Abstract
Atopic dermatitis (AD) is an inflamed skin condition with relapsing pruritus and cutaneous physiological dysfunction. This skin disorder is widespread around the world and frequently affects infants, children and adults. Natural products with bioactive lead compounds are the source of natural medicines for complementary and alternative therapy in managing AD. Cassia alata has been used traditionally as a remedy for a variety of health issues. In Asian countries, it is used as an ethnomedicine to treat skin conditions such pityriasis versicolor, ringworm, scabies, shingles, urticaria and itching. According to previously published studies, the phytochemicals in C. alata may have a wide range of significant pharmacological effects. AD management is highlighted here, as this review explores the literature on the pharmacological effects of C. alata and its phytochemical content. Specifically, antibacterial, wound healing, anti-inflammatory and antioxidant effects are reviewed and discussed in relation to AD management.
1. Introduction
Atopic dermatitis (AD), also referred to as eczema, is a chronic inflammatory skin condition that affects 1–3% of adults and 15–20% of children worldwide [1]. This chronic skin condition is frequently linked to allergic rhinitis, asthma and food allergies. The defining characteristic of AD is itchiness, which causes scratching, which further irritates the skin and may potentially make AD worse, because itchiness is a crucial component of the cycle of itch–scratch behaviour [2]. Due to its chronic nature, AD negatively impacts the sufferer’s quality of life by increasing medical costs, causing absence from work and school, decreasing productivity and disrupting sleep [1,3].
The onset of AD is reported to mainly be at five years of age, often disappearing by adulthood. It is estimated that in the United States, 17% of children in the 6–12 year old age group suffer from AD [4]. However, the disease may persist in 10–30% of cases. There is a high percentage of adults in the United States who experience the onset of the disease during adulthood (≥18 years of age) (41.9%) and 24.4% after the age of 50 [5]. In Singapore, Malaysia and north-eastern Thailand, the lifetime prevalence of chronic rash in 5–15 year old children is about 12.5%, 17.6%, and 17.2%, respectively. The prevalence stabilised at the age of 12–15 in Singapore and 13–14 in Malaysia and north-eastern Thailand [4,6,7]. Hadi et al. reported the prevalence among children and adolescents in Taiwan, China, Japan, South Korea, and Malaysia, which differed according to age group and country (Table 1). Since the studies examined different age groups in populations, direct comparisons of the prevalence among children in these counties are difficult.

Table 1.
Prevalence of atopic dermatitis in Asian countries.
Traditional medicine is believed to be generally safer when compared with synthetic medications and modern treatments due to fewer side effects. Traditional medicine mainly comprises plants or plant extracts as a natural source of medication arising from the wide range of phytochemicals and bioactive molecules present. Cassia alata (Figure 1), from the Leguminosae family, has been traditionally used to treat skin disorders. The plant is antipruritic and has traditionally been used to treat ringworm, eczema, and scabies [20]. The plant is also well known for its laxative effect to treat constipation and gastrointestinal disorders [20]. It has potential as an alternative for managing AD due to the presence of phytochemicals such as carotenoids [21,22], polyphenols [23,24,25], flavonoids [23,26], alkaloids [23,26], terpenoids [21], anthraquinone derivatives [21,23,26], glycosides [23,26], fatty acids [23,27], and phytosterols [21]. These can have antimicrobial, antioxidant, anti-inflammatory and wound healing effects that can heal and alleviate AD. These pharmacological properties are extremely important in facilitating AD management.

Figure 1.
Cassia alata plant. The figure is adapted from Chew et al. [6], under the Creative Commons Attribution License.
2. Botanical Description and Classification
C. alata (Senna alata) is also known as ringworm cassia (Figure 1). This plant is perennial and is known as a ‘candle bush’ due to its shape and inflorescences [6]. The term ringworm plant recognises the effectiveness and popularity of its leaves in the traditional treatment of ringworm [28]. This plant is also known as a craw-craw plant, ringworm bush, empress candle plant, emperor’s candlesticks, yellowtop weed or seven golden candles, in various communities [26,29,30,31].
C. alata is a tropical shrub of 3 to 4 m tall. The flower is yellow and blooms from the bottom to the top. The petals are arranged in a vertical column. Each dark purple to black-coloured winged seed pod has four sides. The pod is 25 cm long and 2.8 cm in width. It holds 50–60 triangular to square-shaped seeds. The seeds are green in colour when they are unripe, turning black when they ripen [32]. C. alata has a rachis as the main stem of a compound leaf. The leaf has an oblong shape about 5–16 cm long and 3–8 cm wide. They exist in 6–12 pairs [33].
3. Ethnomedical Uses
C. alata offers many ethnomedicinal benefits. Various communities across many countries have used it as a traditional medicine to treat various diseases and complications, and for various purposes (Table 2).

Table 2.
Ethnomedicinal uses of Cassia alata.
4. Phytochemistry
Numerous phytochemical compounds have been reported in C. alata, such as carotenoids, four phenolic acids, 20 flavonoids, two alkaloids, seven terpenoids, 17 anthraquinones, four glycosides, 28 fatty acids and two phytosterols (Table 3). Not all phytochemicals exist in all parts of the plant. Some are variously present in seeds, roots, and flowers, but most are reported in the leaves. Consequently, the leaves are the most used part of the plant for disease treatments. These phytochemicals exhibit valuable pharmacological activities that support the management of AD. Selected phytochemicals with documented beneficial pharmacological activities are highlighted and discussed in this review.

Table 3.
Phytochemical compounds in Cassia alata.
5. Pharmacological Properties
C. alata possesses numerous pharmacological properties, including antioxidant, antimicrobial, anti-inflammatory and wound healing. These pharmacological activities are essential in managing AD. In general, the antimicrobial activity of C. alata could prevent secondary infections caused by microbes, especially Staphylococcus aureus [40,41,42]. The antimicrobial activity also contributes to wound healing, closure, and recovery of AD skin lesions [43]. The plant has also been shown to inhibit inflammation through suppression of pro-inflammatory cytokines and through upregulation of the levels of anti-inflammatory-related transcription factors, enzymes, and cytokines [44]. Antioxidants such as phenolics, flavonoids and anthraquinones protect the skin from damage, scavenge free radicals, overcome oxidative stress and inhibit inflammation [45,46,47].
5.1. Antimicrobial Activity
AD patients often have dry and weakened skin barriers. The weakened barriers allow penetration of microorganisms and allergens through the skin. Microorganisms, including dermatophytes, parasitise the keratinised tissue of the skin. They exacerbate the condition due to secondary infection and play a significant role in the aetiology of AD. The skin condition is worsened due to microbial invasion and infection. According to many research studies, S. aureus is the most common microorganism that causes skin infection in AD [40,41,42]. This bacterium is closely associated with the pathogenesis and severity of the disease. When the patient’s skin is massively colonised by S. aureus, it triggers a cascade of inflammatory responses on the skin by releasing high amounts of allergens [48]. As such, antibiotic therapy is one of the essential elements in managing AD [49]. However, the overuse of existing antibiotics could cause the emergence of antibiotic-resistant bacteria. The popularity of plant products with antimicrobial properties has increased in the past decade. They are rich in numerous phytochemicals, including tannins, terpenoids, alkaloids, and flavonoids, which exhibit antimicrobial properties. Some patients are turning to alternative therapies to manage the disease by using plant-derived ingredients as complementary and alternative treatments for AD.
C. alata has been used traditionally to treat various microbial infections. Several studies have proven that it has excellent antimicrobial activity against S. aureus. The alcoholic and aqueous extracts of roots, barks, stems and leaves exhibit moderate antimicrobial activity toward S. aureus [50,51,52]. Iraqui et al. formulated leaf fractions into a hydrogel and the antimicrobial activity was tested against S. aureus [53]. The formulated hydrogel exhibited prominent antimicrobial efficacy. Its antimicrobial effect was better than Renicol and Daktarin, two commercial antimicrobial formulations selected in the study [53]. The authors also performed the hydrogel’s bioburden study in an in vivo model. They discovered that the tissue homogenate from the treatment group consisted of fewer microbial cells. This showed that it killed the wound-causing microbe and reduced the bacteria and fungus-causing infections. Since the reduction in the number of microbial cells in the excision wound was positively correlated with the number of infections [53], the wound would heal faster than the group treated with commercial antimicrobial formulations.
Several studies have investigated the antimicrobial activity of the phytochemicals recovered from C. alata, particularly towards S. aureus. Kaempferol and aloe emodin from C. alata exhibited excellent antimicrobial activity toward multidrug-resistant S. aureus (MIC50 13.0 ± 1.5 μg/mL and 12.0 ± 1.5 μg/mL, respectively). In comparison with the other two flavonoids found in this plant, kaempferol 3-O-β-glucopyranoside (Astragalin) and kaempferol 3-O-gentiobioside had lower potency (MIC50 values of 83.0 ± 0.9 μg/mL and 560.0 ± 1.2 μg/mL, respectively) [54]. On the other hand, gallic acid, caffeic acid, cannabinoid dronabinol, rhein and fatty acids in the leaves and seeds could also exhibit antibacterial activity against S. aureus [24,39,55,56].
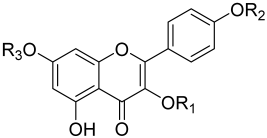
Some studies have reported the antimicrobial mechanism and the structure–activity relationship of C. alata phytochemicals. Phenolic acids in C. alata exhibited significant antimicrobial activity, either alone or in combination with antibiotics [56,57]. Gallic acid and caffeic acid of C. alata targeted the bacterial surface, affecting bacterial susceptibility, altering the membrane fluidity and integrity, and changing the physiochemical properties of the bacteria, resulting in potassium leakage and hence cell death [57,58,59,60]. Various studies have reported that the alkyl chain length of the phenolic acids is correlated with antimicrobial activity [56,57,58,61]. The longer the alkyl chain is, the stronger the antimicrobial activity toward S. aureus will be [56,57,58,61]. Other studies have also reported that the structure–activity relationship of the phytochemicals is closely associated with antimicrobial activity. For instance, a study by Hazni et al. evaluated the structure–activity relationship of kaempferol and its derivatives with antimicrobial potency against S. aureus. It was reported that the free hydroxyl group at the C-3 position of kaempferol and its derivatives is essential for exhibiting antimicrobial activity. In addition, the size of the R1 side group of the kaempferol derivatives may affect the antimicrobial potency (Table 4). The bigger the group size at the R1 position is, the weaker the antimicrobial potency [54].

Table 4.
Structure of kaempferol, kaempferol 3-O-β-glucopyranoside and kaempferol 3-O-gentiobioside and their antimicrobial potency against MRSA.
Quercetin in C. alata exhibited promising antibacterial activity. The activity was demonstrated by the multiple hydroxyl groups in the chemical structure [62,63,64,65]. Phosphorylation and sulfation of the hydroxyl groups exhibited antimicrobial activity affecting the functioning of peptides, proteins, and ion channels embedded in the membrane [64,66]. In addition, quercetin could also disrupt the cell wall and alter the bacterial cell membrane integrity, and causing the cell to die [67]. Rhein in C. alata also exhibited relatively strong antibacterial activity against S. aureus (MIC 4 µg/mL; MIC90 8 µg/mL). It acts as the substrate for many transporters, where it regulates the expression of transporter genes [68], altering the bacteria’s biological mechanism. The genes regulated by rhein include ferrichrome, siderophore, and heme transport. Upregulation of these genes suppressed the proliferation and killed the bacteria. Furthermore, rhein also inhibits aerobic and anaerobic respiration of bacteria. It regulates the metabolism of amino acids, detoxification, and pathogenic factors [68] which ultimately inhibit the growth of S. aureus.
5.2. Wound Healing
Wound healing is a natural process of regenerating dermal and epidermal tissue. A normal wound healing process goes through three phases in response to tissue injury: inflammatory, proliferative and remodelling phases [69]. Normal wound healing response is initiated immediately when the platelets come in contact with the exposed collagen at the injury site. Patients with AD flare-ups on their skin experience an itch–scratch cycle. Excessive scratching of the rash due to itchiness causes skin damage and injury [70] and slow wound recovery. Open wounds allow bacteria, viruses, and fungi to enter the skin, resulting in persistent infections. Consequently, wound healing for AD patients tends to be more challenging and complicated than for healthy individuals.
Wound healing is related to antimicrobial activity. Caffeic acid in C. alata has a broad spectrum of antibacterial activity, fighting both Gram-positive and Gram-negative bacteria. Antibacterial activity favours the wound-healing process [43]. An earlier study by Palanichamy et al. evaluated the antimicrobial effect of C. alata ethanolic leaf extracts in a rabbit wound healing model. The wound was inoculated with S. aureus so that the efficacy of the antimicrobial activity of the extract in wound closure could be evaluated. C. alata leaf extract was prepared in an ointment and applied to the treatment group animals for 21 days. The authors reported that the application of the ointment showed satisfactory wound healing. The wound surface area decreased significantly during the 21 days treatment period (58.8, 79.0, and 88.9% reduction in wound surface area after 7, 14, and 21 days, respectively) [71]. The negative control group (treated with ointment but without any antimicrobial agent) only showed a 57.1% decrease in wound surface area after 21 days. The positive control group treated with 0.2% nitrofurazone ointment had a 98.8% reduction in wound surface area after 14 days. Although the antimicrobial and wound-healing activities of C. alata leaf extract were not as prominent as those of nitrofurazone, it is an effective natural antimicrobial agent for AD wound management.
Another wound-healing study conducted by Midawa et al. also supported evidence that C. alata leaf extract facilitated wound closure in animal models [72]. Excision wounds in a rat model were treated with 125, 250, and 500 mg of leaf extracts twice daily until complete wound closure. They discovered that wound recovery had improved significantly. The wound healing rate was positively correlated with the amount of extract. The duration of epithelialisation (ca. 16 days) was significantly shorter than that of the negative control group (ca. 23 days). The authors commented that the promotion of wound healing could be due to the antimicrobial effect of C. alata, which is in agreement with Palanichamy et al. [71] and Iraqui et al. [53]. Iraqui et al. observed that wound recovery and epithelialisation improved significantly with the application of C. alata leaf hydrogel [53]. The authors also agreed that the antimicrobial properties promoted wound recovery. Wound infection by bacterial and fungal cells was also significantly reduced. Thus, whilst the wound-healing properties of C. alata have apparently been proven, the mechanism of tissue contraction during wound healing is not completely understood.
Alkaloids, terpenoids, flavonoids, anthraquinones and tannins could modulate the activity of fibroblasts and keratinocytes, promote collagen synthesis, and regulate the expression of cytokines and growth factors [6]. Oleic acid and linoleic acid in C. alata leaves modulate the inflammatory responses for tissue repair and wound closure [6,73,74]. They inhibit the accumulation of leukocytes at lesions and cationic serine protease activity in wounds, regulate the expression of metalloproteinase (MMP) and tissue inhibitor of MMP (TIMP) and promote wound closure in tissue remodelling [6,73,74]. The expression of TIMPs counter-regulate the MMPs and prevent exacerbated injury during inflammation [75]. Oleic acid also promotes angiogenesis, increases the collagen III expression during the final inflammatory phase of tissue repair and accelerates wound healing [73]. Wound healing is an essential aspect of managing AD.
5.3. Anti-Inflammatory
C. alata exhibits promising anti-inflammatory activity in both in vitro and in vivo models (Table 5). Excellent anti-inflammatory activity was noted in the carrageenan-induced mouse paw oedema model. Mice in the treatment group were fed orally with 5 mg/20 g C. alata extracts. Results showed a significant reduction in inflammation of the mouse paw after the treatment [76]. This finding is also supported by Lewis et al. [77], who studied the anti-inflammatory action of C. alata using the in vivo rheumatic arthritis model. C. alata leaves were found to inhibit the production of tumour necrosis factor-alpha (TNF-α) by immature dendritic cells in a dose-dependent manner. TNF-α regulates pro-inflammatory cytokine release and increases lipid signal transduction mediators, prostaglandins, and platelet-activating factors. It is crucial in the development of chronic inflammatory diseases. The leaves of C. alata have the ability to inhibit the inflammatory pathway, which includes TNF-α and other inflammatory cytokines. Astragalin in C. alata leaves regulates the anti-inflammatory pathways through controlling the levels of anti-inflammatory-related transcription factors, enzymes, and cytokines such as TNF-α, inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), prostaglandin E2 (PGE2), matrix metalloproteinase-1 (MMP-1), MMP-3, interleukin-1β (IL-1β), IL-4, IL-6, IL-8, IL-13 and interferon-gamma (IFN-γ) [44]. Other studies reported the anti-inflammatory activity of astragalin; some studies used in in vitro models, such as RAW 264.7, human gingival epithelial cells, and mouse uterine endometrial epithelial cells. Some studies of the anti-inflammatory action using animal models such as diabetic rats, BALB/c, and NC/Nga mice are reported in Table 5.

Table 5.
Anti-inflammatory activities of astragalin in vitro and in vivo, reproduced from Riaz et al. [44]. The table reproduced is under the Creative Commons Attribution License.
5.4. Antioxidant Activities
Increased oxidative stress and a reduction in antioxidants are significant contributing factors in the pathogenesis of AD [78]. Free radicals are believed to disrupt the defence and restoration mechanisms, increasing lipid peroxidation and decreasing the levels of antioxidants. These contribute to skin damage and disorders. The supplementation of natural antioxidants such as phenolic compounds in AD management are expected to significantly improve the skin’s condition, protect the skin from oxidative stress and inhibit the inflammatory response.
C. alata is rich in polyphenols, compounds that comprise aromatic rings bearing one or more hydroxyl groups. Some are simple phenols, i.e., phenolic acids and phenol derivatives. Some have complex structures, including flavones, flavonoids, anthocyanins, and anthraquinones. Polyphenols exhibit strong free radical and oxidative agent scavenging activity against nitric oxide, hydrogen peroxide, the superoxide anion, 1, 1-diphenyl-2-picrylhydrazyl (DPPH), and 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) [23,79,80,81,82]. Aqueous extract of C. alata showed promising antioxidant activities. The extract inhibited 50% of the DPPH free radical at IC50 2.25 ± 0.28 μg/mL. The scavenging activity was even more potent than the antioxidant standards, ascorbic acid (IC50 = 3.99 ±0.09 μg/mL) and Trolox (IC50 = 4.50 ± 0.08 μg/mL) [36]. Besides free radical scavenging, studies have also reported that C. alata showed promising reducing power and lipid peroxidation inhibition effects [23,80]. Increased oxidative stress and free radical action can overwhelm the skin’s defence and contribute to skin disorders.
Malondialdehyde (MDA) is an oxidant and a product of lipid peroxidation. MDA is the most frequently measured marker of oxidative stress. A significantly higher level of MDA and a lower level of antioxidant enzymes and antioxidants were noted in AD patients compared with healthy controls [45]. High serum MDA causes redox imbalances. Thus, restoring the redox balance is crucial for treating skin inflammation. Reduction of MDA may be achieved by supplementation of antioxidants with reducing power. Phenolics and flavonoids present in C. alata potentially exhibit good antioxidant activities, such as free radical scavenging, hydrogen donating ability, and reducing power. Numerous antioxidants are present in C. alata, including gallic acid, caffeic acid, kaempferol and its derivatives, quercetin, luteolin, apigenin, naringenin, rhein, emodin and aloe-emodin. As reported by Vargas et al., hydroxyanthraquinones such as rhein, emodin, and aloe emodin are prominent antioxidative compounds [46]. The free radical and reactive oxygen species’ scavenging abilities were evaluated using isoluminol-enhanced chemiluminescence with horseradish peroxidase and luminol-enhanced chemiluminescence with hydrogen peroxide or ferrous iron. The reactive oxygen species released were reduced by these hydroxyanthraquinones. Emodin had the highest reactive oxygen species scavenging activity, followed by rhein and aloe emodin [46]. The free radical scavenging activity of these three hydroxyanthraquinones was also studied, with the scavenging activity being concentration dependent. Rhein and emodin exhibited more potent activity than vitamins C, and E. Lipid peroxidation is closely associated with the level of MDA and severity of AD [45]. The antioxidative activity delays lipid peroxidation by 10–15%.
Phenolic acids such as gallic acid, caffeic acid, quercetin, and naringenin were observed to reduce oxidative stress and improve skin condition and texture [47]. Nowak et al. recently reported that phenolic acids including gallic acid, chlorogenic acid, 3,4-dihydroxybenzoic acid, 4-hydroxybenzoic acid and caffeic acid could penetrate and accumulate in porcine skin tested via Franz diffusion cell measurements [83]. The concentration of antioxidants and free radical scavenging activity evaluated after skin extraction was higher than the acceptor fluid, which supported the proposal that phenolic acids could penetrate the skin, accumulate and exhibit antioxidant activity, suggesting improvement in the endogenous cutaneous protection system and a reduction in skin oxidative stress through the targeting of multiple pathways to overcome skin inflammation, manage bacterial skin infections and accelerate wound recovery. Sikora et al. developed a topical hydrating product formulated with C. alata [84]. A pilot clinical study tested the product’s efficacy on human subjects. Subjects were exposed to harmful ultraviolet rays to induce oxidative stress in the skin. The study showed that antioxidants in C. alata could protect the skin against oxidative stress. Skin conditions improved significantly throughout the 12 weeks of treatment, including skin inflammation (24%), skin dryness (30%), and skin texture (18%). The antioxidative protection was mainly exhibited by the water-soluble, lipid-soluble and enzymatic antioxidants [84]. It is believed that the antioxidative effects would also effectively manage the skin condition in AD.
6. Conclusions
Based on this literature review, it is confirmed that C. alata exhibits various pharmacological properties supporting AD management and healing. Although numerous studies have reported various activities that could effectively manage skin conditions in AD, no AD-related model studies have been reported. It is recommended that the relevant AD models, such as keratinocyte monolayer cultures, immune cells, reconstructed human epidermis and full-thickness human skin equivalents, and a 3-dimensional challenged model should be used in in vitro studies to further determine the effects of C. alata in skin disorder management. In addition, it is proposed that studies using in vivo models, such as inbred mice with spontaneous eczematous dermatitis, NC/NGA mutant mice and sensitised mice models, could be investigated to confirm the effectiveness of this plant and its phytochemicals in treatment. These studies could unlock the potential of C. alata and its phytochemicals as evidence-based complementary and alternative medicine in AD treatment.
Author Contributions
Conceptualization, Y.-L.C. and J.-A.-L.Y.; writing—original draft preparation, J.-A.-L.Y., S.-K.L. and Y.-L.C.; writing—review and editing, J.-A.-L.Y., Y.-L.C., S.-K.L. and J.-W.K.; review and correction, S.-K.L., J.-W.K., K.-B.L., S.-C.C., S.-S.T., W.M.S.M., P.J.M., G.A.A., L.C.M. and B.H.G. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to acknowledge the support of the Fundamental Research Grant Scheme (FRGS) (FRGS/1/2021/STG02/UCSI/02/1) from the Ministry of Higher Education (MOHE), Malaysia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Goh, Y.-Y.; Keshavarzi, F.; Chew, Y.L. Prevalence of atopic dermatitis and pattern of drug therapy in Malaysian children. Dermatitis 2018, 29, 151–161. [Google Scholar] [CrossRef]
- Buddenkotte, J.; Steinhoff, M. Pathophysiology and therapy of pruritus in allergic and atopic diseases. Allergy 2010, 65, 805–821. [Google Scholar] [CrossRef]
- Avena-Woods, C. Overview of atopic dermatitis. Am. J. Manag. Care 2017, 23, S115–S123. [Google Scholar]
- Chew, Y.-L.; Al-Nema, M.; Ong, V.W.-M. Management and treatment of atopic dermatitis with modern therapies, complementary and alternative medicines: A review. Orient. Pharm. Exp. Med. 2018, 18, 67–76. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Vakharia, P.P.; Chopra, R.; Sacotte, R.; Patel, N.; Immaneni, S.; White, T.; Kantor, R.; Hsu, D.Y. Phenotypical differences of childhood-and adult-onset atopic dermatitis. J. Allergy Clin. Immunol. Pract. 2018, 6, 1306–1312. [Google Scholar] [CrossRef]
- Chew, Y.-L.; Khor, M.-A.; Xu, Z.; Lee, S.-K.; Keng, J.-W.; Sang, S.-H.; Akowuah, G.A.; Goh, K.W.; Liew, K.B.; Ming, L.C. Cassia alata, Coriandrum sativum, Curcuma longa and Azadirachta indica: Food ingredients as complementary and alternative therapies for atopic dermatitis—A comprehensive review. Molecules 2022, 27, 5475. [Google Scholar] [CrossRef]
- Chew, Y.-L. The beneficial properties of virgin coconut oil in management of atopic dermatitis. Pharmacogn. Rev. 2019, 13, 24. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Seok, J.; Kim, B. Correlation between socio-economic status and atopic dermatitis in Korean adults: The Korea national health and nutrition examination survey (2007–2014). J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1509–1515. [Google Scholar] [CrossRef]
- Lee, K.-S.; Rha, Y.-H.; Oh, I.-H.; Choi, Y.-S.; Choi, S.-H. Socioeconomic and sociodemographic factors related to allergic diseases in Korean adolescents based on the Seventh Korea Youth Risk Behavior Web-based Survey: A cross-sectional study. BMC Pediatr. 2016, 16, 1–9. [Google Scholar] [CrossRef]
- Ho, C.-L.; Chang, L.-I.; Wu, W.-F. The prevalence and risk factors of atopic dermatitis in 6–8 year-old first graders in Taipei. Pediatr. Neonatol. 2019, 60, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.H.; Hsieh, C.J.; Caffrey, J.L.; Lin, Y.S.; Wang, I.J.; Ho, W.C.; Chen, P.C.; Wu, T.N.; Lin, R.S. Fetal growth, obesity, and atopic disorders in adolescence: A retrospective birth cohort study. Paediatr. Perinat. Epidemiol. 2015, 29, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.-Y.; Chen, Y.-J.; Lin, M.-W.; Chen, T.-J.; Chu, S.-Y.; Chen, C.-C.; Lee, D.-D.; Chang, Y.-T.; Wang, W.-J.; Liu, H.-N. Prevalence of atopic dermatitis, allergic rhinitis and asthma in Taiwan: A national study 2000 to 2007. Acta Derm. Venereol. 2010, 90, 589–594. [Google Scholar]
- Xiao, Y.; Huang, X.; Jing, D.; Huang, Y.; Chen, L.; Zhang, X.; Zhao, S.; Zhang, M.; Luo, Z.; Su, J. The prevalence of atopic dermatitis and chronic spontaneous urticaria are associated with parental socioeconomic status in adolescents in China. Acta Derm. Venereol. 2019, 99, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, P.; Tang, J.; Han, X.; Zou, X.; Xu, G.; Xu, Z.; Wei, F.; Liu, Q.; Wang, M. Prevalence of atopic dermatitis in Chinese children aged 1–7 ys. Sci. Rep. 2016, 6, 29751. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, L.-F.; Zhao, D.-Y.; Shen, Y.-W. Prevalence and clinical features of atopic dermatitis in China. BioMed Res. Int. 2016, 2016, 2568301. [Google Scholar] [CrossRef]
- Okada, Y.; Kumagai, H.; Morikawa, Y.; Akasawa, A. Epidemiology of pediatric allergic diseases in the Ogasawara Islands. Allergol. Int. 2016, 65, 37–43. [Google Scholar] [CrossRef][Green Version]
- Futamura, M.; Ohya, Y.; Akashi, M.; Adachi, Y.; Odajima, H.; Akiyama, K.; Akasawa, A. Age-related prevalence of allergic diseases in Tokyo schoolchildren. Allergol. Int. 2011, 60, 509–515. [Google Scholar] [CrossRef]
- Yura, A.; Kouda, K.; Iki, M.; Shimizu, T. Trends of allergic symptoms in school children: Large-scale long-term consecutive cross-sectional studies in Osaka Prefecture, Japan. Pediatr. Allergy Immunol. 2011, 22, 631–637. [Google Scholar] [CrossRef]
- Lee, K.S.; Oh, I.-H.; Choi, S.H.; Rha, Y.-H. Analysis of epidemiology and risk factors of atopic dermatitis in Korean children and adolescents from the 2010 Korean national health and nutrition examination survey. BioMed Res. Int. 2017, 2017, 5142754. [Google Scholar] [CrossRef]
- Parveen, S.; Shahzad, A. A review on in vitro culture of Cassia alata Linn. (Senna alata): Analysis of metabolites and biological activities. J. Funct. Environ. Bot. 2015, 5, 78. [Google Scholar] [CrossRef]
- Abille, J. A Review on the Medical Plant Cassia alata (L.) Roxb. (Fabaceae). Available online: https://www.researchgate.net/profile/Jaydhelle-Mae-Abille/publication/334680364_A_REVIEW_ON_THE_MEDICINAL_PLANT_CASSIA_ALATA_L_ROXB_FABACEAE/links/5d39c06da6fdcc370a5fcfae/A-REVIEW-ON-THE-MEDICINAL-PLANT-CASSIA-ALATA-L-ROXB-FABACEAE.pdf (accessed on 25 September 2022).
- Abdulwaliyu, I.; Arekemase, S.; Bala, S.; Ibraheem, A.; Dakare, A.; Sangodare, R.; Gero, M. Nutritional Properties of Senna alata linn leaf and flower. Int. J. Mod. Biol. Med. 2013, 4, 1–11. [Google Scholar]
- Fatmawati, S.; Purnomo, A.S.; Bakar, M.F.A. Chemical constituents, usage and pharmacological activity of Cassia alata. Heliyon 2020, 6, e04396. [Google Scholar] [CrossRef]
- Meenupriya, J.; Vinisha, A.S.; Priya, P. Cassia alata and Cassia auriculata—Review of their bioactive potential. World J. Pharm. Sci. 2014, 1760–1769. [Google Scholar]
- Onyegeme-Okerenta, B.; Nwosu, T.; Wegwu, M. Proximate and phytochemical composition of leaf extract of Senna alata (L.) Roxb. J. Pharmacogn. Phytochem. 2017, 6, 320–326. [Google Scholar]
- Oladeji, O.S.; Adelowo, F.E.; Oluyori, A.P.; Bankole, D.T. Ethnobotanical description and biological activities of Senna alata. Evid. Based Complement. Altern. Med. 2020, 2020, 2580259. [Google Scholar] [CrossRef]
- Adigun, R.A.; Faruq, U.Z.; Birnin Yauri, U.A.; Oyeniyi, Y.J. Analysis of amino and fatty acids composition of Senna alata seed. Am. Chem. Sci. J. 2015, 7, 1–6. [Google Scholar] [CrossRef]
- Missouri Botanical Garden. Senna alata. Available online: https://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=280477&isprofile=0&cv=5 (accessed on 21 June 2022).
- Oladeji, S.O.; Adelowo, F.E.; Odelade, K.A. Mass spectroscopic and phytochemical screening of phenolic compounds in the leaf extract of Senna alata (L.) Roxb.(Fabales: Fabaceae). Braz. J. Biol. Sci. 2016, 3, 209–219. [Google Scholar] [CrossRef]
- Hennebelle, T.; Weniger, B.; Joseph, H.; Sahpaz, S.; Bailleul, F. Senna alata. Fitoterapia 2009, 80, 385–393. [Google Scholar] [CrossRef]
- Ranjanie, D.; Yuhanis, F.; Mohammed Ali, N.; Fouad Saleih, R.; Aman Shah, A. A review on Cassia alata: Pharmacological, traditional and medicinal aspects. Aust. Herb. Insight 2019, 2, E016–E021. [Google Scholar]
- National Park of Singapore. Senna alata (Linnaeus) Roxburgh. Available online: https://www.nparks.gov.sg/florafaunaweb/flora/2/4/2450 (accessed on 22 September 2022).
- West African Health Organization. West African Herbal Pharmacopoeia. Available online: https://www.wahooas.org/web-ooas/sites/default/files/publications/2185/west-african-herbal-pharmacopoeiaok.pdf (accessed on 24 September 2022).
- Schmelzer, G.H.; Gurib-Fakim, A.E. Medicinal Plants; Prota Foundation: Wageningen, The Netherlands, 2008; Volume 11. [Google Scholar]
- Gritsanapan, W.; Mangmeesri, P. Standardized Senna alata leaf extract. J. Health Res. 2009, 23, 59–64. [Google Scholar]
- Globinmed. Senna alata (L.) Roxb. Available online: https://www.globinmed.com/index.php?option=com_content&view=article&id=106145:senna-alata-l-roxb&catid=286&Itemid=357 (accessed on 23 June 2021).
- Phansawan, B.; Pongsabangpho, S. Determination of gallic acid and rutin in extracts Cassia alata and Andrographis paniculata. Sci. Asia 2014, 40, 414–419. [Google Scholar] [CrossRef]
- Rahman, M.; Ali, M.; Ali, M.; Moynul Hasan, A. Studies on the lipid and glyceride compositions of Cassia alata seed oil. Bangladesh J. Sci. Ind. Res. 2006, 41, 83–88. [Google Scholar] [CrossRef]
- Muhammad, S.L.; Wada, Y.; Mohammed, M.; Ibrahim, S.; Musa, K.Y.; Olonitola, O.S.; Ahmad, M.H.; Mustapha, S.; Abdul Rahman, Z.; Sha’aban, A. Bioassay-guided identification of bioactive compounds from Senna alata L. against methicillin-resistant Staphylococcus aureus. Appl. Microbiol. 2021, 1, 520–536. [Google Scholar] [CrossRef]
- Geoghegan, J.A.; Irvine, A.D.; Foster, T.J. Staphylococcus aureus and atopic dermatitis: A complex and evolving relationship. Trends Microbiol. 2018, 26, 484–497. [Google Scholar] [CrossRef] [PubMed]
- Blicharz, L.; Rudnicka, L.; Samochocki, Z. Staphylococcus aureus: An underestimated factor in the pathogenesis of atopic dermatitis? Postep. Dermatol. Alergol. 2019, 36, 11. [Google Scholar] [CrossRef]
- Simpson, E.L.; Villarreal, M.; Jepson, B.; Rafaels, N.; David, G.; Hanifin, J.; Taylor, P.; Boguniewicz, M.; Yoshida, T.; De Benedetto, A. Patients with atopic dermatitis colonized with Staphylococcus aureus have a distinct phenotype and endotype. J. Investig. Dermatol. 2018, 138, 2224–2233. [Google Scholar] [CrossRef]
- Kanedi, M. Healing effect of leaf extract of candlebush (Cassia alata L.) on cutaneous wound infected with Trichophyton rubrum. World J. Pharm. Life Sci. 2016, 2, 42–50. [Google Scholar]
- Riaz, A.; Rasul, A.; Hussain, G.; Zahoor, M.K.; Jabeen, F.; Subhani, Z.; Younis, T.; Ali, M.; Sarfraz, I.; Selamoglu, Z. Astragalin: A bioactive phytochemical with potential therapeutic activities. Adv. Pharmacol. Sci. 2018, 2018, 9794625. [Google Scholar] [CrossRef]
- Bertino, L.; Guarneri, F.; Cannavò, S.P.; Casciaro, M.; Pioggia, G.; Gangemi, S. Oxidative stress and atopic dermatitis. Antioxidants 2020, 9, 196. [Google Scholar] [CrossRef]
- Vargas, F.; Díaz, Y.; Carbonell, K. Antioxidant and scavenging activity of emodin, aloe-emodin, and rhein on free-radical and reactive oxygen species. Pharm. Biol. 2004, 42, 342–348. [Google Scholar] [CrossRef]
- Xu, Y.; Tang, G.; Zhang, C.; Wang, N.; Feng, Y. Gallic acid and diabetes mellitus: Its association with oxidative stress. Molecules 2021, 26, 7115. [Google Scholar] [CrossRef]
- Ogonowska, P.; Gilaberte, Y.; Barańska-Rybak, W.; Nakonieczna, J. Colonization with Staphylococcus aureus in atopic dermatitis patients: Attempts to reveal the unknown. Front. Microbiol. 2021, 11, 3468. [Google Scholar] [CrossRef] [PubMed]
- Lübbe, J. Secondary infections in patients with atopic dermatitis. Am. J. Clin. Dermatol. 2003, 4, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Karim, M.; Khan, S.N. Antibacterial activity of different organic extracts of Achyranthes aspera and Cassia alata. J. Sci. Res. 2009, 1, 393–398. [Google Scholar] [CrossRef]
- Somchit, M.; Reezal, I.; Nur, I.E.; Mutalib, A. In vitro antimicrobial activity of ethanol and water extracts of Cassia alata. J. Ethnopharmacol. 2003, 84, 1–4. [Google Scholar] [CrossRef]
- El-Mahmood, A.; Doughari, J. Phytochemical screening and antibacterial evaluation of the leaf and root extracts of Cassia alata Linn. Afr. J. Pharm. Pharmacol. 2008, 2, 124–129. [Google Scholar]
- Iraqui, P.; Chakraborty, T.; Das, M.K.; Yadav, R. Herbal antimicrobial gel with leaf extract of Cassia alata L. J. Drug Deliv. Ther. 2019, 9, 82–94. [Google Scholar] [CrossRef]
- Hazni, H.; Ahmad, N.; Hitotsuyanagi, Y.; Takeya, K.; Choo, C.-Y. Phytochemical constituents from Cassia alata with inhibition against methicillin-resistant Staphylococcus aureus (MRSA). Planta Med. 2008, 74, 1802–1805. [Google Scholar] [CrossRef]
- Paul, B.; Mitra, P.; Ghosh, T.; Salhan, R.; Singh, T.A.; Chakrabarti, A.; Gupta, S.; Basu, B.; Mitra, P.K. Isolation and structural determination of an anti bacterial constituent from the leaves of Cassia alata Linn. J. Pharmacogn. Phytochem. 2013, 2, 326–333. [Google Scholar]
- Kępa, M.; Miklasińska-Majdanik, M.; Wojtyczka, R.D.; Idzik, D.; Korzeniowski, K.; Smoleń-Dzirba, J.; Wąsik, T.J. Antimicrobial potential of caffeic acid against Staphylococcus aureus clinical strains. BioMed. Res. Int. 2018, 2018, 7413504. [Google Scholar] [CrossRef]
- Chew, Y.L.; Mahadi, A.M.; Wong, K.M.; Goh, J.K. Anti-methicillin-resistance Staphylococcus aureus (MRSA) compounds from Bauhinia kockiana Korth. And their mechanism of antibacterial activity. BMC Complement. Altern. Med. 2018, 18, 70. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.; Benfeito, S.; Soares, P.; Magalhães e Silva, D.; Loureiro, J.; Borges, A.; Borges, F.; Simoes, M. Fine-tuning of the hydrophobicity of caffeic acid: Studies on the antimicrobial activity against Staphylococcus aureus and Escherichia coli. RSC Adv. 2015, 5, 53915–53925. [Google Scholar] [CrossRef]
- Chew, Y.L.; Goh, J.K.; Arasi, C. Investigation on antibacterial activity of pyrogallol in methicillin resistant Staphylococcus aureus. Curr. Trends Biotechnol. Pharm. 2020, 14, 176–180. [Google Scholar] [CrossRef]
- Chew, Y.-L.; Arasi, C.; Goh, J.-K. Pyrogallol Induces Antimicrobial Effect and Cell Membrane Disruption on Methicillin-Resistant Staphylococcus aureus (MRSA). Curr. Bioact. Compd. 2022, 18, 38–46. [Google Scholar] [CrossRef]
- Shi, Y.-g.; Zhang, R.-r.; Zhu, C.-m.; Liang, X.-r.; Ettelaie, R.; Jiang, L.; Lin, S. On the mechanism behind enhanced antibacterial activity of alkyl gallate esters against foodborne pathogens and its application in Chinese icefish preservation. Food Microbiol. 2021, 99, 103817. [Google Scholar] [CrossRef]
- Hirai, I.; Okuno, M.; Katsuma, R.; Arita, N.; Tachibana, M.; Yamamoto, Y. Characterisation of anti-Staphylococcus aureus activity of quercetin. Int. J. Food Sci. Technol. 2010, 45, 1250–1254. [Google Scholar] [CrossRef]
- Betts, J.W.; Sharili, A.S.; Phee, L.M.; Wareham, D.W. In vitro activity of epigallocatechin gallate and quercetin alone and in combination versus clinical isolates of methicillin-resistant Staphylococcus aureus. J. Nat. Prod. 2015, 78, 2145–2148. [Google Scholar] [CrossRef]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial activity of quercetin: An approach to its mechanistic principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef]
- Fras-Zemljič, L.; Kokol, V.; Čakara, D. Antimicrobial and antioxidant properties of chitosan-based viscose fibres enzymatically functionalized with flavonoids. Text. Res. J. 2011, 81, 1532–1540. [Google Scholar] [CrossRef]
- Ahmad, A.; Kaleem, M.; Ahmed, Z.; Shafiq, H. Therapeutic potential of flavonoids and their mechanism of action against microbial and viral infections—A review. Food Res. Int. 2015, 77, 221–235. [Google Scholar] [CrossRef]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxidative Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xiang, H.; Fan, J.; Wang, D.; Yang, F.; Guo, N.; Jin, Q.; Deng, X. Global transcriptional response of Staphylococcus aureus to rhein, a natural plant product. J. Biotechnol. 2008, 135, 304–308. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Tokura, Y. Extrinsic and intrinsic types of atopic dermatitis. J. Dermatol. Sci. 2010, 58, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Palanichamy, S.; Bhaskar, E.A.; Bakthavathsalam, R.; Nagarajan, S. Wound healing activity of Cassia alata. Fitoterapia 1991, 62, 153–156. [Google Scholar]
- Midawa, S.; Ali, B.; Mshelia, B.; Johnson, J. Cutaneous wound healing activity of the ethanolic extracts of the leaf of Senna alata L. (Fabaceae). J. Biol. Sci. Conserv. 2010, 2, 63–68. [Google Scholar]
- Cardoso, C.R.; Favoreto, S., Jr.; Oliveira, L.L.; Vancim, J.O.; Barban, G.B.; Ferraz, D.B.; Silva, J.S. Oleic acid modulation of the immune response in wound healing: A new approach for skin repair. Immunobiology 2011, 216, 409–415. [Google Scholar] [CrossRef]
- Mena, S.J.; Manosalva, C.; Carretta, M.D.; Teuber, S.; Olmo, I.; Burgos, R.A.; Hidalgo, M.A. Differential free fatty acid receptor-1 (FFAR1/GPR40) signalling is associated with gene expression or gelatinase granule release in bovine neutrophils. Innate Immun. 2016, 22, 479–489. [Google Scholar] [CrossRef]
- Knight, B.E.; Kozlowski, N.; Havelin, J.; King, T.; Crocker, S.J.; Young, E.E.; Baumbauer, K.M. TIMP-1 attenuates the development of inflammatory pain through MMP-dependent and receptor-mediated cell signaling mechanisms. Front. Mol. Neurosci. 2019, 12, 220. [Google Scholar] [CrossRef]
- Villaseñor, I.M.; Canlas, A.P.; Pascua, M.P.I.; Sabando, M.N.; Soliven, L.A.P. Bioactivity studies on Cassia alata Linn. leaf extracts. Phytother. Res. 2002, 16, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.; Levy, A. Anti-inflammatory activities of Cassia alata leaf extract in complete Freund’s adjuvant arthritis in rats. West Indian Med. J. 2011, 60, 615–621. [Google Scholar] [PubMed]
- Sivaranjani, N.; Rao, S.V.; Rajeev, G. Role of reactive oxygen species and antioxidants in atopic dermatitis. J. Clin. Diagn. Res. 2013, 7, 2683–2685. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Chatterjee, S.; Dey, K.; Dutta, S. Study of antioxidant activity and immune stimulating potency of the ethnomedicinal plant, Cassia alata (L.) Roxb. Med. Aromat. Plants 2013, 2, 1000131. [Google Scholar] [CrossRef]
- Chua, L.Y.W.; Chua, B.L.; Figiel, A.; Chong, C.H.; Wojdyło, A.; Szumny, A.; Lech, K. Characterisation of the convective hot-air drying and vacuum microwave drying of Cassia alata: Antioxidant activity, essential oil volatile composition and quality studies. Molecules 2019, 24, 1625. [Google Scholar] [CrossRef]
- Sagnia, B.; Fedeli, D.; Casetti, R.; Montesano, C.; Falcioni, G.; Colizzi, V. Antioxidant and anti-inflammatory activities of extracts from Cassia alata, Eleusine indica, Eremomastax speciosa, Carica papaya and Polyscias fulva medicinal plants collected in Cameroon. PLoS ONE 2014, 9, e103999. [Google Scholar] [CrossRef]
- Casetti, F.; Bartelke, S.; Biehler, K.; Augustin, M.; Schempp, C.; Frank, U. Antimicrobial activity against bacteria with dermatological relevance and skin tolerance of the essential oil from Coriandrum sativum L. fruits. Phytother. Res. 2012, 26, 420–424. [Google Scholar] [CrossRef]
- Nowak, A.; Cybulska, K.; Makuch, E.; Kucharski, Ł.; Różewicka-Czabańska, M.; Prowans, P.; Czapla, N.; Bargiel, P.; Petriczko, J.; Klimowicz, A. In vitro human skin penetration, antioxidant and antimicrobial activity of ethanol-water extract of fireweed (Epilobium angustifolium L.). Molecules 2021, 26, 329. [Google Scholar] [CrossRef]
- Sikora, B.C.; Wortzman, M.; Nelson, D.B.; Dover, J.S. A pilot study evaluating the efficacy and tolerability of a comprehensive, hydrating topical antioxidant developed specifically for men. J. Cosmet. Dermatol. 2021, 20, 2816. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).