fibers. J. Cosmet. Sci. 2009, 60, 437–465. [Google Scholar] [CrossRef]
- Naito, S.; Takahashi, T.; Hattori, M.; Arai, K. Histochemical observation of the cell membrane complex of hair. Sen-I Gakkaishi 1992, 48, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Sideris, V.; Holt, L.A.; Leaver, I.H. A microscopical study of the pathway for diffusion of rhodamine B and octadecylrhodamine B into wool fibers. J. Soc. Dyers Color. 1990, 106, 131–135. [Google Scholar] [CrossRef]
- Nishimura, K.; Nishino, M.; Inaoka, Y.; Kitada, Y.; Fukushima, M. Interrelationship between the hair lipids and the hair moisture. Nippon. Koshohin Kagakkaishi 1989, 13, 134–139. [Google Scholar]
- Philippe, M.; Garson, J.C.; Gilard, P.; Hocquaux, M.; Hussler, G.; Leroy, F.; Mahieu, C.; Semeria, D.; Vanlerberghe, G. Synthesis of 2-N-oleoylamino-octadecane-1,3-diol: A new ceramide highly effective for the treatment of skin and hair. Int. J. Cosmet. Sci. 1995, 17, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.N.; Rivett, D.E. 18-Methyleicosanoic acid in the structure and formation of mammalian hair fibres. Micron 1997, 28, 469–485. [Google Scholar] [CrossRef] [PubMed]
- Coderch, L.; Méndez, S.; Barba, C.; Pons, R.; Martí, M.; Parra, J.L. Lamellar rearrangement of internal lipids from human hair. Chem. Phys. Lipids 2008, 155, 1–6. [Google Scholar] [CrossRef]
- Smith, J.; Swift, J. Maple syrup urine disease hair reveals the importance of 18-methyleicosanoic acid in cuticular delamination. Micron 2005, 36, 261–266. [Google Scholar] [CrossRef]
- Ishihara, A.; Tsukamoto, Y.; Inoue, H.; Noda, Y.; Koizumi, S.; Joko, K. Analysis of water distribution in delipidated human hair by small-angle neutron scattering (SANS). Int. J. Cosmet. Sci. 2021, 43, 653–661. [Google Scholar] [CrossRef]
- Evans, T. Measuring the water content of hair. Cosmet. Toil 2014, 129, 1–5. [Google Scholar]
- Duvel, L.; Chun, H.; Deppa, D.; Wertz, P.W. Analysis of hair lipids and tensile properties as a function of distance from scalp. Int. J. Cosmet. Sci. 2005, 27, 193–197. [Google Scholar] [CrossRef]
- Coderch, L.; Oliver, M.A.; Carrer, V.; Manich, A.M.; Martí, M. External lipid function in ethnic hairs. J. Cosmet. Dermatol. 2019, 18, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- Tanamachi, H.; Tokunaga, S.; Tanji, N.; Oguri, M.; Inoue, S. 18-MEA and hair appearance. J. Cosmet. Sci. 2010, 61, 147–160. [Google Scholar]
- Daniels, G.; Fraser, A.; Westgate, G.E. How different is human hair? A critical appraisal of the reported differences in global hair fibre characteristics and properties towards defining a more relevant framework for hair type classification. Int. J. Cosmet. Sci. 2023, 45, 50–61. [Google Scholar] [CrossRef]
- Martí, M.; Barba, C.; Manich, A.M.; Rubio, L.; Alonso, C.; Coderch, L. The influence of hair lipids in ethnic hair properties. Int. J. Cosmet. Sci. 2016, 38, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Oliver, M.A.; Coderch, L.; Carrer, V.; Barba, C.; Martí, M. Ethnic hair: Thermoanalytical and spectroscopic differences. Skin. Res. Technol. 2020, 26, 617–626. [Google Scholar] [CrossRef]
- Bildstein, L.; Deniset-Besseau, A.; Pasini, I.; Mazilier, C.; Keuong, Y.W.; Dazzi, A.; Baghdadli, N. Discrete nanoscale distribution of hair lipids fails to provide humidity resistance. Anal. Chem. 2020, 92, 11498–11504. [Google Scholar] [CrossRef]
- Cruz, C.F.; Fernandes, M.M.; Gomes, A.C.; Coderch, L.; Martí, M.; Méndez, S.; Gales, L.; Azoia, G.G.; Shimanovich, U.; Cavaco-Paulo, A. Keratins and lipids in ethnic hair. Int. J. Cosmet. Sci. 2013, 35, 244–249. [Google Scholar] [CrossRef]
- Maymone, M.B.; Laughter, M.; Pollock, S.; Khan, I.; Marques, T.; Abdat, R.; Vashi, N.A. Hair aging in different races and ethnicities. J. Clin. Aesthetic Dermatol. 2021, 14, 38. [Google Scholar]
- Van Wicklin, S.A. Hair Aging in Different Races and Ethnicities. Plast. Aesthetic Nurs. 2023, 43, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Oliver, M.A.; Martí, M.; Coderch, L.; Carrer, V.; Kreuzer, M.; Barba, C. Lipid loses and barrier function modifications of the brown-to-white hair transition. Skin. Res. Technol. 2019, 25, 517–525. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, Y.D.; Pi, L.-Q.; Lee, S.Y.; Hong, H.; Lee, W.-S. Comparison of hair shaft damage after chemical treatment in Asian, White European, and African hair. Int. J. Dermatol. 2014, 53, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Masukawa, Y.; Tsujimura, H.; Narita, H. Liquid chromatographymass spectrometry for comprehensive profiling of ceramide molecules in human hair. J. Lipid Res. 2006, 47, 1559–1571. [Google Scholar] [CrossRef] [PubMed]
- Coderch, L.; Lorenzo, R.D.; Mussone, M.; Alonso, C.; Martí, M. The Role of Lipids in the Process of Hair Ageing. Cosmetics 2022, 9, 124. [Google Scholar] [CrossRef]
- Takahashi, T.; Mamada, A.; Breakspear, S.; Itou, T.; Tanji, N. Age-dependent changes in damage processes of hair cuticle. J. Cosmet. Dermatol. 2015, 14, 2–8. [Google Scholar] [CrossRef]
- Vyumvuhore, R.; Verzeaux, L.; Gilardeau, S.; Bordes, S.; Aymard, E.; Manfait, M.; Closs, B. Investigation of the molecular signature of greying hair shafts. Int. J. Cosmet. Sci. 2021, 14, 2–8. [Google Scholar] [CrossRef]
- Ma, Y.; He, C. Exploration of potential lipid biomarkers for age-induced hair graying by lipidomic analyses of hair shaft roots with follicular tissue attached. J. Cosmet. Dermatol. 2022, 21, 6118–6123. [Google Scholar] [CrossRef] [PubMed]
- Habe, T.; Tanji, N.; Inoue, S.; Okamoto, M.; Tokunaga, S.; Tanamachi, H. ToF-SIMS characterization of the lipid layer on the hair surface. I: The damage caused by chemical treatments and UV radiation. Surf. Interface Anal. 2011, 43, 410–412. [Google Scholar] [CrossRef]
- Ji, J.H.; Park, T.S.; Lee, H.-J.; Kim, Y.-D.; Pi, L.-Q.; Jin, X.-H.; Lee, W.-S. The ethnic differences of the damage of hair and integral hair lipid after ultra violet radiation. Ann. Dermatol. 2013, 25, 54–60. [Google Scholar] [CrossRef]
- Richena, M.; Rezende, C.A. Structure of photo-damaged white and naturally pigmented human hair. J. Photochem. Photobiol. B Biol. 2020, 202, 111673. [Google Scholar] [CrossRef]
- Ross, A.B.; Maes, E.; Lee, E.J.; Homewood, I.; Marsh, J.M.; Davis, S.L.; Willicut, R.J. UV and visible light exposure to hair leads to widespread changes in the hair lipidome. Int. J. Cosmet. Sci. 2022, 44, 672–684. [Google Scholar] [CrossRef]
- Sandt, C.; Borondics, F. Super-resolution infrared microspectroscopy reveals heterogeneous distribution of photosensitive lipids in human hair medulla. Talanta 2023, 254, 124152. [Google Scholar] [CrossRef]
- Tokunaga, S.; Tanamachi, H.; Ishikawa, K. Degradation of hair surface: Importance of 18-MEA and epicuticle. Cosmetics 2019, 6, 31. [Google Scholar] [CrossRef]
- Reis, M.F.; Dias, G. Hair Cosmetics: An Overview. Int. J. Trichology 2015, 7, 2–15. [Google Scholar]
- Deeksha; Malviya, R.; Sharma, P.K. Advancement in shampoo (a dermal care product): Preparation methods, patents and commercial utility. Recent. Pat. Inflamm. Allergy Drug Discov. 2014, 8, 48–58. [Google Scholar] [CrossRef]
- Draelos, Z.D. Essentials of Hair Care often Neglected: Hair Cleansing. Int. J. Trichology 2010, 2, 24–29. [Google Scholar] [CrossRef]
- Robbins, C.R. Chapter 6. Interactions of Shampoo and Conditioner Ingredients with Hair. In Chemical and Physical Behavior of Human Hair, 5th ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 329–436. [Google Scholar]
- Ajayi, O.; Davies, A.; Amin, S. Impact of Processing Conditions on Rheology, Tribology and Wet Lubrication Performance of a Novel Amino Lipid Hair Conditioner. Cosmetics 2021, 8, 77. [Google Scholar] [CrossRef]
- Luengo, G.S.; Fameau, A.L.; Leonforte, F.; Greaves, A.J. Surface science of cosmetic substrates, cleansing actives and formulations. Adv. Colloid Interface Sci. 2021, 290, 102383. [Google Scholar] [CrossRef]
- Fernandes, C.; Medronho, B.; Alves, L.; Rasteiro, M.G. On Hair Care Physicochemistry: From Structure and Degradation to Novel Biobased Conditioning Agents. Polymers 2023, 15, 608. [Google Scholar] [CrossRef]
- La Torre, C.; Bhushan, B. Nanotribological effects of silicone type, silicone deposition level, and surfactant type on human hair using atomic force microscopy. J. Cosmet. Sci. 2006, 57, 37–56. [Google Scholar]
- Nazir, H.; Lv, P.; Wang, L.; Lian, G.; Zhu, S.; Ma, G. Uniform-sized silicone oil microemulsions: Preparation, investigation of stability and deposition on hair surface. J. Colloid. Interface Sci. 2011, 364, 56–64. [Google Scholar] [CrossRef]
- Nazir, H.; Wang, L.; Lian, G.; Zhu, S.; Zhang, Y.; Liu, Y.; Ma, G. Multilayered silicone oil droplets of narrow size distribution: Preparation and improved deposition on hair. Colloids Surf. B Biointerfaces 2012, 10, 42–49. [Google Scholar] [CrossRef] [PubMed]
- O’Lenick, T. Anionic/cationic complexes in hair care. J. Cosmet. Sci. 2011, 62, 209–228. [Google Scholar] [PubMed]
- Kourbaj, G.; Bielfeldt, S.; Kruse, I.; Wilhelm, K.P. Confocal Raman spectroscopy is suitable to assess hair cleansing-derived skin dryness on human scalp. Ski. Res. Technol. 2022, 28, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.A. Hair lipid and surfactants. Extraction of lipid by surfactants and lack of effect of shampooing on rate of re-fatting of hair. Int. J. Cosmet. Sci. 1979, 1, 317–328. [Google Scholar] [CrossRef]
- Koch, J.; Aitzetmuller, K.; Bittorf, G.; Waibel, J. Hair lipids and their contribution to the perception of hair oiliness. Part I: Surface and internal lipids in hair. J. Soc. Cosmet. Chem. 1982, 33, 317–326. [Google Scholar]
- Capablanca, J.S.; Watt, I. Factors affecting the zeta potential at wool fiber surfaces. Text. Res. J. 1986, 56, 49–55. [Google Scholar] [CrossRef]
- Zhang, Y.; Alsop, R.J.; Soomro, A.; Yang, F.C.; Rheinstädter, M.C. Effect of shampoo, conditioner and permanent waving on the molecular structure of human hair. PeerJ 2015, 3, e1296. [Google Scholar] [CrossRef]
- Masukawa, Y.; Tsujimura, H. Highly sensitive determination of diverse ceramides in human hair using reversed-phase high performance liquid chromatography-electrospray ionization mass spectrometry. Lipids 2007, 42, 275–290. [Google Scholar] [CrossRef]
- Masukawa, Y.; Tsujimura, H.; Tanamachi, H.; Narita, H.; Imokawa, G. Damage to human hair caused by repeated bleaching combined with daily weathering during daily life activities. Exog. Dermatol. 2004, 3, 273–281. [Google Scholar] [CrossRef]
- Song, S.-H.; Son, S.K.; Kang, N.-G. Archives of clinical and medical case reports 573 case report dual case reports for lipid loss from human hair. Arch. Clin. Med. Case Rep. 2019, 53, 1103–1110. [Google Scholar]
- Marsh, J.M.; Brown, M.A.; Felts, T.J.; Hutton, H.D.; Vatter, M.L.; Whitaker, S.; Wireko, F.C.; Styczynski, P.B.; Henry, I.D. Gel network shampoo formulation and hair health benefits. Int. J. Cosmet. Sci. 2017, 39, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Šindelka, K.; Kowalski, A.; Cooke, M.; Mendoza, C.; Lísal, M. Interactions of cationic surfactant-fatty alcohol monolayers with natural human hair surface: Insights from dissipative particle dynamics. J. Mol. Liq. 2023, 375, 121385. [Google Scholar] [CrossRef]
- Ahmad, A.; Ahsan, H. Lipid-based formulations in cosmeceuticals and biopharmaceuticals. Biomed. Dermatol. 2020, 4, 12. [Google Scholar] [CrossRef]
| Shampoo Surfactants | ||
|---|---|---|
| Class | Example | Characteristics |
| Anionic | Ammonium lauryl sulfate, sodium laureth sulfate, sodium lauryl sarcosinate, sodium myreth sulfate, sodium pareth sulfate, sodium stearte, sodium lauryl sulphate, alpha-olefin sulfonate, ammonium laureth sulphate | Deep cleansing |
| Cationic | Trimethylalkylammonium chlorides and the chlorides or bromides of benzalkonium and alkylpyridinium ions | Hair softener |
| Nonionic | ethoxylated fatty alcohols, cocamide diethanolamine, coco glucosides, polysorbate 20, PEG-80 sorbitan laurate | Mild cleansing |
| Amphoteric | Alkyl iminopropionates (amido) betaines | Mild cleansing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coderch, L.; Alonso, C.; García, M.T.; Pérez, L.; Martí, M. Hair Lipid Structure: Effect of Surfactants. Cosmetics 2023, 10, 107. https://doi.org/10.3390/cosmetics10040107
Coderch L, Alonso C, García MT, Pérez L, Martí M. Hair Lipid Structure: Effect of Surfactants. Cosmetics. 2023; 10(4):107. https://doi.org/10.3390/cosmetics10040107
Chicago/Turabian StyleCoderch, Luisa, Cristina Alonso, M. Teresa García, Lourdes Pérez, and Meritxell Martí. 2023. "Hair Lipid Structure: Effect of Surfactants" Cosmetics 10, no. 4: 107. https://doi.org/10.3390/cosmetics10040107
APA StyleCoderch, L., Alonso, C., García, M. T., Pérez, L., & Martí, M. (2023). Hair Lipid Structure: Effect of Surfactants. Cosmetics, 10(4), 107. https://doi.org/10.3390/cosmetics10040107







