Chemical and Rheological Characterization of a Facial Mask Containing an Olive Pomace Fraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Methods
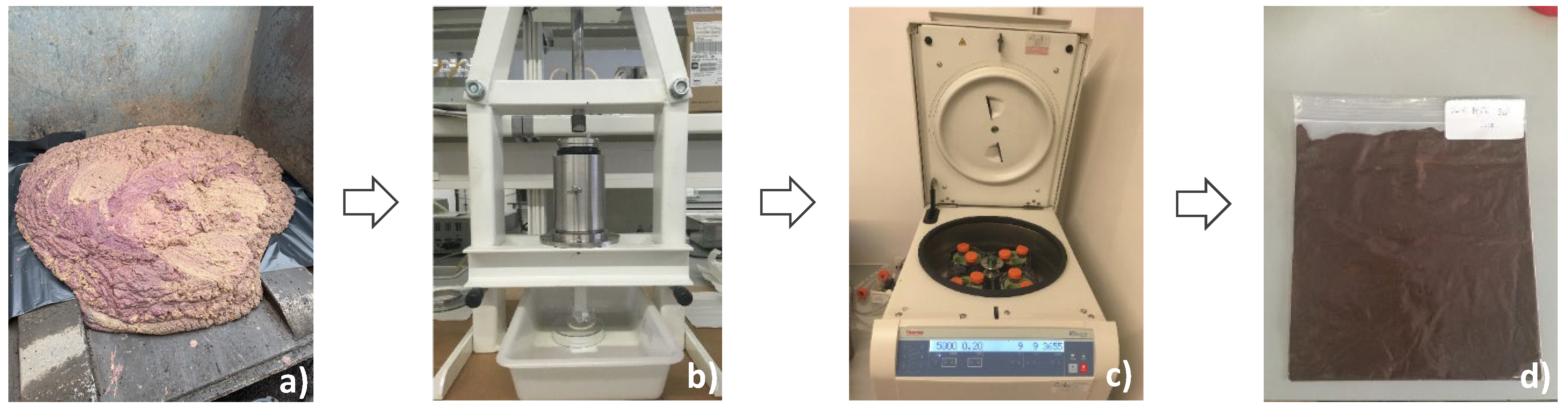
2.2.1. Sample and Sample Preparation
2.2.2. Proximate Analysis
2.2.3. Extracts Preparation
2.2.4. Antioxidant Activity
Ferric Reducing Antioxidant Power (FRAP)
2,2-diphenyl-1-picrylhydrazyl radical (DPPH•) Scavenging Ability
2.2.5. Phenolic and Flavonoid Content
2.2.6. Vitamin E Profile
2.2.7. Facial Mask Formulation
2.2.8. pH Measurement
2.2.9. Texture and Rheological Analysis
2.2.10. Statistical Analysis
3. Results and Discussion
3.1. Proximate Analysis
3.2. Vitamin E Profile
3.3. Phytochemicals and Antioxidant Activity
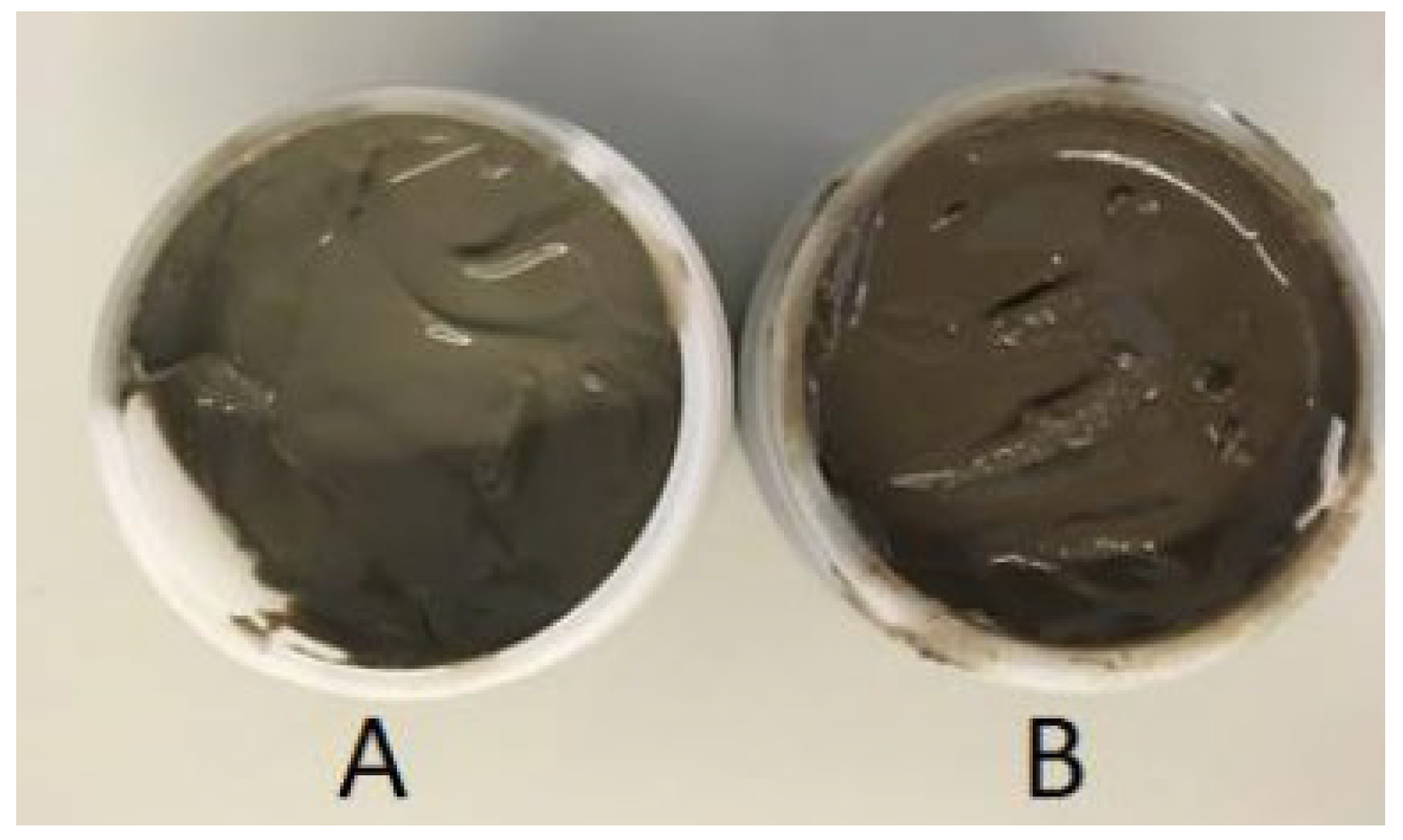
3.4. Facial Masks’ Organoleptic Characterization
3.5. pH Measurement
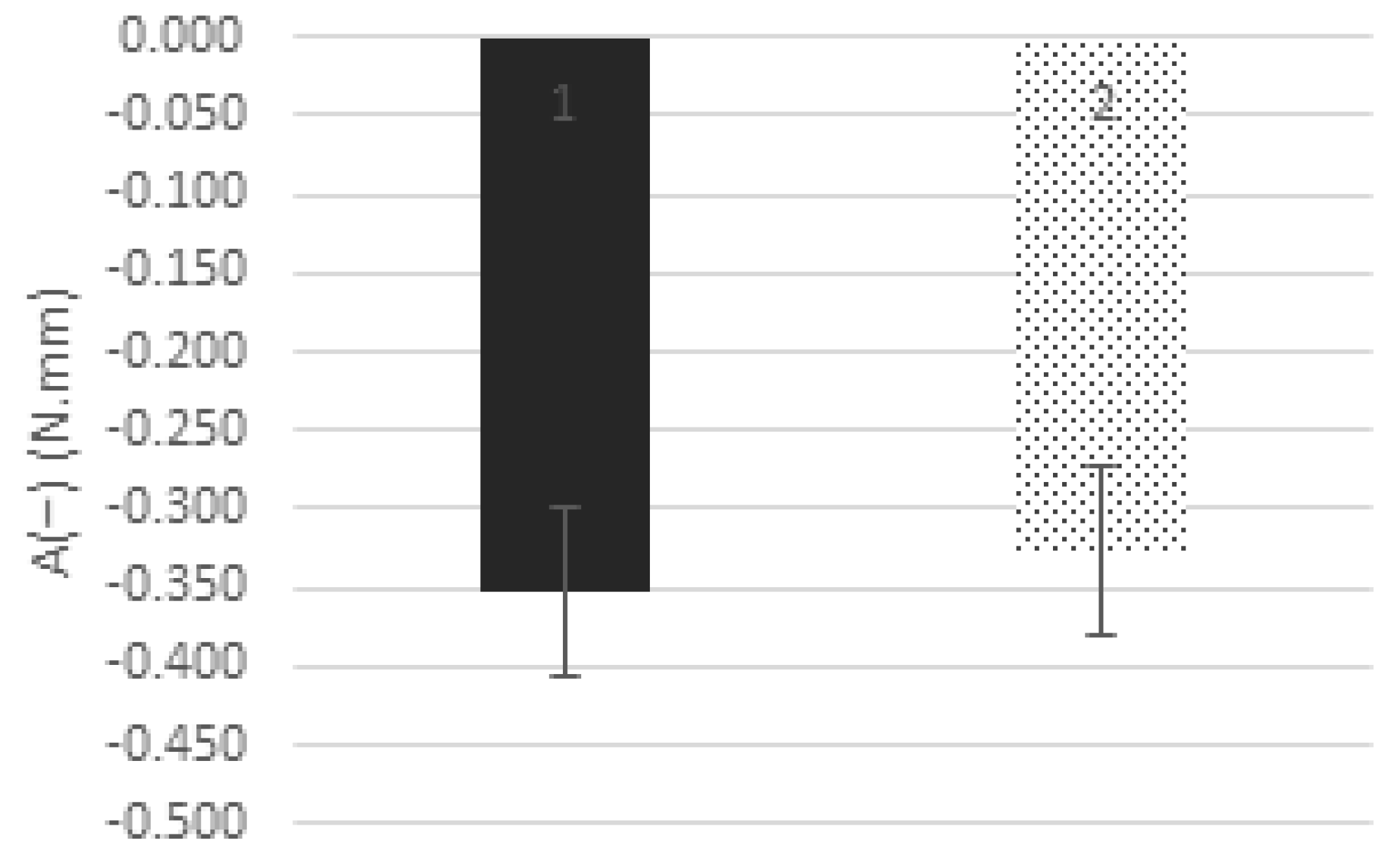
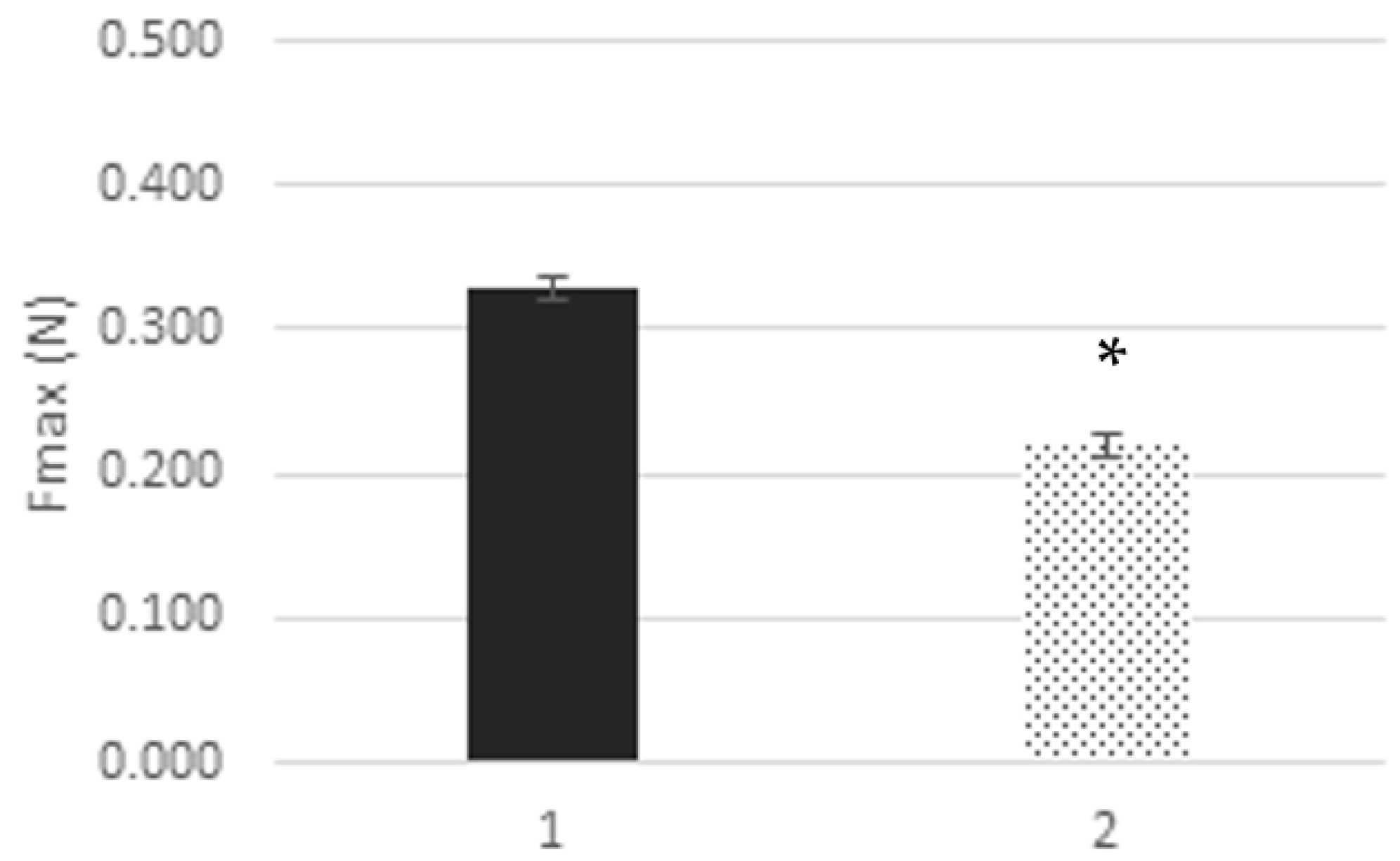
3.6. Textural and Rheological Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mellou, F.; Varvaresou, A.; Papageorgiou, S. Renewable Sources: Applications in Personal Care Formulations. Int. J. Cosmet. Sci. 2019, 41, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Pinelli, P.; Ieri, F.; Bernini, R. Sustainability, Innovation, and Green Chemistry in the Production and Valorization of Phenolic Extracts from Olea europaea L. Sustainability 2016, 8, 1002. [Google Scholar] [CrossRef]
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Rodrigues, F.; Pimentel, F.B.; Oliveira, M.B.P.P. Olive by-products: Challenge Application in Cosmetic Industry. Ind. Crops Prod. 2015, 70, 116–124. [Google Scholar] [CrossRef]
- Nunes, A.; Goncalves, L.; Marto, J.; Martins, A.M.; Silva, A.N.; Pinto, P.; Martins, M.; Fraga, C.; Ribeiro, H.M. Investigations of Olive Oil Industry By-Products Extracts with Potential Skin Benefits in Topical Formulations. Pharmaceutics 2021, 13, 465. [Google Scholar] [CrossRef]
- Nunes, M.A.; Pimentel, F.B.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Olive By-Products for Functional and Food Applications: Challenging Opportunities to Face Environmental Constraints. Innov. Food Sci. Emerg. Technol. 2016, 35, 139–148. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Oliveira, A.; Coelho, M.; Veiga, M.; Costa, E.M.; Silva, S.; Nunes, J.; Vicente, A.A.; Pintado, M. Are Olive Pomace Powders a Safe Source of Bioactives and Nutrients? J. Sci. Food Agric. 2021, 101, 1963–1978. [Google Scholar] [CrossRef]
- Granados-Principal, S.; Quiles, J.L.; Ramirez-Tortosa, C.L.; Sanchez-Rovira, P.; Ramirez-Tortosa, M.C. Hydroxytyrosol: From Laboratory Investigations to Future Clinical Trials. Nutr. Rev. 2010, 68, 191–206. [Google Scholar] [CrossRef]
- Nunes, A.; Marto, J.; Gonçalves, L.; Martins, A.M.; Fraga, C.; Ribeiro, H.M. Potential Therapeutic of Olive Oil Industry By-Products in Skin Health: A Review. Int. J. Food Sci. Technol. 2021, 57, 173–187. [Google Scholar] [CrossRef]
- Kesente, M.; Kavetsou, E.; Roussaki, M.; Blidi, S.; Loupassaki, S.; Chanioti, S.; Siamandoura, P.; Stamatogianni, C.; Philippou, E.; Papaspyrides, C.; et al. Encapsulation of Olive Leaves Extracts in Biodegradable PLA Nanoparticles for Use in Cosmetic Formulation. Bioengineering 2017, 4, 75. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Tsatalas, P.; Galanakis, I.M. Implementation of Phenols Recovered from Olive Mill Wastewater as UV Booster in Cosmetics. Ind. Crops Prod. 2018, 111, 30–37. [Google Scholar] [CrossRef]
- Nilforoushzadeh, M.A.; Amirkhani, M.A.; Zarrintaj, P.; Salehi Moghaddam, A.; Mehrabi, T.; Alavi, S.; Mollapour Sisakht, M. Skin Care and Rejuvenation by Cosmeceutical Facial Mask. J. Cosmet. Dermatol. 2018, 17, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Juliano, C.; Magrini, G. Cosmetic Functional Ingredients from Botanical Sources for Anti-Pollution Skincare Products. Cosmetics 2018, 5, 19. [Google Scholar] [CrossRef]
- Liu, J.K. Natural Products in Cosmetics. Nat. Prod. Bioprospect. 2022, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2010. [Google Scholar]
- Tontisirin, K. Chapter 2: Methods of food analysis. In Food Energy: Methods of Analysis and Conversion Factors: Report of a Technical Workshop; Food and Agriculture Organization of the United Nations: Rome, Italy, 2002; pp. 7–17. [Google Scholar]
- Costa, C.S.G.; Nunes, M.A.; Almeida, A.A.; Santos-Silva, A.; Oliveira, M. Nutritional, Chemical and Antioxidant/Pro-Oxidant Profiles of Silverskin, a Coffee Roasting By-Product. Food Chem. 2018, 267, 28–35. [Google Scholar] [CrossRef]
- Costa, A.S.G.; Alves, R.C.; Vinha, A.F.; Barreira, S.V.P.; Nunes, M.A.; Cunha, L.M.; Oliveira, M.B.P.P. Optimization of Antioxidants Extraction from Coffee Silverskin, a Roasting By-Product, Having in View a Sustainable Process. Ind. Crops Prod. 2014, 53, 350–357. [Google Scholar] [CrossRef]
- Alves, R.C.; Casal, S.; Oliveira, M.B.P.P. Determination of Vitamin E in Coffee Beans by HPLC Using a Micro-extraction Method. Food Sci. Technol. Int. 2009, 15, 57–63. [Google Scholar] [CrossRef]
- Carretero, M.I. Clay Minerals and Their Beneficial Effects Upon Human Health. A Review. Appl. Clay Sci. 2002, 21, 155–163. [Google Scholar] [CrossRef]
- Benson, H.A.E.; Roberts, M.S.; Leite-Silva, V.R.; Walters, K. Cosmetic Formulation: Principles and Practice; CRC Press: Boca Raton, FL, USA, 2019; p. 230. [Google Scholar]
- Antónia Nunes, M.; Costa, A.S.G.; Bessada, S.; Santos, J.; Puga, H.; Alves, R.C.; Freitas, V.; Oliveira, M. Olive Pomace as a Valuable Source of Bioactive Compounds: A Study Regarding its Lipid- and Water-Soluble Components. Sci. Total Environ. 2018, 644, 229–236. [Google Scholar] [CrossRef]
- Jeon, S.; Choi, M. Anti-Inflammatory and Anti-Aging Effects of Hydroxytyrosol on Human Dermal Fibroblasts (HDFs). Biomed. Dermatol. 2018, 2, 21. [Google Scholar] [CrossRef]
- Uchida, R.; Ishikawa, S.; Tomoda, H. Inhibition of Tyrosinase Activity and Melanine Pigmentation by 2-Hydroxytyrosol. Acta Pharm. Sin. B 2014, 4, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Nunes, M.A.; Palmeira, J.D.; Melo, D.; Machado, S.; Lobo, J.C.; Costa, A.S.G.; Alves, R.C.; Ferreira, H.; Oliveira, M. Chemical Composition and Antimicrobial Activity of a New Olive Pomace Functional Ingredient. Pharmaceuticals 2021, 14, 913. [Google Scholar] [CrossRef] [PubMed]
- Paini, M.; Aliakbarian, B.; Casazza, A.A.; Lagazzo, A.; Botter, R.; Perego, P. Microencapsulation of Phenolic Compounds From Olive Pomace Using Spray Drying: A study of operative parameters. LWT Food Sci. Technol. 2015, 62, 177–186. [Google Scholar] [CrossRef]
- Barel, A.O.; Paye, M.; Maibach, H.I. Handbook of Cosmetic Science and Technology, 3rd ed.; Informa Healthcare Inc.: New York, NY, USA, 2009; pp. 221–232. [Google Scholar]
- Klimaszewska, E.; Zieba, M.; Gregorczyk, K.; Markuszewski, L. Application of Blue Honeysuckle Powder Obtained by an Innovative Method of Low-Temperature Drying in Skincare Face Masks. Molecules 2021, 26, 7184. [Google Scholar] [CrossRef]
- Semenzato, A.; Costantini, A.; Meloni, M.; Maramaldi, G.; Meneghin, M.; Baratto, G. Formulating O/W Emulsions with Plant-Based Actives: A Stability Challenge for an Effective Product. Cosmetics 2018, 5, 59. [Google Scholar] [CrossRef]
- Di Mambro, V.M.; Fonseca, M.J. Assays of Physical Stability and Antioxidant Activity of a Topical Formulation Added with Different Plant Extracts. J. Pharm. Biomed. Anal. 2005, 37, 287–295. [Google Scholar] [CrossRef]
- da Silva Favero, J.; dos Santos, V.; Weiss-Angeli, V.; Gomes, L.B.; Veras, D.G.; Dani, N.; Mexias, A.S.; Bergmann, C.P. Evaluation and Characterization of Melo Bentonite Clay for Cosmetic Applications. Appl. Clay Sci. 2019, 175, 40–46. [Google Scholar] [CrossRef]

| Composition (INCI) | Control | SSP Mask |
|---|---|---|
| Bentonite | 38.0 | 33.0 |
| Glycerin | 23.1 | 23.1 |
| Alcohol | 23.1 | 23.1 |
| Panthenol | 10.0 | 10.0 |
| SSP | - | 5 |
| Purified water (Aqua) | 5.8 | 5.8 |
| Moisture | Total Fat | Total Protein | Ash |
|---|---|---|---|
| 68.1 ± 0.2 | 9.6 ± 1.3 | 9.9 ± 0.9 | 5.2 ± 0.1 |
| TPC (g GAE/100 g) | TF (g CE/100 g) | Vitamin E (mg/100 g) | DPPH• Inhibition (g TE/100 g) | FRAP (g FSE/100 g) | |
|---|---|---|---|---|---|
| 3.57 ± 0.15 | 3.18 ± 0.17 | Total α-tocopherol β-tocopherol γ-tocopherol α-tocotrienol | 7.04 ± 0.10 6.83 ± 0.11 0.08 ± 0.01 0.12 ± 0.01 0.01 ± 0.01 | 4.95 ± 0.28 | 6.79 ± 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, R.; Lobo, J.C.; Ferreira, D.M.; Senderowicz, E.; Nunes, M.A.; Amaral, M.H.; Alves, R.C.; Oliveira, M.B.P.P. Chemical and Rheological Characterization of a Facial Mask Containing an Olive Pomace Fraction. Cosmetics 2023, 10, 64. https://doi.org/10.3390/cosmetics10020064
Rodrigues R, Lobo JC, Ferreira DM, Senderowicz E, Nunes MA, Amaral MH, Alves RC, Oliveira MBPP. Chemical and Rheological Characterization of a Facial Mask Containing an Olive Pomace Fraction. Cosmetics. 2023; 10(2):64. https://doi.org/10.3390/cosmetics10020064
Chicago/Turabian StyleRodrigues, Raquel, Joana C. Lobo, Diana M. Ferreira, Ewa Senderowicz, M. Antónia Nunes, M. Helena Amaral, Rita C. Alves, and M. Beatriz P. P. Oliveira. 2023. "Chemical and Rheological Characterization of a Facial Mask Containing an Olive Pomace Fraction" Cosmetics 10, no. 2: 64. https://doi.org/10.3390/cosmetics10020064
APA StyleRodrigues, R., Lobo, J. C., Ferreira, D. M., Senderowicz, E., Nunes, M. A., Amaral, M. H., Alves, R. C., & Oliveira, M. B. P. P. (2023). Chemical and Rheological Characterization of a Facial Mask Containing an Olive Pomace Fraction. Cosmetics, 10(2), 64. https://doi.org/10.3390/cosmetics10020064









