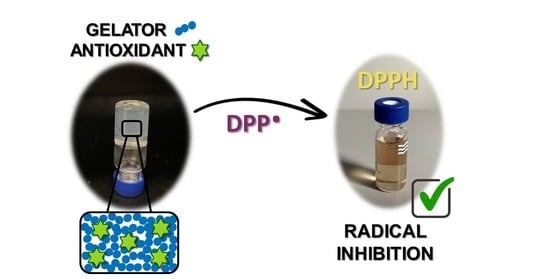
Low-Molecular-Weight Gels as Smart Materials for the Enhancement of Antioxidants Activity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Chemicals
2.1.2. Postbiotics
2.2. Methods
2.2.1. Solutions of Tested Substances
2.2.2. Synthesis and Characterization of Boc-L-DOPA(Bn)2-OH
2.2.3. Preparation of Gels with α-Tocopherol
2.2.4. Preparation of Gels with Postbiotics
2.2.5. Optical Microscopy
2.2.6. Rheological Properties
2.2.7. Determination of DPPH Radical Scavenging Activity (in Gel/in Solution)
2.2.8. Statistical Analysis
3. Results and Discussion
3.1. LMW Gels with α-Tocopherol
3.2. LMW Gel with Postbiotics (PB)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Draper, E.R.; Adams, D.J. Low-Molecular-Weight Gels: The State of the Art. Chem 2017, 3, 390–410. [Google Scholar] [CrossRef]
- Wang, J.; Liu, K.; Xing, R.; Yan, X. Peptide self-assembly: Thermodynamics and kinetics. Chem. Soc. Rev. 2016, 45, 5589–5604. [Google Scholar] [CrossRef]
- Hanabusa, K.; Suzuki, M. Development of low-molecular-weight gelators and polymer-based gelators. Polym. J. 2014, 46, 776–782. [Google Scholar] [CrossRef]
- Dastidar, P.; Roy, R.; Parveen, R.; Sarkar, K. Supramolecular Synthon Approach in Designing Molecular Gels for Advanced Therapeutics. Adv. Ther. 2019, 2, 1800061. [Google Scholar] [CrossRef]
- Nicastro, G.; Black, L.M.; Ravarino, P.; Agostino, S.; Faccio, D.; Tomasini, C.; Giuri, D. Controlled Hydrolysis of Odorants Schiff Bases in Low-Molecular-Weight Gels. Int. J. Mol. Sci. 2022, 23, 3105. [Google Scholar] [CrossRef]
- Du, H.; Liu, J.; Pan, B.; Yang, H.-Y.; Liu, G.-B.; Lu, K. Fabrication of the low molecular weight peptide-based hydrogels and analysis of gelation behaviors. Food Hydrocoll. 2022, 131, 107751. [Google Scholar] [CrossRef]
- Oliveira, C.B.P.; Gomes, V.; Ferreira, P.M.T.; Martins, J.A.; Jervis, P.J. Peptide-Based Supramolecular Hydrogels as Drug Delivery Agents: Recent Advances. Gels 2022, 8, 706. [Google Scholar] [CrossRef]
- Okesola, B.O.; Smith, D.K. Applying low-molecular weight supramolecular gelators in an environmental setting-self-assembled gels as smart materials for pollutant removal. Chem. Soc. Rev. 2016, 45, 4226–4251. [Google Scholar] [CrossRef]
- Giuri, D.; D’Agostino, S.; Ravarino, P.; Faccio, D.; Falini, G.; Tomasini, C. Water Remediation from Pollutants Agents by the Use of an Environmentally Friendly Supramolecular Hydrogel. ChemNanoMat 2022, 8, e202200093. [Google Scholar] [CrossRef]
- Giuri, D.; Zanna, N.; Tomasini, C. Low Molecular Weight Gelators Based on Functionalized l-Dopa Promote Organogels Formation. Gels 2019, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, G.; Giuri, D.; Zanna, N.; Calvaresi, M.; Montalti, M.; Tomasini, C. Biocompatible and Light-Penetrating Hydrogels for Water Decontamination. ACS Omega 2018, 3, 8122–8128. [Google Scholar] [CrossRef]
- Skilling, K.J.; Citossi, F.; Bradshaw, T.D.; Ashford, M.; Kellam, B.; Marlow, M. Insights into low molecular mass organic gelators: A focus on drug delivery and tissue engineering applications. Soft Matter 2014, 10, 237–256. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, S.; Oh, I. Development of Antioxidant-Fortified Oleogel and Its Application as a Solid Fat Replacer to Muffin. Foods 2021, 10, 3059. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhao, Y.; Lu, Z.; Tang, Y. Amino Acid-Modified Conjugated Oligomer Self-Assembly Hydrogel for Efficient Capture and Specific Killing of Antibiotic-Resistant Bacteria. ACS Appl. Mater. Interfaces 2019, 11, 16320–16327. [Google Scholar] [CrossRef]
- Hadian, M.; Rajaei, A.; Mohsenifar, A.; Tabatabaei, M. Encapsulation of Rosmarinus officinalis essential oils in chitosan-benzoic acid nanogel with enhanced antibacterial activity in beef cutlet against Salmonella typhimurium during refrigerated storage. Lwt 2017, 84, 394–401. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Rodrigues, S.; Carvalho, A.M.; Ferreira, I.C.F.R. Development of hydrosoluble gels with Crataegus monogyna extracts for topical application: Evaluation of antioxidant activity of the final formulations. Ind. Crops Prod. 2013, 42, 175–180. [Google Scholar] [CrossRef]
- Maqsoudlou, A.; Assadpour, E.; Mohebodini, H.; Jafari, S.M. Improving the efficiency of natural antioxidant compounds via different nanocarriers. Adv. Colloid Interface Sci. 2020, 278, 102122. [Google Scholar] [CrossRef] [PubMed]
- Villalva, M.; Jaime, L.; Arranz, E.; Zhao, Z.; Corredig, M.; Reglero, G.; Santoyo, S. Nanoemulsions and acidified milk gels as a strategy for improving stability and antioxidant activity of yarrow phenolic compounds after gastrointestinal digestion. Food Res. Int. 2020, 130, 108922. [Google Scholar] [CrossRef]
- Lee, S.H.; Chow, P.S.; Yagnik, C.K. Developing Eco-Friendly Skin Care Formulations with Microemulsions of Essential Oil. Cosmetics 2022, 9, 30. [Google Scholar] [CrossRef]
- Salazar-Bautista, S.C.; Chebil, A.; Pickaert, G.; Gaucher, C.; Jamart-Gregoire, B.; Durand, A.; Leonard, M. Encapsulation and release of hydrophobic molecules from particles of gelled triglyceride with aminoacid-based low-molecular weight gelators. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 514, 11–20. [Google Scholar] [CrossRef]
- Wei, Q.; Duan, J.; Ma, G.; Zhang, W.; Wang, Q.; Hu, Z. Enzymatic crosslinking to fabricate antioxidant peptide-based supramolecular hydrogel for improving cutaneous wound healing. J. Mater. Chem. B 2019, 7, 2220–2225. [Google Scholar] [CrossRef]
- Butkeviciute, A.; Ramanauskiene, K.; Janulis, V. Formulation of Gels and Emulgels with Malus domestica Borkh: Apple Extracts and Their Biopharmaceutical Evaluation In Vitro. Antioxidants 2022, 11, 373. [Google Scholar] [CrossRef]
- Lutchmanen Kolanthan, V.; Brown, A.; Soobramaney, V.; Philibert, E.G.; Francois Newton, V.; Hosenally, M.; Sokeechand, B.N.; Petkar, G.; Moga, A.; Andres, P.; et al. Clinical Evaluation of Indian Sandalwood Oil and Its Protective Effect on the Skin against the Detrimental Effect of Exposome. Cosmetics 2022, 9, 35. [Google Scholar] [CrossRef]
- Wortzman, M.; Nelson, D.B. A comprehensive topical antioxidant inhibits oxidative stress induced by blue light exposure and cigarette smoke in human skin tissue. J. Cosmet. Dermatol. 2021, 20, 1160–1165. [Google Scholar] [CrossRef]
- Shahzad, A.; Hussain, S.; Anwar, N.; Karim, A.; Aeman, U.; Iqbal, M.J. An overview of free Radicals & antioxidants and its Deletenous actions. Front. Chem. Sci. 2021, 2, 147–164. [Google Scholar] [CrossRef]
- Temova Rakuša, Ž.; Roškar, R. Quality Control of Vitamins A and E and Coenzyme Q10 in Commercial Anti-Ageing Cosmetic Products. Cosmetics 2021, 8, 61. [Google Scholar] [CrossRef]
- Akhtar, N.; Akhtar, N. Development of stable tocopherol succinate-loaded ethosomes to enhance transdermal permeation: In vitro and in vivo characterizations. J. Cosmet. Dermatol. 2022, 21, 4942–4955. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.N.; Gupta, G.; Sharma, P. A comprehensive review of free radicals, antioxidants and their relationship with human ailments. Crit. Rev. Eukaryot. Gene Expr. 2018, 28, 139–154. [Google Scholar] [CrossRef]
- Chaudhari, A.; Dwivedi, M.K. Chapter 1—The concept of probiotics, prebiotics, postbiotics, synbiotics, nutribiotics, and pharmabiotics. In Probiotics in the Prevention and Management of Human Diseases; Dwivedi, M.K., Amaresan, N., Sankaranarayanan, A., Kemp, E.H., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 1–11. ISBN 978-0-12-823733-5. [Google Scholar]
- Zawistowska-Rojek, A.; Zaręba, T.; Tyski, S. Microbiological Testing of Probiotic Preparations. Int. J. Environ. Res. Public Health 2022, 19, 5701. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes 2020, 12, 1801944. [Google Scholar] [CrossRef]
- Ácsová, A.; Hojerová, J.; Martiniaková, S. Efficacy of postbiotics against free radicals and UV radiation. Chem. Pap. 2022, 76, 2357–2364. [Google Scholar] [CrossRef]
- Giuri, D.; Jurković, L.; Fermani, S.; Kralj, D.; Falini, G.; Tomasini, C. Supramolecular Hydrogels with Properties Tunable by Calcium Ions: A Bio-Inspired Chemical System. ACS Appl. Bio Mater. 2019, 2, 5819–5828. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, M.F.; Giuri, D.; Marchiori, G.; Maglio, M.; Pagani, S.; Fini, M.; Tomasini, C.; Panzavolta, S. Self-assembling of fibers inside an injectable calcium phosphate bone cement: A feasibility study. Mater. Today Chem. 2022, 24, 100991. [Google Scholar] [CrossRef]
- Gaucher, A.; Dutot, L.; Barbeau, O.; Hamchaoui, W.; Wakselman, M.; Mazaleyrat, J.P. Synthesis of terminally protected (S)-β3-H-DOPA by Arndt-Eistert homologation: An approach to crowned β-peptides. Tetrahedron Asymmetry 2005, 16, 857–864. [Google Scholar] [CrossRef]
- Tangpromphan, P.; Duangsrisai, S.; Jaree, A. Development of separation method for Alpha-Tocopherol and Gamma-Oryzanol extracted from rice bran oil using Three-Zone simulated moving bed process. Sep. Purif. Technol. 2021, 272, 118930. [Google Scholar] [CrossRef]
- Patel, A.R.; Remijn, C.; Heussen, P.C.M.; Den Adel, R.; Velikov, K.P. Novel low-molecular-weight-gelator-based microcapsules with controllable morphology and temperature responsiveness. ChemPhysChem 2013, 14, 305–310. [Google Scholar] [CrossRef]
- Sadeer, N.B.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The versatility of antioxidant assays in food science and safety—Chemistry, applications, strengths, and limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef] [PubMed]
- Nikiforidis, C.V.; Scholten, E. Self-assemblies of lecithin and α-tocopherol as gelators of lipid material. RSC Adv. 2014, 4, 2466–2473. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, T.; Chang, M.; Lu, M.; Liu, R.; Jin, Q.; Wang, X. Effects of interaction between α-tocopherol, oryzanol, and phytosterol on the antiradical activity against DPPH radical. LWT 2019, 112, 108206. [Google Scholar] [CrossRef]
- Müller, L.; Theile, K.; Böhm, V. In vitro antioxidant activity of tocopherols and tocotrienols and comparison of vitamin E concentration and lipophilic antioxidant capacity in human plasma. Mol. Nutr. Food Res. 2010, 54, 731–742. [Google Scholar] [CrossRef]
- Iwatsuki, M.; Tsuchiya, J.; Komuro, E.; Yamamoto, Y.; Niki, E. Effects of solvents and media on the antioxidant activity of α-tocopherol. Biochim. Biophys. Acta—Gen. Subj. 1994, 1200, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Averina, O.V.; Poluektova, E.U.; Marsova, M.V.; Danilenko, V.N. Biomarkers and Utility of the Antioxidant Potential of Probiotic Lactobacilli and Bifidobacteria as Representatives of the Human Gut Microbiota. Biomedicines 2021, 9, 1340. [Google Scholar] [CrossRef] [PubMed]
- Bourebaba, Y.; Marycz, K.; Mularczyk, M.; Bourebaba, L. Postbiotics as potential new therapeutic agents for metabolic disorders management. Biomed. Pharmacother. 2022, 153, 113138. [Google Scholar] [CrossRef] [PubMed]
- Ahire, J.J.; Jakkamsetty, C.; Kashikar, M.S.; Lakshmi, S.G.; Madempudi, R.S. In Vitro Evaluation of Probiotic Properties of Lactobacillus plantarum UBLP40 Isolated from Traditional Indigenous Fermented Food. Probiotics Antimicrob. Proteins 2021, 13, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Hajipour, N.; Hasannezhad, P.; Baghbanzadeh, A.; Aghebati-Maleki, L. Potential in vivo delivery routes of postbiotics. Crit. Rev. Food Sci. Nutr. 2022, 62, 3345–3369. [Google Scholar] [CrossRef]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Taccardi, D.; Scribante, A. Home Oral Care of Periodontal Patients Using Antimicrobial Gel with Postbiotics, Lactoferrin, and Aloe Barbadensis Leaf Juice Powder vs. Conventional Chlorhexidine Gel: A Split-Mouth Randomized Clinical Trial. Antibiotics 2022, 11, 118. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toronyi, A.Á.; Giuri, D.; Martiniakova, S.; Tomasini, C. Low-Molecular-Weight Gels as Smart Materials for the Enhancement of Antioxidants Activity. Cosmetics 2023, 10, 38. https://doi.org/10.3390/cosmetics10020038
Toronyi AÁ, Giuri D, Martiniakova S, Tomasini C. Low-Molecular-Weight Gels as Smart Materials for the Enhancement of Antioxidants Activity. Cosmetics. 2023; 10(2):38. https://doi.org/10.3390/cosmetics10020038
Chicago/Turabian StyleToronyi, Aneta Ácsová, Demetra Giuri, Silvia Martiniakova, and Claudia Tomasini. 2023. "Low-Molecular-Weight Gels as Smart Materials for the Enhancement of Antioxidants Activity" Cosmetics 10, no. 2: 38. https://doi.org/10.3390/cosmetics10020038
APA StyleToronyi, A. Á., Giuri, D., Martiniakova, S., & Tomasini, C. (2023). Low-Molecular-Weight Gels as Smart Materials for the Enhancement of Antioxidants Activity. Cosmetics, 10(2), 38. https://doi.org/10.3390/cosmetics10020038