Abstract
Exosomes are small extracellular nanovesicles that are released by cells, and their potential has been explored for use in cosmetics, skin care, tissue regeneration, and dermatological diseases. The therapeutic value of exosomes lies in their ability to modulate the microenvironment of cells, regulate gene expression, and induce cell differentiation, which can have a positive impact on skin health. In terms of cosmetics, exosomes have been used to reduce wrinkles, improve skin texture and hydration, and enhance skin elasticity, as well as to reduce inflammation and damage caused by UV radiation. Furthermore, exosomes have been used to promote tissue regeneration in skin wounds and to treat dermatological diseases such as systemic lupus erythematosus, psoriasis, atopic dermatitis, systemic sclerosis, pigment regulation, vitiligo, and hair growth. In this review, the therapeutic value of exosomes in the field of cosmetics, skin care, tissue regeneration, and dermatological diseases, has been elaborated. The existing literature demonstrated that with further research, exosomes may become a viable therapeutic option for many skin conditions.
1. Introduction
Exosomes are nano-sized vesicles that are typically 30–200 nanometers in diameter, and they contain a variety of proteins, lipids, and genetic material such as mRNA and miRNA [1,2,3,4]. Exosomes are released from many different types of cells, including stem cells, and they can circulate in the bloodstream. Exosomes originate from the inward budding of multivesicular bodies (MVBs) containing intraluminal vesicles (ILVs) and they are released via the endosomal-lysosomal pathway. They are thought to play a role in intercellular communication. They engage in many biological processes including cell-to-cell communication, tissue repair, and immune system modulation. They contain a variety of molecules, including proteins and nucleic acids [5,6], and exhibit a variety of functions, including immune modulation and the delivery of messages between cells. Exosomes are also known to possess anti-inflammatory and immunomodulatory properties, which make them attractive for use in skin flap reconstruction. The cosmetics industry has seen a surge in demand for skin care products in recent years. This is driven by several factors including an increased focus on self-care, the rising popularity of social media, and the growing awareness of the dangers of sun exposure. The self-care trend is partly driven by the idea that people should take care of their skin since it’s the largest organ in the body [7]. Consumers are increasingly looking for natural, organic, and cruelty-free products that are formulated with ingredients that are safe and beneficial for their skin. Products like facial moisturizers, serums, and masks are becoming more popular, as they provide a way to nourish and protect the skin from environmental damage. Social media has also had a major impact on the cosmetics industry [8]. From influencers showcasing their beauty routines to popular makeup tutorials, people now have easy access to information about skin care products and trends. This has helped to create a demand for products that can help individuals achieve a certain look or level of beauty. Finally, the growing awareness of the dangers of sun exposure has led to an increased demand for sun protection products. Consumers are looking for sunscreen, moisturizers, and other products that are specifically designed to protect their skin from the sun’s harmful rays [8,9]. Overall, the cosmetics industry has seen a steady increase in demand for skin care products. This is driven by a combination of factors, including self-care, social media, and the need for sun protection. As consumers become more conscious of the importance of taking care of their skin, this trend is likely to continue. Though we have seen and read multiple literature reviews on the broad topic covering one area or another, in this review, we focused on all the possible areas where exosomes have been used and cited in the last decade.
2. Exosomes in Cosmetics Industry
Exosomes have recently been gaining attention in the cosmetic world [10]. Used in topical creams, serums, and masks, exosomes have been found to have a number of therapeutic and anti-aging benefits [11]. Exosomes have been found to be beneficial for skin care, as they are filled with proteins, lipids, and other molecules that can help to promote healing, hydration, and the protection of the skin. These molecules can help to boost collagen production, reduce inflammation, and protect the skin from environmental stressors. Additionally, exosomes can help to increase the efficacy of other active ingredients, such as hyaluronic acid, peptides, and antioxidants [11]. Figure 1 depicts Adipose stem cell derived-condition media (ASC-CM), Bone marrow stem cell derived (BMSC)-exosomes, decreased reactive oxygen species (ROS), as well as TNF-α and increased TGF-β, resulting in higher MMP-1 and pro-collagen type I. This led to the increase of collagen synthesis, the improvement of elasticity, and the reduction of wrinkles, an effective anti-aging therapy [12]. Filled with molecules that help stimulate collagen production, exosomes can help to reduce wrinkles and fine lines [13]. Additionally, exosomes can help to repair skin damage such as sun damage and acne scars. Exosomal proteins and lipids can help to plump and hydrate the skin, which can help to improve skin texture. Ingredients of exosomes such as cytokines, nucleic acids, proteins, and other bioactive compounds can also help to protect the skin from environmental stressors and reduce the appearance of dark spots and other discoloration. With their ability to help improve skin tone, texture, and appearance, exosomes provide several promising therapeutic and anti-aging benefits.
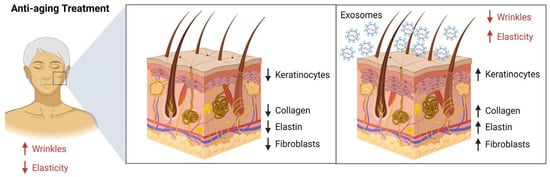
Figure 1.
Role of exosomes for anti-aging treatment. The administration of ASC-CM and BMSC-exos had the effect of decreasing the production of reactive oxygen species (ROS) to a low level, decreasing the expression of tumor necrosis factor-alpha (TNF-α) but increasing the expression of transforming growth factor-beta (TGF-β), leading to an increase in the production of matrix metalloproteinase-1 (MMP-1) and pro-collagen type I, which ultimately enhanced the synthesis of collagen in the skin, improving its elasticity and reducing the appearance of wrinkles, making it an effective anti-aging therapy. Figure was created using Biorender.
3. Role of Exosomes in Wound Healing
Exosomes have been studied extensively in the context of wound healing, particularly in the context of burn wounds [14]. During injury, the cells release exosomes to help the wound heal. These immune cells help to clean the wound and reduce the risk of infection. Exosomes also contain pro-inflammatory proteins that stimulate the release of cytokines and chemokines. These cytokines and chemokines initiate the recruitment of immune cells to the wound site and activate wound healing. In addition, exosomes contain growth factors that stimulate the growth of fibroblasts, which helps to close the wound [15]. Exosomes also promote cell-to-cell communication. They contain miRNAs that are proven to regulate gene expression in the cells around the wound [16]. This promotes the formation of new blood vessels, increasing the blood flow to the wound site. This increased blood flow then delivers oxygen and nutrients to the wound, which further speeds up the healing process [17]. Adipose-derived stem cells (ASCs) and their exosomes have been shown to have a therapeutic role in treating aberrant skin conditions, including wound healing, acne, and alopecia. ASC-exosomes have been shown to reduce inflammation and increase the production of extracellular matrix and growth factors, resulting in improved skin quality and healing (Figure 2). Overall, exosomes play an important role in wound healing.
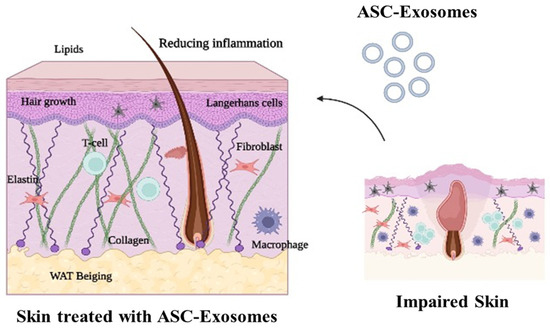
Figure 2.
Therapeutic role of adipose-derived stem cells (ASC-exosomes) in aberrant skin conditions. Exosomes released from ASC-exosomes have been found to have regenerative and anti-inflammatory effects on the skin, helping to improve its appearance and texture. These exosomes are thought to reduce wrinkles and pigmentation, as well as promote collagen production. Figure was created using Biorender.
4. Role of Exosomes in the Reconstruction of Skin Flaps
The potential use of exosomes in skin flap reconstruction has been studied in recent years, with promising results in studies conducted on rats [17]. The use of exosomes in skin flap reconstruction involves the injection of exosomes directly into the skin flap in order to promote angiogenesis and wound healing. Studies have shown that the injection of adipose mesenchymal stem cell (MSC)-derived exosomes into a skin flap can significantly improve the survival rate of the flap, as well as reduce the amount of scarring that occurs [18,19]. In addition to their potential use in skin flap reconstruction, exosomes have also been studied for their potential to promote skin regeneration. Studies have shown that the injection of exosomes into the skin can stimulate the production of collagen and elastin, two substances that are essential for skin regeneration [20]. This is important for skin flap reconstruction, as these substances are necessary for the formation of the new skin that will form during the reconstruction process. Overall, the unique properties of exosomes, including their anti-inflammatory and immunomodulatory properties, their ability to promote angiogenesis and wound healing, and their potential to promote skin regeneration, make them an attractive option for reconstructive surgery. Further research is needed to fully understand their role in skin flap reconstruction and to determine the most effective ways to use them.
5. Role of Exosomes in Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) is an autoimmune disorder that affects multiple organs, including the skin, muscles, and joints. The cause of SLE is unknown, but it is believed to be the result of an environmental trigger, combined with genetic and immunological factors [21]. In recent years, researchers have begun to focus on the role of exosomes in SLE. In SLE, exosomes have been found to play an important role in the development and progression of the disease. Exosomes from SLE patients have been found to contain higher levels of cytokines, chemokines, and other molecules that can promote inflammation [22]. These molecules can also activate other cells of the immune system, leading to increased production of antibodies that can attack the body’s tissues. Exosomes can also be used as biomarkers to diagnose and monitor SLE [23]. Serum exosomes have been found to contain a variety of molecules that can be used to distinguish SLE from other autoimmune disorders [24,25,26]. SLE patients-derived exosomes have also been found to contain higher levels of certain molecules, such as interferon-gamma and interleukin-17, which can be used to monitor the progress of the disease. In addition, exosomes can be used to deliver therapeutic molecules to SLE patients. For example, exosomes loaded with anti-inflammatory molecules can be used to reduce inflammation in SLE patients. Similarly, exosomes loaded with immunosuppressive molecules can be used to reduce the production of antibodies that can attack the body’s tissues. Evidently, exosomes can be used to diagnose and monitor SLE, as well as to deliver therapeutic molecules to SLE patients. As researchers continue to explore the role of exosomes in SLE, they may be able to develop new treatments that can improve the lives of SLE patients [24,25,26].
6. Role of Exosomes in Psoriasis
Psoriasis is a chronic inflammatory skin disease that affects over 125 million people worldwide [27]. It is characterized by patches of red, scaly skin and is caused by an overactive immune system. While there is no cure for psoriasis, recent research has shown that exosomes may play a role in its treatment. In the case of psoriasis, exosomes are believed to play a role in the overactive immune response that leads to psoriasis. The exact mechanism by which exosomes contribute to psoriasis is still not fully understood. However, some studies have suggested that exosomes could be used to modulate the immune system to reduce inflammation and improve skin health. For example, exosomes derived from a certain type of immune cell (regulatory T cells) have been shown to suppress inflammation in mice with psoriasis-like skin conditions. Exosomes could also be used to deliver therapeutic agents directly to the affected areas. For example, exosomes loaded with anti-inflammatory drugs have been shown to reduce psoriasis-like skin lesions in mice [28]. In addition, exosomes loaded with small interfering RNAs (siRNAs) have been shown to reduce skin inflammation in a mouse model of psoriasis [29]. Overall, exosomes appear to be promising candidates for the treatment of psoriasis. While further research is needed to identify their role in psoriasis, exosomes could provide a novel way to target and modulate the immune system to reduce inflammation and improve skin health.
7. Role of Exosomes in Atopic Dermatitis
Atopic dermatitis (AD), also known as eczema, is a chronic skin inflammation caused by an underlying immune system disorder. It is characterized by red, itchy, and scaly rashes [27,30]. The exact cause of AD is not known, but it is believed to be caused by a combination of genetic and environmental factors [31]. Exosomes released from T cells, mast cells, and keratinocytes have been found to contain inflammatory mediators that can induce or aggravate AD [32]. For example, exosomes released from T cells can activate mast cells in the skin, leading to the release of inflammatory mediators such as histamine, which can induce itching and inflammation. Exosomes released from keratinocytes can also contain inflammatory mediators such as IL-4 and IL-13, which can promote the recruitment of inflammatory cells and exacerbate atopic dermatitis. Exosomes can also play a role in modulating the immune system in atopic dermatitis. For instance, exosomes released from keratinocytes have been found to contain anti-inflammatory mediators such as transforming growth factor beta (TGF-β), which can suppress the activation of T cells and reduce inflammation. Exosomes released from mast cells can also contain regulatory cytokines such as IL-10 and TGF-β, which can suppress the activation of T cells and reduce inflammation. In addition, exosomes have been found to contain immunomodulatory molecules such as microRNAs, that are shown to regulate the expression of genes involved in immune responses. For example, exosomes released from mast cells have been found to contain microRNAs that stimulate the downregulation of pro-inflammatory cytokines expressions such as IL-4 and IL-13 which have been shown to reduce inflammation. Overall, exosomes play a key role in the development and progression of atopic dermatitis. Shin et al. showed that ASC-exosomes have been found to reduce symptoms of AD. Studies have demonstrated that these exosomes can reduce inflammation and promote skin barrier restoration by increasing ceramide and dihydroceramide production. This suggests ASC-exosomes could be a viable treatment option for AD, as there are limited available treatments. Therefore, this cell-free therapy could provide much-needed relief for those suffering from this condition (Figure 3) [33]. Further research is needed to better understand the role of exosomes in AD and to develop potential therapeutic strategies to target these exosomes [33,34].
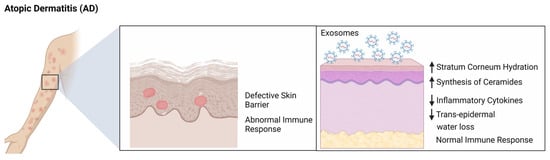
Figure 3.
Role of exosomes in ameliorating atopic dermatitis (AD). ASC-exosomes improved hydration, lowered cytokines IL-4, IL-5, IL-13, IFN-γ, and TNF-α, reduced mast cells and DECs in lesions, and made ceramides to repair the epidermis, thereby alleviating AD. Figure was created using Biorender.
8. Role of Exosomes in Systemic Sclerosis
Systemic sclerosis (SSc) is a chronic autoimmune disorder characterized by progressive fibrosis of the skin and internal organs. Patients with SSc suffer from a variety of symptoms, including Raynaud’s phenomenon, skin thickening, and organ fibrosis [34,35]. While the etiology of SSc is still unknown, recent evidence has suggested that exosomes may play an important role in the progression of this disease. Exosomes are small vesicles released by cells that contain a variety of molecules, including proteins, lipids, and RNA. In SSc, exosomes are thought to contribute to the development of fibrosis by releasing pro-fibrotic molecules, such as transforming growth factor-beta (TGF-β). In addition, exosomes from SSc patients have been found to contain increased levels of pro-inflammatory cytokines, such as interleukin-6 (IL-6), which can promote the development of sclerosis [36]. Exosomes from SSc patients have also been found to contain specific microRNAs (miRNAs) that are associated with the development of fibrosis. These miRNAs are thought to be involved in the regulation of gene expression and can thus contribute to the progression of fibrosis. In addition, exosomes from SSc patients have been found to contain increased levels of matrix metalloproteinases (MMPs), which are enzymes involved in the breakdown of an extracellular matrix [25]. The increased levels of these enzymes are thought to contribute to the development of skin and organ fibrosis. In summary, exosomes have been implicated in the progression of SSc. They are thought to be involved in the release of pro-fibrotic molecules as well as increased levels of pro-inflammatory cytokines and MMPs. In addition, they contain specific miRNAs that can regulate gene expression and thus contribute to the progression of the disease [34]. Further research is needed to better understand the role of exosomes in SSc and to develop potential therapies targeting these exosomes.
9. Role of Exosomes in Scar Removal
In recent years, researchers have been exploring the potential of exosome therapy for scar removal. Exosome-based scar removal works by delivering therapeutic agents directly to the affected area. Exosomes can deliver cytokines and growth factors that stimulate the production of new collagen and elastin, which is shown to simulate the reduction of scars [11]. Exosome therapy can be applied topically or injected directly into the scar tissue. Injections are often used to treat deeper scars, as they can effectively penetrate the dermis where the scar tissue is located. Topical applications may be more suitable for superficial scars, as they can provide a more even distribution of the therapeutic agents. In clinical studies, exosome therapy has been found to be safe and effective for scar removal [37]. It can reduce the appearance of scars, improve skin hydration and elasticity, and reduce inflammation. It can also help to strengthen the skin’s natural defenses against infection. Exosomes derived from umbilical cord-derived MSCs (uMSCs) are enriched in specific miR-21, miR-23a, miR-125b, and miR-145 [38]. A skin-defect mouse model showed that these exosomes could suppress fibrosis and scar formation by interfering with the TGF-β2/SMAD2 pathway. Additionally, exosomes high in miR-21-3p were shown to accelerate re-epithelialization, reduce scar widths, and promote angiogenesis via inhibition of PTEN and SPRY1 [39]. These findings suggest that MSC-derived exosomes, including ADSC-exos, may regulate fibroblast function and collagen deposition to promote scarless healing. Thus, ADSC-exos represent a potential therapeutic approach for improving wound healing and scar prevention (Figure 4). Evidently, exosome therapy is a promising treatment for scar removal, but further research is needed to understand its full potential. If proven effective, it could provide an alternative to more invasive treatments, such as laser resurfacing and chemical peels. As the technology continues to evolve, it may become a viable option for those looking to reduce the appearance of their scars [39].
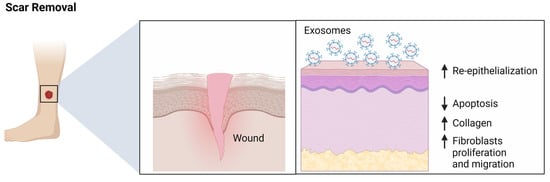
Figure 4.
Role of exosomes in the treatment of scars. MSC-derived exosomes from umbilical cords showed enrichment in specific miRNAs, including miR-21, -23a, -125b, and -145. Their application to a mouse skin-defect model reduced fibrosis, resulting in less scarring. Additionally, human umbilical cord-derived exosomes containing miR-21–3p accelerated re-epithelialization, reduced scar widths, and boosted angiogenesis. Thus, MSC-derived exosomes could regulate fibroblasts, collagen deposition, and promote scarless wound healing. Figure was created using Biorender.
10. Role of Exosomes in the Rejuvenation of the Face
The field of facial rejuvenation has seen a wide variety of treatments, from laser resurfacing to chemical peels. In the midst of this, a new technique is gaining traction: exosome facial rejuvenation. This innovative procedure uses stem cells and the body’s natural healing powers to produce long-lasting, natural-looking results. Exosomes are thought to help with tissue regeneration and cell rejuvenation and are being used as a way to promote facial rejuvenation [40]. The process begins with a biopsy of fat from the patient, which is then processed to extract the stem cells and exosomes. The stem cells and their exosomes are then injected into the facial skin, which is shown to stimulate the body’s natural healing process. The exosome facial rejuvenation process can help to reduce wrinkles, dark circles, and age spots. The process also helps to improve skin tone and texture, as well as increase collagen production. Exosome injections have been part of clinical studies but have yet to be granted FDA approval for use as injections [41].
11. Role of Exosomes in the Regulation of Pigmentation
Recent studies have identified that exosomes may play an important role in the regulation of pigmentation in multiple organisms, including humans, through several different mechanisms. Exosomes have been shown to regulate the expression of genes involved in pigmentation and provide an efficient way for cells to transfer melanin-producing enzymes to other cells, enabling them to synthesize and produce melanin [42]. Studies have shown that exosomes can stimulate the activity of tyrosinase, leading to an increase in melanin production [43]. Additionally, exosomes can regulate the expression of tyrosinase, which is important for modulating the production of melanin. Keratinocytes-derived exosomes have been shown to activate melanocyte precursors and induce their differentiation into mature melanocytes. Furthermore, exosomes can help to stimulate the migration of melanocytes to areas of the skin where pigment production is needed. In conclusion, exosomes can help to regulate the expression of genes involved in pigmentation, provide a way for cells to transfer melanin-producing enzymes, and regulate the activity and expression of tyrosinase. Additionally, exosomes can help to regulate the development and migration of melanocytes. Therefore, a better understanding of the role of exosomes in pigmentation could be beneficial for the development of therapeutic agents to treat skin disorders.
12. Role of Stem Cell-Derived Exosomes in Cosmetic Dermatology
Stem cell-derived exosomes are from pluripotent stem cells, mesenchymal stem cells, and adipose stem cells [44]. The stem-cell-derived exosomes are like the other exosomes derived from other cells, morphologically and structurally, and their difference is between their surface proteins and the information transmitted within the membrane [44,45]. Stem cell exosomes possess unique abilities for cell proliferation, regeneration, and wound healing [45]. One such example is an alteration of extracellular matrix compositions and fibroblast proliferation [45]. Thus, because of these properties, stem cell-derived exosomes can play a vital role in skin rejuvenation, skin microenvironment, and wound repair during acne healing [44,45]. As we know, skin aging results from both internal and external factors. Internally it is based on gene dependence for aging and skin texture [44,45]. However, externally, multiple factors are responsible for this, such as ultraviolet B (280–320 nm) (UV-B) light, which induces DNA mutations and increases oxidative stress on the cells which leads to skin aging [46]. Apart from this, UV-B can also simulate MAPK signaling pathways, further enhancing the expression of MMPs and leading to downregulating collagen type I by activator protein-1 [47,48]. Through other research, we know that collagen type I and III syntheses are essential aspects of ECM, as they eventually make skin look younger and fuller, leading to skin aging [45,49]. Apart from this, as the dermis fibroblast ages, it will ultimately lead to reduced collagen [12] and, along with the expression of MMPs, it will lead to cleavage of existing collagen which leads to skin aging symptoms such as wrinkles and pigmentation [50,51,52]. Senescence studies on the skin have proven that stem cell-derived exosomes are vital in reducing skin aging (Figure 5). Exosomes derived from human induced pluripotent stem cells (iPSCs) can reduce the aging of human dermal fibroblasts. It has been shown that iPSCs expression can regulate the expression level of MMP-1/3 and thus, in turn, increase the expression of type 1 collagen and reduced skin aging [50,51,52]. Furthermore, this will reduce the expression of senescence-associated-β-gaalctosidase (SA-β-Gal) [45]. Apart from a reduction in senescence, stem cell-derived exosomes play an essential role in wound healing [53,54]. As wound healing involves inflammation, hyperplasia, and remodeling, inflammation is regulated by the polarization of macrophages into an M2 phenotype [53]. It has been observed that exosomes secreted by human mesenchymal stem cells (hMSCs) can decrease the number of neutrophils and, in turn, decrease the macrophage population during the inflammatory response [54]. Another study observed conveys that exosomes impact various pathways to wound repairs, such as the Wnt4/β-catenin signaling pathway [55], Erk1/2 signaling pathway [56], NF-κB signaling pathway [55], and Notch signaling pathway [57]. Similarly, stem cells derived exosomes play a vital role in scarless healing [57]. The presence of fine reticular collagen, lesser inflammation at the recovery site, fewer myofibroblasts, and fewer cross-linking define scarless healing [58]. Apart from the presence of higher type 1 collagen, TGF β1, and matrix metalloproteinase tissue inhibitors compared to type III collagen, TGF β3 and MMPs expression is required for scarless healing [58,59]. Studies have shown that exosomes derived from human adipose mesenchymal stem cells can regulate the latter expression and thus help in scarless recovery [59].
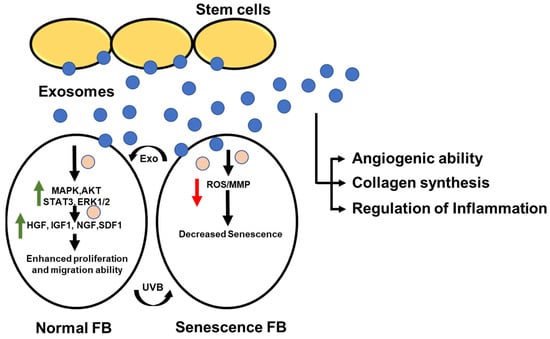
Figure 5.
Stem cell derived exosomes enhance the proliferation and migration of skin fibroblast. Exosomes from stem cells are shown to enhance proliferation and increase migration ability by increasing MAPK and AKT etc. in the case of normal fibroblast cells. However, in the case of senescence fibroblast (induced due to exposure to UVB), the same exosomes are found to decrease senescence. Stem cell exosomes are found to have an impact on angiogenic ability, collagen synthesis, and regulation of inflammation in skin disorders. The green up-arrow depicts increased expression, and the red colored down-arrow depicts decreased expression.
13. Role of Exosomes in Vitiligo
Vitiligo is a skin condition that causes the loss of skin pigment, resulting in white patches on the skin. It affects both men and women of all ages and is more common in people with dark skin [60]. While the cause of vitiligo is still unknown, there is evidence that suggests it is an autoimmune disorder [60]. Exosomes are thought to be involved in the development of vitiligo by regulating the activity of genes involved in melanin production [43]. Exosomes also contain antioxidants, which may help to protect against oxidative damage to the skin. While it is not clear how these molecules may play a role in vitiligo, some studies have suggested that they may help to protect the skin from UV radiation, which is thought to be a factor in the development of vitiligo. In addition to their role in the regulation of the immune system and in protecting the skin from oxidative damage, exosomes may also be involved in the repair of damaged skin cells [61]. By delivering the necessary molecules to damaged cells, exosomes may help to stimulate the reparative process and promote the formation of new skin cells. Mesenchymal stem cells (MSCs) are capable of immunomodulatory and regenerative properties, which make them a potential treatment option for vitiligo. By secreting immunosuppressive cytokines and growth factors, MSCs can modulate the immune response and increase the re-pigmentation of vitiligo lesions. Clinical and pre-clinical studies have already demonstrated promising results of MSC-based therapies for vitiligo treatment (Figure 6). Overall, exosomes appear to play a role in the development and progression of vitiligo. While more research is needed to understand their exact role, they may be a potential target for therapies that could help to improve this condition [61].
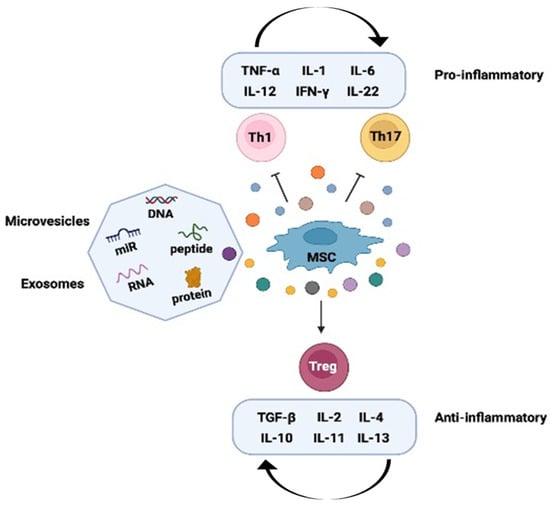
Figure 6.
Immunomodulation by mesenchymal stem cells (MSCs) in vitiligo. MSCs have been shown to have immunomodulatory effects in vitiligo. The mechanisms of MSC-mediated immunomodulation are complex and involve both cell-cell contact-dependent mechanisms and the secretion of various soluble mediators. These mediators can affect the activity of regulatory T cells (Tregs), T helper 1 (Th1) cells, T helper 2 (Th2) cells, T helper 17 (Th17) cells, dendritic cells (DCs), and natural killer (NK) cells. The mediators include interleukins (ILs), interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α) and transforming growth factor-beta (TGF-β). Figure was created using Biorender.
14. Role of Exosomes in Hair Growth
Hair growth is a complex biological process regulated by numerous mechanisms, including genetics, hormones, and other external factors [62]. Recent studies have discovered that exosomes, small vesicles released by cells, may play an important role in this process [63]. Li et al. showed that ADSC-exosomes had a positive effect on DPC proliferation, migration, and hair growth. It decreased apoptosis and increased HFs and dermis thickness. RNA-seq showed that miR-22 and TNF-α were inhibited. qRT-PCR and western blotting showed the Wnt/β-catenin pathway was activated in the skin of ADSC-exosomes-treated mice. Overall, ADSC-exosomes had positive effects on DPCs and hair growth in mice. This suggests that ADSC-exosome therapy improved hair regrowth by controlling miR-22, Wnt/β-catenin, and TNF-α pathways, suggesting it could be an effective cell-free treatment for alopecia caused by the immune system [64]. Kwack et al. showed that exosomes from 3D DP (3D DP-Exos) enhanced the proliferation of dermal papilla (DP) and outer root sheath (ORS) cells and increased the expression of growth factors (IGF-1, KGF, and HGF) in DP cells. When used to treat cultured human hair follicles, 3D DP-Exo treatment increased hair shaft elongation. Local injections of 3D DP-Exos also triggered anagen from telogen and prolonged anagen in mice. Moreover, when DP spheres treated with Exo were implanted with mouse epidermal cells, hair follicle neogenesis was enhanced. Similar outcomes were observed when Exos derived from 2D-cultured DP cells (2D DP-Exo) were used. Their findings suggest that Exos derived from DP cells can promote hair growth and regeneration by regulating the activity of follicular dermal and epidermal cells. These results have implications for the development of therapeutic strategies for hair loss [65]. Wu et al. also showed that exosomes from adipose-derived stem cells could promote hair regeneration [66]. As such, exosomes may play an important role in hair growth and hair follicle regeneration. Further research is needed to better understand the role of exosomes in this process.
15. Conclusions and Outlook
The cosmetics industry is constantly evolving as it is driven by consumer demand for new and innovative products. Exosomes have been identified as potential new ingredients in cosmetics, with the potential to provide significant cosmetic benefits. Exosomes have been found to have a therapeutic role in wound healing, skin flap reconstruction, systemic lupus erythematosus, psoriasis, atopic dermatitis, systemic sclerosis, scar removal, facial rejuvenation, pigmentation regulation, vitiligo, and hair growth. Exosomes have tremendous potential to revolutionize the cosmetics industry and provide more effective skin care products. The unique properties of exosomes, such as their ability to penetrate the skin, their high concentration of bioactive molecules, and their ability to interact with and modulate the skin’s cells, make them desirable ingredients for cosmetic products.
The future of exosomes in the cosmetics industry appears to be very promising. As research continues to uncover the potential of exosomes, more and more products will be developed to take advantage of their unique properties. In addition, the use of exosomes in combination with traditional cosmetic ingredients is likely to lead to more effective skin care products. Finally, as technology advances, exosomes may be modified to provide even more specific benefits, such as targeted delivery of active ingredients. In conclusion, exosomes have the potential to revolutionize the cosmetics industry. With the help of exosomes, the cosmetics industry is sure to continue to develop innovative and effective skin care products in the future.
Despite the tremendous benefits of exosomes, their regulatory aspects have been a topic of debate. The US Food and Drug Administration (FDA) has yet to officially classify exosomes for cosmetics; therefore, it is not yet known what type of regulation should be imposed. In the EU, exosomes for cosmetic use may be subject to regulation by the European Commission, which could include restrictions on manufacturing, labeling, and advertising. As exosomes are a relatively new technology, further research and discussion are needed to understand the regulatory implications of their use.
Author Contributions
Conceptualization, A.T.; validation, A.T., D.S. and D.R.; formal analysis, A.T.; investigation, A.T., D.S. and D.R.; resources, D.S., D.R., D.C.P., S.P., S.K. and A.C.; data curation, D.R., D.C.P., S.P. and S.K.; writing—original draft preparation, A.T.; writing—review and editing, D.S., D.R. and S.K.; visualization, A.T., D.R., D.C.P. and S.P.; supervision, A.T.; project administration, A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and exosomal microRNA: Trafficking, sorting, and function. Genom. Proteom. Bioinform. 2015, 13, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Thakur, A.; Xu, C.; Ng, S.-P.; Lee, Y.; Wu, C.-M.L. Detection of Glioma-Derived Exosomes with the Biotinylated Antibody-Functionalized Titanium Nitride Plasmonic Biosensor. Adv. Funct. Mater. 2019, 29, 1806761. [Google Scholar] [CrossRef]
- Thakur, A.; Qiu, G.; Xu, C.; Han, X.; Yang, T.; Ng, S.P.; Chan, K.W.Y.; Wu, C.M.L.; Lee, Y. Label-free sensing of exosomal MCT1 and CD147 for tracking metabolic reprogramming and malignant progression in glioma. Sci. Adv. 2020, 6, eaaz6119. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Mishra, A.P.; Panda, B.; Sweta, K.; Majhi, B. Detection of Disease-Specific Parent Cells via Distinct Population of Nano-Vesicles by Machine Learning. Curr. Pharm. Des. 2020, 26, 3985–3996. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, Y.; Peng, J.; Thakur, A.; Bai, N.; Yang, K.; Xu, Z. Decoding Roles of Exosomal lncRNAs in Tumor-Immune Regulation and Therapeutic Potential. Cancers 2022, 15, 286. [Google Scholar] [CrossRef]
- Thakur, A.; Liang, L.; Ghosh, D.; Cili, A.; Zhang, K. Identification and functional analysis of exosomal miR-16-5p, miR-6721-5p, and miR-486-5p associated with immune infiltration for potential vitiligo theranostics. Clin. Immunol. Commun. 2022, 2, 110–117. [Google Scholar] [CrossRef]
- Pop, R.-A.; Săplăcan, Z.; Alt, M.-A. Social Media Goes Green—The Impact of Social Media on Green Cosmetics Purchase Motivation and Intention. Information 2020, 11, 447. [Google Scholar] [CrossRef]
- Limbu, Y.B.; Pham, L.; Nguyen, T.T.T. Predictors of Green Cosmetics Purchase Intentions among Young Female Consumers in Vietnam. Sustainability 2022, 14, 12599. [Google Scholar] [CrossRef]
- Britton, A.M. The Beauty Industry’s Influence on Women in Society. Bachelor’s Thesis, University of New Hampshire, Durham, NH, USA, 2012. [Google Scholar]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef]
- Shi, H.; Wang, M.; Sun, Y.; Yang, D.; Xu, W.; Qian, H. Exosomes: Emerging Cell-Free Based Therapeutics in Dermatologic Diseases. Front. Cell Dev. Biol. 2021, 9, 736022. [Google Scholar] [CrossRef]
- Hu, S.; Li, Z.; Cores, J.; Huang, K.; Su, T.; Dinh, P.-U.; Cheng, K. Needle-Free Injection of Exosomes Derived from Human Dermal Fibroblast Spheroids Ameliorates Skin Photoaging. ACS Nano 2019, 13, 11273–11282. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Zhang, Q.; Hu, W.; Zhao, C.; Lv, W.; Yi, Y.; Wang, Y.; Tang, H.; Wu, M.; Wu, Y. The novel mechanisms and applications of exosomes in dermatology and cutaneous medical aesthetics. Pharmacol. Res. 2021, 166, 105490. [Google Scholar] [CrossRef]
- Bo, Y.; Yang, L.; Liu, B.; Tian, G.; Li, C.; Zhang, L.; Yan, Y. Exosomes from human induced pluripotent stem cells-derived keratinocytes accelerate burn wound healing through miR-762 mediated promotion of keratinocytes and endothelial cells migration. J. Nanobiotechnol. 2022, 20, 291. [Google Scholar] [CrossRef]
- Xia, W.; Li, M.; Jiang, X.; Huang, X.; Gu, S.; Ye, J.; Zhu, L.; Hou, M.; Zan, T. Young fibroblast-derived exosomal microRNA-125b transfers beneficial effects on aged cutaneous wound healing. J. Nanobiotechnol. 2022, 20, 144. [Google Scholar] [CrossRef]
- Dai, W.; Dong, Y.; Han, T.; Wang, J.; Gao, B.; Guo, H.; Xu, F.; Li, J.; Ma, Y. Microenvironmental cue-regulated exosomes as therapeutic strategies for improving chronic wound healing. NPG Asia Mater. 2022, 14, 75. [Google Scholar] [CrossRef]
- Guo, L.; Chen, Y.; Feng, X.; Sun, D.; Sun, J.; Mou, S.; Zhao, K.; An, R. Oxidative stress-induced endothelial cells-derived exosomes accelerate skin flap survival through Lnc NEAT1-mediated promotion of endothelial progenitor cell function. Stem Cell Res. Ther. 2022, 13, 325. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wei, S.; Xu, Q.; Sun, Y.; Ning, X.; Wang, Z. Application of ADSCs and their Exosomes in Scar Prevention. Stem Cell Rev. Rep. 2022, 18, 952–967. [Google Scholar] [CrossRef]
- Hong, P.; Yang, H.; Wu, Y.; Li, K.; Tang, Z. The functions and clinical application potential of exosomes derived from adipose mesenchymal stem cells: A comprehensive review. Stem Cell Res. Ther. 2019, 10, 242. [Google Scholar] [CrossRef]
- Yang, G.H.; Lee, Y.B.; Kang, D.; Choi, E.; Nam, Y.; Lee, K.H.; You, H.-J.; Kang, H.J.; An, S.H.; Jeon, H. Overcome the barriers of the skin: Exosome therapy. Biomater. Res. 2021, 25, 22. [Google Scholar] [CrossRef]
- Cojocaru, M.; Cojocaru, I.M.; Silosi, I.; Vrabie, C.D. Manifestations of systemic lupus erythematosus. Maedica 2011, 6, 330–336. [Google Scholar]
- Lee, J.Y.; Park, J.K.; Lee, E.Y.; Lee, E.B.; Song, Y.W. Circulating exosomes from patients with systemic lupus erythematosus induce an proinflammatory immune response. Arthritis Res. Ther. 2016, 18, 264. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.; Liu, Q.; Peng, N.; Yang, G.; Shen, Z.; Hong, P.; Wang, S.; Rui, K.; Cui, D. Exosomes as Crucial Players in Pathogenesis of Systemic Lupus Erythematosus. J. Immunol. Res. 2022, 2022, 8286498. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Liu, Q.; Wu, K.; Liu, L.; Zhao, M.; Yang, H.; Wang, X.; Wang, W. Extracellular vesicles as potential biomarkers and therapeutic approaches in autoimmune diseases. J. Transl. Med. 2020, 18, 432. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Wang, Y.; Jin, H.; Li, L. The role of exosome in autoimmune connective tissue disease. Ann. Med. 2019, 51, 101–108. [Google Scholar] [CrossRef]
- Miao, C.; Wang, X.; Zhou, W.; Huang, J. The emerging roles of exosomes in autoimmune diseases, with special emphasis on microRNAs in exosomes. Pharmacol. Res. 2021, 169, 105680. [Google Scholar] [CrossRef]
- Bu, J.; Ding, R.; Zhou, L.; Chen, X.; Shen, E. Epidemiology of Psoriasis and Comorbid Diseases: A Narrative Review. Front. Immunol. 2022, 13, 880201. [Google Scholar] [CrossRef]
- Zhang, B.; Lai, R.C.; Sim, W.K.; Choo, A.B.H.; Lane, E.B.; Lim, S.K. Topical Application of Mesenchymal Stem Cell Exosomes Alleviates the Imiquimod Induced Psoriasis-Like Inflammation. Int. J. Mol. Sci. 2021, 22, 720. [Google Scholar] [CrossRef]
- Nemati, H.; Ghahramani, M.H.; Faridi-Majidi, R.; Izadi, B.; Bahrami, G.; Madani, S.H.; Tavoosidana, G. Using siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation in psoriasis. J. Control. Release 2017, 268, 259–268. [Google Scholar] [CrossRef]
- Lu, Z.; Zeng, N.; Cheng, Y.; Chen, Y.; Li, Y.; Lu, Q.; Xia, Q.; Luo, D. Atopic dermatitis and risk of autoimmune diseases: A systematic review and meta-analysis. Allergy Asthma Clin. Immunol. 2021, 17, 96. [Google Scholar] [CrossRef]
- Wang, W.M.; Wu, C.; Jin, H.Z. Exosomes in chronic inflammatory skin diseases and skin tumors. Exp. Dermatol. 2019, 28, 213–218. [Google Scholar] [CrossRef]
- Shao, S.; Fang, H.; Li, Q.; Wang, G. Extracellular vesicles in Inflammatory Skin Disorders: From Pathophysiology to Treatment. Theranostics 2020, 10, 9937–9955. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.-O.; Ha, D.H.; Kim, J.O.; Crumrine, D.A.; Meyer, J.M.; Wakefield, J.S.; Lee, Y.; Kim, B.; Kim, S.; Kim, H.-k.; et al. Exosomes from Human Adipose Tissue-Derived Mesenchymal Stem Cells Promote Epidermal Barrier Repair by Inducing de Novo Synthesis of Ceramides in Atopic Dermatitis. Cells 2020, 9, 680. [Google Scholar] [CrossRef] [PubMed]
- Colletti, M.; Galardi, A.; De Santis, M.; Guidelli, G.M.; Di Giannatale, A.; Di Luigi, L.; Antinozzi, C. Exosomes in Systemic Sclerosis: Messengers Between Immune, Vascular and Fibrotic Components? Int. J. Mol. Sci. 2019, 20, 4337. [Google Scholar] [CrossRef] [PubMed]
- Sobolewski, P.; Maślińska, M.; Wieczorek, M.; Łagun, Z.; Malewska, A.; Roszkiewicz, M.; Nitskovich, R.; Szymańska, E.; Walecka, I. Systemic sclerosis—Multidisciplinary disease: Clinical features and treatment. Reumatologia 2019, 57, 221–233. [Google Scholar] [CrossRef]
- O’Reilly, S.; Cant, R.; Ciechomska, M.; van Laar, J.M. Interleukin-6: A new therapeutic target in systemic sclerosis? Clin. Transl. Immunol. 2013, 2, e4. [Google Scholar] [CrossRef]
- Quiñones-Vico, M.I.; Sanabria-de la Torre, R.; Sánchez-Díaz, M.; Sierra-Sánchez, Á.; Montero-Vílchez, T.; Fernández-González, A.; Arias-Santiago, S. The Role of Exosomes Derived From Mesenchymal Stromal Cells in Dermatology. Front. Cell Dev. Biol. 2021, 9, 647012. [Google Scholar] [CrossRef]
- Fang, S.; Xu, C.; Zhang, Y.; Xue, C.; Yang, C.; Bi, H.; Qian, X.; Wu, M.; Ji, K.; Zhao, Y.; et al. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomal MicroRNAs Suppress Myofibroblast Differentiation by Inhibiting the Transforming Growth Factor-β/SMAD2 Pathway During Wound Healing. Stem Cells Transl. Med. 2016, 5, 1425–1439. [Google Scholar] [CrossRef]
- Hu, Y.; Rao, S.S.; Wang, Z.X.; Cao, J.; Tan, Y.J.; Luo, J.; Li, H.M.; Zhang, W.S.; Chen, C.Y.; Xie, H. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics 2018, 8, 169–184. [Google Scholar] [CrossRef]
- Vyas, K.S.; Kaufman, J.; Munavalli, G.S.; Robertson, K.; Behfar, A.; Wyles, S.P. Exosomes: The latest in regenerative aesthetics. Regen. Med. 2023, 18, 181–194. [Google Scholar] [CrossRef]
- Zhang, B.; Gong, J.; He, L.; Khan, A.; Xiong, T.; Shen, H.; Li, Z. Exosomes based advancements for application in medical aesthetics. Front. Bioeng. Biotechnol. 2022, 10, 1083640. [Google Scholar] [CrossRef]
- Lo Cicero, A.; Delevoye, C.; Gilles-Marsens, F.; Loew, D.; Dingli, F.; Guéré, C.; André, N.; Vié, K.; van Niel, G.; Raposo, G. Exosomes released by keratinocytes modulate melanocyte pigmentation. Nat. Commun. 2015, 6, 7506. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.M.; Yang, L.; Yang, L.; Wu, H.; Li, W.; Ma, X.; Katayama, I.; Zhang, H. New insight into the role of exosomes in vitiligo. Autoimmun. Rev. 2020, 19, 102664. [Google Scholar] [CrossRef] [PubMed]
- McCabe, M.C.; Hill, R.C.; Calderone, K.; Cui, Y.; Yan, Y.; Quan, T.; Fisher, G.J.; Hansen, K.C. Alterations in extracellular matrix composition during aging and photoaging of the skin. Matrix Biol. Plus 2020, 8, 100041. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Lee, J.; Kim, Y.J.; Rhee, W.J.; Park, J.H. Exosomes Derived from Human Induced Pluripotent Stem Cells Ameliorate the Aging of Skin Fibroblasts. Int. J. Mol. Sci. 2018, 19, 1715. [Google Scholar] [CrossRef]
- Zhong, Q.Y.; Lin, B.; Chen, Y.T.; Huang, Y.P.; Feng, W.P.; Wu, Y.; Long, G.H.; Zou, Y.N.; Liu, Y.; Lin, B.Q.; et al. Gender differences in UV-induced skin inflammation, skin carcinogenesis and systemic damage. Environ. Toxicol. Pharmacol. 2021, 81, 103512. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.J.; Bowden, G.T. Ultraviolet B regulation of transcription factor families: Roles of nuclear factor-kappa B (NF-kappaB) and activator protein-1 (AP-1) in UVB-induced skin carcinogenesis. Curr. Cancer Drug Targets 2007, 7, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef]
- Byron, A.; Humphries, J.D.; Humphries, M.J. Defining the extracellular matrix using proteomics. Int. J. Exp. Pathol. 2013, 94, 75–92. [Google Scholar] [CrossRef]
- Li, L.; Ngo, H.T.T.; Hwang, E.; Wei, X.; Liu, Y.; Liu, J.; Yi, T.H. Conditioned Medium from Human Adipose-Derived Mesenchymal Stem Cell Culture Prevents UVB-Induced Skin Aging in Human Keratinocytes and Dermal Fibroblasts. Int. J. Mol. Sci. 2019, 21, 49. [Google Scholar] [CrossRef]
- Fafián-Labora, J.A.; Rodríguez-Navarro, J.A.; O’Loghlen, A. Small Extracellular Vesicles Have GST Activity and Ameliorate Senescence-Related Tissue Damage. Cell Metab. 2020, 32, 71–86.e75. [Google Scholar] [CrossRef]
- Tallant, C.; Marrero, A.; Gomis-Rüth, F.X. Matrix metalloproteinases: Fold and function of their catalytic domains. Biochim. Biophys. Acta 2010, 1803, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Juhl, P.; Bondesen, S.; Hawkins, C.L.; Karsdal, M.A.; Bay-Jensen, A.-C.; Davies, M.J.; Siebuhr, A.S. Dermal fibroblasts have different extracellular matrix profiles induced by TGF-β, PDGF and IL-6 in a model for skin fibrosis. Sci. Rep. 2020, 10, 17300. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Gálvez, A.B.; Gálvez-Peisl, S.; González-Correa, J.E.; de Haro-Carrillo, M.; Ayllón, V.; Carmona-Sáez, P.; Ramos-Mejía, V.; Galindo-Moreno, P.; Cara, F.E.; Granados-Principal, S.; et al. GARP is a key molecule for mesenchymal stromal cell responses to TGF-β and fundamental to control mitochondrial ROS levels. Stem Cells Transl. Med. 2020, 9, 636–650. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, X.; Zhang, X.; Sun, Y.; Yan, Y.; Shi, H.; Zhu, Y.; Wu, L.; Pan, Z.; Zhu, W.; et al. Human umbilical cord mesenchymal stem cell exosomes enhance angiogenesis through the Wnt4/β-catenin pathway. Stem Cells Transl. Med. 2015, 4, 513–522. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Hu, B.; Niu, X.; Liu, X.; Zhang, G.; Zhang, C.; Li, Q.; Wang, Y. Exosomes Derived from Human Endothelial Progenitor Cells Accelerate Cutaneous Wound Healing by Promoting Angiogenesis Through Erk1/2 Signaling. Int. J. Biol. Sci. 2016, 12, 1472–1487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, M.; Gong, A.; Zhang, X.; Wu, X.; Zhu, Y.; Shi, H.; Wu, L.; Zhu, W.; Qian, H.; et al. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells 2015, 33, 2158–2168. [Google Scholar] [CrossRef] [PubMed]
- Dalirfardouei, R.; Jamialahmadi, K.; Jafarian, A.H.; Mahdipour, E. Promising effects of exosomes isolated from menstrual blood-derived mesenchymal stem cell on wound-healing process in diabetic mouse model. J. Tissue Eng. Regen. Med. 2019, 13, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiao, Y.; Pan, Y.; Zhang, L.; Gong, H.; Qi, Y.; Wang, M.; Gong, H.; Shao, M.; Wang, X.; et al. Fetal Dermal Mesenchymal Stem Cell-Derived Exosomes Accelerate Cutaneous Wound Healing by Activating Notch Signaling. Stem Cells Int. 2019, 2019, 2402916. [Google Scholar] [CrossRef]
- Picardo, M.; Dell’Anna, M.L.; Ezzedine, K.; Hamzavi, I.; Harris, J.E.; Parsad, D.; Taieb, A. Vitiligo. Nat. Rev. Dis. Prim. 2015, 1, 15011. [Google Scholar] [CrossRef]
- Rashighi, M.; Harris, J.E. Vitiligo Pathogenesis and Emerging Treatments. Dermatol. Clin. 2017, 35, 257–265. [Google Scholar] [CrossRef]
- Grymowicz, M.; Rudnicka, E.; Podfigurna, A.; Napierala, P.; Smolarczyk, R.; Smolarczyk, K.; Meczekalski, B. Hormonal Effects on Hair Follicles. Int. J. Mol. Sci. 2020, 21, 5342. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Ntege, E.H.; Sunami, H.; Inoue, Y. Regenerative medicine strategies for hair growth and regeneration: A narrative review of literature. Regen. Ther. 2022, 21, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, G.; Wang, Q.; Zhang, Y.; Cui, L.; Huang, X. Exosomes Secreted from Adipose-Derived Stem Cells Are a Potential Treatment Agent for Immune-Mediated Alopecia. J. Immunol. Res. 2022, 2022, 7471246. [Google Scholar] [CrossRef]
- Kwack, M.H.; Seo, C.H.; Gangadaran, P.; Ahn, B.-C.; Kim, M.K.; Kim, J.C.; Sung, Y.K. Exosomes derived from human dermal papilla cells promote hair growth in cultured human hair follicles and augment the hair-inductive capacity of cultured dermal papilla spheres. Exp. Dermatol. 2019, 28, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yang, Q.; Wu, S.; Yuan, R.; Zhao, X.; Li, Y.; Wu, W.; Zhu, N. Adipose-Derived Stem Cell Exosomes Promoted Hair Regeneration. Tissue Eng. Regen. Med. 2021, 18, 685–691. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).