Abstract
Melasma is a fairly common condition that is the result of hyperpigmentation caused by increased melanin secretion. In the course of melasma, certain areas of the skin become darker than the rest of the epidermis. Although the pathogenesis remains incompletely clarified, several contributing factors have been identified, namely exposure to ultraviolet and visible light, family predisposition, pregnancy, and the use of exogenous hormones. Since current beauty standards associate healthy skin with its flawless and uniform color, people strive to eliminate any unaesthetic discoloration. Cosmetic and pharmaceutical products containing active substances with a whitening effect then become helpful. The most commonly used for this purpose are hydroquinone, arbutin, retinoids, organic acids (e.g., kojic, azelaic, and ellagic), and vitamins (B3, C, and E). However, the undesirable side effects they cause and the drive to replace synthetic chemicals with their natural counterparts have resulted in numerous reports on extracts of natural origin that exhibit skin-whitening effects. The purpose of this paper is to review the most recent scientific literature, which presents active substances of natural and synthetic origin with potential for the treatment of melasma. In addition, analytical techniques that can be used for qualitative and quantitative analysis of these substances present in cosmetic and pharmaceutical products will also be presented.
1. Introduction
Current beauty canons in some cultures particularly value light skin color since it is associated with health, beauty, and prosperity, while darker skin tones may be correlated with a lower social class [1]. Sometimes the lightening of skin pigmentation has a medical justification—this is especially the case with dermatological conditions and dysfunctions based on excessive melanin synthesis. Skin afflictions such as melasma, freckles, birthmarks, senile/solar lentigo, pigmented acne scars, post-inflammatory hyperpigmentation, and lentigines are among the most commonly diagnosed skin conditions based on the process of hyperpigmentation. They are caused by a disruption of the melanogenesis process that occurs in human skin. During this process, melanin is produced, the light-absorbing pigment that determines the coloration of human skin and hair. Melanin is produced in melanocytes, which are specialized cells located mostly in the epidermal-dermal junction. From melanocytes, melanin is distributed by melanosomes (specialized lysosome-lineage organelles) and through the elongated dendrites to neighboring keratinocytes [2].
The precursor in melanogenesis is L-tyrosine, the amino acid that, through a series of enzymatic reactions, including the hydroxylation of L-tyrosine to L-3,4,-dihydroxyphenylalanine (L-DOPA) and the oxidation of L-DOPA to dopachinone, is eventually converted to brown-black eumelanin or yellow-red pheomelanin. One of the enzymes involved in the conversion of L-tyrosine to melanin is tyrosinase. Inhibiting its activity is the main target of many topically applied whitening preparations. Important regulators of melanogenesis are also melanotropin, adrenocorticotropic hormone (ACTH), and some cytokines. The processes leading to the onset of pigmentary disorders are very complex and have still not been fully clarified. Three mechanisms are considered the main triggers for the lesions named hypermelanosis and include: (1) an increase in the number of melanocytes; (2) a disturbance in the melanin synthesis process; and (3) a disturbance in the growth, transport, and transfer of melanosomes to keratinocytes. Uncontrolled melanocyte proliferation is a feature of melanomas, pigmented nevi, and lentigines, among others. In the cases of melasma, post-inflammatory hyperpigmentation, and freckles, it is currently accepted that the predominant pathological process is excessive melanin production. Hypermelanosis can be caused by several genetic and environmental factors, the most important of which are inflammation, hormonal factors, drugs, exposure to UV radiation, visible light and heat, and mechanical trauma [3].
Currently, the scientific literature presents numerous possibilities for the use of active substances with whitening effects, including hydroquinone, kojic acid, azelaic acid, retinoids (isotretinoin, tretinoin), arbutin, and vitamins (B3, C and E) in the treatment of skin hyperpigmentation of various origins [4]. At the same time, the literature also reports adverse effects of this type of therapy, which most commonly include skin drying, irritation, peeling, or hypopigmentation [5]. This, in turn, stimulates the search for more natural substitutes and directs researchers’ attention toward plant extracts [2,6,7], substances of marine origin [8,9], and even compounds extracted from mushrooms/fungi [10], which show the potential to lighten skin hyperpigmentation.
Surprisingly, whitening agents may also be helpful in the treatment of vitiligo, which is a chronic skin condition manifested by discoloration of large fragments of the skin and caused by a permanent dysfunction of skin pigment cells. In this case, whitening substances are applied to unaffected parts of the skin, allowing them to be matched to the fragments affected by vitiligo [11].
Nonetheless, it should not be forgotten that the etiology of skin hyperpigmentation is still not fully recognized, and it is difficult to select an effective therapy to permanently remove hyperpigmentation. Therefore, very often, in addition to topical treatments, other procedures are used, which can independently affect hyperpigmentation or support the action of products applied directly to the skin. These include peels (mainly chemical) as well as laser and light therapy. Frequently used chemical peels are based on glycolic, salicylic, and trichloroacetic acids, while laser- and light-based therapies involve, among others, intense pulsed light (IPL), Q-switched neodymium-doped yttrium aluminum garnet (QS-Nd:YAG) lasers, pulsed-dye lasers (PDL), and fractionated lasers [5].
An extremely important aspect of the study of the effectiveness of new cosmetic and pharmaceutical preparations containing skin-whitening agents is the quantification of the active substances in both the extracts prepared from natural sources and the final cosmetic or pharmaceutical product. For this purpose, methods based on chromatographic techniques (including TLC, GC, HPLC, and UHPLC) are used. Nevertheless, it frequently becomes a challenge to adequately separate the tested ingredients from the complex matrix of cosmetic and pharmaceutical products. In such cases, various extraction techniques assisted by ultrasound or microwave radiation are often used.
Taking into account all of the aforementioned aspects, this paper presents a compilation of scientific publications published over the past five years that describe the therapeutic effects of already well-known skin-whitening substances, as well as those that suggest potential for such use and which are derived from plant extracts and medicinal and edible mushrooms. This paper will also describe the analytical techniques currently available for the qualitative and quantitative analysis of skin-lightening substances, both those present in extracts of natural origin and those introduced as a component into a cosmetic or pharmaceutical product.
2. Melasma and Other Common Hyperpigmentation-Based Skin Disorders
Melasma, also referred to as chloasma or “the mask of pregnancy” because it is often associated with pregnant women, is one of the most common hyperpigmentation-based skin disorders. It manifests itself as light to dark brown, clearly delineated stains on the skin, usually located symmetrically on the face but sometimes taking unusual shapes. It usually occurs on the forehead, cheeks, nose, and area above the upper lip, sometimes also affecting the neck, neckline, and forearms. Melasma may be categorized by both location and depth of involvement. The most common types of melasma that describe the facial location are: (1) centrofacial, (2) malar, and (3) mandibular. On the other hand, considering the depth of involvement, melasma can be further divided into four categories: (1) epidermal, (2) dermal, (3) mixed, and (4) indeterminate. This is evaluated by illumination of the skin with long-wave ultraviolet light (Wood’s lamp) [12].
Melasma mainly affects women of childbearing age and less frequently affects men and postmenopausal women. It is most common in people with a high skin phototype (with Fitzpatrick skin types III-IV), corresponding to European and North African Mediterranean populations. Histologically, melasma is characterized by an elevated concentration of melanin in the epidermis and/or dermis. The pathophysiology of chloasma is still not fully elucidated, but the most significant factors influencing the onset of symptoms include exposure to sunlight, family predisposition, and hormonal factors (pregnancy, hormonal contraception, premenstrual period, and less commonly hormone-producing tumors such as ovarian cancer). In addition, it was also determined that melasma may originate from vascular disorders since some studies reported that melasma-affected skin had increased vascularity [13]. It is possible for melasma to resolve completely on its own (especially if it is caused by pregnancy). However, it is not uncommon for this condition to show resistance to treatment and a tendency to recur under the influence of various factors, such as even minor exposure to sunlight [5].
Sunlight, with its UV radiation component, is referred to as one of the main causes of melasma formation. One possible mechanism in this case is the induction of the synthesis of higher amounts of tyrosinase, an enzyme that promotes melanogenesis in the skin. It is widely accepted that the influence of genes is also of similar importance since tyrosinase-encoding genes and tyrosinase-related proteins are involved in pigmentation disorders caused by many exogenous and endogenous factors. However, there have been few reports that straightforwardly associate genetic polymorphisms with melasma—one showed that almost 279 genes are involved in the development of melasma [14]. It is more noticeable in the epidemiological studies that patients’ racial differences and positive family history may play a vital role in the occurrence of melasma [15]. Furthermore, several studies have revealed the influence of sex steroids on the development of melasma, suggesting a possible role of hormones in melasma pathogenesis as elevated expression of estrogen receptors in the dermis and progesterone receptors in the epidermis was observed. This may be related, for example, to pregnancy or the use of oral contraceptives. In contrast, male hormones seem not to play any role in hyperpigmentation; therefore, UV radiation is considered a main factor for elevated melanogenesis in the male skin [16].
The other most common skin pigmentation disorder is post-inflammatory hyperpigmentation. It usually arises as a consequence of acute and chronic inflammatory skin conditions, which may be caused by a number of inflammatory dermatological diseases, some procedures in cosmetic or aesthetic medicine, certain medications, and accidental mechanical, thermal, or chemical traumas to the skin. The location and severity of post-inflammatory hyperpigmentation depend on the nature of the triggering factor. It may occur at any age, regardless of gender, but people with phototypes IV–VI according to the Fitzpatrick classification are more susceptible to its occurrence. It may undergo gradual lightening (spontaneous or treatment-related), which usually lasts 6–12 months but may also persist for many years. Moreover, post-inflammatory hyperpigmentation sometimes shows a tendency to recur [17].
Another skin condition based on hyperpigmentation is lentigines, also known as actinic lentigines, liver spots, or age/sun spots. Lentigines take the form of brown, clearly demarcated spots that are sometimes clinically similar to freckles but can reach larger sizes. They do not lighten with limited exposure to sunlight and have a particular tendency to appear at an older age. They come in several clinical variants, the most common of which are (1) lentigo simplex, unrelated to sun exposure or systemic disease, and (2) lentigo solaris, which is considered a marker of photodamage to the skin and occurs mainly on the face, back of the hands, arms, and upper torso, that is, parts of the body particularly exposed to sunlight. The development of multiple lentigines spots can also be a symptom of rare and genetically determined conditions, such as Peutz–Jeghers, LEOPARD, or Laugier–Hunziker syndrome [18].
3. Current Methods of Melasma Treatment
In general, hyperpigmentation can noticeably affect the quality of life, although it cannot be considered a detrimental or lethal disorder. There are several options for hyperpigmentation treatment available nowadays. They include mainly topical routes in the form of creams, gels, or ointments. However, quite often they are accompanied by various side effects such as drying, irritation, peeling, or hypopigmentation of the skin. Additionally, even prolonged treatment, which can last up to several years, may produce poor results and low patient satisfaction [3].
To avoid this situation, there is a drive to develop methods based on so-called combination therapies, which use peels and light- and laser-based treatments in addition to topical therapy. This is particularly advisable for dermal melasma, which is less likely to respond to topical therapy. Triple combination cream (TCC) is one of the primary topical treatments for melasma. In general, it consists of hydroquinone, a retinoid, and a fluorinated corticosteroid and is widely regarded as a safe and effective treatment for melasma. There are several options for the composition of TCC, such as 4% hydroquinone, 0.05% tretinoin, and 0.01% fluocinolone acetonide, with the so-called Kligman–Willis formula (5% hydroquinone, 0.1% tretinoin, and 0.1% dexamethasone) that has been used for hyperpigmentation treatment for more than 30 years [19,20].
Chemical peels are commonly used for several skin disorders, even though they may cause skin irritation and post-inflammatory hyperpigmentation. Glycolic acid seems to be the most broadly used for chemical peeling, whereas salicylic acid represents a safer choice for patients with sensitive skin and dark phenotypes [21]. Additionally, Jessner’s solution (an alcohol solution containing 14% resorcinol, salicylic acid, and lactic acid) could also be effective [22]. Looking for other options in the treatment of melasma, a study was conducted comparing the effectiveness of chemical peels based on 70% glycolic acid and 1% tretinoin, which showed that the efficiency of peels based on tretinoin was similar to those based on glycolic acid. Furthermore, the side effects in both groups of patients were rather negligible [23]. Quite frequently, attempts are also made to combine chemical peels with topical therapy. Chaudhary et al. described a study showing that the combination of topical application of 2% hydroquinone, 1% hydrocortisone, and 0.05% tretinoin with sequential use of glycolic acid-based peeling significantly improves the therapeutic efficiency in the treatment of melasma in Indian patients [24]. Hagag et al. conducted a similar study in which the efficiency of topically delivered nano-vitamin C enhanced with iontophoresis was compared with that of a chemical peel containing 20% trichloroacetic acid. The authors concluded that nanosomal vitamin C supported by iontophoresis may be an easy, safe, effective, painless, and non-invasive alternative in the treatment of melasma since its effectiveness was found to be as effective as trichloroacetic acid peelings [25].
In recent years, it has been well recognized that intense pulsed light, fractional and pigment lasers, or radiofrequency may be successfully used in melasma treatment [26,27,28,29]. However, it has also been documented that therapies based on laser and light sources can lead to adverse effects, namely paradoxical hyperpigmentation resulting from direct damage to the skin, especially for patients with high skin phototypes. Hence, it has also been suggested to limit this therapeutic approach to patients with disorders resistant to topical treatment [5]. It should also be taken into consideration that non-ablative fractional lasers are the only laser-based systems approved by the FDA (since 2003), producing well-documented results in the treatment of melasma [30].
Intense pulsed light uses a flash lamp light source to emit noncoherent light with wavelengths between 515 nm and 1200 nm. The application of filters allows the targeting of a particular chromophore, namely melanin, in the case of melasma [31]. Q-switched lasers generate high-intensity laser beams with very short pulse intervals. These types of lasers target melanin and are available in multiple wavelengths, with the most commonly used being 532 nm or 1064 nm from a neodymium-doped yttrium aluminum garnet (QS-Nd:YAG) laser [32]. Recently, comparative studies were also performed on 1064 nm QS-Nd:YAG laser and Jessner’s peeling, proving that these therapies are equally effective in the treatment of melasma [33]. Moreover, the skin-lightening effect achieved by laser therapy can be further sustained by using an appropriate combination therapy based on classical whitening substances, namely serums containing vitamin C, ferulic acid, and phloretin [34]. Pulsed-dye lasers target hemoglobin, which may be considered the vascular component of melasma [35]. In contrast, fractional lasers act by creating thousands of microthermal treatment zones with each pulse. These microthermal damages are able to penetrate into the deeper layers of the skin, influencing dermal melanin. The most important advantage of fractional laser therapy is that it does not create open wounds, allowing for faster recovery and reducing the risk of scarring or pigmentary alterations [36]. Elmorsy et al. showed that low-power fractional laser can serve as a safe and effective melasma treatment in patients with different skin types, especially Fitzpatrick skin type III. Additionally, its combination with Jessner’s peel gives faster improvement and higher patient satisfaction compared to separate treatments with laser or Jessner’s solution [37].
In addition to the laser-based equipment, devices using radiofrequency may also be used in the treatment of melasma. For example, a monopolar radiofrequency was successfully applied to facilitate the drug delivery of a phytocomplex of 1% kojic acid [38].
In the context of treating hyperpigmentation-based conditions, one of the most important aspects is preventive action, which in this case is mainly based on photoprotective measures. A natural factor that protects against the harmful effects of sunlight is eumelanin, one of the natural pigments of the skin, which is associated with dark skin pigmentation. The light phototype skin preferentially contains pheomelanin; the amount of eumelanin is not sufficient to provide full photoprotection, and therefore the application of complementary sunscreens is essential [2]. Today, many cosmetic UV filters with a wide range of photoprotection are available. In addition, much research is currently being conducted on new sunscreens and novel methods to incorporate them into cosmetic formulations and extend their durability after application to the skin [39]. It was found that the best solution for patients with hyperpigmentation-based skin conditions such as melasma is the use of broad spectrum sunscreens along with protection against visible light [40].
4. Chemicals Commonly Used in the Treatment of Melasma
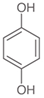
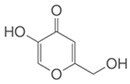
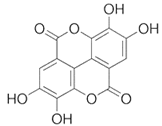
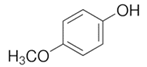
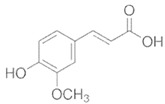
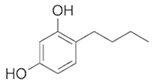
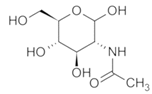
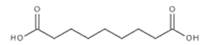
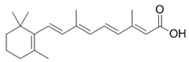
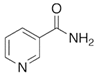
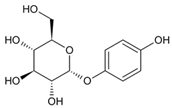
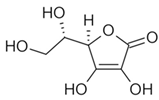
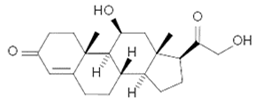
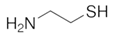
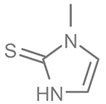
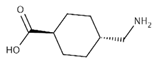
To date, a number of chemicals have been used in the treatment of melasma, primarily aimed at abolishing excessive tyrosinase production (so-called tyrosinase inhibitors). They can be administered through the skin or orally. An additional determining factor in the usefulness of a given substance is the duration of therapy [16]. The most common chemicals used in topical melasma treatment are shown in Figure 1, and examples of publications from the last five years describing this type of research are included in Table 1.
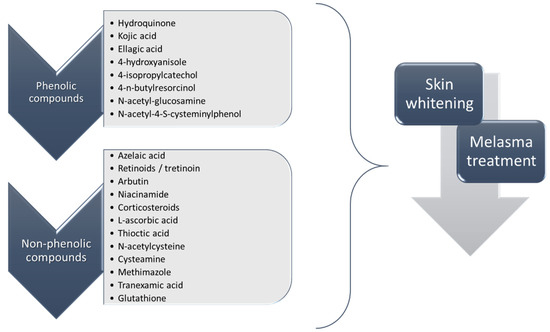
Figure 1.
The most common chemical compounds in the treatment of melasma.

Table 1.
The most common chemical compounds with skin-whitening properties published in the last five years.
Current research indicates that these substances can act as individual therapies or can be used in combination with other chemicals or other techniques, such as peels and/or laser therapy, as already outlined in Section 3 of this paper. Consequently, the literature reports from the last few years show that research on treatments based on combinations of several different ingredients administered topically is still ongoing [62,63,64,65,66]. An interesting approach was proposed by Martínez-Gutiérrez et al., who used artificial intelligence-based tools to determine the optimal combination of substances with depigmentation effects. The mathematical models they developed indicated that the optimal combination for skin whitening would be a mixture of retinol, diosmin, and ferulic acid, which would have synergistic effects in the treatment of melasma [67].
5. Substances of Natural Origin Prepared on the Basis of Plant and Mushroom/Fungi Extracts Showing Skin-Whitening Properties
Treatment of melasma is a challenging task due to the recurrent nature of this condition. Current trends in the treatment of melasma involve several procedures, namely the application of sunscreens, the use of topical whitening agents such as hydroquinone alone or in combination with tretinoin, and a corticosteroid. Superficial chemical peels and laser therapy are also recommended. However, the use of hydroquinone in cosmetic products is currently forbidden in many regions, as its application can be associated with many undesirable side effects, such as skin irritations, cytotoxicity, mutagenicity of melanocytes, contact dermatitis, or exogenous ochronosis [68]. Therefore, natural compounds with an anti-pigmentary action are currently in great demand, as they are expected to exhibit comparable efficiency with lower toxicity than existing skin-whitening agents [7,69,70,71]. The scientific literature of the past few years abounds with examples of studies on plant extracts that may have the potential to alleviate skin conditions based on hyperpigmentation (Table 2). Of course, it is important to keep in mind that these studies are in various stages of development and are often based only on in silico tests, requiring further testing before they can be introduced as an official therapy for the treatment of melasma.

Table 2.
Selected substances of plant origin recently tested as topical whitening agents with potential in the melasma treatment.
Among substances of natural origin, a widely studied group of compounds with hypopigmentation potential are polyphenols and their derivatives, which additionally exhibit antioxidant activity, thus counteracting skin aging processes [131]. Within the range of antioxidants that show inhibitory activity against tyrosinase, resveratrol, a compound present in a wide variety of plants such as berries or grapes (Vitis vinifera), has gained great interest among researchers in recent years. Studies have shown that this compound and its derivatives exhibit not only anti-inflammatory, anti-cancer, and anti-oxidant properties but may also be used as a skin-whitening agent [132,133,134,135]. Resveratrol derivatives characterized by better bioavailability, namely resveratrol glucosides, which were obtained by transglycosylation by amylosucrase of Deinococcus geothermalis, showed activity similar to that of arbutin in suppression of melanin synthesis and tyrosinase activity [136]. Higher bioavailability of resveratrol in the control of skin pigmentation can also be achieved by incorporating this stilbene derivative into a suitable carrier that will allow better penetration through the epidermal layers and thus a better whitening effect. An example is the use of microemulsion gel, which was able to significantly enhance the capability of resveratrol to inhibit melanin formation when compared to resveratrol-containing suspension and microemulsion [137]. Furthermore, Sheweita et al., in preclinical studies conducted on melanogenesis proteins, showed that trans-resveratrol introduced into emulsion systems also had the ability to suppress the protein expressions of tyrosinase and microphthalmia-associated transcription factors [138]. Due to the low absorption and poor solubility of resveratrol, studies have also been conducted to increase the bioavailability of this compound through the use of dissolving microneedle patches. Both in vitro and in vivo studies revealed that the use of these patches effectively improved intradermal resveratrol delivery compared to a cream formulation; therefore, it can be concluded that this approach is a promising way for the effective local delivery of whitening ingredients in the melasma treatment [139].
Vitis vinifera and other grape varieties are not only rich in stilbene derivatives but also in flavonoids, phenolic acids, anthocyanins, and vitamins [140]. Most of these compounds show the ability to inhibit tyrosinase production and may therefore be attractive in the cosmetics and medicinal industries as depigmentation agents [141]. Malinowska and co-workers tested extracts from canes of five selected varieties of V. vinifera, namely Villard Noir, Sauvignon, Savagnin, Riesling, and Magdeleine Noire des Charentes, for their ability to inhibit tyrosinase production. All extracts were found to be relatively efficient tyrosinase inhibitors, with the Riesling-based extract being the most potent [142].
Many plants contain carotenoids, which are well-known for their antioxidant activity [143]. Recent studies have shown that cosmetic formulations containing 0.05% w/w tomato lycopene and 3.45% w/w wheat bran extract can be successfully used in the treatment of skin hyperpigmentation without causing side effects [144]. In addition, a recent study on Z-isomer-rich lycopene and β-carotene showed that carotenoid Z-isomers exhibit powerful skin-whitening action, promising their future application in skin care supplements and cosmetic products. This skin-whitening action may result from the regulation of gene expressions of tyrosinase-related enzymes; nevertheless, the exact mechanism still needs to be clarified [145].
The current literature also offers many suggestions for combining plant extracts with classic anti-pigmentation agents. As an example, there are studies on a French maritime pine bark extract containing pycnogenol in combination with triple combination cream [146], on an oral nutritional supplement containing extracts from Pinus pinaster and grape seed extract, used along with a high SPF sunscreen [147], and on a tomato extract supplement applied together with a topical sunscreen and cream containing 4% hydroquinone [148]. Licorice extract was also used in the tests on combined therapy based on dissolving microneedle patches containing tranexamic acid. It has been found that dissolving microneedles based on polyvinyl alcohol and polyvinylpyrrolidone can be successfully applied as a delivery platform for the combination of tranexamic acid and licorice extract in synergistic melasma therapy [149].
Not only extracts may be applied as a potential source of skin-whitening agents, but fermented plant materials can also serve as such. For example, Liu et al. have shown that ethanol extracts from Lactobacillus plantarum TWK10 fermented-nongerminated black soy milk and fermented-germinated black soy milk could inhibit melanogenesis, indicating their potential as whitening agents in cosmetic products [150]. The extracts of Aloe vera leaf skin fermented with Lactobacillus plantarum BN41 were tested for their whitening properties. According to the studies of Jeon et al., the inhibition of tyrosinase activity and melanin secretion was much higher for the fermented extracts than those of commercial skin-lightening ingredients, namely arbutin and aloesin [151]. The fermented by-product from aloe processing also contains compounds that have the potential to be used as tyrosinase-suppressing agents [152]. Lin and co-workers positively verified the skin-whitening properties of Chenopodium formosanum leaf extract fermented with the filamentous fungus Aspergillus oryzae [153].
Botanical products are constantly gaining interest in topical therapies for melasma and could potentially reach comparable efficacy with active compounds of pharmaceutical origin. Undoubtedly, the biggest advantage is that they are well tolerated, with no serious adverse effects reported. Thus, the use of substances from botanical sources may be a promising option for patients seeking alternative therapeutic options for hyperpigmentation-based conditions [154].
Although a lot of tests evaluating the skin-whitening effect of plant extracts have been described in the literature, many still lack sufficient evidence on safety and effectiveness in the treatment of hyperpigmentation. Only selected plant extracts with skin-lightening potential are currently used in cosmetic products legally available in the European market. Vitis vinifera seed extracts are present, among others, in products designed for skin treatment in the eye area, while ginseng extracts are used in nourishing formulations that retard the onset of aging. Licorice extracts, which are rich in glabridin, are also used in cosmetic and medicinal skin care products, such as creams, lotions, and body washes, which are designed for hyperpigmented skin. Olive extracts are used to help protect skin from UV damage and regenerate it afterward. Correspondingly, mulberry extracts are used as skin-lightening ingredients in products protecting against pigmentation disorders and skin damage caused by overexposure to the sun. Extracts from the Rosacea family are used, for example, in cleansing and toning products, body care, and after-sun care formulations with depigmenting properties. Pomegranate extracts are used in formulations with skin-whitening properties, such as radiant and glowing cleansers, eye contour lighteners, spot fading treatments, and skin tone eveners.
Not only botanical extracts but also mushroom components and their secondary metabolites contain many compounds with potential antimicrobial, antiviral, anti-cancer, anti-inflammatory, anti-aging, antioxidant, anti-wrinkle, moisturizing, or skin-whitening activity. Edible mushrooms are considered valuable sources of new bioactive compounds with potential applications in cosmetics, as they are expected to exhibit low toxicity. Biologically active compounds of mushroom origin have been studied for skincare applications [10,155,156].
In recent years, there have been reports on the potential use of extracts derived from naturally occurring edible and medicinal mushrooms as cosmetic ingredients with potential skin-lightening effects [157]. Recently, Angelini et al. conducted a study on extracts of the edible mushroom Tricholosporum goniospermum (Bres.) Guzmán ex T.J. Baroni. According to their results, ethyl acetate extract acts as an anti-tyrosinase agent, mainly due to the presence of catechin [158]. Neolentinus lepideus (Fr.) Redhead and Ginns, a wood-decaying mushroom and one of the most popular edible mushrooms in East Asian countries, was also tested by Ishihara et al. for its tyrosinase-inhibiting activity. The results showed that the extracts contain 1,3-dihydroisobenzofuran and 4,5,7-triol-5-methoxy-1,3-dihydroisobenzofuran-4,7-diol, with IC50 values significantly lower than those calculated for hydroquinone and arbutin, giving them the ability to be used as active ingredients in skin-whitening formulations [159]. The same authors reported that 6-hydroxy-L-tryptophan isolated with hot water from the lyophilized fruiting body of Lyophyllum decastes may also act as a tyrosinase inhibitor with low IC50 values [160]. In vivo tests (zebrafish embryo model as a preclinical animal platform) of ethanol extracts from five edible mushrooms (Lethiporus sulphureus, Agaricus silvaticus, Agrocybe aegerita, Pleurotus ostreatus, and Polyporus squamosus) have been performed by Pavic and co-workers. They comprehensively evaluated their potential for use as topical depigmenting agents and concluded that extracts of A. silvaticus and L. sulphureus have a potent depigmenting activity based on inhibition of tyrosinase activity and melanin synthesis in skin melanocytes of zebrafish [161]. Methanol and hot water extracts from the fruiting bodies of Phellinus vaninii, a mushroom that grows on wood, were also investigated for their potential for depigmentation. The results obtained by Hoan et al. suggest that methanol extract was significantly more active in inhibiting melanin synthesis in B16F10 melanoma cells than arbutin [162]. The anti-tyrosinase effect was also verified for different extracts of cultivated edible mushrooms: Schizophyllum commune [163], Pleurotus ostreatus, Ganoderma lucidum, Auricularia polytricha, and Schizophyllum commune [164], Polyozellus multiplex [165], Lentinula edodes (Berk.) Pegler [166], Ganoderma lucidum [167], Agaricus bisporus (brown) [168], and Inonotus obliquus [169]. Sangthong and co-workers studied polysaccharides from Volvariella volvacea obtained by different extraction techniques. According to their results, a gel cream containing 0.2% mushroom extract may be considered a multi-functional cosmetic with whitening properties [170]. An interesting approach to obtaining skin-whitening agents was demonstrated by Pintathong et al., who used for this purpose solid-based residues from harvesting the fruiting bodies of cultured Cordyceps militaris. These residue parts were used to prepare extracts by solid-state fermentation with solid media containing a mixture of defatted rice bran, barley, white rice, riceberry rice, and wheat. The obtained extracts were characterized, among others, by excellent tyrosinase inhibitory activity [171].
In addition to mushroom-based materials (a fruiting body of certain fungi), research has also been conducted on a number of fermentative fungi species, which exhibit antityrosinase activities and can serve as a source of substances to aid in the therapy of melasma [172,173,174,175,176,177].
6. Analytical Methods for Determining the Content of Active Substances Present in Skin-Whitening Formulations
The precise qualitative and quantitative determination of active substances in skin-whitening preparations is a very important issue, not only for the effectiveness of the product but also for the safety of its use. Products containing skin-whitening ingredients, depending on the manufacturer’s declaration, are subject to different regulations. As far as the European market is concerned, these are the regulations EC 1223/2009 or EC 726/2004 for cosmetic or pharmaceutical products, respectively. Cosmetic products are used for aesthetic reasons on large skin areas and are freely available, while for pharmaceutical products, the application and availability are quite different: they are used to correct skin disorders in small areas and are available only by prescription. There is an official analytical procedure for the determination of skin-whitening ingredients, which was published in 1995 under the EU framework (Sixth Commission Directive 95/32/EC). It combines thin-layer chromatography (TLC), followed by quantitative determination using liquid chromatography (LC) with ultraviolet/visible (UV-Vis) detection, and it primarily concerns the determination of hydroquinone, hydroquinone monomethyl ether, hydroquinone monoethyl ether, and hydroquinone monobenzyl ether. There is also an extension of this method in which a procedure is described for analyzing not only hydroquinone and its esters but also the most common corticosteroids used illegally in cosmetic products [178].
Until now, many different analytical techniques have been applied to determine the chemical composition of extracts used in studies on skin-lightening potential, namely HPLC, UHPLC, or UV-Vis. However, the most commonly used are combined systems, such as GC-MS, LC-MS, or LC-MS/MS, which allow the chemical composition of extracts with the potential to counteract skin hyperpigmentation to be determined with a high degree of precision (Table 3).

Table 3.
Techniques for qualitative analysis of extracts with skin-lightening potential.
While in the case of extract samples, analyses can be carried out almost immediately, the analysis of cosmetic or pharmaceutical products containing whitening agents requires a much more complicated sample preparation procedure as well as the use of appropriate validation procedures. Often, the preparation of a cosmetic product sample requires appropriate procedures for extracting analytes from the matrix. These are often based on solvent extraction assisted by ultrasound or microwave radiation.
Cosmetic products usually consist of mixtures of whitening agents, so their direct analysis by, e.g., UV-Vis spectroscopy may be difficult without a previous separation step. Therefore, chromatographic techniques are usually employed to enable the determination of a high number of these types of cosmetic ingredients. Nevertheless, Li and co-workers put forward a proposal for the quantitative analysis of five skin-whitening agents, namely arbutin, nicotinamide, kojic acid, hydroquinone, and phenol, in various cosmetic products (lotion, emulsion, and cream), based on the conventional UV-Vis determination with a Tchebichef curve moment approach, reaching the leave-one-out correlation coefficients of the established models with values higher than 0.9948 within the linear ranges [188].
The literature of recent years has been dominated by studies on the simultaneous determination of several whitening ingredients present in a cosmetic or pharmaceutical product. They are mainly based on chromatographic techniques and concern the simultaneous detection of chemicals such as hydroquinone and retinoic acid [189], hydroquinone, hydrocortisone acetate, and tretinoin [190], hydroquinone, tretinoin, and betamethasone [191], arbutin, niacinamide, and 3-O-ethyl ascorbic acid [192], hydroquinone, resorcinol, catechol, and 3,3′-dichlorobenzidine [193], as well as two glucocorticoids, namely clobetasol 17-propionate and betamethasone 17-valerate [194], present in various forms of cosmetic and pharmaceutical products.
In addition to the chromatographic separation-based methods mentioned above, there are also reports on electrochemical sensors that would allow rapid and simultaneous analysis of skin-whitening substances. An example of such an approach is the research presented by Butwong et al., who developed a glassy carbon electrode modified with a composite of Ag@AgCl, Ag2S, carbon nanotubes, and chitosan for the simultaneous analysis of hydroquinone, arbutin, and ascorbyl glucoside [195]. Another highly effective and promising analytic option for skin-whitening agents in cosmetic products was suggested by Wang et al., who established a simple, reliable, sensitive, and selective method for the detection of ellagic acid used in whitening cosmetics. This method is based on the seed-mediated growth of Au@Ag bimetallic core-shell nanorods and the monitoring of blue shifts in surface plasmon resonance [196]. In turn, Li and co-workers developed an electrochemical sensor based on nitrogen and sulfur co-doped Fe-Ni alloy (N,S-FeNi3/C) nanoparticles obtained via hydrothermal synthesis and high-temperature carbonization, which turned out to be an efficient tool for simultaneous analysis of arbutin and hydroquinone content in cosmetic products [197].
Arbutin, which is commonly used in skin-whitening products, has limitations on its allowable content due to the fact that it can generate hydroquinone during the decomposition process. Therefore, appropriate analytical techniques based on chromatography [198], fluorescence spectroscopy [199], and cyclic voltammetry [200] have been developed to precisely control the content of arbutin in cosmetic products.
It is worth mentioning here that hydroquinone is a banned ingredient in the EU for use in cosmetic topical preparations [201], and its use as an anti-hyperpigmentation agent is limited to pharmaceutical products. Therefore, it is very common in the literature to find descriptions of various methods for determining this substance in cosmetic products from non-EU countries or from illegal trade, which are widely available in online stores. Among them, chromatographic methods predominate, such as reversed-phase high-performance thin-layer chromatography (RP-HPTLC) [202] and reversed-phase high-performance liquid chromatography (RP-HPLC) [203,204]. However, there are also reports of methods based on electrochemical sensors [205,206,207,208] and descriptions of comprehensive analytical procedures to quantitatively verify the amount of hydroquinone in dermatological products [209].
A detailed review of the analytical techniques developed between 2006 and 2016, including a description of the preparation of cosmetic product samples, was published by Chisvert et al. [178]. Among them, the method developed and validated by Desmedt et al., based on ultra-high pressure liquid chromatography (UHPLC), allowed qualitative and quantitative analysis of eight illegal (hydroquinone, tretinoin, and six active dermatologic corticosteroids) and four legal (kojic acid, arbutin, nicotinamide, and salicylic acid) skin-whitening agents applied in different types of cosmetic preparations, namely creams, lotions, and soaps [210]. UHPLC analysis was preceded by the ultrasound-assisted extraction procedure performed with acetonitrile at 50 °C for 30 min. Importantly, this method of detection can be used for a market survey of suspected illegal whitening cosmetics products, which may pose a threat to public health.
7. Summary
This paper presents the currently used options in the treatment of melasma, a skin condition that is quite complex in its genesis and whose main symptom is hyperpigmentation of the skin. All therapeutic options, i.e., skin-lightening chemicals, laser light-based therapies, as well as chemical peels, are briefly characterized. What are also presented are the literature reports from the past five years describing research on plant and edible mushroom extracts with potentially skin-whitening ingredients. Despite the abundance of information on potential replacements for therapies based on synthetically derived chemicals used to date, it should be kept in mind that most of the studies presented in the literature are at a very early stage, and it will be a long time before these extracts can be officially incorporated into therapeutic regimes for the treatment of skin hyperpigmentation, including melasma.
An important element in the search for natural substitutes in the treatment of melasma is the development of appropriate analytical techniques and procedures that will allow full qualitative and quantitative characterization of the extracts obtained, thus ensuring the therapeutic efficacy and safety of their use. With the continuous improvement of the technique of obtaining natural extracts, the possibility of accurate qualitative and quantitative determination of the active ingredients present in these extracts will probably be achieved in the near future. In addition, it will be possible to precisely determine the effect of the active ingredients present in the extracts and find the substances with the best whitening effect. The continuous improvement of analytical techniques will also allow for the control of the composition of cosmetic preparations for patients with conditions based on hyperpigmentation, which are already available on the market.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declare no conflict of interest.
References
- Naidoo, L.; Khoza, N.; Dlova, N.C. A Fairer Face, a Fairer Tomorrow? A Review of Skin Lighteners. Cosmetics 2016, 3, 33. [Google Scholar] [CrossRef]
- Solano, F. Photoprotection and Skin Pigmentation: Melanin-Related Molecules and Some Other New Agents Obtained from Natural Sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, A.; Wairkar, S. Management of hyperpigmentation: Current treatments and emerging therapies. Pigment. Cell Melanoma Res. 2021, 34, 1000–1014. [Google Scholar] [CrossRef] [PubMed]
- AlSalem, S.; Alexis, A. Melasma hyperpigmentation: An overview of current topical therapeutics. Dermatol. Rev. 2023, 4, 38–52. [Google Scholar] [CrossRef]
- McKesey, J.; Tovar-Garza, A.; Pandya, A.G. Melasma Treatment: An Evidence-Based Review. Am. J. Clin. Dermatol. 2020, 21, 173–225. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, A.; Wang, J.; Huang, D.; Deng, Y.; Zhang, X.; Qu, Q.; Ma, W.; Xiong, R.; Zhu, M.; et al. Potential application of natural bioactive compounds as skin-whitening agents: A review. J. Cosmet. Dermatol. 2022, 21, 6669–6687. [Google Scholar] [CrossRef]
- Hollinger, J.C.; Angra, K.; Halder, R.M. Are Natural Ingredients Effective in the Management of Hyperpigmentation? A Systematic Review. J. Clin. Aesthetic Dermatol. 2018, 11, 28–37. [Google Scholar]
- Siahaan, E.A.; Agusman; Pangestuti, R.; Shin, K.-H.; Kim, S.-K. Potential Cosmetic Active Ingredients Derived from Marine By-Products. Mar. Drugs 2022, 20, 734. [Google Scholar] [CrossRef]
- Agrawal, S.; Barrow, C.J.; Adholeya, A.; Deshmukh, S.K. Unveiling the dermatological potential of marine fungal species components: Antioxidant and inhibitory capacities over tyrosinase. Biotechnol. Appl. Biochem. 2022, 69, 1252–1266. [Google Scholar] [CrossRef]
- Taofiq, O.; González-Paramás, A.M.; Martins, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Mushrooms extracts and compounds in cosmetics, cosmeceuticals and nutricosmetics—A review. Ind. Crop. Prod. 2016, 90, 38–48. [Google Scholar] [CrossRef]
- Burger, P.; Landreau, A.; Azoulay, S.; Michel, T.; Fernandez, X. Skin Whitening Cosmetics: Feedback and Challenges in the Development of Natural Skin Lighteners. Cosmetics 2016, 3, 36. [Google Scholar] [CrossRef]
- Mahajan, V.K.; Patil, A.; Blicharz, L.; Kassir, M.; Konnikov, N.; Gold, M.H.; Goldman, M.P.; Galadari, H.; Goldust, M. Medical therapies for melasma. J. Cosmet. Dermatol. 2022, 21, 3707–3728. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-H.; Hwang, Y.-J.; Lee, S.-K.; Park, K.-C. Heterogeneous Pathology of Melasma and Its Clinical Implications. Int. J. Mol. Sci. 2016, 17, 824. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.Y.; Suzuki, I.; Lee, D.J.; Ha, J.; Reiniche, P.; Aubert, J.; Deret, S.; Zugaj, D.; Voegel, J.J.; Ortonne, J.-P. Transcriptional Profiling Shows Altered Expression of Wnt Pathway– and Lipid Metabolism–Related Genes as Well as Melanogenesis-Related Genes in Melasma. J. Investig. Dermatol. 2011, 131, 1692–1700. [Google Scholar] [CrossRef]
- Lee, A.-Y. Recent progress in melasma pathogenesis. Pigment. Cell Melanoma Res. 2015, 28, 648–660. [Google Scholar] [CrossRef]
- Maddaleno, A.S.; Camargo, J.; Mitjans, M.; Vinardell, M.P. Melanogenesis and Melasma Treatment. Cosmetics 2021, 8, 82. [Google Scholar] [CrossRef]
- Chaowattanapanit, S.; Silpa-archa, N.; Kohli, I.; Lim, H.W.; Hamzavi, I. Postinflammatory hyperpigmentation: A comprehensive overview: Treatment options and prevention. J. Am. Acad. Dermatol. 2017, 77, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Neumann, L. Lentigo and Solar Lentigines. In Dermatological Cryosurgery and Cryotherapy; Abramovits, W., Graham, G., Har-Shai, Y., Strumia, R., Eds.; Springer: London, UK, 2016. [Google Scholar]
- Gong, Z.; Lai, W.; Zhao, G.; Wang, X.; Zheng, M.; Li, L.; Yang, Q.; Dang, Y.; Liu, L.; Zou, Y. Efficacy and safety of fluocinolone acetonide, hydroquinone, and tretinoin cream in Chinese patients with melasma: A randomized, double-blind, placebo-controlled, multicenter, parallel-group study. Clin. Drug Investig. 2015, 35, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Pandya, A.P. Melasma: Clinical diagnosis and management options. Australas. J. Dermatol. 2015, 56, 151–163. [Google Scholar] [CrossRef]
- Sarkar, R.; Garg, V.; Bansal, S.; Sethi, S.; Gupta, C. Comparative evaluation of efficacy and tolerability of glycolic acid, salicylic mandelic acid, and phytic acid combination peels in melasma. Dermatol. Surg. 2016, 42, 384–391. [Google Scholar] [CrossRef]
- Dorgham, N.A.; Dorgham, D.A.; Hegazy, R.A.; Sharobim, A.K. Efficacy and Tolerability of Chemical Peeling as A Single Agent for Melasma in Dark-Skinned Patients: A Systematic Review and Meta-analysis of Comparative Trials. J. Cosmet. Dermatol. 2020, 19, 2812–2819. [Google Scholar] [CrossRef]
- Faghihi, G.; Shahingohar, A.; Siadat, A.H. Comparison between 1% tretinoin peeling versus 70% glycolic acid peeling in the treatment of female patients with melasma. J. Drugs Dermatol. 2011, 10, 1439–1442. [Google Scholar] [PubMed]
- Chaudhary, S.; Dayal, S. Efficacy of combination of glycolic acid peeling with topical regimen in treatment of melasma. J. Drugs Dermatol. 2013, 12, 1149–1153. [Google Scholar] [PubMed]
- Hagag Sara, M.M.; Abd Allah, S.H. The effect of topical nano vitamin-C iontophoresis versus the effect of trichloroacetic acid 20% peel in treatment of melasma. Menoufia Med. J. 2022, 35, 489–495. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Yang, F.C.; Cho, B.K. A review of laser and light therapy in melasma. Int. J. Women’s Dermatol. 2017, 3, 11–20. [Google Scholar] [CrossRef]
- Shah, S.D.; Aurangabadkar, S.J. Laser Toning in Melasma. J. Cutan. Aesthetic Surg. 2019, 12, 76–84. [Google Scholar] [CrossRef]
- Mehrabi, J.N.; Bar-Ilan, E.; Wasim, S.; Koren, A.; Zusmanovitch, L.; Salameh, F.; Nelkenbaum, G.I.; Horovitz, T.; Zur, E.; Lim, T.S.; et al. A review of combined treatments for melasma involving energy-based devices and proposed pathogenesis-oriented combinations. J. Cosmet. Dermatol. 2021, 21, 461–472. [Google Scholar] [CrossRef]
- Feng, J.; Shen, S.; Song, X.; Xiang, W. Efficacy and safety of picosecond laser for the treatment of melasma: A systematic review and meta-analysis. Lasers Med. Sci. 2023, 38, 84. [Google Scholar] [CrossRef]
- Küçük, Ö.S. Current treatment approaches for melasma. Bezmialem Sci. 2018, 6, 54–62. [Google Scholar] [CrossRef]
- Tirico, M.C.C.P.; Jensen, D.; Green, C.; Ross, E.V. Short pulse intense pulsed light versus pulsed dye laser for the treatment of facial redness. J. Cosmet. Laser Ther. 2020, 22, 60–64. [Google Scholar] [CrossRef]
- Kaminaka, C.; Furukawa, F.; Yamamoto, Y. The clinical and histological effect of a low-fluence Q-Switched 1064-nm neodymium:Yttrium-aluminum-garnet laser for the treatment of melasma and solar lentigines in asians prospective, randomized, and split-face comparative study. Dermatol. Surg. 2017, 43, 1120–1133. [Google Scholar] [CrossRef]
- Sagduyu, I.E.; Marakli, O.; Oraloglu, G.; Okut, E.B.; Unal, I. Comparison of 1064 nm Q-switched Nd:YAG laser and Jessner peeling in melasma treatment. Dermatol. Ther. 2022, 35, e15970. [Google Scholar] [CrossRef]
- Campos, V. 28379 Case report of effect of a topical antioxidant serum containing vitamin C, ferulic acid, and phloretin after Q-switched laser for treatment of melasma. J. Am. Acad. Dermatol. 2021, 85, AB186. [Google Scholar] [CrossRef]
- Reynal, S.; Martin, E.; Munavalli, G. Energy-based devices for melasma and postinflammatory hyperpigmentation. Dermatol. Rev. 2023, 4, 58–66. [Google Scholar] [CrossRef]
- Chalermchai, T.; Rummaneethorn, P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasma. J. Cosmet. Laser Ther. 2018, 20, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Elmorsy, E.; Aboukhadr, N.; Tayyeb, M.; Taha, A.A.A. Low-power Fractional Carbon Dioxide Laser Followed by Jessner’s Peel versus Jessner’s Peel Alone for the Treatment of Melasma. J. Clin. Aesthetic Dermatol. 2021, 14, 61–67. [Google Scholar]
- Cameli, N.; Abril, E.; Mariano, M.; Berardesca, E. Combined use of monopolar radiofrequency and transdermal drug delivery in the treatment of melasma. Dermatol. Surg. 2014, 40, 748–755. [Google Scholar]
- Wawrzyńczak, A.; Feliczak-Guzik, A.; Nowak, I. Nanosunscreens: From Nanoencapsulated to Nanosized Cosmetic Active Forms. In Nanobiomaterials in Galenic Formulations and Cosmetics; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 25–46. [Google Scholar] [CrossRef]
- Boukari, F.; Jourdan, E.; Fontas, E.; Montaudié, H.; Castela, E.; Lacour, J.P.; Passeron, T. Prevention of melasma relapses with sunscreen combining protection against UV and short wavelengths of visible light: A prospective randomized comparative trial. J. Am. Acad. Dermatol. 2015, 72, 189–190. [Google Scholar] [CrossRef]
- Searle, T.; Al-Niaimi, F.; Ali, F.R. Hydroquinone: Myths and reality. Clin. Exp. Dermatol. 2021, 46, 636–640. [Google Scholar] [CrossRef]
- Schwartz, C.; Jan, A.; Zito, P.M. Hydroquinone. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539693/ (accessed on 29 April 2023).
- Phasha, V.; Senabe, J.; Ndzotoyi, P.; Okole, B.; Fouche, G.; Chuturgoon, A. Review on the Use of Kojic Acid-A Skin-Lightening Ingredient. Cosmetics 2022, 9, 64. [Google Scholar] [CrossRef]
- Yang, H.-L.; Lin, C.-P.; Gowrisankar, Y.V.; Huang, P.-J.; Chang, W.-L.; Shrestha, S.; Hseu, Y.-C. The anti-melanogenic effects of ellagic acid through induction of autophagy in melanocytes and suppression of UVA-activated α-MSH pathways via Nrf2 activation in keratinocytes. Biochem. Pharmacol. 2021, 185, 114454. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Cho, J.H.; Hong, S.H.; Kim, D.-H.; Jung, H.-Y.; Kang, I.-K.; Cho, Y.-J. Whitening and anti-wrinkle activities of ferulic acid isolated from Tetragonia tetragonioides in B16F10 melanoma and CCD-986sk fibroblast cells. J. Nat. Med. 2018, 72, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-H.; Yang, J.H.; Shin, J.-W.; Park, K.-C.; Huh, C.-H.; Na, J.-I. Efficacy of liposome-encapsulated 4-n-butylresorcinol and resveratrol cream in the treatment of melasma. J. Cosmet. Dermatol. 2020, 19, 891–895. [Google Scholar] [CrossRef]
- Tokudome, Y.; Hoshi, T.; Mori, S.; Hijikuro, I. Synthesis of Resorcinol Derivatives and their Effects on Melanin Production. Cosmetics 2020, 7, 55. [Google Scholar] [CrossRef]
- Bissett, D.L.; Robinson, L.R.; Raleigh, P.S.; Miyamoto, K.; Hakozaki, T.; Li, J.; Kelm, G.R. Reduction in the appearance of facial hyperpigmentation by topical N-acetyl glucosamine. J. Cosmet. Dermatol. 2007, 6, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Searle, T.; Ali, F.R.; Al-Niaimi, F. The versatility of azelaic acid in dermatology. J. Dermatol. Treat. 2022, 33, 722–732. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, R.; Yadav, P. Azelaic Acid: A Promising Agent for Dermatological Applications. Curr. Drug Ther. 2020, 15, 181–193. [Google Scholar] [CrossRef]
- da Silva Bergmann, C.L.M.; Pochmann, D.; Bergmann, J.; Brasil Bocca, F.; Proença, I.; Marinho, J.; Mello, A.; Dani, C. The use of retinoic acid in association with microneedling in the treatment of epidermal melasma: Efficacy and oxidative stress parameters. Arch. Dermatol. Res. 2021, 313, 695–704. [Google Scholar] [CrossRef]
- Al Hamzawi, N.K. Nicotinamide as a Skin Whitener: Evidence and Controversies. J. Pharm. Res. Int. 2021, 33, 300–305. [Google Scholar] [CrossRef]
- Pedroso, A.G.; Furtado, G.R.D.; Barbosa, K.L. Niacinamide for the treatment of melasma: An integrative review of randomized clinical trials. Res. Soc. Dev. 2022, 11, e198111133581. [Google Scholar] [CrossRef]
- Boo, Y.C. Arbutin as a Skin Depigmenting Agent with Antimelanogenic and Antioxidant Properties. Antioxidants 2021, 10, 1129. [Google Scholar] [CrossRef]
- Saeedi, M.; Khezri, K.; Seyed Zakaryaei, A.; Mohammadamini, H. A comprehensive review of the therapeutic potential of α-arbutin. Phytother. Res. 2021, 35, 4136–4154. [Google Scholar] [CrossRef] [PubMed]
- Zerbinati, N.; Sommatis, S.; Maccario, C.; Di Francesco, S.; Capillo, M.C.; Rauso, R.; Herrera, M.; Bencini, P.L.; Guida, S.; Mocchi, R. The Anti-Ageing and Whitening Potential of a Cosmetic Serum Containing 3-O-ethyl-l-ascorbic Acid. Life 2021, 11, 406. [Google Scholar] [CrossRef] [PubMed]
- Nassar, A.A.E.; Ibrahim, A.-S.M.; Mahmoud, A.A. Efficacy and safety of intralesional steroid injection in the treatment of melasma. J. Cosmet. Dermatol. 2021, 20, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Ahramiyanpour, N.; Saki, N.; Akbari, Z.; Shamsi-Meymandi, S.; Amiri, R.; Heiran, A. Efficacy of topical cysteamine hydrochloride in treating melasma: A systematic review. J. Cosmet. Dermatol. 2021, 20, 3593–3602. [Google Scholar] [CrossRef]
- Niazi, S.; Gheisari, M.; Moravvej, H.; Doroodgar, F.; Niazi, F. Efficacy of cysteamine and methimazole in treating melasma: A comparative narrative review. J. Cosmet. Dermatol. 2022, 21, 3867–3875. [Google Scholar] [CrossRef]
- Sitohang, I.B.S.; Ninditya, S. Systemic Glutathione as a Skin-Whitening Agent in Adult. Dermatol. Res. Pract. 2020, 2020, 8547960. [Google Scholar] [CrossRef]
- Konisky, H.; Balazic, E.; Jaller, J.A.; Khanna, U.; Kobets, K. Tranexamic acid in melasma: A focused review on drug administration routes. J. Cosmet. Dermatol. 2023, 22, 1197–1206. [Google Scholar] [CrossRef]
- Chang, Y.-F.; Lee, T.L.; Oyerinde, O.; Desai, S.R.; Aljabban, A.; Bay, C.P.; Bain, P.A.; Chung, H.J. Efficacy and safety of topical agents in the treatment of melasma: What’s evidence? A systematic review and meta-analysis. J. Cosmet. Dermatol. 2023, 22, 1168–1176. [Google Scholar] [CrossRef]
- de Freitas, A.C.P.; Rigon, R.B.; Bagatin, E.; Leonardi, G.R. Perspectives of topical formulations for melasma. Int. J. Dermatol. 2023, 62, 260–268. [Google Scholar] [CrossRef]
- González-Molina, V.; Martí-Pineda, A.; González, N. Topical Treatments for Melasma and Their Mechanism of Action. J. Clin. Aesthetic Dermatol. 2022, 15, 19–28. [Google Scholar]
- Cassiano, D.P.; Espósito, A.C.C.; da Silva, C.N.; Lima, P.B.; Dias, J.A.F.; Hassun, K.; Miot, L.D.B.; Miot, H.A.; Bagatin, E. Update on Melasma-Part II: Treatment. Dermatol. Ther. 2022, 12, 1989–2012. [Google Scholar] [CrossRef] [PubMed]
- Berardesca, E.; Rigoni, C.; Cantù, A.; Laureti, T. Effectiveness of a new cosmetic treatment for melasma. J. Cosmet. Dermatol. 2020, 19, 1684–1690. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gutiérrez, A.; Pérez-Martínez, M.; Pena-Rodríguez, E.; Gómez-Escalante, S.; Luis, L.G.S.; González, M.C. Depigmenting topical therapy based on a synergistic combination of compounds targeting the key pathways involved in melasma pathophysiology. Exp. Dermatol. 2023, 32, 611–619. [Google Scholar] [CrossRef]
- Juliano, C.C.A. Spreading of Dangerous Skin-Lightening Products as a Result of Colourism: A Review. Appl. Sci. 2022, 12, 3177. [Google Scholar] [CrossRef]
- Nomakhosi, M.; Heidi, A. Natural options for management of melasma, a review. J. Cosmet. Laser Ther. 2018, 20, 470–481. [Google Scholar] [CrossRef]
- Kim, K.; Huh, Y.; Lim, K.-M. Anti-Pigmentary Natural Compounds and Their Mode of Action. Int. J. Mol. Sci. 2021, 22, 6206. [Google Scholar] [CrossRef]
- Goelzer Neto, C.F.; do Nascimento, P.; da Silveira, V.C.; de Mattos, A.B.N.; Bertol, C.D. Natural sources of melanogenic inhibitors: A systematic review. Int. J. Cosmet. Sci. 2022, 44, 143–153. [Google Scholar] [CrossRef]
- Lee, J.-O.; Kim, E.; Kim, J.H.; Hong, Y.H.; Kim, H.G.; Jeong, D.; Kim, J.; Kim, S.H.; Park, C.; Seo, D.B.; et al. Antimelanogenesis and skin-protective activities of Panax ginseng calyx ethanol extract. J. Ginseng Res. 2018, 42, 389–399. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, K.T.; Kim, S.S.; Hur, J.; Ha, S.K.; Cho, C.W.; Choi, S.Y. Inhibitory effects of ginseng seed on melanin biosynthesis. Pharmacogn. Mag. 2014, 10 (Suppl. S2), S272–S275. [Google Scholar]
- Lee, D.Y.; Lee, J.; Jeong, Y.T.; Byun, G.H.; Kim, J.H. Melanogenesis inhibition activity of floralginsenoside A from Panax ginseng berry. J. Ginseng Res. 2017, 41, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-S.; Nam, G.; Bae, I.-H.; Park, J. Whitening efficacy of ginsenoside F1 through inhibition of melanin transfer in cocultured human melanocytes–keratinocytes and three-dimensional human skin equivalent. J. Ginseng Res. 2019, 43, 300. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Kim, J.H.; Hong, H.-D.; Kwon, J.; Lee, E.J.; Jang, M.; Lee, S.-Y.; Han, A.-R.; Nam, T.G.; Hong, S.K.; et al. Ginsenosides Rg5 and Rk1, the skin-whitening agents in black ginseng. J. Funct. Foods 2018, 45, 67–74. [Google Scholar] [CrossRef]
- Celina, Y.; Viardo, V.; Villafuerte, L. A Pilot Study on Aloe vera Leaf Extract in Cream Base for the Clinical Improvement of Melasma: A Split-Face Trial. J. Clin. Investig. Dermatol. 2020, 8, 5. [Google Scholar]
- Hikmawati, D.; Respati, T.; Yuniarti, Y.; Yuniarti, L. In silico analysis of multi-target antimelasma aloe vera compound. In Medical Technology and Environmental Health; Abdullah, A.G., Widiaty, I., Abdullah, C.U., Eds.; CRC Press: London, UK, 2020; pp. 136–140. [Google Scholar] [CrossRef]
- Mikayoulou, M.; Mayr, F.; Temml, V.; Pandian, A.; Vermaak, I.; Chen, W.; Komane, B.; Stuppner, H.; Viljoen, A. Anti-tyrosinase activity of South African Aloe species and isolated compounds plicataloside and aloesin. Fitoterapia 2021, 150, 104828. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.H.; Jang, G.Y.; Ji, Y.-J.; Lee, J.H.; Choi, S.J.; Hyun, T.K.; Kim, H.D. Antioxidant and Anti-Melanogenic Activities of Heat-Treated Licorice (Wongam, Glycyrrhiza glabra × G. uralensis) Extract. Curr. Issues Mol. Biol. 2021, 43, 1171–1187. [Google Scholar] [CrossRef]
- Cerulli, A.; Masullo, M.; Montoro, P.; Piacente, S. Licorice (Glycyrrhiza glabra, G. uralensis, and G. inflata) and Their Constituents as Active Cosmeceutical Ingredients. Cosmetics 2022, 9, 7. [Google Scholar] [CrossRef]
- Lv, J.; Fu, Y.; Cao, Y.; Jiang, S.; Yang, Y.; Song, G.; Yun, C.; Gao, R. Isoliquiritigenin inhibits melanogenesis, melanocyte dendricity and melanosome transport by regulating ERK-mediated MITF degradation. Exp. Dermatol. 2020, 29, 149–157. [Google Scholar] [CrossRef]
- de Toledo Bagatin, J.; Bagatin, E.; Campos, P.M.B.G.M. A pilot clinical study to evaluate the effectiveness of olive extract containing hydroxytyrosol for oral and topical treatment of melasma. Biomed. Biopharm. Res. 2020, 17, 48–62. [Google Scholar] [CrossRef]
- Byeon, J.-H.; Alam, M.B.; Kim, K.-C.; Heo, S.; Lim, J.; Kwon, Y.-G.; Zhao, P.; Cha, Y.-H.; Choi, H.-J.; Lee, S.-H. Anti-Melanogenic Effect of Chestnut Spike Extract through Downregulation of Tyrosinase-Related Proteins and Activation of ERK ½. Nat. Prod. Commun. 2018, 13, 1023–1026. [Google Scholar] [CrossRef]
- Bahadori, M.B.; Kirkan, B.; Sarikurkcu, C. Phenolic ingredients and therapeutic potential of Stachys cretica subsp. smyrnaea for the management of oxidative stress, Alzheimer’s disease, hyperglycemia, and melasma. Ind. Crop. Prod. 2019, 127, 82–87. [Google Scholar] [CrossRef]
- Goh, C.L.; Chuah, S.Y.; Tien, S.; Thng, G.; Vitale, M.A.; Delgado-Rubin, A. Double-blind, Placebo-controlled Trial to Evaluate the Effectiveness of Polypodium leucotomos Extract in the Treatment of Melasma in Asian Skin: A Pilot Study. J. Clin. Aesthetic Dermatol. 2018, 11, 14–19. [Google Scholar]
- Parrado, C.; Nicolas, J.; Juarranz, A.; Gonzalez, S. The role of the aqueous extract Polypodium leucotomos in photoprotection. Photochem. Photobiol. Sci. 2020, 19, 831–843. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, E.J.; Ahn, Y.; Park, S.J.; Kim, S.H.; Oh, S.H. A chemical compound from fruit extract of Juglans mandshurica inhibits melanogenesis through p-ERK-associated MITF degradation. Phytomedicine 2019, 57, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Javedan, K.; Hydarpur, F.; Mohammadi Pour, P.; Najafi, F.; Mirzaeei, S.; Rahimi, R.; Gravandi, M.M.; Farzaei, M.H. The formulation and efficacy of topical Dorema ammoniacum in treating Melasma: A randomized double-blind, placebo-controlled trial. J. Complement. Integr. Med. 2022, 19, 743–751. [Google Scholar] [CrossRef]
- Gryn-Rynko, A.; Sperkowska, B.; Majewski, M.S. Screening and Structure–Activity Relationship for Selective and Potent Anti-Melanogenesis Agents Derived from Species of Mulberry (Genus Morus). Molecules 2022, 27, 9011. [Google Scholar] [CrossRef]
- Jeon, Y.-H.; Choi, S.-W. Isolation, Identification, and Quantification of Tyrosinase and α-Glucosidase Inhibitors from UVC-Irradiated Mulberry (Morus alba L.) Leaves. Prev. Nutr. Food Sci. 2019, 24, 84–94. [Google Scholar] [CrossRef]
- Li, H.X.; Park, J.U.; Su, X.D.; Kim, K.T.; Kang, J.S.; Kim, Y.R.; Kim, Y.H.; Yang, S.Y. Identification of Anti-Melanogenesis Constituents from Morus alba L. Leaves. Molecules 2018, 23, 2559. [Google Scholar] [CrossRef]
- Nguyen, L.T.H. Biological Activities of Paper Mulberry (Broussonetia papyrifera): More than a Skin-Lightening Agent. Cosmetics 2022, 9, 112. [Google Scholar] [CrossRef]
- Zhu, W.-F.; Wang, C.-L.; Ye, F.; Sun, H.-P.; Ma, C.-Y.; Liu, W.-Y.; Feng, F.; Abe, M.; Akihisa, T.; Zhang, J. Chemical Constituents of the Seed Cake of Camellia oleifera and Their Antioxidant and Antimelanogenic Activities. Chem. Biodivers. 2018, 15, e1800137. [Google Scholar] [CrossRef]
- Wang, Y.; Du, G.-Y.; Guo, T.; Zou, H.-M.; Jia, D. Skin-whitening mechanism of cumin (Cuminum cyminum L.) extract. Pak. J. Pharm. Sci. 2021, 34, 077–084. [Google Scholar] [CrossRef]
- Vijayakumar, R.; Abd Gani, S.S.; Zaidan, U.H.; Halmi, M.I.E. Optimization of the Antioxidant Potentials of Red Pitaya Peels and Its In Vitro Skin Whitening Properties. Appl. Sci. 2018, 8, 1516. [Google Scholar] [CrossRef]
- Song, Y.-R.; Lim, W.-C.; Han, A.; Lee, M.-h.; Shin, E.J.; Lee, K.-M.; Nam, T.-G.; Lim, T.-G. Rose Petal Extract (Rosa gallica) Exerts Skin Whitening and Anti-Skin Wrinkle Effects. J. Med. Food 2020, 23, 870–878. [Google Scholar] [CrossRef]
- Shin, E.J.; Han, A.; Lee, M.; Song, Y.-R.; Lee, K.M.; Nam, T.-G.; Lee, P.; Lee, S.-Y.; Lim, T.-G. Extraction conditions for Rosa gallica petal extracts with anti-skin aging activities. Food Sci. Biotechnol. 2019, 28, 1439–1446. [Google Scholar] [CrossRef]
- Li, M.-X.; Xie, J.; Bai, X.; Du, Z.-Z. Anti-aging potential, anti-tyrosinase and antibacterial activities of extracts and compounds isolated from Rosa chinensis cv. ‘JinBian’. Ind. Crop. Prod. 2021, 159, 113059. [Google Scholar] [CrossRef]
- Kaushik, N.; Kim, J.-H.; Nguyen, L.N.; Kaushik, N.K.; Choi, K.-A. Characterization of Bioactive Compounds Having Antioxidant and Anti-Inflammatory Effects of Liliaceae Family Flower Petal Extracts. J. Funct. Biomater. 2022, 13, 284. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-G.; Park, G.-K.; Jang, W.; Kim, B.-Y.; Kim, S.-K.; Kim, Y.-A.; Park, S.-H.; Park, B. Skin-Whitening and Antiwrinkle Proprieties of Maackia amurensis Methanolic Extract Lead Compounds. Processes 2022, 10, 855. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, Y.; Li, Y. Solvent-Free Microwave Extraction of Essential Oils from Litsea cubeba (Lour.) Pers. at Different Harvesting Times and Their Skin-Whitening Cosmetic Potential. Antioxidants 2022, 11, 2389. [Google Scholar] [CrossRef]
- Gogoi, R.; Sarma, N.; Pandey, S.K.; Lal, M. Phytochemical constituents and pharmacological potential of Solanum khasianum C.B. Clarke., extracts: Special emphasis on its skin whitening, anti-diabetic, acetylcholinesterase and genotoxic activities. Trends Phytochem. Res. 2021, 5, 47–61. [Google Scholar] [CrossRef]
- Chatatikun, M.; Supjaroen, P.; Promlat, P.; Chantarangkul, C.; Waranuntakul, S.; Nawarat, J.; Tangpong, J.; Chiabchalard, A. Antioxidant and Tyrosinase Inhibitory Properties of an Aqueous Extract of Garcinia atroviridis Griff. ex. T. Anderson Fruit Pericarps. Pharmacogn. J. 2020, 12, 71–78. [Google Scholar] [CrossRef]
- Kanlayavattanakul, M.; Chongnativisit, W.; Chaikul, P.; Lourith, N. Phenolic-rich Pomegranate Peel Extract: In Vitro, Cellular, and In Vivo Activities for Skin Hyperpigmentation Treatment. Planta Med. 2020, 86, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Mat Saad, H.; Tan, C.H.; Lim, S.H.; Manickam, S.; Sim, K.S. Evaluation of anti-melanogenesis and free radical scavenging activities of five Artocarpus species for cosmeceutical applications. Ind. Crop. Prod. 2021, 161, 113184. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Liu, I.-H.; Huang, X.-Z.; Chen, H.-J.; Chang, S.-T.; Chang, M.-L.; Ho, Y.-T.; Chang, H.-T. Antimelanogenesis Effects of Leaf Extract and Phytochemicals from Ceylon Olive (Elaeocarpus serratus) in Zebrafish Model. Pharmaceutics 2021, 13, 1059. [Google Scholar] [CrossRef]
- Smeriglio, A.; D’Angelo, V.; Denaro, M.; Trombetta, D.; Raimondo, F.M.; Germanò, M.P. Polyphenol Characterization, Antioxidant and Skin Whitening Properties of Alnus cordata Stem Bark. Chem. Biodivers. 2019, 16, e1900314. [Google Scholar] [CrossRef]
- Li, M.-X.; Bai, X.; Ma, Y.-P.; Zhang, H.-X.; Nama, N.; Pei, S.-J.; Du, Z.-Z. Cosmetic potentials of extracts and compounds from Zingiber cassumunar Roxb. rhizome. Ind. Crop. Prod. 2019, 141, 111764. [Google Scholar] [CrossRef]
- Ko, G.; Kang, H.R.; Moon, J.Y.; Ediriweera, M.K.; Eum, S.; Bach, T.T.; Cho, S.K. Annona squamosa L. leaves inhibit alpha-melanocyte-stimulating hormone (α-MSH) stimulated melanogenesis via p38 signaling pathway in B16F10 melanoma cells. J. Cosmet. Dermatol. 2019, 19, 1785–1792. [Google Scholar] [CrossRef]
- Deniz, F.S.S.; Orhan, I.E.; Duman, H. Profiling cosmeceutical effects of various herbal extracts through elastase, collagenase, tyrosinase inhibitory and antioxidant assays. Phytochem. Lett. 2021, 45, 171–183. [Google Scholar] [CrossRef]
- Sultanov, A.; Lee, E.-H.; Park, H.-J.; Kim, S.-R.; Cho, Y.-J. Antioxidant and skin health-enhancing activities of wild indigo (Baptisia tinctoria) root extracts. Korean J. Food Preserv. 2022, 29, 367–380. [Google Scholar] [CrossRef]
- Younis, M.M.; Ayoub, I.M.; Mostafa, N.M.; El Hassab, M.A.; Eldehna, W.M.; Al-Rashood, S.T.; Eldahshan, O.A. GC/MS Profiling, Anti-Collagenase, Anti-Elastase, Anti-Tyrosinase and Anti-Hyaluronidase Activities of a Stenocarpus sinuatus Leaves Extract. Plants 2022, 11, 918. [Google Scholar] [CrossRef] [PubMed]
- Meziant, L.; Bachir-bey, M.; Bensouici, C.; Saci, F.; Boutiche, M.; Louaileche, H. Assessment of inhibitory properties of flavonoid-rich fig (Ficus carica L.) peel extracts against tyrosinase, α-glucosidase, urease and cholinesterases enzymes, and relationship with antioxidant activity. Eur. J. Integr. Med. 2021, 43, 101272. [Google Scholar] [CrossRef]
- Oh, K.-E.; Shin, H.; Lee, M.K.; Park, B.; Lee, K.Y. Characterization and Optimization of the Tyrosinase Inhibitory Activity of Vitis amurensis Root Using LC-Q-TOF-MS Coupled with a Bioassay and Response Surface Methodology. Molecules 2021, 26, 446. [Google Scholar] [CrossRef]
- Ersoy, E.; Ozkan, E.E.; Boga, M.; Yilmaz, M.A.; Mat, A. Anti-aging potential and anti-tyrosinase activity of three Hypericum species with focus on phytochemical composition by LC–MS/MS. Ind. Crop. Prod. 2019, 141, 111735. [Google Scholar] [CrossRef]
- Gao, D.; Kim, J.H.; Kim, C.T.; Jeong, W.S.; Kim, H.M.; Sim, J.; Kang, J.S. Evaluation of Anti-Melanogenesis Activity of Enriched Pueraria lobata Stem Extracts and Characterization of Its Phytochemical Components Using HPLC–PDA–ESI–MS/MS. Int. J. Mol. Sci. 2021, 22, 8105. [Google Scholar] [CrossRef] [PubMed]
- Taddeo, V.A.; Epifano, F.; Preziuso, F.; Fiorito, S.; Caron, N.; Rives, A.; de Medina, P.; Poirot, M.; Silvente-Poirot, S.; Genovese, S. HPLC Analysis and Skin Whitening Effects of Umbelliprenin-containing Extracts of Anethum graveolens, Pimpinella anisum, and Ferulago campestris. Molecules 2019, 24, 501. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Jeon, Y.D.; Cha, J.Y.; Hwang, S.-W.; Lee, H.-Y.; Park, M.; Lee, B.-R.; Shin, M.-K.; Kim, S.-J.; Shin, S.-M.; et al. Antioxidant and skin-whitening effects of aerial part of Euphorbia supina Raf. Extract. BMC Complement. Altern. Med. 2018, 18, 256. [Google Scholar] [CrossRef] [PubMed]
- Sarikurkcu, C.; Sahinler, S.S.; Ceylan, O.; Tepe, B. Onosma pulchra: Phytochemical composition, antioxidant, skin-whitening and anti-diabetic activity. Ind. Crop. Prod. 2020, 154, 112632. [Google Scholar] [CrossRef]
- Ma, X.; Shao, S.; Xiao, F.; Zhang, H.; Zhang, R.; Wang, M.; Li, G.; Yan, M. Platycodon grandiflorum extract: Chemical composition and whitening, antioxidant, and anti-inflammatory effects. RSC Adv. 2021, 11, 10814–10826. [Google Scholar] [CrossRef]
- Han, H.J.; Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Heo, H.J. Anti-Melanogenic Effect of Ethanolic Extract of Sorghum bicolor on IBMX–Induced Melanogenesis in B16/F10 Melanoma Cells. Nutrients 2020, 12, 832. [Google Scholar] [CrossRef]
- Sim, Y.Y.; Nyam, K.L. Application of Hibiscus cannabinus L. (kenaf) leaves extract as skin whitening and anti-aging agents in natural cosmetic prototype. Ind. Crop. Prod. 2021, 167, 113491. [Google Scholar] [CrossRef]
- Lin, D.; Wang, S.-H.; Song, T.-Y.; Hsieh, C.-W.; Tsai, M.-S. Safety and efficacy of tyrosinase inhibition of Paeonia suffruticosa Andrews extracts on human melanoma cells. J. Cosmet. Dermatol. 2019, 18, 1921–1929. [Google Scholar] [CrossRef]
- Liu, W.; Wang, M.; Xu, S.; Gao, C.; Liu, J. Inhibitory effects of shell of Camellia oleifera Abel extract on mushroom tyrosinase and human skin melanin. J. Cosmet. Dermatol. 2019, 18, 1955–1960. [Google Scholar] [CrossRef]
- Huang, H.-C.; Wang, S.-S.; Tsai, T.-C.; Ko, W.-P.; Chang, T.-M. Phoenix dactylifera L. Seed Extract Exhibits Antioxidant Effects and Attenuates Melanogenesis in B16F10 Murine Melanoma Cells by Downregulating PKA Signaling. Antioxidants 2020, 9, 1270. [Google Scholar] [CrossRef] [PubMed]
- Chowjarean, V.; Phiboonchaiyanan, P.P.; Harikarnpakdee, S. Skin Brightening Efficacy of Grammatophyllum speciosum: A Prospective, Split-Face, Randomized Placebo-Controlled Study. Sustainability 2022, 14, 16829. [Google Scholar] [CrossRef]
- Chen, Y.H.; Yan, S.L.; Wu, J.Y.; Hsieh, C.W.; Wang, S.H.; Tsai, M.S. Analyses of the Compositions, Antioxidant Capacities, and Tyrosinase-Inhibitory Activities of Extracts from Two New Varieties of Chrysanthemum morifolium Ramat Using Four Solvents. Appl. Sci. 2021, 11, 7631. [Google Scholar] [CrossRef]
- Murata, K.; Suzuki, S.; Miyamoto, A.; Horimoto, M.; Nanko, S.; Mori, D.; Kanamaru, H.; Endo, Y. Tyrosinase Inhibitory Activity of Extracts from Prunus persica. Separations 2022, 9, 107. [Google Scholar] [CrossRef]
- Bilhman, S.; Ramanathan, S.; Dumjun, K.; Wunnoo, S.; Lethongkam, S.; Waen-ngoen, T.; Kaewnopparat, N.; Paosen, S.; Voravuthikunchai, S.P. Value-Added from Microwave-Assisted Extraction of Musa sapientum Waste as an Alternative Safe and Effective Agent for the Treatment of Hyperpigmentation. Waste Biomass Valoriz. 2023, 14, 1477–1488. [Google Scholar] [CrossRef]
- Babbush, K.M.; Babbush, R.A.; Khachemoune, A. Treatment of melasma: A review of less commonly used antioxidants. Int. J. Dermat. 2020, 60, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Na, J.-I.; Shin, J.-W.; Choi, H.-R.; Kwon, S.-H.; Park, K.-C. Resveratrol as a Multifunctional Topical Hypopigmenting Agent. Int. J. Mol. Sci. 2019, 20, 956. [Google Scholar] [CrossRef]
- Boo, Y.C. Human Skin Lightening Efficacy of Resveratrol and Its Analogs: From In Vitro Studies to Cosmetic Applications. Antioxidants 2019, 8, 332. [Google Scholar] [CrossRef]
- Lin, M.-H.; Hung, C.-F.; Sung, H.-C.; Yang, S.-C.; Yu, H.-P.; Fang, J.-Y. The bioactivities of resveratrol and its naturally occurring derivatives on skin. J. Food Drug Anal. 2021, 29, 15–38. [Google Scholar] [CrossRef]
- Liu, F.; Qu, L.; Li, H.; He, J.; Wang, L.; Fang, Y.; Yan, X.; Yang, Q.; Peng, B.; Wu, W.; et al. Advances in Biomedical Functions of Natural Whitening Substances in the Treatment of Skin Pigmentation Diseases. Pharmaceutics 2022, 14, 2308. [Google Scholar] [CrossRef]
- Moon, K.; Lee, S.; Park, H.; Cha, J. Enzymatic Synthesis of Resveratrol α-Glucoside by Amylosucrase of Deinococcus geothermalis. J. Microbiol. Biotechnol. 2021, 31, 1692–1700. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Li, X.; Zhao, J.; Du, Q.; Dun, J. Skin pigmentation improvement with resveratrol microemulsion gel using polyoxyethylene hydrogenated castor oil. Drug Dev. Ind. Pharm. 2023, 49, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Sheweita, S.A.; El-Masry, Y.M.; Zaghloul, T.I.; Mostafa, S.K.; Elgindy, N.A. Preclinical studies on melanogenesis proteins using a resveratrol-nanoformula as a skin whitener. Int. J. Biol. Macromol. 2022, 223, 870–881. [Google Scholar] [CrossRef]
- Aung, N.N.; Pengnam, S.; Rojanarata, T.; Patrojanasophon, P.; Opanasopit, P.; Ngawhirunpat, T.; Pamornpathomkul, B. Fabrication of polyvinyl pyrrolidone-K90/Eudragit RL100-based dissolving microneedle patch loaded with alpha-arbutin and resveratrol for skin depigmentation. Biomater. Sci. 2023. accepted manuscript. [Google Scholar] [CrossRef] [PubMed]
- Sharafan, M.; Malinowska, M.A.; Ekiert, H.; Kwaśniak, B.; Sikora, E.; Szopa, A. Vitis vinifera (Vine Grape) as a Valuable Cosmetic Raw Material. Pharmaceutics 2023, 15, 1372. [Google Scholar] [CrossRef] [PubMed]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Malinowska, M.A.; Billet, K.; Drouet, S.; Munsch, T.; Unlubayir, M.; Tungmunnithum, D.; Giglioli-Guivarc’h, N.; Hano, C.; Lanoue, A. Grape Cane Extracts as Multifunctional Rejuvenating Cosmetic Ingredient: Evaluation of Sirtuin Activity, Tyrosinase Inhibition and Bioavailability Potential. Molecules 2020, 25, 2203. [Google Scholar] [CrossRef]
- Igielska-Kalwat, J.; Wawrzyńczak, A.; Nowak, I. β-Carotene as an exemplary carotenoid and its application in cosmetic industry. Chemik 2012, 66, 140–144. [Google Scholar]
- Bavarsad, N.; Ali Mapar, M.; Safaezadeh, M.; Latifi, S.M. A double-blind, placebo-controlled randomized trial of skin-lightening cream containing lycopene and wheat bran extract on melasma. J. Cosmet. Dermatol. 2020, 20, 1795–1800. [Google Scholar] [CrossRef]
- Honda, M. Z-Isomers of lycopene and β-carotene exhibit greater skin-quality improving action than their all-E-isomers. Food Chem. 2023, 421, 135954. [Google Scholar] [CrossRef]
- Lima, P.B.; Dias, J.A.F.; Esposito, A.C.C.; Miot, L.D.B.; Miot, H.A. French maritime pine bark extract (pycnogenol) in association with triple combination cream for the treatment of facial melasma in women: A double-blind, randomized, placebo-controlled trial. J. Eur. Acad. Dermatol. Venereol. 2020, 35, 502–508. [Google Scholar] [CrossRef]
- Aladrén, S.; Garre, A.; Valderas-Martínez, P.; Piquero-Casals, J.; Granger, C. Efficacy and Safety of an Oral Nutritional (Dietary) Supplement Containing Pinus pinaster Bark Extract and Grape Seed Extract in Combination with a High SPF Sunscreen in the Treatment of Mild-to-Moderate Melasma: A Prospective Clinical Study. Cosmetics 2019, 6, 15. [Google Scholar] [CrossRef]
- Avianggi, H.D.; Indar, R.; Adriani, D.; Riyanto, P.; Muslimin, M.; Afriliana, L.; Kabulrachman, M. The effectiveness of tomato extract on superoxide dismutase (SOD) and severity degree of patients with melasma. Ital. J. Dermatol. Venerol. 2022, 157, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Wang, X.; Zhao, L.; Zhou, Z.; Liu, H.; Wang, B.; Cheng, A.; Zhang, S.; Gao, Y. Novel dissolving microneedles preparation for synergistic melasma therapy: Combined effects of tranexamic acid and licorice extract. Int. J. Pharm. 2021, 600, 120406. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-H.; Chiang, W.-T.; Cheng, M.-C.; Tsai, T.-Y. Effects of Germination Black Soy Milk Fermented with Lactobacillus plantarum TWK10 on Anti-Oxidative and Anti-Melanogenesis. Appl. Sci. 2022, 12, 277. [Google Scholar] [CrossRef]
- Jeon, G.; Ro, H.-S.; Kim, G.-R.; Lee, H.-Y. Enhancement of Melanogenic Inhibitory Effects of the Leaf Skin Extracts of Aloe barbadensis Miller by the Fermentation Process. Fermentation 2022, 8, 580. [Google Scholar] [CrossRef]
- Lee, S.-H.; Eun, C.-H.; Kwon, Y.-S.; Baek, J.-H.; Kim, I.-J. Evaluation of Fermented Extracts of Aloe vera Processing Byproducts as Potential Functional Ingredients. Fermentation 2021, 7, 269. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Chung, Y.-C.; Chen, P.-Y.; Chang, Y.-C.; Chen, W.-L. Fermentation of Chenopodium formosanum Leaf Extract with Aspergillus oryzae Significantly Enhanced Its Physiological Activities. Appl. Sci. 2023, 13, 2917. [Google Scholar] [CrossRef]
- Tianyun, W.; Youmei, W.; Jue, W.; Hongwei, C.; Biao, Q.; Zheng, L. Efficacy and Safety of Topical Therapy with Botanical Products for Melasma: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Med. 2022, 8, 797890. [Google Scholar] [CrossRef]
- Wu, Y.; Choi, M.-H.; Li, J.; Yang, H.; Shin, H.-J. Mushroom Cosmetics: The Present and Future. Cosmetics 2016, 3, 22. [Google Scholar] [CrossRef]
- Badalyan, S.M.; Barkhudaryan, A.; Rapior, S. Medicinal Macrofungi as Cosmeceuticals: A Review. Int. J. Med. Mushrooms 2022, 24, 1–13. [Google Scholar] [CrossRef]
- Taofiq, O.; Heleno, S.A.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Barreiro, M.F.; González-Paramás, A.M.; Ferreira, I.C.F.R. Development of Mushroom-Based Cosmeceutical Formulations with Anti-Inflammatory, Anti-Tyrosinase, Antioxidant, and Antibacterial Properties. Molecules 2016, 21, 1372. [Google Scholar] [CrossRef]
- Angelini, P.; Venanzoni, R.; Angeles Flores, G.; Tirillini, B.; Orlando, G.; Recinella, L.; Chiavaroli, A.; Brunetti, L.; Leone, S.; Di Simone, S.C.; et al. Evaluation of Antioxidant, Antimicrobial and Tyrosinase Inhibitory Activities of Extracts from Tricholosporum goniospermum, an Edible Wild Mushroom. Antibiotics 2020, 9, 513. [Google Scholar] [CrossRef]
- Ishihara, A.; Ide, Y.; Bito, T.; Ube, N.; Endo, N.; Sotome, K.; Maekawa, N.; Ueno, K.; Nakagiri, A. Novel tyrosinase inhibitors from liquid culture of Neolentinus lepideus. Biosci. Biotechnol. Biochem. 2018, 82, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, A.; Sugai, N.; Bito, T.; Ube, N.; Ueno, K.; Okuda, Y.; Fukushima-Sakuno, E. Isolation of 6-hydroxy-L-tryptophan from the fruiting body of Lyophyllum decastes for use as a tyrosinase inhibitor. Biosci. Biotechnol. Biochem. 2019, 83, 1800–1806. [Google Scholar] [CrossRef]
- Pavic, A.; Ilic-Tomic, T.; Glamočlija, J. Unravelling Anti-Melanogenic Potency of Edible Mushrooms Laetiporus sulphureus and Agaricus silvaticus In Vivo Using the Zebrafish Model. J. Fungi 2021, 7, 834. [Google Scholar] [CrossRef]
- Im, K.H.; Baek, S.A.; Choi, J.; Lee, T.S. Antioxidant, Anti-Melanogenic and Anti-Wrinkle Effects of Phellinus vaninii. Mycobiology 2019, 47, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Razak, D.L.A.; Fadzil, N.H.M.; Jamaluddin, A.; Rashid, N.Y.A.; Sani, N.A.; Manan, M.A. Effects of different extracting conditions on anti-tyrosinase and antioxidant activities of Schizophyllum commune fruit bodies. Biocatal. Agric. Biotechnol. 2019, 19, 101116. [Google Scholar] [CrossRef]
- Abd Razak, D.L.; Jamaluddin, A.; Abd Rashid, N.Y.; Sani, N.A.; Abdul Manan, M. Assessment of Cosmeceutical Potentials of Selected Mushroom Fruitbody Extracts through Evaluation of Antioxidant, Anti-Hyaluronidase and Anti-Tyrosinase Activity. J. Multidiscip. Res. 2020, 3, 329–342. [Google Scholar] [CrossRef]
- Jeon, D.-H.; Lee, E.-H.; Park, H.-J.; Sultanov, A.; Jung, H.-Y.; Kang, I.-K.; Cho, Y.-J. Antioxidant activity and inhibitory effects of whitening and wrinkle-related enzymes of Polyozellus multiplex extracts. Food Measure. 2023, 17, 1279–1288. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, V.P. Isolation of Bioactive Compounds from Fruit Body of Lentinula edodes (Berk.) Pegler and In Silico Approach using Tyrosinase Target Protein Involved in Melanin Production. Indian J. Pharm. Sci. 2022, 84, 1026–1040. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Jakovljević, D.; Todorović, N.; Wan-Mohtar, W.A.A.Q.I.; Nikšić, M. Ganoderma lucidum as a cosmeceutical: Antiradical potential and inhibitory effect on hyperpigmentation and skin extracellular matrix degradation enzymes. Arch. Biol. Sci. 2019, 71, 253–264. [Google Scholar] [CrossRef]
- Saad, H.M.; Sim, K.S.; Tan, Y.S. Antimelanogenesis and Anti-Inflammatory Activity of Selected Culinary-Medicinal Mushrooms. Int. J. Med. Mushrooms 2018, 20, 141–153. [Google Scholar] [CrossRef]
- Lee, E.J.; Cha, H.J. Inonotus obliquus Extract as An Inhibitor of α-MSH-Induced Melanogenesis in B16F10 Mouse Melanoma Cells. Cosmetics 2019, 6, 9. [Google Scholar] [CrossRef]
- Sangthong, S.; Pintathong, P.; Pongsua, P.; Jirarat, A.; Chaiwut, P. Polysaccharides from Volvariella volvacea Mushroom: Extraction, Biological Activities and Cosmetic Efficacy. J. Fungi 2022, 8, 572. [Google Scholar] [CrossRef] [PubMed]
- Pintathong, P.; Chomnunti, P.; Sangthong, S.; Jirarat, A.; Chaiwut, P. The Feasibility of Utilizing Cultured Cordyceps militaris Residues in Cosmetics: Biological Activity Assessment of Their Crude Extracts. J. Fungi 2021, 7, 973. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Cheng, K.-C.; Wang, H.-T.; Hsieh, C.-W.; Lai, Y.-J. Extracts of Antrodia cinnamomea mycelium as a highly potent tyrosinase inhibitor. J. Cosmet. Dermatol. 2020, 20, 2341–2349. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.; Cao, H.; Cui, X.; Guo, H.; Zheng, W.; Zhong, X.; Zhang, Y.; Han, C. Comparing the Cosmetic Effects of Liquid-Fermented Culture of Some Medicinal Mushrooms Including Antioxidant, Moisturizing, and Whitening Activities. Int. J. Med. Mushrooms 2020, 22, 693–703. [Google Scholar] [CrossRef]
- Wu, H.-C.; Chen, Y.-F.; Cheng, M.-J.; Wu, M.-D.; Chen, Y.-L.; Chang, H.-S. Investigations into Chemical Components from Monascus purpureus with Photoprotective and Anti-Melanogenic Activities. J. Fungi 2021, 7, 619. [Google Scholar] [CrossRef]
- Sułkowska-Ziaja, K.; Grabowska, K.; Apola, A.; Kryczyk-Poprawa, A.; Muszyńska, B. Mycelial culture extracts of selected wood-decay mushrooms as a source of skin-protecting factors. Biotechnol. Lett. 2021, 43, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- Zerva, A.; Tsafantakis, N.; Topakas, E. Evaluation of Basidiomycetes Wild Strains Grown in Agro-Industrial Residues for Their Anti-Tyrosinase and Antioxidant Potential and for the Production of Biocatalysts. Fermentation 2021, 7, 19. [Google Scholar] [CrossRef]
- Georgousaki, K.; González-Menéndez, V.; Tormo, J.R.; Tsafantakis, N.; Mackenzie, T.A.; Martín, J.; Gumeni, S.; Trougakos, I.P.; Reyes, F.; Fokialakis, N.; et al. Comoclathrin, a novel potent skin-whitening agent produced by endophytic Comoclathris strains associated with Andalusia desert plants. Sci. Rep. 2022, 12, 1649. [Google Scholar] [CrossRef]
- Chisvert, A.; Benedé, J.L.; Salvador, A. Tanning and Whitening Agents in Cosmetics: Regulatory Aspects and Analytical Methods. In Analysis of Cosmetic Products, 2nd ed.; Salvador, A., Chisvert, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 6; pp. 107–121. [Google Scholar] [CrossRef]
- Ludek, S.; Wawrzyńczak, A.; Nowak, I.; Feliczak-Guzik, A. Synthesis of Lipid Nanoparticles Incorporated with Ferula assa-foetida L. Extract. Cosmetics 2022, 9, 129. [Google Scholar] [CrossRef]
- Lee, K.B.; Choi, J.; Ahn, S.K.; Na, J.-K.; Shrestha, K.K.; Nguon, S.; Park, S.U.; Choi, S.; Kim, J.K. Quantification of Arbutin in Plant Extracts by Stable Isotope Dilution Gas Chromatography-Mass Spectrometry. Chromatographia 2018, 81, 533–538. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Wójciak, M.; Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Hoian, U.; Klimczak, K.; Szczepanek, D.; Sowa, I. Evaluation of the Antioxidant, Cytoprotective and Antityrosinase Effects of Schisandra chinensis Extracts and Their Applicability in Skin Care Product. Molecules 2022, 27, 8877. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Kim, J.K.; Kim, J.; Jung, S.-H.; Lee, K. Characterization of Caffeoylquinic Acids from Lepisorus thunbergianus and Their Melanogenesis Inhibitory Activity. ACS Omega 2020, 5, 30946–30955. [Google Scholar] [CrossRef]
- Tlili, N.; Sarikurkcu, R.T.; Ozer, M.S.; Sarikurkcu, C. Liquid Chromatography–Electrospray Ionization Tandem Mass Spectrometry (LC-ESI-MS/MS) Identification of Phytochemicals and the Effects of Solvents on Phenolic Constituents, Antioxidant Capacity, Skin-Whitening and anti-Diabetic Activity of Onosma mitis. Anal. Lett. 2022, 55, 32–46. [Google Scholar] [CrossRef]
- Song, W.; Zhao, Y.-Y.; Ren, Y.-J.; Liu, L.-L.; Wei, S.-D.; Yang, H.-B. Proanthocyanidins isolated from the leaves of Photinia × fraseri block the cell cycle and induce apoptosis by inhibiting tyrosinase activity in melanoma cells. Food Funct. 2021, 12, 3978–3991. [Google Scholar] [CrossRef]
- Gao, D.; Cho, C.-W.; Kim, J.-H.; Kim, C.-T.; Jeong, W.-S.; Wang, Y.; Li, X.; Kang, J.-S. Extraction and Concentration of Waste Pueraria lobata Stems with Antioxidants and Anti-Melanogenesis Activity as a Novel Skin Whitening Agent for Natural Cosmetic Prototypes. Int. J. Mol. Sci. 2022, 23, 10352. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Strzępek-Gomółka, M.; Czop, M.; Sakipova, Z.; Głowniak, K.; Kukula-Koch, W. Achillea millefolium L. and Achillea biebersteinii Afan. Hydroglycolic Extracts–Bioactive Ingredients for Cosmetic Use. Molecules 2020, 25, 3368. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Li, J.; Yang, F.; Yang, K.; Liu, B.; Tong, S.; Yan, J.; Chen, S. A novel high-resolution monophenolase/diphenolase/radical scavenging profiling for the rapid screening of natural whitening candidates from Peaonia lactiflora root and their mechanism study with molecular docking. J. Ethnopharmacol. 2022, 282, 114607. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Yin, B.; Zhai, H.L.; Lua, S.H.; Mi, J.Y. An effective approach to the quantitative analysis of skin-whitening agents in cosmetics with different substrates based on conventional UV-Vis determination. Anal. Methods 2019, 11, 1500–1507. [Google Scholar] [CrossRef]
- Martono, S.; Febriani, I.; Rohman, A. Application of liquid chromatography-photodiode array detector for analysis of whitening agents in cream cosmetics. J. Appl. Pharm. Sci. 2018, 8, 143–147. [Google Scholar] [CrossRef]
- Ibrahim, F.; Sharaf El-Din, M.K.; El-Deen, A.K.; Shimizu, K. A new HPLC-DAD method for the concurrent determination of hydroquinone, hydrocortisone acetate and tretinoin in different pharmaceuticals for melasma treatment. J. Chromatogr. Sci. 2019, 57, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Maggadani, B.; Harmita, H.; Harahap, Y.; Hutabalian, H.L.N. Simultaneous identification and quantification of hydroquinone, tretinoin and betamethasone in cosmetic products by isocratic reversed phase high performance liquid chromatography. Int. J. Appl. Pharm. 2019, 11, 181–185. [Google Scholar] [CrossRef]
- Permana, B.; Tursino, M.H. Simultaneous HPLC Determination of Arbutin, Niacinamide and 3-O-Ethyl Ascorbic Acid in Whitening Cream Products in the Presence of Parabens. J. Chromatogr. Sci. 2023, 61, 241–248. [Google Scholar] [CrossRef]
- Pahade, P.; Bose, D.; Peris-Vicente, J.; Carda-Broch, S.; Durgbanshi, A. Simultaneous detection of hazardous skin whitening agents in Indian cosmetic products using a green chromatographic technique. J. Chromatogr. Open 2021, 1, 100010. [Google Scholar] [CrossRef]
- Sahib, M.N. Screening of two glucocorticoids in non-prescription skin whitening creams purchased via internet in Iraq by HPLC method. J. Appl. Pharm. Sci. 2018, 8, 78–84. [Google Scholar] [CrossRef]
- Butwong, N.; Kunawong, T.; Luong, J.H.T. Simultaneous Analysis of Hydroquinone, Arbutin, and Ascorbyl Glucoside Using a Nanocomposite of Ag@AgCl Nanoparticles, Ag2S Nanoparticles, Multiwall Carbon Nanotubes, and Chitosan. Nanomaterials 2020, 10, 1583. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, Y.; Fu, W.; Zhang, P.; Li, L.; Ye, C.; Yu, L.; Zhu, X.; Zhao, S. Seed-mediated growth of Au@Ag core-shell nanorods for the detection of ellagic acid in whitening cosmetics. Anal. Chim. Acta 2018, 1002, 97–104. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Q.; Feng, Y.; Ye, B.-C. A robust electrochemical sensor based on N,S-FeNi3/C for simultaneous detection of hydroquinone and arbutin in cosmetics. Microchim. Acta 2023, 190, 150. [Google Scholar] [CrossRef] [PubMed]
- Repert, S.; Matthes, S.; Rozhon, W. Quantification of Arbutin in Cosmetics, Drugs and Food Supplements by Hydrophilic-Interaction Chromatography. Molecules 2022, 27, 5673. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, D.; Xie, Z. Rapid and Specific Fluorescence Method for the Quantification of Arbutin in Cosmetics. Anal. Lett. 2022, 55, 318–326. [Google Scholar] [CrossRef]
- Khatoon, A.; Syed, J.A.; Buledi, J.A.; Shakeel, S.; Mallah, A.; Solangi, A.R.; Sirajuddin; Sherazi, S.T.H.; Shah, M.R. Bio-green fabrication of bell pepper mediated silver nanoparticles: An efficient material for electrochemical sensing of arbutin in cosmetics. J. Iran. Chem. Soc. 2022, 19, 3659–3672. [Google Scholar] [CrossRef]
- EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02009R1223-20221217 (accessed on 29 April 2023).
- Alqarni, M.H.; Alam, P.; Shakeel, F.; Foudah, A.I.; Alshehri, S. Highly Sensitive and Ecologically Sustainable Reversed-Phase HPTLC Method for the Determination of Hydroquinone in Commercial Whitening Creams. Processes 2021, 9, 1631. [Google Scholar] [CrossRef]
- Irfan, M.; Shafeeq, A.; Siddiq, U.; Bashir, F.; Ahmad, T.; Athar, M.; Butt, M.T.; Ullah, S.; Mukhtar, A.; Hussien, M.; et al. A mechanistic approach for toxicity and risk assessment of heavy metals, hydroquinone and microorganisms in cosmetic creams. J. Hazard. Mater. 2022, 433, 128806. [Google Scholar] [CrossRef]
- Arshad, M.; Sadef, Y.; Shakoor, M.B.; Naeem, M.; Bashir, F.; Ahmad, S.R.; Ali, S.; Abid, I.; Khan, N.; Alyemeni, M.N. Quantitative Estimation of the Hydroquinone, Mercury and Total Plate Count in Skin-Lightening Creams. Sustainability 2021, 13, 8786. [Google Scholar] [CrossRef]
- Chuenjitt, S.; Kongsuwan, A.; Phua, C.H.; Saichanapan, J.; Soleh, A.; Saisahas, K.; Samoson, K.; Wangchuk, S.; Promsuwan, K.; Limbut, W. A poly(neutral red)/porous graphene modified electrode for a voltammetric hydroquinone sensor. Electrochim. Acta 2022, 434, 141272. [Google Scholar] [CrossRef]
- Pato, A.H.; Balouch, A.; Alveroglu, E.; Buledi, J.A.; Lal, S.; Mal, D. A Practical Non-Enzymatic, Ultra-Sensitive Molybdenum Oxide (MoO3) Electrochemical Nanosensor for Hydroquinone. J. Electrochem. Soc. 2021, 168, 056503. [Google Scholar] [CrossRef]
- Cotchim, S.; Promsuwan, K.; Dueramae, M.; Duerama, S.; Dueraning, A.; Thavarungkul, P.; Kanatharana, P.; Limbut, W. Development and Application of an Electrochemical Sensor for Hydroquinone in Pharmaceutical Products. J. Electrochem. Soc. 2020, 167, 155528. [Google Scholar] [CrossRef]
- Soltani, H.; Pardakhty, A.; Ahmadzadeh, S. Determination of hydroquinone in food and pharmaceutical samples using a voltammetric based sensor employing NiO nanoparticle and ionic liquids. J. Mol. Liq. 2016, 219, 63–67. [Google Scholar] [CrossRef]
- de Oliveira Moreira, O.B.; de Faria, L.V.; Matos, R.C.; Enes, K.B.; Couri, M.R.C.; de Oliveira, M.A.L. Determination of hydroquinone and benzoquinone in pharmaceutical formulations: Critical considerations on quantitative analysis of easily oxidized compounds. Anal. Methods 2022, 14, 4784–4794. [Google Scholar] [CrossRef] [PubMed]
- Desmedt, B.; Rogiers, V.; Courselle, P.; De Beer, J.O.; De Paepe, K.; Deconinck, E. Development and validation of a fast chromatographic method for screening and quantification of legal and illegal skin whitening agents. J. Pharm. Biomed. Anal. 2013, 83, 82–88. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).