Follicular Delivery of Caffeine from a Shampoo for Hair Retention
Abstract
1. Introduction
2. Materials and Methods
2.1. Shampoo Formulations
2.2. Study Characteristics and Volunteers
2.3. Experimental Setup and Application of the Formulations
2.4. Differential Stripping of the Treatment Areas
2.5. Extraction of Caffeine from the Tapes
2.5.1. Cyanoacrylate Tape Strip
2.5.2. Stratum Corneum Tape Strips
2.6. Analysis of Caffeine Content by Isotope-Dilution Liquid Chromatography Coupled with Tandem-Mass Spectrometry (LC-MS/MS)
2.7. Statistical Analysis
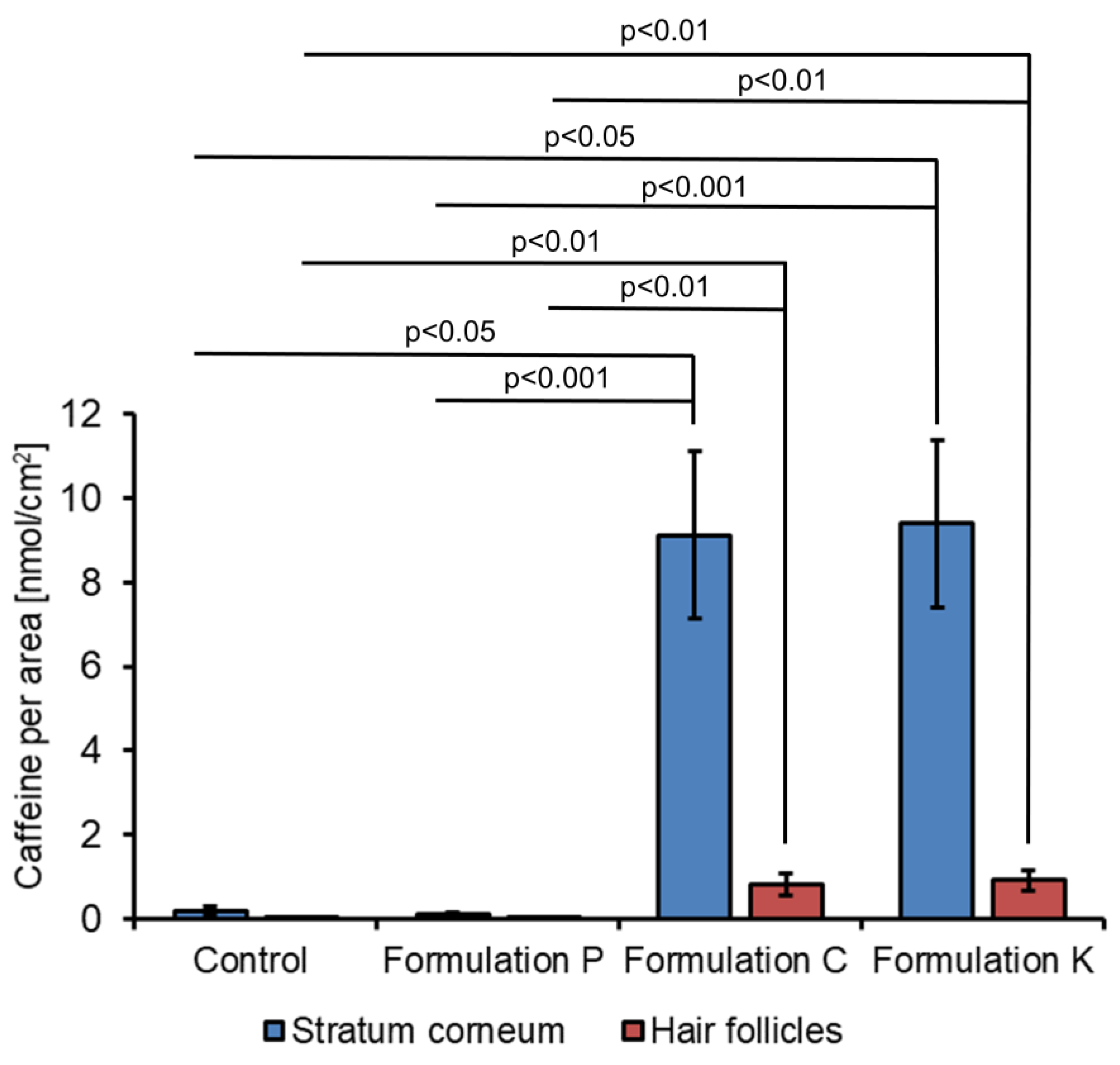
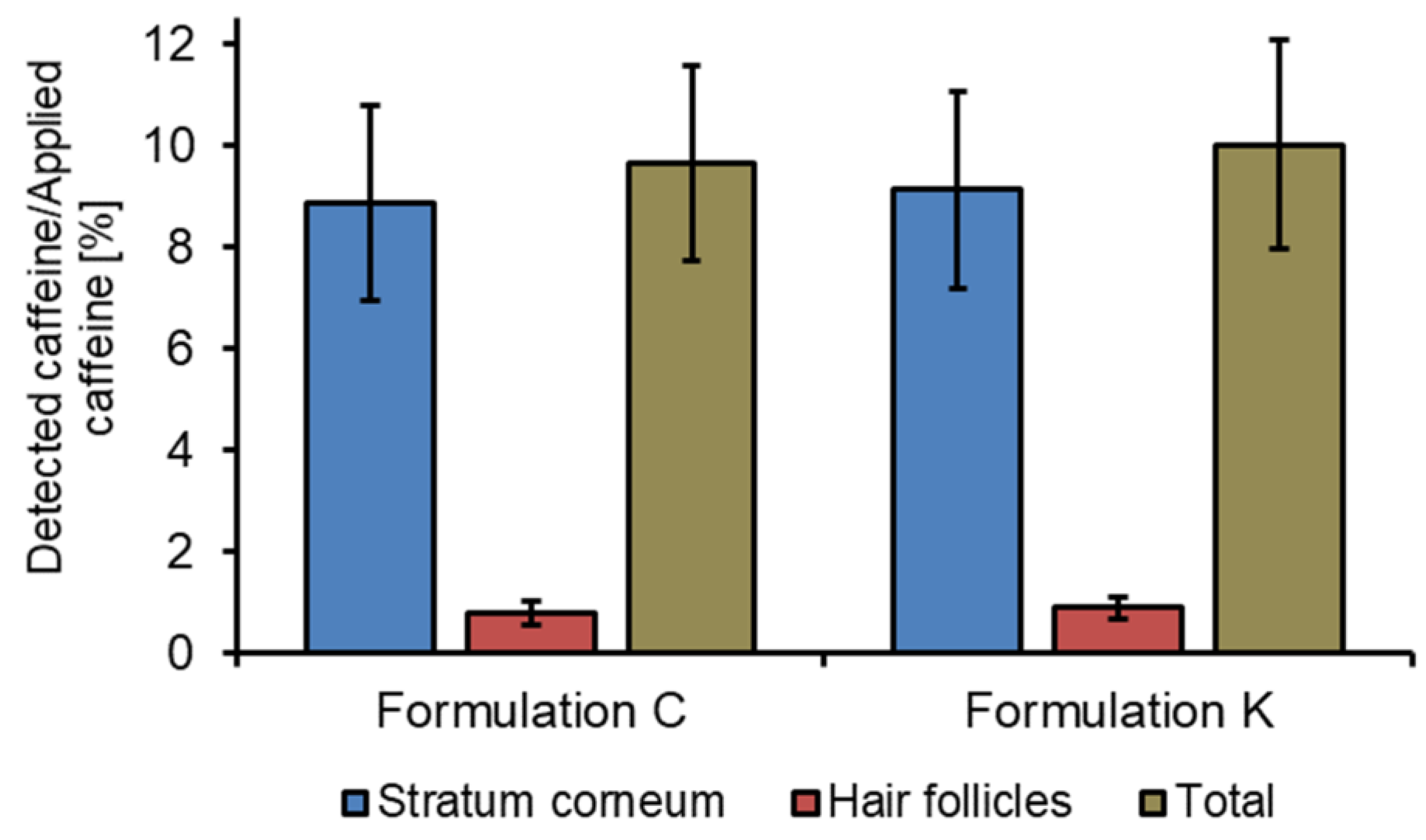
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mulinari-Brenner, F.; Bergfeld, W.F. Hair loss: An overview. Dermatol. Nurs. 2001, 13, 269. [Google Scholar] [PubMed]
- Alessandrini, A.; Bruni, F.; Piraccini, B.M.; Starace, M. Common causes of hair loss–clinical manifestations, trichoscopy and therapy. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Robbins, C.; Mirmirani, P.; Messenger, A.G.; Birch, M.P.; Youngquist, R.S.; Tamura, M.; Filloon, T.; Luo, F.; Dawson, T.L., Jr. What women want–quantifying the perception of hair amount: An analysis of hair diameter and density changes with age in Caucasian women. Br. J. Dermatol. 2012, 167, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Otberg, N.; Restrepo, I.; Shapiro, J. Possibilities and limitations for reversal of age-related hair loss. In Aging Hair; Springer: Berlin/Heidelberg, Germany, 2010; pp. 193–205. [Google Scholar]
- Kaufman, K.D.; Olsen, E.A.; Whiting, D.; Savin, R.; DeVillez, R.; Bergfeld, W.; Price, V.H.; Van Neste, D.; Roberts, J.L.; Hordinsky, M.; et al. Finasteride in the treatment of men with androgenetic alopecia. J. Am. Acad. Dermatol. 1998, 39, 578–589. [Google Scholar] [CrossRef]
- E Buhl, A.; Waldon, D.J.; A Baker, C.; A Johnson, G. Minoxidil sulfate is the active metabolite that stimulates hair follicles. J. Investig. Dermatol. 1990, 95, 553–557. [Google Scholar] [CrossRef]
- Tosti, A.; Schwartz, J.R. Role of scalp health in achieving optimal hair growth and retention. Int. J. Cosmet. Sci. 2021, 43, S1–S8. [Google Scholar] [CrossRef]
- Trüeb, R.M. Oxidative stress and its impact on skin, scalp and hair. Int. J. Cosmet. Sci. 2021, 43, S9–S13. [Google Scholar] [CrossRef]
- Trüeb, R.M.; Henry, J.P.; Davis, M.G.; Schwartz, J.R. Scalp condition impacts hair growth and retention via oxidative stress. Int. J. Trichol. 2018, 10, 262. [Google Scholar] [CrossRef]
- Schwartz, J.R.; Henry, J.P.; Kerr, K.M.; Mizoguchi, H.; Li, L. The role of oxidative damage in poor scalp health: Ramifications to causality and associated hair growth. Int. J. Cosmet. Sci. 2015, 37 (Suppl. S2), 9–15. [Google Scholar] [CrossRef]
- Schwartz, J.R.; Henry, J.P.; Kerr, K.M.; Flagler, M.J.; Page, S.H.; Redman-Furey, N. Incubatory environment of the scalp impacts pre-emergent hair to affect post-emergent hair cuticle integrity. J. Cosmet. Dermatol. 2018, 17, 105–111. [Google Scholar] [CrossRef]
- Niki, E. Lipid oxidation in the skin. Free Radic. Res. 2015, 49, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Ambaw, Y.A.; Pagac, M.P.; Irudayaswamy, A.S.; Raida, M.; Bendt, A.K.; Torta, F.T.; Wenk, M.R.; Dawson, T.L., Jr. Host/malassezia interaction: A quantitative, non-invasive method profiling oxylipin production associates human skin eicosanoids with malassezia. Metabolites 2021, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Meyer, L.E.; Otberg, N.; Tietz, H.-J.; Sterry, W.; Lademann, J. In vivo imaging of Malassezia yeasts on human skin using confocal laser scanning microscopy. Laser Phys. Lett. 2005, 2, 148–152. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.S.; Chang, D.W.; Schwartz, J.R.; Blume-Peytavi, U.; Henry, J.P.; Caterino, T.L.; Talley, A.L. Enhanced piroctone olamine retention from shampoo for superior anti-dandruff efficacy. Int. J. Cosmet. Sci. 2023, 45, 236–245. [Google Scholar] [CrossRef]
- Davis, M.G.; Piliang, M.P.; Bergfeld, W.F.; Caterino, T.L.; Fisher, B.K.; Sacha, J.P.; Carr, G.J.; Moulton, L.T.; Whittenbarger, D.J.; Punyani, S.; et al. Scalp application of the antioxidant piroctone olamine reduces hair shedding in an 8-week randomized, double-blind, placebo-controlled clinical study. Int. J. Cosmet. Sci. 2021, 43, S26–S33. [Google Scholar] [CrossRef]
- Shi, X.; Dalal, N.S.; Jain, A.C. Antioxidant behavior of caffeine: Efficient scavenging of hydroxyl radicals. Food Chem. Toxicol. 1991, 29, 1–6. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Caffeine’s mechanisms of action and its cosmetic use. Skin Pharmacol. Physiol. 2012, 26, 8–14. [Google Scholar] [CrossRef]
- Devasagayam, T.; Kamat, J.; Mohan, H.; Kesavan, P. Caffeine as an antioxidant: Inhibition of lipid peroxidation induced by reactive oxygen species. Biochim. Biophys Acta 1996, 1282, 63–70. [Google Scholar] [CrossRef]
- Otberg, N.; Teichmann, A.; Rasuljev, U.; Sinkgraven, R.; Sterry, W.; Lademann, J. Follicular penetration of topically applied caffeine via a shampoo formulation. Skin Pharm. Physiol. 2007, 20, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Teichmann, A.; Richter, H.; Knorr, F.; Antoniou, C.; Sterry, W.; Lademann, J. Investigation of the penetration and storage of a shampoo formulation containing caffeine into the hair follicles by in vivo laser scanning microscopy. Laser Phys. Lett. 2007, 4, 464–468. [Google Scholar] [CrossRef]
- Lademann, J.; Richter, H.; Schanzer, S.; Klenk, A.; Sterry, W.; Patzelt, A. Analysis of the penetration of a caffeine containing shampoo into the hair follicles by in vivo laser scanning microscopy. Laser Phys. 2010, 20, 551–556. [Google Scholar] [CrossRef]
- Teichmann, A.; Jacobi, U.; Ossadnik, M.; Richter, H.; Koch, S.; Sterry, W.; Lademann, J. Differential stripping: Determination of the amount of topically applied substances penetrated into the hair follicles. J. Investig. Dermatol. 2005, 125, 264–269. [Google Scholar] [CrossRef]
- Knorr, F.; Lademann, J.; Patzelt, A.; Sterry, W.; Blume-Peytavi, U.; Vogt, A. Follicular transport route–research progress and future perspectives. Eur. J. Pharm. Biopharm. 2009, 71, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Blume-Peytavi, U.; Vogt, A. Human hair follicle: Reservoir function and selective targeting. Br. J. Dermatol. 2011, 165, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.O.H.; Kligman, A.M. The follicular biopsy. Dermatology 1983, 167, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Hadshiew, I.M.; Foitzik, K.; Arck, P.C.; Paus, R. Burden of hair loss: Stress and the underestimated psychosocial impact of telogen effluvium and androgenetic alopecia. J. Investig. Dermatol. 2004, 123, 455–457. [Google Scholar] [CrossRef]
- Davis, M.G.; Piliang, M.P.; Bergfeld, W.F.; Caterino, T.L.; Fisher, B.K.; Sacha, J.P.; Carr, G.J.; Moulton, L.T.; Whittenbarger, D.J.; Schwartz, J.R. Scalp application of antioxidants improves scalp condition and reduces hair shedding in a 24-week randomized, double-blind, placebo-controlled clinical trial. Int. J. Cosmet. Sci. 2021, 43, S14–S25. [Google Scholar] [CrossRef]
- Gehring, W. Nicotinic acid/niacinamide and the skin. J. Cosm. Dermatol. 2004, 3, 88–93. [Google Scholar] [CrossRef]
- Burnett, C.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Hydrolyzed Wheat Protein and Hydrolyzed Wheat Gluten as Used in Cosmetics. Int. J. Toxicol. 2018, 37 (Suppl. S1), 55S–66S. [Google Scholar] [CrossRef]
- Scott, L.N.; Fiume, M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; et al. Safety Assessment of Panthenol, Pantothenic Acid, and Derivatives as Used in Cosmetics. Int. J. Toxicol. 2022, 41 (Suppl. S2), 77–128. [Google Scholar] [CrossRef] [PubMed]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.C.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Glycerin as Used in Cosmetics. Int. J. Toxicol. 2019, 38 (Suppl. S3), 6S–22S. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, F.; Ruifernández, J.M. Distribution of human hair in follicular units: A mathematical model for estimating the donor size in follicular unit transplantation. Dermatol. Surg. 1999, 25, 294–298. [Google Scholar] [CrossRef]



| Formula P | Formula C | Formula K | |
|---|---|---|---|
| Caffeine (%) | None | 1% | 1% |
| Surfactants | |||
| Sodium Alkyl Sulfates | X | X | X |
| Cocamidopropyl Betaine | X | X | |
| Disodium Laureth Sulfosuccinate | X | ||
| Sodium Lauroyl Glutamate | X | ||
| Nonionic Surfactants | X | X | |
| Hair Benefit Agents | |||
| Panthenol, Panthenyl Ethyl Ether | X | X | X |
| Hydrolyzed Wheat Protein | X | ||
| Skin Benefit Agents | |||
| Niacinamide | X | X | |
| Tocopherol | X | ||
| Zinc PCA | X | ||
| Glycerin | X | ||
| Piroctone Olamine | X | ||
| Ginger Root Extract | X | ||
| Cationic Polymer Deposition Aids | X | X | X |
| Sensates | |||
| Menthol | X |
| Control | Formula P | Formula C | Formula K | |
|---|---|---|---|---|
| Caffeine Level (nmoL/cm2) | ||||
| Stratum corneum | 0.18 ± 0.11 | 0.09 ± 0.03 | 9.13 ± 1.98 | 9.40 ± 1.99 |
| Hair Follicles | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.80 ± 0.26 | 0.91 ± 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Busch, L.; Klein, A.L.; Schwartz, J.R.; Pearson, K.; Richter, H.; Schanzer, S.; Lohan, S.B.; Schumacher, F.; Kleuser, B.; Meinke, M.C. Follicular Delivery of Caffeine from a Shampoo for Hair Retention. Cosmetics 2023, 10, 104. https://doi.org/10.3390/cosmetics10040104
Busch L, Klein AL, Schwartz JR, Pearson K, Richter H, Schanzer S, Lohan SB, Schumacher F, Kleuser B, Meinke MC. Follicular Delivery of Caffeine from a Shampoo for Hair Retention. Cosmetics. 2023; 10(4):104. https://doi.org/10.3390/cosmetics10040104
Chicago/Turabian StyleBusch, Loris, Anna Lena Klein, James R. Schwartz, Kathleen Pearson, Heike Richter, Sabine Schanzer, Silke B. Lohan, Fabian Schumacher, Burkhard Kleuser, and Martina C. Meinke. 2023. "Follicular Delivery of Caffeine from a Shampoo for Hair Retention" Cosmetics 10, no. 4: 104. https://doi.org/10.3390/cosmetics10040104
APA StyleBusch, L., Klein, A. L., Schwartz, J. R., Pearson, K., Richter, H., Schanzer, S., Lohan, S. B., Schumacher, F., Kleuser, B., & Meinke, M. C. (2023). Follicular Delivery of Caffeine from a Shampoo for Hair Retention. Cosmetics, 10(4), 104. https://doi.org/10.3390/cosmetics10040104









