Post-Instrumentation Dentinal Microcracks Induced by Two NiTi Rotary Systems with Increased Super Elasticity and Shape Memory: A MicroCT Comparative and Methodological Ex Vivo Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Selection and Root Canal Preparation
2.2. MicroCT Scanning
2.3. Root Canal Preparation
2.4. Sectioning and Microscope Analysis
2.5. Evaluation of the Microcracks
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Endodontists, A.A. Endodontics: Colleagues for Excellence-Cracking the Cracked Tooth Code; Fall-Winter: Chicago, IL, USA, 2008. [Google Scholar]
- Chai, H.; Tamse, A. Vertical Root Fracture in Buccal Roots of Bifurcated Maxillary Premolars from Condensation of Gutta-Percha. J. Endod. 2018, 44, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Afrashtehfar, K.I.; MacDonald, D. Vertical tooth root fracture detection through cone-beam computed tomography: An umbrella review protocol testing four hypotheses. Open Dent. J. 2019, 13, 449–453. [Google Scholar] [CrossRef]
- Al-Jadaa, A.; Alsmadi, R.F.; Salem, W.M.; Abdulridha, A.A.; Afrashtehfar, K.I. Radicular Intracanal Splitting Forces and Cutting Efficiency of NiTi Rotary versus Reciprocating Systems: A Comparative In Vitro Study. Cosmetics 2023, 10, 23. [Google Scholar] [CrossRef]
- Roylance, D. Introduction to Fracture Mechanics; Elsevier: Cambridge, MA, USA, 2001. [Google Scholar]
- Kishen, A. Biomechanics of Fractures in Endodontically Treated Teeth. Endod. Topics 2015, 33, 3–13. [Google Scholar] [CrossRef]
- Yoldas, O.; Yilmaz, S.; Atakan, G.; Kuden, C.; Kasan, Z. Dentinal Microcrack Formation during Root Canal Preparations by Different NiTi Rotary Instruments and the Self-Adjusting File. J. Endod. 2012, 38, 232–235. [Google Scholar] [CrossRef]
- Adorno, C.G.; Yoshioka, T.; Suda, H. The Effect of Root Preparation Technique and Instrumentation Length on the Development of Apical Root Cracks. J. Endod. 2009, 35, 389–392. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Afrashtehfar, K.I.; Corbella, S.; El-Kabbaney, A.; Perondi, I.; Taschieri, S. In Vivo and in vitro effectiveness of ro-tary nickel-titanium vs manual stainless steel instruments for root canal therapy: Systematic review and meta-analysis. J. Evid. Based Dent. Pract. 2018, 18, 59–69. [Google Scholar] [CrossRef]
- De-Deus, G.; Cavalcante, D.M.; Belladonna, F.G.; Carvalhal, J.; Souza, E.M.; Lopes, R.T.; Versiani, M.A.; Silva, E.J.N.L.; Dummer, P.M.H. Root Dentinal Microcracks: A Post-Extraction Experimental Phenomenon? Int. Endod. J. 2019, 52, 857–865. [Google Scholar] [CrossRef]
- De-Deus, G.; César de Azevedo Carvalhal, J.; Belladonna, F.G.; Silva, E.J.N.L.; Lopes, R.T.; Moreira Filho, R.E.; Souza, E.M.; Provenzano, J.C.; Versiani, M.A. Dentinal Microcrack Development after Canal Preparation: A Longitudinal In Situ Micro–Computed Tomography Study Using a Cadaver Model. J. Endod. 2017, 43, 1553–1558. [Google Scholar] [CrossRef]
- PradeepKumar, A.R.; Shemesh, H.; Archana, D.; Versiani, M.A.; Sousa-Neto, M.D.; Leoni, G.B.; Silva-Sousa, Y.T.C.; Kishen, A. Root Canal Preparation Does Not Induce Dentinal Microcracks In Vivo. J. Endod. 2019, 45, 1258–1264. [Google Scholar] [CrossRef]
- de Oliveira, B.P.; Câmara, A.C.; Duarte, D.A.; Heck, R.J.; Antonino, A.C.D.; Aguiar, C.M. Micro–Computed Tomographic Analysis of Apical Microcracks before and after Root Canal Preparation by Hand, Rotary, and Reciprocating Instruments at Different Working Lengths. J. Endod. 2017, 43, 1143–1147. [Google Scholar] [CrossRef] [PubMed]
- Kansal, R.; Rajput, A.; Talwar, S.; Roongta, R.; Verma, M. Assessment of Dentinal Damage during Canal Preparation Using Reciprocating and Rotary Files. J. Endod. 2014, 40, 1443–1446. [Google Scholar] [CrossRef] [PubMed]
- Peters, O.A.; Arias, A.; Paqué, F. A Micro-Computed Tomographic Assessment of Root Canal Preparation with a Novel Instrument, TRUShape, in Mesial Roots of Mandibular Molars. J. Endod. 2015, 41, 1545–1550. [Google Scholar] [CrossRef] [PubMed]
- Arias, A.; Paqué, F.; Shyn, S.; Murphy, S.; Peters, O.A. Effect of Canal Preparation with TRUShape and Vortex Rotary Instruments on Three-Dimensional Geometry of Oval Root Canals. Aust. Endod. J. 2018, 44, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Uslu, G.; Özyürek, T.; Yılmaz, K.; Gündoğar, M.; Plotino, G. Apically Extruded Debris during Root Canal Instrumentation with Reciproc Blue, HyFlex EDM, and XP-Endo Shaper Nickel-Titanium Files. J. Endod. 2018, 44, 856–859. [Google Scholar] [CrossRef] [PubMed]
- TruShape|Dentsply Sirona. Available online: https://www.dentsplysirona.com/en-us/categories/endodontics/trushape.html (accessed on 12 July 2022).
- XP-Endo Shaper|XP-Endo Solutions. Available online: https://www.fkg.ch/xpendo/shaper (accessed on 12 July 2022).
- Aksoy, Ç.; Keriş, E.Y.; Yaman, S.D.; Ocak, M.; Geneci, F.; Çelik, H.H. Evaluation of XP-Endo Shaper, Reciproc Blue, and ProTaper Universal NiTi Systems on Dentinal Microcrack Formation Using Micro–Computed Tomography. J. Endod. 2019, 45, 338–342. [Google Scholar] [CrossRef]
- Zuolo, M.L.; De-Deus, G.; Belladonna, F.G.; da Silva, E.J.N.L.; Lopes, R.T.; Souza, E.M.; Versiani, M.A.; Zaia, A.A. Micro–Computed Tomography Assessment of Dentinal Micro-Cracks after Root Canal Preparation with TRUShape and Self-Adjusting File Systems. J. Endod. 2017, 43, 619–622. [Google Scholar] [CrossRef]
- Bayram, H.M.; Bayram, E.; Ocak, M.; Uzuner, M.B.; Geneci, F.; Celik, H.H. Micro–Computed Tomographic Evaluation of Dentinal Microcrack Formation after Using New Heat-Treated Nickel-Titanium Systems. J. Endod. 2017, 43, 1736–1739. [Google Scholar] [CrossRef]
- Alkahtany, S.M.; Al-Madi, E.M. Dentinal Microcrack Formation after Root Canal Instrumentation by XP-Endo Shaper and ProTaper Universal: A Microcomputed Tomography Evaluation. Int. J. Dent. 2020, 2020, 4030194. [Google Scholar] [CrossRef]
- Coelho, M.S.; Card, S.J.; Tawil, P.Z. Light-Emitting Diode Assessment of Dentinal Defects after Root Canal Preparation with Profile, TRUShape, and WaveOne Gold Systems. J. Endod. 2016, 42, 1393–1396. [Google Scholar] [CrossRef]
- Çiçek, E.; Koçak, M.M.; Saʇlam, B.C.; Koçak, S. Evaluation of Microcrack Formation in Root Canals after Instrumentation with Different NiTi Rotary File Systems: A Scanning Electron Microscopy Study. Scanning 2015, 37, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Dowker, S.E.P.; Davis, G.R.; Elliott, J.C. X-ray Microtomography Nondestructive Three-Dimensional Imaging for In Vitro Endodontic Studies. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 1997, 83, 510–516. [Google Scholar] [CrossRef] [PubMed]
- De-Deus, G.; Belladonna, F.G.; Souza, E.M.; Silva, E.J.N.L.; Neves, A.D.A.; Alves, H.; Lopes, R.T.; Versiani, M.A. Micro-Computed Tomographic Assessment on the Effect of ProTaper Next and Twisted File Adaptive Systems on Dentinal Cracks. J. Endod. 2015, 41, 1116–1119. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.S.; Pitt Ford, T.R.; Lynch, J.A.; Liepins, P.J.; Curtis, R.V. Micro-Computed Tomography: A New Tool for Experimental Endodontology. Int. Endod. J. 1999, 32, 165–170. [Google Scholar] [CrossRef]
- Siqueira, J.F.; Alves, F.R.F.; Versiani, M.A.; Rôças, I.N.; Almeida, B.M.; Neves, M.A.S.; Sousa-Neto, M.D. Correlative Bacteriologic and Micro-Computed Tomographic Analysis of Mandibular Molar Mesial Canals Prepared by Self-Adjusting File, Reciproc, and Twisted File Systems. J. Endod. 2013, 39, 1044–1050. [Google Scholar] [CrossRef]
- Ashwinkumar, V.; Krithikadatta, J.; Surendran, S.; Velmurugan, N. Effect of reciprocating file motion on microcrack formation in root canals: An SEM study. Int. Endod. J. 2014, 47, 622–627. [Google Scholar] [CrossRef]
- World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [CrossRef]
- de las Perez Morales, M.N.; González Sánchez, J.A.; Olivieri Fernández, J.G.; Laperre, K.; Abella Sans, F.; Jaramillo, D.E.; Terol, F.D.S. TRUShape Versus XP-Endo Shaper: A Micro–Computed Tomographic Assessment and Comparative Study of the Shaping Ability—An In Vitro Study. J. Endod. 2020, 46, 271–276. [Google Scholar] [CrossRef]
- Schneider, S.W.; Austin, D.D.S. A Comparison of Canal Preparations in Straight and Curved Root Canals. Oral Surg. Oral Med. Oral Pathol. 1971, 32, 271–275. [Google Scholar] [CrossRef]
- Ratcliff, S.; Becker, I.M.; Quinn, L. Type and Incidence of Cracks in Posterior Teeth. J. Prosthet. Dent. 2001, 86, 168–172. [Google Scholar] [CrossRef]
- PradeepKumar, A.R.; Shemesh, H.; Chang, J.W.W.; Bhowmik, A.; Sibi, S.; Gopikrishna, V.; Lakshmi-Narayanan, L.; Kishen, A. Preexisting Dentinal Microcracks in Nonendodontically Treated Teeth: An Ex Vivo Micro–Computed Tomographic Analysis. J. Endod. 2017, 43, 896–900. [Google Scholar] [CrossRef] [PubMed]
- Miguéns-Vila, R.; Martín-Biedma, B.; De-Deus, G.; Belladonna, F.G.; Peña-López, A.; Castelo-Baz, P. Micro-computed Tomographic Evaluation of Dentinal Microcracks after Preparation of Curved Root Canals with ProTaper Gold, WaveOne Gold, and ProTaper Next Instruments. J. Endod. 2021, 47, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Uğur Aydın, Z.; Keskin, N.B.; Özyürek, T. Effect of Reciproc Blue, XP-Endo Shaper, and WaveOne Gold Instruments on Dentinal Microcrack Formation: A Micro-Computed Tomographic Evaluation. Microsc. Res. Tech. 2019, 82, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Arashiro, F.N.; De-Deus, G.; Belladonna, F.G.; Cavalcante, D.M.; Coelho, M.S.; Silva, E.J.N.L.; Pereira, K.F.S.; da Silva, P.G.; Lopes, R.T.; Souza, E.M. Dentinal Microcracks on Freshly Extracted Teeth: The Impact of the Extraction Technique. Int. Endod. J. 2020, 53, 440–446. [Google Scholar] [CrossRef]
- Çapar, İ.D.; Gök, T.; Uysal, B.; Keleş, A. Comparison of Microcomputed Tomography, Cone Beam Tomography, Stereomicroscopy, and Scanning Electron Microscopy Techniques for Detection of Microcracks on Root Dentin and Effect of Different Apical Sizes on Microcrack Formation. Microsc. Res. Tech. 2019, 82, 1748–1755. [Google Scholar] [CrossRef]
- Harper, R.A.; Shelton, R.M.; James, J.D.; Salvati, E.; Besnard, C.; Korsunsky, A.M.; Landini, G. Acid-Induced Demineralisation of Human Enamel as a Function of Time and PH Observed Using X-ray and Polarised Light Imaging. Acta Biomater. 2021, 120, 240–248. [Google Scholar] [CrossRef]
- Floratos, S.; Kim, S. Modern Endodontic Microsurgery Concepts: A Clinical Update. Dent. Clin. N. Am. 2017, 61, 81–91. [Google Scholar] [CrossRef]
- Ghorbanzadeh, A.; Aminifar, S.; Shadan, L.; Ghanati, H.; Aminifar, S. Evaluation of Three Methods in the Diagnosis of Dentin Cracks Caused by Apical Resection. J. Dent. 2013, 10, 175. [Google Scholar]
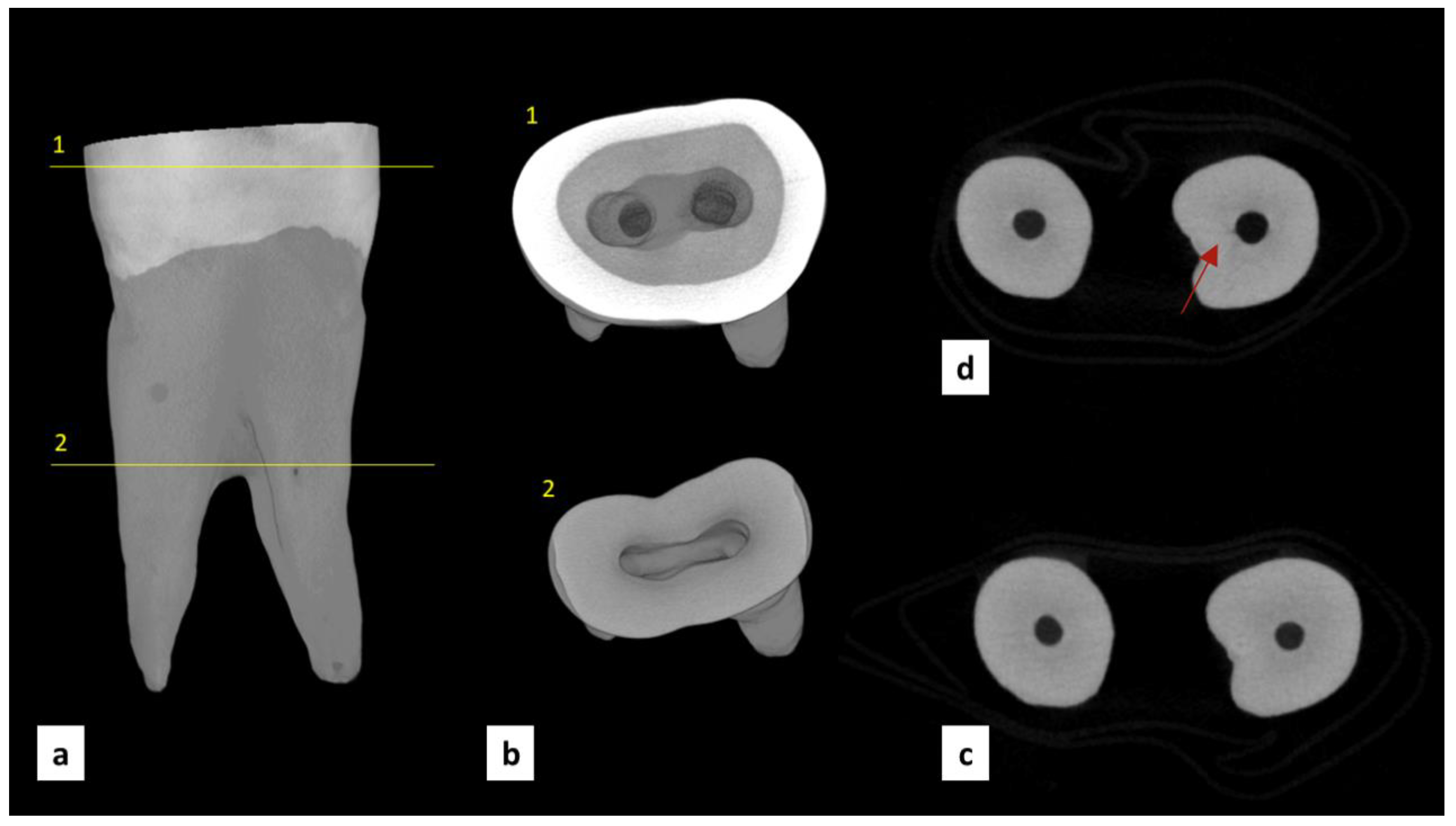
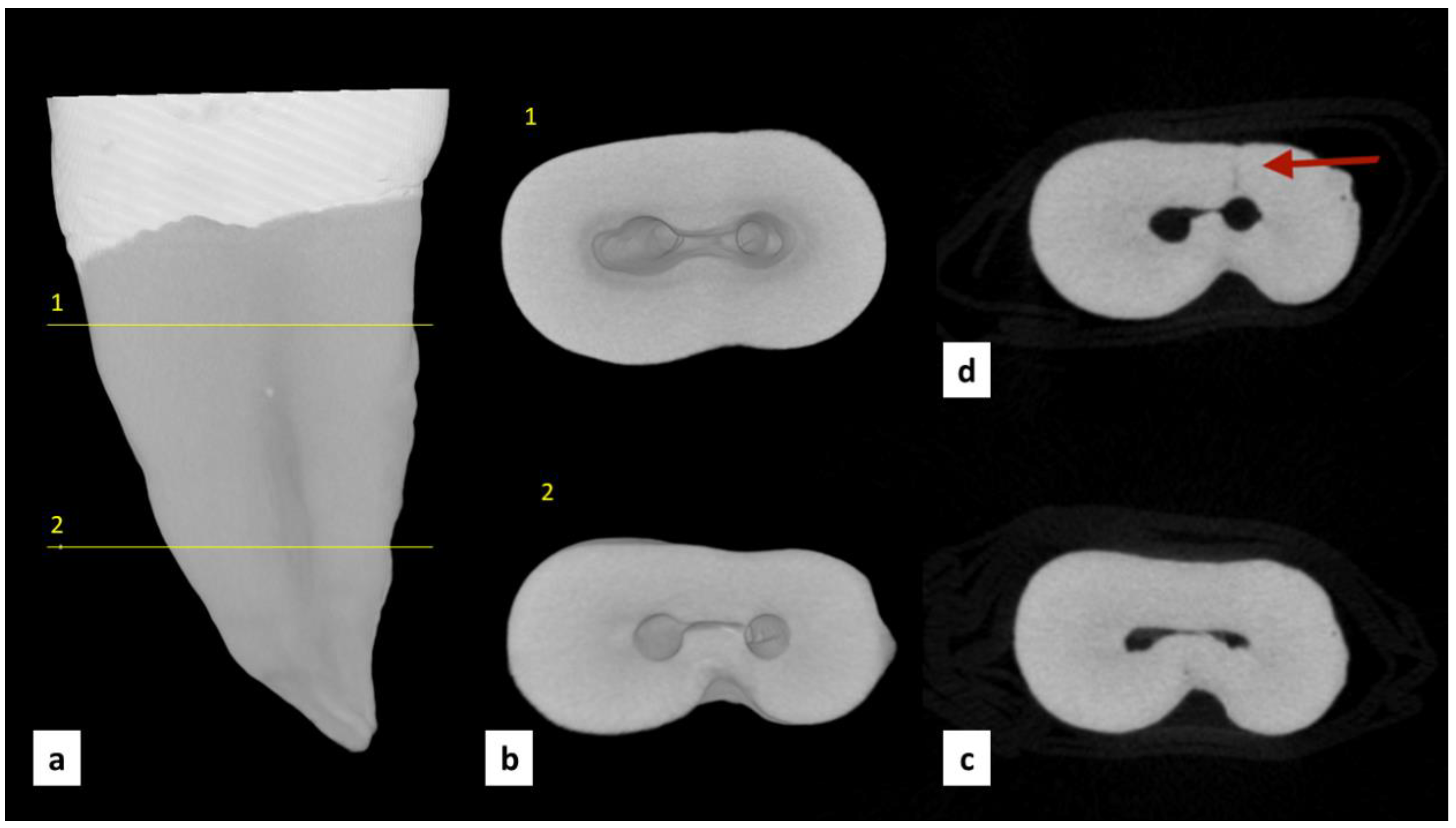
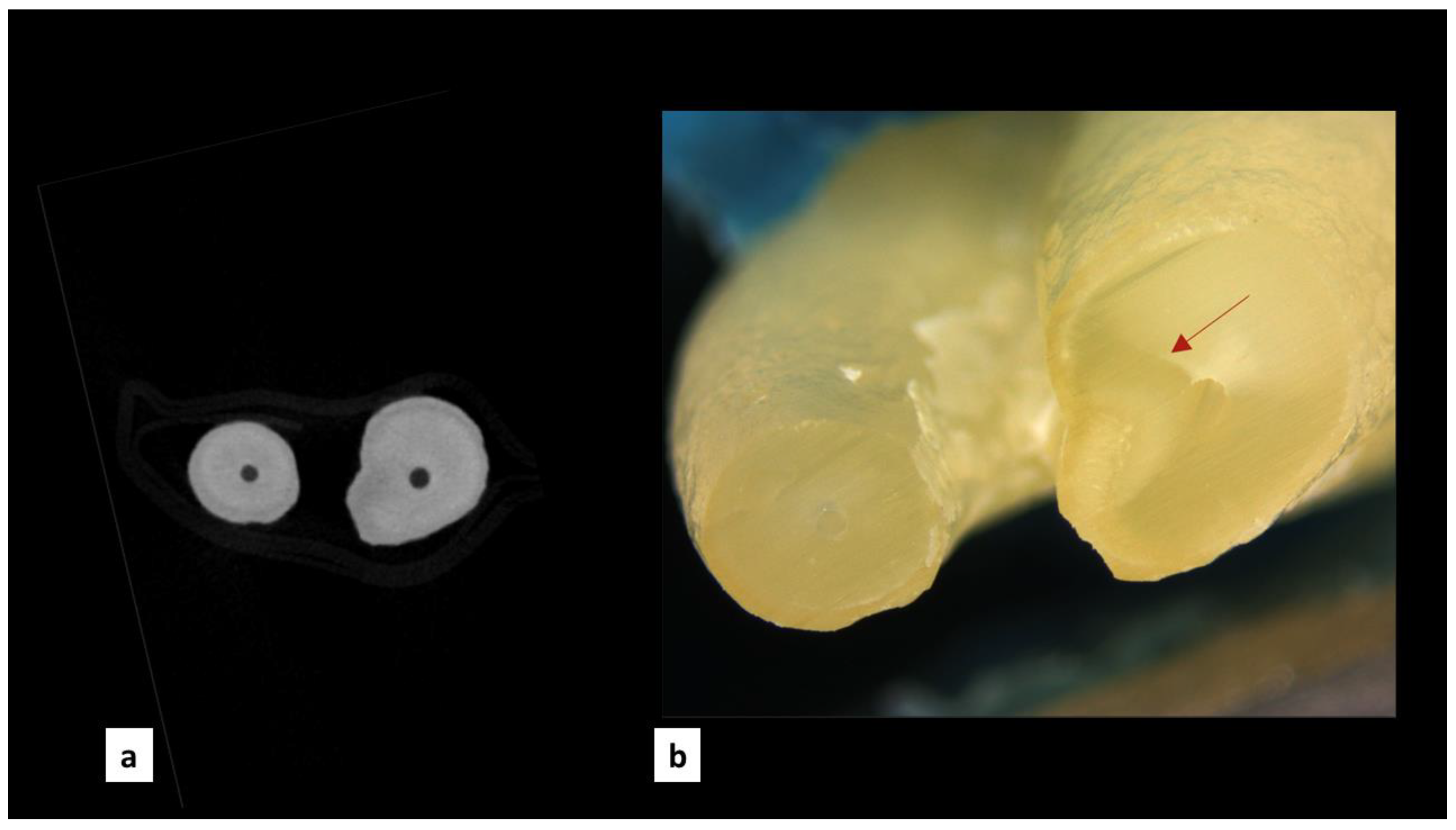
| Group | MicroCT Scans Examined (n) | Pre-Existing Dentinal Microcracks | Newly Formed Dentinal Microcracks (After Instrumentation) | ||
|---|---|---|---|---|---|
| Scans (n) | Percentage (%) | Scans (n) | Percentage (%) | ||
| TRUshape | 27,061 | 1709 | 6.3 | 30 | 0.11 |
| XP-Endo shaper | 27,061 | 2911 | 10.7 | 235 | 0.87 |
| Total | 54,122 | 4620 | 8.5 | 265 | 0.49 |
| Group | Images Obtained with Stereomicroscope after Sectioning (n) | Dentinal Microcracks Detected Only under Stereomicroscope and Not in Their Counterpart MicroCT Scans | |
|---|---|---|---|
| Images (n) | Percentage (%) | ||
| TRUshape | 33 | 5 | 15.1 |
| XP-Endo shaper | 33 | 6 | 18.1 |
| Total | 66 | 11 | 16.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmsmari, F.; Prina, J.N.; Morales, M.d.l.N.P.; Olivieri, J.G.; Durán-Sindreu, F.; González Sánchez, J.A.; Afrashtehfar, K.I. Post-Instrumentation Dentinal Microcracks Induced by Two NiTi Rotary Systems with Increased Super Elasticity and Shape Memory: A MicroCT Comparative and Methodological Ex Vivo Study. Cosmetics 2023, 10, 37. https://doi.org/10.3390/cosmetics10010037
Elmsmari F, Prina JN, Morales MdlNP, Olivieri JG, Durán-Sindreu F, González Sánchez JA, Afrashtehfar KI. Post-Instrumentation Dentinal Microcracks Induced by Two NiTi Rotary Systems with Increased Super Elasticity and Shape Memory: A MicroCT Comparative and Methodological Ex Vivo Study. Cosmetics. 2023; 10(1):37. https://doi.org/10.3390/cosmetics10010037
Chicago/Turabian StyleElmsmari, Firas, João Nuno Prina, Maria de las Nieves Perez Morales, Juan Gonzalo Olivieri, Fernando Durán-Sindreu, José Antonio González Sánchez, and Kelvin I. Afrashtehfar. 2023. "Post-Instrumentation Dentinal Microcracks Induced by Two NiTi Rotary Systems with Increased Super Elasticity and Shape Memory: A MicroCT Comparative and Methodological Ex Vivo Study" Cosmetics 10, no. 1: 37. https://doi.org/10.3390/cosmetics10010037
APA StyleElmsmari, F., Prina, J. N., Morales, M. d. l. N. P., Olivieri, J. G., Durán-Sindreu, F., González Sánchez, J. A., & Afrashtehfar, K. I. (2023). Post-Instrumentation Dentinal Microcracks Induced by Two NiTi Rotary Systems with Increased Super Elasticity and Shape Memory: A MicroCT Comparative and Methodological Ex Vivo Study. Cosmetics, 10(1), 37. https://doi.org/10.3390/cosmetics10010037







