Natural Antioxidant-Loaded Nanoemulsions for Sun Protection Enhancement
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
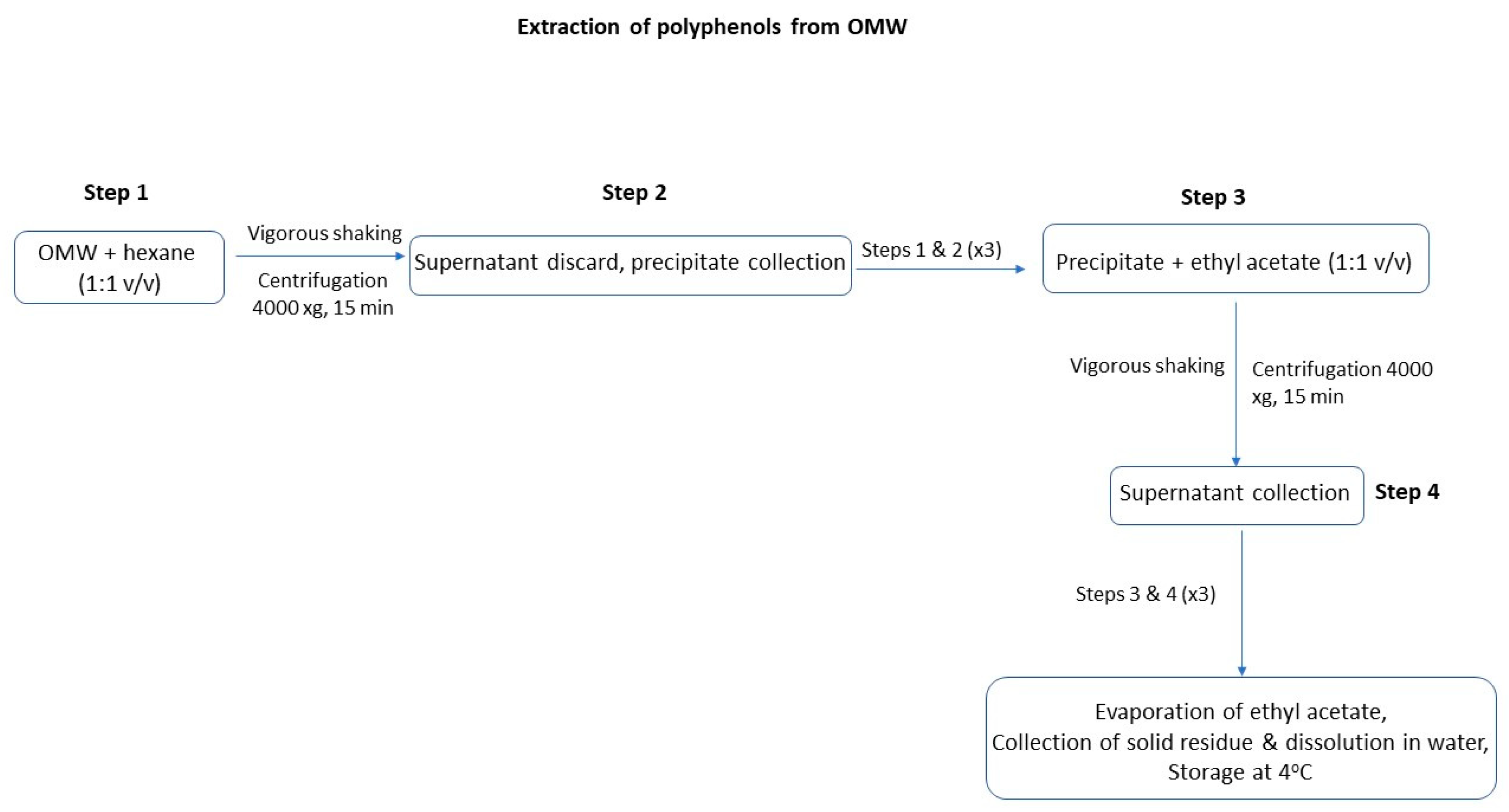
2.2.1. Extraction of Antioxidants from OMW
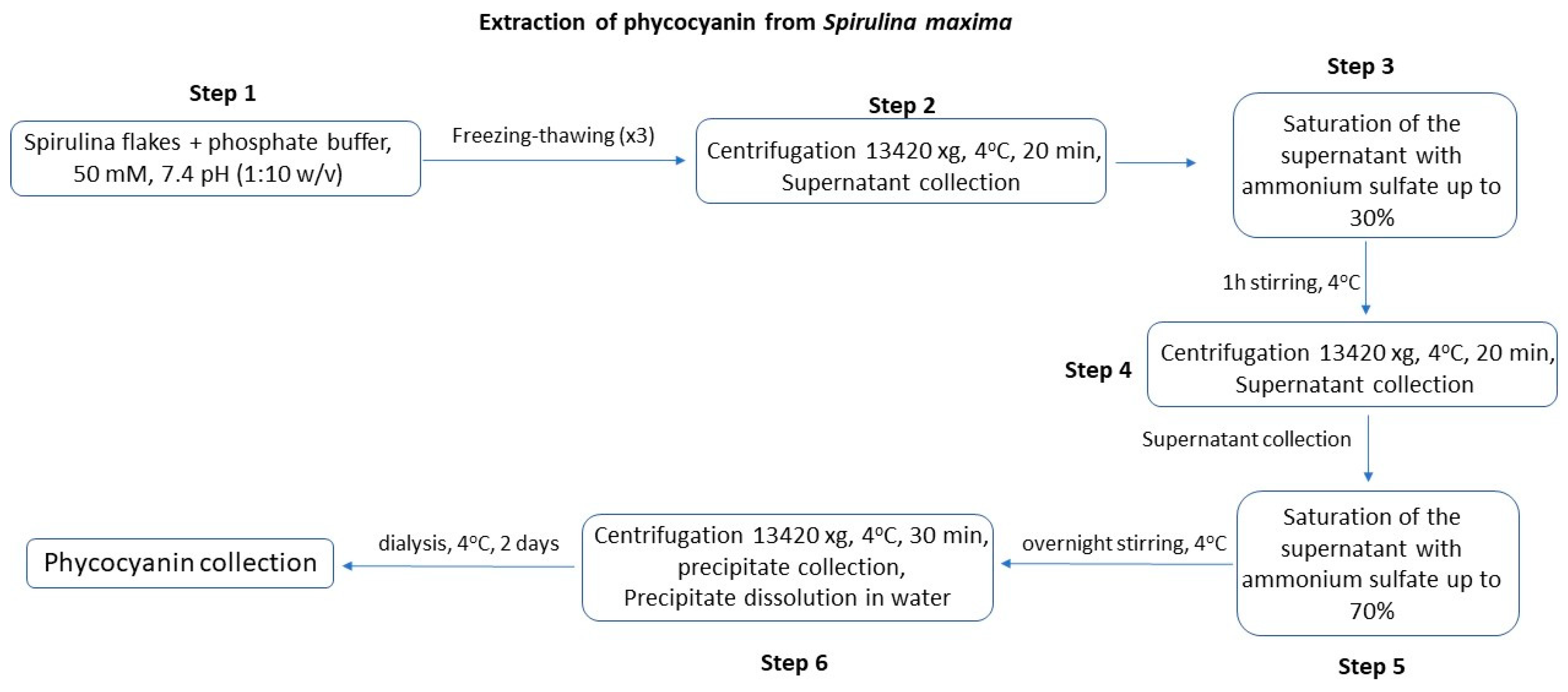
2.2.2. Extraction of Phycocyanin from Spirulina maxima
2.2.3. Determination of the OMW Extracts’ Total Phenol Content
2.2.4. Determination of the Spirulina maxima Extract’s Phycocyanin Content
2.2.5. Preparation of W/O NEs Using the Low-Energy Method
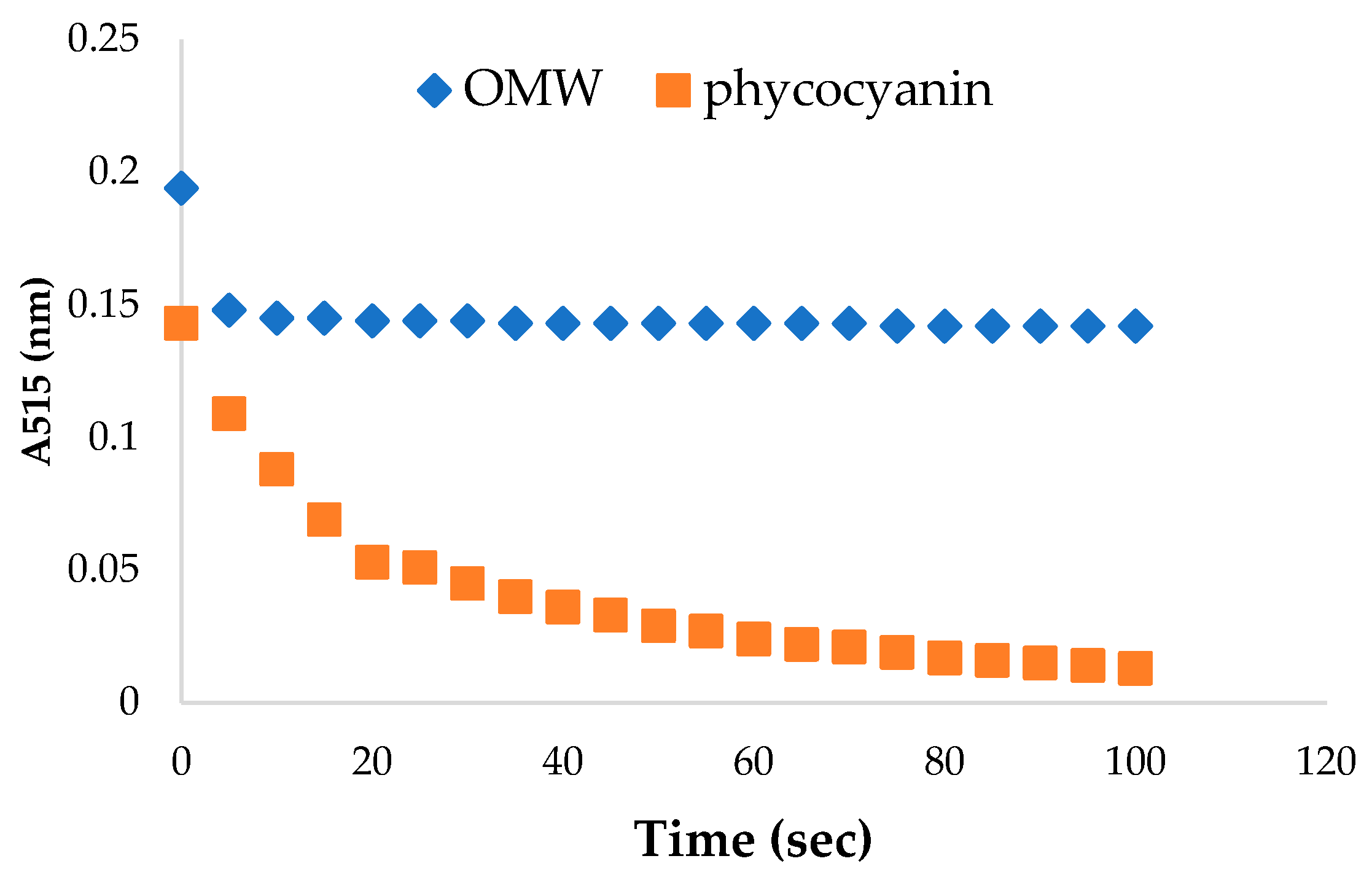
2.2.6. The Antioxidant Assessment of the OMW and Spirulina maxima Extracts via DPPH Scavenging
2.2.7. Determination of the Antioxidant Activity Using the Galvinoxyl Free Radical Detected via Electron Paramagnetic Resonance (EPR) Spectroscopy
2.2.8. Dynamic Light Scattering (DLS) Measurements
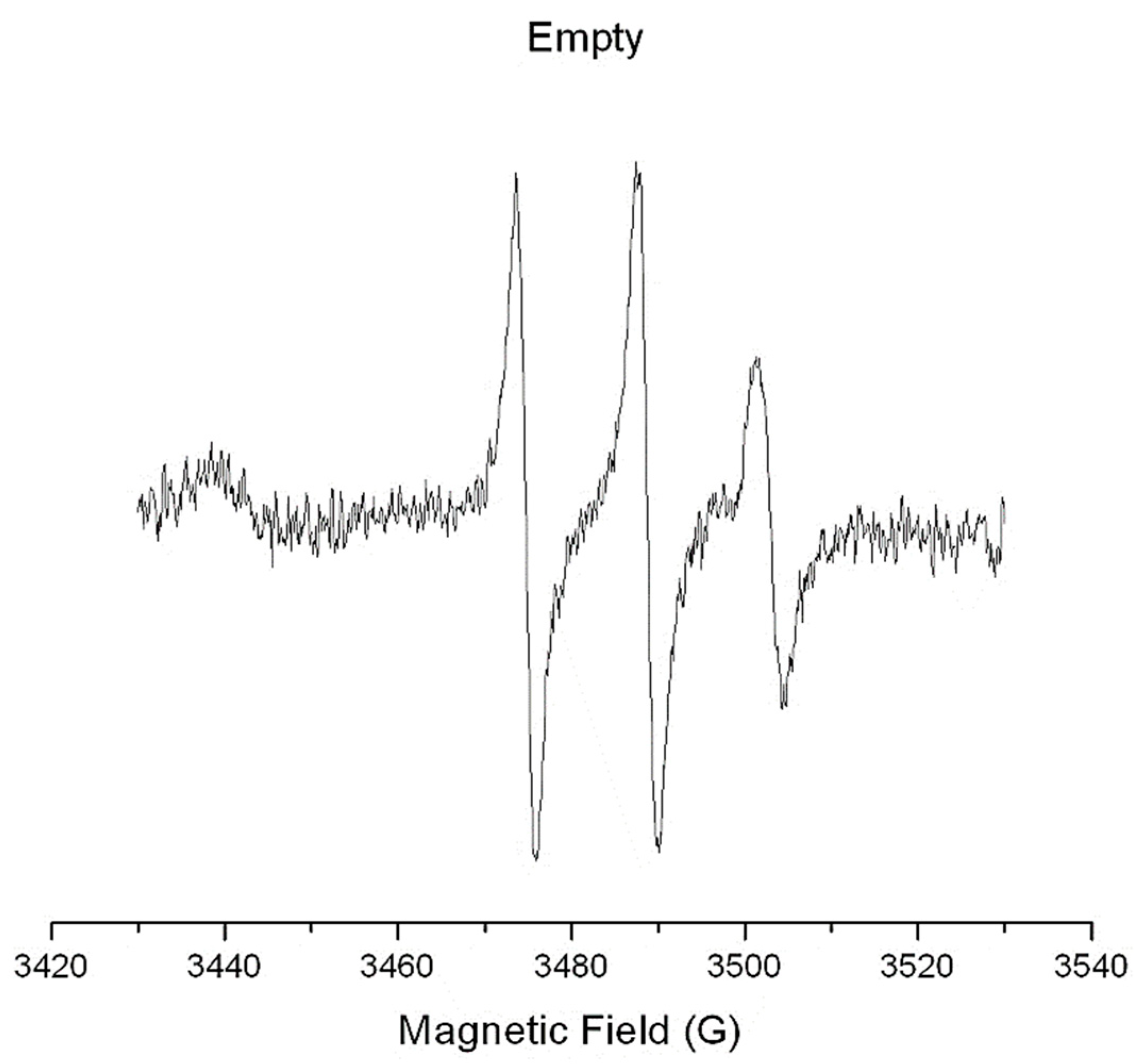
2.2.9. Surfactant Membrane Dynamics Using Electron Paramagnetic Resonance (EPR) Spectroscopy
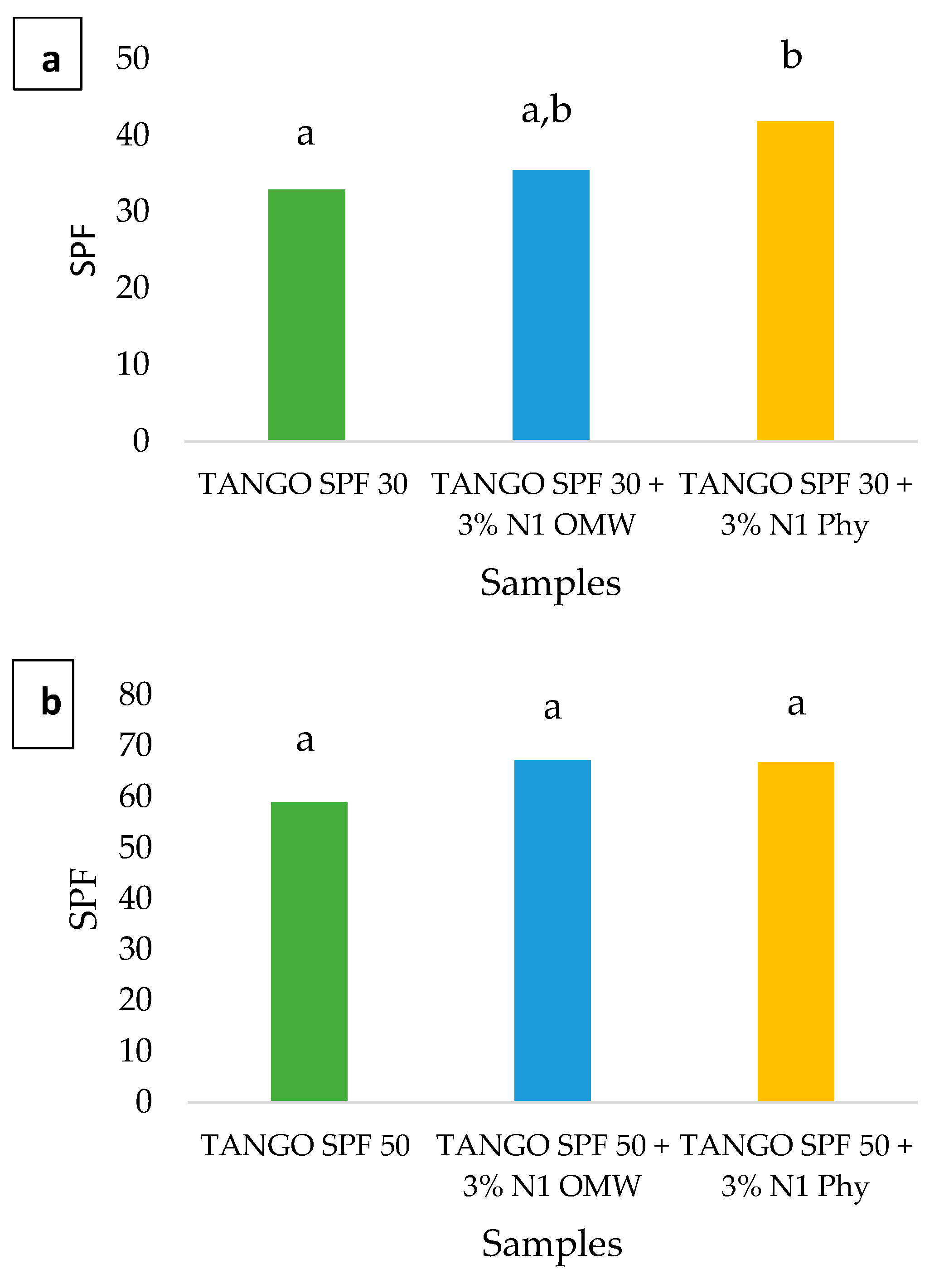
2.2.10. Clinical Pre-Assessment of the Sun Protection Factor (SPF)
- Children and persons below the local legal age of consent or >70 years;
- Pregnant or lactating women;
- Subjects using medication with photo-sensitizing potential;
- Subjects using anti-inflammatory medication;
- Subjects with systemic dermatological conditions;
- Subjects with a history of abnormal response to the sun;
- Subjects who used tanning beds in the previous eight weeks prior to SPF testing;
- Subjects with sun exposure on the back area in the previous eight weeks prior to SPF testing;
- Subjects with marks, blemishes, or nevi in the test area;
- Subjects presenting with existing sun damage in the test area;
- Subjects with excessive hair in the area on the test on the day of testing;
- Subjects with skeletal protrusions and extreme areas of curvature in the test area.
3. Results and Discussion
3.1. Determination of the OMW Extracts’ Total Phenol Content
3.2. Extraction of Phycocyanin from Spirulina maxima
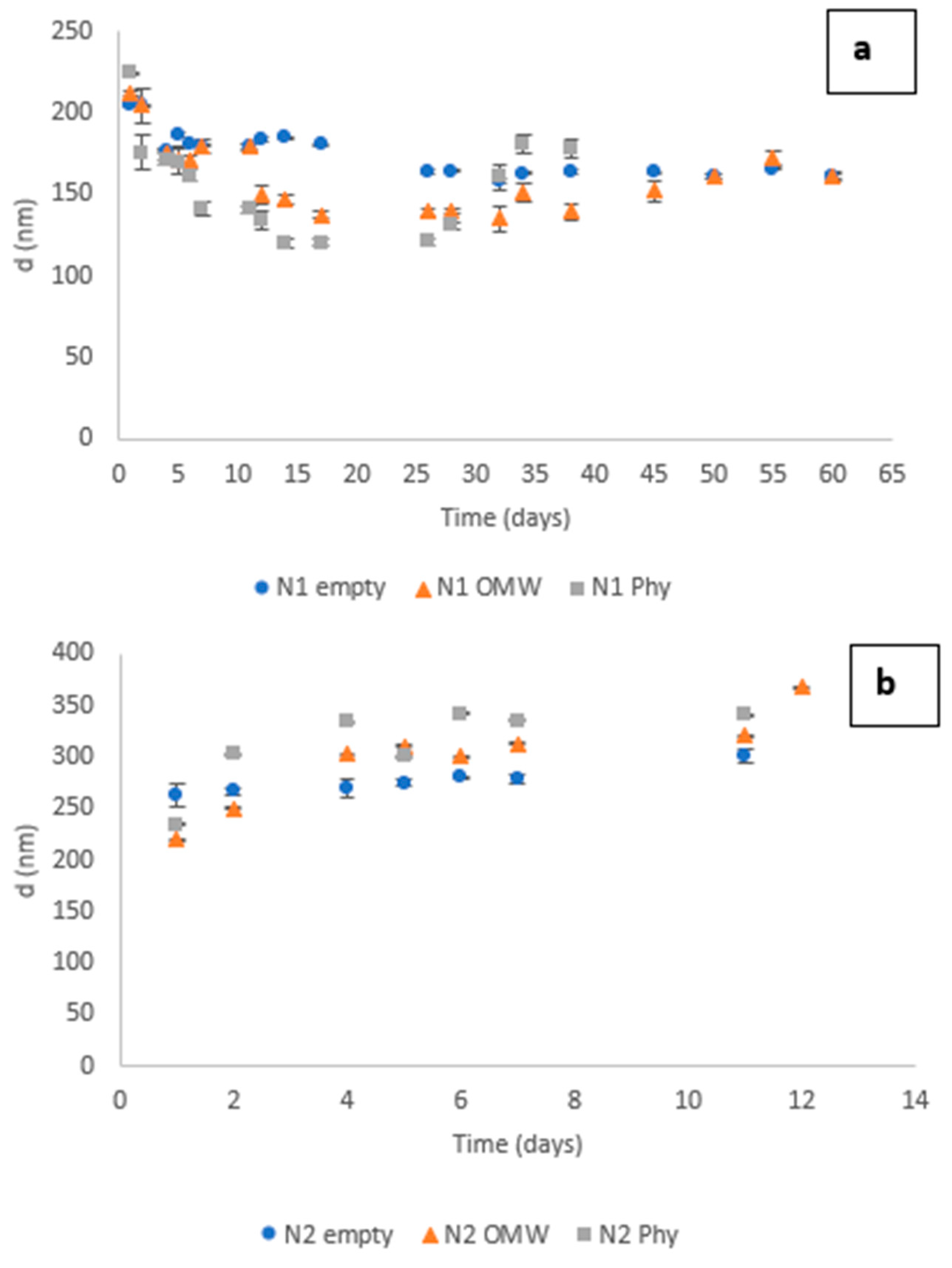
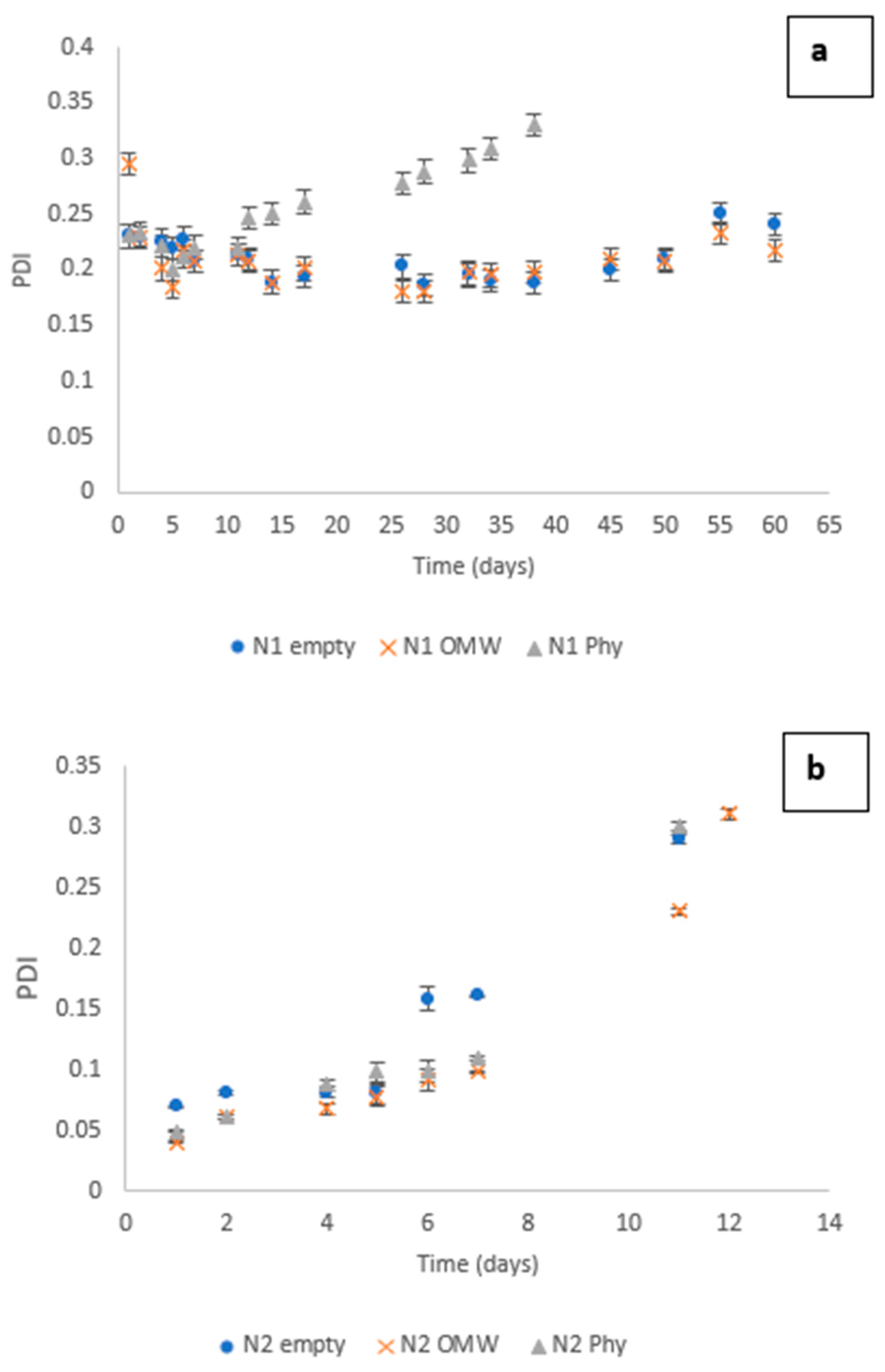
3.3. Dynamic Light Scattering (DLS) Measurements
3.4. Structural Characterization Using Electron Paramagnetic Resonance (EPR) Spectroscopy
3.5. Antioxidant Assessment of the OMW and Spirulina maxima Extracts via DPPH Scavenging
3.6. Determination of the Antioxidant Activity Using the Galvinoxyl Free Radical Detected via Electron Paramagnetic Resonance (EPR) Spectroscopy
3.7. Clinical Pre-Assessment of the Sun Protection Factor (SPF)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leiter, U.; Keim, U.; Garbe, C. Epidemiology of Skin Cancer: Update 2019. Adv. Exp. Med. Biol. 2020, 1268, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Douki, T. Formation of UV-Induced DNA Damage Contributing to Skin Cancer Development. Photochem. Photobiol. Sci. 2018, 17, 1816–1841. [Google Scholar] [CrossRef]
- Oral, D.; Yirun, A.; Erkekoglu, P. Safety Concerns of Organic Ultraviolet Filters: Special Focus on Endocrine-Disrupting Properties. J. Environ. Pathol. Toxicol. Oncol. 2020, 39, 201–212. [Google Scholar] [CrossRef]
- Chatzigianni, M.; Pavlou, P.; Siamidi, A.; Vlachou, M.; Varvaresou, A.; Papageorgiou, S. Environmental Impacts Due to the Use of Sunscreen Products: A Mini-Review. Ecotoxicology 2022, 31, 1331–1345. [Google Scholar] [CrossRef]
- Jesus, A.; Sousa, E.; Cruz, M.T.; Cidade, H.; Lobo, J.M.S.; Almeida, I.F. UV Filters: Challenges and Prospects. Pharmaceuticals 2022, 15, 263. [Google Scholar] [CrossRef]
- Levine, A. Reducing the Prevalence of Chemical UV Filters from Sunscreen in Aquatic Environments: Regulatory, Public Awareness, and Other Considerations. Integr. Environ. Assess. Manag. 2021, 17, 982–988. [Google Scholar] [CrossRef]
- Yarovaya, L.; Khunkitti, W. The Effect of Grape Seed Extract as a Sunscreen Booster Effect of Grape Seed Extract as a Sunscreening Booster. Songklanakarin J. Sci. Technol. 2019, 41, 708–715. [Google Scholar] [CrossRef]
- Aguilera, J.; Vicente-Manzanares, M.; de Gálvez, M.V.; Herrera-Ceballos, E.; Rodríguez-Luna, A.; González, S. Booster Effect of a Natural Extract of Polypodium Leucotomos (Fernblock®) That Improves the UV Barrier Function and Immune Protection Capability of Sunscreen Formulations. Front. Med. 2021, 8, 684665. [Google Scholar] [CrossRef]
- Radice, M.; Manfredini, S.; Ziosi, P.; Dissette, V.; Buso, P.; Fallacara, A.; Vertuani, S. Herbal Extracts, Lichens and Biomolecules as Natural Photo-Protection Alternatives to Synthetic UV Filters. A Systematic Review. Fitoterapia 2016, 114, 144–162. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Tsatalas, P.; Galanakis, I.M. Phenols from Olive Mill Wastewater and Other Natural Antioxidants as UV Filters in Sunscreens. Environ. Technol. Innov. 2018, 9, 160–168. [Google Scholar] [CrossRef]
- Babić, S.; Malev, O.; Pflieger, M.; Lebedev, A.T.; Mazur, D.M.; Kužić, A.; Čož-Rakovac, R.; Trebše, P. Toxicity Evaluation of Olive Oil Mill Wastewater and Its Polar Fraction Using Multiple Whole-Organism Bioassays. Sci. Total Environ. 2019, 686, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Rahmanian, N.; Jafari, S.M.; Galanakis, C.M. Recovery and Removal of Phenolic Compounds from Olive Mill Wastewater. JAOCS 2014, 91, 1–18. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, J.; Yang, Z.; Xiong, L.; Li, L.; Gu, Z.; Li, Y. Polyphenolic Sunscreens for Photoprotection. Green Chem. 2022, 24, 3605–3622. [Google Scholar] [CrossRef]
- de Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as Natural Antioxidants in Cosmetics Applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Wu, Q.; Kuča, K. Chapter 42—Spirulina. In Nutraceuticals; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 569–583. ISBN 9780128021477. [Google Scholar] [CrossRef]
- Yarkent, Ç.; Gürlek, C.; Oncel, S.S. Potential of Microalgal Compounds in Trending Natural Cosmetics: A Review. Sustain. Chem. Pharm. 2020, 17, 100304. [Google Scholar] [CrossRef]
- Sarada, R.; Pillai, M.G.; Ravishankar, G.A. Phycocyanin from Spirulina Sp: Influence of Processing of Biomass on Phycocyanin Yield, Analysis of Efficacy of Extraction Methods and Stability Studies on Phycocyanin. Process. Biochem. 1999, 34, 795–801. [Google Scholar] [CrossRef]
- Jang, Y.A.; Kim, B.A. Protective Effect of Spirulina-Derived C-Phycocyanin against Ultraviolet B-Induced Damage in HaCaT Cells. Medicina 2021, 57, 273. [Google Scholar] [CrossRef]
- Vega, J.; Bonomi-Barufi, J.; Gómez-Pinchetti, J.L.; Figueroa, F.L. Cyanobacteria and Red Macroalgae as Potential Sources of Antioxidants and UV Radiation-Absorbing Compounds for Cosmeceutical Applications. Mar. Drugs 2020, 18, 659. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Nanoemulsions versus Microemulsions: Terminology, Differences, and Similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Silva, H.D.; Cerqueira, M.Â.; Vicente, A.A. Nanoemulsions for Food Applications: Development and Characterization. Food Bioprocess Technol. 2012, 5, 854–867. [Google Scholar] [CrossRef]
- Golfomitsou, I.; Mitsou, E.; Xenakis, A.; Papadimitriou, V. Development of Food Grade O/W Nanoemulsions as Carriers of Vitamin D for the Fortification of Emulsion Based Food Matrices: A Structural and Activity Study. J. Mol. Liq. 2018, 268, 734–742. [Google Scholar] [CrossRef]
- Demisli, S.; Theochari, I.; Christodoulou, P.; Zervou, M.; Xenakis, A.; Papadimitriou, V. Structure, Activity and Dynamics of Extra Virgin Olive Oil-in-Water Nanoemulsions Loaded with Vitamin D3 and Calcium Citrate. J. Mol. Liq. 2020, 306, 112908. [Google Scholar] [CrossRef]
- Pathak, K.; Pattnaik, S.; Swain, K. Application of Nanoemulsions in Drug Delivery; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 415–433. ISBN 9780128118399. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Guerra, M.; Dias-Ferreira, J.; Lopez-Machado, A.; Ettcheto, M.; Cano, A.; Espina, M.; Camins, A.; Garcia, M.L.; Souto, E.B. Current Applications of Nanoemulsions in Cancer Therapeutics. Nanomaterials 2019, 9, 821. [Google Scholar] [CrossRef]
- Mitsou, E.; Pletsa, V.; Sotiroudis, G.T.; Panine, P.; Zoumpanioti, M.; Xenakis, A. Development of a Microemulsion for Encapsulation and Delivery of Gallic Acid. The Role of Chitosan. Colloids Surf. B Biointerfaces 2020, 190, 110974. [Google Scholar] [CrossRef]
- Mitsou, E.; Kalogianni, E.P.; Georgiou, D.; Stamatis, H.; Xenakis, A.; Zoumpanioti, M. Formulation and Structural Study of a Biocompatible Water-in-Oil Microemulsion as an Appropriate Enzyme Carrier: The Model Case of Horseradish Peroxidase. Langmuir 2019, 35, 150–160. [Google Scholar] [CrossRef]
- Theochari, I.; Xenakis, A.; Papadimitriou, V. Nanocarriers for Effective Drug Delivery. In Smart Nanocontainers Micro Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 315–341. [Google Scholar] [CrossRef]
- Mansur, M.C.P.P.R.; Campos, C.; Vermelho, A.B.; Nobrega, J.; da Cunha Boldrini, L.; Balottin, L.; Lage, C.; Rosado, A.S.; Ricci-Júnior, E.; dos Santos, E.P. Photoprotective Nanoemulsions Containing Microbial Carotenoids and Buriti Oil: Efficacy and Safety Study. Arab. J. Chem. 2020, 13, 6741–6752. [Google Scholar] [CrossRef]
- Faria-Silva, A.C.; Costa, A.M.; Ascenso, A.; Ribeiro, H.M.; Marto, J.; Gonçalves, L.M.; Carvalheiro, M.; Simões, S. Nanoemulsions for Cosmetic Products. Nanocosmetics 2020, 59–77. [Google Scholar] [CrossRef]
- Reis-Mansur, M.C.P.P.; Firmino Gomes, C.C.; Nigro, F.; Ricci-Júnior, E.; de Freitas, Z.M.F.; dos Santos, E.P. Nanotechnology as a Tool for Optimizing Topical Photoprotective Formulations Containing Buriti Oil (Mauritia flexuosa) and Dry Aloe vera Extracts: Stability and Cytotoxicity Evaluations. Pharmaceuticals 2023, 16, 292. [Google Scholar] [CrossRef]
- Yang, C.C.; Hung, C.F.; Chen, B.H. Preparation of coffee oil-algae oil-based nanoemulsions and the study of their inhibition effect on UVA-induced skin damage in mice and melanoma cell growth. Int. J. Nanomed. 2017, 12, 6559–6580. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Tsatalas, P.; Galanakis, I.M. Implementation of Phenols Recovered from Olive Mill Wastewater as UV Booster in Cosmetics. Ind. Crops Prod. 2018, 111, 30–37. [Google Scholar] [CrossRef]
- Wiseva, K.O.; Widyastuti, F.; Faadhilah, H.; Wathoni, N. A review on various formulation of nanoemulsions in cosmetics with plant extracts as the active ingredients. Int. J. Appl. Pharm. 2021, 13, 34–40. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Saadaoui, N.; Kiai, H.; Raiti, J.; Hafidi, A. Potential Applications of Olive Mill Wastewater as Biopesticide for Crops Protection. Sci. Total Environ. 2017, 576, 10–21. [Google Scholar] [CrossRef]
- Aissam, H.; Penninckx, M.J.; Benlemlih, M. Reduction of Phenolics Content and COD in Olive Oil Mill Wastewaters by Indigenous Yeasts and Fungi. World J. Microbiol. Biotechnol. 2007, 23, 1203–1208. [Google Scholar] [CrossRef]
- Hawker, M.; Wang, F.; Mouzakitis, Y.; Adamides, E.D. Techno-Economic Assessment of an Olive Mill Wastewater (OMWW) Biorefinery in the Context of Circular Bioeconomy. Eng 2022, 3, 488–503. [Google Scholar] [CrossRef]
- Economic Importance of Algae for NEET—Features, Types, Importance and Effects. Available online: https://www.vedantu.com/neet/economic-importance-of-algae (accessed on 31 March 2023).
- Pereira, A.G.; Fraga-Corral, M.; Garcia-Oliveira, P.; Otero, P.; Soria-Lopez, A.; Cassani, L.; Cao, H.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Single-Cell Proteins Obtained by Circular Economy Intended as a Feed Ingredient in Aquaculture. Foods 2022, 11, 2831. [Google Scholar] [CrossRef] [PubMed]
- Leouifoudi, I.; Zyad, A.; Amechrouq, A.; Oukerrou, M.A.; Mouse, H.A.; Mbarki, M. Identification and Characterisation of Phenolic Compounds Extracted from Moroccan Olive Mill Wastewater. Food Sci. Technol. 2014, 34, 249–257. [Google Scholar] [CrossRef]
- Silveira, S.T.; Burkert, J.F.M.; Costa, J.A.V.; Burkert, C.A.V.; Kalil, S.J. Optimization of Phycocyanin Extraction from Spirulina Platensis Using Factorial Design. Bioresour. Technol. 2007, 98, 1629–1634. [Google Scholar] [CrossRef]
- Koukouraki, P.; Tsoupras, A.; Sotiroudis, G.; Demopoulos, C.A.; Sotiroudis, T.G. Antithrombotic Properties of Spirulina Extracts against Platelet-Activating Factor and Thrombin. Food Biosci. 2020, 37, 100686. [Google Scholar] [CrossRef]
- Olmo-García, L.; Fernández-Fernández, C.; Hidalgo, A.; Vílchez, P.; Fernández-Gutiérrez, A.; Marchal, R.; Carrasco-Pancorbo, A. Evaluating the Reliability of Specific and Global Methods to Assess the Phenolic Content of Virgin Olive Oil: Do They Drive to Equivalent Results? J. Chromatogr. A 2019, 1585, 56–69. [Google Scholar] [CrossRef]
- Patil, G.; Chethana, S.; Sridevi, A.S.; Raghavarao, K.S.M.S. Method to Obtain C-Phycocyanin of High Purity. J. Chromatogr. A 2006, 1127, 76–81. [Google Scholar] [CrossRef]
- Schwarz, K.; Bertelsen, G.; Nissen, L.R.; Gardner, P.T.; Heinonen, M.I.; Hopia, A.; Huynh-Ba, T.; Lambelet, P.; McPhail, D.; Skibsted, L.H.; et al. Investigation of plant extracts for the protection of processed foods against lipid oxidation. Comparison of antioxidant assays based on radical scavenging, lipid oxidation and analysis of the principal antioxidant compounds. Eur. Food Res. Technol. 2001, 212, 319–328. [Google Scholar] [CrossRef]
- Papadimitriou, V.; Sotiroudis, T.G.; Xenakis, A.; Sofikiti, N.; Stavyiannoudaki, V.; Chaniotakis, N.A. Oxidative Stability and Radical Scavenging Activity of Extra Virgin Olive Oils: An Electron Paramagnetic Resonance Spectroscopy Study. Anal. Chim. Acta 2006, 573–574, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, I.; Mitsou, E.; Damjanovic, A.; Papadimitriou, V.; Antic-Stankovic, J.; Stanojevic, B.; Xenakis, A.; Savic, S. Curcumin-Loaded Low-Energy Nanoemulsions: Linking EPR Spectroscopy-Analysed Microstructure and Antioxidant Potential with in Vitro Evaluated Biological Activity. J. Mol. Liq. 2020, 301, 112479. [Google Scholar] [CrossRef]
- Chatzidaki, M.D.; Papadimitriou, K.; Alexandraki, V.; Tsirvouli, E.; Chakim, Z.; Ghazal, A.; Mortensen, K.; Yaghmur, A.; Salentinig, S.; Papadimitriou, V.; et al. Microemulsions as Potential Carriers of Nisin: Effect of Composition on Structure and Efficacy. Langmuir 2016, 32, 8988–8998. [Google Scholar] [CrossRef]
- He, J.; Alister-Briggs, M.; de Lyster, T.; Jones, G.P. Stability and Antioxidant Potential of Purified Olive Mill Wastewater Extracts. Food Chem. 2012, 131, 1312–1321. [Google Scholar] [CrossRef]
- ISO 24444:2019 (E); Cosmetics—Sun Protection Test Methods—In Vivo Determination of the Sun Protection Factor (SPF). ISO: Geneva, Switzerland, 2019.
- Lafka, T.I.; Lazou, A.E.; Sinanoglou, V.J.; Lazos, E.S. Phenolic and Antioxidant Potential of Olive Oil Mill Wastes. Food Chem. 2011, 1, 92–98. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Kiai, H.; Hafidi, A. Phenolic Profile and Antioxidant Activities of Olive Mill Wastewater. Food Chem. 2012, 132, 406–412. [Google Scholar] [CrossRef]
- Roig, A.; Cayuela, M.L.; Sánchez-Monedero, M.A. An Overview on Olive Mill Wastes and Their Valorisation Methods. Waste Manag. 2006, 26, 960–969. [Google Scholar] [CrossRef]
- Jerman Klen, T.; Mozetič Vodopivec, B. The Fate of Olive Fruit Phenols during Commercial Olive Oil Processing: Traditional Press versus Continuous Two- and Three-Phase Centrifuge. LWT Food Sci. Technol. 2012, 49, 267–274. [Google Scholar] [CrossRef]
- Khdair, A.; Abu-Rumman, G. Sustainable environmental management and valorization options for olive mill byproducts in the Middle East and North Africa (MENA) region. Processes 2020, 8, 671. [Google Scholar] [CrossRef]
- Adjali, A.; Clarot, I.; Chen, Z.; Marchioni, E.; Boudier, A. Physicochemical degradation of phycocyanin and means to improve its stability: A short review. J. Pharm. Anal. 2022, 12, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Almog, R.; Marsilio, F.; Berns, D.S. Interaction of C-Phycocyanin with Lipid Monolayers under Nitrogen and in the Presence of Air. Arch. Biochem. Biophys. 1988, 260, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Reitzer, F.; Allais, M.; Ball, V.; Meyer, F. Polyphenols at Interfaces. Adv. Colloid Interface Sci. 2018, 257, 31–41. [Google Scholar] [CrossRef]
- Sotiroudis, T.G.; Sotiroudis, G.T.; Varkas, N.; Xenakis, A. The Role of Endogenous Amphiphiles on the Stability of Virgin Olive Oil-in-Water Emulsions. JAOCS J. Am. Oil Chem. Soc. 2005, 82, 415–420. [Google Scholar] [CrossRef]
- Demisli, S.; Galani, E.; Goulielmaki, M.; Kyrilis, F.L.; Ilić, T.; Hamdi, F.; Crevar, M.; Kastritis, P.L.; Pletsa, V.; Nallet, F.; et al. Encapsulation of Cannabidiol in Oil-in-Water Nanoemulsions and Nanoemulsion-Filled Hydrogels: A Structure and Biological Assessment Study. J. Colloid Interface Sci. 2023, 634, 300–313. [Google Scholar] [CrossRef]
- Zhou, H.; Yue, Y.; Liu, G.; Li, Y.; Zhang, J.; Gong, Q.; Yan, Z.; Duan, M. Preparation and Characterization of a Lecithin Nanoemulsion as a Topical Delivery System. Nanoscale Res. Lett. 2010, 5, 224–230. [Google Scholar] [CrossRef]
- Sakulku, U.; Nuchuchua, O.; Uawongyart, N.; Puttipipatkhachorn, S.; Soottitantawat, A.; Ruktanonchai, U. Characterization and Mosquito Repellent Activity of Citronella Oil Nanoemulsion. Int. J. Pharm. 2009, 372, 105–111. [Google Scholar] [CrossRef]
- Saberi, A.H.; Fang, Y.; McClements, D.J. Effect of Glycerol on Formation, Stability, and Properties of Vitamin-E Enriched Nanoemulsions Produced Using Spontaneous Emulsification. J. Colloid Interface Sci. 2013, 411, 105–113. [Google Scholar] [CrossRef]
- Hategekimana, J.; Chamba, M.V.M.; Shoemaker, C.F.; Majeed, H.; Zhong, F. Vitamin E Nanoemulsions by Emulsion Phase Inversion: Effect of Environmental Stress and Long-Term Storage on Stability and Degradation in Different Carrier Oil Types. Colloids Surf. A Physicochem. Eng. Asp. 2015, 483, 70–80. [Google Scholar] [CrossRef]
- Katsouli, M.; Tzia, C. Effect of Lipid Type, Dispersed Phase Volume Fraction and Emulsifier on the Physicochemical Properties of Nanoemulsions Fortified with Conjugated Linoleic Acid (CLA): Process Optimization and Stability Assessment during Storage Conditions. J. Mol. Liq. 2019, 292, 111397. [Google Scholar] [CrossRef]
- Chatzidaki, M.D.; Mitsou, E.; Yaghmur, A.; Xenakis, A.; Papadimitriou, V. Formulation and Characterization of Food-Grade Microemulsions as Carriers of Natural Phenolic Antioxidants. Colloids Surf. A Physicochem. Eng. Asp. 2015, 483, 130–136. [Google Scholar] [CrossRef]
- Saberi, A.H.; Fang, Y.; McClements, D.J. Fabrication of Vitamin E-Enriched Nanoemulsions by Spontaneous Emulsification: Effect of Propylene Glycol and Ethanol on Formation, Stability, and Properties. Food Res. Int. 2013, 54, 812–820. [Google Scholar] [CrossRef]
- Montes de Oca-Ávalos, J.M.; Candal, R.J.; Herrera, M.L. Nanoemulsions: Stability and Physical Properties. Curr. Opin. Food Sci. 2017, 16, 1–6. [Google Scholar] [CrossRef]
- Visioli, F.; Romani, A.; Mulinacci, N.; Zarini, S.; Conte, D.; Vincieri, F.F.; Galli, C. Antioxidant and Other Biological Activities of Olive Mill Waste Waters. J. Agric. Food Chem. 1999, 47, 3397–3401. [Google Scholar] [CrossRef]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef] [PubMed]
- de Marco, E.; Savarese, M.; Paduano, A.; Sacchi, R. Characterization and Fractionation of Phenolic Compounds Extracted from Olive Oil Mill Wastewaters. Food Chem. 2007, 104, 858–867. [Google Scholar] [CrossRef]
- Wu, H.L.; Wang, G.H.; Xiang, W.Z.; Li, T.; He, H. Stability and Antioxidant Activity of Food-Grade Phycocyanin Isolated from Spirulina Platensis. Int. J. Food Prop. 2016, 19, 2349–2362. [Google Scholar] [CrossRef]
- Renugadevi, K.; Valli Nachiyar, C.; Sowmiya, P.; Sunkar, S. Antioxidant Activity of Phycocyanin Pigment Extracted from Marine Filamentous Cyanobacteria Geitlerinema Sp TRV57. Biocatal. Agric. Biotechnol. 2018, 16, 237–242. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Samborska, K.; Lee, C.C.; Tomas, M.; Capanoglu, E.; Tarhan, Ö.; Taze, B.; Jafari, S.M. Phycocyanin, a Super Functional Ingredient from Algae; Properties, Purification Characterization, and Applications. Int. J. Biol. Macromol. 2021, 193, 2320–2331. [Google Scholar] [CrossRef]
- Wang, L.; Qu, Y.; Fu, X.; Zhao, M.; Wang, S.; Sun, L. Isolation, Purification and Properties of an R-Phycocyanin from the Phycobilisomes of a Marine Red Macroalga Polysiphonia Urceolata. PLoS ONE 2014, 9, e87833. [Google Scholar] [CrossRef]
- Fernández-Rojas, B.; Hernández-Juárez, J.; Pedraza-Chaverri, J. Nutraceutical Properties of Phycocyanin. J. Funct. Foods 2014, 11, 375–392. [Google Scholar] [CrossRef]
- Huang, Z.; Guo, B.J.; Wong, R.N.S.; Jiang, Y. Characterization and Antioxidant Activity of Selenium-Containing Phycocyanin Isolated from Spirulina Platensis. Food Chem. 2007, 100, 1137–1143. [Google Scholar] [CrossRef]
- Romay, C.; Gonzalez, R.; Pizarro, M.; Lissi, E. Kinetics of C-Phycocyanin Reaction with Hypochlorite. J. Protein Chem. 2000, 19, 151–155. [Google Scholar] [CrossRef]
- Herrling, T.; Seifert, M.; Sandig, G.; Jung, K. The Determination of the Radical Power—An in Vitro Test for the Evaluation of Cosmetic Products. Int. J. Cosmet. Sci. 2016, 38, 232–237. [Google Scholar] [CrossRef]
- Quiñones, R.; Kolling, D.R.J.; Shoup, D.; Smythers, A.L.; Nickel, S.; Westfall, T.D.; Epperly, C.; Coplin, M. Comparing Free Radicals in Sunscreen-Treated Pig Skin by Using Electron Paramagnetic Resonance Spectroscopy. J. Chem. Educ. 2019, 96, 2021–2028. [Google Scholar] [CrossRef]
- Markou, G.; Kougia, E.; Kefalogianni, I.; Tsagou, V.; Arapoglou, D.; Chatzipavlidis, I. Effect of Glycerol Concentration and Light Intensity on Growth and Biochemical Composition of Arthrospira (Spirulina) Platensis: A Study in Semi-Continuous Mode with Non-Aseptic Conditions. Appl. Sci. 2019, 9, 4703. [Google Scholar] [CrossRef]
- Wang, S.Q.; Lim, H.W. Current Status of the Sunscreen Regulation in the United States: 2011 Food and Drug Administration’s Final Rule on Labeling and Effectiveness Testing. J. Am. Acad. Dermatol. 2011, 65, 863–869. [Google Scholar] [CrossRef]
- Sayre, R.M.; Dowdy, J.C.; Lott, D.L.; Marlowe, E. Commentary on ‘UVB-SPF’: The SPF Labels of Sunscreen Products Convey More than Just UVB Protection. Photodermatol. Photoimmunol. Photomed. 2008, 24, 218–220. [Google Scholar] [CrossRef]
- Carrara, M.; Kelly, M.T.; Roso, F.; Larroque, M.; Margout, D. Potential of Olive Oil Mill Wastewater as a Source of Polyphenols for the Treatment of Skin Disorders: A Review. J. Agric. Food Chem. 2021, 69, 7268–7284. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.A.; Katiyar, S.K. Skin Photoprotection by Natural Polyphenols: Anti-Inflammatory, Antioxidant and DNA Repair Mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef]
- Choquenet, B.; Couteau, C.; Paparis, E.; Coiffard, L.J.M. Flavonoids and Polyphenols, Molecular Families with Sunscreen Potential: Determining Effectiveness with an in vitro Method. Nat. Prod. Commun. 2009, 4, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Shin Yi, N.; Gew, L.T.; Eh Suk, V.R. Plant Polyphenols as Green Sunscreen Ingredients: A Systematic Review. J. Cosmet. Dermatol. 2022, 21, 5409–5444. [Google Scholar] [CrossRef]
- Puglisi, R.; Biazzi, E.; Gesmundo, D.; Vanni, R.; Tava, A.; Cenadelli, S. The Antioxidant Activity of a Commercial and a Fractionated Phycocyanin on Human Skin Cells In Vitro. Molecules 2022, 27, 5276. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Sakamoto, T.; Matsugo, S. Multiple Roles of Photosynthetic and Sunscreen Pigments in Cyanobacteria Focusing on the Oxidative Stress. Metabolites 2013, 3, 463–483. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Liu, L. Fabrication and Characterization of Chitosan Nanoemulsions Loading Thymol or Thyme Essential Oil for the Preservation of Refrigerated Pork. Int. J. Biol. Macromol. 2020, 162, 1509–1515. [Google Scholar] [CrossRef]
- Lohith Kumar, D.H.; Sarkar, P. Encapsulation of Bioactive Compounds Using Nanoemulsions. Environ. Chem. Lett. 2017, 16, 59–70. [Google Scholar] [CrossRef]
- Pandey, P.; Gulati, N.; Makhija, M.; Purohit, D.; Dureja, H. Nanoemulsion: A Novel Drug Delivery Approach for Enhancement of Bioavailability. Recent Pat. Nanotechnol. 2020, 14, 276–293. [Google Scholar] [CrossRef]
- Choi, S.J.; McClements, D.J. Nanoemulsions as Delivery Systems for Lipophilic Nutraceuticals: Strategies for Improving Their Formulation, Stability, Functionality and Bioavailability. Food Sci. Biotechnol. 2020, 29, 149–168. [Google Scholar] [CrossRef]
- Sonneville-Aubrun, O.; Yukuyama, M.N.; Pizzino, A. Application of Nanoemulsions in Cosmetics. Nanoemuls. Formul. Appl. Charact. 2018, 435–475. [Google Scholar] [CrossRef]

| Composition (% w/w) | Nanoemulsions | |
|---|---|---|
| N1 | N2 | |
| IPM | 86.25 | 81.25 |
| Water | 10 | 10 |
| Glycerol | - | 5 |
| Plurol Oleique | 1.5 | 1.5 |
| Plurol Diisostearique | 0.75 | 0.75 |
| Labrasol | 0.75 | 0.75 |
| Dehymuls PGPH | 0.75 | 0.75 |
| Reference Sunscreen Formulation | Mean SPF | Acceptance Range | |
|---|---|---|---|
| Lower Limit | Upper Limit | ||
| P6 | 43.0 | 31.0 | 54.9 |
| P8 | 63.1 | 43.9 | 82.3 |
| Sample | Total Phenols (mg/L of Extract) | Total Phenols (mg/L of Wastewater) |
|---|---|---|
| Three-phase OMW (first-stage waste) | 3131.9 ± 0.8 | 1565.5 ± 0.5 |
| Three-phase OMW (second-stage waste) | 115.4 ± 0.9 | 57.7 ± 0.3 |
| Two-phase OMW | 24.6 ± 0.2 | 12.3 ± 0.9 |
| System | 5-DSA | |
|---|---|---|
| τR (ns) | S | |
| Ν1 empty | 0.57 ± 0.08 | 0.05 ± 0.01 |
| N1 OMW | 0.59 ± 0.02 | 0.06 ± 0.01 |
| N1 Phy | 0.63 ± 0.06 | 0.05 ± 0.01 |
| N2 empty | 0.67 ± 0.04 | 0.05 ± 0.01 |
| N2 OMW | 0.54 ± 0.03 | 0.05 ± 0.01 |
| N2 Phy | 0.58 ± 0.05 | 0.05 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galani, E.; Galatis, D.; Tzoka, K.; Papadimitriou, V.; Sotiroudis, T.G.; Bonos, A.; Xenakis, A.; Chatzidaki, M.D. Natural Antioxidant-Loaded Nanoemulsions for Sun Protection Enhancement. Cosmetics 2023, 10, 102. https://doi.org/10.3390/cosmetics10040102
Galani E, Galatis D, Tzoka K, Papadimitriou V, Sotiroudis TG, Bonos A, Xenakis A, Chatzidaki MD. Natural Antioxidant-Loaded Nanoemulsions for Sun Protection Enhancement. Cosmetics. 2023; 10(4):102. https://doi.org/10.3390/cosmetics10040102
Chicago/Turabian StyleGalani, Eleni, Dimitrios Galatis, Kyriaki Tzoka, Vassiliki Papadimitriou, Theodore G. Sotiroudis, Antonios Bonos, Aristotelis Xenakis, and Maria D. Chatzidaki. 2023. "Natural Antioxidant-Loaded Nanoemulsions for Sun Protection Enhancement" Cosmetics 10, no. 4: 102. https://doi.org/10.3390/cosmetics10040102
APA StyleGalani, E., Galatis, D., Tzoka, K., Papadimitriou, V., Sotiroudis, T. G., Bonos, A., Xenakis, A., & Chatzidaki, M. D. (2023). Natural Antioxidant-Loaded Nanoemulsions for Sun Protection Enhancement. Cosmetics, 10(4), 102. https://doi.org/10.3390/cosmetics10040102







